User login
Risks vs Benefits for SGLT2 Inhibitor Medications
Diabetes mellitus (DM) is a metabolic disorder affecting about 5% to 13% of the population in the US.1 Since 1552, the earliest record of a person with DM, many treatment advances have been made.2Sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the newest antidiabetic pharmaceuticals on the market. The SGLT2 inhibitor drugs include canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin; however, only canagliflozin, dapagliflozin, and empagliflozin have been approved by the US Food and Drug Administration (FDA). These pharmaceuticals promote glycosuria via the kidneys and enhance sugar excretion from the body. Along with lifestyle changes and self-care measures, such as healthful eating and increased physical activity, SGLT2 inhibitor pharmaceuticals provide antidiabetic efficacy by facilitating normoglycemia and minimizing vascular pathology.
Although SGLT2 inhibitor pharmaceuticals are newly introduced into the market, their discovery dates to 1835.3 Phlorizin, a nonselective SGLT inhibitor, was first isolated by French chemists from the bark of an apple tree.4 Phlorizin inhibits SGLT1 mostly in small intestinal cells, and SGLT2 similarly affects the kidney.4 Renal SGLT2 is the primary therapeutic target. Canagliflozin was the first pharmaceutical SGLT2 inhibitor approved by the FDA in 2013. Dapagliflozin’s FDA approval followed in 2013 and empagliflozin in 2014.5
Mechanism Of Action
In healthy individuals, tubular glucose is absorbed, resulting in no urinary glucose excretion. Sodium-glucose cotransporters 1 and 2 contribute to the renal absorption of glucose. A SGLT2 is responsible for 90% of the glucose reuptake in the segment 1 of the proximal tubule, while SGLT 1 is accountable for the remaining 10%.3 Unlike other antidiabetic medications, which act by increasing insulin secretion or improving insulin sensitivity for the receptors, SGLT2 inhibitor drugs prevent the reuptake of glucose into the bloodstream. This selective action spares the inhibition of SGLT1 present in other tissues, avoiding gastrointestinal effects.6
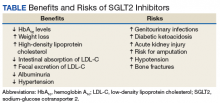
Benefits
The SGLT2 inhibitor action is focused on renal excretion of glucose and is independent of insulin action.
Hemoglobin A1c Levels
Canagliflozin, dapagliflozin, and empagliflozin reduce hemoglobin A1c (HbA1c) levels.5 Inagaki and colleagues found significant reductions in HbA1c and weight gain with > 100 mg canagliflozin compared with that of placebo when used for 12 weeks.7 In a study where 2.5-mg, 5-mg, and 10-mg dapagliflozin was compared with placebo, the mean HbA1c change from the baseline was -0.23% with placebo; -0.58% at 2.5 mg; -0.77% at 5 mg; and -0.89% at 10 mg.8 Empagliflozin was more effective in reducing HbA1c levels than was sitagliptin.9 When patients were treated with 10-mg empagliflozin, 25-mg empagliflozin, and sitagliptin, HbA1c levels dropped -1.44%, -1.43%, and -1.04%, respectively.9
Cholesterol
Sodium-glucose cotransporter 2 inhibitors have the beneficial effect of reducing vascular disease risk factors.10,11 A study by Hayashi and colleagues found that dapagliflozin decreases harmful atherogenic small, low-density lipoprotein-cholesterol (LDL-C), increases less atherogenic large, buoyant LDL-C, and increases high-density, lipoprotein-2 cholesterol (HDL-2C).10 Empagliflozin, however, can cause a small dose-dependent increase in HDL-C and LDL-C.11 Although there is an increase in serum LDL-C concentrations, empagliflozin can induce a decrease in intestinal absorption of cholesterol, thus promoting fecal excretion of LDL-C and macrophage-derived cholesterol.11
Weight Loss
A study by Weber and colleagues found that the SGLT2 inhibitor dapagloflozin lead to a reduction in body weight from -1.0 kg to -0.3 kg compared with placebo.12 Cefalu and colleagues found that daily prescribing of 100 mg and/or 300 mg of canagliflozin evidenced dose-dependent loss of weight.13 Neeland and colleagues found that empagliflozin utilization resulted in less adiposity indices in 3,300 subjects.14
Albuminuria
Sodium-glucose cotransporter 2 inhibitors have a reno-protective role in patients with type 2 DM (T2DM). In those receiving renin-angiotensin blockers with T2DM and hypertension, dapagliflozin decreased their albuminuria.15 Canagliflozin has a similar potential.16 Empagliflozin reduced the urine albumin-creatinine ratio in patients with macro- or micro-albuminuria, supporting a direct renal effect by SGLT2 inhibitors.17
Systolic Blood Pressure
Sodium-glucose cotransporter 2 inhibitors can have beneficial effects on physiologic vascular outcomes. In patients with T2DM and hypertension, dapagliflozin reduced mean systolic blood pressure (SBP) compared with placebo: -7.3 mm Hg vs -10.4 mm Hg, respectively.12 Prescribing canagliflozin treatment at 100 mg or 300 mg reduced SBP (-4.3 mm Hg and -5.0 mm Hg, respectively, vs placebo at -0.3 mm Hg).18 Subjects taking empagliflozin 10 mg or 25 mg exhibited an adjusted mean BP change from baseline of -4.60 mm Hg and -5.47mm Hg, respectively, whereas placebo induced a -0.67 mm Hg decline.19
Risks
Nausea, fatigue, polyuria, polydipsia, and xerostomia are common SGLT2 AEs. Use of SGLT2 inhibitors can induce certain other more serious AEs as well.
Increased Risk for Amputations
The Canagliflozin Cardiovascular Assessment Study (CANVAS) and the Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) documented that canagliflozin doubled the incidence of leg and foot amputations in research participants compared with placebo (6.3 vs 3.4 per 1,000 patient-years).16 Therefore, canagliflozin should be prescribed with caution in persons with a prior history of foot ulceration, neuropathy, and/or vascular diseases.20
Acute Renal Injury
The mechanism of kidney damage by SGLT2 inhibitor drugs is not completely understood. About 100 patients experienced renal failure after the intake of SGLT2 inhibitor drugs.21 Among them, more than half reported symptom onset within a month of starting the medication, and their symptoms improved after discontinuing the SGLT2 medication. As a result, the FDA issued a warning to monitor renal function before initiating and during such pharmacotherapy.21
Ketoacidosis
Sodium-glucose cotransporter 2 inhibitors might lead to elevated ketone body levels22 and euglycemic ketoacidosis;23 however, this risk reportedly is negligible.24 Use of SGLT2 inhibitors is not recommended for patients evidencing the presence of precipitating factors like acute gastroenteritis or insulin pump failure.25
Genitourinary Infections
About 10% to 15% of women taking SGLT2 inhibitor medications developed urinary tract infections and vulvovaginitis.26 This could be because of a glycosuria effect caused by SGLT2 inhibitors.27
Hypotension
Sodium-glucose cotransporter 2 inhibitors cause contraction of intravascular volume. Therefore, patients taking SGLT2 inhibitors are at risk for hypotension, leading to dizziness and potentially dangerous falls. Patients already taking volume-depleting medications, such as diuretics, should be advised to use this group of medications with caution and report these AEs.28
Bone Fractures
A clinical trial revealed that SGLT2 inhibitors, such as canagliflozin, decrease bone mineral density possibly leading to bone fractures.29 Bone fractures occurred in about 1.5% of cases of patients taking 100 mg and 300 mg of canagliflozin compared with a 1.1% fracture rate among the placebo group.29
Conclusion
Since the FDA approval of SGLT2 inhibitor medications, their usage has increased. The American Diabetes Association first recommends nonpharmacologic approaches, such as diet modification, exercise, and weight loss for patients diagnosed with DM, followed by a medicinal intervention with metformin if required. Sodium-glucose cotransporter 2 inhibitors are suggested as an additional medication in dual or triple pharmacotherapies when metformin alone fails to achieve normoglycemia.
Prior to starting a patient on SGLT2 inhibitor medication, clinicians should monitor hydration adequacy, check bone density, review the patient’s cardiac profile, and assess hepatic and renal function. Prescribing SGLT2 inhibitors should be restricted if the patient has a history of type 1 DM, ketosisprone T2DM, and in those with a glomerular filtration rate of < 60 mL/min. Considering the preexisting medical conditions of the patient and monitoring the blood glucose levels, renal function, and volume status at every visit should minimize risks and enhance the benefits of prescribing this new medication class.
1. Li C, Balluz LS, Okoro CA, et al; Centers for Disease Control and Prevention. Surveillance of certain health behaviors and condition among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60(9):1-250.
2. Loriaux DL. Diabetes and the ebers papyrus: 1552 BC. Endocrinologist. 2006;16(2):55-56.
3. Malhotra A, Kudyar S, Gupta AK, Kudyar RP, Malhotra P. Sodium glucose cotransporter inhibitors—a new class of old drugs. Int J Appl Basic Med Res. 2015;5(3):161-163.
4. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
5. Mosley JF II, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451-462.
6. Bays H. Sodium glucose cotransporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195-220.
7. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136-1145.
8. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
9. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
10. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetology. 2017;16:8.
11. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose cotransporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399-408.
12. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Press. 2016;25(2):93-103.
13. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183-1187.
14. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126.
15. Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597.
16. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7): 644-657.
17. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870.
18. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
19. Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
20. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
21. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711-712.
22. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852.
23. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
24. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53-60.
25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187-194.
26. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
27. Chaplin S. SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26-30.
28. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16(12):875-882.
29. Watts NB, Bilezkian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellietus. J Clin Endocrinol Metab. 2016;101(1):157-166.
Diabetes mellitus (DM) is a metabolic disorder affecting about 5% to 13% of the population in the US.1 Since 1552, the earliest record of a person with DM, many treatment advances have been made.2Sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the newest antidiabetic pharmaceuticals on the market. The SGLT2 inhibitor drugs include canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin; however, only canagliflozin, dapagliflozin, and empagliflozin have been approved by the US Food and Drug Administration (FDA). These pharmaceuticals promote glycosuria via the kidneys and enhance sugar excretion from the body. Along with lifestyle changes and self-care measures, such as healthful eating and increased physical activity, SGLT2 inhibitor pharmaceuticals provide antidiabetic efficacy by facilitating normoglycemia and minimizing vascular pathology.
Although SGLT2 inhibitor pharmaceuticals are newly introduced into the market, their discovery dates to 1835.3 Phlorizin, a nonselective SGLT inhibitor, was first isolated by French chemists from the bark of an apple tree.4 Phlorizin inhibits SGLT1 mostly in small intestinal cells, and SGLT2 similarly affects the kidney.4 Renal SGLT2 is the primary therapeutic target. Canagliflozin was the first pharmaceutical SGLT2 inhibitor approved by the FDA in 2013. Dapagliflozin’s FDA approval followed in 2013 and empagliflozin in 2014.5
Mechanism Of Action
In healthy individuals, tubular glucose is absorbed, resulting in no urinary glucose excretion. Sodium-glucose cotransporters 1 and 2 contribute to the renal absorption of glucose. A SGLT2 is responsible for 90% of the glucose reuptake in the segment 1 of the proximal tubule, while SGLT 1 is accountable for the remaining 10%.3 Unlike other antidiabetic medications, which act by increasing insulin secretion or improving insulin sensitivity for the receptors, SGLT2 inhibitor drugs prevent the reuptake of glucose into the bloodstream. This selective action spares the inhibition of SGLT1 present in other tissues, avoiding gastrointestinal effects.6
Benefits
The SGLT2 inhibitor action is focused on renal excretion of glucose and is independent of insulin action.
Hemoglobin A1c Levels
Canagliflozin, dapagliflozin, and empagliflozin reduce hemoglobin A1c (HbA1c) levels.5 Inagaki and colleagues found significant reductions in HbA1c and weight gain with > 100 mg canagliflozin compared with that of placebo when used for 12 weeks.7 In a study where 2.5-mg, 5-mg, and 10-mg dapagliflozin was compared with placebo, the mean HbA1c change from the baseline was -0.23% with placebo; -0.58% at 2.5 mg; -0.77% at 5 mg; and -0.89% at 10 mg.8 Empagliflozin was more effective in reducing HbA1c levels than was sitagliptin.9 When patients were treated with 10-mg empagliflozin, 25-mg empagliflozin, and sitagliptin, HbA1c levels dropped -1.44%, -1.43%, and -1.04%, respectively.9
Cholesterol
Sodium-glucose cotransporter 2 inhibitors have the beneficial effect of reducing vascular disease risk factors.10,11 A study by Hayashi and colleagues found that dapagliflozin decreases harmful atherogenic small, low-density lipoprotein-cholesterol (LDL-C), increases less atherogenic large, buoyant LDL-C, and increases high-density, lipoprotein-2 cholesterol (HDL-2C).10 Empagliflozin, however, can cause a small dose-dependent increase in HDL-C and LDL-C.11 Although there is an increase in serum LDL-C concentrations, empagliflozin can induce a decrease in intestinal absorption of cholesterol, thus promoting fecal excretion of LDL-C and macrophage-derived cholesterol.11
Weight Loss
A study by Weber and colleagues found that the SGLT2 inhibitor dapagloflozin lead to a reduction in body weight from -1.0 kg to -0.3 kg compared with placebo.12 Cefalu and colleagues found that daily prescribing of 100 mg and/or 300 mg of canagliflozin evidenced dose-dependent loss of weight.13 Neeland and colleagues found that empagliflozin utilization resulted in less adiposity indices in 3,300 subjects.14
Albuminuria
Sodium-glucose cotransporter 2 inhibitors have a reno-protective role in patients with type 2 DM (T2DM). In those receiving renin-angiotensin blockers with T2DM and hypertension, dapagliflozin decreased their albuminuria.15 Canagliflozin has a similar potential.16 Empagliflozin reduced the urine albumin-creatinine ratio in patients with macro- or micro-albuminuria, supporting a direct renal effect by SGLT2 inhibitors.17
Systolic Blood Pressure
Sodium-glucose cotransporter 2 inhibitors can have beneficial effects on physiologic vascular outcomes. In patients with T2DM and hypertension, dapagliflozin reduced mean systolic blood pressure (SBP) compared with placebo: -7.3 mm Hg vs -10.4 mm Hg, respectively.12 Prescribing canagliflozin treatment at 100 mg or 300 mg reduced SBP (-4.3 mm Hg and -5.0 mm Hg, respectively, vs placebo at -0.3 mm Hg).18 Subjects taking empagliflozin 10 mg or 25 mg exhibited an adjusted mean BP change from baseline of -4.60 mm Hg and -5.47mm Hg, respectively, whereas placebo induced a -0.67 mm Hg decline.19
Risks
Nausea, fatigue, polyuria, polydipsia, and xerostomia are common SGLT2 AEs. Use of SGLT2 inhibitors can induce certain other more serious AEs as well.
Increased Risk for Amputations
The Canagliflozin Cardiovascular Assessment Study (CANVAS) and the Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) documented that canagliflozin doubled the incidence of leg and foot amputations in research participants compared with placebo (6.3 vs 3.4 per 1,000 patient-years).16 Therefore, canagliflozin should be prescribed with caution in persons with a prior history of foot ulceration, neuropathy, and/or vascular diseases.20
Acute Renal Injury
The mechanism of kidney damage by SGLT2 inhibitor drugs is not completely understood. About 100 patients experienced renal failure after the intake of SGLT2 inhibitor drugs.21 Among them, more than half reported symptom onset within a month of starting the medication, and their symptoms improved after discontinuing the SGLT2 medication. As a result, the FDA issued a warning to monitor renal function before initiating and during such pharmacotherapy.21
Ketoacidosis
Sodium-glucose cotransporter 2 inhibitors might lead to elevated ketone body levels22 and euglycemic ketoacidosis;23 however, this risk reportedly is negligible.24 Use of SGLT2 inhibitors is not recommended for patients evidencing the presence of precipitating factors like acute gastroenteritis or insulin pump failure.25
Genitourinary Infections
About 10% to 15% of women taking SGLT2 inhibitor medications developed urinary tract infections and vulvovaginitis.26 This could be because of a glycosuria effect caused by SGLT2 inhibitors.27
Hypotension
Sodium-glucose cotransporter 2 inhibitors cause contraction of intravascular volume. Therefore, patients taking SGLT2 inhibitors are at risk for hypotension, leading to dizziness and potentially dangerous falls. Patients already taking volume-depleting medications, such as diuretics, should be advised to use this group of medications with caution and report these AEs.28
Bone Fractures
A clinical trial revealed that SGLT2 inhibitors, such as canagliflozin, decrease bone mineral density possibly leading to bone fractures.29 Bone fractures occurred in about 1.5% of cases of patients taking 100 mg and 300 mg of canagliflozin compared with a 1.1% fracture rate among the placebo group.29
Conclusion
Since the FDA approval of SGLT2 inhibitor medications, their usage has increased. The American Diabetes Association first recommends nonpharmacologic approaches, such as diet modification, exercise, and weight loss for patients diagnosed with DM, followed by a medicinal intervention with metformin if required. Sodium-glucose cotransporter 2 inhibitors are suggested as an additional medication in dual or triple pharmacotherapies when metformin alone fails to achieve normoglycemia.
Prior to starting a patient on SGLT2 inhibitor medication, clinicians should monitor hydration adequacy, check bone density, review the patient’s cardiac profile, and assess hepatic and renal function. Prescribing SGLT2 inhibitors should be restricted if the patient has a history of type 1 DM, ketosisprone T2DM, and in those with a glomerular filtration rate of < 60 mL/min. Considering the preexisting medical conditions of the patient and monitoring the blood glucose levels, renal function, and volume status at every visit should minimize risks and enhance the benefits of prescribing this new medication class.
Diabetes mellitus (DM) is a metabolic disorder affecting about 5% to 13% of the population in the US.1 Since 1552, the earliest record of a person with DM, many treatment advances have been made.2Sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the newest antidiabetic pharmaceuticals on the market. The SGLT2 inhibitor drugs include canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin; however, only canagliflozin, dapagliflozin, and empagliflozin have been approved by the US Food and Drug Administration (FDA). These pharmaceuticals promote glycosuria via the kidneys and enhance sugar excretion from the body. Along with lifestyle changes and self-care measures, such as healthful eating and increased physical activity, SGLT2 inhibitor pharmaceuticals provide antidiabetic efficacy by facilitating normoglycemia and minimizing vascular pathology.
Although SGLT2 inhibitor pharmaceuticals are newly introduced into the market, their discovery dates to 1835.3 Phlorizin, a nonselective SGLT inhibitor, was first isolated by French chemists from the bark of an apple tree.4 Phlorizin inhibits SGLT1 mostly in small intestinal cells, and SGLT2 similarly affects the kidney.4 Renal SGLT2 is the primary therapeutic target. Canagliflozin was the first pharmaceutical SGLT2 inhibitor approved by the FDA in 2013. Dapagliflozin’s FDA approval followed in 2013 and empagliflozin in 2014.5
Mechanism Of Action
In healthy individuals, tubular glucose is absorbed, resulting in no urinary glucose excretion. Sodium-glucose cotransporters 1 and 2 contribute to the renal absorption of glucose. A SGLT2 is responsible for 90% of the glucose reuptake in the segment 1 of the proximal tubule, while SGLT 1 is accountable for the remaining 10%.3 Unlike other antidiabetic medications, which act by increasing insulin secretion or improving insulin sensitivity for the receptors, SGLT2 inhibitor drugs prevent the reuptake of glucose into the bloodstream. This selective action spares the inhibition of SGLT1 present in other tissues, avoiding gastrointestinal effects.6
Benefits
The SGLT2 inhibitor action is focused on renal excretion of glucose and is independent of insulin action.
Hemoglobin A1c Levels
Canagliflozin, dapagliflozin, and empagliflozin reduce hemoglobin A1c (HbA1c) levels.5 Inagaki and colleagues found significant reductions in HbA1c and weight gain with > 100 mg canagliflozin compared with that of placebo when used for 12 weeks.7 In a study where 2.5-mg, 5-mg, and 10-mg dapagliflozin was compared with placebo, the mean HbA1c change from the baseline was -0.23% with placebo; -0.58% at 2.5 mg; -0.77% at 5 mg; and -0.89% at 10 mg.8 Empagliflozin was more effective in reducing HbA1c levels than was sitagliptin.9 When patients were treated with 10-mg empagliflozin, 25-mg empagliflozin, and sitagliptin, HbA1c levels dropped -1.44%, -1.43%, and -1.04%, respectively.9
Cholesterol
Sodium-glucose cotransporter 2 inhibitors have the beneficial effect of reducing vascular disease risk factors.10,11 A study by Hayashi and colleagues found that dapagliflozin decreases harmful atherogenic small, low-density lipoprotein-cholesterol (LDL-C), increases less atherogenic large, buoyant LDL-C, and increases high-density, lipoprotein-2 cholesterol (HDL-2C).10 Empagliflozin, however, can cause a small dose-dependent increase in HDL-C and LDL-C.11 Although there is an increase in serum LDL-C concentrations, empagliflozin can induce a decrease in intestinal absorption of cholesterol, thus promoting fecal excretion of LDL-C and macrophage-derived cholesterol.11
Weight Loss
A study by Weber and colleagues found that the SGLT2 inhibitor dapagloflozin lead to a reduction in body weight from -1.0 kg to -0.3 kg compared with placebo.12 Cefalu and colleagues found that daily prescribing of 100 mg and/or 300 mg of canagliflozin evidenced dose-dependent loss of weight.13 Neeland and colleagues found that empagliflozin utilization resulted in less adiposity indices in 3,300 subjects.14
Albuminuria
Sodium-glucose cotransporter 2 inhibitors have a reno-protective role in patients with type 2 DM (T2DM). In those receiving renin-angiotensin blockers with T2DM and hypertension, dapagliflozin decreased their albuminuria.15 Canagliflozin has a similar potential.16 Empagliflozin reduced the urine albumin-creatinine ratio in patients with macro- or micro-albuminuria, supporting a direct renal effect by SGLT2 inhibitors.17
Systolic Blood Pressure
Sodium-glucose cotransporter 2 inhibitors can have beneficial effects on physiologic vascular outcomes. In patients with T2DM and hypertension, dapagliflozin reduced mean systolic blood pressure (SBP) compared with placebo: -7.3 mm Hg vs -10.4 mm Hg, respectively.12 Prescribing canagliflozin treatment at 100 mg or 300 mg reduced SBP (-4.3 mm Hg and -5.0 mm Hg, respectively, vs placebo at -0.3 mm Hg).18 Subjects taking empagliflozin 10 mg or 25 mg exhibited an adjusted mean BP change from baseline of -4.60 mm Hg and -5.47mm Hg, respectively, whereas placebo induced a -0.67 mm Hg decline.19
Risks
Nausea, fatigue, polyuria, polydipsia, and xerostomia are common SGLT2 AEs. Use of SGLT2 inhibitors can induce certain other more serious AEs as well.
Increased Risk for Amputations
The Canagliflozin Cardiovascular Assessment Study (CANVAS) and the Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) documented that canagliflozin doubled the incidence of leg and foot amputations in research participants compared with placebo (6.3 vs 3.4 per 1,000 patient-years).16 Therefore, canagliflozin should be prescribed with caution in persons with a prior history of foot ulceration, neuropathy, and/or vascular diseases.20
Acute Renal Injury
The mechanism of kidney damage by SGLT2 inhibitor drugs is not completely understood. About 100 patients experienced renal failure after the intake of SGLT2 inhibitor drugs.21 Among them, more than half reported symptom onset within a month of starting the medication, and their symptoms improved after discontinuing the SGLT2 medication. As a result, the FDA issued a warning to monitor renal function before initiating and during such pharmacotherapy.21
Ketoacidosis
Sodium-glucose cotransporter 2 inhibitors might lead to elevated ketone body levels22 and euglycemic ketoacidosis;23 however, this risk reportedly is negligible.24 Use of SGLT2 inhibitors is not recommended for patients evidencing the presence of precipitating factors like acute gastroenteritis or insulin pump failure.25
Genitourinary Infections
About 10% to 15% of women taking SGLT2 inhibitor medications developed urinary tract infections and vulvovaginitis.26 This could be because of a glycosuria effect caused by SGLT2 inhibitors.27
Hypotension
Sodium-glucose cotransporter 2 inhibitors cause contraction of intravascular volume. Therefore, patients taking SGLT2 inhibitors are at risk for hypotension, leading to dizziness and potentially dangerous falls. Patients already taking volume-depleting medications, such as diuretics, should be advised to use this group of medications with caution and report these AEs.28
Bone Fractures
A clinical trial revealed that SGLT2 inhibitors, such as canagliflozin, decrease bone mineral density possibly leading to bone fractures.29 Bone fractures occurred in about 1.5% of cases of patients taking 100 mg and 300 mg of canagliflozin compared with a 1.1% fracture rate among the placebo group.29
Conclusion
Since the FDA approval of SGLT2 inhibitor medications, their usage has increased. The American Diabetes Association first recommends nonpharmacologic approaches, such as diet modification, exercise, and weight loss for patients diagnosed with DM, followed by a medicinal intervention with metformin if required. Sodium-glucose cotransporter 2 inhibitors are suggested as an additional medication in dual or triple pharmacotherapies when metformin alone fails to achieve normoglycemia.
Prior to starting a patient on SGLT2 inhibitor medication, clinicians should monitor hydration adequacy, check bone density, review the patient’s cardiac profile, and assess hepatic and renal function. Prescribing SGLT2 inhibitors should be restricted if the patient has a history of type 1 DM, ketosisprone T2DM, and in those with a glomerular filtration rate of < 60 mL/min. Considering the preexisting medical conditions of the patient and monitoring the blood glucose levels, renal function, and volume status at every visit should minimize risks and enhance the benefits of prescribing this new medication class.
1. Li C, Balluz LS, Okoro CA, et al; Centers for Disease Control and Prevention. Surveillance of certain health behaviors and condition among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60(9):1-250.
2. Loriaux DL. Diabetes and the ebers papyrus: 1552 BC. Endocrinologist. 2006;16(2):55-56.
3. Malhotra A, Kudyar S, Gupta AK, Kudyar RP, Malhotra P. Sodium glucose cotransporter inhibitors—a new class of old drugs. Int J Appl Basic Med Res. 2015;5(3):161-163.
4. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
5. Mosley JF II, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451-462.
6. Bays H. Sodium glucose cotransporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195-220.
7. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136-1145.
8. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
9. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
10. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetology. 2017;16:8.
11. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose cotransporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399-408.
12. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Press. 2016;25(2):93-103.
13. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183-1187.
14. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126.
15. Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597.
16. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7): 644-657.
17. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870.
18. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
19. Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
20. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
21. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711-712.
22. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852.
23. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
24. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53-60.
25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187-194.
26. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
27. Chaplin S. SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26-30.
28. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16(12):875-882.
29. Watts NB, Bilezkian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellietus. J Clin Endocrinol Metab. 2016;101(1):157-166.
1. Li C, Balluz LS, Okoro CA, et al; Centers for Disease Control and Prevention. Surveillance of certain health behaviors and condition among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60(9):1-250.
2. Loriaux DL. Diabetes and the ebers papyrus: 1552 BC. Endocrinologist. 2006;16(2):55-56.
3. Malhotra A, Kudyar S, Gupta AK, Kudyar RP, Malhotra P. Sodium glucose cotransporter inhibitors—a new class of old drugs. Int J Appl Basic Med Res. 2015;5(3):161-163.
4. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
5. Mosley JF II, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451-462.
6. Bays H. Sodium glucose cotransporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195-220.
7. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136-1145.
8. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
9. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
10. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetology. 2017;16:8.
11. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose cotransporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399-408.
12. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Press. 2016;25(2):93-103.
13. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183-1187.
14. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126.
15. Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597.
16. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7): 644-657.
17. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870.
18. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
19. Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
20. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
21. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711-712.
22. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852.
23. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
24. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53-60.
25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187-194.
26. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
27. Chaplin S. SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26-30.
28. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16(12):875-882.
29. Watts NB, Bilezkian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellietus. J Clin Endocrinol Metab. 2016;101(1):157-166.