User login
A Better Approach to the Diagnosis of PE
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.

Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).

Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
Can Vitamin D Prevent Acute Respiratory Infections?

Ms. M, a generally healthy 55-year-old woman, was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level of 8 ng/mL). She presents with her second episode of acute viral bronchitis in the past 6 months. She has no history of significant smoking or exposure or history of asthma and does not take respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/wk in bolus dosing—but is that your best option for the patient?
ARTIs include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common reason for ambulatory care visits, accounting for almost 120 million (about 10% of all) visits per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D is protective in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N = 10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk for bias. The Cochrane risk-for-bias tool was used to address threats to validity.
The study included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI; and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR], 0.88; number needed to treat [NNT], 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR, 0.81), but bolus dosing (≥ 30,000 IU) was not (AOR, 0.97).
In 2-step analysis, patients benefited if they had baseline circulating 25(OH)D concentrations < 10 ng/mL (AOR, 0.30; NNT, 4); had baseline circulating 25(OH)D levels of 10 to 28 ng/mL (AOR, 0.75; NNT, 15); were ages 1.1 to 15.9 (AOR, 0.59); were ages 16 to 65 (AOR, 0.79); or had a BMI < 25 (AOR, 0.82).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25(OH)D ≥ 30 ng/mL did not appear to provide benefit (AOR, 0.96). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR, 0.98).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25(OH)D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect was noted in those who were most severely vitamin D deficient (those with circulating 25(OH)D levels < 10 ng/mL [NNT, 4] and those with circulating 25(OH)D levels 10-28 ng/mL [NNT, 15]). There was no demonstrable effect once circulating 25(OH)D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs. However, the doses studied were much lower than those commonly used (10,000 to 50,000 IU bolus), which were ineffective in reducing ARTIs in this meta-analysis. Changing from bolus dosing may prove challenging, a
In addition, the authors of the study suggest that one way to provide this level of vitamin D is through food fortification. But this method is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[4]:230-231).
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. CDC National Center for Health Statistics. National Health Care Surveys. www.cdc.gov/nchs/dhcs.htm. Accessed September 5, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.

Ms. M, a generally healthy 55-year-old woman, was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level of 8 ng/mL). She presents with her second episode of acute viral bronchitis in the past 6 months. She has no history of significant smoking or exposure or history of asthma and does not take respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/wk in bolus dosing—but is that your best option for the patient?
ARTIs include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common reason for ambulatory care visits, accounting for almost 120 million (about 10% of all) visits per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D is protective in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N = 10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk for bias. The Cochrane risk-for-bias tool was used to address threats to validity.
The study included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI; and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR], 0.88; number needed to treat [NNT], 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR, 0.81), but bolus dosing (≥ 30,000 IU) was not (AOR, 0.97).
In 2-step analysis, patients benefited if they had baseline circulating 25(OH)D concentrations < 10 ng/mL (AOR, 0.30; NNT, 4); had baseline circulating 25(OH)D levels of 10 to 28 ng/mL (AOR, 0.75; NNT, 15); were ages 1.1 to 15.9 (AOR, 0.59); were ages 16 to 65 (AOR, 0.79); or had a BMI < 25 (AOR, 0.82).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25(OH)D ≥ 30 ng/mL did not appear to provide benefit (AOR, 0.96). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR, 0.98).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25(OH)D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect was noted in those who were most severely vitamin D deficient (those with circulating 25(OH)D levels < 10 ng/mL [NNT, 4] and those with circulating 25(OH)D levels 10-28 ng/mL [NNT, 15]). There was no demonstrable effect once circulating 25(OH)D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs. However, the doses studied were much lower than those commonly used (10,000 to 50,000 IU bolus), which were ineffective in reducing ARTIs in this meta-analysis. Changing from bolus dosing may prove challenging, a
In addition, the authors of the study suggest that one way to provide this level of vitamin D is through food fortification. But this method is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[4]:230-231).

Ms. M, a generally healthy 55-year-old woman, was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level of 8 ng/mL). She presents with her second episode of acute viral bronchitis in the past 6 months. She has no history of significant smoking or exposure or history of asthma and does not take respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/wk in bolus dosing—but is that your best option for the patient?
ARTIs include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common reason for ambulatory care visits, accounting for almost 120 million (about 10% of all) visits per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D is protective in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N = 10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk for bias. The Cochrane risk-for-bias tool was used to address threats to validity.
The study included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI; and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR], 0.88; number needed to treat [NNT], 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR, 0.81), but bolus dosing (≥ 30,000 IU) was not (AOR, 0.97).
In 2-step analysis, patients benefited if they had baseline circulating 25(OH)D concentrations < 10 ng/mL (AOR, 0.30; NNT, 4); had baseline circulating 25(OH)D levels of 10 to 28 ng/mL (AOR, 0.75; NNT, 15); were ages 1.1 to 15.9 (AOR, 0.59); were ages 16 to 65 (AOR, 0.79); or had a BMI < 25 (AOR, 0.82).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25(OH)D ≥ 30 ng/mL did not appear to provide benefit (AOR, 0.96). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR, 0.98).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25(OH)D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect was noted in those who were most severely vitamin D deficient (those with circulating 25(OH)D levels < 10 ng/mL [NNT, 4] and those with circulating 25(OH)D levels 10-28 ng/mL [NNT, 15]). There was no demonstrable effect once circulating 25(OH)D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs. However, the doses studied were much lower than those commonly used (10,000 to 50,000 IU bolus), which were ineffective in reducing ARTIs in this meta-analysis. Changing from bolus dosing may prove challenging, a
In addition, the authors of the study suggest that one way to provide this level of vitamin D is through food fortification. But this method is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[4]:230-231).
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. CDC National Center for Health Statistics. National Health Care Surveys. www.cdc.gov/nchs/dhcs.htm. Accessed September 5, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. CDC National Center for Health Statistics. National Health Care Surveys. www.cdc.gov/nchs/dhcs.htm. Accessed September 5, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
A better approach to the diagnosis of PE
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
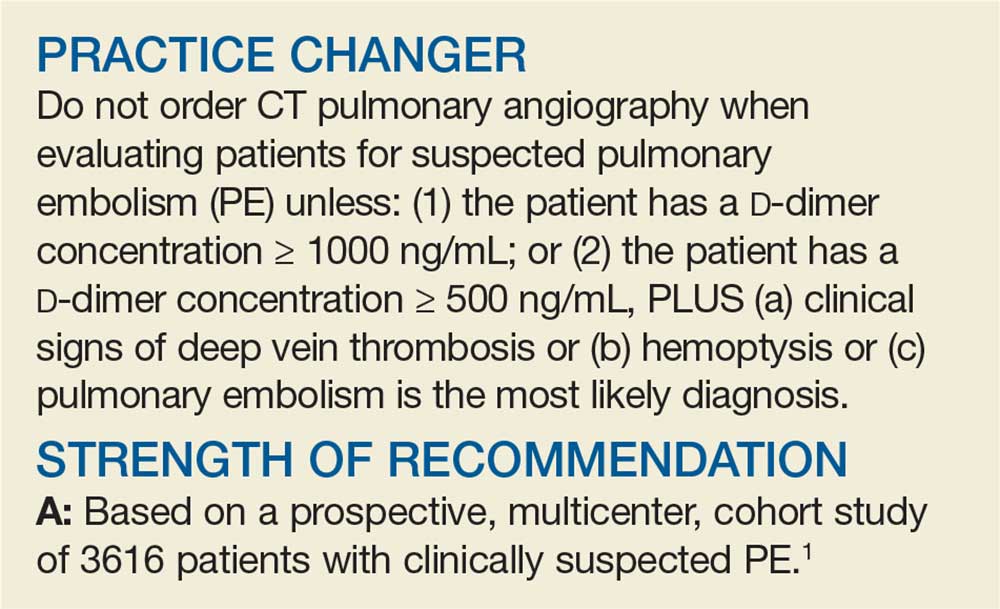
PRACTICE CHANGER
Do not order computed tomography pulmonary angiography when evaluating patients for suspected pulmonary embolism unless: (1) the patient has a
STRENGTH OF RECOMMENDATION
A: Based on a prospective, multicenter, cohort study of 3616 patients with clinically suspected pulmonary embolism.1
van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
Can vitamin D prevent acute respiratory infections?
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
PRACTICE CHANGER
Reduce acute respiratory tract infections in those with significant vitamin D deficiency (circulating 25-hydroxyvitamin D levels < 10 ng/mL) with daily or weekly vitamin D supplementation—not bolus vitamin D treatment.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 25 trials.
Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.