User login
Painful Ulcerating Lesions on the Breast
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
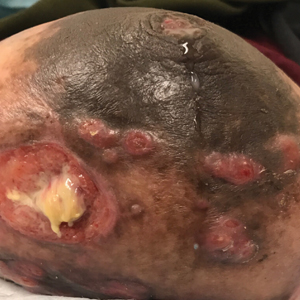
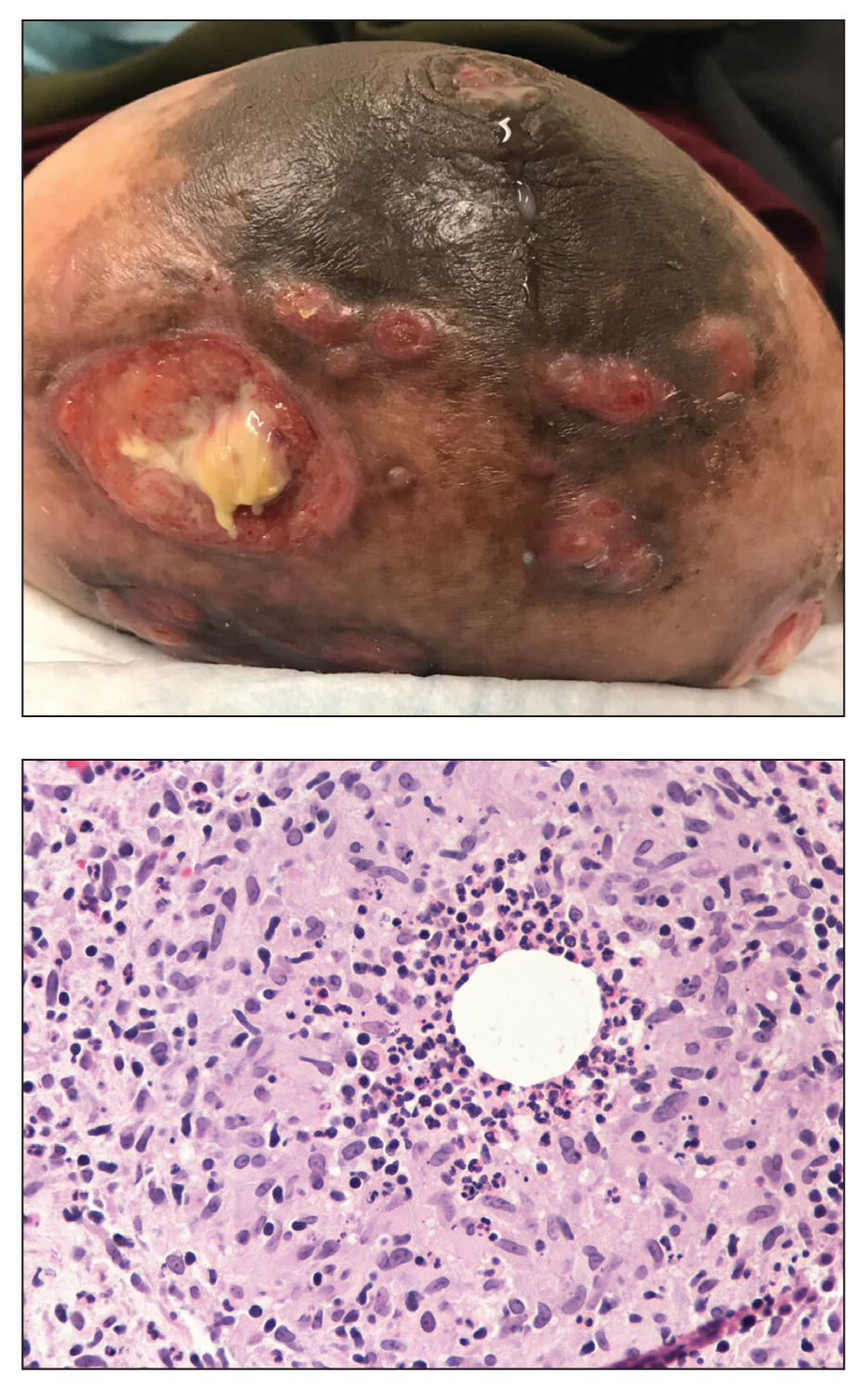
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
A 36-year-old puerperal woman presented with painful, unilateral, ulcerating breast lesions (top) of 3 months’ duration that developed during pregnancy and drained pus with blood. No improvement was seen with antibiotics or incision and drainage. Biopsy of a lesion showed stellate granulomas with cystic spaces and suppurative lipogranulomas where central lipid vacuoles were rimmed by neutrophils and an outer cuff of epithelioid histiocytes (bottom). Acid-fast bacilli, Grocott-Gomori methenamine-silver, Gram, and Steiner staining did not reveal any microorganisms. Additionally, wound cultures were negative.
Unilateral Verrucous Psoriasis
Case Report
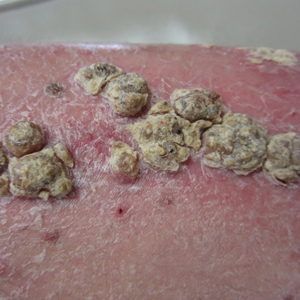
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.
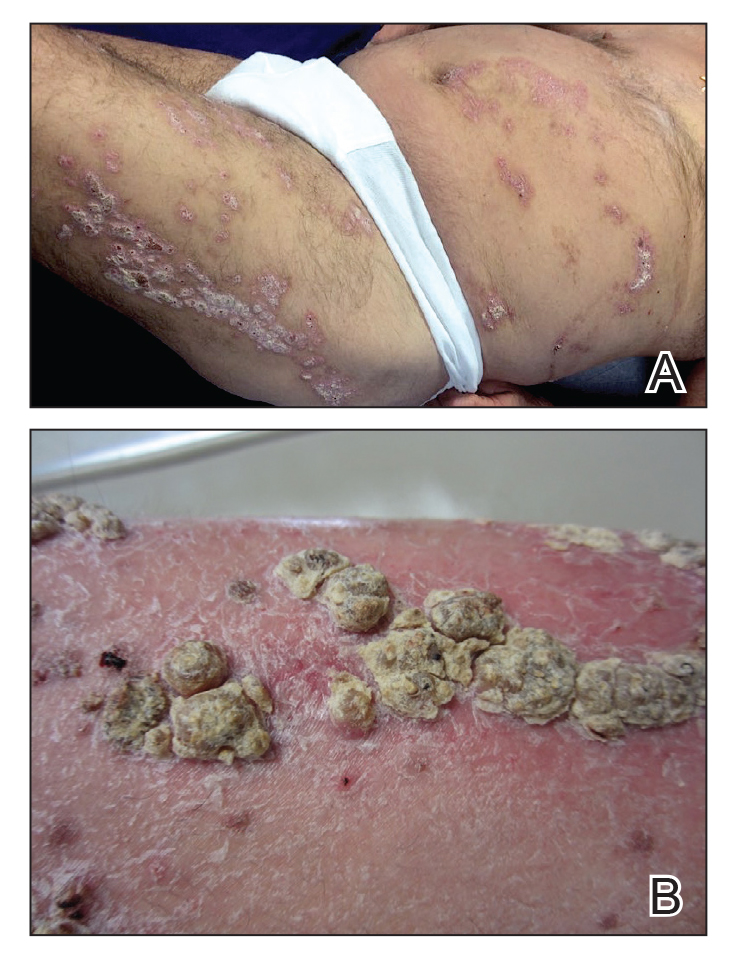
At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
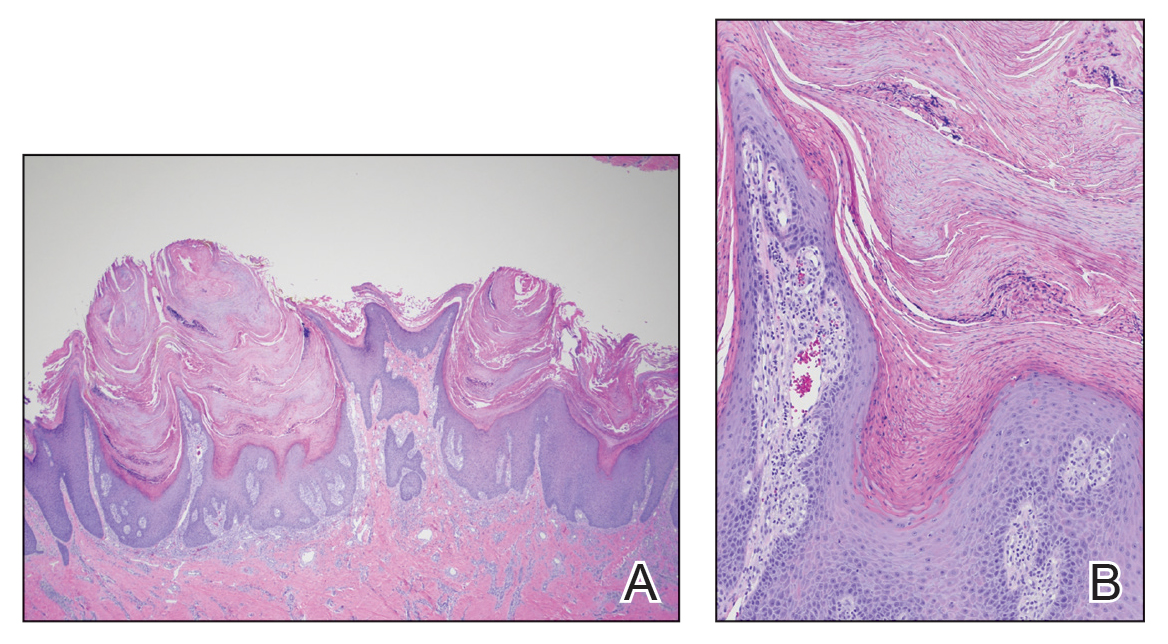
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.
Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.

At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.

Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.

At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.

Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
Practice Points
- Verrucous psoriasis is a rare variant of psoriasis characterized by hypertrophic verrucous papules and plaques on an erythematous base.
- Histologically, verrucous psoriasis presents with overlapping features of verruca and psoriasis.
- Although psoriasis typically presents in a symmetric distribution, unilateral psoriasis can occur either de novo in younger patients or after surgical trauma in older patients.
Widespread Poikilodermatous Dermatomyositis Associated With Chronic Lymphocytic Leukemia
To the Editor:
Dermatomyositis represents a rare idiopathic inflammatory process presenting with cutaneous lesions and muscular weakness. It often represents a paraneoplastic syndrome. We report the case of a 62-year-old man with a history of total-body poikiloderma and a recent diagnosis of chronic lymphocytic leukemia (CLL). Despite lacking typical features of the disease, a diagnosis of dermatomyositis was made. Our patient may represent a distinct poikilodermatous variant of dermatomyositis, sharing the generalized distribution of the erythrodermic subtype.
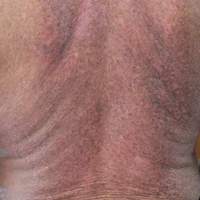
A 62-year-old man presented with pruritic poikiloderma involving the neck, arms, legs, abdomen, chest, and back of 2 years’ duration (Figure). He also experienced dysphagia and weakness of the legs. The rash was previously treated by other dermatologists with a combination of high-potency topical steroids and topical tacrolimus 0.1% without success. His history was notable for CLL, which had been diagnosed by a dermatologist 6 months prior to the current presentation. Prior to his visit to the dermatologist, the patient had received 6 chemotherapeutic sessions with a combination of rituximab and cyclophosphamide for the treatment of CLL. The rash did not improve with chemotherapy.

Repeat biopsies of affected regions only demonstrated features of mild interface dermatitis. Direct immunofluorescence studies showed scattered colloid body fluorescence for IgM. Because of bilateral weakness of the legs, a muscle biopsy was taken, which demonstrated severe atrophy and interstitial fibrosis, with neurogenic abnormalities detected in areas of lesser atrophy via abnormal muscle fiber–type grouping. Metabolic panel showed elevated muscle enzymes in the blood: creatine kinase, 243 U/L (reference range, 10–225 U/L); serum aldolase, 16 U/L (reference range, ≤8.1 U/L); lactate dehydrogenase, 314 U/L (reference range, 60–200 U/L). An autoimmune panel was negative for Jo-1, Scl-70, U1 ribonucleoprotein, DNA, desmoglein 1 and 3, and antiacetylcholine receptor antibodies. An elevated erythrocyte sedimentation rate was measured at 16 mm/h (reference range, 0–10 mm/h). Given these findings, the lesions were confirmed as a widespread poikilodermatous variant of dermatomyositis.
The patient was placed on a daily 50-mg dose of prednisone, which produced rapid improvement in scaling and erythema. Creatine kinase and serum aldolase levels normalized and motor strength increased. After 1 week the prednisone dosage was reduced to a daily 30-mg dose, and then 20 mg a week later. The skin lesions completely resolved within 4 to 5 months and the patient is currently on a prednisone dose of 5 mg, alternating with 2.5 mg of prednisone and rituximab infusion every 2 months.
Dermatomyositis is a rare entity with an incidence of approximately 0.5 to 1 per 100,000 individuals.1 It presents with a characteristic rash composed of Gottron papules; pathognomonic flat violaceous papules on the dorsal interphalangeal joints, elbows, or knees; and a heliotrope rash, a violaceous erythema involving the eyelids. Poikiloderma frequently is reported to present in a shawl-like distribution, encompassing the shoulders, arms, and upper back.1,2 Dermatomyositis of the poikilodermatous type can present in nonphotoexposed areas and photoexposed areas. The unusual feature is the total-body involvement, which is analogous to erythroderma.3
Our case may represent a distinct poikilodermatous manifestation sharing the distribution of the erythrodermic subtype. We believe that the skin lesions may have represented a paraneoplastic event presenting prior to diagnosis with CLL. Dermatomyositis has a strong association with cancer, with patients 3 times more likely to develop internal malignancy.4 Association is strongest for non-Hodgkin lymphoma, as well as ovarian, lung, colorectal, pancreatic, and gastric cancer. When associated with malignancy, symptoms of dermatomyositis or myositis typically precede the discovery of malignancy by an average of 1.9 years.5 Dermatomyositis has been previously reported to present as a paraneoplastic manifestation of CLL.6 One case has been reported of a patient with CLL who developed leukemia cutis presenting with poikiloderma in the characteristic dermatomyositis shawl-like distribution.7 The lack of dermal infiltration with leukemic cells in our patient, however, makes a paraneoplastic etiology much more likely.
Our patient’s rash did not initially improve with treatment of CLL, but dermatomyositis associated with hematological malignancy may precede, occur simultaneously, or follow the diagnosis of malignancy.8 Additionally, symptoms of dermatomyositis do not always parallel the course of hematological malignancy outcome. However, rituximab has been used as a treatment of dermatomyositis and may have contributed some synergistic effect in combination with prednisone in our patient.9
- Dourmishev LA, Dourmishev AL, Schwartz RA. Dermatomyositis: cutaneous manifestations of its variants. Int J Dermatol. 2002;41:625-630.
- Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899-920; quiz 921-992.
- Liu ZH, Wang XD. Acute-onset adult dermatomyositis presenting with erythroderma and diplopia. Clin Exp Dermatol. 2007;32:751-752.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Bohan A, Peter JB, Bowman RL, et al. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore). 1977;56:255-286.
- Ishida T, Aikawa K, Tamura T, et al. Chronic lymphocytic leukemia associated with nephrotic syndrome and dermatomyositis. Intern Med. 1995;34:15-17.
- Nousari HC, Kimyai-Asadi A, Huang CH, et al. T-cell chronic lymphocytic leukemia mimicking dermatomyositis. Int J Dermatol. 2000;39:144-146.
- Marie I, Guillevin L, Menard JF, et al. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun Rev. 2012;11:615-620.
- Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005;52:601-607.
To the Editor:
Dermatomyositis represents a rare idiopathic inflammatory process presenting with cutaneous lesions and muscular weakness. It often represents a paraneoplastic syndrome. We report the case of a 62-year-old man with a history of total-body poikiloderma and a recent diagnosis of chronic lymphocytic leukemia (CLL). Despite lacking typical features of the disease, a diagnosis of dermatomyositis was made. Our patient may represent a distinct poikilodermatous variant of dermatomyositis, sharing the generalized distribution of the erythrodermic subtype.
A 62-year-old man presented with pruritic poikiloderma involving the neck, arms, legs, abdomen, chest, and back of 2 years’ duration (Figure). He also experienced dysphagia and weakness of the legs. The rash was previously treated by other dermatologists with a combination of high-potency topical steroids and topical tacrolimus 0.1% without success. His history was notable for CLL, which had been diagnosed by a dermatologist 6 months prior to the current presentation. Prior to his visit to the dermatologist, the patient had received 6 chemotherapeutic sessions with a combination of rituximab and cyclophosphamide for the treatment of CLL. The rash did not improve with chemotherapy.

Repeat biopsies of affected regions only demonstrated features of mild interface dermatitis. Direct immunofluorescence studies showed scattered colloid body fluorescence for IgM. Because of bilateral weakness of the legs, a muscle biopsy was taken, which demonstrated severe atrophy and interstitial fibrosis, with neurogenic abnormalities detected in areas of lesser atrophy via abnormal muscle fiber–type grouping. Metabolic panel showed elevated muscle enzymes in the blood: creatine kinase, 243 U/L (reference range, 10–225 U/L); serum aldolase, 16 U/L (reference range, ≤8.1 U/L); lactate dehydrogenase, 314 U/L (reference range, 60–200 U/L). An autoimmune panel was negative for Jo-1, Scl-70, U1 ribonucleoprotein, DNA, desmoglein 1 and 3, and antiacetylcholine receptor antibodies. An elevated erythrocyte sedimentation rate was measured at 16 mm/h (reference range, 0–10 mm/h). Given these findings, the lesions were confirmed as a widespread poikilodermatous variant of dermatomyositis.
The patient was placed on a daily 50-mg dose of prednisone, which produced rapid improvement in scaling and erythema. Creatine kinase and serum aldolase levels normalized and motor strength increased. After 1 week the prednisone dosage was reduced to a daily 30-mg dose, and then 20 mg a week later. The skin lesions completely resolved within 4 to 5 months and the patient is currently on a prednisone dose of 5 mg, alternating with 2.5 mg of prednisone and rituximab infusion every 2 months.
Dermatomyositis is a rare entity with an incidence of approximately 0.5 to 1 per 100,000 individuals.1 It presents with a characteristic rash composed of Gottron papules; pathognomonic flat violaceous papules on the dorsal interphalangeal joints, elbows, or knees; and a heliotrope rash, a violaceous erythema involving the eyelids. Poikiloderma frequently is reported to present in a shawl-like distribution, encompassing the shoulders, arms, and upper back.1,2 Dermatomyositis of the poikilodermatous type can present in nonphotoexposed areas and photoexposed areas. The unusual feature is the total-body involvement, which is analogous to erythroderma.3
Our case may represent a distinct poikilodermatous manifestation sharing the distribution of the erythrodermic subtype. We believe that the skin lesions may have represented a paraneoplastic event presenting prior to diagnosis with CLL. Dermatomyositis has a strong association with cancer, with patients 3 times more likely to develop internal malignancy.4 Association is strongest for non-Hodgkin lymphoma, as well as ovarian, lung, colorectal, pancreatic, and gastric cancer. When associated with malignancy, symptoms of dermatomyositis or myositis typically precede the discovery of malignancy by an average of 1.9 years.5 Dermatomyositis has been previously reported to present as a paraneoplastic manifestation of CLL.6 One case has been reported of a patient with CLL who developed leukemia cutis presenting with poikiloderma in the characteristic dermatomyositis shawl-like distribution.7 The lack of dermal infiltration with leukemic cells in our patient, however, makes a paraneoplastic etiology much more likely.
Our patient’s rash did not initially improve with treatment of CLL, but dermatomyositis associated with hematological malignancy may precede, occur simultaneously, or follow the diagnosis of malignancy.8 Additionally, symptoms of dermatomyositis do not always parallel the course of hematological malignancy outcome. However, rituximab has been used as a treatment of dermatomyositis and may have contributed some synergistic effect in combination with prednisone in our patient.9
To the Editor:
Dermatomyositis represents a rare idiopathic inflammatory process presenting with cutaneous lesions and muscular weakness. It often represents a paraneoplastic syndrome. We report the case of a 62-year-old man with a history of total-body poikiloderma and a recent diagnosis of chronic lymphocytic leukemia (CLL). Despite lacking typical features of the disease, a diagnosis of dermatomyositis was made. Our patient may represent a distinct poikilodermatous variant of dermatomyositis, sharing the generalized distribution of the erythrodermic subtype.
A 62-year-old man presented with pruritic poikiloderma involving the neck, arms, legs, abdomen, chest, and back of 2 years’ duration (Figure). He also experienced dysphagia and weakness of the legs. The rash was previously treated by other dermatologists with a combination of high-potency topical steroids and topical tacrolimus 0.1% without success. His history was notable for CLL, which had been diagnosed by a dermatologist 6 months prior to the current presentation. Prior to his visit to the dermatologist, the patient had received 6 chemotherapeutic sessions with a combination of rituximab and cyclophosphamide for the treatment of CLL. The rash did not improve with chemotherapy.

Repeat biopsies of affected regions only demonstrated features of mild interface dermatitis. Direct immunofluorescence studies showed scattered colloid body fluorescence for IgM. Because of bilateral weakness of the legs, a muscle biopsy was taken, which demonstrated severe atrophy and interstitial fibrosis, with neurogenic abnormalities detected in areas of lesser atrophy via abnormal muscle fiber–type grouping. Metabolic panel showed elevated muscle enzymes in the blood: creatine kinase, 243 U/L (reference range, 10–225 U/L); serum aldolase, 16 U/L (reference range, ≤8.1 U/L); lactate dehydrogenase, 314 U/L (reference range, 60–200 U/L). An autoimmune panel was negative for Jo-1, Scl-70, U1 ribonucleoprotein, DNA, desmoglein 1 and 3, and antiacetylcholine receptor antibodies. An elevated erythrocyte sedimentation rate was measured at 16 mm/h (reference range, 0–10 mm/h). Given these findings, the lesions were confirmed as a widespread poikilodermatous variant of dermatomyositis.
The patient was placed on a daily 50-mg dose of prednisone, which produced rapid improvement in scaling and erythema. Creatine kinase and serum aldolase levels normalized and motor strength increased. After 1 week the prednisone dosage was reduced to a daily 30-mg dose, and then 20 mg a week later. The skin lesions completely resolved within 4 to 5 months and the patient is currently on a prednisone dose of 5 mg, alternating with 2.5 mg of prednisone and rituximab infusion every 2 months.
Dermatomyositis is a rare entity with an incidence of approximately 0.5 to 1 per 100,000 individuals.1 It presents with a characteristic rash composed of Gottron papules; pathognomonic flat violaceous papules on the dorsal interphalangeal joints, elbows, or knees; and a heliotrope rash, a violaceous erythema involving the eyelids. Poikiloderma frequently is reported to present in a shawl-like distribution, encompassing the shoulders, arms, and upper back.1,2 Dermatomyositis of the poikilodermatous type can present in nonphotoexposed areas and photoexposed areas. The unusual feature is the total-body involvement, which is analogous to erythroderma.3
Our case may represent a distinct poikilodermatous manifestation sharing the distribution of the erythrodermic subtype. We believe that the skin lesions may have represented a paraneoplastic event presenting prior to diagnosis with CLL. Dermatomyositis has a strong association with cancer, with patients 3 times more likely to develop internal malignancy.4 Association is strongest for non-Hodgkin lymphoma, as well as ovarian, lung, colorectal, pancreatic, and gastric cancer. When associated with malignancy, symptoms of dermatomyositis or myositis typically precede the discovery of malignancy by an average of 1.9 years.5 Dermatomyositis has been previously reported to present as a paraneoplastic manifestation of CLL.6 One case has been reported of a patient with CLL who developed leukemia cutis presenting with poikiloderma in the characteristic dermatomyositis shawl-like distribution.7 The lack of dermal infiltration with leukemic cells in our patient, however, makes a paraneoplastic etiology much more likely.
Our patient’s rash did not initially improve with treatment of CLL, but dermatomyositis associated with hematological malignancy may precede, occur simultaneously, or follow the diagnosis of malignancy.8 Additionally, symptoms of dermatomyositis do not always parallel the course of hematological malignancy outcome. However, rituximab has been used as a treatment of dermatomyositis and may have contributed some synergistic effect in combination with prednisone in our patient.9
- Dourmishev LA, Dourmishev AL, Schwartz RA. Dermatomyositis: cutaneous manifestations of its variants. Int J Dermatol. 2002;41:625-630.
- Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899-920; quiz 921-992.
- Liu ZH, Wang XD. Acute-onset adult dermatomyositis presenting with erythroderma and diplopia. Clin Exp Dermatol. 2007;32:751-752.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Bohan A, Peter JB, Bowman RL, et al. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore). 1977;56:255-286.
- Ishida T, Aikawa K, Tamura T, et al. Chronic lymphocytic leukemia associated with nephrotic syndrome and dermatomyositis. Intern Med. 1995;34:15-17.
- Nousari HC, Kimyai-Asadi A, Huang CH, et al. T-cell chronic lymphocytic leukemia mimicking dermatomyositis. Int J Dermatol. 2000;39:144-146.
- Marie I, Guillevin L, Menard JF, et al. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun Rev. 2012;11:615-620.
- Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005;52:601-607.
- Dourmishev LA, Dourmishev AL, Schwartz RA. Dermatomyositis: cutaneous manifestations of its variants. Int J Dermatol. 2002;41:625-630.
- Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899-920; quiz 921-992.
- Liu ZH, Wang XD. Acute-onset adult dermatomyositis presenting with erythroderma and diplopia. Clin Exp Dermatol. 2007;32:751-752.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Bohan A, Peter JB, Bowman RL, et al. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore). 1977;56:255-286.
- Ishida T, Aikawa K, Tamura T, et al. Chronic lymphocytic leukemia associated with nephrotic syndrome and dermatomyositis. Intern Med. 1995;34:15-17.
- Nousari HC, Kimyai-Asadi A, Huang CH, et al. T-cell chronic lymphocytic leukemia mimicking dermatomyositis. Int J Dermatol. 2000;39:144-146.
- Marie I, Guillevin L, Menard JF, et al. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun Rev. 2012;11:615-620.
- Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005;52:601-607.
Practice Points
- Poikiloderma, even with an unusual clinical presentation, can be a useful clinical clue for the diagnosis of dermatomyositis or other collagen vascular disease.
- Dermatomyositis can be paraneoplastic and though often associated with epithelial malignancies and solid tumors can also be associated with leukemias.