User login
Allergic Contact Dermatitis
THE COMPARISON
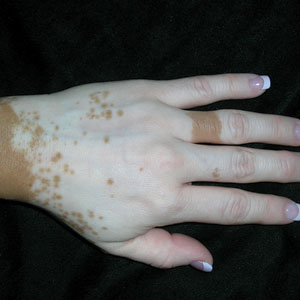
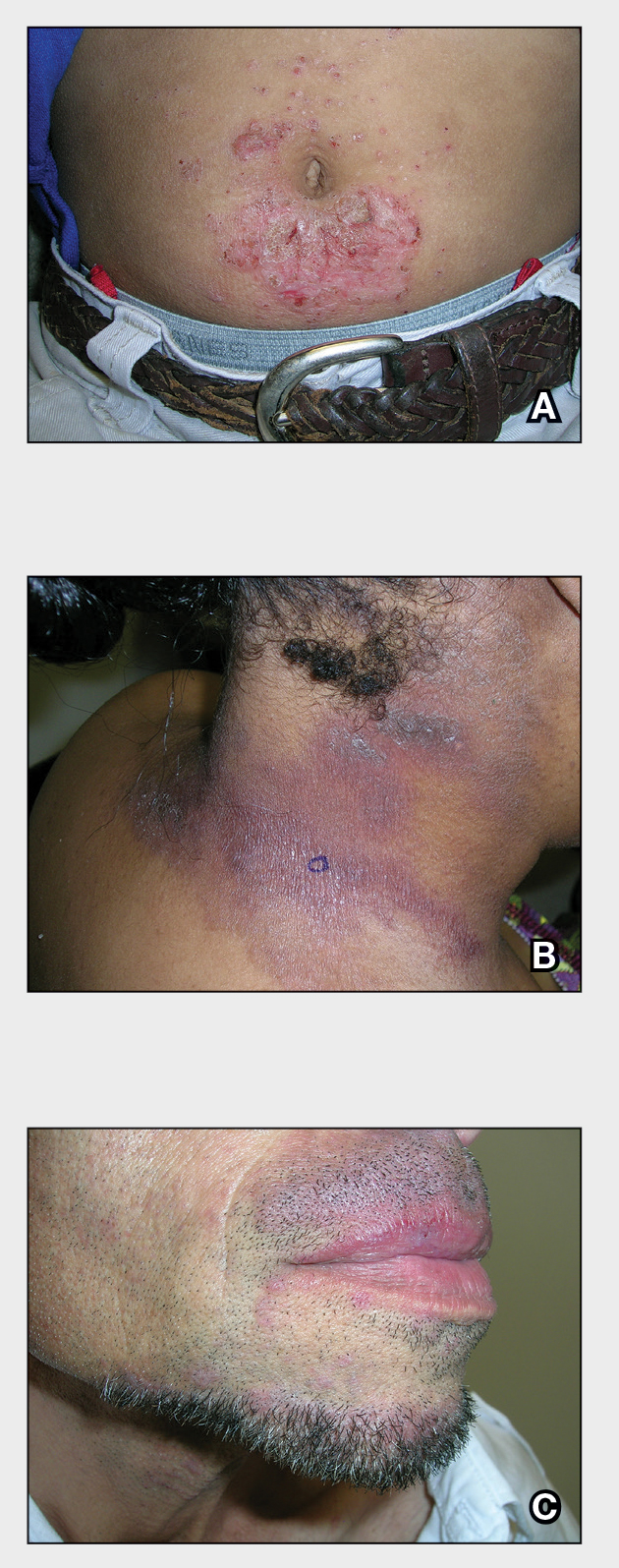
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
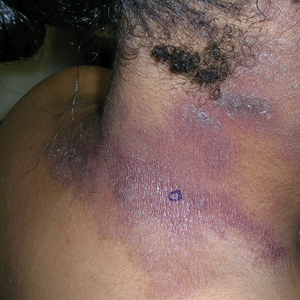
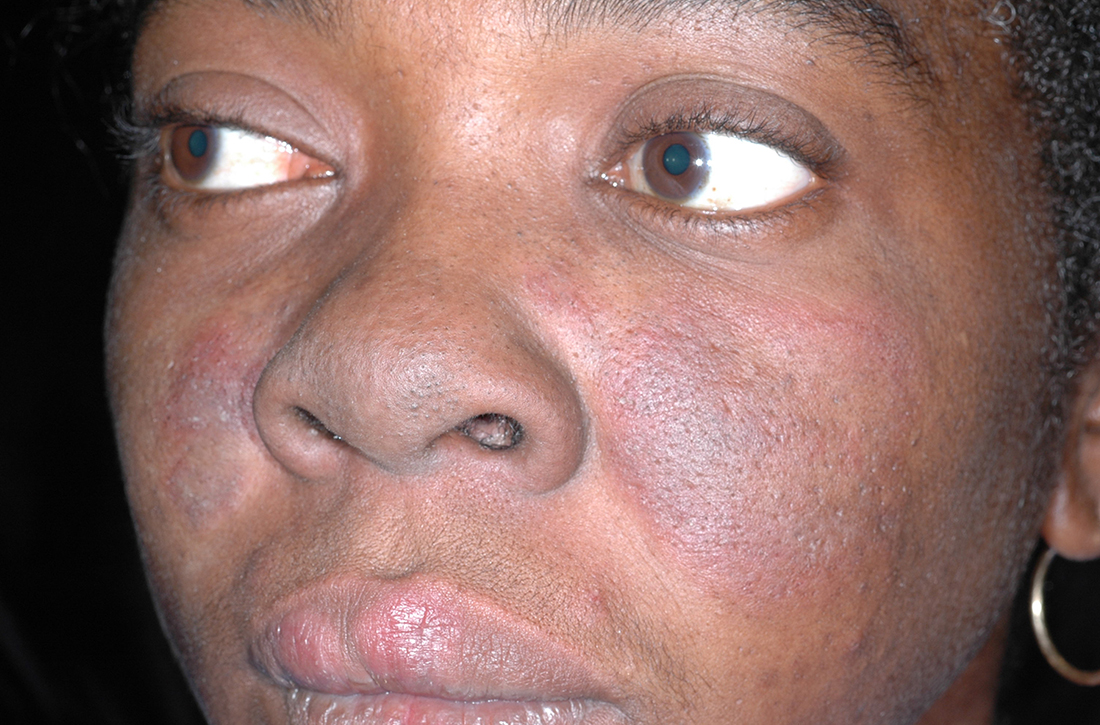
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
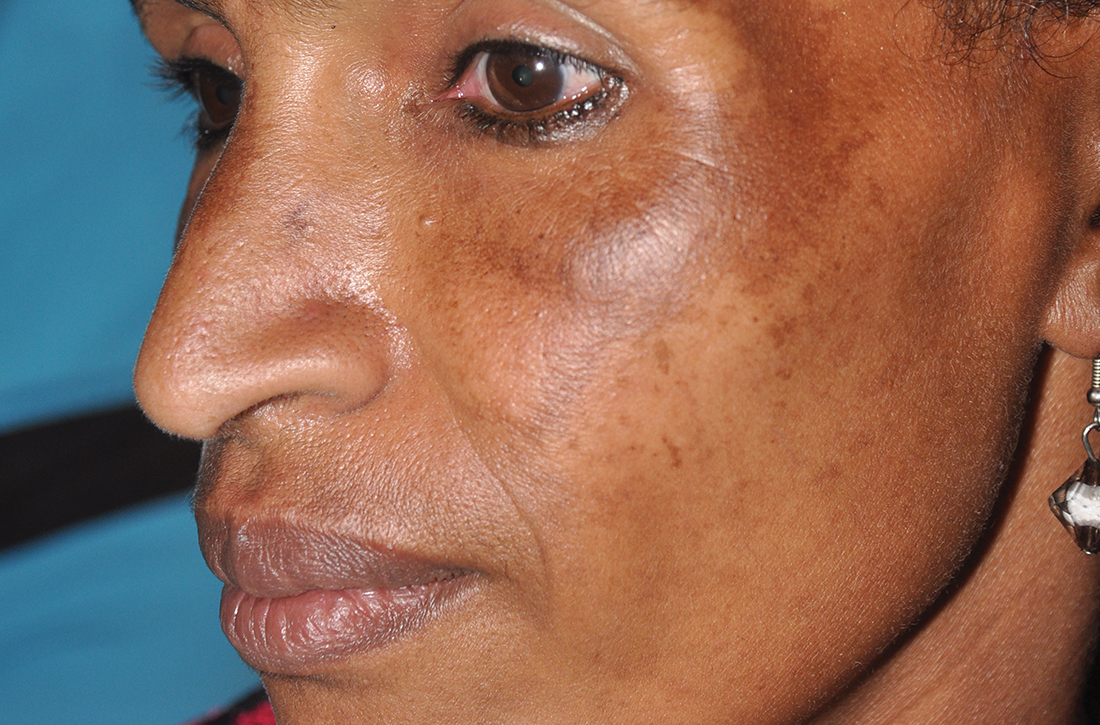
C A Hispanic man with ACD on hair-bearing areas on the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to one or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is reexposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 Allergic contact dermatitis is a challenge to manage, as complete avoidance of the allergen may not be possible.8
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%- 23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N=19,457); 17,803 (92.9%) of these patients were White and only 1360 (7.1%) were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
Allergic contact dermatitis is more common in women, with nickel being the most frequently identified allergen (Figure, A).10 Personal care products often are linked to ACD (Figure, B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD)(a common component of hair dye) (Figure, C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative) (9.1% vs 2.6%) compared to White men.13
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. Allergic contact dermatitis due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on day 1 and covered. Then, on day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N=1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children aged 0–12 years) were significantly lower than for other groups when ACD was suspected (P<.0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23
The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74: 1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi:10.1016/j.jaci.2022.02.002
- Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi:10.1007/s11882-023-01070-5
- Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi:10.1111/j .1365-2133.2005.06415.x
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Nielsen NH, Menne T. The relationship between IgE‐mediated and cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi:10.1111/j.1365-2133.1996.tb06967.x
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998- 2006. Dermatitis. 2016;27:288-292. doi:10.1097/DER.0000000000000220
- Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi:10.1016/j.jaad.2020.10.003
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi:10.1053/ajcd.2001.20110
- DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi:10.1067/mjd.2002.120792
- Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi:10.12788/cutis.0292
- Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi:10.1111/pde.14578
- Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. StatPearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459230/
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi:10.1016/j.jaad.2018.08.049
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi:10.1097 /DER.0000000000000581
- Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi:10.1016/j.jaad.2021.09.022
- Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi:10.1016/j.jaad.2022.08.041
- Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi:10.1016/j.jaad.2022.11.031
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas on the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).

Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to one or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is reexposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 Allergic contact dermatitis is a challenge to manage, as complete avoidance of the allergen may not be possible.8
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%- 23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N=19,457); 17,803 (92.9%) of these patients were White and only 1360 (7.1%) were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
Allergic contact dermatitis is more common in women, with nickel being the most frequently identified allergen (Figure, A).10 Personal care products often are linked to ACD (Figure, B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD)(a common component of hair dye) (Figure, C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative) (9.1% vs 2.6%) compared to White men.13
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. Allergic contact dermatitis due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on day 1 and covered. Then, on day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N=1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children aged 0–12 years) were significantly lower than for other groups when ACD was suspected (P<.0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23
The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas on the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).

Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to one or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is reexposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 Allergic contact dermatitis is a challenge to manage, as complete avoidance of the allergen may not be possible.8
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%- 23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N=19,457); 17,803 (92.9%) of these patients were White and only 1360 (7.1%) were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
Allergic contact dermatitis is more common in women, with nickel being the most frequently identified allergen (Figure, A).10 Personal care products often are linked to ACD (Figure, B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD)(a common component of hair dye) (Figure, C).12
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative) (9.1% vs 2.6%) compared to White men.13
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. Allergic contact dermatitis due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on day 1 and covered. Then, on day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N=1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children aged 0–12 years) were significantly lower than for other groups when ACD was suspected (P<.0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23
The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74: 1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi:10.1016/j.jaci.2022.02.002
- Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi:10.1007/s11882-023-01070-5
- Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi:10.1111/j .1365-2133.2005.06415.x
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Nielsen NH, Menne T. The relationship between IgE‐mediated and cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi:10.1111/j.1365-2133.1996.tb06967.x
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998- 2006. Dermatitis. 2016;27:288-292. doi:10.1097/DER.0000000000000220
- Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi:10.1016/j.jaad.2020.10.003
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi:10.1053/ajcd.2001.20110
- DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi:10.1067/mjd.2002.120792
- Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi:10.12788/cutis.0292
- Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi:10.1111/pde.14578
- Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. StatPearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459230/
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi:10.1016/j.jaad.2018.08.049
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi:10.1097 /DER.0000000000000581
- Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi:10.1016/j.jaad.2021.09.022
- Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi:10.1016/j.jaad.2022.08.041
- Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi:10.1016/j.jaad.2022.11.031
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74: 1029-1040. doi:10.1016/j.jaad.2015.02.1139
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi:10.1016/j.jaci.2022.02.002
- Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi:10.1007/s11882-023-01070-5
- Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi:10.1111/j .1365-2133.2005.06415.x
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Nielsen NH, Menne T. The relationship between IgE‐mediated and cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi:10.1111/j.1365-2133.1996.tb06967.x
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998- 2006. Dermatitis. 2016;27:288-292. doi:10.1097/DER.0000000000000220
- Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi:10.1016/j.jaad.2020.10.003
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi:10.1053/ajcd.2001.20110
- DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi:10.1067/mjd.2002.120792
- Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi:10.12788/cutis.0292
- Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi:10.1111/pde.14578
- Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. StatPearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459230/
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi:10.1016/j.jaad.2018.08.049
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi:10.1097 /DER.0000000000000581
- Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi:10.1016/j.jaad.2021.09.022
- Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi:10.1016/j.jaad.2022.08.041
- Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi:10.1016/j.jaad.2022.11.031
- Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
Squamous Cell Carcinoma
THE COMPARISON
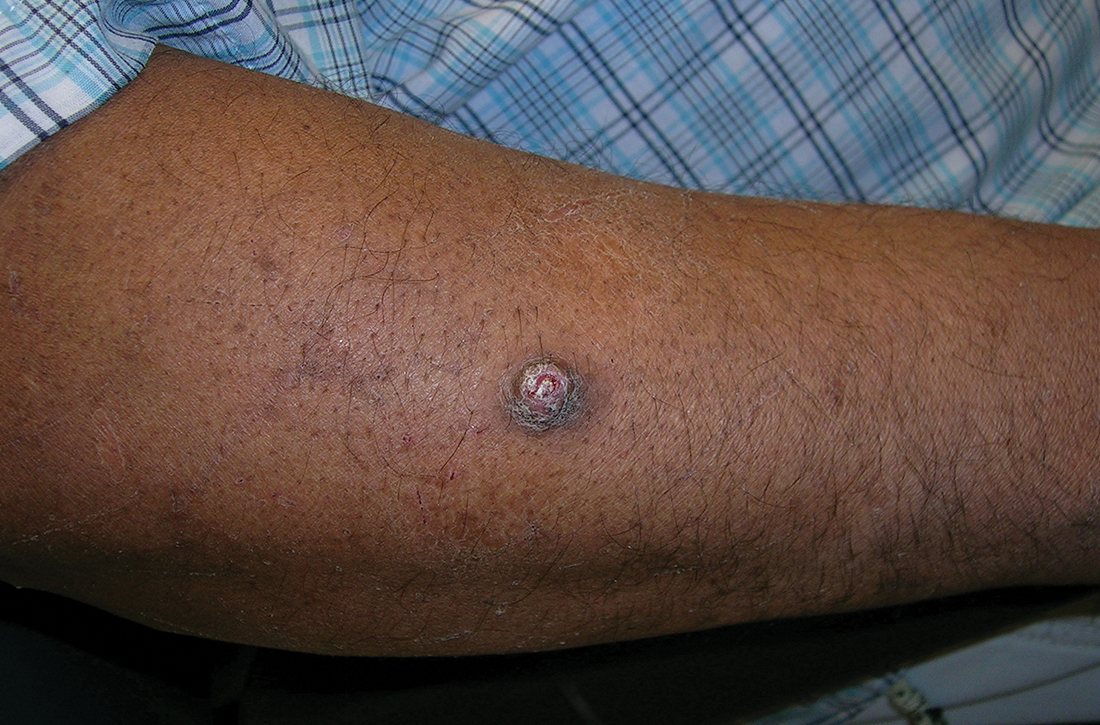
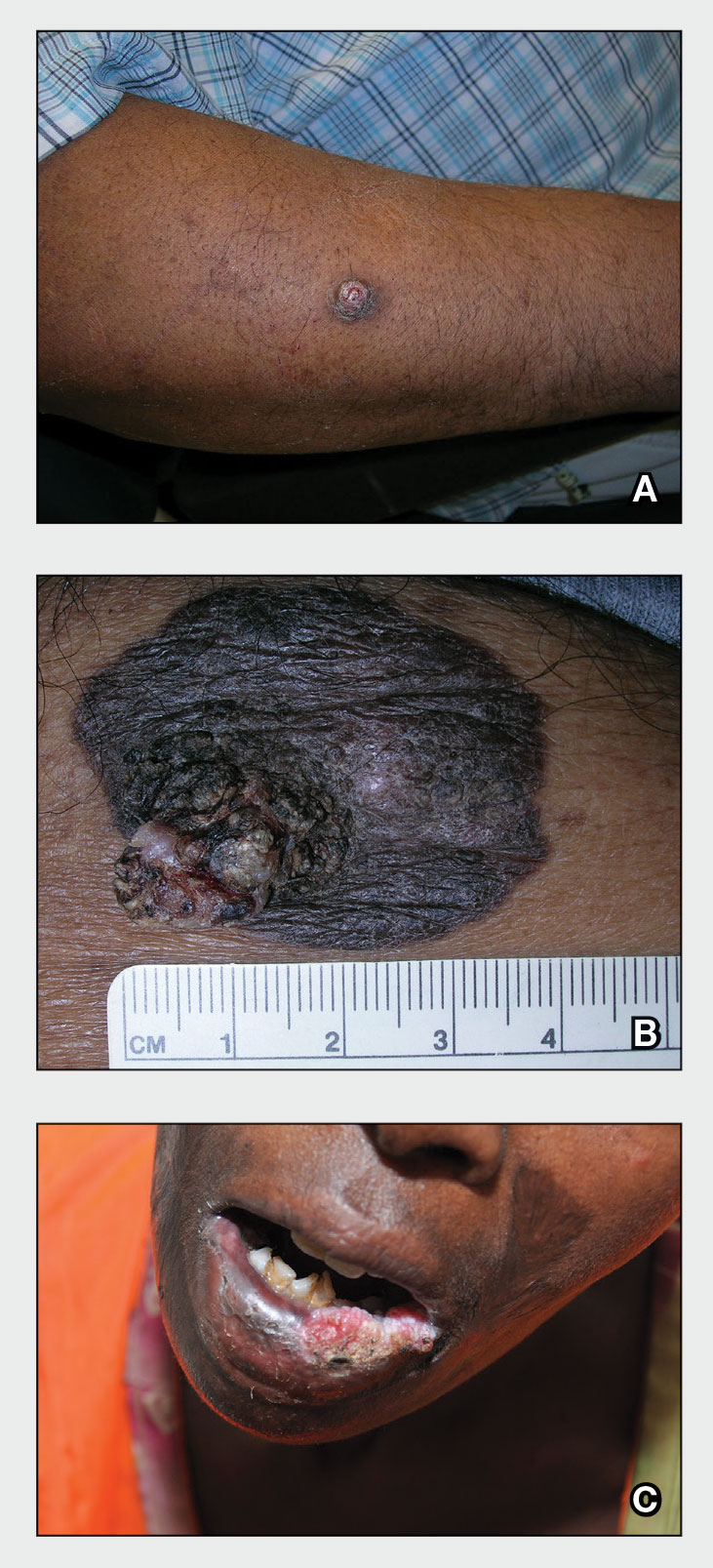
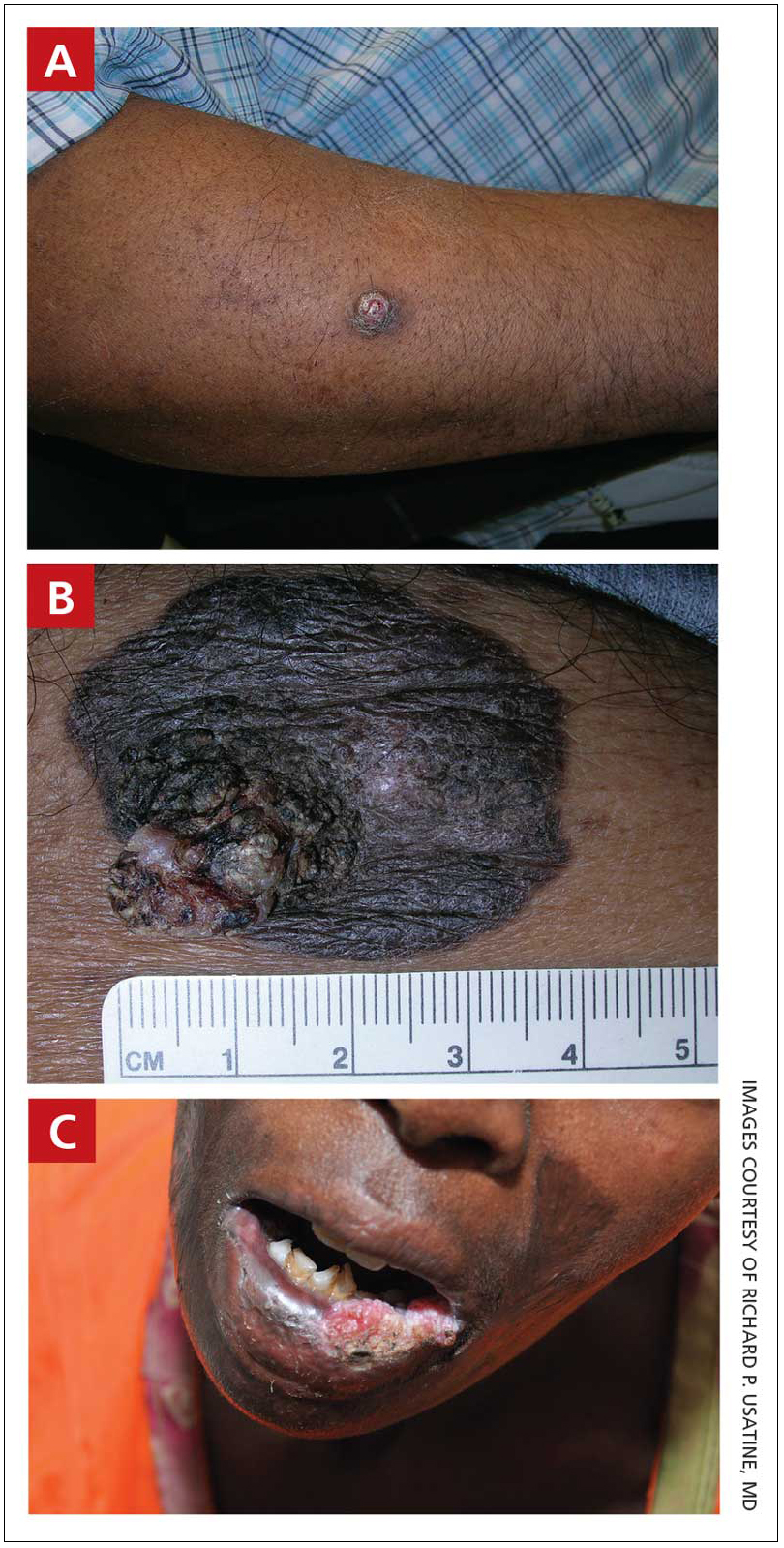
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
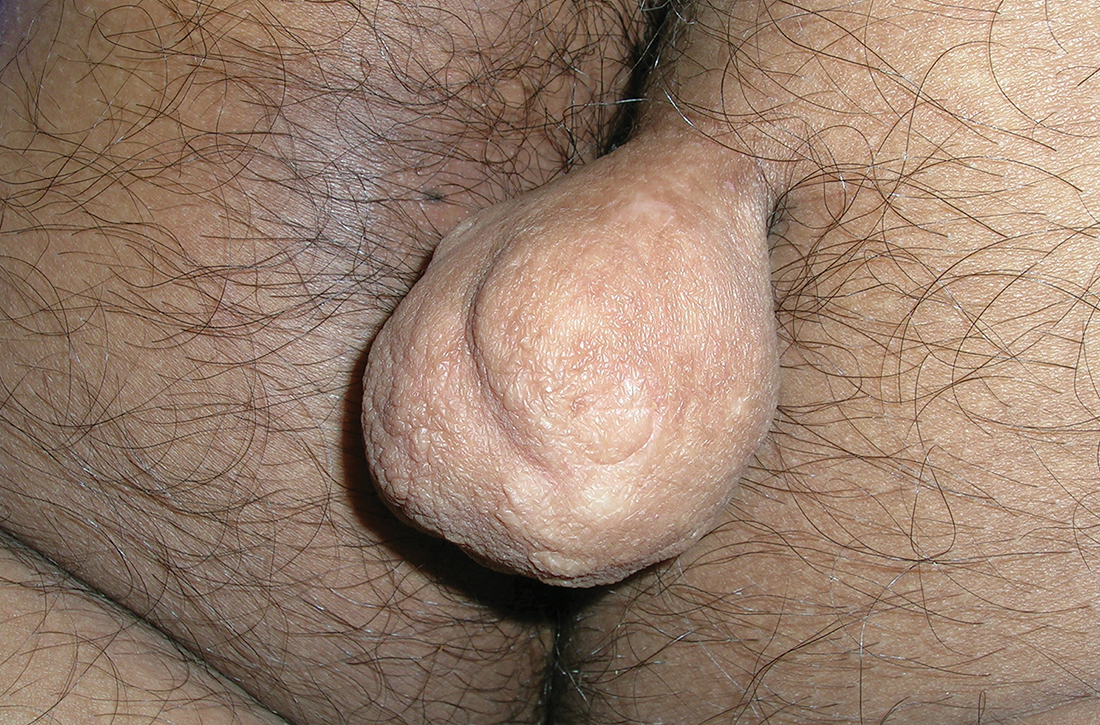
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
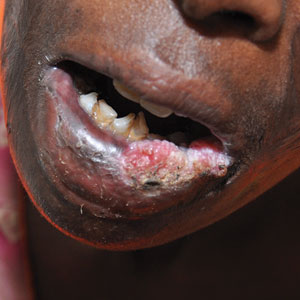
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3
Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Squamous cell carcinoma
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (FIGURE A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (FIGURE B). SCC is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3
Epidemiology
SCC is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N = 413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see highrisk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17
Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 SCC arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (FIGURE C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
SCC is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
Continue to: The risk for SCC...
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
ACKNOWLEDGMENT
The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
1. Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi: 10.1097/ DSS.0000000000000292
2. Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi: 10.1002/1096-9071(200007)61:3<289::aidjmv2> 3.0.co;2-z
3. Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. doi: 10.1111/ijd.12553.
4. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public J Am Acad Dermatol. 2014;70:748-762. doi: 10.1016/j.jaad.2013.11.038
5. Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
6. Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
7. Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. doi: 10.1016/ j.ijwd.2021.01.017
8. Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
9. Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi: 10.1001/jamadermatol.2016.3328
10. Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi: 10.1093/jnci/ djj092
11. Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173:17-21. doi: 10.1111/bjd.13380
12. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi: 10.1016/ s1011-1344(01)00198-1
13. Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
14. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi: 10.1016/s0190-9622 (81)70113-0
15. Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi: 10.5826/dpc.0902a09
16. Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
17. Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635 (03)00085-8
18. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
19. Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61:426-432.
20. Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
21. Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. doi: 10.1016/j.jaad.2021. 12.062
22. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137- 151. doi: 10.1007/s40257-021-00662-z
23. Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi: 10.1046/j.1365- 2230.2003.01210.x
24. Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi: 10.1200/ GO.20.00094
25. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi: 10.1016/j.det.2019.05.009
26. Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
27. Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (FIGURE A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (FIGURE B). SCC is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
SCC is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N = 413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see highrisk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17
Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 SCC arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (FIGURE C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
SCC is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
Continue to: The risk for SCC...
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
ACKNOWLEDGMENT
The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (FIGURE A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (FIGURE B). SCC is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
SCC is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N = 413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see highrisk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17
Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 SCC arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (FIGURE C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
SCC is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
Continue to: The risk for SCC...
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
ACKNOWLEDGMENT
The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
1. Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi: 10.1097/ DSS.0000000000000292
2. Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi: 10.1002/1096-9071(200007)61:3<289::aidjmv2> 3.0.co;2-z
3. Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. doi: 10.1111/ijd.12553.
4. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public J Am Acad Dermatol. 2014;70:748-762. doi: 10.1016/j.jaad.2013.11.038
5. Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
6. Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
7. Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. doi: 10.1016/ j.ijwd.2021.01.017
8. Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
9. Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi: 10.1001/jamadermatol.2016.3328
10. Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi: 10.1093/jnci/ djj092
11. Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173:17-21. doi: 10.1111/bjd.13380
12. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi: 10.1016/ s1011-1344(01)00198-1
13. Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
14. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi: 10.1016/s0190-9622 (81)70113-0
15. Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi: 10.5826/dpc.0902a09
16. Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
17. Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635 (03)00085-8
18. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
19. Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61:426-432.
20. Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
21. Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. doi: 10.1016/j.jaad.2021. 12.062
22. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137- 151. doi: 10.1007/s40257-021-00662-z
23. Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi: 10.1046/j.1365- 2230.2003.01210.x
24. Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi: 10.1200/ GO.20.00094
25. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi: 10.1016/j.det.2019.05.009
26. Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
27. Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
1. Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi: 10.1097/ DSS.0000000000000292
2. Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi: 10.1002/1096-9071(200007)61:3<289::aidjmv2> 3.0.co;2-z
3. Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. doi: 10.1111/ijd.12553.
4. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public J Am Acad Dermatol. 2014;70:748-762. doi: 10.1016/j.jaad.2013.11.038
5. Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
6. Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
7. Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. doi: 10.1016/ j.ijwd.2021.01.017
8. Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
9. Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi: 10.1001/jamadermatol.2016.3328
10. Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi: 10.1093/jnci/ djj092
11. Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173:17-21. doi: 10.1111/bjd.13380
12. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi: 10.1016/ s1011-1344(01)00198-1
13. Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
14. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi: 10.1016/s0190-9622 (81)70113-0
15. Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi: 10.5826/dpc.0902a09
16. Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
17. Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635 (03)00085-8
18. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
19. Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61:426-432.
20. Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
21. Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. doi: 10.1016/j.jaad.2021. 12.062
22. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137- 151. doi: 10.1007/s40257-021-00662-z
23. Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi: 10.1046/j.1365- 2230.2003.01210.x
24. Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi: 10.1200/ GO.20.00094
25. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi: 10.1016/j.det.2019.05.009
26. Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
27. Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Pedunculated gluteal mass
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.
On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.

On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.

On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
Systemic lupus erythematosus
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare.
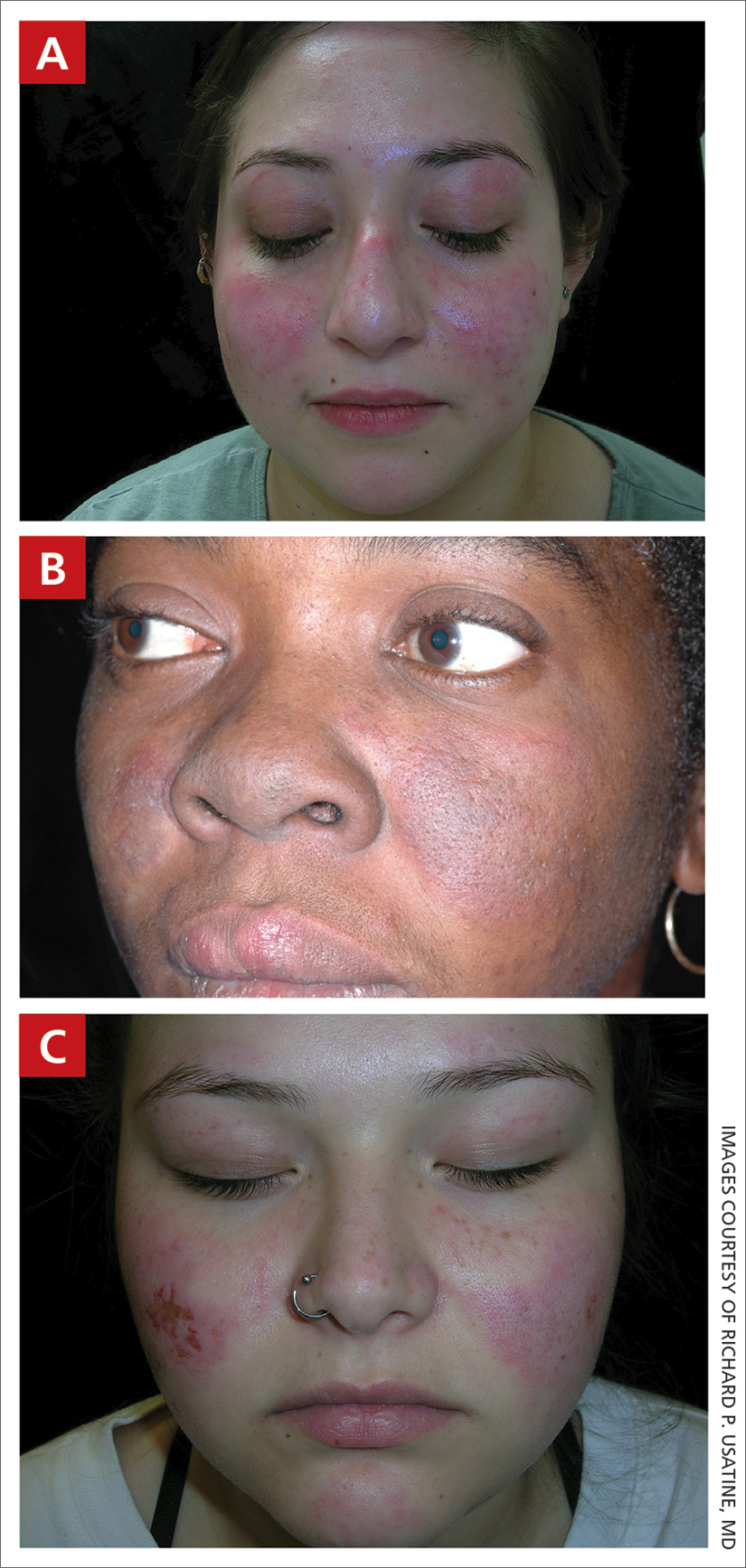
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, although it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE (particularly in those with skin of color), as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/ Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4
Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphospholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
- Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
- The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have farreaching effects, negatively impacting quality of life and even mortality.
1. Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
2. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021;73:991-996. doi: 10.1002/art.41632
3. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi: 10.1002/art.40930
4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
5. Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
6. Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
7. Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
8. Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
9. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi: 10.1080/17446 66X.2018.1538789
10. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi: 10.1038/nrrheum.2016.137
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare.

Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, although it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE (particularly in those with skin of color), as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/ Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4
Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphospholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
- Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
- The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have farreaching effects, negatively impacting quality of life and even mortality.
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare.

Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, although it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE (particularly in those with skin of color), as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/ Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4
Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphospholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
- Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
- The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have farreaching effects, negatively impacting quality of life and even mortality.
1. Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
2. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021;73:991-996. doi: 10.1002/art.41632
3. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi: 10.1002/art.40930
4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
5. Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
6. Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
7. Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
8. Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
9. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi: 10.1080/17446 66X.2018.1538789
10. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi: 10.1038/nrrheum.2016.137
1. Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
2. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021;73:991-996. doi: 10.1002/art.41632
3. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi: 10.1002/art.40930
4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
5. Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
6. Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
7. Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
8. Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
9. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi: 10.1080/17446 66X.2018.1538789
10. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi: 10.1038/nrrheum.2016.137
Systemic Lupus Erythematosus
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
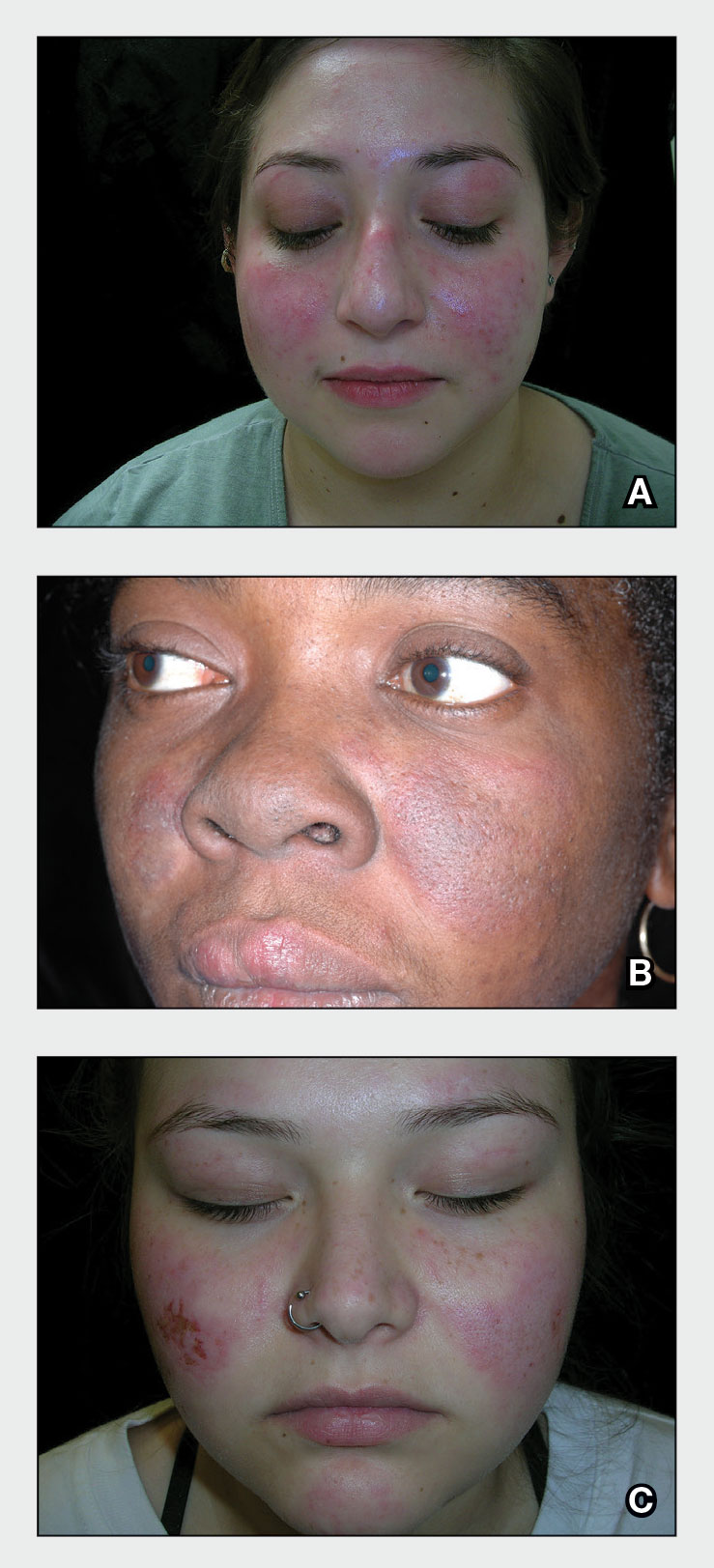
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.

Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.

Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
Melasma
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.
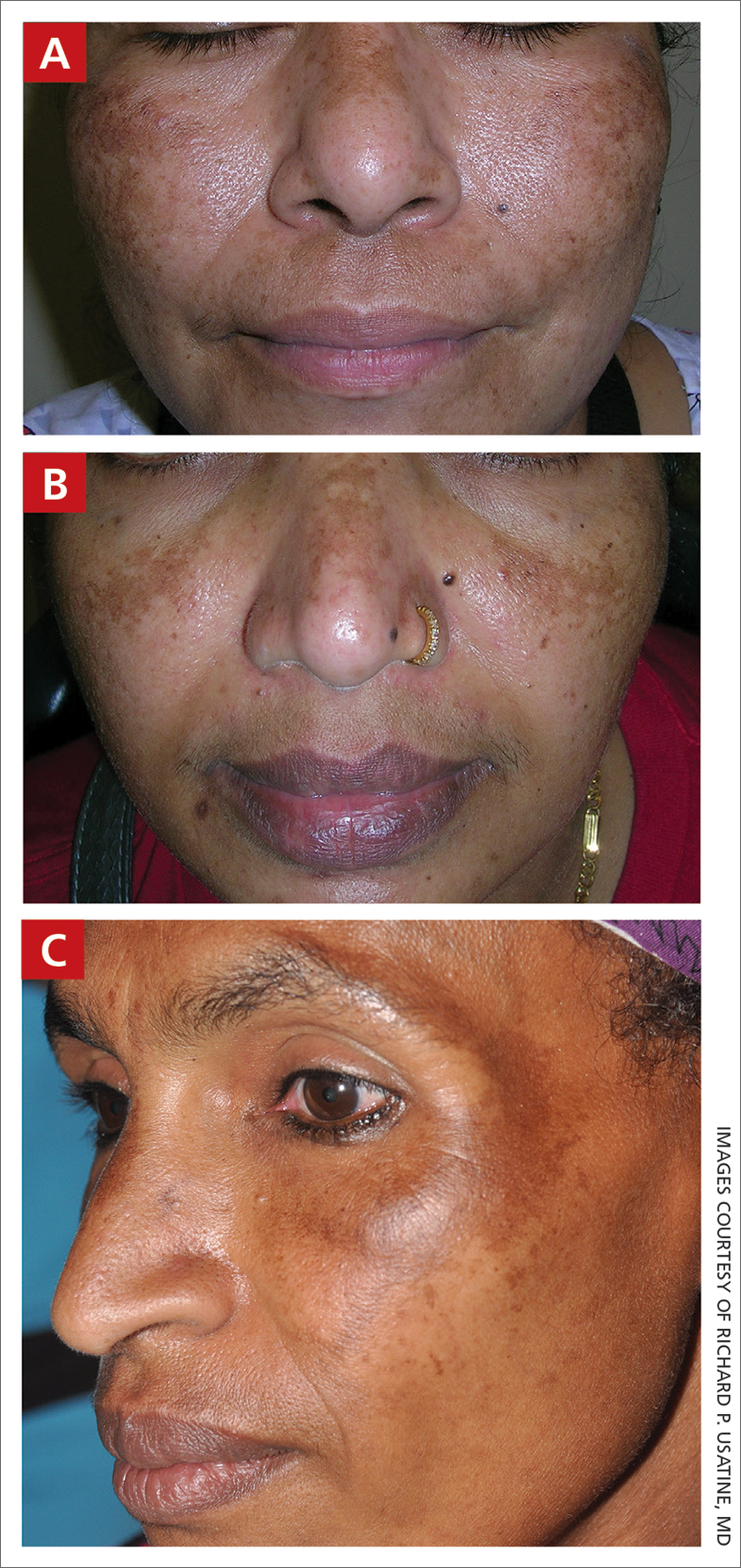
Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women ages 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
- Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
- Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
- Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
- First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
- Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or a-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
- Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or secondline topical therapy.
- Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots, and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
- Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
- Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and self-esteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out of pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
1. Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
2. Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
3. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
4. Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
5. Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
6. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
7. Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30(3). doi:10.1111/dth.12465
8. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
9. Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
10. Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
11. Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
12. Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
13. Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
Melasma
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.
Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
Vitiligo
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Infant with red eyelid lesion
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.
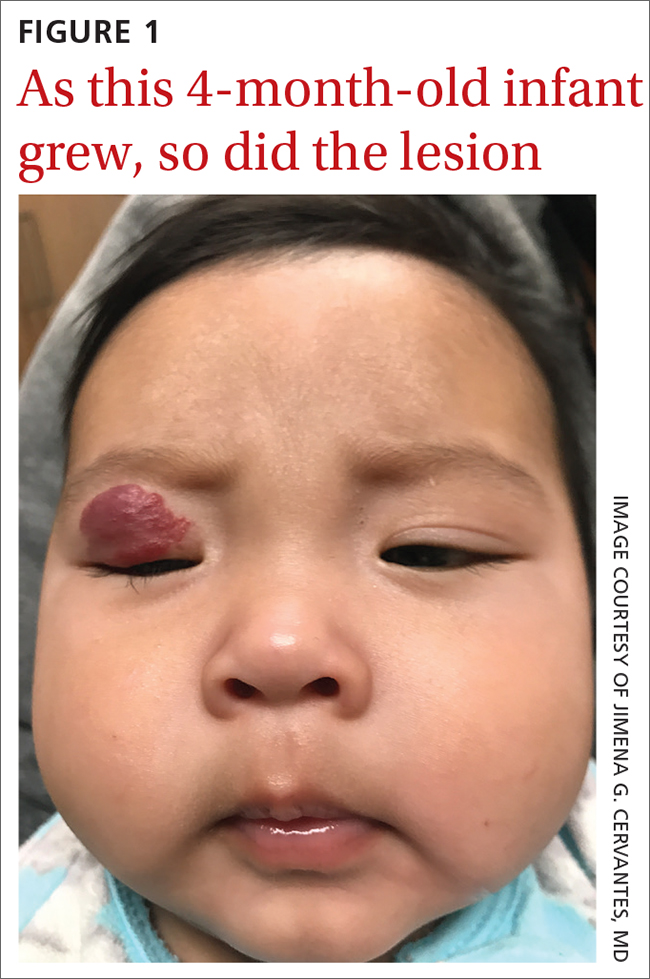
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.
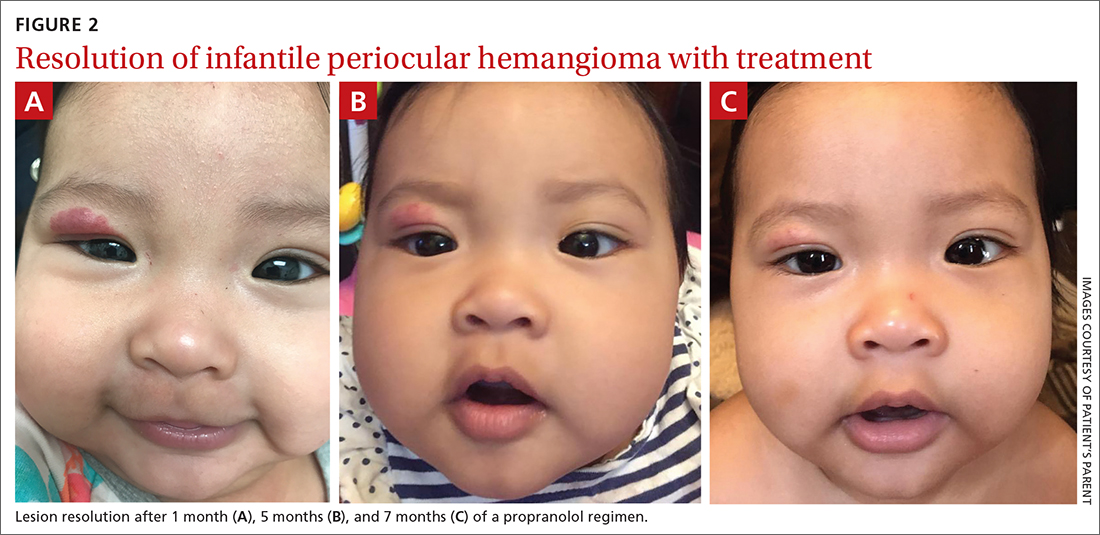
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525