User login
Moving Beyond Traditional Methods for Treatment of Acne Keloidalis Nuchae
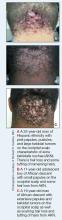
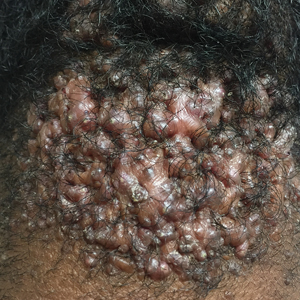
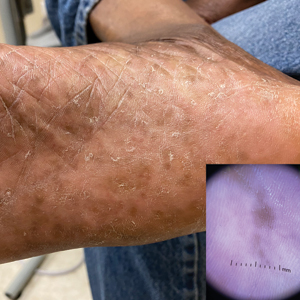
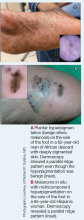
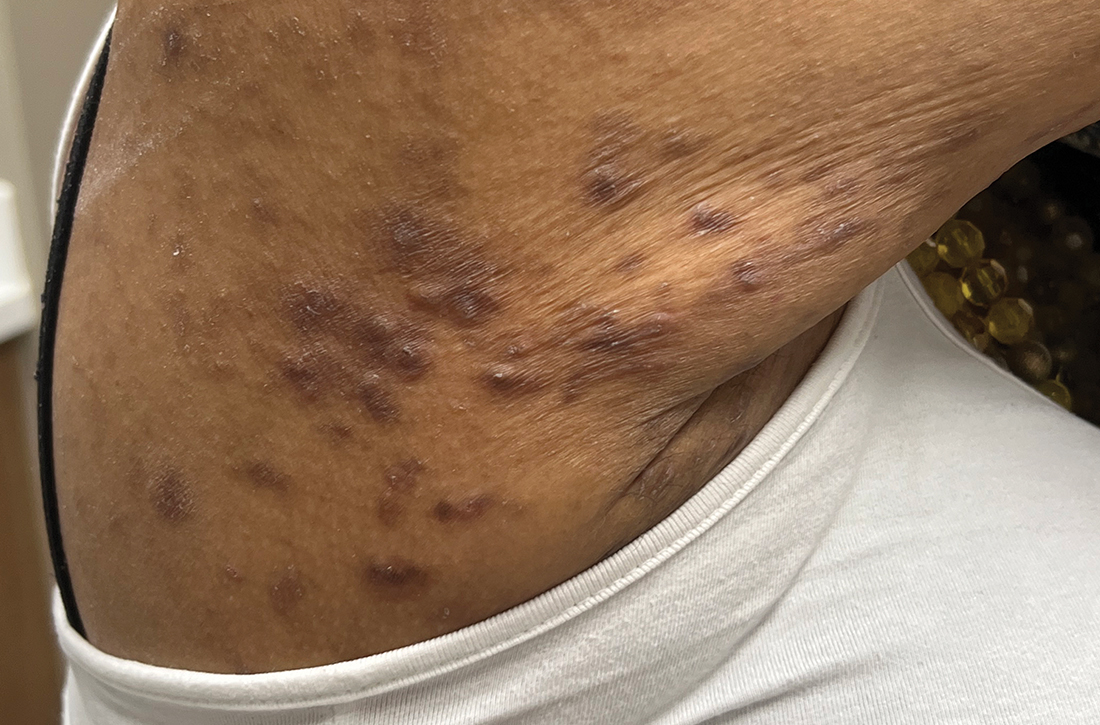
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.16
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace, which leads to hairstyling practices that may increase the risk for AKN.21
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.16
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace, which leads to hairstyling practices that may increase the risk for AKN.21
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.16
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace, which leads to hairstyling practices that may increase the risk for AKN.21
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
Moving Beyond Traditional Methods for Treatment of Acne Keloidalis Nuchae
The Comparison
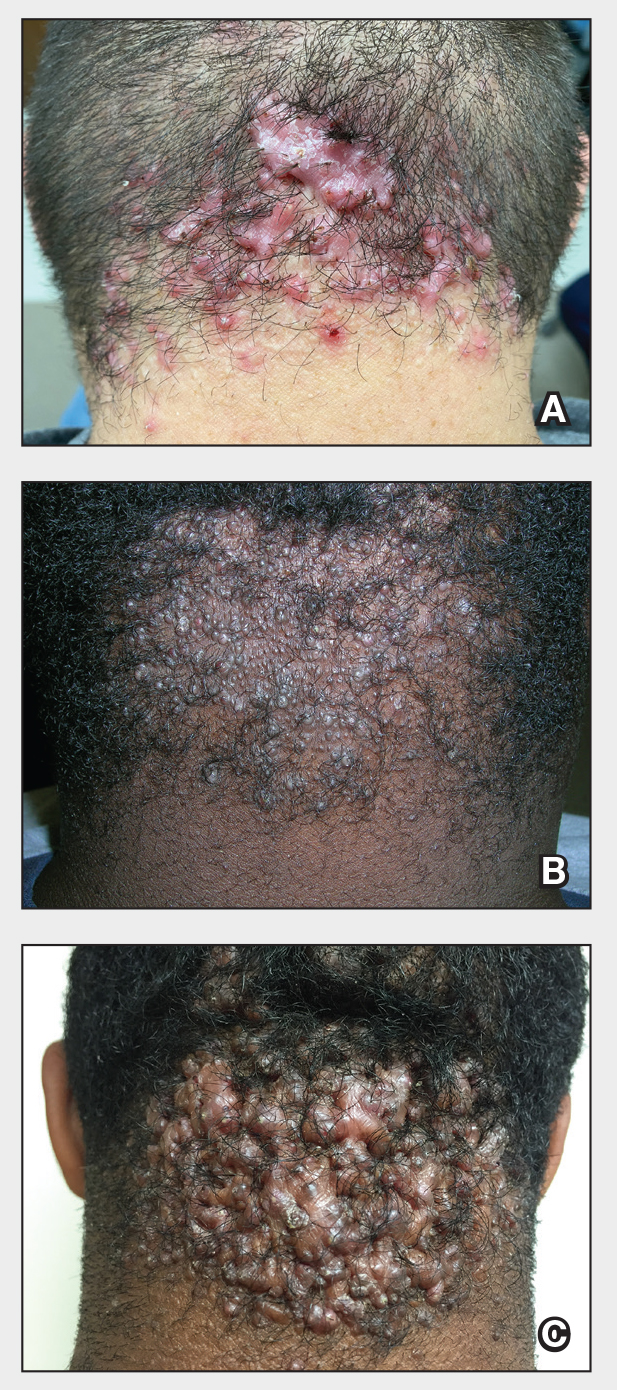
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
The Comparison

Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
The Comparison

Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
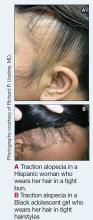
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
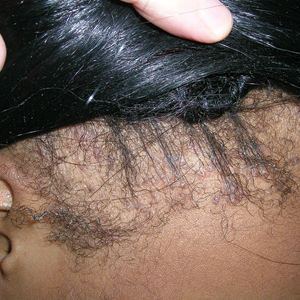
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8 Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
1. Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
2. James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
3. Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
4. Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
5. Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
6. Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
7. Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
8. Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
9. Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
10. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
11. Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
12. Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
13. Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10.1111/ajd.13187
14. Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
15. Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
16. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
17. Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
18. Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
19. Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
20. Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
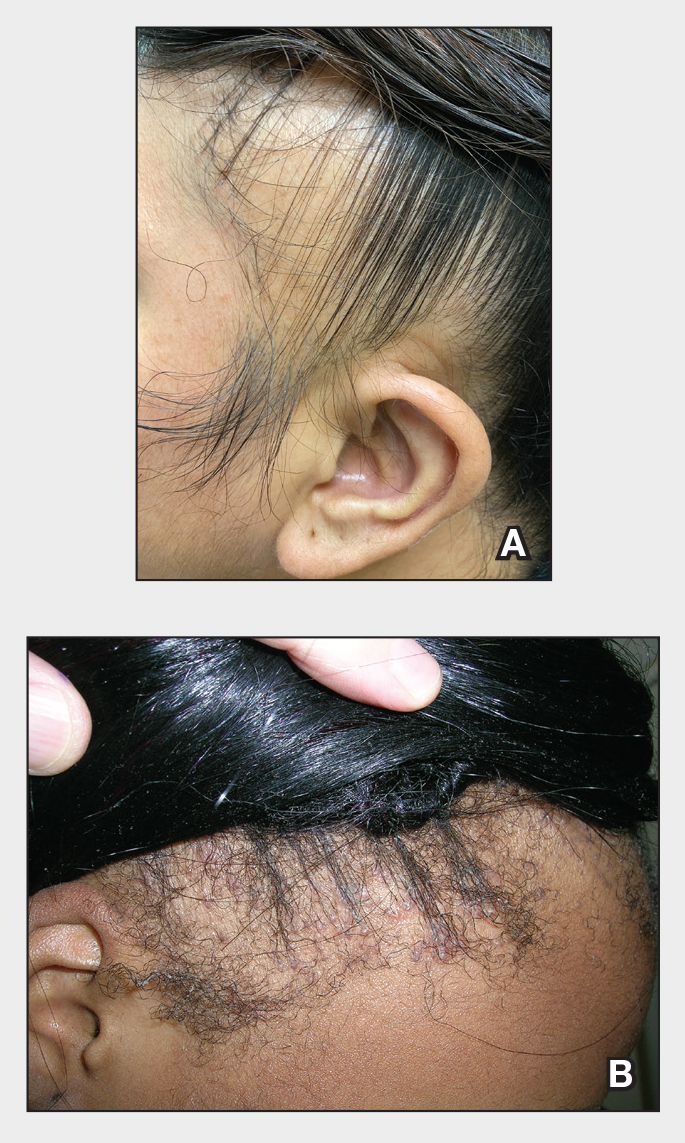
The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Plantar Hyperpigmentation
The Comparison
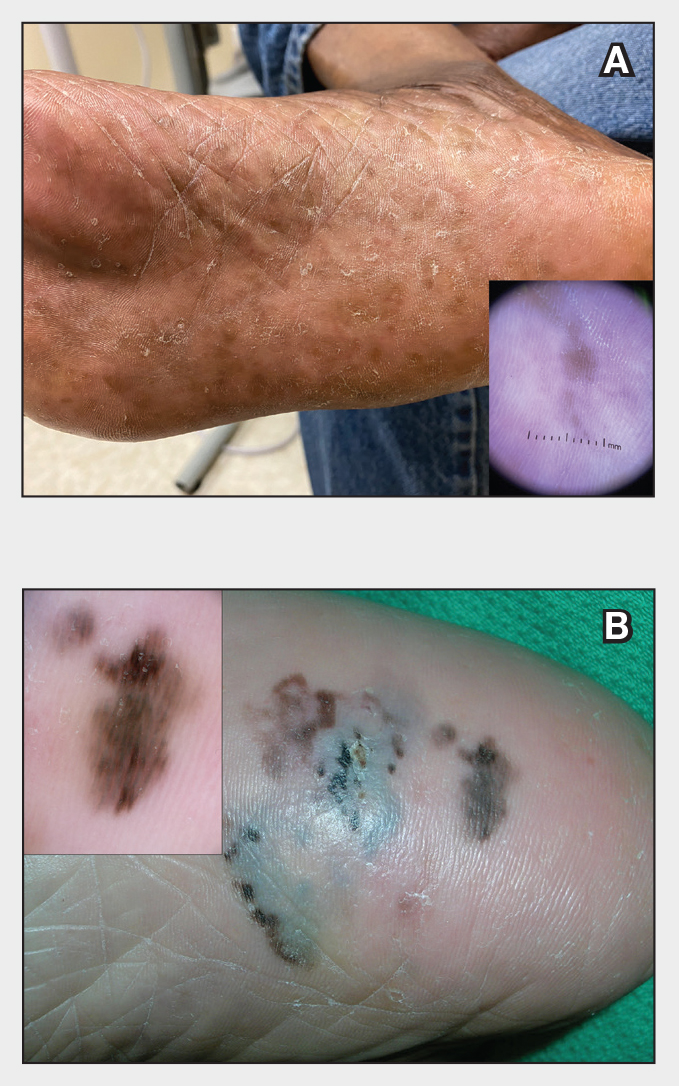
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Plantar Hyperpigmentation
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Dx Across the Skin Color Spectrum: Longitudinal Melanonychia
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal Melanonychia
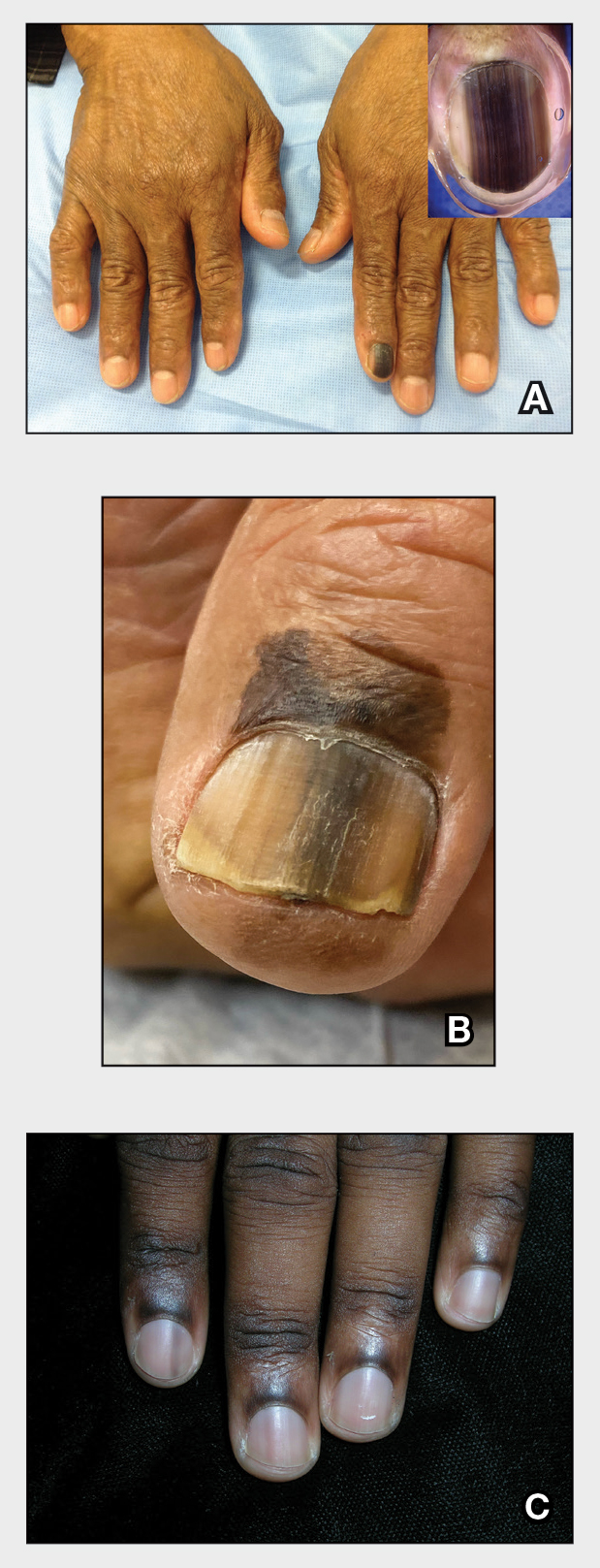
THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Pruritic rash and nocturnal itching
A 62-YEAR-OLD HISPANIC WOMAN with a history of well-controlled diabetes and hypertension presented with an intensely pruritic rash of 3 months’ duration. She reported poor sleep due to scratching throughout the night. She denied close contact with individuals with similar rashes or itching, new intimate partners, or recent travel. She worked in an office setting and had stable, noncrowded housing.
A physical exam revealed brown and purple scaly papules and many excoriation marks. The rash was concentrated along clothing lines, around intertriginous areas, and on her ankles, wrists, and the interdigital spaces (FIGURE 1A and 1B).
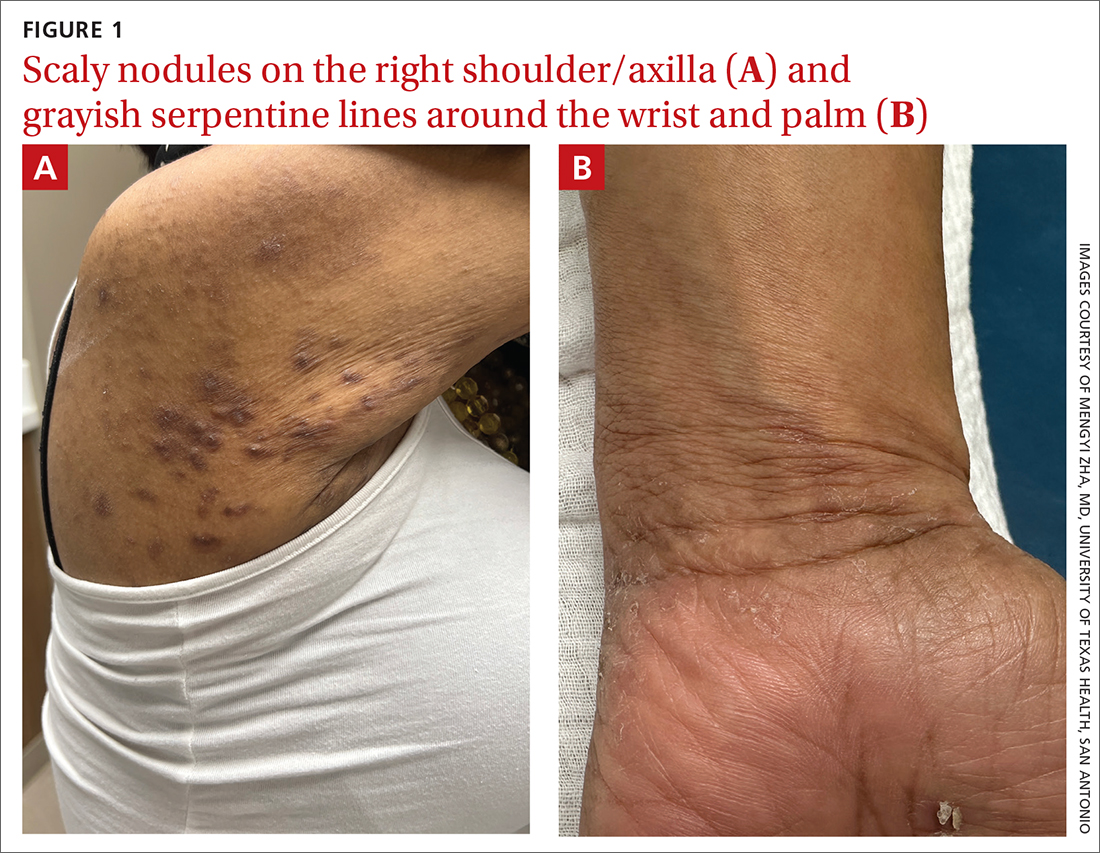
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scabies
Scabies is a diagnosis that should be considered in any patient with new-onset, widespread, nocturnal-dominant pruritus1 and it was suspected, in this case, after the initial history taking and physical exam. (See “Consider these diagnoses in cases of pruritic skin conditions” for more on lichen planus and prurigo nodularis, which were also included in the differential diagnosis.)
SIDEBAR
Consider these diagnoses in cases of pruritic skin conditions
Lichen planus is a chronic inflammatory condition that mostly affects the skin and mucosa. Characteristic findings are groups of shiny, flat-topped, firm papules. This patient’s widespread nodular lesions with rough scales were not typical of lichen planus, which usually manifests with flat (hence the name “planus”) and shiny lesions.
Prurigo nodularis is a chronic condition that manifests as intensely itchy, firm papules. The lesions can appear anywhere on the body, but more commonly are found on the extremities, back, and torso. The recent manifestation of the patient’s lesions and her lack of a history of chronic dermatitis argued against this diagnosis.
The use of a handheld dermatoscope confirmed the diagnosis by revealing white to yellow scales following the serpiginous lines. These serpiginous lines resembled scabies burrows, and at the end of some burrows, small triangular and hyperpigmented structures resembling “delta-winged jets” were seen. These “delta-winged jets” were the mite’s pigmented mouth parts and anterior legs. The burrows, which contain eggs and feces, have been described as the “contrails” behind the jets (FIGURE 2).
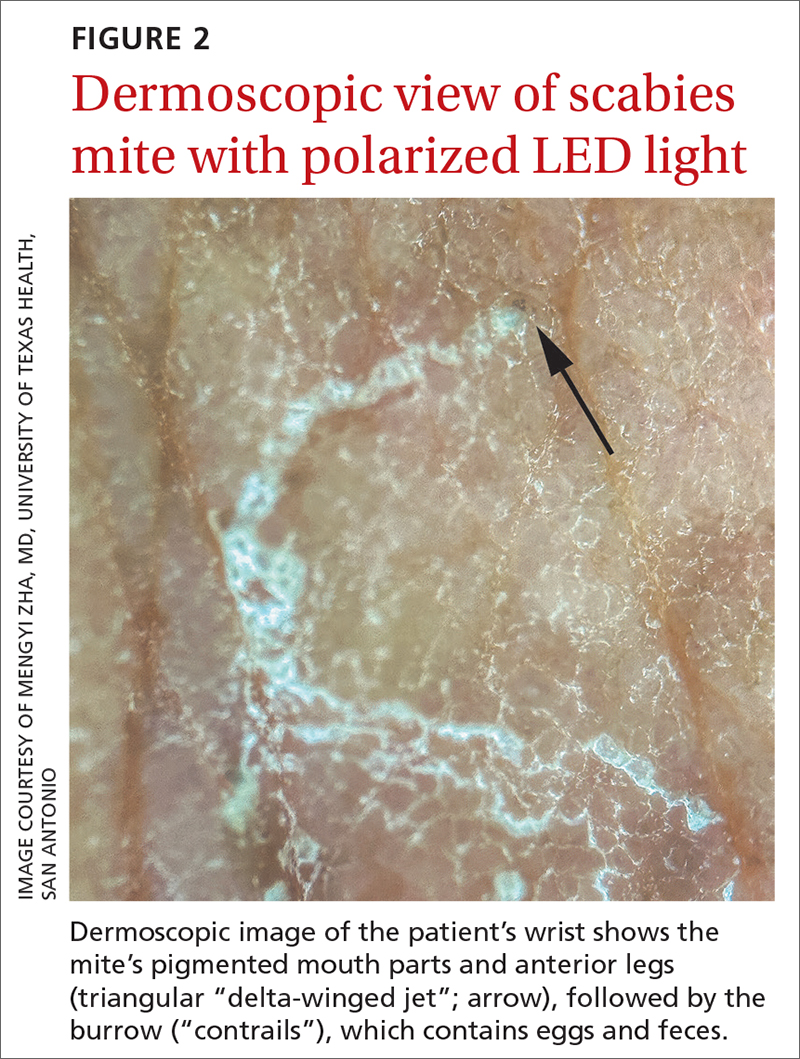
The use of a new UV illumination feature on our dermatoscope (which we’ll describe shortly) made for an even more dramatic diagnostic visual. With the click of a button, the mites fluoresced green to yellow and the burrows fluoresced white to blue (FIGURE 3).
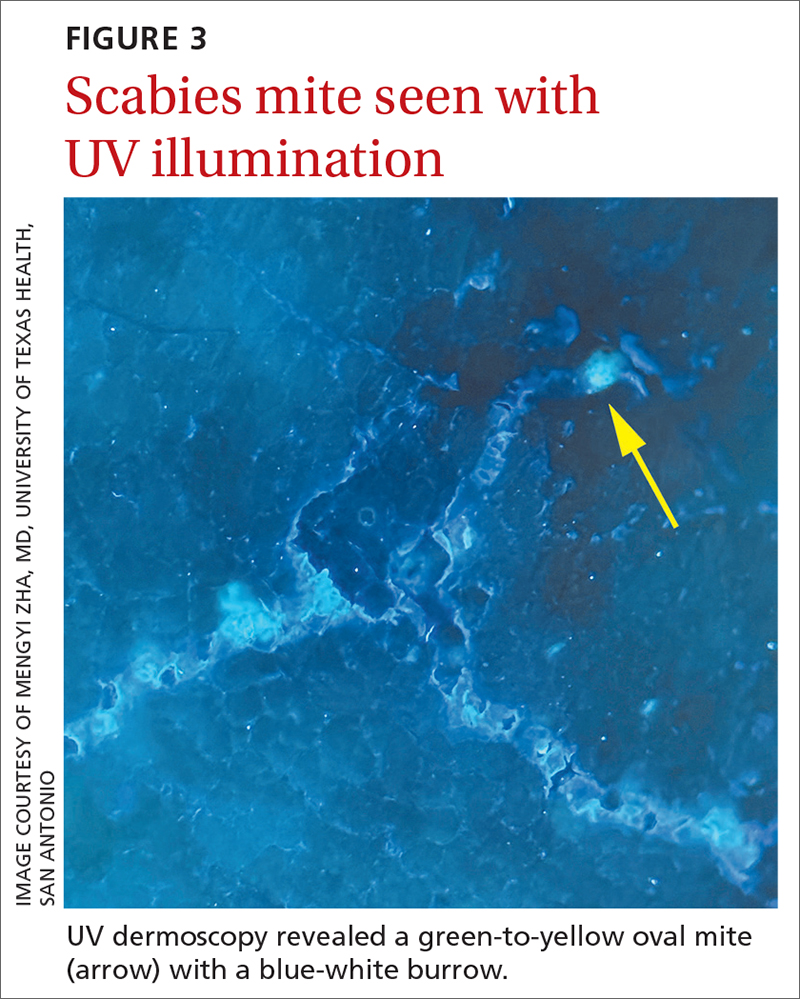
Meeting the criteria. The clinical and dermoscopic findings met the 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies,2 confirming the diagnosis in this patient. Scabies infestation poses a significant public health burden globally, with an estimated incidence of more than 454 million in 2016.3
Visualization is key to the diagnosis
Traditionally, the diagnosis of scabies infestation is made by direct visualization of mites via microscopy of skin scrapings.4 However, this approach is seldom feasible in a family medicine office. Fortunately, the 2020 IACS criteria included dermoscopy as a Level A diagnostic method for confirmed scabies.
Continue to: The pros and cons of dermoscopy
The pros and cons of dermoscopy. A handheld dermatoscope is an accessible, convenient tool for any clinician who treats the skin. It has been demonstrated that, in the hands of experts and novices alike, dermoscopy has a sensitivity of 91% and specificity of 86% for the diagnosis of scabies.5
However, accurate identification of the dermoscopic findings can depend on the operator and can be harder to achieve in patients who have skin of color.2 This is largely because the mite’s brown-to-black triangular head is small (sometimes hidden under skin scales) and easy to miss, especially against darker skin.
A new technologic feature helps. In this case, we used the built-in 365-nm UV illumination feature of our handheld dermatoscope (Dermlite-5) and both mites and burrows fluoresced intensely (FIGURE 3). A skin scraping at the location of the fluorescent body under microscopic examination confirmed that the organism was a Sarcoptes scabiei mite (FIGURE 4).
UV light dermoscopy can decrease operator error and ameliorate the challenge of diagnosing scabies in skin of color. Specifically, when using UV dermoscopy it’s easier to:
- locate mites, regardless of the patient’s skin color
- see the mite’s entire body, rather than just a small portion (thus increasing diagnostic certainty).
New diagnostic feature, classic treatment
Due to the severity of the patient’s scabies, she was prescribed both permethrin 5% cream and oral ivermectin 200 mcg/kg, both to be used immediately and repeated in 1 week. Notably, a systematic review indicated that topical permethrin is a superior treatment to oral ivermectin.6 However, in cases of widespread scabies and crusted scabies, it is standard of care to treat with both medications.
The patient’s pruritus was treated with cetirizine as needed. She was told that the itching might persist for a few weeks after treatment was completed.
Reinfestation was a concern with this patient because she was unable to identify a source for the mites. To minimize the likelihood of reinfestation, we advised her to decontaminate her bedding, clothing, and towels by washing them in hot water (≥ 122° F) or placing in a sealed plastic bag for at least 1 week.1 For crusted scabies cases, thorough vacuuming of a patient’s furniture and carpets is recommended.
1. Gunning K, Kiraly B, Pippitt K. Lice and scabies: treatment update. Am Fam Physician. 2019;99:635-642.
2. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br J Dermatol. 2020;183:808-820. doi: 10.1111/bjd.18943
A 62-YEAR-OLD HISPANIC WOMAN with a history of well-controlled diabetes and hypertension presented with an intensely pruritic rash of 3 months’ duration. She reported poor sleep due to scratching throughout the night. She denied close contact with individuals with similar rashes or itching, new intimate partners, or recent travel. She worked in an office setting and had stable, noncrowded housing.
A physical exam revealed brown and purple scaly papules and many excoriation marks. The rash was concentrated along clothing lines, around intertriginous areas, and on her ankles, wrists, and the interdigital spaces (FIGURE 1A and 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scabies
Scabies is a diagnosis that should be considered in any patient with new-onset, widespread, nocturnal-dominant pruritus1 and it was suspected, in this case, after the initial history taking and physical exam. (See “Consider these diagnoses in cases of pruritic skin conditions” for more on lichen planus and prurigo nodularis, which were also included in the differential diagnosis.)
SIDEBAR
Consider these diagnoses in cases of pruritic skin conditions
Lichen planus is a chronic inflammatory condition that mostly affects the skin and mucosa. Characteristic findings are groups of shiny, flat-topped, firm papules. This patient’s widespread nodular lesions with rough scales were not typical of lichen planus, which usually manifests with flat (hence the name “planus”) and shiny lesions.
Prurigo nodularis is a chronic condition that manifests as intensely itchy, firm papules. The lesions can appear anywhere on the body, but more commonly are found on the extremities, back, and torso. The recent manifestation of the patient’s lesions and her lack of a history of chronic dermatitis argued against this diagnosis.
The use of a handheld dermatoscope confirmed the diagnosis by revealing white to yellow scales following the serpiginous lines. These serpiginous lines resembled scabies burrows, and at the end of some burrows, small triangular and hyperpigmented structures resembling “delta-winged jets” were seen. These “delta-winged jets” were the mite’s pigmented mouth parts and anterior legs. The burrows, which contain eggs and feces, have been described as the “contrails” behind the jets (FIGURE 2).

The use of a new UV illumination feature on our dermatoscope (which we’ll describe shortly) made for an even more dramatic diagnostic visual. With the click of a button, the mites fluoresced green to yellow and the burrows fluoresced white to blue (FIGURE 3).

Meeting the criteria. The clinical and dermoscopic findings met the 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies,2 confirming the diagnosis in this patient. Scabies infestation poses a significant public health burden globally, with an estimated incidence of more than 454 million in 2016.3
Visualization is key to the diagnosis
Traditionally, the diagnosis of scabies infestation is made by direct visualization of mites via microscopy of skin scrapings.4 However, this approach is seldom feasible in a family medicine office. Fortunately, the 2020 IACS criteria included dermoscopy as a Level A diagnostic method for confirmed scabies.
Continue to: The pros and cons of dermoscopy
The pros and cons of dermoscopy. A handheld dermatoscope is an accessible, convenient tool for any clinician who treats the skin. It has been demonstrated that, in the hands of experts and novices alike, dermoscopy has a sensitivity of 91% and specificity of 86% for the diagnosis of scabies.5
However, accurate identification of the dermoscopic findings can depend on the operator and can be harder to achieve in patients who have skin of color.2 This is largely because the mite’s brown-to-black triangular head is small (sometimes hidden under skin scales) and easy to miss, especially against darker skin.
A new technologic feature helps. In this case, we used the built-in 365-nm UV illumination feature of our handheld dermatoscope (Dermlite-5) and both mites and burrows fluoresced intensely (FIGURE 3). A skin scraping at the location of the fluorescent body under microscopic examination confirmed that the organism was a Sarcoptes scabiei mite (FIGURE 4).

UV light dermoscopy can decrease operator error and ameliorate the challenge of diagnosing scabies in skin of color. Specifically, when using UV dermoscopy it’s easier to:
- locate mites, regardless of the patient’s skin color
- see the mite’s entire body, rather than just a small portion (thus increasing diagnostic certainty).
New diagnostic feature, classic treatment
Due to the severity of the patient’s scabies, she was prescribed both permethrin 5% cream and oral ivermectin 200 mcg/kg, both to be used immediately and repeated in 1 week. Notably, a systematic review indicated that topical permethrin is a superior treatment to oral ivermectin.6 However, in cases of widespread scabies and crusted scabies, it is standard of care to treat with both medications.
The patient’s pruritus was treated with cetirizine as needed. She was told that the itching might persist for a few weeks after treatment was completed.
Reinfestation was a concern with this patient because she was unable to identify a source for the mites. To minimize the likelihood of reinfestation, we advised her to decontaminate her bedding, clothing, and towels by washing them in hot water (≥ 122° F) or placing in a sealed plastic bag for at least 1 week.1 For crusted scabies cases, thorough vacuuming of a patient’s furniture and carpets is recommended.
A 62-YEAR-OLD HISPANIC WOMAN with a history of well-controlled diabetes and hypertension presented with an intensely pruritic rash of 3 months’ duration. She reported poor sleep due to scratching throughout the night. She denied close contact with individuals with similar rashes or itching, new intimate partners, or recent travel. She worked in an office setting and had stable, noncrowded housing.
A physical exam revealed brown and purple scaly papules and many excoriation marks. The rash was concentrated along clothing lines, around intertriginous areas, and on her ankles, wrists, and the interdigital spaces (FIGURE 1A and 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scabies
Scabies is a diagnosis that should be considered in any patient with new-onset, widespread, nocturnal-dominant pruritus1 and it was suspected, in this case, after the initial history taking and physical exam. (See “Consider these diagnoses in cases of pruritic skin conditions” for more on lichen planus and prurigo nodularis, which were also included in the differential diagnosis.)
SIDEBAR
Consider these diagnoses in cases of pruritic skin conditions
Lichen planus is a chronic inflammatory condition that mostly affects the skin and mucosa. Characteristic findings are groups of shiny, flat-topped, firm papules. This patient’s widespread nodular lesions with rough scales were not typical of lichen planus, which usually manifests with flat (hence the name “planus”) and shiny lesions.
Prurigo nodularis is a chronic condition that manifests as intensely itchy, firm papules. The lesions can appear anywhere on the body, but more commonly are found on the extremities, back, and torso. The recent manifestation of the patient’s lesions and her lack of a history of chronic dermatitis argued against this diagnosis.
The use of a handheld dermatoscope confirmed the diagnosis by revealing white to yellow scales following the serpiginous lines. These serpiginous lines resembled scabies burrows, and at the end of some burrows, small triangular and hyperpigmented structures resembling “delta-winged jets” were seen. These “delta-winged jets” were the mite’s pigmented mouth parts and anterior legs. The burrows, which contain eggs and feces, have been described as the “contrails” behind the jets (FIGURE 2).

The use of a new UV illumination feature on our dermatoscope (which we’ll describe shortly) made for an even more dramatic diagnostic visual. With the click of a button, the mites fluoresced green to yellow and the burrows fluoresced white to blue (FIGURE 3).

Meeting the criteria. The clinical and dermoscopic findings met the 2020 International Alliance for the Control of Scabies (IACS) Consensus Criteria for the Diagnosis of Scabies,2 confirming the diagnosis in this patient. Scabies infestation poses a significant public health burden globally, with an estimated incidence of more than 454 million in 2016.3
Visualization is key to the diagnosis
Traditionally, the diagnosis of scabies infestation is made by direct visualization of mites via microscopy of skin scrapings.4 However, this approach is seldom feasible in a family medicine office. Fortunately, the 2020 IACS criteria included dermoscopy as a Level A diagnostic method for confirmed scabies.
Continue to: The pros and cons of dermoscopy
The pros and cons of dermoscopy. A handheld dermatoscope is an accessible, convenient tool for any clinician who treats the skin. It has been demonstrated that, in the hands of experts and novices alike, dermoscopy has a sensitivity of 91% and specificity of 86% for the diagnosis of scabies.5
However, accurate identification of the dermoscopic findings can depend on the operator and can be harder to achieve in patients who have skin of color.2 This is largely because the mite’s brown-to-black triangular head is small (sometimes hidden under skin scales) and easy to miss, especially against darker skin.
A new technologic feature helps. In this case, we used the built-in 365-nm UV illumination feature of our handheld dermatoscope (Dermlite-5) and both mites and burrows fluoresced intensely (FIGURE 3). A skin scraping at the location of the fluorescent body under microscopic examination confirmed that the organism was a Sarcoptes scabiei mite (FIGURE 4).

UV light dermoscopy can decrease operator error and ameliorate the challenge of diagnosing scabies in skin of color. Specifically, when using UV dermoscopy it’s easier to:
- locate mites, regardless of the patient’s skin color
- see the mite’s entire body, rather than just a small portion (thus increasing diagnostic certainty).
New diagnostic feature, classic treatment
Due to the severity of the patient’s scabies, she was prescribed both permethrin 5% cream and oral ivermectin 200 mcg/kg, both to be used immediately and repeated in 1 week. Notably, a systematic review indicated that topical permethrin is a superior treatment to oral ivermectin.6 However, in cases of widespread scabies and crusted scabies, it is standard of care to treat with both medications.
The patient’s pruritus was treated with cetirizine as needed. She was told that the itching might persist for a few weeks after treatment was completed.
Reinfestation was a concern with this patient because she was unable to identify a source for the mites. To minimize the likelihood of reinfestation, we advised her to decontaminate her bedding, clothing, and towels by washing them in hot water (≥ 122° F) or placing in a sealed plastic bag for at least 1 week.1 For crusted scabies cases, thorough vacuuming of a patient’s furniture and carpets is recommended.
1. Gunning K, Kiraly B, Pippitt K. Lice and scabies: treatment update. Am Fam Physician. 2019;99:635-642.
2. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br J Dermatol. 2020;183:808-820. doi: 10.1111/bjd.18943
1. Gunning K, Kiraly B, Pippitt K. Lice and scabies: treatment update. Am Fam Physician. 2019;99:635-642.
2. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br J Dermatol. 2020;183:808-820. doi: 10.1111/bjd.18943
Allergic contact dermatitis
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12
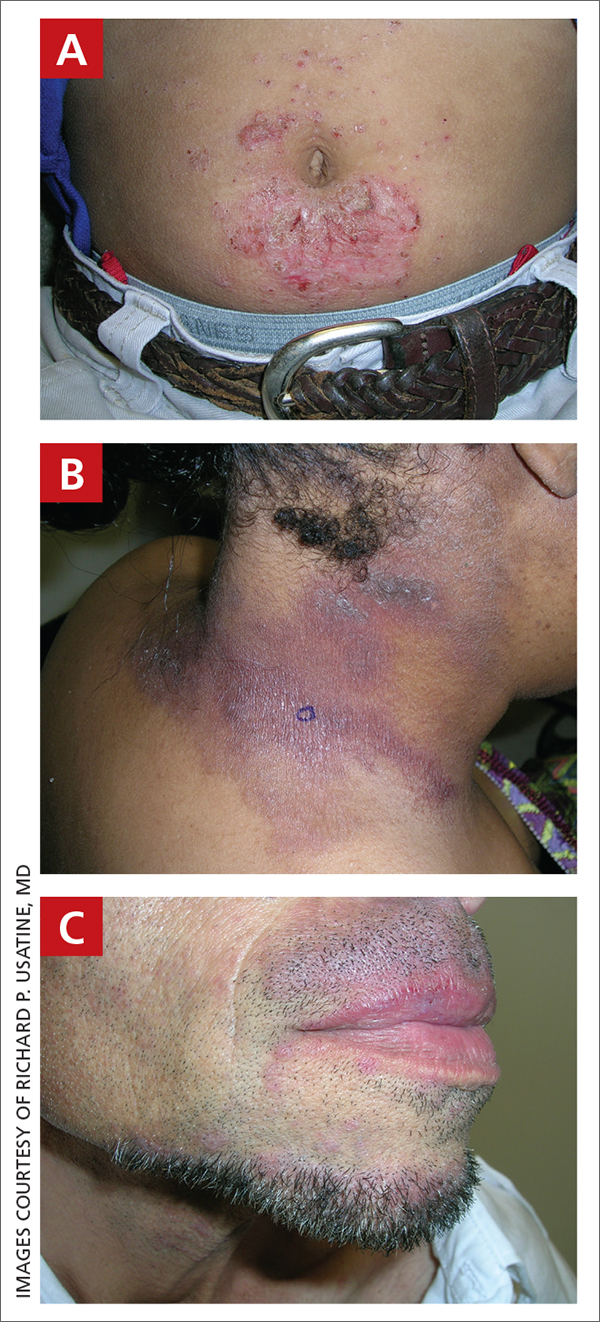
There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12

There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
THE COMPARISON
A An 11-year-old Hispanic boy with allergic contact dermatitis (ACD) on the abdomen. The geometric nature of the eruption and proximity to the belt buckle were highly suggestive of ACD to nickel; patch testing was not needed.
B A Black woman with ACD on the neck. A punch biopsy demonstrated spongiotic dermatitis that was typical of ACD. The diagnosis was supported by the patient’s history of dermatitis that developed after new products were applied to the hair. The patient declined patch testing.
C A Hispanic man with ACD on hair-bearing areas of the face where hair dye was used. The patient’s history of dermatitis following the application of hair dye was highly suggestive of ACD; patch testing confirmed the allergen was paraphenylenediamine (PPD).
Allergic contact dermatitis (ACD) is an inflammatory condition of the skin caused by an immunologic response to 1 or more identifiable allergens. A delayed-type immune response (type IV hypersensitivity reaction) occurs after the skin is re-exposed to an offending allergen.1 Severe pruritus is the main symptom of ACD in the early stages, accompanied by erythema, vesicles, and scaling in a distinct pattern corresponding to the allergen’s contact with the skin.2 Delayed widespread dermatitis after exposure to an allergen—a phenomenon known as autoeczematization (id reaction)—also may occur.3
The gold-standard diagnostic tool for ACD is patch testing, in which the patient is re-exposed to the suspected contact allergen(s) and observed for the development of dermatitis.4 However, ACD can be diagnosed with a detailed patient history including occupation, hobbies, personal care practices, and possible triggers with subsequent rashes. Thorough clinical examination of the skin is paramount. Indicators of possible ACD include dermatitis that persists despite use of appropriate treatment, an unexplained flare of previously quiescent dermatitis, and a diagnosis of dermatitis without a clear cause.1
Hairdressers, health care workers, and metal workers are at higher risk for ACD.5 Occupational ACD has notable socioeconomic implications, as it can result in frequent sick days, inability to perform tasks at work, and in some cases job loss.6
Patients with atopic dermatitis have impaired barrier function of the skin, permitting the entrance of allergens and subsequent sensitization.7 ACD is a challenge to manage, as complete avoidance of the allergen may not be possible.8
Continue to: The underrepresentation of patients...
The underrepresentation of patients with skin of color (SOC) in educational materials as well as socioeconomic health disparities may contribute to the lower rates of diagnosis, patch testing, and treatment of ACD in this patient population.
Epidemiology
An ACD prevalence of 15.2% was reported in a study of 793 Danish patients who underwent skin prick and patch testing.9 Alinaghi et al10 conducted a meta-analysis of 20,107 patients across 28 studies who were patch tested to determine the prevalence of ACD in the general population. The researchers concluded that 20.1% (95% CI, 16.8%-23.7%) of the general population experienced ACD. They analyzed 22 studies to determine the prevalence of ACD based on specific geographic area, including 18,709 individuals from Europe with a prevalence of 19.5% (95% CI, 15.8%-23.4%), 1639 individuals from North America with a prevalence of 20.6% (95% CI, 9.2%-35.2%), and 2 studies from China (no other studies from Asia found) with a prevalence of 20.6% (95% CI, 17.4%-23.9%). Researchers did not find data from studies conducted in Africa or South America.10
The current available epidemiologic data on ACD are not representative of SOC populations. DeLeo et al11 looked at patch test reaction patterns in association with race and ethnicity in a large sample size (N = 19,457); 92.9% of these patients were White and only 7.1% were Black. Large-scale, inclusive studies are needed, which can only be achieved with increased suspicion for ACD and increased access to patch testing.
ACD is more common in women, with nickel being the most frequently identified allergen (FIGURE A).10 Personal care products often are linked to ACD (FIGURE B). An analysis of data from the North American Contact Dermatitis Group revealed that the top 5 personal care product allergens were methylisothiazolinone (a preservative), fragrance mix I, balsam of Peru, quaternium-15 (a preservative), and paraphenylenediamine (PPD; a common component of hair dye) (FIGURE C).12

There is a paucity of epidemiologic data among various ethnic groups; however, a few studies have suggested that there is no difference in the frequency rates of positive patch test results in Black vs White populations.11,13,14 One study of patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic Foundation in Ohio demonstrated a similar allergy frequency of 43.0% and 43.6%, respectively.13 However, there were differences in the types of allergen sensitization. Black patients had higher positive patch test rates for PPD than White patients (10.6% vs 4.5%). Black men had a higher frequency of sensitivity to PPD (21.2% vs 4.2%) and imidazolidinyl urea (a formaldehyde-releasing preservative; 9.1% vs 2.6%) compared to White men.13
Continue to: Ethnicity and cultural practices...
Ethnicity and cultural practices influence epidemiologic patterns of ACD. Darker hair dyes used in Black patients14 and deeply pigmented PPD dye found in henna tattoos used in Indian and Black patients15 may lead to increased sensitization to PPD. ACD due to formaldehyde is more common in White patients, possibly due to more frequent use of formaldehyde-containing moisturizers, shampoos, and creams.15
Key clinical features in people with darker skin tones
In patients with SOC, the clinical features of ACD vary, posing a diagnostic challenge. Hyperpigmentation, lichenification, and induration are more likely to be seen than the papules, vesicles, and erythematous dermatitis often described in lighter skin tones or acute ACD. Erythema can be difficult to assess on darker skin and may appear violaceous or very faint pink.16
Worth noting
A high index of suspicion is necessary when interpreting patch tests in patients with SOC, as patch test kits use a reading plate with graduated intensities of erythema, papulation, and vesicular reactions to determine the likelihood of ACD. The potential contact allergens are placed on the skin on Day 1 and covered. Then, on Day 3 the allergens are removed. The skin is clinically evaluated using visual assessment and skin palpation. The reactions are graded as negative, irritant reaction, equivocal, weak positive, strong positive, or extreme reaction at around Days 3 and 5 to capture both early and delayed reactions.17 A patch test may be positive even if obvious signs of erythema are not appreciated as expected.
Adjusting the lighting in the examination room, including side lighting, or using a blue background can be helpful in identifying erythema in darker skin tones.15,16,18 Palpation of the skin also is useful, as even slight texture changes and induration are indicators of a possible skin reaction to the test allergen.15
Health disparity highlight
Clinical photographs of ACD and patch test results in patients with SOC are not commonplace in the literature. Positive patch test results in patients with darker skin tones vary from those of patients with lighter skin tones, and if the clinician reading the patch test result is not familiar with the findings in darker skin tones, the diagnosis may be delayed or missed.15
Continue to: Furthermore, Scott et al...
Furthermore, Scott et al15 highlighted that many dermatology residency training programs have a paucity of SOC education in their curriculum. This lack of representation may contribute to the diagnostic challenges encountered by health care providers.
Timely access to health care and education as well as economic stability are essential for the successful management of patients with ACD. Some individuals with SOC have been disproportionately affected by social determinants of health. Rodriguez-Homs et al19 demonstrated that the distance needed to travel to a clinic and the poverty rate of the county the patient lives in play a role in referral to a clinician specializing in contact dermatitis.
A retrospective registry review of 2310 patients undergoing patch testing at the Massachusetts General Hospital in Boston revealed that 2.5% were Black, 5.5% were Latinx, 8.3% were Asian, and the remaining 83.7% were White.20 Qian et al21 also looked at patch testing patterns among various sociodemographic groups (N = 1,107,530) and found that 69% of patients were White and 59% were female. Rates of patch testing among patients who were Black, lesser educated, male, lower income, and younger (children ages 0-12 years) were significantly lower than for other groups when ACD was suspected (P < .0001).21 The lower rates of patch testing in patients with SOC may be due to low suspicion of diagnosis, low referral rates due to limited medical insurance, and financial instability, as well as other socioeconomic factors.20
Tamazian et al16 reviewed pediatric populations at 13 US centers and found that Black children received patch testing less frequently than White and Hispanic children. Another review of pediatric patch testing in patients with SOC found that a less comprehensive panel of allergens was used in this population.22
The key to resolution of ACD is removal of the offending antigen, and if patients are not being tested, then they risk having a prolonged and complicated course of ACD with a poor prognosis. Patients with SOC also experience greater negative psychosocial impact due to ACD disease burden.21,23 The lower rates of patch testing in Black patients cannot solely be attributed to difficulty diagnosing ACD in darker skin tones; it is likely due to the impact of social determinants of health. Alleviating health disparities will improve patient outcomes and quality of life.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
2. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
3. Bertoli MJ, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi: 10.12788/cutis.0342
4. Johansen JD, Bonefeld CM, Schwensen JFB, et al. Novel insights into contact dermatitis. J Allergy Clin Immunol. 2022;149:1162-1171. doi: 10.1016/j.jaci.2022.02.002
5. Karagounis TK, Cohen DE. Occupational hand dermatitis. Curr Allergy Asthma Rep. 2023;23:201-212. doi: 10.1007/s11882-023- 01070-5
6. Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol. 2005;152:93-98. doi: 10.1111/j.1365-2133.2005.06415.x
7. Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi: 10.1007/s40257-017-0340-7
8. Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi: 10.1016/ j.phrs.2020.105282
9. Nielsen NH, Menne T. The relationship between IgE‐mediatedand cell‐mediated hypersensitivities in an unselected Danish population: the Glostrup Allergy Study, Denmark. Br J Dermatol. 1996;134:669-672. doi: 10.1111/j.1365-2133.1996.tb06967.x
10. Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80:77-85. doi: 10.1111/cod.13119
11. DeLeo VA, Alexis A, Warshaw EM, et al. The association of race/ ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292. doi: 10.1097/ DER.0000000000000220
12. Warshaw EM, Schlarbaum JP, Silverberg JI, et al. Contact dermatitis to personal care products is increasing (but different!) in males and females: North American Contact Dermatitis Group data, 1996-2016. J Am Acad Dermatol. 2021;85:1446-1455. doi: 10.1016/j jaad.2020.10.003
13. Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermatol. 2001;12:77-82. doi: 10.1053/ajcd.2001.20110
14. DeLeo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl):S107-S112. doi: 10.1067/mjd.2002.120792
15. Scott I, Atwater AR, Reeder M. Update on contact dermatitis and patch testing in patients with skin of color. Cutis. 2021;108:10-12. doi: 10.12788/cutis.0292
16. Tamazian S, Oboite M, Treat JR. Patch testing in skin of color: a brief report. Pediatr Dermatol. 2021;38:952-953. doi: 10.1111/ pde.14578
17. Litchman G, Nair PA, Atwater AR, et al. Contact dermatitis. Stat- Pearls [Internet]. Updated February 9, 2023. Accessed September 25, 2023. www.ncbi.nlm.nih.gov/books/NBK459230/
18. Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729. doi: 10.1016/j.jaad.2018.08.049
19. Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264. doi: 10.1097/DER.0000000000000581
20. Foschi CM, Tam I, Schalock PC, et al. Patch testing results in skin of color: a retrospective review from the Massachusetts General Hospital contact dermatitis clinic. J Am Acad Dermatol. 2022;87:452-454. doi: 10.1016/j.jaad.2021.09.022
21. Qian MF, Li S, Honari G, et al. Sociodemographic disparities in patch testing for commercially insured patients with dermatitis: a retrospective analysis of administrative claims data. J Am Acad Dermatol. 2022;87:1411-1413. doi: 10.1016/j.jaad.2022.08.041
22. Young K, Collis RW, Sheinbein D, et al. Retrospective review of pediatric patch testing results in skin of color. J Am Acad Dermatol. 2023;88:953-954. doi: 10.1016/j.jaad.2022.11.031
23. Kadyk DL, Hall S, Belsito DV. Quality of life of patients with allergic contact dermatitis: an exploratory analysis by gender, ethnicity, age, and occupation. Dermatitis. 2004;15:117-124.