User login
A practical guide to community-acquired MRSA
› Treat a simple cutaneous abscess from a methicillin-resistant Staphylococcus
aureus (MRSA) infection with incision and drainage alone. A
› Treat minor MRSA skin lesions in children with mupirocin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
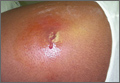
CASE › A 21-year-old man seeks care at his family physician (FP)’s office for a painful, draining lesion that’s been on his left thigh for 5 days. He reports that he was bitten by a spider during one of his weekly football games with his fraternity brothers, but doesn’t recall seeing a spider. He has been applying an over-the-counter topical antibiotic without any improvement, and reports that the area of redness has tripled in size within the last 24 hours.
His past medical history is unremarkable except for an allergy to sulfa drugs that was discovered during treatment for a skin infection 2 years ago. He takes no regular medications.
The patient is afebrile and in no distress. The skin overlying his left thigh has a 1 cm oozing lesion with pus evident and surrounding erythema. The wound is warm and tender to the touch.
Based on the patient’s history, the FP suspects methicillin-resistant Staphylococcus aureus (MRSA) and obtains a swab for bacterial culture and sensitivities.
What the surveillance data tell us about MRSA
MRSA infections—a subset of staph infections that are resistant to beta-lactam antibiotics and cephalosporins—continue to be a major source of infection in the community.1 The prevalence of both community-acquired (CA) and hospital acquired (HA) MRSA infections worldwide has continued to grow despite improvements in controlling nosocomial spread.2,3 Data from the early 2000s found an approximate 1% to 2% MRSA colonization rate in the United States, but other countries have had rates as high as 50%.3 Rates across countries have consistently been higher in children and adolescents. A recent surveillance study suggests a decline in the incidence of the most serious types of MRSA infections in major metropolitan areas, the significance of which is still under investigation.4
CA-MRSA and HA-MRSA have different in vitro sensitivities to antimicrobials, different virulence factors, and different epidemiologic profiles.
CA-MRSA occurs in people who have not been recently hospitalized or had any recent medical procedures. These infections usually develop on skin and soft tissue. CA-MRSA typically contains the genes for the Panton-Valentine Leukocidin (PVL) toxin, which is a virulence factor that leads to increased interleukin-8 secretion and skin necrosis.5,6 In addition, CA-MRSA usually does not have genes associated with multidrug-resistant strains.
HA-MRSA occurs in people who have recently been hospitalized, had recent medical procedures, or have been treated in a longterm care setting. HA-MRSA is associated with multidrug-resistant strains; however, it usually does not have the genes for PVL toxin.7
Factors that put patients at risk for CA-MRSA skin infections
As many as 90% of CA-MRSA infections present as skin and soft tissue infections (SSTIs) that have the potential to become invasive if not managed appropriately.3,8 There are a number of factors that put patients at risk for these SSTIs (TABLE 1)7-9—chief among them, intrafamilial or close contact transmission. People living in close quarters with colonized individuals are 14 times more likely to be carriers than a matched unexposed population.9 Similarly, other environments that typically involve close quarters or overcrowding, including military installations, prisons, long-term care facilities, and daycare centers, generally see higher rates of MRSA colonization.10
Researchers also hypothesize that repeated skin trauma is another risk factor for CA-MRSA SSTIs. This may explain the increased rates of CA-MRSA infections seen in athletes and military recruits undergoing basic training, as they are prone to skin abrasions.8 Certain ethnic groups have a higher prevalence of CA-MRSA infections as well, including Native Americans, Pacific Islanders, and African Americans.9,11 It is not entirely clear if there is anything unique predisposing these populations to MRSA or if this might be attributed to living in tight communities with close household contacts.
High-risk groups that have elevated rates of CA-MRSA SSTIs and are more likely to be carriers include intravenous drug users, men who have sex with men, immunocompromised individuals (including those with human immunodeficiency virus), and the homeless.8,9 Several studies have also reported higher rates of MRSA colonization and CA-MRSA infections in individuals who have come into contact with the health care system. A meta-analysis indicated that nasal swabs taken from patients at health care facilities were 2.35 times more likely to be positive for MRSA than those taken from individuals as nonhealth care locations.9 Risk factors such as antibiotic use or one or more physician visits in the past year have been associated with higher rates of infections, as well.2
Diagnosis requires careful history and exam
Community-acquired MRSA SSTIs are often diagnosed based on the patient’s history and risk factors, along with physical exam findings. Common findings include single or multiple erythematous pustules, furuncles, carbuncles, cellulitis, and abscesses. Patients may confuse the initial lesion with an insect or spider bite,12 as occurred with the patient in our opening scenario.
If purulent drainage is present, perform a swab for culture and sensitivity to confirm the diagnosis of MRSA and assist in antibiotic selection.13 If a patient with an SSTI does not respond to beta-lactam antibiotics, consider MRSA until proven otherwise.
Once confirmed by microbiological assessment, follow Centers for Disease Control and Prevention guidelines to differentiate CA-MRSA from HA-MRSA.14 It is important to distinguish between the two, as patients with HA-MRSA are at greater risk for antibiotic failure and progression to more invasive infections.
Confirmation of a CA-MRSA infection requires all of the following criteria:14
• Diagnosis was made in the outpatient setting or by positive culture within 48 hours of admission to the hospital
• No history of MRSA infection or colonization
• No history in the past year of hospitalization; admission to a skilled nursing facility or hospice; dialysis; or surgery
• No permanent indwelling catheters or medical devices that go through the skin.
Confirmation of an HA-MRSA infection requires at least one of the following criteria:14
• It occurs more than 48 hours after admission to the hospital.
• There is a prior history of MRSA infection or colonization.
• There is a history in the past year of hospitalization; admission to a skilled
nursing facility, nursing home, or hospice; dialysis; or surgery.
• The presence of any indwelling catheter or medical device that passes through the skin into the body.
Rely on evidence-based treatment protocols
In 2011, the Infectious Disease Society of America (IDSA) published consensus guidelines for CA-MRSA to assist with evidencebased decision making. Primary treatment of cutaneous abscesses remains incision and drainage alone without antibiotics, except under certain circumstances (TABLE 2).13 The guidelines recommend antibiotic treatment with empiric coverage for CA-MRSA when purulent cellulitis exists. However, if cellulitis exists without purulence or abscess formation, antibiotic coverage for CA-MRSA is not encouraged for initial treatment.13
Empiric antibiotic coverage for uncomplicated CA-MRSA SSTIs managed in the outpatient setting should include clindamycin, trimethoprim/sulfamethoxazole (TMPSMX), a tetracycline, or linezolid for 5 to 10 days. Factors including cost, patient age and comorbidities, drug allergies, and local resistance patterns should guide the initial antibiotic you choose. TMP-SMX and tetracyclines are relatively inexpensive (<$20), clindamycin is more costly, and linezolid is often costprohibitive as standard treatment.
Do not prescribe tetracyclines for children younger than 8 years of age because of the risk of permanent tooth discoloration. Clindamycin is the only category “B” antibiotic in the group that can be used during pregnancy, and renal impairment needs to be taken into consideration with TMP-SMX and linezolid. For very minor skin lesions in children, mupirocin 2% topical ointment appears to be the therapy of choice.13
Although IDSA has not provided strong recommendations about treatment of asymptomatic close household contacts, this may be considered as another means of attempting to control the spread of MRSA in the community.
Provide patient education. Patient education is paramount to successful treatment and prevention. Explain to patients that people living in close quarters with individuals who already have the bacteria on the skin are far more likely to be carriers. Overcrowding situations that pose a risk include military installations, prisons, long-term care facilities, athletic teams, and daycare centers.
To prevent MRSA, encourage patients to wash their hands often and shower regularly, especially after exercise. Also keep cuts, scrapes, and wounds clean and covered until they heal. Avoid sharing personal items, such as towel and razors.
Finally, remind patients to get care early if they think they may be infected. Symptoms suggestive of MRSA include a red, swollen, painful area on the skin that may look like a spider bite. The area may contain pus and be accompanied by a fever.
Provide close outpatient follow-up within a few days of initiating therapy to document clinical response and aid in further decision-making. Signs of more extensive cellulitis may signal a need for a change of antibiotics or parenteral therapy. Similarly, joint or bone pain underlying areas of cellulitis, significant myalgias or muscle pain out of proportion to exam, or systemic symptoms such as fever, chills, nausea, or lethargy might indicate more extensive infection requiring inpatient treatment.
How to manage recurrent infections
Management of recurrent CA-MRSA SSTIs in the outpatient setting poses a challenge. Following successful treatment of active infection, you may want to attempt decolonization in select patients. Those with repeated MRSA infections despite adequate hygiene measures or with a high probability of reexposure to colonized close contacts may be treated, although the evidence supporting such protocols is lacking.8,13
Acceptable procedures described by IDSA include nasal mupirocin twice daily for 5 to 10 days, mupirocin plus topical antiseptic solution (eg, chlorhexidine, triclosan, or povidone-iodine) for 5 to 14 days, or mupirocin plus dilute bleach baths (1 teaspoon bleach/gallon of water) twice weekly for 15 minutes over 3 months.13 Although antibiotics are generally only recommended for active infection, the combination of rifampin and an antibiotic with MRSA coverage may be used for 1 to 2 weeks in cases of recurrent infections despite recommended hygiene and topical decolonization measures.13,15,16 Rifampin is not recommended as monotherapy for MRSA infection or decolonization.13
Unfortunately, even in cases where eradication is initially successful, about half of those with subsequent negative MRSA cultures will test positive before the end of a year.16 With recurrent or severe infections or immunocompromised patients, it’s advisable to consider an infectious disease consult.
CASE › The patient’s lesion did not require incision as it was already draining. He received a prescription for doxycycline hyclate 100 mg BID for 7 days (since there was evidence of rapidly progressing cellulitis) and was instructed to return to the clinic in 48 hours.
When he returned to the clinic, the patient stated that the pain had improved and the wound was no longer oozing. Culture results confirmed MR SA sensitive to TMP-SMX, doxycycline, and clindamycin. Examination showed improved erythema, a dry wound, and no pain on palpation. He was given a patient information handout on MRSA infection and advised to return to the clinic if the wound did not completely heal within the next 7 days.
1. Özel G, Aslan V, Bahar Erdem G, et al. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyol Bul. 2011;45:258-265.
2. Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951.
3. Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39:193-200.
4. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. September 16, 2013. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1738718. Accessed October 15, 2013.
5. Kawaguchiya M, Urushibara N, Kuwahara O, et al. Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 2011;17:241-250.
6. Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32:228-235.
7. Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263-272.
8. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1-16; quiz 17-20.
9. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
10. Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1-7.
11. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core Surveillance (ABCs) MRSA Investigators. Invasive methicillinresistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
12. NeVille-Swensen M, Clayton M. Outpatient management of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infection. J Pediatr Health Care. 2011;25:308-315.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
14. Diagnosis and testing for MRSA infections. Centers for Disease Control and Infection Web site. Available at: http://www.cdc.gov/mrsa/diagnosis/index.html. Accessed September 30, 2013.
15. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510-516.
16. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178-185.
› Treat a simple cutaneous abscess from a methicillin-resistant Staphylococcus
aureus (MRSA) infection with incision and drainage alone. A
› Treat minor MRSA skin lesions in children with mupirocin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 21-year-old man seeks care at his family physician (FP)’s office for a painful, draining lesion that’s been on his left thigh for 5 days. He reports that he was bitten by a spider during one of his weekly football games with his fraternity brothers, but doesn’t recall seeing a spider. He has been applying an over-the-counter topical antibiotic without any improvement, and reports that the area of redness has tripled in size within the last 24 hours.
His past medical history is unremarkable except for an allergy to sulfa drugs that was discovered during treatment for a skin infection 2 years ago. He takes no regular medications.
The patient is afebrile and in no distress. The skin overlying his left thigh has a 1 cm oozing lesion with pus evident and surrounding erythema. The wound is warm and tender to the touch.
Based on the patient’s history, the FP suspects methicillin-resistant Staphylococcus aureus (MRSA) and obtains a swab for bacterial culture and sensitivities.
What the surveillance data tell us about MRSA
MRSA infections—a subset of staph infections that are resistant to beta-lactam antibiotics and cephalosporins—continue to be a major source of infection in the community.1 The prevalence of both community-acquired (CA) and hospital acquired (HA) MRSA infections worldwide has continued to grow despite improvements in controlling nosocomial spread.2,3 Data from the early 2000s found an approximate 1% to 2% MRSA colonization rate in the United States, but other countries have had rates as high as 50%.3 Rates across countries have consistently been higher in children and adolescents. A recent surveillance study suggests a decline in the incidence of the most serious types of MRSA infections in major metropolitan areas, the significance of which is still under investigation.4
CA-MRSA and HA-MRSA have different in vitro sensitivities to antimicrobials, different virulence factors, and different epidemiologic profiles.
CA-MRSA occurs in people who have not been recently hospitalized or had any recent medical procedures. These infections usually develop on skin and soft tissue. CA-MRSA typically contains the genes for the Panton-Valentine Leukocidin (PVL) toxin, which is a virulence factor that leads to increased interleukin-8 secretion and skin necrosis.5,6 In addition, CA-MRSA usually does not have genes associated with multidrug-resistant strains.
HA-MRSA occurs in people who have recently been hospitalized, had recent medical procedures, or have been treated in a longterm care setting. HA-MRSA is associated with multidrug-resistant strains; however, it usually does not have the genes for PVL toxin.7
Factors that put patients at risk for CA-MRSA skin infections
As many as 90% of CA-MRSA infections present as skin and soft tissue infections (SSTIs) that have the potential to become invasive if not managed appropriately.3,8 There are a number of factors that put patients at risk for these SSTIs (TABLE 1)7-9—chief among them, intrafamilial or close contact transmission. People living in close quarters with colonized individuals are 14 times more likely to be carriers than a matched unexposed population.9 Similarly, other environments that typically involve close quarters or overcrowding, including military installations, prisons, long-term care facilities, and daycare centers, generally see higher rates of MRSA colonization.10
Researchers also hypothesize that repeated skin trauma is another risk factor for CA-MRSA SSTIs. This may explain the increased rates of CA-MRSA infections seen in athletes and military recruits undergoing basic training, as they are prone to skin abrasions.8 Certain ethnic groups have a higher prevalence of CA-MRSA infections as well, including Native Americans, Pacific Islanders, and African Americans.9,11 It is not entirely clear if there is anything unique predisposing these populations to MRSA or if this might be attributed to living in tight communities with close household contacts.
High-risk groups that have elevated rates of CA-MRSA SSTIs and are more likely to be carriers include intravenous drug users, men who have sex with men, immunocompromised individuals (including those with human immunodeficiency virus), and the homeless.8,9 Several studies have also reported higher rates of MRSA colonization and CA-MRSA infections in individuals who have come into contact with the health care system. A meta-analysis indicated that nasal swabs taken from patients at health care facilities were 2.35 times more likely to be positive for MRSA than those taken from individuals as nonhealth care locations.9 Risk factors such as antibiotic use or one or more physician visits in the past year have been associated with higher rates of infections, as well.2
Diagnosis requires careful history and exam
Community-acquired MRSA SSTIs are often diagnosed based on the patient’s history and risk factors, along with physical exam findings. Common findings include single or multiple erythematous pustules, furuncles, carbuncles, cellulitis, and abscesses. Patients may confuse the initial lesion with an insect or spider bite,12 as occurred with the patient in our opening scenario.
If purulent drainage is present, perform a swab for culture and sensitivity to confirm the diagnosis of MRSA and assist in antibiotic selection.13 If a patient with an SSTI does not respond to beta-lactam antibiotics, consider MRSA until proven otherwise.
Once confirmed by microbiological assessment, follow Centers for Disease Control and Prevention guidelines to differentiate CA-MRSA from HA-MRSA.14 It is important to distinguish between the two, as patients with HA-MRSA are at greater risk for antibiotic failure and progression to more invasive infections.
Confirmation of a CA-MRSA infection requires all of the following criteria:14
• Diagnosis was made in the outpatient setting or by positive culture within 48 hours of admission to the hospital
• No history of MRSA infection or colonization
• No history in the past year of hospitalization; admission to a skilled nursing facility or hospice; dialysis; or surgery
• No permanent indwelling catheters or medical devices that go through the skin.
Confirmation of an HA-MRSA infection requires at least one of the following criteria:14
• It occurs more than 48 hours after admission to the hospital.
• There is a prior history of MRSA infection or colonization.
• There is a history in the past year of hospitalization; admission to a skilled
nursing facility, nursing home, or hospice; dialysis; or surgery.
• The presence of any indwelling catheter or medical device that passes through the skin into the body.
Rely on evidence-based treatment protocols
In 2011, the Infectious Disease Society of America (IDSA) published consensus guidelines for CA-MRSA to assist with evidencebased decision making. Primary treatment of cutaneous abscesses remains incision and drainage alone without antibiotics, except under certain circumstances (TABLE 2).13 The guidelines recommend antibiotic treatment with empiric coverage for CA-MRSA when purulent cellulitis exists. However, if cellulitis exists without purulence or abscess formation, antibiotic coverage for CA-MRSA is not encouraged for initial treatment.13
Empiric antibiotic coverage for uncomplicated CA-MRSA SSTIs managed in the outpatient setting should include clindamycin, trimethoprim/sulfamethoxazole (TMPSMX), a tetracycline, or linezolid for 5 to 10 days. Factors including cost, patient age and comorbidities, drug allergies, and local resistance patterns should guide the initial antibiotic you choose. TMP-SMX and tetracyclines are relatively inexpensive (<$20), clindamycin is more costly, and linezolid is often costprohibitive as standard treatment.
Do not prescribe tetracyclines for children younger than 8 years of age because of the risk of permanent tooth discoloration. Clindamycin is the only category “B” antibiotic in the group that can be used during pregnancy, and renal impairment needs to be taken into consideration with TMP-SMX and linezolid. For very minor skin lesions in children, mupirocin 2% topical ointment appears to be the therapy of choice.13
Although IDSA has not provided strong recommendations about treatment of asymptomatic close household contacts, this may be considered as another means of attempting to control the spread of MRSA in the community.
Provide patient education. Patient education is paramount to successful treatment and prevention. Explain to patients that people living in close quarters with individuals who already have the bacteria on the skin are far more likely to be carriers. Overcrowding situations that pose a risk include military installations, prisons, long-term care facilities, athletic teams, and daycare centers.
To prevent MRSA, encourage patients to wash their hands often and shower regularly, especially after exercise. Also keep cuts, scrapes, and wounds clean and covered until they heal. Avoid sharing personal items, such as towel and razors.
Finally, remind patients to get care early if they think they may be infected. Symptoms suggestive of MRSA include a red, swollen, painful area on the skin that may look like a spider bite. The area may contain pus and be accompanied by a fever.
Provide close outpatient follow-up within a few days of initiating therapy to document clinical response and aid in further decision-making. Signs of more extensive cellulitis may signal a need for a change of antibiotics or parenteral therapy. Similarly, joint or bone pain underlying areas of cellulitis, significant myalgias or muscle pain out of proportion to exam, or systemic symptoms such as fever, chills, nausea, or lethargy might indicate more extensive infection requiring inpatient treatment.
How to manage recurrent infections
Management of recurrent CA-MRSA SSTIs in the outpatient setting poses a challenge. Following successful treatment of active infection, you may want to attempt decolonization in select patients. Those with repeated MRSA infections despite adequate hygiene measures or with a high probability of reexposure to colonized close contacts may be treated, although the evidence supporting such protocols is lacking.8,13
Acceptable procedures described by IDSA include nasal mupirocin twice daily for 5 to 10 days, mupirocin plus topical antiseptic solution (eg, chlorhexidine, triclosan, or povidone-iodine) for 5 to 14 days, or mupirocin plus dilute bleach baths (1 teaspoon bleach/gallon of water) twice weekly for 15 minutes over 3 months.13 Although antibiotics are generally only recommended for active infection, the combination of rifampin and an antibiotic with MRSA coverage may be used for 1 to 2 weeks in cases of recurrent infections despite recommended hygiene and topical decolonization measures.13,15,16 Rifampin is not recommended as monotherapy for MRSA infection or decolonization.13
Unfortunately, even in cases where eradication is initially successful, about half of those with subsequent negative MRSA cultures will test positive before the end of a year.16 With recurrent or severe infections or immunocompromised patients, it’s advisable to consider an infectious disease consult.
CASE › The patient’s lesion did not require incision as it was already draining. He received a prescription for doxycycline hyclate 100 mg BID for 7 days (since there was evidence of rapidly progressing cellulitis) and was instructed to return to the clinic in 48 hours.
When he returned to the clinic, the patient stated that the pain had improved and the wound was no longer oozing. Culture results confirmed MR SA sensitive to TMP-SMX, doxycycline, and clindamycin. Examination showed improved erythema, a dry wound, and no pain on palpation. He was given a patient information handout on MRSA infection and advised to return to the clinic if the wound did not completely heal within the next 7 days.
› Treat a simple cutaneous abscess from a methicillin-resistant Staphylococcus
aureus (MRSA) infection with incision and drainage alone. A
› Treat minor MRSA skin lesions in children with mupirocin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 21-year-old man seeks care at his family physician (FP)’s office for a painful, draining lesion that’s been on his left thigh for 5 days. He reports that he was bitten by a spider during one of his weekly football games with his fraternity brothers, but doesn’t recall seeing a spider. He has been applying an over-the-counter topical antibiotic without any improvement, and reports that the area of redness has tripled in size within the last 24 hours.
His past medical history is unremarkable except for an allergy to sulfa drugs that was discovered during treatment for a skin infection 2 years ago. He takes no regular medications.
The patient is afebrile and in no distress. The skin overlying his left thigh has a 1 cm oozing lesion with pus evident and surrounding erythema. The wound is warm and tender to the touch.
Based on the patient’s history, the FP suspects methicillin-resistant Staphylococcus aureus (MRSA) and obtains a swab for bacterial culture and sensitivities.
What the surveillance data tell us about MRSA
MRSA infections—a subset of staph infections that are resistant to beta-lactam antibiotics and cephalosporins—continue to be a major source of infection in the community.1 The prevalence of both community-acquired (CA) and hospital acquired (HA) MRSA infections worldwide has continued to grow despite improvements in controlling nosocomial spread.2,3 Data from the early 2000s found an approximate 1% to 2% MRSA colonization rate in the United States, but other countries have had rates as high as 50%.3 Rates across countries have consistently been higher in children and adolescents. A recent surveillance study suggests a decline in the incidence of the most serious types of MRSA infections in major metropolitan areas, the significance of which is still under investigation.4
CA-MRSA and HA-MRSA have different in vitro sensitivities to antimicrobials, different virulence factors, and different epidemiologic profiles.
CA-MRSA occurs in people who have not been recently hospitalized or had any recent medical procedures. These infections usually develop on skin and soft tissue. CA-MRSA typically contains the genes for the Panton-Valentine Leukocidin (PVL) toxin, which is a virulence factor that leads to increased interleukin-8 secretion and skin necrosis.5,6 In addition, CA-MRSA usually does not have genes associated with multidrug-resistant strains.
HA-MRSA occurs in people who have recently been hospitalized, had recent medical procedures, or have been treated in a longterm care setting. HA-MRSA is associated with multidrug-resistant strains; however, it usually does not have the genes for PVL toxin.7
Factors that put patients at risk for CA-MRSA skin infections
As many as 90% of CA-MRSA infections present as skin and soft tissue infections (SSTIs) that have the potential to become invasive if not managed appropriately.3,8 There are a number of factors that put patients at risk for these SSTIs (TABLE 1)7-9—chief among them, intrafamilial or close contact transmission. People living in close quarters with colonized individuals are 14 times more likely to be carriers than a matched unexposed population.9 Similarly, other environments that typically involve close quarters or overcrowding, including military installations, prisons, long-term care facilities, and daycare centers, generally see higher rates of MRSA colonization.10
Researchers also hypothesize that repeated skin trauma is another risk factor for CA-MRSA SSTIs. This may explain the increased rates of CA-MRSA infections seen in athletes and military recruits undergoing basic training, as they are prone to skin abrasions.8 Certain ethnic groups have a higher prevalence of CA-MRSA infections as well, including Native Americans, Pacific Islanders, and African Americans.9,11 It is not entirely clear if there is anything unique predisposing these populations to MRSA or if this might be attributed to living in tight communities with close household contacts.
High-risk groups that have elevated rates of CA-MRSA SSTIs and are more likely to be carriers include intravenous drug users, men who have sex with men, immunocompromised individuals (including those with human immunodeficiency virus), and the homeless.8,9 Several studies have also reported higher rates of MRSA colonization and CA-MRSA infections in individuals who have come into contact with the health care system. A meta-analysis indicated that nasal swabs taken from patients at health care facilities were 2.35 times more likely to be positive for MRSA than those taken from individuals as nonhealth care locations.9 Risk factors such as antibiotic use or one or more physician visits in the past year have been associated with higher rates of infections, as well.2
Diagnosis requires careful history and exam
Community-acquired MRSA SSTIs are often diagnosed based on the patient’s history and risk factors, along with physical exam findings. Common findings include single or multiple erythematous pustules, furuncles, carbuncles, cellulitis, and abscesses. Patients may confuse the initial lesion with an insect or spider bite,12 as occurred with the patient in our opening scenario.
If purulent drainage is present, perform a swab for culture and sensitivity to confirm the diagnosis of MRSA and assist in antibiotic selection.13 If a patient with an SSTI does not respond to beta-lactam antibiotics, consider MRSA until proven otherwise.
Once confirmed by microbiological assessment, follow Centers for Disease Control and Prevention guidelines to differentiate CA-MRSA from HA-MRSA.14 It is important to distinguish between the two, as patients with HA-MRSA are at greater risk for antibiotic failure and progression to more invasive infections.
Confirmation of a CA-MRSA infection requires all of the following criteria:14
• Diagnosis was made in the outpatient setting or by positive culture within 48 hours of admission to the hospital
• No history of MRSA infection or colonization
• No history in the past year of hospitalization; admission to a skilled nursing facility or hospice; dialysis; or surgery
• No permanent indwelling catheters or medical devices that go through the skin.
Confirmation of an HA-MRSA infection requires at least one of the following criteria:14
• It occurs more than 48 hours after admission to the hospital.
• There is a prior history of MRSA infection or colonization.
• There is a history in the past year of hospitalization; admission to a skilled
nursing facility, nursing home, or hospice; dialysis; or surgery.
• The presence of any indwelling catheter or medical device that passes through the skin into the body.
Rely on evidence-based treatment protocols
In 2011, the Infectious Disease Society of America (IDSA) published consensus guidelines for CA-MRSA to assist with evidencebased decision making. Primary treatment of cutaneous abscesses remains incision and drainage alone without antibiotics, except under certain circumstances (TABLE 2).13 The guidelines recommend antibiotic treatment with empiric coverage for CA-MRSA when purulent cellulitis exists. However, if cellulitis exists without purulence or abscess formation, antibiotic coverage for CA-MRSA is not encouraged for initial treatment.13
Empiric antibiotic coverage for uncomplicated CA-MRSA SSTIs managed in the outpatient setting should include clindamycin, trimethoprim/sulfamethoxazole (TMPSMX), a tetracycline, or linezolid for 5 to 10 days. Factors including cost, patient age and comorbidities, drug allergies, and local resistance patterns should guide the initial antibiotic you choose. TMP-SMX and tetracyclines are relatively inexpensive (<$20), clindamycin is more costly, and linezolid is often costprohibitive as standard treatment.
Do not prescribe tetracyclines for children younger than 8 years of age because of the risk of permanent tooth discoloration. Clindamycin is the only category “B” antibiotic in the group that can be used during pregnancy, and renal impairment needs to be taken into consideration with TMP-SMX and linezolid. For very minor skin lesions in children, mupirocin 2% topical ointment appears to be the therapy of choice.13
Although IDSA has not provided strong recommendations about treatment of asymptomatic close household contacts, this may be considered as another means of attempting to control the spread of MRSA in the community.
Provide patient education. Patient education is paramount to successful treatment and prevention. Explain to patients that people living in close quarters with individuals who already have the bacteria on the skin are far more likely to be carriers. Overcrowding situations that pose a risk include military installations, prisons, long-term care facilities, athletic teams, and daycare centers.
To prevent MRSA, encourage patients to wash their hands often and shower regularly, especially after exercise. Also keep cuts, scrapes, and wounds clean and covered until they heal. Avoid sharing personal items, such as towel and razors.
Finally, remind patients to get care early if they think they may be infected. Symptoms suggestive of MRSA include a red, swollen, painful area on the skin that may look like a spider bite. The area may contain pus and be accompanied by a fever.
Provide close outpatient follow-up within a few days of initiating therapy to document clinical response and aid in further decision-making. Signs of more extensive cellulitis may signal a need for a change of antibiotics or parenteral therapy. Similarly, joint or bone pain underlying areas of cellulitis, significant myalgias or muscle pain out of proportion to exam, or systemic symptoms such as fever, chills, nausea, or lethargy might indicate more extensive infection requiring inpatient treatment.
How to manage recurrent infections
Management of recurrent CA-MRSA SSTIs in the outpatient setting poses a challenge. Following successful treatment of active infection, you may want to attempt decolonization in select patients. Those with repeated MRSA infections despite adequate hygiene measures or with a high probability of reexposure to colonized close contacts may be treated, although the evidence supporting such protocols is lacking.8,13
Acceptable procedures described by IDSA include nasal mupirocin twice daily for 5 to 10 days, mupirocin plus topical antiseptic solution (eg, chlorhexidine, triclosan, or povidone-iodine) for 5 to 14 days, or mupirocin plus dilute bleach baths (1 teaspoon bleach/gallon of water) twice weekly for 15 minutes over 3 months.13 Although antibiotics are generally only recommended for active infection, the combination of rifampin and an antibiotic with MRSA coverage may be used for 1 to 2 weeks in cases of recurrent infections despite recommended hygiene and topical decolonization measures.13,15,16 Rifampin is not recommended as monotherapy for MRSA infection or decolonization.13
Unfortunately, even in cases where eradication is initially successful, about half of those with subsequent negative MRSA cultures will test positive before the end of a year.16 With recurrent or severe infections or immunocompromised patients, it’s advisable to consider an infectious disease consult.
CASE › The patient’s lesion did not require incision as it was already draining. He received a prescription for doxycycline hyclate 100 mg BID for 7 days (since there was evidence of rapidly progressing cellulitis) and was instructed to return to the clinic in 48 hours.
When he returned to the clinic, the patient stated that the pain had improved and the wound was no longer oozing. Culture results confirmed MR SA sensitive to TMP-SMX, doxycycline, and clindamycin. Examination showed improved erythema, a dry wound, and no pain on palpation. He was given a patient information handout on MRSA infection and advised to return to the clinic if the wound did not completely heal within the next 7 days.
1. Özel G, Aslan V, Bahar Erdem G, et al. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyol Bul. 2011;45:258-265.
2. Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951.
3. Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39:193-200.
4. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. September 16, 2013. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1738718. Accessed October 15, 2013.
5. Kawaguchiya M, Urushibara N, Kuwahara O, et al. Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 2011;17:241-250.
6. Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32:228-235.
7. Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263-272.
8. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1-16; quiz 17-20.
9. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
10. Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1-7.
11. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core Surveillance (ABCs) MRSA Investigators. Invasive methicillinresistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
12. NeVille-Swensen M, Clayton M. Outpatient management of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infection. J Pediatr Health Care. 2011;25:308-315.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
14. Diagnosis and testing for MRSA infections. Centers for Disease Control and Infection Web site. Available at: http://www.cdc.gov/mrsa/diagnosis/index.html. Accessed September 30, 2013.
15. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510-516.
16. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178-185.
1. Özel G, Aslan V, Bahar Erdem G, et al. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyol Bul. 2011;45:258-265.
2. Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943-951.
3. Skov R, Christiansen K, Dancer SJ, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39:193-200.
4. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. September 16, 2013. Available at: http://archinte.jamanetwork.com/article.aspx?articleid=1738718. Accessed October 15, 2013.
5. Kawaguchiya M, Urushibara N, Kuwahara O, et al. Molecular characteristics of community-acquired methicillin-resistant Staphylococcus aureus in Hokkaido, northern main island of Japan: identification of sequence types 6 and 59 Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 2011;17:241-250.
6. Wiener-Kronish JP, Pittet JF. Therapies against virulence products of Staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2011;32:228-235.
7. Hansra NK, Shinkai K. Cutaneous community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus. Dermatol Ther. 2011;24:263-272.
8. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1-16; quiz 17-20.
9. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-139.
10. Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1-7.
11. Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core Surveillance (ABCs) MRSA Investigators. Invasive methicillinresistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-1771.
12. NeVille-Swensen M, Clayton M. Outpatient management of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infection. J Pediatr Health Care. 2011;25:308-315.
13. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
14. Diagnosis and testing for MRSA infections. Centers for Disease Control and Infection Web site. Available at: http://www.cdc.gov/mrsa/diagnosis/index.html. Accessed September 30, 2013.
15. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510-516.
16. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178-185.