User login
The State of Hepatitis C Care in the VA
As the largest single provider of hepatitis C virus infection (HCV) care in the U.S., the VA provided care to > 174,000 veterans with chronic HCV in 2013. Identifying veterans most likely to be infected with HCV, particularly those in the highrisk birth cohort born between 1945 and 1965, is a priority given recent CDC and U.S. Preventive Services Task Force (USPSTF) recommendations.1,2 The availability of new, all-oral HCV antiviral regimens with shorter treatment durations and improved tolerability are expected to greatly increase the number of veterans with HCV who could be treated successfully. In order to effectively reach those who are undiagnosed and to ensure that those diagnosed with HCV are evaluated and offered treatment, expanded reliance on primary care providers (PCPs) is essential. This article provides a population view of the current state of VA care for this large HCV-infected population and the important role PCPs share in disease identification and management.
Data Source
Data regarding the state of HCV care in the VA comes from the VA National Clinical Case Registry (CCR) for HCV.3 The VA HCV CCR is an extract of the VA electronic medical record that contains laboratory results, pharmacy information, provider information, and ICD-9 diagnosis codes from inpatient hospitalizations, outpatient visits, and problem lists of veterans with HCV seen at all VAMCs.
Screening and Prevalence of HCV
It is estimated that 2.3 to 2.7 million Americans are living with HCV, with 45% to 85% of those unaware of their infection.4,5 Nearly 75% of those infected are expected to have been born between 1945 and 1965; thus, the CDC and USPSTF now recommend onetime HCV screening for this birth cohort.1,2 Among nearly 5.6 million veterans with a VA outpatient visit in 2013, 56% have been screened for HCV. The HCV screening rate was 42% for those born prior to 1945, 65% for those born during 1945-1965, and 59% for those born after 1965. HCV infection prevalence overall in the VA was 5.8% but differed markedly among the birth cohorts: 1.6% for those born prior to 1945, 9.5% for those born during 1945-1965, and 1.2% for those born after 1965. The prevalence rate of veterans born in the 1945-1965 birth cohort (9.5%) is almost 3 times higher than that of the general U.S. population in this birth cohort (2.4%). The high prevalence serves as a reminder of the greater HCV disease burden in veterans and largely represents Vietman era veterans. Although HCV screening rates in VA have increased over 25% since 2002, the high prevalence among veterans in this birth cohort underscores the importance of continued screening efforts.
Veterans with Chronic HCV Infection
The VA Office of Public Health/Population Health generates national HCV reports annually from the HCV CCR describing the population of veterans with chronic HCV infection receiving VA care. These reports are intended to inform about patient care activities, clinician and patient education, prevention activities, and research directed at continuous improvement of veteran care. The first step in providing responsive care is understanding the affected population, and summarized herein is a description of the veterans with chronic HCV who received VA care in 2013.
In 2013, 174,302 veterans had laboratory evidence of HCV viremia and could be characterized as having chronic HCV. HCV treatment regimens and response depend on the viral genotype. Among veterans with genotype testing, 107,144 (80%) have genotype 1; 15,486 (12%) genotype 2; 9,745 (7%) genotype 3; 1333 (1%) genotype 4; and 63 (< 1%) genotype 5 or 6.
In terms of demographics, most veterans with chronic HCV in VA care in 2013 were men (97%); however, > 5,000 women veterans with chronic HCV received care from the VA. Over half (54%) of veterans with chronic HCV are white, and about one-third (34%) are black. The proportion of blacks within the HCV-infected veteran population is substantially greater than the proportion of blacks in the overall veteran population in VA care (15%) and highlights the disproportionately large burden of HCV that black veterans bear. A smaller proportion of the VA HCV population is Hispanic (6%), and the remaining veterans are other races, multiple races, or unknown.
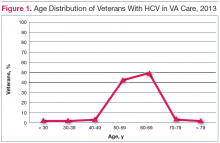
The HCV-infected veteran population is aging. The mean age of veterans with chronic HCV in 2013 was 59.7 years and for the first time, more veterans with HCV were aged 60 years (Figure 1).
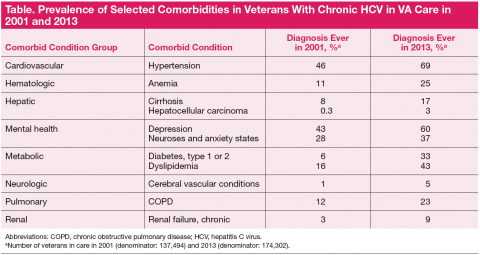
Among the comorbidities that may have historically prevented veterans from receiving HCV antiviral therapy, 2 of the most pervasive are mental health conditions and alcohol use. The rates of mental illness among veterans overall is high, but mental illness is particularly high in veterans with HCV. Depression has affected 60%; of this population anxiety, 37%; posttraumatic stress disorder, 28%; and schizophrenia, 10%. Alcohol use disorders are also common among veterans with HCV in care. Active mental health conditions and substance use may affect medication adherence or follow-up visit adherence thereby limiting treatment candidacy. Integrating care of these individuals with mental health providers and substance-use treatment specialists is an important aspect of HCV care and is a priority in VA.
Three-quarters (76%) of the HCV-infected veteran population has been screened for HIV and HIV-HCV co-infection is present in 5733 (3%) of veterans with HCV. HIV-HCV co-infection is associated with an increased progression of liver disease and may have implications for the selection of HCV antiviral agents because of drug interactions. Hepatitis B virus (HBV)-HCV co-infection rates are higher at 7%. HBV vaccination or documentation of HBV immunity in those without HBV infection is 78%.
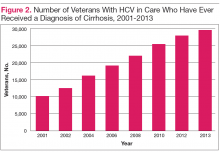
With regard to specific liver complications, 5% to 20% of those infected with chronic HCV will develop cirrhosis over a period of 20 to 30 years, and 1% to 5% will die of hepatocellular carcinoma (HCC) or cirrhosis.6 Given the natural history of chronic HCV and the aging HCV veteran cohort, increasing numbers of conditions related to progression of liver disease are expected over time. This is most evident in the number of veterans with a diagnosis of cirrhosis, which has increased from approximately 10,000 veterans (8%) in care in 2001 to nearly 30,000 veterans (17%) in care in 2013 (Figure 2).
Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR.7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin.9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.
The Role of Primary Care in HCV
Primary care providers have held the responsibility for multiple roles in HCV care since the discovery of the virus—particularly for HCV risk factor assessment, screening, and diagnosis. HCV antiviral treatment, however, was largely placed in the hands of specialists, given the complexities of patient selection, frequent reliance on a liver biopsy for determining need for treatment, and the toxicities of peginterferon and ribavirin therapy.
There are discussions both inside and outside the VA about potentially expanding the role of PCPs in HCV care. First, primary care is the major setting where the CDC and USPSTF recommendations for birth cohort screening are being implemented, and thus PCPs will be identifying veterans previously undiagnosed with HCV.1,2 Second, the ease and tolerability of the new all-oral combination regimens is causing a shift in the paradigm for HCV treatment, from a highly individualized approach, toward a more uniform approach.
Expanding the role of primary care would have multiple benefits to patients and the health care system as a whole. Only approximately 9% of HCV-infected veterans in VA care have been successfully treated at this time, largely due to low eligibility rates and the poor response rates, but other barriers have also contributed to the low success rate, one of which has been limited access to specialists. Furthermore, veterans who are referred to specialists are often noncompliant with the referral.10 If seeing an HCV specialist is required for treatment, the time to treat the HCV population will be much greater, more costly, and less efficient. Therefore, if the prospect of delivering HCV treatment to the majority of HCV patients is to be accomplished, it is necessary to consider providing treatment in the primary care setting as well as the specialist setting.
Treatment provided by nonspecialists has been evaluated in patients receiving peginterferon and ribavirin regimens and has shown that with adequate education and support, SVR rates were equivalent in the specialist and nonspecialist setting.11 To develop programs to provide primary care with such support, the VA has implemented the Specialty Care Access Network-Extension of Community Healthcare Outcomes program initiative, with casebased learning along with real-time consultation.
Currently, the majority of HCV-infected patients have never seen an HCV specialist, thus PCPs are already providing the majority of HCV care beyond HCV antiviral
treatment.12 Primary care providers are, therefore, key to addressing multiple important aspects of HCV care, including (1) counseling patients on transmission, prevention, lifestyle, and the role of substance use; (2) providing hepatitis A and B vaccination as well as appropriate general vaccinations for any patient with chronic liver disease; (3) modifying comorbidities that could accelerate fibrosis progression, such as diabetes mellitus, obesity and hyperlipidemia; (4) reducing risk from ongoing alcohol, drug, and tobacco use; (5) monitoring patients for fibrosis progression and identifying the presence of cirrhosis; and (6) providing general care for patients with cirrhosis, including HCC screening. These are critical aspects of HCV care, and many PCPs may still need additional education for these roles. The VA provides education and support for PCPs in their current role and is enhancing efforts to expand delivery of HCV treatment to the primary care setting as well.
Conclusions
In 2013, the typical veteran with chronic HCV was white, aged 60 years, and male, with a history of comorbidities, including hypertension, depression, and current or prior alcohol abuse. The proportion of veterans with advanced liver disease including cirrhosis (17%) and HCC (3%), has grown significantly over the past 10 years. By the end of 2013, almost 40,000 veterans had received antiviral therapy for HCV, more than 5,700 of whom had received DAAs. Overall SVR rates have been about 42% among those who were treated. Of veterans who are still potential treatment candidates, 85% are treatment-naive and about one-quarter have advanced liver disease.
Although HCV screening rates in veterans are higher than reported in other health care settings, particularly among those in the critical 1945-1965 birth cohort (65% screening rate), substantial numbers of veterans still require testing. The burden of disease, the lack of specialists, the ease and tolerability of new HCV antiviral medications, and the interplay of HCV with other traditional primary care efforts underly an increased role for PCPs in the care of veterans with HCV. Together, this information helps to construct a view of historical, current, and future HCV care in veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patient.
1. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965 [published correction appears in MMWR Recomm Rep. 2012;61(43):886]. MMWR Recomm Rep. 2012;61(RR-4):1-32.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
3. Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: Simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775-783.
4. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: Model-based predictions. Ann Intern Med. 2014;161(3):170-180
5. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300
6. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127(5):1372-1380.
7. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958-965.
8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
9. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
10. Brady CW, Coffman CJ, Provenzale D. Compliance with referral for hepatitis C evaluation among veterans. J Clin Gastroenterol. 2007;41(10):927-931.
11. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
12. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859-1861.
As the largest single provider of hepatitis C virus infection (HCV) care in the U.S., the VA provided care to > 174,000 veterans with chronic HCV in 2013. Identifying veterans most likely to be infected with HCV, particularly those in the highrisk birth cohort born between 1945 and 1965, is a priority given recent CDC and U.S. Preventive Services Task Force (USPSTF) recommendations.1,2 The availability of new, all-oral HCV antiviral regimens with shorter treatment durations and improved tolerability are expected to greatly increase the number of veterans with HCV who could be treated successfully. In order to effectively reach those who are undiagnosed and to ensure that those diagnosed with HCV are evaluated and offered treatment, expanded reliance on primary care providers (PCPs) is essential. This article provides a population view of the current state of VA care for this large HCV-infected population and the important role PCPs share in disease identification and management.
Data Source
Data regarding the state of HCV care in the VA comes from the VA National Clinical Case Registry (CCR) for HCV.3 The VA HCV CCR is an extract of the VA electronic medical record that contains laboratory results, pharmacy information, provider information, and ICD-9 diagnosis codes from inpatient hospitalizations, outpatient visits, and problem lists of veterans with HCV seen at all VAMCs.
Screening and Prevalence of HCV
It is estimated that 2.3 to 2.7 million Americans are living with HCV, with 45% to 85% of those unaware of their infection.4,5 Nearly 75% of those infected are expected to have been born between 1945 and 1965; thus, the CDC and USPSTF now recommend onetime HCV screening for this birth cohort.1,2 Among nearly 5.6 million veterans with a VA outpatient visit in 2013, 56% have been screened for HCV. The HCV screening rate was 42% for those born prior to 1945, 65% for those born during 1945-1965, and 59% for those born after 1965. HCV infection prevalence overall in the VA was 5.8% but differed markedly among the birth cohorts: 1.6% for those born prior to 1945, 9.5% for those born during 1945-1965, and 1.2% for those born after 1965. The prevalence rate of veterans born in the 1945-1965 birth cohort (9.5%) is almost 3 times higher than that of the general U.S. population in this birth cohort (2.4%). The high prevalence serves as a reminder of the greater HCV disease burden in veterans and largely represents Vietman era veterans. Although HCV screening rates in VA have increased over 25% since 2002, the high prevalence among veterans in this birth cohort underscores the importance of continued screening efforts.
Veterans with Chronic HCV Infection
The VA Office of Public Health/Population Health generates national HCV reports annually from the HCV CCR describing the population of veterans with chronic HCV infection receiving VA care. These reports are intended to inform about patient care activities, clinician and patient education, prevention activities, and research directed at continuous improvement of veteran care. The first step in providing responsive care is understanding the affected population, and summarized herein is a description of the veterans with chronic HCV who received VA care in 2013.
In 2013, 174,302 veterans had laboratory evidence of HCV viremia and could be characterized as having chronic HCV. HCV treatment regimens and response depend on the viral genotype. Among veterans with genotype testing, 107,144 (80%) have genotype 1; 15,486 (12%) genotype 2; 9,745 (7%) genotype 3; 1333 (1%) genotype 4; and 63 (< 1%) genotype 5 or 6.
In terms of demographics, most veterans with chronic HCV in VA care in 2013 were men (97%); however, > 5,000 women veterans with chronic HCV received care from the VA. Over half (54%) of veterans with chronic HCV are white, and about one-third (34%) are black. The proportion of blacks within the HCV-infected veteran population is substantially greater than the proportion of blacks in the overall veteran population in VA care (15%) and highlights the disproportionately large burden of HCV that black veterans bear. A smaller proportion of the VA HCV population is Hispanic (6%), and the remaining veterans are other races, multiple races, or unknown.
The HCV-infected veteran population is aging. The mean age of veterans with chronic HCV in 2013 was 59.7 years and for the first time, more veterans with HCV were aged 60 years (Figure 1).
Among the comorbidities that may have historically prevented veterans from receiving HCV antiviral therapy, 2 of the most pervasive are mental health conditions and alcohol use. The rates of mental illness among veterans overall is high, but mental illness is particularly high in veterans with HCV. Depression has affected 60%; of this population anxiety, 37%; posttraumatic stress disorder, 28%; and schizophrenia, 10%. Alcohol use disorders are also common among veterans with HCV in care. Active mental health conditions and substance use may affect medication adherence or follow-up visit adherence thereby limiting treatment candidacy. Integrating care of these individuals with mental health providers and substance-use treatment specialists is an important aspect of HCV care and is a priority in VA.
Three-quarters (76%) of the HCV-infected veteran population has been screened for HIV and HIV-HCV co-infection is present in 5733 (3%) of veterans with HCV. HIV-HCV co-infection is associated with an increased progression of liver disease and may have implications for the selection of HCV antiviral agents because of drug interactions. Hepatitis B virus (HBV)-HCV co-infection rates are higher at 7%. HBV vaccination or documentation of HBV immunity in those without HBV infection is 78%.
With regard to specific liver complications, 5% to 20% of those infected with chronic HCV will develop cirrhosis over a period of 20 to 30 years, and 1% to 5% will die of hepatocellular carcinoma (HCC) or cirrhosis.6 Given the natural history of chronic HCV and the aging HCV veteran cohort, increasing numbers of conditions related to progression of liver disease are expected over time. This is most evident in the number of veterans with a diagnosis of cirrhosis, which has increased from approximately 10,000 veterans (8%) in care in 2001 to nearly 30,000 veterans (17%) in care in 2013 (Figure 2).
Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR.7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin.9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.
The Role of Primary Care in HCV
Primary care providers have held the responsibility for multiple roles in HCV care since the discovery of the virus—particularly for HCV risk factor assessment, screening, and diagnosis. HCV antiviral treatment, however, was largely placed in the hands of specialists, given the complexities of patient selection, frequent reliance on a liver biopsy for determining need for treatment, and the toxicities of peginterferon and ribavirin therapy.
There are discussions both inside and outside the VA about potentially expanding the role of PCPs in HCV care. First, primary care is the major setting where the CDC and USPSTF recommendations for birth cohort screening are being implemented, and thus PCPs will be identifying veterans previously undiagnosed with HCV.1,2 Second, the ease and tolerability of the new all-oral combination regimens is causing a shift in the paradigm for HCV treatment, from a highly individualized approach, toward a more uniform approach.
Expanding the role of primary care would have multiple benefits to patients and the health care system as a whole. Only approximately 9% of HCV-infected veterans in VA care have been successfully treated at this time, largely due to low eligibility rates and the poor response rates, but other barriers have also contributed to the low success rate, one of which has been limited access to specialists. Furthermore, veterans who are referred to specialists are often noncompliant with the referral.10 If seeing an HCV specialist is required for treatment, the time to treat the HCV population will be much greater, more costly, and less efficient. Therefore, if the prospect of delivering HCV treatment to the majority of HCV patients is to be accomplished, it is necessary to consider providing treatment in the primary care setting as well as the specialist setting.
Treatment provided by nonspecialists has been evaluated in patients receiving peginterferon and ribavirin regimens and has shown that with adequate education and support, SVR rates were equivalent in the specialist and nonspecialist setting.11 To develop programs to provide primary care with such support, the VA has implemented the Specialty Care Access Network-Extension of Community Healthcare Outcomes program initiative, with casebased learning along with real-time consultation.
Currently, the majority of HCV-infected patients have never seen an HCV specialist, thus PCPs are already providing the majority of HCV care beyond HCV antiviral
treatment.12 Primary care providers are, therefore, key to addressing multiple important aspects of HCV care, including (1) counseling patients on transmission, prevention, lifestyle, and the role of substance use; (2) providing hepatitis A and B vaccination as well as appropriate general vaccinations for any patient with chronic liver disease; (3) modifying comorbidities that could accelerate fibrosis progression, such as diabetes mellitus, obesity and hyperlipidemia; (4) reducing risk from ongoing alcohol, drug, and tobacco use; (5) monitoring patients for fibrosis progression and identifying the presence of cirrhosis; and (6) providing general care for patients with cirrhosis, including HCC screening. These are critical aspects of HCV care, and many PCPs may still need additional education for these roles. The VA provides education and support for PCPs in their current role and is enhancing efforts to expand delivery of HCV treatment to the primary care setting as well.
Conclusions
In 2013, the typical veteran with chronic HCV was white, aged 60 years, and male, with a history of comorbidities, including hypertension, depression, and current or prior alcohol abuse. The proportion of veterans with advanced liver disease including cirrhosis (17%) and HCC (3%), has grown significantly over the past 10 years. By the end of 2013, almost 40,000 veterans had received antiviral therapy for HCV, more than 5,700 of whom had received DAAs. Overall SVR rates have been about 42% among those who were treated. Of veterans who are still potential treatment candidates, 85% are treatment-naive and about one-quarter have advanced liver disease.
Although HCV screening rates in veterans are higher than reported in other health care settings, particularly among those in the critical 1945-1965 birth cohort (65% screening rate), substantial numbers of veterans still require testing. The burden of disease, the lack of specialists, the ease and tolerability of new HCV antiviral medications, and the interplay of HCV with other traditional primary care efforts underly an increased role for PCPs in the care of veterans with HCV. Together, this information helps to construct a view of historical, current, and future HCV care in veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patient.
As the largest single provider of hepatitis C virus infection (HCV) care in the U.S., the VA provided care to > 174,000 veterans with chronic HCV in 2013. Identifying veterans most likely to be infected with HCV, particularly those in the highrisk birth cohort born between 1945 and 1965, is a priority given recent CDC and U.S. Preventive Services Task Force (USPSTF) recommendations.1,2 The availability of new, all-oral HCV antiviral regimens with shorter treatment durations and improved tolerability are expected to greatly increase the number of veterans with HCV who could be treated successfully. In order to effectively reach those who are undiagnosed and to ensure that those diagnosed with HCV are evaluated and offered treatment, expanded reliance on primary care providers (PCPs) is essential. This article provides a population view of the current state of VA care for this large HCV-infected population and the important role PCPs share in disease identification and management.
Data Source
Data regarding the state of HCV care in the VA comes from the VA National Clinical Case Registry (CCR) for HCV.3 The VA HCV CCR is an extract of the VA electronic medical record that contains laboratory results, pharmacy information, provider information, and ICD-9 diagnosis codes from inpatient hospitalizations, outpatient visits, and problem lists of veterans with HCV seen at all VAMCs.
Screening and Prevalence of HCV
It is estimated that 2.3 to 2.7 million Americans are living with HCV, with 45% to 85% of those unaware of their infection.4,5 Nearly 75% of those infected are expected to have been born between 1945 and 1965; thus, the CDC and USPSTF now recommend onetime HCV screening for this birth cohort.1,2 Among nearly 5.6 million veterans with a VA outpatient visit in 2013, 56% have been screened for HCV. The HCV screening rate was 42% for those born prior to 1945, 65% for those born during 1945-1965, and 59% for those born after 1965. HCV infection prevalence overall in the VA was 5.8% but differed markedly among the birth cohorts: 1.6% for those born prior to 1945, 9.5% for those born during 1945-1965, and 1.2% for those born after 1965. The prevalence rate of veterans born in the 1945-1965 birth cohort (9.5%) is almost 3 times higher than that of the general U.S. population in this birth cohort (2.4%). The high prevalence serves as a reminder of the greater HCV disease burden in veterans and largely represents Vietman era veterans. Although HCV screening rates in VA have increased over 25% since 2002, the high prevalence among veterans in this birth cohort underscores the importance of continued screening efforts.
Veterans with Chronic HCV Infection
The VA Office of Public Health/Population Health generates national HCV reports annually from the HCV CCR describing the population of veterans with chronic HCV infection receiving VA care. These reports are intended to inform about patient care activities, clinician and patient education, prevention activities, and research directed at continuous improvement of veteran care. The first step in providing responsive care is understanding the affected population, and summarized herein is a description of the veterans with chronic HCV who received VA care in 2013.
In 2013, 174,302 veterans had laboratory evidence of HCV viremia and could be characterized as having chronic HCV. HCV treatment regimens and response depend on the viral genotype. Among veterans with genotype testing, 107,144 (80%) have genotype 1; 15,486 (12%) genotype 2; 9,745 (7%) genotype 3; 1333 (1%) genotype 4; and 63 (< 1%) genotype 5 or 6.
In terms of demographics, most veterans with chronic HCV in VA care in 2013 were men (97%); however, > 5,000 women veterans with chronic HCV received care from the VA. Over half (54%) of veterans with chronic HCV are white, and about one-third (34%) are black. The proportion of blacks within the HCV-infected veteran population is substantially greater than the proportion of blacks in the overall veteran population in VA care (15%) and highlights the disproportionately large burden of HCV that black veterans bear. A smaller proportion of the VA HCV population is Hispanic (6%), and the remaining veterans are other races, multiple races, or unknown.
The HCV-infected veteran population is aging. The mean age of veterans with chronic HCV in 2013 was 59.7 years and for the first time, more veterans with HCV were aged 60 years (Figure 1).
Among the comorbidities that may have historically prevented veterans from receiving HCV antiviral therapy, 2 of the most pervasive are mental health conditions and alcohol use. The rates of mental illness among veterans overall is high, but mental illness is particularly high in veterans with HCV. Depression has affected 60%; of this population anxiety, 37%; posttraumatic stress disorder, 28%; and schizophrenia, 10%. Alcohol use disorders are also common among veterans with HCV in care. Active mental health conditions and substance use may affect medication adherence or follow-up visit adherence thereby limiting treatment candidacy. Integrating care of these individuals with mental health providers and substance-use treatment specialists is an important aspect of HCV care and is a priority in VA.
Three-quarters (76%) of the HCV-infected veteran population has been screened for HIV and HIV-HCV co-infection is present in 5733 (3%) of veterans with HCV. HIV-HCV co-infection is associated with an increased progression of liver disease and may have implications for the selection of HCV antiviral agents because of drug interactions. Hepatitis B virus (HBV)-HCV co-infection rates are higher at 7%. HBV vaccination or documentation of HBV immunity in those without HBV infection is 78%.
With regard to specific liver complications, 5% to 20% of those infected with chronic HCV will develop cirrhosis over a period of 20 to 30 years, and 1% to 5% will die of hepatocellular carcinoma (HCC) or cirrhosis.6 Given the natural history of chronic HCV and the aging HCV veteran cohort, increasing numbers of conditions related to progression of liver disease are expected over time. This is most evident in the number of veterans with a diagnosis of cirrhosis, which has increased from approximately 10,000 veterans (8%) in care in 2001 to nearly 30,000 veterans (17%) in care in 2013 (Figure 2).
Antiviral Therapy for Chronic HCV
Prior to mid-2011, the standard of care for HCV treatment was the combination of pegylated interferon and ribavirin. From 2011 through 2013, direct-acting antiviral (DAA) regimens containing boceprevir and telaprevir in combination with pegylated interferon and ribavirin became standard of care for genotype 1 while
the standard of care remained pegylated interferon and ribavirin for genotypes other than genotype 1. Recent advances in HCV antiviral therapy offer higher cure rates and fewer adverse events (AEs) compared with peginterferon-containing treatment. The expected ease and tolerability of these all-oral combination regimens is anticipated to greatly increase the number of veterans with HCV who could be treated successfully.
Because of the poor tolerability, prolonged treatment durations, serious AEs, and relative or absolute contraindications to peginterferon-based therapy, many veterans were not previously candidates for treatment. Of the 174,302 veterans with chronic HCV in care in 2013, 39,388 (23%) had received at least 1 course of HCV antiviral treatment. This largely reflects the time when peginterferon-based therapy was the standard of care. Since the approval of boceprevir and telaprevir 5,732 veterans (5.8%) in care in 2013 had ever received boceprevir or telaprevir-based regimens.
While recognizing that all veterans should be considered for HCV treatment, the urgency for treatment may be greater in those with advanced liver disease, because these patients are at the highest risk of developing decompensated cirrhosis or dying of liver-related disease. In 2013, there were 28,945 veterans in care that had advanced liver disease who might be considered potential HCV treatment candidates with an urgency to treat.
Duration of treatment and anticipated rates of treatment success with the all-oral regimens depend in part on a patient’s prior treatment status in addition to whether the patient has a diagnosis of advanced liver disease/cirrhosis. Regardless of HCV genotype, among all veterans approximately 85% are treatment-naïve and 15% are treatment-experienced. Advanced liver disease is present in 24% of treatment-naïve and 31% of treatment-experienced veterans with HCV genotype 1; 23% and 24% of veterans with HCV genotype 2, respectively; and 34% and 43% of veterans with HCV genotype 3, respectively.
Further understanding the population of veterans with HCV, including prior treatment status and stage of liver disease, is useful in identifying the target population for treatment. The VA uses these data to project treatment costs and assess capacity across the system in preparation for expected uptake of new regimens.
Sustained Virologic Response After HCV Antiviral Treatment
The goal of HCV antiviral therapy is to eradicate HCV and reduce the progression of liver disease and death from HCV infection. Successful antiviral treatment of HCV is determined by achieving a sustained virologic response (SVR) defined as an undetectable HCV viral load 12 weeks after the end of treatment. Of the 39,388 veterans in VA care in 2013 who have ever received antiviral therapy, SVR could be assessed in 32,815 veterans, and the overall SVR rate in this population was 42%. This SVR rate is similar to that observed in phase III trials of pegylated interferon-based regimens, where 42% to 46% of those infected with HCV genotype 1 achieved SVR.7,8 Although most veterans with genotype 1 infection received boceprevir or telaprevir-based regimens in 2013 and achieved higher SVRs of 50% to 52%, the overwhelming majority of veterans in care in 2013 received prior treatment with only peginterferon and ribavirin.9 Although SVR rates are expected to increase with newer all-oral HCV regimens, differences between clinical efficacy and real-world effectiveness will continue to be apparent,
and patient and provider expectations should be tempered accordingly.
The Role of Primary Care in HCV
Primary care providers have held the responsibility for multiple roles in HCV care since the discovery of the virus—particularly for HCV risk factor assessment, screening, and diagnosis. HCV antiviral treatment, however, was largely placed in the hands of specialists, given the complexities of patient selection, frequent reliance on a liver biopsy for determining need for treatment, and the toxicities of peginterferon and ribavirin therapy.
There are discussions both inside and outside the VA about potentially expanding the role of PCPs in HCV care. First, primary care is the major setting where the CDC and USPSTF recommendations for birth cohort screening are being implemented, and thus PCPs will be identifying veterans previously undiagnosed with HCV.1,2 Second, the ease and tolerability of the new all-oral combination regimens is causing a shift in the paradigm for HCV treatment, from a highly individualized approach, toward a more uniform approach.
Expanding the role of primary care would have multiple benefits to patients and the health care system as a whole. Only approximately 9% of HCV-infected veterans in VA care have been successfully treated at this time, largely due to low eligibility rates and the poor response rates, but other barriers have also contributed to the low success rate, one of which has been limited access to specialists. Furthermore, veterans who are referred to specialists are often noncompliant with the referral.10 If seeing an HCV specialist is required for treatment, the time to treat the HCV population will be much greater, more costly, and less efficient. Therefore, if the prospect of delivering HCV treatment to the majority of HCV patients is to be accomplished, it is necessary to consider providing treatment in the primary care setting as well as the specialist setting.
Treatment provided by nonspecialists has been evaluated in patients receiving peginterferon and ribavirin regimens and has shown that with adequate education and support, SVR rates were equivalent in the specialist and nonspecialist setting.11 To develop programs to provide primary care with such support, the VA has implemented the Specialty Care Access Network-Extension of Community Healthcare Outcomes program initiative, with casebased learning along with real-time consultation.
Currently, the majority of HCV-infected patients have never seen an HCV specialist, thus PCPs are already providing the majority of HCV care beyond HCV antiviral
treatment.12 Primary care providers are, therefore, key to addressing multiple important aspects of HCV care, including (1) counseling patients on transmission, prevention, lifestyle, and the role of substance use; (2) providing hepatitis A and B vaccination as well as appropriate general vaccinations for any patient with chronic liver disease; (3) modifying comorbidities that could accelerate fibrosis progression, such as diabetes mellitus, obesity and hyperlipidemia; (4) reducing risk from ongoing alcohol, drug, and tobacco use; (5) monitoring patients for fibrosis progression and identifying the presence of cirrhosis; and (6) providing general care for patients with cirrhosis, including HCC screening. These are critical aspects of HCV care, and many PCPs may still need additional education for these roles. The VA provides education and support for PCPs in their current role and is enhancing efforts to expand delivery of HCV treatment to the primary care setting as well.
Conclusions
In 2013, the typical veteran with chronic HCV was white, aged 60 years, and male, with a history of comorbidities, including hypertension, depression, and current or prior alcohol abuse. The proportion of veterans with advanced liver disease including cirrhosis (17%) and HCC (3%), has grown significantly over the past 10 years. By the end of 2013, almost 40,000 veterans had received antiviral therapy for HCV, more than 5,700 of whom had received DAAs. Overall SVR rates have been about 42% among those who were treated. Of veterans who are still potential treatment candidates, 85% are treatment-naive and about one-quarter have advanced liver disease.
Although HCV screening rates in veterans are higher than reported in other health care settings, particularly among those in the critical 1945-1965 birth cohort (65% screening rate), substantial numbers of veterans still require testing. The burden of disease, the lack of specialists, the ease and tolerability of new HCV antiviral medications, and the interplay of HCV with other traditional primary care efforts underly an increased role for PCPs in the care of veterans with HCV. Together, this information helps to construct a view of historical, current, and future HCV care in veterans.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patient.
1. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965 [published correction appears in MMWR Recomm Rep. 2012;61(43):886]. MMWR Recomm Rep. 2012;61(RR-4):1-32.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
3. Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: Simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775-783.
4. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: Model-based predictions. Ann Intern Med. 2014;161(3):170-180
5. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300
6. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127(5):1372-1380.
7. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958-965.
8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
9. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
10. Brady CW, Coffman CJ, Provenzale D. Compliance with referral for hepatitis C evaluation among veterans. J Clin Gastroenterol. 2007;41(10):927-931.
11. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
12. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859-1861.
1. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965 [published correction appears in MMWR Recomm Rep. 2012;61(43):886]. MMWR Recomm Rep. 2012;61(RR-4):1-32.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
3. Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: Simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775-783.
4. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: Model-based predictions. Ann Intern Med. 2014;161(3):170-180
5. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300
6. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology. 2004;127(5):1372-1380.
7. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958-965.
8. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
9. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
10. Brady CW, Coffman CJ, Provenzale D. Compliance with referral for hepatitis C evaluation among veterans. J Clin Gastroenterol. 2007;41(10):927-931.
11. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207.
12. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859-1861.