User login
Ruling out PE in pregnancy
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
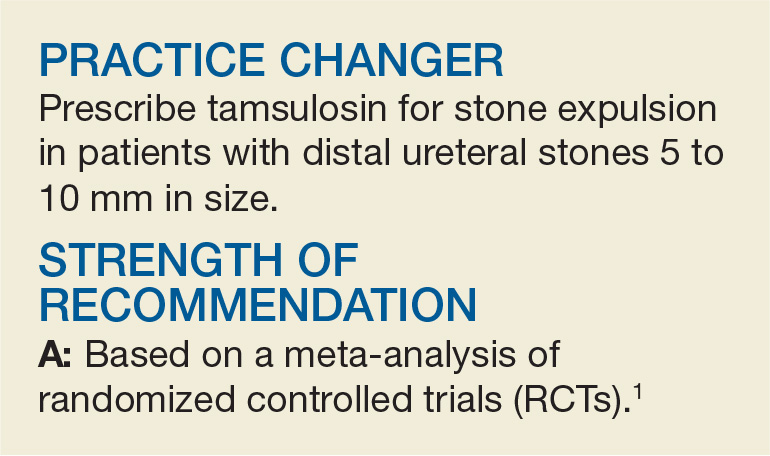
PRACTICE CHANGER
Use a clinical probability score to identify patients at low or intermediate risk for pulmonary embolism (PE) and combine that with a high-sensitivity D-dimer test to rule out PE in pregnant women.
STRENGTH OF RECOMMENDATION
B: Prospective diagnostic management outcome study.1
Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.1
Tamsulosin for Patients With Ureteral Stones?
A 54-year-old man presents to the emergency department (ED) with acute-onset left flank pain that radiates to the groin. CT of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed an appropriate candidate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8%, with a self-reported prevalence of 10.6% in men and 7.1% in women.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with NSAIDs as firstline treatment and opioids as a second-line option.3
In addition, α-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the α-blocker tamsulosin. Two systematic reviews (limited by heterogeneity because some of the studies lacked a placebo control and blinding) concluded that α-blockers increased stone passage within one to six weeks when compared with placebo or no additional therapy.4,5 However, a recent large, multicenter RCT revealed no difference between tamsulosin and nifedipine, or either one compared with placebo, at decreasing the need for further treatment to achieve stone passage within four weeks.6
STUDY SUMMARY
Results broken down by stone size
This meta-analysis, comprising eight double-blind RCTs, examined the effect of oral tamsulosin (0.4 mg/d; average course, 28 d) on distal ureteral stone passage in adult patients (N = 1,384).1 A subgroup analysis comparing stone size (< 5 mm and 5-10 mm) was also conducted to determine whether size modified the effect of tamsulosin.
The eight selected studies were published between 2009 and 2015; the trials were conducted in multiple countries, in ED and outpatient urology settings. The main outcome measure was the risk difference (RD) in stone passage between the tamsulosin group and placebo group after follow-up imaging at three weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk for stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; RD, 17%), but significant heterogeneity existed across the trials (I2, 80.2%). Subgroup analysis by stone size (< 5 mm vs 5-10 mm) revealed that, compared to placebo, tamsulosin was beneficial for larger stones (6 trials, N = 514; RD, 22%; number needed to treat, 5) but not for smaller stones (4 trials, N = 533; RD, –0.3%). The 5-to-10–mm subgroup had a less heterogeneous population of studies than did the < 5-mm subgroup (I2, 33% and 0% respectively).
In terms of adverse events, tamsulosin did not increase the risk for dizziness (RD, 0.2%) or postural hypotension (RD, 0.1%), compared with placebo.
WHAT’S NEW
Increased passage of larger stones
This meta-analysis included only double-blind RCTs; prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Clinician Reviews. 2016;26[4]:20,44), which recommended against the use of α-blockers tamsulosin and nifedipine for ureteral stones measuring < 10 mm.6,7
But the subgroup analysis in this review went one step further by examining passage rates by stone size (< 5 mm vs 5-10 mm) and revealing that passage of larger stones increased with tamsulosin use. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
What about proximal or XL stones?
Only distal stones were included in seven of the eight trials in this analysis. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones > 10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[1]:37-38).
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1): 160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015; 386(9991):341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65(2): 118-120.
A 54-year-old man presents to the emergency department (ED) with acute-onset left flank pain that radiates to the groin. CT of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed an appropriate candidate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8%, with a self-reported prevalence of 10.6% in men and 7.1% in women.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with NSAIDs as firstline treatment and opioids as a second-line option.3
In addition, α-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the α-blocker tamsulosin. Two systematic reviews (limited by heterogeneity because some of the studies lacked a placebo control and blinding) concluded that α-blockers increased stone passage within one to six weeks when compared with placebo or no additional therapy.4,5 However, a recent large, multicenter RCT revealed no difference between tamsulosin and nifedipine, or either one compared with placebo, at decreasing the need for further treatment to achieve stone passage within four weeks.6
STUDY SUMMARY
Results broken down by stone size
This meta-analysis, comprising eight double-blind RCTs, examined the effect of oral tamsulosin (0.4 mg/d; average course, 28 d) on distal ureteral stone passage in adult patients (N = 1,384).1 A subgroup analysis comparing stone size (< 5 mm and 5-10 mm) was also conducted to determine whether size modified the effect of tamsulosin.
The eight selected studies were published between 2009 and 2015; the trials were conducted in multiple countries, in ED and outpatient urology settings. The main outcome measure was the risk difference (RD) in stone passage between the tamsulosin group and placebo group after follow-up imaging at three weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk for stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; RD, 17%), but significant heterogeneity existed across the trials (I2, 80.2%). Subgroup analysis by stone size (< 5 mm vs 5-10 mm) revealed that, compared to placebo, tamsulosin was beneficial for larger stones (6 trials, N = 514; RD, 22%; number needed to treat, 5) but not for smaller stones (4 trials, N = 533; RD, –0.3%). The 5-to-10–mm subgroup had a less heterogeneous population of studies than did the < 5-mm subgroup (I2, 33% and 0% respectively).
In terms of adverse events, tamsulosin did not increase the risk for dizziness (RD, 0.2%) or postural hypotension (RD, 0.1%), compared with placebo.
WHAT’S NEW
Increased passage of larger stones
This meta-analysis included only double-blind RCTs; prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Clinician Reviews. 2016;26[4]:20,44), which recommended against the use of α-blockers tamsulosin and nifedipine for ureteral stones measuring < 10 mm.6,7
But the subgroup analysis in this review went one step further by examining passage rates by stone size (< 5 mm vs 5-10 mm) and revealing that passage of larger stones increased with tamsulosin use. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
What about proximal or XL stones?
Only distal stones were included in seven of the eight trials in this analysis. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones > 10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[1]:37-38).
A 54-year-old man presents to the emergency department (ED) with acute-onset left flank pain that radiates to the groin. CT of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed an appropriate candidate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8%, with a self-reported prevalence of 10.6% in men and 7.1% in women.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with NSAIDs as firstline treatment and opioids as a second-line option.3
In addition, α-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the α-blocker tamsulosin. Two systematic reviews (limited by heterogeneity because some of the studies lacked a placebo control and blinding) concluded that α-blockers increased stone passage within one to six weeks when compared with placebo or no additional therapy.4,5 However, a recent large, multicenter RCT revealed no difference between tamsulosin and nifedipine, or either one compared with placebo, at decreasing the need for further treatment to achieve stone passage within four weeks.6
STUDY SUMMARY
Results broken down by stone size
This meta-analysis, comprising eight double-blind RCTs, examined the effect of oral tamsulosin (0.4 mg/d; average course, 28 d) on distal ureteral stone passage in adult patients (N = 1,384).1 A subgroup analysis comparing stone size (< 5 mm and 5-10 mm) was also conducted to determine whether size modified the effect of tamsulosin.
The eight selected studies were published between 2009 and 2015; the trials were conducted in multiple countries, in ED and outpatient urology settings. The main outcome measure was the risk difference (RD) in stone passage between the tamsulosin group and placebo group after follow-up imaging at three weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk for stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; RD, 17%), but significant heterogeneity existed across the trials (I2, 80.2%). Subgroup analysis by stone size (< 5 mm vs 5-10 mm) revealed that, compared to placebo, tamsulosin was beneficial for larger stones (6 trials, N = 514; RD, 22%; number needed to treat, 5) but not for smaller stones (4 trials, N = 533; RD, –0.3%). The 5-to-10–mm subgroup had a less heterogeneous population of studies than did the < 5-mm subgroup (I2, 33% and 0% respectively).
In terms of adverse events, tamsulosin did not increase the risk for dizziness (RD, 0.2%) or postural hypotension (RD, 0.1%), compared with placebo.
WHAT’S NEW
Increased passage of larger stones
This meta-analysis included only double-blind RCTs; prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Clinician Reviews. 2016;26[4]:20,44), which recommended against the use of α-blockers tamsulosin and nifedipine for ureteral stones measuring < 10 mm.6,7
But the subgroup analysis in this review went one step further by examining passage rates by stone size (< 5 mm vs 5-10 mm) and revealing that passage of larger stones increased with tamsulosin use. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
What about proximal or XL stones?
Only distal stones were included in seven of the eight trials in this analysis. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones > 10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[1]:37-38).
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1): 160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015; 386(9991):341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65(2): 118-120.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1): 160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015; 386(9991):341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65(2): 118-120.
Tamsulosin for patients with ureteral stones?
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe tamsulosin for stone expulsion in patients with distal ureteral stones 5 to 10 mm in size.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.