User login
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Bilateral Ankle Ulcerations and Gangrene of the Toes
Bilateral Ankle Ulcerations and Gangrene of the Toes
THE DIAGNOSIS: Rheumatoid Vasculitis
A diagnosis of rheumatoid vasculitis (RV) was made based on the clinical features, histopathology, and laboratory results in the setting of rheumatoid arthritis (RA). The distal gangrene was surgically managed with bilateral transmetatarsal amputation followed by ankle collagen graft placement. The patient was started on a prednisone taper for 1 month (40 mg/d for 3 days, then 30 mg/d for 3 days, then 20 mg/d for 24 days) before transitioning to rituximab (375 mg/m2 once weekly for 4 weeks), which improved the size and depth of the ulcers.
Rheumatoid vasculitis is an inflammatory disease that affects small- to medium-sized blood vessels in patients with RA. The pathogenesis involves immune complex deposition and complement system activation, leading to vessel wall destruction.1 Rheumatoid vasculitis is an extra-articular complication of RA that primarily is observed in seropositive patients with long-standing severe disease.1,2 The mean duration between RA diagnosis and RV onset is 10 to 14 years.2 Rheumatoid vasculitis manifests heterogeneously and can affect many organs; however, it most frequently affects the skin. Cutaneous manifestations vary in severity. Palpable purpura, pyoderma gangrenosum, and distal ulcers can be seen in addition to extensive digital ischemia with necrosis, as was present in our patient.1
When RA patients present with skin changes that are concerning for vasculitis, RV should be suspected. Currently, there are no validated diagnostic criteria for RV. Diagnosis is made based on clinical presentation and tissue biopsy. Histopathology shows small- and medium-sized vessel wall destruction with neutrophilic, granulomatous, or lymphocytic infiltration, which may be observed only in the lower dermis sparing superficial vessels.3 Direct immunofluorescence shows IgM, IgA, and C3 deposition within and around vessels.3,4 Laboratory findings including elevated inflammatory markers, positive rheumatoid factor, positive anti–cyclic citrullinated peptide, and hypocomplementemia support a diagnosis of RV.1,2
Mortality rates for RV remain high, necessitating aggressive treatment. High-dose corticosteroids typically are combined with immunosuppressant or biologic agents, frequently cyclophosphamide or rituximab.1 Consistent with other reported cases, our patient’s ulcers improved with rituximab and oral steroids.
The differential diagnosis for our patient included type I cryoglobulinemia, cutaneous polyarteritis nodosa (CPAN), peripheral vascular disease (PVD), and nonuremic calciphylaxis. Type I cryoglobulinemia manifests due to direct occlusion of vessels by precipitation of monoclonal immunoglobulin.5 It commonly is associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and multiple myeloma. While our patient’s history of RA was a risk factor for mixed cryoglobulinemia as opposed to type I cryoglobulinemia, the clinical presentation aligned more closely with type I cryoglobulinemia. The clinical manifestations of type I cryoglobulinemia are related to intravascular obstruction, including Raynaud phenomenon, retiform purpura, ischemic ulcers, distal gangrene, and cold-induced urticaria.6-8 Type I cryoglobulinemia also frequently has neurologic and renal manifestations. Histopathology, along with the detection of serum cryoglobulins, is the gold standard for diagnosing cryoglobulinemia.6 On histopathology, type I cryoglobulinemia typically shows a thrombotic vasculopathy with amorphous eosinophilic periodic acid–Schiff–positive thrombi.7 False-negative results are particularly common with serum cryoglobulins, so repeat testing often is needed. While many clinical features overlap, RV is the most likely diagnosis in a patient with long-standing RA who is negative for cryoglobulins and has no history of lymphoproliferative disorders.
Cutaneous polyarteritis nodosa is a necrotizing vasculitis that similarly affects small- and medium-sized vessels. The exact etiology is unknown, but the high prevalence of anti–phosphatidylserine/prothrombin complex antibodies among patients with CPAN suggests that prothrombin bound to apoptotic endothelial cells may initiate the immune response.9 Underlying infection and inflammatory and autoimmune diseases (including group A beta-hemolytic streptococcus, hepatitis B, inflammatory bowel disease, myasthenia gravis, and RA) also may trigger CPAN.9,10,11 The most common clinical manifestations of CPAN are tender subcutaneous nodules, livedo reticularis, leg ulcers, and cutaneous necrosis. Extracutaneous symptoms such as myalgias and arthralgias also can be associated with CPAN. There is no specific serologic test to diagnose CPAN; the diagnosis is made based on clinicopathologic correlation, with characteristic histopathology showing leukocytoclastic vasculitis in the small- and medium-sized arteries of the deep dermis or hypodermis.9
Peripheral vascular disease is a manifestation of atherosclerosis that affects the legs. Risk factors for atherosclerosis, especially smoking and diabetes mellitus, similarly increase the risk for PVD.12 The most common clinical manifestation of PVD is intermittent claudication, but rarely PVD can progress to critical limb ischemia, which is characterized by pain at rest, nonhealing ulcers, or gangrene of the legs.12 Common findings on physical examination include diminished or absent pedal pulses, abnormal skin color, and skin that is cool to the touch.12 The standard diagnostic test for PVD affecting the legs is evaluation via the ankle-brachial index, with a score of 0.90 or lower being diagnostic of PVD, a score of 0.91 to 1.00 being borderline, and a score of 1.01 to 1.40 being normal.13
Calciphylaxis most frequently is seen in patients with end-stage kidney disease; however, it also has been less commonly reported in patients with normal kidney function, known as nonuremic calciphylaxis. It is characterized by calcification of arteries, arterioles, and soft tissues, which can lead to thrombosis and eventually ischemia and necrosis of the skin.14 Calciphylaxis initially causes tender, indurated, erythematous to purpuric plaques that quickly progress to retiform and stellate ulcers with overlying necrotic eschars.15 Disease typically occurs on the legs and areas that are rich in adipose tissue, such as the abdomen and thighs.16 Skin biopsy is needed for diagnosis of calciphylaxis. Characteristic histopathologic findings include calcification, microvascular thrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles.16
We present a rare case of RV in a patient with well-controlled RA. While the incidence of RV is decreasing in the United States and United Kingdom due to the initiation of earlier and more aggressive RA therapies, mortality remains high.1 Thus, it is important to include RV in the differential diagnosis when there are skin changes concerning vasculitis in patients with seropositive, longstanding RA, even if the RA is well controlled.
- Kishore S, Maher L, Majithia V. Rheumatoid vasculitis: a diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19:39. doi:10.1007/s11926-017-0667-3
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol. 2015;27:63-70. doi:10.1097 /BOR.0000000000000126
- Patterson J. The vasculopathic reaction pattern. In: Patterson J, ed. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:241-301.
- Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243-255. doi:10.23736 /S0392-0488.18.05872-8
- Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. 2021;33:1-7. doi:10.1097/BOR.0000000000000757
- Silva F, Pinto C, Barbosa A, et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi:10.1016 /j.jaut.2019.102313
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j.jbspin.2019.01.016
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756. doi:10.1111/j.1365-4632.2010.04522.
- Criado PR, Marques GF, Morita TC, et al. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558-563. doi:10.1016/j.autrev.2016.02.010
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706-713.
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133-1141. doi:10.1016/j.amjmed.2019.04.043
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013 Jan 1;127:e264]. Circulation. 2012;126:2890-2909. doi:10.1161/CIR.0b013e318276fbcb
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Gomes F, La Feria P, Costa C, et al. Non-uremic calciphylaxis: a rare diagnosis with limited therapeutic strategies. Eur J Case Rep Intern Med.
THE DIAGNOSIS: Rheumatoid Vasculitis
A diagnosis of rheumatoid vasculitis (RV) was made based on the clinical features, histopathology, and laboratory results in the setting of rheumatoid arthritis (RA). The distal gangrene was surgically managed with bilateral transmetatarsal amputation followed by ankle collagen graft placement. The patient was started on a prednisone taper for 1 month (40 mg/d for 3 days, then 30 mg/d for 3 days, then 20 mg/d for 24 days) before transitioning to rituximab (375 mg/m2 once weekly for 4 weeks), which improved the size and depth of the ulcers.
Rheumatoid vasculitis is an inflammatory disease that affects small- to medium-sized blood vessels in patients with RA. The pathogenesis involves immune complex deposition and complement system activation, leading to vessel wall destruction.1 Rheumatoid vasculitis is an extra-articular complication of RA that primarily is observed in seropositive patients with long-standing severe disease.1,2 The mean duration between RA diagnosis and RV onset is 10 to 14 years.2 Rheumatoid vasculitis manifests heterogeneously and can affect many organs; however, it most frequently affects the skin. Cutaneous manifestations vary in severity. Palpable purpura, pyoderma gangrenosum, and distal ulcers can be seen in addition to extensive digital ischemia with necrosis, as was present in our patient.1
When RA patients present with skin changes that are concerning for vasculitis, RV should be suspected. Currently, there are no validated diagnostic criteria for RV. Diagnosis is made based on clinical presentation and tissue biopsy. Histopathology shows small- and medium-sized vessel wall destruction with neutrophilic, granulomatous, or lymphocytic infiltration, which may be observed only in the lower dermis sparing superficial vessels.3 Direct immunofluorescence shows IgM, IgA, and C3 deposition within and around vessels.3,4 Laboratory findings including elevated inflammatory markers, positive rheumatoid factor, positive anti–cyclic citrullinated peptide, and hypocomplementemia support a diagnosis of RV.1,2
Mortality rates for RV remain high, necessitating aggressive treatment. High-dose corticosteroids typically are combined with immunosuppressant or biologic agents, frequently cyclophosphamide or rituximab.1 Consistent with other reported cases, our patient’s ulcers improved with rituximab and oral steroids.
The differential diagnosis for our patient included type I cryoglobulinemia, cutaneous polyarteritis nodosa (CPAN), peripheral vascular disease (PVD), and nonuremic calciphylaxis. Type I cryoglobulinemia manifests due to direct occlusion of vessels by precipitation of monoclonal immunoglobulin.5 It commonly is associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and multiple myeloma. While our patient’s history of RA was a risk factor for mixed cryoglobulinemia as opposed to type I cryoglobulinemia, the clinical presentation aligned more closely with type I cryoglobulinemia. The clinical manifestations of type I cryoglobulinemia are related to intravascular obstruction, including Raynaud phenomenon, retiform purpura, ischemic ulcers, distal gangrene, and cold-induced urticaria.6-8 Type I cryoglobulinemia also frequently has neurologic and renal manifestations. Histopathology, along with the detection of serum cryoglobulins, is the gold standard for diagnosing cryoglobulinemia.6 On histopathology, type I cryoglobulinemia typically shows a thrombotic vasculopathy with amorphous eosinophilic periodic acid–Schiff–positive thrombi.7 False-negative results are particularly common with serum cryoglobulins, so repeat testing often is needed. While many clinical features overlap, RV is the most likely diagnosis in a patient with long-standing RA who is negative for cryoglobulins and has no history of lymphoproliferative disorders.
Cutaneous polyarteritis nodosa is a necrotizing vasculitis that similarly affects small- and medium-sized vessels. The exact etiology is unknown, but the high prevalence of anti–phosphatidylserine/prothrombin complex antibodies among patients with CPAN suggests that prothrombin bound to apoptotic endothelial cells may initiate the immune response.9 Underlying infection and inflammatory and autoimmune diseases (including group A beta-hemolytic streptococcus, hepatitis B, inflammatory bowel disease, myasthenia gravis, and RA) also may trigger CPAN.9,10,11 The most common clinical manifestations of CPAN are tender subcutaneous nodules, livedo reticularis, leg ulcers, and cutaneous necrosis. Extracutaneous symptoms such as myalgias and arthralgias also can be associated with CPAN. There is no specific serologic test to diagnose CPAN; the diagnosis is made based on clinicopathologic correlation, with characteristic histopathology showing leukocytoclastic vasculitis in the small- and medium-sized arteries of the deep dermis or hypodermis.9
Peripheral vascular disease is a manifestation of atherosclerosis that affects the legs. Risk factors for atherosclerosis, especially smoking and diabetes mellitus, similarly increase the risk for PVD.12 The most common clinical manifestation of PVD is intermittent claudication, but rarely PVD can progress to critical limb ischemia, which is characterized by pain at rest, nonhealing ulcers, or gangrene of the legs.12 Common findings on physical examination include diminished or absent pedal pulses, abnormal skin color, and skin that is cool to the touch.12 The standard diagnostic test for PVD affecting the legs is evaluation via the ankle-brachial index, with a score of 0.90 or lower being diagnostic of PVD, a score of 0.91 to 1.00 being borderline, and a score of 1.01 to 1.40 being normal.13
Calciphylaxis most frequently is seen in patients with end-stage kidney disease; however, it also has been less commonly reported in patients with normal kidney function, known as nonuremic calciphylaxis. It is characterized by calcification of arteries, arterioles, and soft tissues, which can lead to thrombosis and eventually ischemia and necrosis of the skin.14 Calciphylaxis initially causes tender, indurated, erythematous to purpuric plaques that quickly progress to retiform and stellate ulcers with overlying necrotic eschars.15 Disease typically occurs on the legs and areas that are rich in adipose tissue, such as the abdomen and thighs.16 Skin biopsy is needed for diagnosis of calciphylaxis. Characteristic histopathologic findings include calcification, microvascular thrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles.16
We present a rare case of RV in a patient with well-controlled RA. While the incidence of RV is decreasing in the United States and United Kingdom due to the initiation of earlier and more aggressive RA therapies, mortality remains high.1 Thus, it is important to include RV in the differential diagnosis when there are skin changes concerning vasculitis in patients with seropositive, longstanding RA, even if the RA is well controlled.
THE DIAGNOSIS: Rheumatoid Vasculitis
A diagnosis of rheumatoid vasculitis (RV) was made based on the clinical features, histopathology, and laboratory results in the setting of rheumatoid arthritis (RA). The distal gangrene was surgically managed with bilateral transmetatarsal amputation followed by ankle collagen graft placement. The patient was started on a prednisone taper for 1 month (40 mg/d for 3 days, then 30 mg/d for 3 days, then 20 mg/d for 24 days) before transitioning to rituximab (375 mg/m2 once weekly for 4 weeks), which improved the size and depth of the ulcers.
Rheumatoid vasculitis is an inflammatory disease that affects small- to medium-sized blood vessels in patients with RA. The pathogenesis involves immune complex deposition and complement system activation, leading to vessel wall destruction.1 Rheumatoid vasculitis is an extra-articular complication of RA that primarily is observed in seropositive patients with long-standing severe disease.1,2 The mean duration between RA diagnosis and RV onset is 10 to 14 years.2 Rheumatoid vasculitis manifests heterogeneously and can affect many organs; however, it most frequently affects the skin. Cutaneous manifestations vary in severity. Palpable purpura, pyoderma gangrenosum, and distal ulcers can be seen in addition to extensive digital ischemia with necrosis, as was present in our patient.1
When RA patients present with skin changes that are concerning for vasculitis, RV should be suspected. Currently, there are no validated diagnostic criteria for RV. Diagnosis is made based on clinical presentation and tissue biopsy. Histopathology shows small- and medium-sized vessel wall destruction with neutrophilic, granulomatous, or lymphocytic infiltration, which may be observed only in the lower dermis sparing superficial vessels.3 Direct immunofluorescence shows IgM, IgA, and C3 deposition within and around vessels.3,4 Laboratory findings including elevated inflammatory markers, positive rheumatoid factor, positive anti–cyclic citrullinated peptide, and hypocomplementemia support a diagnosis of RV.1,2
Mortality rates for RV remain high, necessitating aggressive treatment. High-dose corticosteroids typically are combined with immunosuppressant or biologic agents, frequently cyclophosphamide or rituximab.1 Consistent with other reported cases, our patient’s ulcers improved with rituximab and oral steroids.
The differential diagnosis for our patient included type I cryoglobulinemia, cutaneous polyarteritis nodosa (CPAN), peripheral vascular disease (PVD), and nonuremic calciphylaxis. Type I cryoglobulinemia manifests due to direct occlusion of vessels by precipitation of monoclonal immunoglobulin.5 It commonly is associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and multiple myeloma. While our patient’s history of RA was a risk factor for mixed cryoglobulinemia as opposed to type I cryoglobulinemia, the clinical presentation aligned more closely with type I cryoglobulinemia. The clinical manifestations of type I cryoglobulinemia are related to intravascular obstruction, including Raynaud phenomenon, retiform purpura, ischemic ulcers, distal gangrene, and cold-induced urticaria.6-8 Type I cryoglobulinemia also frequently has neurologic and renal manifestations. Histopathology, along with the detection of serum cryoglobulins, is the gold standard for diagnosing cryoglobulinemia.6 On histopathology, type I cryoglobulinemia typically shows a thrombotic vasculopathy with amorphous eosinophilic periodic acid–Schiff–positive thrombi.7 False-negative results are particularly common with serum cryoglobulins, so repeat testing often is needed. While many clinical features overlap, RV is the most likely diagnosis in a patient with long-standing RA who is negative for cryoglobulins and has no history of lymphoproliferative disorders.
Cutaneous polyarteritis nodosa is a necrotizing vasculitis that similarly affects small- and medium-sized vessels. The exact etiology is unknown, but the high prevalence of anti–phosphatidylserine/prothrombin complex antibodies among patients with CPAN suggests that prothrombin bound to apoptotic endothelial cells may initiate the immune response.9 Underlying infection and inflammatory and autoimmune diseases (including group A beta-hemolytic streptococcus, hepatitis B, inflammatory bowel disease, myasthenia gravis, and RA) also may trigger CPAN.9,10,11 The most common clinical manifestations of CPAN are tender subcutaneous nodules, livedo reticularis, leg ulcers, and cutaneous necrosis. Extracutaneous symptoms such as myalgias and arthralgias also can be associated with CPAN. There is no specific serologic test to diagnose CPAN; the diagnosis is made based on clinicopathologic correlation, with characteristic histopathology showing leukocytoclastic vasculitis in the small- and medium-sized arteries of the deep dermis or hypodermis.9
Peripheral vascular disease is a manifestation of atherosclerosis that affects the legs. Risk factors for atherosclerosis, especially smoking and diabetes mellitus, similarly increase the risk for PVD.12 The most common clinical manifestation of PVD is intermittent claudication, but rarely PVD can progress to critical limb ischemia, which is characterized by pain at rest, nonhealing ulcers, or gangrene of the legs.12 Common findings on physical examination include diminished or absent pedal pulses, abnormal skin color, and skin that is cool to the touch.12 The standard diagnostic test for PVD affecting the legs is evaluation via the ankle-brachial index, with a score of 0.90 or lower being diagnostic of PVD, a score of 0.91 to 1.00 being borderline, and a score of 1.01 to 1.40 being normal.13
Calciphylaxis most frequently is seen in patients with end-stage kidney disease; however, it also has been less commonly reported in patients with normal kidney function, known as nonuremic calciphylaxis. It is characterized by calcification of arteries, arterioles, and soft tissues, which can lead to thrombosis and eventually ischemia and necrosis of the skin.14 Calciphylaxis initially causes tender, indurated, erythematous to purpuric plaques that quickly progress to retiform and stellate ulcers with overlying necrotic eschars.15 Disease typically occurs on the legs and areas that are rich in adipose tissue, such as the abdomen and thighs.16 Skin biopsy is needed for diagnosis of calciphylaxis. Characteristic histopathologic findings include calcification, microvascular thrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles.16
We present a rare case of RV in a patient with well-controlled RA. While the incidence of RV is decreasing in the United States and United Kingdom due to the initiation of earlier and more aggressive RA therapies, mortality remains high.1 Thus, it is important to include RV in the differential diagnosis when there are skin changes concerning vasculitis in patients with seropositive, longstanding RA, even if the RA is well controlled.
- Kishore S, Maher L, Majithia V. Rheumatoid vasculitis: a diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19:39. doi:10.1007/s11926-017-0667-3
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol. 2015;27:63-70. doi:10.1097 /BOR.0000000000000126
- Patterson J. The vasculopathic reaction pattern. In: Patterson J, ed. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:241-301.
- Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243-255. doi:10.23736 /S0392-0488.18.05872-8
- Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. 2021;33:1-7. doi:10.1097/BOR.0000000000000757
- Silva F, Pinto C, Barbosa A, et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi:10.1016 /j.jaut.2019.102313
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j.jbspin.2019.01.016
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756. doi:10.1111/j.1365-4632.2010.04522.
- Criado PR, Marques GF, Morita TC, et al. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558-563. doi:10.1016/j.autrev.2016.02.010
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706-713.
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133-1141. doi:10.1016/j.amjmed.2019.04.043
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013 Jan 1;127:e264]. Circulation. 2012;126:2890-2909. doi:10.1161/CIR.0b013e318276fbcb
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Gomes F, La Feria P, Costa C, et al. Non-uremic calciphylaxis: a rare diagnosis with limited therapeutic strategies. Eur J Case Rep Intern Med.
- Kishore S, Maher L, Majithia V. Rheumatoid vasculitis: a diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19:39. doi:10.1007/s11926-017-0667-3
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol. 2015;27:63-70. doi:10.1097 /BOR.0000000000000126
- Patterson J. The vasculopathic reaction pattern. In: Patterson J, ed. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:241-301.
- Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243-255. doi:10.23736 /S0392-0488.18.05872-8
- Kolopp-Sarda MN, Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. 2021;33:1-7. doi:10.1097/BOR.0000000000000757
- Silva F, Pinto C, Barbosa A, et al. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi:10.1016 /j.jaut.2019.102313
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j.jbspin.2019.01.016
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756. doi:10.1111/j.1365-4632.2010.04522.
- Criado PR, Marques GF, Morita TC, et al. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558-563. doi:10.1016/j.autrev.2016.02.010
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706-713.
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133-1141. doi:10.1016/j.amjmed.2019.04.043
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association [published correction appears in Circulation. 2013 Jan 1;127:e264]. Circulation. 2012;126:2890-2909. doi:10.1161/CIR.0b013e318276fbcb
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Gomes F, La Feria P, Costa C, et al. Non-uremic calciphylaxis: a rare diagnosis with limited therapeutic strategies. Eur J Case Rep Intern Med.
Bilateral Ankle Ulcerations and Gangrene of the Toes
Bilateral Ankle Ulcerations and Gangrene of the Toes
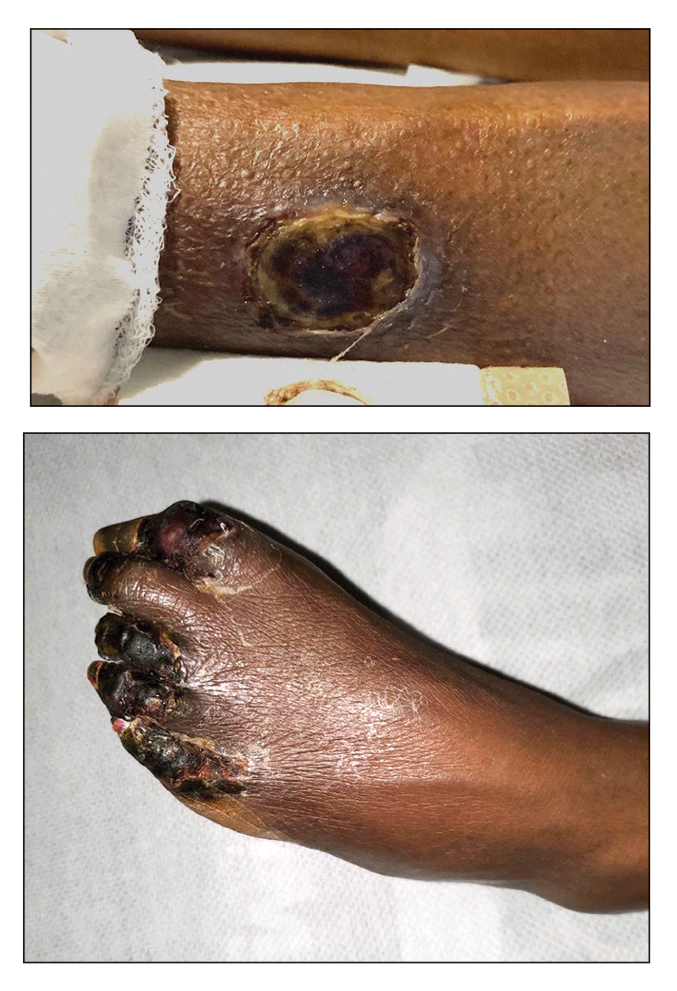
A 74-year-old woman presented to the hospital with large tender ulcerations on both ankles as well as gangrene of the toes of 6 to 8 weeks’ duration. The patient had a history of hypertension as well as seropositive nonerosive rheumatoid arthritis that had been diagnosed 8 years prior and was well controlled with leflunomide and prednisone as needed for flares. She denied any history of similar ulcers as well as any recent illnesses, medication changes, or joint pain or swelling. She was evaluated by vascular surgery 1 week prior to the current presentation, at which time her ankle-brachial index score was normal. Skin examination revealed noninflammatory retiform purpura surrounding ulcerations on both ankles (top) and necrosis of all toes (bottom) with peripheral retiform purpura. Joint examination revealed swan neck deformities of multiple fingers with normal range of motion, and there was no effusion or tenderness of the joints of the fingers on palpation. No rheumatoid nodules were present. Laboratory testing revealed elevated rheumatoid factor, anti–cyclic citrullinated peptide, C-reactive protein, and anti–Sjögren syndrome–related antigen A levels and low C4 levels. Cryoglobulins, antineutrophil cytoplasmic antibodies, and serum protein electrophoresis were negative. Biopsy of an ulcer on the right ankle showed medium-sized vessel vasculitis with fibrinoid necrosis, including endothelium necrosis and a perivascular lymphocytic infiltrate. Direct immunofluorescence demonstrated dense, granular, intraperivascular deposition of IgM and IgG with slightly weaker deposition of IgA, C3, and C5b-9 in the dermis and subcutis with a greater effect on medium-sized vessels.