User login
In reply: ‘Non-criteria’ antiphospholipid antibodies and thrombosis
In Reply: We appreciate the response of Drs. Maharaj, Chang, and Shaikh. Antiphospholipid antibody testing and the diagnosis of antiphospholipid antibody syndrome are quite complex. We recognize that there is controversy with regard to the role of antiphosphatidylserine (aPS) antibodies, antiprothrombin antibodies, (aPT-A), and antibodies to the antiphosphatidylserine-prothrombin complex (aPS/PT).
In the systematic review cited, the authors concluded that measurement of aPS/PT may be helpful in determining the thrombotic risk in a subset of patients with prior thrombosis and systemic lupus erythematosus (SLE).1 However, the majority of the studies included in the systematic review enrolled patients with antiphospholipid antibody syndrome and SLE. Our patient did not have SLE. Additionally, most of the studies were small. Therefore, the independent association between aPS/PT and thrombosis in patients without known SLE or previously known antiphospholipid antibody syndrome is challenging to infer on the basis of available data.1
At our institution, we do not routinely test for these “non-criteria” antibodies as part of our evaluation of suspected antiphospholipid antibody syndrome. However, we agree that this is an area that warrants further investigation. There is a need for prospective trials or, more likely, longitudinal observational studies to further delineate the association of aPT-A, aPS, or aPS/PT with clinical features of antiphospholipid antibody syndrome.2
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111(2):354–364. doi:10.1160/TH13-06-0509
- Miyakis S, Lockshin MD, Atsumi T et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
In Reply: We appreciate the response of Drs. Maharaj, Chang, and Shaikh. Antiphospholipid antibody testing and the diagnosis of antiphospholipid antibody syndrome are quite complex. We recognize that there is controversy with regard to the role of antiphosphatidylserine (aPS) antibodies, antiprothrombin antibodies, (aPT-A), and antibodies to the antiphosphatidylserine-prothrombin complex (aPS/PT).
In the systematic review cited, the authors concluded that measurement of aPS/PT may be helpful in determining the thrombotic risk in a subset of patients with prior thrombosis and systemic lupus erythematosus (SLE).1 However, the majority of the studies included in the systematic review enrolled patients with antiphospholipid antibody syndrome and SLE. Our patient did not have SLE. Additionally, most of the studies were small. Therefore, the independent association between aPS/PT and thrombosis in patients without known SLE or previously known antiphospholipid antibody syndrome is challenging to infer on the basis of available data.1
At our institution, we do not routinely test for these “non-criteria” antibodies as part of our evaluation of suspected antiphospholipid antibody syndrome. However, we agree that this is an area that warrants further investigation. There is a need for prospective trials or, more likely, longitudinal observational studies to further delineate the association of aPT-A, aPS, or aPS/PT with clinical features of antiphospholipid antibody syndrome.2
In Reply: We appreciate the response of Drs. Maharaj, Chang, and Shaikh. Antiphospholipid antibody testing and the diagnosis of antiphospholipid antibody syndrome are quite complex. We recognize that there is controversy with regard to the role of antiphosphatidylserine (aPS) antibodies, antiprothrombin antibodies, (aPT-A), and antibodies to the antiphosphatidylserine-prothrombin complex (aPS/PT).
In the systematic review cited, the authors concluded that measurement of aPS/PT may be helpful in determining the thrombotic risk in a subset of patients with prior thrombosis and systemic lupus erythematosus (SLE).1 However, the majority of the studies included in the systematic review enrolled patients with antiphospholipid antibody syndrome and SLE. Our patient did not have SLE. Additionally, most of the studies were small. Therefore, the independent association between aPS/PT and thrombosis in patients without known SLE or previously known antiphospholipid antibody syndrome is challenging to infer on the basis of available data.1
At our institution, we do not routinely test for these “non-criteria” antibodies as part of our evaluation of suspected antiphospholipid antibody syndrome. However, we agree that this is an area that warrants further investigation. There is a need for prospective trials or, more likely, longitudinal observational studies to further delineate the association of aPT-A, aPS, or aPS/PT with clinical features of antiphospholipid antibody syndrome.2
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111(2):354–364. doi:10.1160/TH13-06-0509
- Miyakis S, Lockshin MD, Atsumi T et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111(2):354–364. doi:10.1160/TH13-06-0509
- Miyakis S, Lockshin MD, Atsumi T et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
A 75-year-old with abdominal pain, hypoxia, and weak pulses in the left leg
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
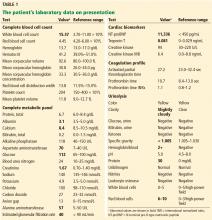
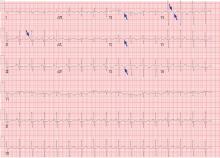
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
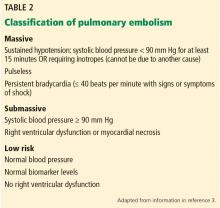
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
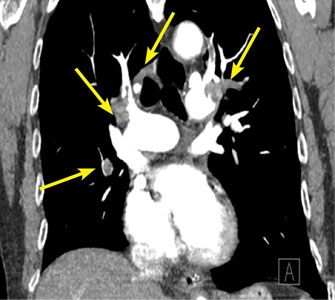
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
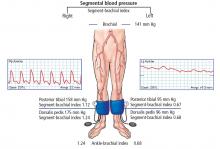
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
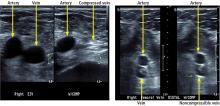
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
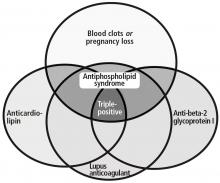
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.