User login
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.
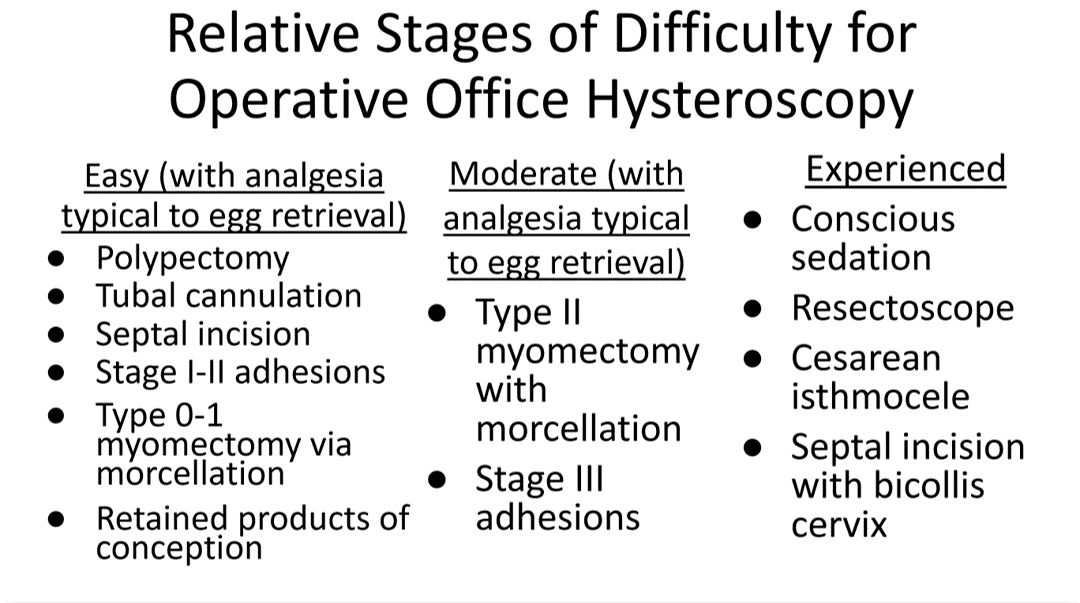
If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
Understanding clinic-reported IVF success rates
The field of assisted reproductive technologies (ART) continues to evolve from its first successful birth in 1978 in England, and then in 1981 in the United States. Over the last 6 years, the total number of cycles in the U.S. has increased by 44% to nearly 370,000.
SART membership consists of more than 350 clinics throughout the United States, representing 80% of ART clinics. Over 95% of ART cycles in 2021 in the United States were performed in SART-member clinics.
SART is an invaluable resource for both patients and physicians. Their website includes a “Predict My Success” calculator that allows patients and physicians to enter individualized data to calculate the chance of having a baby over one or more complete cycles of IVF. To help us understand the pregnancy outcome data from ART – cycles per clinic along with national results – I posed the questions below to Amy Sparks, PhD, HCLD, director of the IVF and Andrology Laboratories and the Center for Advanced Reproductive Care at University of Iowa Hospitals and Clinics, Iowa City. Dr. Sparks is past president of SART and former chairperson of the SART Registry committee when the current Clinic Summary Report format was initially released.
Question: The Fertility Clinic Success Rate and Certification Act (FCSRCA) of 1992 mandated that all ART clinics report success rate data to the federal government, through the Centers for Disease Control and Prevention, in a standardized manner. As ART is the only field in medicine to be required to annually report their patient outcomes, that is, all initiated cycles and live births, why do you believe this law was enacted and is limited to reproductive medicine?
Answer: The FCSRCA of 1992 was enacted in response to the lack of open and reliable pregnancy success rate information for patients seeking infertility care using assisted reproductive technologies. Success rates of 25%-50% were being advertised by independent clinics when, nationally, fewer than 15% of ART procedures led to live births. The Federal Trade Commission said such claims were deceptive and filed charges against five clinics, saying they misrepresented their success in helping women become pregnant. The government won one case by court order and the other four cases were settled out of court.
This field of medicine was in the spotlight as the majority of patients lacked insurance coverage for their ART cycles, and there was a strong desire to protect consumers paying out of pocket for relatively low success. Recognizing that the FTC’s mission is to ensure truth in advertising and not regulate medical care, Congress passed the FCSRCA, mandating that all centers providing ART services report all initiated cycles and their outcomes. The CDC was appointed as the agency responsible for collecting cycle data and reporting outcomes. Centers not reporting their cycles are listed as nonreporting centers.
This act also established standards for accreditation of embryology laboratories including personnel and traditional clinical laboratory management requirements. These standards serve as the foundation for embryology laboratory accrediting agencies.
Q: Why have live-birth rates on SART appeared to be focused on “per IVF cycle” as opposed to the CDC reporting of live births “per embryo transfer?”
A: An ART cycle “start” is defined as the initiation of ovarian stimulation with medication that may or may not include administration of exogenous gonadotropins, followed by oocyte retrieval and embryo transfer. Not every patient beginning a cycle will undergo an oocyte retrieval and not all patients who undergo oocyte retrieval have an embryo transfer. The live-birth rates (LBR) for each of these steps of progression in the ART process are available in the SART and CDC reports.
In 2016, SART recognized that practices were foregoing fresh embryo transfer after oocyte retrieval, opting to cryopreserve all embryos to either accommodate genetic testing of the embryos prior to transfer or to avoid embryo transfer to an unfavorable uterine environment. In response to changes in practice and in an effort to deemphasize live birth per transfer, thereby alleviating a potential motivator or pressure for practitioners to transfer multiple embryos, SART moved to a report that displays the cumulative live-birth rate per cycle start for oocyte retrieval. The cumulative live-birth rate per cycle start for oocyte retrieval is the chance of live birth from transfers of embryos derived from the oocyte retrieval and performed within 1 year of the oocyte retrieval.
This change in reporting further reduced the pressure to transfer multiple embryos and encouraged elective, single-embryo transfer. The outcome per transfer is no longer the report’s primary focus.
Q: The latest pregnancy outcomes statistics are from the year 2020 and are finalized by the CDC. Why does the SART website have this same year labeled “preliminary” outcomes?
A: Shortly after the 2016 SART report change, the CDC made similar changes to their report. The difference is that SART provides a “preliminary” report of outcomes within the year of the cycle start for oocyte retrieval. The cumulative outcome is not “finalized” until the following year as transfers may be performed as late as 12 months after the oocyte retrieval.
SART has opted to report both the “preliminary” or interim outcome and the “final” outcome a year later. The CDC has opted to limit their report to “final” outcomes. I’m happy to report that SART recently released the final report for 2021 cycles.
Q: Have national success rates in the United States continued to rise or have they plateaued?
A: It appears that success rates have plateaued; however, we find ourselves at another point where practice patterns and patients’ approach to using ART for family building have changed.
Recognizing the impact of maternal aging on reproductive potential, patients are opting to undergo multiple ART cycles to cryopreserve embryos for family building before they attempt to get pregnant. This family-building path reduces the value of measuring the LBR per cycle start as we may not know the outcome for many years. SART leaders are deliberating intently as to how to best represent this growing patient population in outcome reporting.
Q: Can you comment on the reduction of multiple gestations with the increasing use of single-embryo transfer?
A: The reduction in emphasis on live births per transfer, emphasis on singleton live-birth rates in both the SART and CDC reports, and American Society for Reproductive Medicine practice committee guidelines strongly supporting single embryo transfer have significantly reduced the rate of multiple gestations.
A decade ago, only a third of the transfers were single-embryo transfers and over 25% of live births resulted in a multiple birth. Today, the majority of embryo transfers are elective, single-embryo transfers, and the multiple birth rate has been reduced by nearly 80%. In 2020, 93% of live births from IVF were singletons.
Q: SART offers an online IVF calculator so both patients and physicians can plug in data for an approximate cumulative success rate for up to three IVF cycles. The calculator pools data from all U.S.-reporting IVF centers. Can you explain what an “IVF cycle” is and what patient information is required? Why do success rates increase over time?
A: Each “IVF cycle” is a cycle start for an oocyte retrieval and all transfers of embryos from that cycle within a year of the oocyte retrieval. If the first cycle and subsequent transfers do not lead to a live birth, patients still have a chance to achieve a live birth with a second or third cycle. The success rate increases over time as it reflects the chance of success for a population of patients, with some achieving a live birth after the first cycle and additional patients who achieve success following their third cycle.
Q: The SART IVF calculator can be used with no prior IVF cycles or following an unsuccessful cycle. Are there data to support an estimation of outcome following two or even more unsuccessful cycles?
A: The variables in the SART IVF calculator are based upon the cycle-specific data from patients seeking care at SART member clinics. The current predictor was built with data from cycles performed in 2015-2016. SART is adjusting the predictor and developing a calculator that will be routinely updated, accordingly.
Q: Only approximately 40% of states have some form of infertility coverage law in place; however the number of IVF cycles in the United States continues to increase on an annual basis. What do you think are the driving factors behind this?
A: Advocacy efforts to improve patients’ access to infertility care have included giving patients tools to encourage their employers to include infertility care in their health care benefits package. More recently, the “Great Resignation” has led to the “Great Recruitment” and employers are recognizing that the addition of infertility care to health care benefits is a powerful recruitment tool.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
The field of assisted reproductive technologies (ART) continues to evolve from its first successful birth in 1978 in England, and then in 1981 in the United States. Over the last 6 years, the total number of cycles in the U.S. has increased by 44% to nearly 370,000.
SART membership consists of more than 350 clinics throughout the United States, representing 80% of ART clinics. Over 95% of ART cycles in 2021 in the United States were performed in SART-member clinics.
SART is an invaluable resource for both patients and physicians. Their website includes a “Predict My Success” calculator that allows patients and physicians to enter individualized data to calculate the chance of having a baby over one or more complete cycles of IVF. To help us understand the pregnancy outcome data from ART – cycles per clinic along with national results – I posed the questions below to Amy Sparks, PhD, HCLD, director of the IVF and Andrology Laboratories and the Center for Advanced Reproductive Care at University of Iowa Hospitals and Clinics, Iowa City. Dr. Sparks is past president of SART and former chairperson of the SART Registry committee when the current Clinic Summary Report format was initially released.
Question: The Fertility Clinic Success Rate and Certification Act (FCSRCA) of 1992 mandated that all ART clinics report success rate data to the federal government, through the Centers for Disease Control and Prevention, in a standardized manner. As ART is the only field in medicine to be required to annually report their patient outcomes, that is, all initiated cycles and live births, why do you believe this law was enacted and is limited to reproductive medicine?
Answer: The FCSRCA of 1992 was enacted in response to the lack of open and reliable pregnancy success rate information for patients seeking infertility care using assisted reproductive technologies. Success rates of 25%-50% were being advertised by independent clinics when, nationally, fewer than 15% of ART procedures led to live births. The Federal Trade Commission said such claims were deceptive and filed charges against five clinics, saying they misrepresented their success in helping women become pregnant. The government won one case by court order and the other four cases were settled out of court.
This field of medicine was in the spotlight as the majority of patients lacked insurance coverage for their ART cycles, and there was a strong desire to protect consumers paying out of pocket for relatively low success. Recognizing that the FTC’s mission is to ensure truth in advertising and not regulate medical care, Congress passed the FCSRCA, mandating that all centers providing ART services report all initiated cycles and their outcomes. The CDC was appointed as the agency responsible for collecting cycle data and reporting outcomes. Centers not reporting their cycles are listed as nonreporting centers.
This act also established standards for accreditation of embryology laboratories including personnel and traditional clinical laboratory management requirements. These standards serve as the foundation for embryology laboratory accrediting agencies.
Q: Why have live-birth rates on SART appeared to be focused on “per IVF cycle” as opposed to the CDC reporting of live births “per embryo transfer?”
A: An ART cycle “start” is defined as the initiation of ovarian stimulation with medication that may or may not include administration of exogenous gonadotropins, followed by oocyte retrieval and embryo transfer. Not every patient beginning a cycle will undergo an oocyte retrieval and not all patients who undergo oocyte retrieval have an embryo transfer. The live-birth rates (LBR) for each of these steps of progression in the ART process are available in the SART and CDC reports.
In 2016, SART recognized that practices were foregoing fresh embryo transfer after oocyte retrieval, opting to cryopreserve all embryos to either accommodate genetic testing of the embryos prior to transfer or to avoid embryo transfer to an unfavorable uterine environment. In response to changes in practice and in an effort to deemphasize live birth per transfer, thereby alleviating a potential motivator or pressure for practitioners to transfer multiple embryos, SART moved to a report that displays the cumulative live-birth rate per cycle start for oocyte retrieval. The cumulative live-birth rate per cycle start for oocyte retrieval is the chance of live birth from transfers of embryos derived from the oocyte retrieval and performed within 1 year of the oocyte retrieval.
This change in reporting further reduced the pressure to transfer multiple embryos and encouraged elective, single-embryo transfer. The outcome per transfer is no longer the report’s primary focus.
Q: The latest pregnancy outcomes statistics are from the year 2020 and are finalized by the CDC. Why does the SART website have this same year labeled “preliminary” outcomes?
A: Shortly after the 2016 SART report change, the CDC made similar changes to their report. The difference is that SART provides a “preliminary” report of outcomes within the year of the cycle start for oocyte retrieval. The cumulative outcome is not “finalized” until the following year as transfers may be performed as late as 12 months after the oocyte retrieval.
SART has opted to report both the “preliminary” or interim outcome and the “final” outcome a year later. The CDC has opted to limit their report to “final” outcomes. I’m happy to report that SART recently released the final report for 2021 cycles.
Q: Have national success rates in the United States continued to rise or have they plateaued?
A: It appears that success rates have plateaued; however, we find ourselves at another point where practice patterns and patients’ approach to using ART for family building have changed.
Recognizing the impact of maternal aging on reproductive potential, patients are opting to undergo multiple ART cycles to cryopreserve embryos for family building before they attempt to get pregnant. This family-building path reduces the value of measuring the LBR per cycle start as we may not know the outcome for many years. SART leaders are deliberating intently as to how to best represent this growing patient population in outcome reporting.
Q: Can you comment on the reduction of multiple gestations with the increasing use of single-embryo transfer?
A: The reduction in emphasis on live births per transfer, emphasis on singleton live-birth rates in both the SART and CDC reports, and American Society for Reproductive Medicine practice committee guidelines strongly supporting single embryo transfer have significantly reduced the rate of multiple gestations.
A decade ago, only a third of the transfers were single-embryo transfers and over 25% of live births resulted in a multiple birth. Today, the majority of embryo transfers are elective, single-embryo transfers, and the multiple birth rate has been reduced by nearly 80%. In 2020, 93% of live births from IVF were singletons.
Q: SART offers an online IVF calculator so both patients and physicians can plug in data for an approximate cumulative success rate for up to three IVF cycles. The calculator pools data from all U.S.-reporting IVF centers. Can you explain what an “IVF cycle” is and what patient information is required? Why do success rates increase over time?
A: Each “IVF cycle” is a cycle start for an oocyte retrieval and all transfers of embryos from that cycle within a year of the oocyte retrieval. If the first cycle and subsequent transfers do not lead to a live birth, patients still have a chance to achieve a live birth with a second or third cycle. The success rate increases over time as it reflects the chance of success for a population of patients, with some achieving a live birth after the first cycle and additional patients who achieve success following their third cycle.
Q: The SART IVF calculator can be used with no prior IVF cycles or following an unsuccessful cycle. Are there data to support an estimation of outcome following two or even more unsuccessful cycles?
A: The variables in the SART IVF calculator are based upon the cycle-specific data from patients seeking care at SART member clinics. The current predictor was built with data from cycles performed in 2015-2016. SART is adjusting the predictor and developing a calculator that will be routinely updated, accordingly.
Q: Only approximately 40% of states have some form of infertility coverage law in place; however the number of IVF cycles in the United States continues to increase on an annual basis. What do you think are the driving factors behind this?
A: Advocacy efforts to improve patients’ access to infertility care have included giving patients tools to encourage their employers to include infertility care in their health care benefits package. More recently, the “Great Resignation” has led to the “Great Recruitment” and employers are recognizing that the addition of infertility care to health care benefits is a powerful recruitment tool.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
The field of assisted reproductive technologies (ART) continues to evolve from its first successful birth in 1978 in England, and then in 1981 in the United States. Over the last 6 years, the total number of cycles in the U.S. has increased by 44% to nearly 370,000.
SART membership consists of more than 350 clinics throughout the United States, representing 80% of ART clinics. Over 95% of ART cycles in 2021 in the United States were performed in SART-member clinics.
SART is an invaluable resource for both patients and physicians. Their website includes a “Predict My Success” calculator that allows patients and physicians to enter individualized data to calculate the chance of having a baby over one or more complete cycles of IVF. To help us understand the pregnancy outcome data from ART – cycles per clinic along with national results – I posed the questions below to Amy Sparks, PhD, HCLD, director of the IVF and Andrology Laboratories and the Center for Advanced Reproductive Care at University of Iowa Hospitals and Clinics, Iowa City. Dr. Sparks is past president of SART and former chairperson of the SART Registry committee when the current Clinic Summary Report format was initially released.
Question: The Fertility Clinic Success Rate and Certification Act (FCSRCA) of 1992 mandated that all ART clinics report success rate data to the federal government, through the Centers for Disease Control and Prevention, in a standardized manner. As ART is the only field in medicine to be required to annually report their patient outcomes, that is, all initiated cycles and live births, why do you believe this law was enacted and is limited to reproductive medicine?
Answer: The FCSRCA of 1992 was enacted in response to the lack of open and reliable pregnancy success rate information for patients seeking infertility care using assisted reproductive technologies. Success rates of 25%-50% were being advertised by independent clinics when, nationally, fewer than 15% of ART procedures led to live births. The Federal Trade Commission said such claims were deceptive and filed charges against five clinics, saying they misrepresented their success in helping women become pregnant. The government won one case by court order and the other four cases were settled out of court.
This field of medicine was in the spotlight as the majority of patients lacked insurance coverage for their ART cycles, and there was a strong desire to protect consumers paying out of pocket for relatively low success. Recognizing that the FTC’s mission is to ensure truth in advertising and not regulate medical care, Congress passed the FCSRCA, mandating that all centers providing ART services report all initiated cycles and their outcomes. The CDC was appointed as the agency responsible for collecting cycle data and reporting outcomes. Centers not reporting their cycles are listed as nonreporting centers.
This act also established standards for accreditation of embryology laboratories including personnel and traditional clinical laboratory management requirements. These standards serve as the foundation for embryology laboratory accrediting agencies.
Q: Why have live-birth rates on SART appeared to be focused on “per IVF cycle” as opposed to the CDC reporting of live births “per embryo transfer?”
A: An ART cycle “start” is defined as the initiation of ovarian stimulation with medication that may or may not include administration of exogenous gonadotropins, followed by oocyte retrieval and embryo transfer. Not every patient beginning a cycle will undergo an oocyte retrieval and not all patients who undergo oocyte retrieval have an embryo transfer. The live-birth rates (LBR) for each of these steps of progression in the ART process are available in the SART and CDC reports.
In 2016, SART recognized that practices were foregoing fresh embryo transfer after oocyte retrieval, opting to cryopreserve all embryos to either accommodate genetic testing of the embryos prior to transfer or to avoid embryo transfer to an unfavorable uterine environment. In response to changes in practice and in an effort to deemphasize live birth per transfer, thereby alleviating a potential motivator or pressure for practitioners to transfer multiple embryos, SART moved to a report that displays the cumulative live-birth rate per cycle start for oocyte retrieval. The cumulative live-birth rate per cycle start for oocyte retrieval is the chance of live birth from transfers of embryos derived from the oocyte retrieval and performed within 1 year of the oocyte retrieval.
This change in reporting further reduced the pressure to transfer multiple embryos and encouraged elective, single-embryo transfer. The outcome per transfer is no longer the report’s primary focus.
Q: The latest pregnancy outcomes statistics are from the year 2020 and are finalized by the CDC. Why does the SART website have this same year labeled “preliminary” outcomes?
A: Shortly after the 2016 SART report change, the CDC made similar changes to their report. The difference is that SART provides a “preliminary” report of outcomes within the year of the cycle start for oocyte retrieval. The cumulative outcome is not “finalized” until the following year as transfers may be performed as late as 12 months after the oocyte retrieval.
SART has opted to report both the “preliminary” or interim outcome and the “final” outcome a year later. The CDC has opted to limit their report to “final” outcomes. I’m happy to report that SART recently released the final report for 2021 cycles.
Q: Have national success rates in the United States continued to rise or have they plateaued?
A: It appears that success rates have plateaued; however, we find ourselves at another point where practice patterns and patients’ approach to using ART for family building have changed.
Recognizing the impact of maternal aging on reproductive potential, patients are opting to undergo multiple ART cycles to cryopreserve embryos for family building before they attempt to get pregnant. This family-building path reduces the value of measuring the LBR per cycle start as we may not know the outcome for many years. SART leaders are deliberating intently as to how to best represent this growing patient population in outcome reporting.
Q: Can you comment on the reduction of multiple gestations with the increasing use of single-embryo transfer?
A: The reduction in emphasis on live births per transfer, emphasis on singleton live-birth rates in both the SART and CDC reports, and American Society for Reproductive Medicine practice committee guidelines strongly supporting single embryo transfer have significantly reduced the rate of multiple gestations.
A decade ago, only a third of the transfers were single-embryo transfers and over 25% of live births resulted in a multiple birth. Today, the majority of embryo transfers are elective, single-embryo transfers, and the multiple birth rate has been reduced by nearly 80%. In 2020, 93% of live births from IVF were singletons.
Q: SART offers an online IVF calculator so both patients and physicians can plug in data for an approximate cumulative success rate for up to three IVF cycles. The calculator pools data from all U.S.-reporting IVF centers. Can you explain what an “IVF cycle” is and what patient information is required? Why do success rates increase over time?
A: Each “IVF cycle” is a cycle start for an oocyte retrieval and all transfers of embryos from that cycle within a year of the oocyte retrieval. If the first cycle and subsequent transfers do not lead to a live birth, patients still have a chance to achieve a live birth with a second or third cycle. The success rate increases over time as it reflects the chance of success for a population of patients, with some achieving a live birth after the first cycle and additional patients who achieve success following their third cycle.
Q: The SART IVF calculator can be used with no prior IVF cycles or following an unsuccessful cycle. Are there data to support an estimation of outcome following two or even more unsuccessful cycles?
A: The variables in the SART IVF calculator are based upon the cycle-specific data from patients seeking care at SART member clinics. The current predictor was built with data from cycles performed in 2015-2016. SART is adjusting the predictor and developing a calculator that will be routinely updated, accordingly.
Q: Only approximately 40% of states have some form of infertility coverage law in place; however the number of IVF cycles in the United States continues to increase on an annual basis. What do you think are the driving factors behind this?
A: Advocacy efforts to improve patients’ access to infertility care have included giving patients tools to encourage their employers to include infertility care in their health care benefits package. More recently, the “Great Resignation” has led to the “Great Recruitment” and employers are recognizing that the addition of infertility care to health care benefits is a powerful recruitment tool.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.




