User login
A career in industry: Is it right for me?
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.
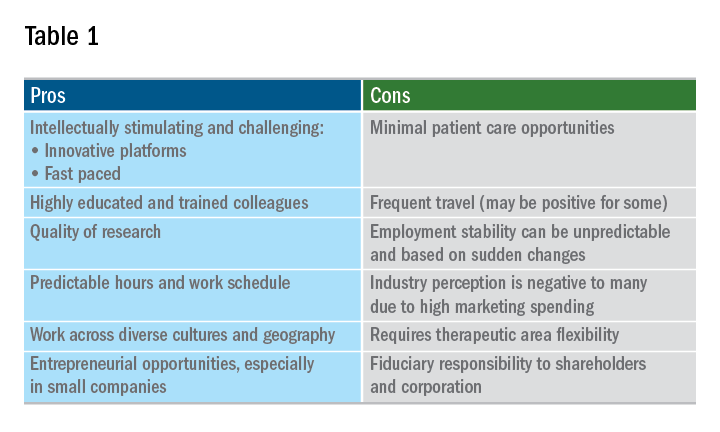
A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.

A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.
As gastroenterology fellows ponder their futures, one career path is often overlooked. Working in the pharmaceutical or biotechnology industry is a path that is not often at the top of career option lists. It is a rare occurrence for fellows to transition immediately into an industry position as opposed to a clinical or academic post. Initial clinical experience, caring for patients, and gaining experience with health economic challenges in today’s complex environment are considered invaluable assets for job applicants seeking industry positions. A minimum of 3-5 years of real-world clinical care experience will greatly enhance applicants’ marketability as “clinical experts” who can provide meaningful value to industry employers.
What exactly does “industry” mean? Traditionally it includes pharmaceutical and/or biotechnology (discovery, development, manufacture, sales, and marketing of small or large molecules), contract research organizations (CROs), and medical device companies. The variety in terms of size, scope, and reach of these companies is truly staggering and includes: entrepreneurial small startups (fewer than 20 employees, one location), midsize companies (more than 200 employees), and global multinational worldwide behemoths (“big pharma” with more than 50,000 employees and numerous facilities with diverse geographic locations). There are certain geographic regions of the United States where many companies’ headquarters are concentrated. At present (although this certainly can change over time), Cambridge, Mass.; New Jersey; Philadelphia; Raleigh-Durham, N.C.; and the San Francisco Bay Area are “hot areas.”
The breadth of “specialty” areas in industry for experienced clinicians is wide and includes: discovery, translational medicine, early- and late-stage clinical development, medical affairs, patient safety, epidemiology, and commercial development. For those interested in transitioning into industry, it is ideal to have a preferred area in mind so that training and education while in fellowship and clinical practice can be directed to that topic.
Discovery and translational medicine
These areas focus on preclinical development of small and large molecules from first concept until first-in-human studies and filing of an investigational new drug application (IND) with regulatory agencies. Translation of basic science concepts into potentially clinically useful “candidate” molecules requires a strong basic knowledge of science in addition to clinical experience. A passion for bridging novel concepts from “bench” to nonhuman studies is critical for success in this area.
Early-stage clinical development
Early-stage clinical development focuses on progressing discovery candidates to first-in-human studies (phase 1 in healthy volunteers) through phase 2 proof-of-concept studies (PoC). PoC studies typically involve first proof in a clinical trial in the target population that the drug under development may provide clinical benefit. These studies typically include 50-200 subjects with tight inclusion and exclusion controls. Intellectual rigor and scientific curiosity, as well as a passion for protecting patient safety, are essential for success as an early-stage drug developer.
Late-stage clinical development
Late-stage clinical development involves designing, conducting, and executing very large clinical studies (typically with hundreds to thousands of patients) that will provide the necessary rigorous pivotal clinical data supporting new drug marketing applications (NDAs). Relatively few drug candidates successfully make it to this stage of development and these studies are extremely expensive (sometimes hundreds of millions of dollars). This stage of development requires close collaboration with numerous company functions including regulatory, biostatistics, patient safety, clinical pharmacology, clinical operations, and manufacturing, as well as commercial colleagues. In addition to strong clinical expertise, this stage of drug development requires excellent communication, with leadership skills and attention to detail as well. Successfully shepherding a drug candidate through to Food and Drug Administration or other regulatory agency approval is an extremely satisfying experience, which can lead to meaningful differences for patients.
Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.

A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.

