User login
A Patient Presenting With Shortness of Breath, Fever, and Eosinophilia
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?
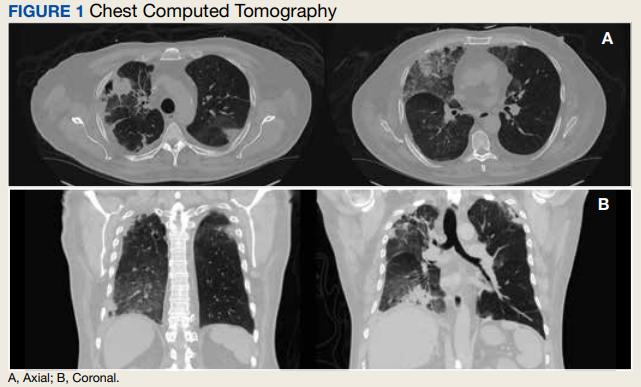
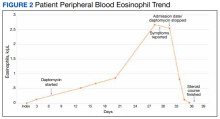
In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
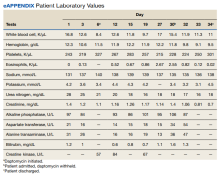
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8