User login
Lichenoid Drug Eruption Secondary to Apalutamide Treatment
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
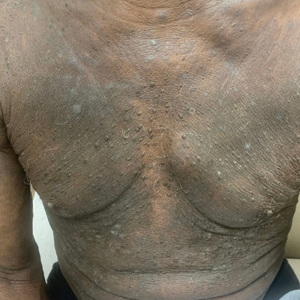
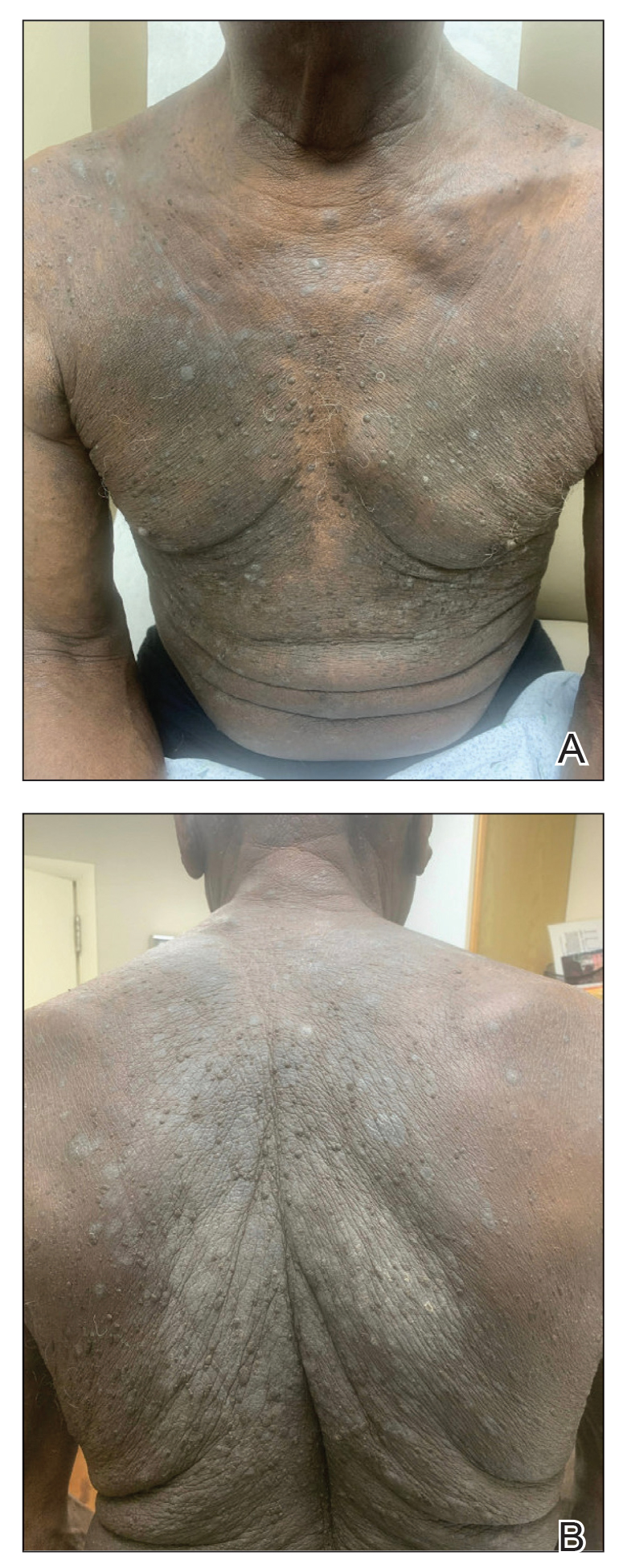
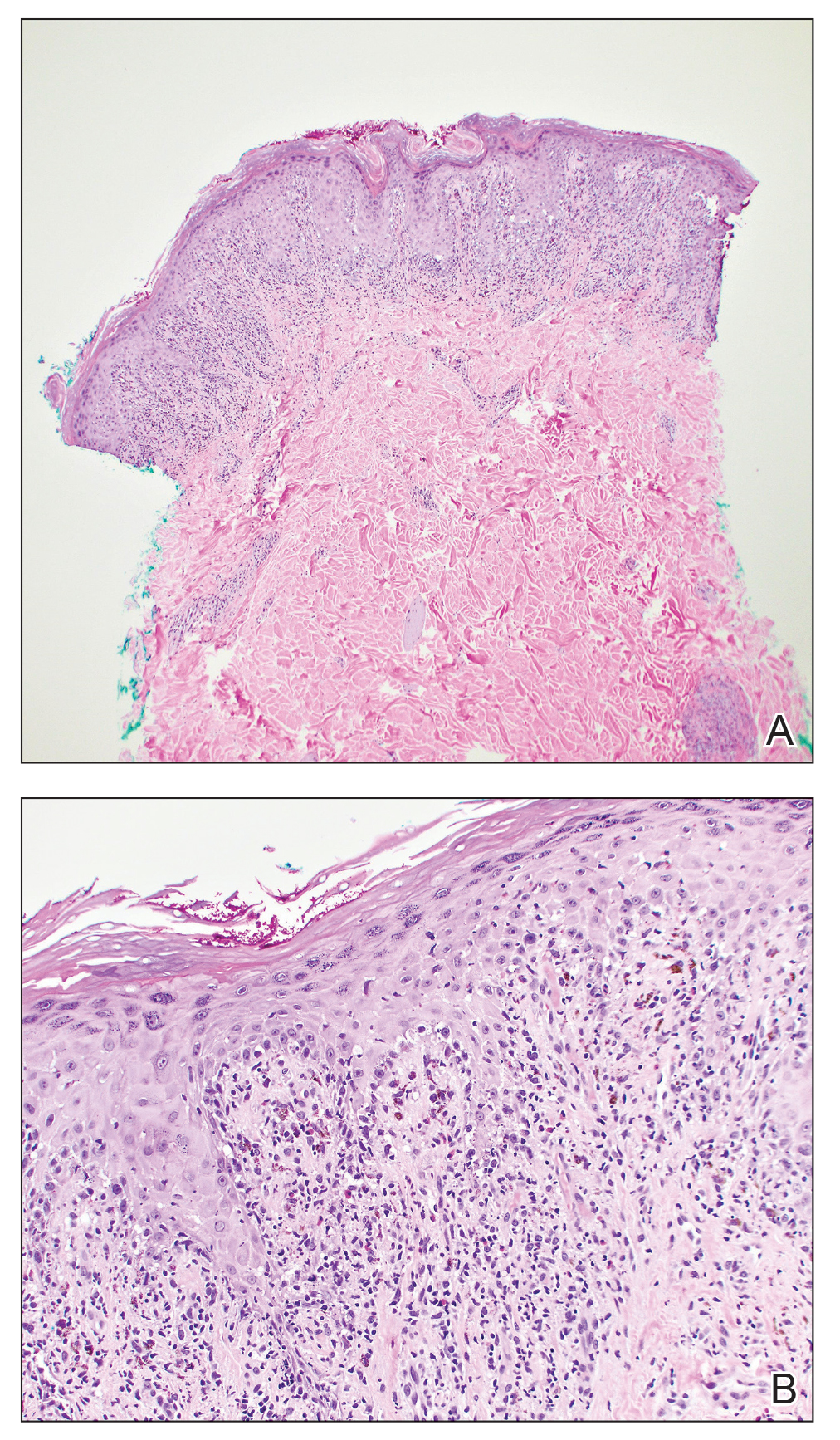
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.
Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.


Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.


Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
Practice Points
- Although it is rare, patients can develop lichenoid drug eruptions secondary to treatment with second-generation nonsteroidal androgen receptor antagonists such as apalutamide.
- If a patient develops a lichenoid drug eruption while taking a specific second-generation nonsteroidal androgen receptor antagonist, the entire class of medications should not be ruled out, as some patients can tolerate other drugs from that class.