User login
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
Supporting meaningful research that has a positive impact on the health and quality of life of veterans is a priority of the US Department of Veterans Affairs Office of Research and Development.1 For nearly a century, VA researchers have been conducting high quality studies. To continue this trajectory, it is imperative to attract, train, and retain exceptional investigators while nurturing their development throughout their careers.2
Mentorship is defined as guidance provided by an experienced and trusted party to another (usually junior) individual with the intent of helping the person succeed. It benefits the mentee, mentor, and their institutions.3 Mentorship is crucial for personal and professional development as well as productivity, which may help reduce clinician burnout.4-7 Conversely, a lack of mentorship could have negative effects on work satisfaction and stagnate career progression.8
Mentorship is vital for developing and advancing a VA investigator’s research agenda. Funding, grant writing, and research design were among the most discussed topics in a large comprehensive mentorship program for academic faculty.9 However, there are several known barriers to effective research mentorship; among them include a lack of resources, time constraints, and competing clinical priorities.10,11
Finding time for effective one-on-one research mentoring is difficult within the time constraints of clinical duties; a group mentorship model may help overcome this barrier. Group mentorship can aid in personal and professional development because no single mentor can effectively meet every mentoring need of an individual.12 Group mentorship also allows for the exchange of ideas among individuals with different backgrounds and the ability to utilize the strengths of each member of the group. For example, a member may have methodological expertise, while another may be skilled in grantsmanship. A team of mentors may be more beneficial for both the mentors (eg, establish a more manageable workload) and the mentee (eg, gains a broader perspective of expertise) when compared to having a single mentor.3
Peer mentorship within the group setting may also yield additional benefits. For example, having a supportive peer group may help reduce stress levels and burnout, while also improving overall well-being.3,13 Formal mentorship programs do not frequently discuss concerns such as work-life balance, so including peers as mentors may help fill this void.9 Peer mentorship has also been found to be beneficial in providing mentees with pooled resources and shared learning.12,13 This article describes the components, benefits, impacts, and challenges of a group research mentorship program for VA clinicians interested in conducting VA-relevant research.
Program Description
The VA Clinical Research Mentorship Program was initiated at the VA Ann Arbor Healthcare System (VAAAHS) in October 2015 by the Chief of Medicine to assist VA clinician investigators with developing and submitting VA clinical science and health services research grant applications. The program offers group and one-on-one consultation services through the expertise of 2 experienced investigators/faculty mentors who also serve as program directors, each of whom devote about 3 to 5 hours per month to activities associated with the mentorship program (eg, attending the meeting, reviewing materials sent by mentees, and one-on-one discussions with mentees).
The program also fostered peer-led mentorship. This encourages all attendees to provide feedback during group sessions and communication by mentees outside the group sessions. An experienced project manager serves as program coordinator and contributes about 4 hours per month for activities such as attending, scheduling, and sending reminders for each meeting, distributing handouts, reviewing materials, and answering mentee’s questions via email. A statistician and additional research staff (ie, an epidemiologist and research assistant) do not attend the recurring meetings, but are available for offline consultation as needed. The program runs on a 12-month cycle with regular meetings occurring twice monthly during the 9-month academic period. Resources to support the program, primarily program director(s) and project coordinator effort, are provided by the Chief of Medicine and through the VAAAHS affiliated VA Health Systems Research (formerly Health Services Research & Development) Center of Innovation.
Invitations for new mentees are sent annually. Mentees expressing interest in the program outside of its annual recruitment period are evaluated for inclusion on a rolling basis. Recruitment begins with the program coordinator sending email notifications to all VAAAHS Medicine Service faculty, section chiefs, and division chiefs at the VAAAHS academic affiliate. Recipients are encouraged to distribute the announcement to eligible applicants and refer them to the application materials for entry consideration into the program. The application consists of the applicant’s curriculum vitae and a 1-page summary that includes a description of their research area of interest, how it is relevant to the VA, in addition to an idea for a research study, its potential significance, and proposed methodology. Applicant materials are reviewed by the program coordinator and program directors. The applicants are evaluated using a simple scoring approach that focuses on the applicant’s research area and agenda, past research training, past research productivity, potential for obtaining VA funding, and whether they have sufficient research time.
Program eligibility initially required being a physician with ≥ 1/8 VA appointment from the Medicine Service. However, clinicians with clinical appointments from other VA services are also accepted for participation as needed. Applicants must have previous research experience and have a career goal to obtain external funding for conducting and publishing original research. Those who have previously served as a principal investigator on a funded VA grant proposal are not eligible as new applicants but can remain in the program as peer mentors. The number of annual applicants varies and ranges from 1 to 11; on average, about 90% of applicants receive invitations to join the program.
Sessions
The program holds recurring meetings twice monthly for 1 hour during the 9-month academic year. However, program directors are available year-round, and mentees are encouraged to communicate questions or concerns via email during nonacademic months. Prior to the COVID-19 pandemic, all meetings were held in-person. However, the group pivoted to virtual meetings and continues to utilize this format. The dedicated program coordinator is responsible for coordinating meetings and distributing meeting materials.
Each session is informal, flexible, and supportive. Attendance is not enforced, and mentees are allowed to join meetings as their schedules permit; however, program directors and program coordinator attend each meeting. In advance of each session, the program coordinator sends out a call for agenda items to all active members invited to discuss any research related items. Each mentee presents their ideas to lead the discussion for their portion of the meeting with no defined format required.
A variety of topics are covered including, but not limited to: (1) grant-specific concerns (eg, questions related to specific aim pages, grantsmanship, postsubmission comments from reviewers, or postaward logistics); (2) research procedures (eg, questions related to methodological practices or institutional review board concerns); (3) manuscript or presentation preparation; and (4) careerrelated issues. The program coordinator distributes handouts prior to meetings and mentees may record their presentations. These handouts may include, but are not limited to, specific aims pages, analytical plans, grant solicitations, and PowerPoint presentations. If a resource that can benefit the entire group is mentioned during the meeting, the program coordinator is responsible for distribution.
The program follows a group facilitated discussion format. Program directors facilitate each meeting, but input is encouraged from all attendees. This model allows for mentees to learn from the faculty mentors as well as peer mentees in a simultaneous and efficient fashion. Group discussions foster collective problem solving, peer support, and resource sharing that would not be possible through individualized mentorship. Participants have access to varied expertise during each session which reduces the need to seek specialized help elsewhere. Participants are also encouraged to contact the program directors or research staff for consultation as needed. Some one-on-one consultations have transitioned to a more sustained and ongoing mentorship relationship between a program director and mentee, but most are often brief email exchanges or a single meeting.
Participants
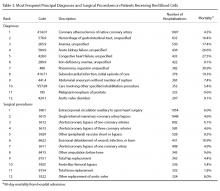
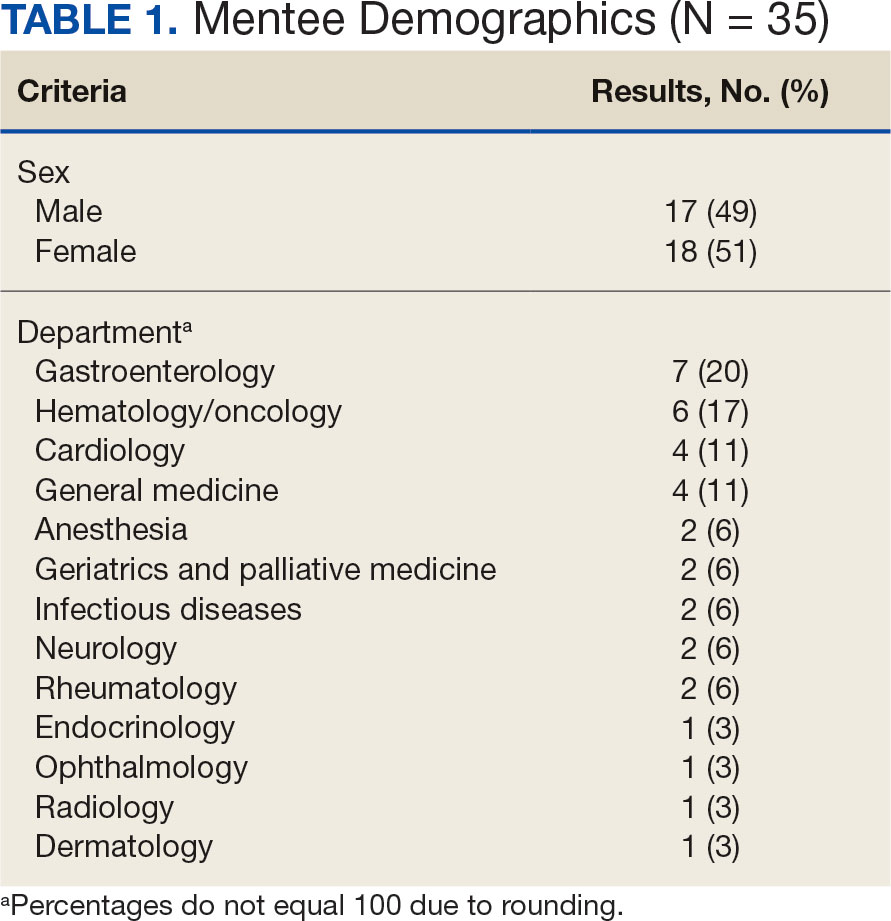
Since its inception in 2015, 35 clinicians have enrolled in the program. The mentees are equally distributed by sex and practice in a variety of disciplines including gastroenterology, hematology/oncology, cardiology, and general medicine (Table 1). Mentees have submitted 33 grant proposals addressing a variety of health care issues to a diverse group of federal and nonfederal funding agencies (Table 2). As of May 15, 2024, 19 (58%) of the submitted applications have been funded.

Many factors contribute to a successfully funded grant application, and several mentees report that participating in the mentorship program was helpful. For example, a mentee became the first lead investigator for a VA Cooperative Studies Program funded at VAAAHS. The VA Cooperative Studies Program, a division of the Office of Research and Development, plans and conducts large multicenter clinical trials and epidemiological studies within the VA via a vast network of clinician investigators, statisticians, and other key research experts.14
Several program mentees have also received VA Clinical Science Research and Development Career Development Awards. The VA Career Development program supports investigators during their early research careers with a goal of retaining talented researchers committed to improving the health and care of veterans.15
Survey Responses
Mentee productivity and updates are tracked through direct mentee input, as requested by the program coordinator. Since 2022, participants could complete an end-of-year survey based on an assessment tool used in a VAAAHS nonresearch mentorship program.16 The survey, distributed to mentees and program directors, requests feedback on logistics (eg, if the meeting was a good use of time and barriers to attendance); perceptions of effectiveness (eg, ability to discuss agenda items, helpfulness with setting and reaching research goals, and quality of mentors’ feedback); and the impact of the mentoring program on work satisfaction and clinician burnout. Respondents are also encouraged to leave open-ended qualitative feedback.
To date the survey has elicited 19 responses. Seventeen (89%) indicated that they agree or strongly agree the meetings were an effective use of their time and 11 (58%) indicated that they were able to discuss all or most of the items they wanted to during the meeting. Sixteen respondents (84%) agreed the program helped them set and achieve their research goals and 14 respondents (74%) agreed the feedback they received during the meeting was specific, actionable, and focused on how to improve their research agenda. Seventeen respondents (89%) agreed the program increased their work satisfaction, while 13 respondents (68%) felt the program reduced levels of clinician burnout.
As attendance was not mandatory, the survey asked participants how often they attended meetings during the past year. Responses were mixed: 4 (21%) respondents attended regularly (12 to 16 times per year) and 8 (42%) attended most sessions (8 to 11 times per year). Noted barriers to attendance included conflicts with patient care activities and conflicts with other high priority meetings.
Mentees also provided qualitive feedback regarding the program. They highlighted the supportive environment, valuable expertise of the mentors, and usefulness of obtaining tailored feedback from the group. “This group is an amazing resource to anyone developing a research career,” a mentee noted, adding that the program directors “fostered an incredibly supportive group where research ideas and methodology can be explored in a nonthreatening and creative environment.”
Conclusions
This mentorship program aims to help aspiring VA clinician investigators develop and submit competitive research grant applications. The addition of the program to the existing robust research environments at VAAAHS and its academic affiliate appears to have contributed to this success, with 58% of applications submitted by program mentees receiving funding.
In addition to funding success, we also found that most participants have a favorable impression of the program. Of the participants who responded to the program evaluation survey, nearly all indicated the program was an effective use of their time. The program also appeared to increase work satisfaction and reduce levels of clinician burnout. Barriers to attendance were also noted, with the most frequent being scheduling conflicts.
This program’s format includes facilitated group discussion as well as peer mentorship. This collaborative structure allows for an efficient and rich learning experience. Feedback from multiple perspectives encourages natural networking and relationship building. Incorporating the collective wisdom of the faculty mentors and peer mentees is beneficial; it not only empowers the mentees but also enriches the experience for the mentors. This program can serve as a model for other VA facilities—or non-VA academic medical centers—to enhance their research programs.
- US Department of Veterans Affairs, Office of Research and Development. Strategic priorities for VA research. Published March 10, 2021. Accessed September 17, 2024. https://www.research.va.gov/about/strategic_priorities.cfm
- US Department of Veterans Affairs, Office of Research and Development. About the Office of Research & Development. Published November 11, 2023. Accessed September 17, 2024. https://www.research.va.gov/about/default.cfm
- Chopra V, Vaughn V, Saint S. The Mentoring Guide: Helping Mentors and Mentees Succeed. Michigan Publishing Services; 2019.
- Gilster SD, Accorinti KL. Mentoring program yields staff satisfaction. Mentoring through the exchange of information across all organizational levels can help administrators retain valuable staff. Provider. 1999;25(10):99-100.
- Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112(4):336-341. doi:10.1016/s0002-9343(02)01032-x
- Sambunjak D, Straus SE, Marusi' A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. doi:10.1001/jama.296.9.1103
- Sambunjak D, Straus SE, Marusi' A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25(1):72-78. doi:10.1007/s11606-009-1165-8
- Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78(3):328-334. doi:10.1097/00001888-200303000-00020
- Feldman MD, Arean PA, Marshall SJ, Lovett M, O’Sullivan P. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:10.3402/meo.v15i0.5063. doi:10.3402/meo.v15i0.5063
- Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hosp Pediatr. 2016;6(4):219-225. doi:10.1542/hpeds.2015-0108
- Rustgi AK, Hecht GA. Mentorship in academic medicine. Gastroenterology. 2011;141(3):789-792. doi:10.1053/j.gastro.2011.07.024
- DeCastro R, Sambuco D, Ubel PA, Stewart A, Jagsi R. Mentor networks in academic medicine: moving beyond a dyadic conception of mentoring for junior faculty researchers. Acad Med. 2013;88(4):488-496. doi:10.1097/ACM.0b013e318285d302
- McDaugall M, Beattie RS. Peer mentoring at work: the nature and outcomes of non-hierarchical developmental relationships. Management Learning. 2016;28(4):423-437. doi:10.1177/1350507697284003
- US Department of Veterans Affairs, Office of Rsearch and Development. VA Cooperative Studies Program (CSP). Updated July 2019. Accessed September 17, 2024. https://www.vacsp.research.va.gov
- US Department of Veterans Affairs, Office of Research and Development. Career development program for biomedical laboratory and clinical science R&D services. Published April 17, 2023. Accessed September 17, 2024. https://www.research.va.gov/services/shared_docs/career_dev.cfm
- Houchens N, Kuhn L, Ratz D, Su G, Saint S. Committed to success: a structured mentoring program for clinically-oriented physicians. Mayo Clin Pro Innov Qual Outcomes. 2024;8(4):356-363. doi:10.1016/j.mayocpiqo.2024.05.002
Supporting meaningful research that has a positive impact on the health and quality of life of veterans is a priority of the US Department of Veterans Affairs Office of Research and Development.1 For nearly a century, VA researchers have been conducting high quality studies. To continue this trajectory, it is imperative to attract, train, and retain exceptional investigators while nurturing their development throughout their careers.2
Mentorship is defined as guidance provided by an experienced and trusted party to another (usually junior) individual with the intent of helping the person succeed. It benefits the mentee, mentor, and their institutions.3 Mentorship is crucial for personal and professional development as well as productivity, which may help reduce clinician burnout.4-7 Conversely, a lack of mentorship could have negative effects on work satisfaction and stagnate career progression.8
Mentorship is vital for developing and advancing a VA investigator’s research agenda. Funding, grant writing, and research design were among the most discussed topics in a large comprehensive mentorship program for academic faculty.9 However, there are several known barriers to effective research mentorship; among them include a lack of resources, time constraints, and competing clinical priorities.10,11
Finding time for effective one-on-one research mentoring is difficult within the time constraints of clinical duties; a group mentorship model may help overcome this barrier. Group mentorship can aid in personal and professional development because no single mentor can effectively meet every mentoring need of an individual.12 Group mentorship also allows for the exchange of ideas among individuals with different backgrounds and the ability to utilize the strengths of each member of the group. For example, a member may have methodological expertise, while another may be skilled in grantsmanship. A team of mentors may be more beneficial for both the mentors (eg, establish a more manageable workload) and the mentee (eg, gains a broader perspective of expertise) when compared to having a single mentor.3
Peer mentorship within the group setting may also yield additional benefits. For example, having a supportive peer group may help reduce stress levels and burnout, while also improving overall well-being.3,13 Formal mentorship programs do not frequently discuss concerns such as work-life balance, so including peers as mentors may help fill this void.9 Peer mentorship has also been found to be beneficial in providing mentees with pooled resources and shared learning.12,13 This article describes the components, benefits, impacts, and challenges of a group research mentorship program for VA clinicians interested in conducting VA-relevant research.
Program Description
The VA Clinical Research Mentorship Program was initiated at the VA Ann Arbor Healthcare System (VAAAHS) in October 2015 by the Chief of Medicine to assist VA clinician investigators with developing and submitting VA clinical science and health services research grant applications. The program offers group and one-on-one consultation services through the expertise of 2 experienced investigators/faculty mentors who also serve as program directors, each of whom devote about 3 to 5 hours per month to activities associated with the mentorship program (eg, attending the meeting, reviewing materials sent by mentees, and one-on-one discussions with mentees).
The program also fostered peer-led mentorship. This encourages all attendees to provide feedback during group sessions and communication by mentees outside the group sessions. An experienced project manager serves as program coordinator and contributes about 4 hours per month for activities such as attending, scheduling, and sending reminders for each meeting, distributing handouts, reviewing materials, and answering mentee’s questions via email. A statistician and additional research staff (ie, an epidemiologist and research assistant) do not attend the recurring meetings, but are available for offline consultation as needed. The program runs on a 12-month cycle with regular meetings occurring twice monthly during the 9-month academic period. Resources to support the program, primarily program director(s) and project coordinator effort, are provided by the Chief of Medicine and through the VAAAHS affiliated VA Health Systems Research (formerly Health Services Research & Development) Center of Innovation.
Invitations for new mentees are sent annually. Mentees expressing interest in the program outside of its annual recruitment period are evaluated for inclusion on a rolling basis. Recruitment begins with the program coordinator sending email notifications to all VAAAHS Medicine Service faculty, section chiefs, and division chiefs at the VAAAHS academic affiliate. Recipients are encouraged to distribute the announcement to eligible applicants and refer them to the application materials for entry consideration into the program. The application consists of the applicant’s curriculum vitae and a 1-page summary that includes a description of their research area of interest, how it is relevant to the VA, in addition to an idea for a research study, its potential significance, and proposed methodology. Applicant materials are reviewed by the program coordinator and program directors. The applicants are evaluated using a simple scoring approach that focuses on the applicant’s research area and agenda, past research training, past research productivity, potential for obtaining VA funding, and whether they have sufficient research time.
Program eligibility initially required being a physician with ≥ 1/8 VA appointment from the Medicine Service. However, clinicians with clinical appointments from other VA services are also accepted for participation as needed. Applicants must have previous research experience and have a career goal to obtain external funding for conducting and publishing original research. Those who have previously served as a principal investigator on a funded VA grant proposal are not eligible as new applicants but can remain in the program as peer mentors. The number of annual applicants varies and ranges from 1 to 11; on average, about 90% of applicants receive invitations to join the program.
Sessions
The program holds recurring meetings twice monthly for 1 hour during the 9-month academic year. However, program directors are available year-round, and mentees are encouraged to communicate questions or concerns via email during nonacademic months. Prior to the COVID-19 pandemic, all meetings were held in-person. However, the group pivoted to virtual meetings and continues to utilize this format. The dedicated program coordinator is responsible for coordinating meetings and distributing meeting materials.
Each session is informal, flexible, and supportive. Attendance is not enforced, and mentees are allowed to join meetings as their schedules permit; however, program directors and program coordinator attend each meeting. In advance of each session, the program coordinator sends out a call for agenda items to all active members invited to discuss any research related items. Each mentee presents their ideas to lead the discussion for their portion of the meeting with no defined format required.
A variety of topics are covered including, but not limited to: (1) grant-specific concerns (eg, questions related to specific aim pages, grantsmanship, postsubmission comments from reviewers, or postaward logistics); (2) research procedures (eg, questions related to methodological practices or institutional review board concerns); (3) manuscript or presentation preparation; and (4) careerrelated issues. The program coordinator distributes handouts prior to meetings and mentees may record their presentations. These handouts may include, but are not limited to, specific aims pages, analytical plans, grant solicitations, and PowerPoint presentations. If a resource that can benefit the entire group is mentioned during the meeting, the program coordinator is responsible for distribution.
The program follows a group facilitated discussion format. Program directors facilitate each meeting, but input is encouraged from all attendees. This model allows for mentees to learn from the faculty mentors as well as peer mentees in a simultaneous and efficient fashion. Group discussions foster collective problem solving, peer support, and resource sharing that would not be possible through individualized mentorship. Participants have access to varied expertise during each session which reduces the need to seek specialized help elsewhere. Participants are also encouraged to contact the program directors or research staff for consultation as needed. Some one-on-one consultations have transitioned to a more sustained and ongoing mentorship relationship between a program director and mentee, but most are often brief email exchanges or a single meeting.

Participants
Since its inception in 2015, 35 clinicians have enrolled in the program. The mentees are equally distributed by sex and practice in a variety of disciplines including gastroenterology, hematology/oncology, cardiology, and general medicine (Table 1). Mentees have submitted 33 grant proposals addressing a variety of health care issues to a diverse group of federal and nonfederal funding agencies (Table 2). As of May 15, 2024, 19 (58%) of the submitted applications have been funded.

Many factors contribute to a successfully funded grant application, and several mentees report that participating in the mentorship program was helpful. For example, a mentee became the first lead investigator for a VA Cooperative Studies Program funded at VAAAHS. The VA Cooperative Studies Program, a division of the Office of Research and Development, plans and conducts large multicenter clinical trials and epidemiological studies within the VA via a vast network of clinician investigators, statisticians, and other key research experts.14
Several program mentees have also received VA Clinical Science Research and Development Career Development Awards. The VA Career Development program supports investigators during their early research careers with a goal of retaining talented researchers committed to improving the health and care of veterans.15
Survey Responses
Mentee productivity and updates are tracked through direct mentee input, as requested by the program coordinator. Since 2022, participants could complete an end-of-year survey based on an assessment tool used in a VAAAHS nonresearch mentorship program.16 The survey, distributed to mentees and program directors, requests feedback on logistics (eg, if the meeting was a good use of time and barriers to attendance); perceptions of effectiveness (eg, ability to discuss agenda items, helpfulness with setting and reaching research goals, and quality of mentors’ feedback); and the impact of the mentoring program on work satisfaction and clinician burnout. Respondents are also encouraged to leave open-ended qualitative feedback.
To date the survey has elicited 19 responses. Seventeen (89%) indicated that they agree or strongly agree the meetings were an effective use of their time and 11 (58%) indicated that they were able to discuss all or most of the items they wanted to during the meeting. Sixteen respondents (84%) agreed the program helped them set and achieve their research goals and 14 respondents (74%) agreed the feedback they received during the meeting was specific, actionable, and focused on how to improve their research agenda. Seventeen respondents (89%) agreed the program increased their work satisfaction, while 13 respondents (68%) felt the program reduced levels of clinician burnout.
As attendance was not mandatory, the survey asked participants how often they attended meetings during the past year. Responses were mixed: 4 (21%) respondents attended regularly (12 to 16 times per year) and 8 (42%) attended most sessions (8 to 11 times per year). Noted barriers to attendance included conflicts with patient care activities and conflicts with other high priority meetings.
Mentees also provided qualitive feedback regarding the program. They highlighted the supportive environment, valuable expertise of the mentors, and usefulness of obtaining tailored feedback from the group. “This group is an amazing resource to anyone developing a research career,” a mentee noted, adding that the program directors “fostered an incredibly supportive group where research ideas and methodology can be explored in a nonthreatening and creative environment.”
Conclusions
This mentorship program aims to help aspiring VA clinician investigators develop and submit competitive research grant applications. The addition of the program to the existing robust research environments at VAAAHS and its academic affiliate appears to have contributed to this success, with 58% of applications submitted by program mentees receiving funding.
In addition to funding success, we also found that most participants have a favorable impression of the program. Of the participants who responded to the program evaluation survey, nearly all indicated the program was an effective use of their time. The program also appeared to increase work satisfaction and reduce levels of clinician burnout. Barriers to attendance were also noted, with the most frequent being scheduling conflicts.
This program’s format includes facilitated group discussion as well as peer mentorship. This collaborative structure allows for an efficient and rich learning experience. Feedback from multiple perspectives encourages natural networking and relationship building. Incorporating the collective wisdom of the faculty mentors and peer mentees is beneficial; it not only empowers the mentees but also enriches the experience for the mentors. This program can serve as a model for other VA facilities—or non-VA academic medical centers—to enhance their research programs.
Supporting meaningful research that has a positive impact on the health and quality of life of veterans is a priority of the US Department of Veterans Affairs Office of Research and Development.1 For nearly a century, VA researchers have been conducting high quality studies. To continue this trajectory, it is imperative to attract, train, and retain exceptional investigators while nurturing their development throughout their careers.2
Mentorship is defined as guidance provided by an experienced and trusted party to another (usually junior) individual with the intent of helping the person succeed. It benefits the mentee, mentor, and their institutions.3 Mentorship is crucial for personal and professional development as well as productivity, which may help reduce clinician burnout.4-7 Conversely, a lack of mentorship could have negative effects on work satisfaction and stagnate career progression.8
Mentorship is vital for developing and advancing a VA investigator’s research agenda. Funding, grant writing, and research design were among the most discussed topics in a large comprehensive mentorship program for academic faculty.9 However, there are several known barriers to effective research mentorship; among them include a lack of resources, time constraints, and competing clinical priorities.10,11
Finding time for effective one-on-one research mentoring is difficult within the time constraints of clinical duties; a group mentorship model may help overcome this barrier. Group mentorship can aid in personal and professional development because no single mentor can effectively meet every mentoring need of an individual.12 Group mentorship also allows for the exchange of ideas among individuals with different backgrounds and the ability to utilize the strengths of each member of the group. For example, a member may have methodological expertise, while another may be skilled in grantsmanship. A team of mentors may be more beneficial for both the mentors (eg, establish a more manageable workload) and the mentee (eg, gains a broader perspective of expertise) when compared to having a single mentor.3
Peer mentorship within the group setting may also yield additional benefits. For example, having a supportive peer group may help reduce stress levels and burnout, while also improving overall well-being.3,13 Formal mentorship programs do not frequently discuss concerns such as work-life balance, so including peers as mentors may help fill this void.9 Peer mentorship has also been found to be beneficial in providing mentees with pooled resources and shared learning.12,13 This article describes the components, benefits, impacts, and challenges of a group research mentorship program for VA clinicians interested in conducting VA-relevant research.
Program Description
The VA Clinical Research Mentorship Program was initiated at the VA Ann Arbor Healthcare System (VAAAHS) in October 2015 by the Chief of Medicine to assist VA clinician investigators with developing and submitting VA clinical science and health services research grant applications. The program offers group and one-on-one consultation services through the expertise of 2 experienced investigators/faculty mentors who also serve as program directors, each of whom devote about 3 to 5 hours per month to activities associated with the mentorship program (eg, attending the meeting, reviewing materials sent by mentees, and one-on-one discussions with mentees).
The program also fostered peer-led mentorship. This encourages all attendees to provide feedback during group sessions and communication by mentees outside the group sessions. An experienced project manager serves as program coordinator and contributes about 4 hours per month for activities such as attending, scheduling, and sending reminders for each meeting, distributing handouts, reviewing materials, and answering mentee’s questions via email. A statistician and additional research staff (ie, an epidemiologist and research assistant) do not attend the recurring meetings, but are available for offline consultation as needed. The program runs on a 12-month cycle with regular meetings occurring twice monthly during the 9-month academic period. Resources to support the program, primarily program director(s) and project coordinator effort, are provided by the Chief of Medicine and through the VAAAHS affiliated VA Health Systems Research (formerly Health Services Research & Development) Center of Innovation.
Invitations for new mentees are sent annually. Mentees expressing interest in the program outside of its annual recruitment period are evaluated for inclusion on a rolling basis. Recruitment begins with the program coordinator sending email notifications to all VAAAHS Medicine Service faculty, section chiefs, and division chiefs at the VAAAHS academic affiliate. Recipients are encouraged to distribute the announcement to eligible applicants and refer them to the application materials for entry consideration into the program. The application consists of the applicant’s curriculum vitae and a 1-page summary that includes a description of their research area of interest, how it is relevant to the VA, in addition to an idea for a research study, its potential significance, and proposed methodology. Applicant materials are reviewed by the program coordinator and program directors. The applicants are evaluated using a simple scoring approach that focuses on the applicant’s research area and agenda, past research training, past research productivity, potential for obtaining VA funding, and whether they have sufficient research time.
Program eligibility initially required being a physician with ≥ 1/8 VA appointment from the Medicine Service. However, clinicians with clinical appointments from other VA services are also accepted for participation as needed. Applicants must have previous research experience and have a career goal to obtain external funding for conducting and publishing original research. Those who have previously served as a principal investigator on a funded VA grant proposal are not eligible as new applicants but can remain in the program as peer mentors. The number of annual applicants varies and ranges from 1 to 11; on average, about 90% of applicants receive invitations to join the program.
Sessions
The program holds recurring meetings twice monthly for 1 hour during the 9-month academic year. However, program directors are available year-round, and mentees are encouraged to communicate questions or concerns via email during nonacademic months. Prior to the COVID-19 pandemic, all meetings were held in-person. However, the group pivoted to virtual meetings and continues to utilize this format. The dedicated program coordinator is responsible for coordinating meetings and distributing meeting materials.
Each session is informal, flexible, and supportive. Attendance is not enforced, and mentees are allowed to join meetings as their schedules permit; however, program directors and program coordinator attend each meeting. In advance of each session, the program coordinator sends out a call for agenda items to all active members invited to discuss any research related items. Each mentee presents their ideas to lead the discussion for their portion of the meeting with no defined format required.
A variety of topics are covered including, but not limited to: (1) grant-specific concerns (eg, questions related to specific aim pages, grantsmanship, postsubmission comments from reviewers, or postaward logistics); (2) research procedures (eg, questions related to methodological practices or institutional review board concerns); (3) manuscript or presentation preparation; and (4) careerrelated issues. The program coordinator distributes handouts prior to meetings and mentees may record their presentations. These handouts may include, but are not limited to, specific aims pages, analytical plans, grant solicitations, and PowerPoint presentations. If a resource that can benefit the entire group is mentioned during the meeting, the program coordinator is responsible for distribution.
The program follows a group facilitated discussion format. Program directors facilitate each meeting, but input is encouraged from all attendees. This model allows for mentees to learn from the faculty mentors as well as peer mentees in a simultaneous and efficient fashion. Group discussions foster collective problem solving, peer support, and resource sharing that would not be possible through individualized mentorship. Participants have access to varied expertise during each session which reduces the need to seek specialized help elsewhere. Participants are also encouraged to contact the program directors or research staff for consultation as needed. Some one-on-one consultations have transitioned to a more sustained and ongoing mentorship relationship between a program director and mentee, but most are often brief email exchanges or a single meeting.

Participants
Since its inception in 2015, 35 clinicians have enrolled in the program. The mentees are equally distributed by sex and practice in a variety of disciplines including gastroenterology, hematology/oncology, cardiology, and general medicine (Table 1). Mentees have submitted 33 grant proposals addressing a variety of health care issues to a diverse group of federal and nonfederal funding agencies (Table 2). As of May 15, 2024, 19 (58%) of the submitted applications have been funded.

Many factors contribute to a successfully funded grant application, and several mentees report that participating in the mentorship program was helpful. For example, a mentee became the first lead investigator for a VA Cooperative Studies Program funded at VAAAHS. The VA Cooperative Studies Program, a division of the Office of Research and Development, plans and conducts large multicenter clinical trials and epidemiological studies within the VA via a vast network of clinician investigators, statisticians, and other key research experts.14
Several program mentees have also received VA Clinical Science Research and Development Career Development Awards. The VA Career Development program supports investigators during their early research careers with a goal of retaining talented researchers committed to improving the health and care of veterans.15
Survey Responses
Mentee productivity and updates are tracked through direct mentee input, as requested by the program coordinator. Since 2022, participants could complete an end-of-year survey based on an assessment tool used in a VAAAHS nonresearch mentorship program.16 The survey, distributed to mentees and program directors, requests feedback on logistics (eg, if the meeting was a good use of time and barriers to attendance); perceptions of effectiveness (eg, ability to discuss agenda items, helpfulness with setting and reaching research goals, and quality of mentors’ feedback); and the impact of the mentoring program on work satisfaction and clinician burnout. Respondents are also encouraged to leave open-ended qualitative feedback.
To date the survey has elicited 19 responses. Seventeen (89%) indicated that they agree or strongly agree the meetings were an effective use of their time and 11 (58%) indicated that they were able to discuss all or most of the items they wanted to during the meeting. Sixteen respondents (84%) agreed the program helped them set and achieve their research goals and 14 respondents (74%) agreed the feedback they received during the meeting was specific, actionable, and focused on how to improve their research agenda. Seventeen respondents (89%) agreed the program increased their work satisfaction, while 13 respondents (68%) felt the program reduced levels of clinician burnout.
As attendance was not mandatory, the survey asked participants how often they attended meetings during the past year. Responses were mixed: 4 (21%) respondents attended regularly (12 to 16 times per year) and 8 (42%) attended most sessions (8 to 11 times per year). Noted barriers to attendance included conflicts with patient care activities and conflicts with other high priority meetings.
Mentees also provided qualitive feedback regarding the program. They highlighted the supportive environment, valuable expertise of the mentors, and usefulness of obtaining tailored feedback from the group. “This group is an amazing resource to anyone developing a research career,” a mentee noted, adding that the program directors “fostered an incredibly supportive group where research ideas and methodology can be explored in a nonthreatening and creative environment.”
Conclusions
This mentorship program aims to help aspiring VA clinician investigators develop and submit competitive research grant applications. The addition of the program to the existing robust research environments at VAAAHS and its academic affiliate appears to have contributed to this success, with 58% of applications submitted by program mentees receiving funding.
In addition to funding success, we also found that most participants have a favorable impression of the program. Of the participants who responded to the program evaluation survey, nearly all indicated the program was an effective use of their time. The program also appeared to increase work satisfaction and reduce levels of clinician burnout. Barriers to attendance were also noted, with the most frequent being scheduling conflicts.
This program’s format includes facilitated group discussion as well as peer mentorship. This collaborative structure allows for an efficient and rich learning experience. Feedback from multiple perspectives encourages natural networking and relationship building. Incorporating the collective wisdom of the faculty mentors and peer mentees is beneficial; it not only empowers the mentees but also enriches the experience for the mentors. This program can serve as a model for other VA facilities—or non-VA academic medical centers—to enhance their research programs.
- US Department of Veterans Affairs, Office of Research and Development. Strategic priorities for VA research. Published March 10, 2021. Accessed September 17, 2024. https://www.research.va.gov/about/strategic_priorities.cfm
- US Department of Veterans Affairs, Office of Research and Development. About the Office of Research & Development. Published November 11, 2023. Accessed September 17, 2024. https://www.research.va.gov/about/default.cfm
- Chopra V, Vaughn V, Saint S. The Mentoring Guide: Helping Mentors and Mentees Succeed. Michigan Publishing Services; 2019.
- Gilster SD, Accorinti KL. Mentoring program yields staff satisfaction. Mentoring through the exchange of information across all organizational levels can help administrators retain valuable staff. Provider. 1999;25(10):99-100.
- Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112(4):336-341. doi:10.1016/s0002-9343(02)01032-x
- Sambunjak D, Straus SE, Marusi' A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. doi:10.1001/jama.296.9.1103
- Sambunjak D, Straus SE, Marusi' A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25(1):72-78. doi:10.1007/s11606-009-1165-8
- Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78(3):328-334. doi:10.1097/00001888-200303000-00020
- Feldman MD, Arean PA, Marshall SJ, Lovett M, O’Sullivan P. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:10.3402/meo.v15i0.5063. doi:10.3402/meo.v15i0.5063
- Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hosp Pediatr. 2016;6(4):219-225. doi:10.1542/hpeds.2015-0108
- Rustgi AK, Hecht GA. Mentorship in academic medicine. Gastroenterology. 2011;141(3):789-792. doi:10.1053/j.gastro.2011.07.024
- DeCastro R, Sambuco D, Ubel PA, Stewart A, Jagsi R. Mentor networks in academic medicine: moving beyond a dyadic conception of mentoring for junior faculty researchers. Acad Med. 2013;88(4):488-496. doi:10.1097/ACM.0b013e318285d302
- McDaugall M, Beattie RS. Peer mentoring at work: the nature and outcomes of non-hierarchical developmental relationships. Management Learning. 2016;28(4):423-437. doi:10.1177/1350507697284003
- US Department of Veterans Affairs, Office of Rsearch and Development. VA Cooperative Studies Program (CSP). Updated July 2019. Accessed September 17, 2024. https://www.vacsp.research.va.gov
- US Department of Veterans Affairs, Office of Research and Development. Career development program for biomedical laboratory and clinical science R&D services. Published April 17, 2023. Accessed September 17, 2024. https://www.research.va.gov/services/shared_docs/career_dev.cfm
- Houchens N, Kuhn L, Ratz D, Su G, Saint S. Committed to success: a structured mentoring program for clinically-oriented physicians. Mayo Clin Pro Innov Qual Outcomes. 2024;8(4):356-363. doi:10.1016/j.mayocpiqo.2024.05.002
- US Department of Veterans Affairs, Office of Research and Development. Strategic priorities for VA research. Published March 10, 2021. Accessed September 17, 2024. https://www.research.va.gov/about/strategic_priorities.cfm
- US Department of Veterans Affairs, Office of Research and Development. About the Office of Research & Development. Published November 11, 2023. Accessed September 17, 2024. https://www.research.va.gov/about/default.cfm
- Chopra V, Vaughn V, Saint S. The Mentoring Guide: Helping Mentors and Mentees Succeed. Michigan Publishing Services; 2019.
- Gilster SD, Accorinti KL. Mentoring program yields staff satisfaction. Mentoring through the exchange of information across all organizational levels can help administrators retain valuable staff. Provider. 1999;25(10):99-100.
- Ramanan RA, Phillips RS, Davis RB, Silen W, Reede JY. Mentoring in medicine: keys to satisfaction. Am J Med. 2002;112(4):336-341. doi:10.1016/s0002-9343(02)01032-x
- Sambunjak D, Straus SE, Marusi' A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103-1115. doi:10.1001/jama.296.9.1103
- Sambunjak D, Straus SE, Marusi' A. A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine. J Gen Intern Med. 2010;25(1):72-78. doi:10.1007/s11606-009-1165-8
- Jackson VA, Palepu A, Szalacha L, Caswell C, Carr PL, Inui T. “Having the right chemistry”: a qualitative study of mentoring in academic medicine. Acad Med. 2003;78(3):328-334. doi:10.1097/00001888-200303000-00020
- Feldman MD, Arean PA, Marshall SJ, Lovett M, O’Sullivan P. Does mentoring matter: results from a survey of faculty mentees at a large health sciences university. Med Educ Online. 2010;15:10.3402/meo.v15i0.5063. doi:10.3402/meo.v15i0.5063
- Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hosp Pediatr. 2016;6(4):219-225. doi:10.1542/hpeds.2015-0108
- Rustgi AK, Hecht GA. Mentorship in academic medicine. Gastroenterology. 2011;141(3):789-792. doi:10.1053/j.gastro.2011.07.024
- DeCastro R, Sambuco D, Ubel PA, Stewart A, Jagsi R. Mentor networks in academic medicine: moving beyond a dyadic conception of mentoring for junior faculty researchers. Acad Med. 2013;88(4):488-496. doi:10.1097/ACM.0b013e318285d302
- McDaugall M, Beattie RS. Peer mentoring at work: the nature and outcomes of non-hierarchical developmental relationships. Management Learning. 2016;28(4):423-437. doi:10.1177/1350507697284003
- US Department of Veterans Affairs, Office of Rsearch and Development. VA Cooperative Studies Program (CSP). Updated July 2019. Accessed September 17, 2024. https://www.vacsp.research.va.gov
- US Department of Veterans Affairs, Office of Research and Development. Career development program for biomedical laboratory and clinical science R&D services. Published April 17, 2023. Accessed September 17, 2024. https://www.research.va.gov/services/shared_docs/career_dev.cfm
- Houchens N, Kuhn L, Ratz D, Su G, Saint S. Committed to success: a structured mentoring program for clinically-oriented physicians. Mayo Clin Pro Innov Qual Outcomes. 2024;8(4):356-363. doi:10.1016/j.mayocpiqo.2024.05.002
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
A Group Approach to Clinical Research Mentorship at a Veterans Affairs Medical Center
A Longitudinal Study of Transfusion Utilization in Hospitalized Veterans
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
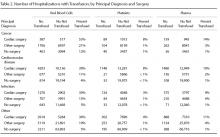
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
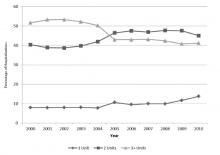
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, maryroge@umich.edu.
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, maryroge@umich.edu.
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, maryroge@umich.edu.
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Estimating Hospital Costs of CAUTI
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).
Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).
After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).

Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).

After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Healthcare‐associated infections affect 5% to 10% of all hospitalized patients each year in the United States, account for nearly $45 billion in direct hospital costs, and cause nearly 100,000 deaths annually.[1, 2] Because catheter‐associated urinary tract infection (CAUTI) is one of the most common healthcare‐associated infections in the United States and is reasonably preventable, the Centers for Medicare and Medicaid Services stopped reimbursing hospitals in 2008 for the additional costs of caring for patients who develop CAUTI during hospitalization.[3] Still, strategies for reducing inappropriate urinary catheterization are infrequently implemented in practice; this is despite a consensus that such strategies are effective.[4]
To help motivate hospitals to reduce inappropriate urinary catheter use, we present a tool for estimating costs of CAUTI for individual hospitals. Although other tools for estimating the excess costs of healthcare‐associated infections are available (eg, the APIC Cost of Healthcare‐Associated Infections Model available at
METHODS
General Setup
We consider 4 possible events after urinary catheter placement: bacteriuria, symptomatic urinary tract infection (SUTI), bloodstream infection (BSI), and catheter removal. Conservatively, assuming that bacteriuria must precede SUTI and BSI, there are 5 possible trajectories for any hospitalized patient (Figure 1): (1) no infection, (2) only bacteriuria, (3) bacteriuria and SUTI, (4) bacteriuria and BSI, or (5) bacteriuria, SUTI, and BSI. The cost of CAUTI for a particular hospital is therefore the per‐patient cost of each trajectory multiplied by the number of patients experiencing each trajectory. Our approach for estimating hospital costs is based on factorizing the number of patients experiencing each trajectory into a product of terms for which estimates are available from the literature (see the Supporting Information, Appendix, in the online version of this article for all technical details).

Deriving Estimates of Current Costs
We start with 2 minor simplifying assumptions. First, because the presence of asymptomatic bacteriuria is typically unknown, we only consider costs to the hospital due to SUTI and BSI[6]; in other words, we assume hospitals do not incur costs for patients with trajectories 1 or 2. This assumption should only bias cost estimates conservatively. Second, we assume that patients with both SUTI and BSI (trajectory 5) incur costs equal to those for patients with only BSI (trajectory 4). Further, because the joint risk of SUTI and BSI is unknown, we conservatively assume SUTI must precede BSI. Under these assumptions we can write: (total CAUTI costs)=(per‐patient SUTI cost) (number with SUTI but no BSI)+(perpatient BSI cost) (number with BSI).
We use per‐patient hospital costs of SUTI and BSI of $911 and $3824, respectively, which were determined using a microcosting approach[6] and adjusted for inflation using the general Consumer Price Index.[7] Although an alternative strategy for estimating costs would be to enter the hospital‐specific, per‐patient costs of SUTI and BSI into the above equation, these quantities are often difficult to measure or otherwise unavailable. Thus, it remains to factorize the number of hospitalized patients who develop SUTI and BSI into component terms for which we have accessible estimates. First note that the number with only SUTI (or any BSI) equals the total number of patients hospitalized times the proportion of hospitalizations with only SUTI (or any BSI). The former quantity depends on the particular hospital and so is specified as an input by the user. The latter quantity can be factorized further under our aforementioned conservative assumption that bacteriuria must precede SUTI and BSI.
Specifically, for SUTI:
(Proportion SUTI but no BSI)={(SUTI risk among those catheterized with bacteriuria)(BSI risk among those catheterized with bacteriuria)} (bacteriuria risk among those catheterized) (proportion catheterized).
And for BSI:
(Proportion BSI)=(BSI risk among those catheterized with bacteriuria) (bacteriuria risk among those catheterized) (proportion catheterized).
The risks of SUTI and BSI among those catheterized with bacteriuria, along with the risk of bacteriuria among those catheterized, have been estimated previously via a meta‐analytic approach.[6] The proportion catheterized depends on the particular hospital, such as the total number of patients hospitalized, and so is also specified as a user input. Therefore, we have now factorized the total hospital costs due to CAUTI as a product of either user‐specified terms or terms for which we have estimates from the literature. All estimates and corresponding standard errors derived from the literature are listed together in Table 1 (see the Supporting Information, Appendix Section 1, for further details in the online version of this article).
| Quantity | Estimate (SE) |
|---|---|
| |
| Overall risk of bacteriuria among those catheterized | 26.0% (1.53%) |
| Per‐day risk of bacteriuria among those catheterized | 5.0% |
| days | 6.68 |
| Risk of SUTI among those catheterized with bacteriuria | 24.0% (4.08%) |
| Risk of BSI among those catheterized with bacteriuria | 3.6% (0.10%) |
| Per‐patient SUTI cost | $911 ($911) |
| Per‐patient BSI cost | $3824 ($3824) |
Deriving Projected Costs After Intervention
The approach described above permits estimation of current costs for managing patients with CAUTI for a particular hospital. To estimate projected costs after participation in an intervention to reduce infection risk, we characterize interventions of interest and introduce additional factorization. Specifically, following previous work,[8] we consider interventions that reduce (1) placement (ie, the proportion catheterized) and (2) duration (ie, the mean duration of catheterization). Incorporating reductions in placement is straightforward, because our above expression for costs already contains a term for the proportion catheterized. However, incorporating reductions in duration requires further factorization. Under the assumptions of constant per‐day risks of bacteriuria and of catheter removal, we can write the postintervention risk of bacteriuria among the catheterized as a function of (1) the percent decrease in mean duration of catheterization due to intervention, and (2) the preintervention risk of bacteriuria among the catheterized (see the Supporting Information, Appendix Section 2, for further details in the online version of this article). This means we can fully characterize postintervention costs as a function of user‐specified quantities, quantities specific to the intervention (which are varied across plausible ranges), and quantities for which we have estimates from the literature. Therefore, we can estimate savings by subtracting postintervention costs from current costs.
Because our estimators of current costs, projected costs, and savings are all formulated as functions of other estimators, we use the standard delta method approach[9] to derive appropriate variance estimates (see the Supporting Information, Appendix Section 3, for further details in the online version of this article).
Online Implementation
Customized results (based on annual admissions, urinary catheter prevalence, and other inputs) can be computed using online implementation of our proposed method at
RESULTS
Figure 2 shows the projected savings in hospital costs due to CAUTI across a range of interventions defined by percent decreases in placement and duration, for a hypothetical hospital with 3000 total patients, 15% with urinary catheters preintervention, and with all other default values listed in Table 1. The current costs for this hospital (ie, the costs when the percent reduction in placement and duration is zero) are estimated to be $37,868 (95% confidence interval [CI]: $9159‐$156,564). After an intervention resulting in 40% reductions in both urinary catheter placement and duration, this hospital would be expected to save $22,653 (95% CI: $5479‐$93,656). A less effective intervention yielding a 10% reduction in both urinary catheter placement and duration would result in more modest savings of $6376 (95% CI: $1542‐$26,360).

After an intervention resulting in 29% and 37% reductions in placement and duration, respectively, reflecting reductions seen in practice,[10, 11] our hypothetical hospital is estimated to save $19,126 (95% CI: $4626‐$79,074). This reflects an estimated savings of nearly 50%.
DISCUSSION
We have presented a tool for estimating customized hospital costs of CAUTI, both before and after a hypothetical intervention to reduce risk of infection. Our approach relies on mostly conservative assumptions, incorporates published risk estimates (properly accounting for their associated variability), and has easy‐to‐use online implementation. We believe this can play an important role in motivating hospitals to reduce inappropriate urinary catheter use.
The methodology employed here does have a few limitations. First and foremost, our results depend on the reliability of the input values, which are either provided by users or are based on estimates from the literature (see Table 1 for a complete list of suggested defaults). New information could potentially be incorporated if and when available. For example, substitution of more precise risk estimates could help reduce confidence interval length. Second, our approach essentially averages over hospital quality; we do not directly take into account quality of care or variation in underlying infection risk across hospitals in computing estimated costs. Finally, we only compute direct costs due to infection; other costs (eg, intervention costs) would typically also need to be considered for decision making.
Despite these limitations, we believe that our tool can help infection control professionals demonstrate the values of CAUTI prevention efforts to key administrators, particularly at a time where it has become increasingly necessary to develop a business case to initiate new interventions or justify the continued support for ongoing programs.[12] Additionally, we believe the proposed approach can be an important supplement to initiatives like the Society of Hospital Medicine's Choosing Wisely campaign, which aims to help reduce inappropriate urinary catheter use. Reducing catheter utilization has the potential to reduce costs associated with caring for CAUTI patients, but more importantly would help reduce CAUTI incidence as well as catheter‐related, noninfectious complications.[13, 14] We hope that our tool will greatly assist hospitals in promoting their CAUTI prevention efforts and improve the overall safety of hospitalized patients.
Disclosures
This project was supported by the Ann Arbor VA Medical Center/University of Michigan Patient Safety Enhancement Program (PSEP) and a subcontract to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Mr. Kennedy has no conflicts of interest to report. Drs. Saint and Greene are subcontracted to implement multistate CAUTI prevention with the Agency for Healthcare Research and Quality/Health Educational and Research Trust. Dr. Saint has received numerous honoraria and speaking fees for lectures on healthcare‐associated infection prevention, implementation science, and patient safety from hospitals, academic medical centers, professional societies, and nonprofit foundations. None of these activities are related to speaker's bureaus. Dr. Saint is also on the medical advisory board of Doximity, a new social networking site for physicians. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.
- The direct medical costs of healthcare‐associated infections in US hospitals and the benefits of prevention. US Centers for Disease Control and Prevention Web site. Published 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed March 24, 2013.
- , , , , . Catheter‐associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150(12):877–884.
- , . Improving use of the other catheter. Arch Intern Med. 2012;172(3):260–261.
- Choosing Wisely: five things patients and physicians should question. Society of Hospital Medicine. Published 2012. Available at: http://www.hospitalmedicine.org/AM/pdf/SHM‐Adult_5things_List_Web.pdf. Accessed March 24, 2013.
- . Clinical and economic consequences of nosocomial catheter‐related bacteriuria. Am J Infect Control. 2000;28(1):68–75.
- CPI Inflation Calculator. United States Department of Labor, Bureau of Labor Statistics Web site. Published 2013. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed March 24, 2013.
- , , , et al. Introducing a population‐based outcome measure to evaluate the effect of interventions to reduce catheter‐associated urinary tract infection. Am J Infect Control. 2012;40(4):359–364.
- . Asymptotic Statistics. Cambridge, UK: Cambridge University Press; 2000.
- , , , et al. Effect of establishing guidelines on appropriate urinary catheter placement. Acad Emerg Med. 2010;17:337–340.
- , , , . Systematic review and meta‐analysis: reminder systems to reduce catheter‐associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560.
- , , , et al. Raising standards while watching the bottom line: making a business case for infection control. Infect Control Hosp Epidemiol. 2007;28:1121–1133.
- , , , , . Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457.
- , , . Indwelling urinary catheters: a one‐point restraint? Ann Intern Med. 2002;137(2):125–127.