User login
A talk about, then a plan for, antidepressants in pregnancy
CAS: Depressive disorder, anticipating a pregnancy
Your patient Megan—well-educated, 29 years old, G0P0—has come to you to discuss her antidepressant (paroxetine [Paxil]) because she is planning her first pregnancy.
Megan has a history of recurrent major depressive disorder (MDD), which is in remission (see “What is MDD?”).
How will you begin the conversation with this patient about keeping MDD in remission during her pregnancy and ensuring the safety of her fetus?
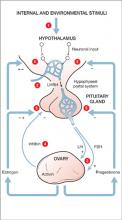
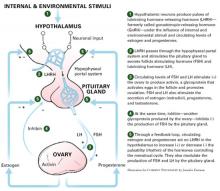
- Major depressive disorder (MDD) is defined by criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)
- The disorder varies in severity, 1) across an affected person’s lifetime and 2) within a depressive episode
- A current or prior episode of depression that includes 1) a significant impact on an individual’s functioning, 2) active suicidality, or 3) hospitalization signals severe MDD
- In women, average age at first episode of depression is 24 years
There is a 20% to 25% lifetime prevalence of depression in women; the disorder peaks during childbearing years, however.1 As of 2003, 13% of pregnant women had taken an antidepressant at some time during their pregnancy, a percentage that has doubled since it was assessed in 1999.2
You are faced with several quandaries in deciding whether to recommend that your patient continue, or discontinue, antidepressant therapy during pregnancy:
- As many as 68% of women who terminate antidepressant treatment before or during pregnancy relapse.
- Even 26% of women who continue antidepressant during pregnancy relapse—requiring a dosage adjustment or change in treatment.3
- Yet the possibly elevated cortisol levels of severe, untreated depression may harm the placenta and fetus.4,5
So, what do you need to know to assess the risks and benefits of “Megan” stopping, or continuing, paroxetine during her anticipated pregnancy? And what are the risks to Megan’s fetus of treating, or not treating, her depression with a serotonin reuptake inhibitor (SRI*)?
“Selective” has been dropped
from “SSRI” to yield simply “SRI.”
Gauging the risks of depression in pregnancy
In any given patient, her history and family history of depression are key to determining the likelihood that she will suffer ongoing or recurrent depression.
CASE continued Repeated treated episodes plus a family history
In obtaining Megan’s history, you learn that she has had three prior episodes of depression, all of which were successfully treated with paroxetine. Megan has been stable on paroxetine for 3 years.
Notably, the second episode of depression was initially treated with a 16-week trial of psychotherapy alone; when depressive symptoms did not remit, paroxetine was added. That episode was considered severe because it included pervasive thoughts of suicide.
You also learn that Megan’s mother suffered from postpartum depression and that her father and paternal grandmother were treated for depression.
Known risk factors for depression during pregnancy include: maternal anxiety; prior diagnosis of depression during pregnancy; history of postpartum anxiety or depression; prior diagnosis of either anxiety or depressive disorder; significant life stress (e.g., divorce, death of a loved one); degree of social support—particularly, intimate social support; “intendedness” of pregnancy; domestic violence; and insurance status.6
You review with Megan her risk factors for depression during pregnancy, namely: three prior episodes of MDD and a strong family history of mood disorder. Her MDD is considered “severe” because she has a history of suicidality. You tell Megan that, given these factors, she is at high risk of a recurrence of her depressive illness during pregnancy.
Megan asks: “Would getting depressed during pregnancy hurt the baby?”
Depression during pregnancy affects both infant and maternal well-being, although studies are in conflict about the extent of that morbidity. Multiple areas of potential risk to mother and infant have been studied, including the effect of depression on:
- maternal well-being
- growth of the infant
- spontaneous abortion
- preterm delivery
- neonatal physiologic and neurobehavioral measures
- long-term considerations for the developing infant and child.
Within these categories of risk, a diagnosis of depression during pregnancy has been associated (in some but not all studies) with a higher risk, or rate, of:
- postpartum depression
- preterm birth
- lower maternal weight gain
- maternal tobacco, alcohol, and other substance use
- lower infant gestational age at birth
- small-for-gestational age infant birth.7-10
In terms of long-term impact on offspring, studies differ in their estimation of risk; however, children exposed to untreated, maternal depression at 18 weeks’ and 32 weeks’ gestation did show a greater degree of developmental delay at 18 months than children who were born to a mother who was not depressed during pregnancy.11
You discuss these risks with Megan. She asks: “What treatment do you recommend for me?” You turn to the 2009 guidelines published jointly by the American Psychiatric Association (APA) and ACOG.
These guidelines recommend that you consider 1) the severity of her current depression, 2) her history of depression severity, and 3) her preference for treatment.12 For mild depression during pregnancy, when there is no history of severe depression, or for a history of depression that responded well to psychotherapy in the past, a trial of psychotherapy without medications is recommended.
But Megan’s history of depression falls into the “severe” category, and a prior episode of depression did not respond well to psychotherapy. Your recommendation to her, therefore, is that she should continue taking an antidepressant—unless she feels strongly that she should discontinue it.

Megan considers what you’ve discussed about her high risk of developing recurrent depression during pregnancy. She decides that she wants to continue taking her antidepressant during pregnancy, but she has concerns—based on what she has been reading on the Internet.
Megan hands you a detailed printout downloaded from a Web site unfamiliar to you and asks about risks to the baby of such medications as paroxetine.
What should you tell Megan about SRIs in pregnancy—paroxetine, specifically?
You preface your remarks to her by noting that the data physicians work with are imperfect—because randomized, controlled clinical trials pose an ethical dilemma as a method of study in pregnant women. You then discuss with her current scientific understanding of potential risks to her fetus.
The difference in the rates of structural malformation among SRI-exposed and SRI-unexposed groups has been studied; most studies have found no increased rate of major or specific cardiac malformations.12 However, first-trimester paroxetine appeared, in some studies, to be associated with an increased rate of cardiac malformations. That led to a category-“D” pregnancy classification in 2005 and an FDA “Public Health Advisory.”
Other large cohort studies have not uncovered such an association. It has been hypothesized that the methodology of data collection may have influenced this finding.13
Other malformations have been implicated in some studies but not others, and have included associations between specific SRIs and cardiac ventricular outflow defects, craniosynostosis, and omphalocele. The absolute risk of these defects remains extremely low, however, and close to the background rate seen in the general population.14
Megan asks: “With that risk-category ‘D’ for paroxetine, do you recommend I continue taking it or should I switch to another medication while I’m pregnant?”
You review again with Megan that, although some studies have linked first-trimester paroxetine to an increased risk of cardiac malformation, that finding has not been replicated in several large cohort studies. You explain that, if she had a history of recurrent depression that had failed to respond to many antidepressants and only paroxetine worked, an attempt at switching the SRI would not be recommended because of the potential for relapse.
Megan tells you that she would feel safer not taking a category-“D” drug. You agree and propose a judicious approach: Because she has come to see you before she became pregnant, with enough time to complete a slow crossover to an alternative SRI, and because she has not had any earlier trials of other SRIs, a slow taper of paroxetine, coupled with a crossover to an alternative SRI, is a reasonable option—with the caution that substitution always carries a risk of relapse.
Problems in newborns
Megan considers the risks you’ve discussed so far. She remembers a recent article in a magazine for pregnant women that described severe “respiratory” and “withdrawal” symptoms in infants who were born to mothers taking an SRI antidepressant. She wonders if she should consider discontinuing her SRI in the third trimester to try to mitigate those risks.
Megan is asking you about an SRI exposure risk that has been fairly consistent across studies, called neonatal abstinence syndrome (NAS) or poor neonatal adaptation.
NAS is a cluster of symptoms that occurs in 15% to 30% of newborns who have been exposed to an SRI during the third trimester of pregnancy.15 Signs include irritability, weak cry, tachypnea, temperature instability, and hypoglycemia—all of which are transient, peak during the first 48 hours after delivery, and resolve in less than 2 weeks.
Multiple hypotheses have been put forward to account for NAS, including the possibilities that it reflects a withdrawal syndrome, pharmacotoxicity, or an underlying gene–SRI interaction. The physiology behind NAS remains unknown, however.12
Megan next asks you about persistent pulmonary hypertension of the newborn (PPHN). You explain that PPHN is of recent concern in women who have been taking an SRI in the latter half of their pregnancy.
The rate of PPHN in the general population is 0.5 to 2 newborns for every 1,000. Associated mortality is approximately 10% to 20%.16-18 This rate is thought to rise to approximately 6 of every 1,000 newborns among those who have been exposed to an SRI in utero—with some evidence of increased risk conferred through SRI exposure during later pregnancy (studies define this as the second half of the pregnancy).15 Although the relative risk of PPHN is increased threefold to sixfold when an SRI is used in pregnancy, absolute risk remains extremely low.
Concerns have been raised over research methodology in the few studies that have looked into SRI exposure and PPHN. Not all such studies found a change in relative risk or absolute risk of PPHN in SRI- exposed infants, compared to what was found in non-SRI–exposed infants.15,19,20
Megan presses you, however, with the understandable question of whether she should taper her SRI during the last trimester (which the Web site she has found recommends). With the above information in mind, you explain that, given current understanding of the low absolute risk of PPHN, and given her illness history and severity of prior depression, you would not recommend that she taper the antidepressant in the third trimester.
Furthermore, the same counsel applies in regard to NAS: Given the risk of psychiatric morbidity caused by discontinuing an SRI during the third trimester, you do not recommend that she taper an SRI during that period to avoid NAS.
You explain that, instead, physicians now counsel women who take an SRI about the signs of NAS so that they can be prepared if they observe any of them in their infant.
Megan has one more question: “Will I be able to breastfeed while I’m taking an antidepressant?”
Given the inherent difficulties and risks of relapse associated with a crossover to an alternative antidepressant postpartum, it makes sense, when possible, for a woman to take an antidepressant during pregnancy that can safely be continued while she is breastfeeding.
You tell Megan that, even though the quality of the data in this area is also thin, SRIs that have a low maternal serum profile are considered safest in breastfeeding.
To date, two SRIs—sertraline and paroxetine—have not been detectable in the breast milk of women taking either of them.21
CASE Appointment concluded, overflowing with information,
advice, and optimism
Megan says that, taking into account all that you and she have talked about, and even though she wants to return with her husband, she would like to switch to sertraline before she becomes pregnant—while she gauges its effectiveness at keeping her disorder in remission.
A good outcome requires you to prevail over obstacles
Because a diagnosis of depression spans a continuum of severity and, often, is not perceived as an acutely life-threatening illness, evaluating the risks and benefits of treatment is a murky undertaking.
Our role as physicians is to, first, educate ourselves and our patients about these variables and, second, support our patients in the decisions that they make. Physicians who care for pregnant women must be aware of the benefits and limitations of treatments as reported in the most current literature if they are going to assist women with decisions about treatment in the best possible way.
Social stigma. There remains the impact of stigma. Depressive and anxiety disorders are often perceived to be either under the control of an affected person’s “free will” or not as serious as other forms of “medical” disease. Consequently, the role that cultural and social pressures play in the risk–benefit analysis conducted by pregnant women and their physicians can’t be discounted.
Customized decision-making. As more data emerge about the treatment of depression in pregnancy, it has become clear: Treatment algorithms meant to simplify our decisions must always be individualized and extended into the postpartum period.
Treatment selection. Management of mild depression during pregnancy does not always require medication. Multiple variables—the list is long, and includes a patient’s psychiatric history, family psychiatric history, response to prior treatment, severity of depression, severity of prior depression, degree of social support, and personal desires—must be considered in determining what treatment is appropriate before, during, and after a pregnancy.
For a woman who suffers mild or moderate depression, with few antenatal depression risk factors, a trial of psychotherapy is recommended as first-line treatment. For a woman suffering from severe depression, or one who has a history of severe depression that has not responded well to psychotherapy alone, continuation or initiation of an SRI antidepressant is the current recommendation.
We want to hear from you! Tell us what you think.
1. Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
2. Cooper W, Willy M, Pont S, Ray W. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-e5.
3. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
4. Kramer MS, Lydon J, Séguin L, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169(11):1319-1326.
5. Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50(3):232-241.
6. Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5-14.
7. Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206-1213.
8. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact no pregnancy and neonatal outcomes.” Am J Psychiatry. 2009;166(5):557-566.
9. Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146-153.
10. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;150(5Pt 1):1107-1111.
11. Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
12. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703-713.
13. Gentile S, Bellantuono C. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of fetal major malformations: focus on paroxetine. J Clin Psychiatry. 2009;70(3):414-422.
14. Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. New Engl J Med. 2007;356(26):2675-2683.
15. Chambers CD, Hernandez-Diaz S, Marter LJV, et al. Selective seroteonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New Engl J Med. 2006;354(6):579-587.
16. Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. New Engl J Med. 1996;335(14):1010-1015.
17. Hageman JR, Adams MA, Gardner TH. Persistent pulmonary hypertension of the newborn. Trends in incidence, diagnosis and management. Am J Dis Child. 1984;137(6):592-595.
18. Fricker J. Nitric oxide may reduce need for extracorporeal membrane oxygenation. Lancet. 1996;347(9012):1397.-
19. Kallen B, Olausson P. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2008;17(8):801-806.
20. Andrade S, McPhillips H, Loren D, et al. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. Lanza di Scalea T, Wisner K. Antidepressant medication use during breastfeeding. Clin Obstet Gynecol. 2009;52(3):483-497.
CAS: Depressive disorder, anticipating a pregnancy
Your patient Megan—well-educated, 29 years old, G0P0—has come to you to discuss her antidepressant (paroxetine [Paxil]) because she is planning her first pregnancy.
Megan has a history of recurrent major depressive disorder (MDD), which is in remission (see “What is MDD?”).
How will you begin the conversation with this patient about keeping MDD in remission during her pregnancy and ensuring the safety of her fetus?
- Major depressive disorder (MDD) is defined by criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)
- The disorder varies in severity, 1) across an affected person’s lifetime and 2) within a depressive episode
- A current or prior episode of depression that includes 1) a significant impact on an individual’s functioning, 2) active suicidality, or 3) hospitalization signals severe MDD
- In women, average age at first episode of depression is 24 years
There is a 20% to 25% lifetime prevalence of depression in women; the disorder peaks during childbearing years, however.1 As of 2003, 13% of pregnant women had taken an antidepressant at some time during their pregnancy, a percentage that has doubled since it was assessed in 1999.2
You are faced with several quandaries in deciding whether to recommend that your patient continue, or discontinue, antidepressant therapy during pregnancy:
- As many as 68% of women who terminate antidepressant treatment before or during pregnancy relapse.
- Even 26% of women who continue antidepressant during pregnancy relapse—requiring a dosage adjustment or change in treatment.3
- Yet the possibly elevated cortisol levels of severe, untreated depression may harm the placenta and fetus.4,5
So, what do you need to know to assess the risks and benefits of “Megan” stopping, or continuing, paroxetine during her anticipated pregnancy? And what are the risks to Megan’s fetus of treating, or not treating, her depression with a serotonin reuptake inhibitor (SRI*)?
“Selective” has been dropped
from “SSRI” to yield simply “SRI.”
Gauging the risks of depression in pregnancy
In any given patient, her history and family history of depression are key to determining the likelihood that she will suffer ongoing or recurrent depression.
CASE continued Repeated treated episodes plus a family history
In obtaining Megan’s history, you learn that she has had three prior episodes of depression, all of which were successfully treated with paroxetine. Megan has been stable on paroxetine for 3 years.
Notably, the second episode of depression was initially treated with a 16-week trial of psychotherapy alone; when depressive symptoms did not remit, paroxetine was added. That episode was considered severe because it included pervasive thoughts of suicide.
You also learn that Megan’s mother suffered from postpartum depression and that her father and paternal grandmother were treated for depression.
Known risk factors for depression during pregnancy include: maternal anxiety; prior diagnosis of depression during pregnancy; history of postpartum anxiety or depression; prior diagnosis of either anxiety or depressive disorder; significant life stress (e.g., divorce, death of a loved one); degree of social support—particularly, intimate social support; “intendedness” of pregnancy; domestic violence; and insurance status.6
You review with Megan her risk factors for depression during pregnancy, namely: three prior episodes of MDD and a strong family history of mood disorder. Her MDD is considered “severe” because she has a history of suicidality. You tell Megan that, given these factors, she is at high risk of a recurrence of her depressive illness during pregnancy.
Megan asks: “Would getting depressed during pregnancy hurt the baby?”
Depression during pregnancy affects both infant and maternal well-being, although studies are in conflict about the extent of that morbidity. Multiple areas of potential risk to mother and infant have been studied, including the effect of depression on:
- maternal well-being
- growth of the infant
- spontaneous abortion
- preterm delivery
- neonatal physiologic and neurobehavioral measures
- long-term considerations for the developing infant and child.
Within these categories of risk, a diagnosis of depression during pregnancy has been associated (in some but not all studies) with a higher risk, or rate, of:
- postpartum depression
- preterm birth
- lower maternal weight gain
- maternal tobacco, alcohol, and other substance use
- lower infant gestational age at birth
- small-for-gestational age infant birth.7-10
In terms of long-term impact on offspring, studies differ in their estimation of risk; however, children exposed to untreated, maternal depression at 18 weeks’ and 32 weeks’ gestation did show a greater degree of developmental delay at 18 months than children who were born to a mother who was not depressed during pregnancy.11
You discuss these risks with Megan. She asks: “What treatment do you recommend for me?” You turn to the 2009 guidelines published jointly by the American Psychiatric Association (APA) and ACOG.
These guidelines recommend that you consider 1) the severity of her current depression, 2) her history of depression severity, and 3) her preference for treatment.12 For mild depression during pregnancy, when there is no history of severe depression, or for a history of depression that responded well to psychotherapy in the past, a trial of psychotherapy without medications is recommended.
But Megan’s history of depression falls into the “severe” category, and a prior episode of depression did not respond well to psychotherapy. Your recommendation to her, therefore, is that she should continue taking an antidepressant—unless she feels strongly that she should discontinue it.

Megan considers what you’ve discussed about her high risk of developing recurrent depression during pregnancy. She decides that she wants to continue taking her antidepressant during pregnancy, but she has concerns—based on what she has been reading on the Internet.
Megan hands you a detailed printout downloaded from a Web site unfamiliar to you and asks about risks to the baby of such medications as paroxetine.
What should you tell Megan about SRIs in pregnancy—paroxetine, specifically?
You preface your remarks to her by noting that the data physicians work with are imperfect—because randomized, controlled clinical trials pose an ethical dilemma as a method of study in pregnant women. You then discuss with her current scientific understanding of potential risks to her fetus.
The difference in the rates of structural malformation among SRI-exposed and SRI-unexposed groups has been studied; most studies have found no increased rate of major or specific cardiac malformations.12 However, first-trimester paroxetine appeared, in some studies, to be associated with an increased rate of cardiac malformations. That led to a category-“D” pregnancy classification in 2005 and an FDA “Public Health Advisory.”
Other large cohort studies have not uncovered such an association. It has been hypothesized that the methodology of data collection may have influenced this finding.13
Other malformations have been implicated in some studies but not others, and have included associations between specific SRIs and cardiac ventricular outflow defects, craniosynostosis, and omphalocele. The absolute risk of these defects remains extremely low, however, and close to the background rate seen in the general population.14
Megan asks: “With that risk-category ‘D’ for paroxetine, do you recommend I continue taking it or should I switch to another medication while I’m pregnant?”
You review again with Megan that, although some studies have linked first-trimester paroxetine to an increased risk of cardiac malformation, that finding has not been replicated in several large cohort studies. You explain that, if she had a history of recurrent depression that had failed to respond to many antidepressants and only paroxetine worked, an attempt at switching the SRI would not be recommended because of the potential for relapse.
Megan tells you that she would feel safer not taking a category-“D” drug. You agree and propose a judicious approach: Because she has come to see you before she became pregnant, with enough time to complete a slow crossover to an alternative SRI, and because she has not had any earlier trials of other SRIs, a slow taper of paroxetine, coupled with a crossover to an alternative SRI, is a reasonable option—with the caution that substitution always carries a risk of relapse.
Problems in newborns
Megan considers the risks you’ve discussed so far. She remembers a recent article in a magazine for pregnant women that described severe “respiratory” and “withdrawal” symptoms in infants who were born to mothers taking an SRI antidepressant. She wonders if she should consider discontinuing her SRI in the third trimester to try to mitigate those risks.
Megan is asking you about an SRI exposure risk that has been fairly consistent across studies, called neonatal abstinence syndrome (NAS) or poor neonatal adaptation.
NAS is a cluster of symptoms that occurs in 15% to 30% of newborns who have been exposed to an SRI during the third trimester of pregnancy.15 Signs include irritability, weak cry, tachypnea, temperature instability, and hypoglycemia—all of which are transient, peak during the first 48 hours after delivery, and resolve in less than 2 weeks.
Multiple hypotheses have been put forward to account for NAS, including the possibilities that it reflects a withdrawal syndrome, pharmacotoxicity, or an underlying gene–SRI interaction. The physiology behind NAS remains unknown, however.12
Megan next asks you about persistent pulmonary hypertension of the newborn (PPHN). You explain that PPHN is of recent concern in women who have been taking an SRI in the latter half of their pregnancy.
The rate of PPHN in the general population is 0.5 to 2 newborns for every 1,000. Associated mortality is approximately 10% to 20%.16-18 This rate is thought to rise to approximately 6 of every 1,000 newborns among those who have been exposed to an SRI in utero—with some evidence of increased risk conferred through SRI exposure during later pregnancy (studies define this as the second half of the pregnancy).15 Although the relative risk of PPHN is increased threefold to sixfold when an SRI is used in pregnancy, absolute risk remains extremely low.
Concerns have been raised over research methodology in the few studies that have looked into SRI exposure and PPHN. Not all such studies found a change in relative risk or absolute risk of PPHN in SRI- exposed infants, compared to what was found in non-SRI–exposed infants.15,19,20
Megan presses you, however, with the understandable question of whether she should taper her SRI during the last trimester (which the Web site she has found recommends). With the above information in mind, you explain that, given current understanding of the low absolute risk of PPHN, and given her illness history and severity of prior depression, you would not recommend that she taper the antidepressant in the third trimester.
Furthermore, the same counsel applies in regard to NAS: Given the risk of psychiatric morbidity caused by discontinuing an SRI during the third trimester, you do not recommend that she taper an SRI during that period to avoid NAS.
You explain that, instead, physicians now counsel women who take an SRI about the signs of NAS so that they can be prepared if they observe any of them in their infant.
Megan has one more question: “Will I be able to breastfeed while I’m taking an antidepressant?”
Given the inherent difficulties and risks of relapse associated with a crossover to an alternative antidepressant postpartum, it makes sense, when possible, for a woman to take an antidepressant during pregnancy that can safely be continued while she is breastfeeding.
You tell Megan that, even though the quality of the data in this area is also thin, SRIs that have a low maternal serum profile are considered safest in breastfeeding.
To date, two SRIs—sertraline and paroxetine—have not been detectable in the breast milk of women taking either of them.21
CASE Appointment concluded, overflowing with information,
advice, and optimism
Megan says that, taking into account all that you and she have talked about, and even though she wants to return with her husband, she would like to switch to sertraline before she becomes pregnant—while she gauges its effectiveness at keeping her disorder in remission.
A good outcome requires you to prevail over obstacles
Because a diagnosis of depression spans a continuum of severity and, often, is not perceived as an acutely life-threatening illness, evaluating the risks and benefits of treatment is a murky undertaking.
Our role as physicians is to, first, educate ourselves and our patients about these variables and, second, support our patients in the decisions that they make. Physicians who care for pregnant women must be aware of the benefits and limitations of treatments as reported in the most current literature if they are going to assist women with decisions about treatment in the best possible way.
Social stigma. There remains the impact of stigma. Depressive and anxiety disorders are often perceived to be either under the control of an affected person’s “free will” or not as serious as other forms of “medical” disease. Consequently, the role that cultural and social pressures play in the risk–benefit analysis conducted by pregnant women and their physicians can’t be discounted.
Customized decision-making. As more data emerge about the treatment of depression in pregnancy, it has become clear: Treatment algorithms meant to simplify our decisions must always be individualized and extended into the postpartum period.
Treatment selection. Management of mild depression during pregnancy does not always require medication. Multiple variables—the list is long, and includes a patient’s psychiatric history, family psychiatric history, response to prior treatment, severity of depression, severity of prior depression, degree of social support, and personal desires—must be considered in determining what treatment is appropriate before, during, and after a pregnancy.
For a woman who suffers mild or moderate depression, with few antenatal depression risk factors, a trial of psychotherapy is recommended as first-line treatment. For a woman suffering from severe depression, or one who has a history of severe depression that has not responded well to psychotherapy alone, continuation or initiation of an SRI antidepressant is the current recommendation.
We want to hear from you! Tell us what you think.
CAS: Depressive disorder, anticipating a pregnancy
Your patient Megan—well-educated, 29 years old, G0P0—has come to you to discuss her antidepressant (paroxetine [Paxil]) because she is planning her first pregnancy.
Megan has a history of recurrent major depressive disorder (MDD), which is in remission (see “What is MDD?”).
How will you begin the conversation with this patient about keeping MDD in remission during her pregnancy and ensuring the safety of her fetus?
- Major depressive disorder (MDD) is defined by criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)
- The disorder varies in severity, 1) across an affected person’s lifetime and 2) within a depressive episode
- A current or prior episode of depression that includes 1) a significant impact on an individual’s functioning, 2) active suicidality, or 3) hospitalization signals severe MDD
- In women, average age at first episode of depression is 24 years
There is a 20% to 25% lifetime prevalence of depression in women; the disorder peaks during childbearing years, however.1 As of 2003, 13% of pregnant women had taken an antidepressant at some time during their pregnancy, a percentage that has doubled since it was assessed in 1999.2
You are faced with several quandaries in deciding whether to recommend that your patient continue, or discontinue, antidepressant therapy during pregnancy:
- As many as 68% of women who terminate antidepressant treatment before or during pregnancy relapse.
- Even 26% of women who continue antidepressant during pregnancy relapse—requiring a dosage adjustment or change in treatment.3
- Yet the possibly elevated cortisol levels of severe, untreated depression may harm the placenta and fetus.4,5
So, what do you need to know to assess the risks and benefits of “Megan” stopping, or continuing, paroxetine during her anticipated pregnancy? And what are the risks to Megan’s fetus of treating, or not treating, her depression with a serotonin reuptake inhibitor (SRI*)?
“Selective” has been dropped
from “SSRI” to yield simply “SRI.”
Gauging the risks of depression in pregnancy
In any given patient, her history and family history of depression are key to determining the likelihood that she will suffer ongoing or recurrent depression.
CASE continued Repeated treated episodes plus a family history
In obtaining Megan’s history, you learn that she has had three prior episodes of depression, all of which were successfully treated with paroxetine. Megan has been stable on paroxetine for 3 years.
Notably, the second episode of depression was initially treated with a 16-week trial of psychotherapy alone; when depressive symptoms did not remit, paroxetine was added. That episode was considered severe because it included pervasive thoughts of suicide.
You also learn that Megan’s mother suffered from postpartum depression and that her father and paternal grandmother were treated for depression.
Known risk factors for depression during pregnancy include: maternal anxiety; prior diagnosis of depression during pregnancy; history of postpartum anxiety or depression; prior diagnosis of either anxiety or depressive disorder; significant life stress (e.g., divorce, death of a loved one); degree of social support—particularly, intimate social support; “intendedness” of pregnancy; domestic violence; and insurance status.6
You review with Megan her risk factors for depression during pregnancy, namely: three prior episodes of MDD and a strong family history of mood disorder. Her MDD is considered “severe” because she has a history of suicidality. You tell Megan that, given these factors, she is at high risk of a recurrence of her depressive illness during pregnancy.
Megan asks: “Would getting depressed during pregnancy hurt the baby?”
Depression during pregnancy affects both infant and maternal well-being, although studies are in conflict about the extent of that morbidity. Multiple areas of potential risk to mother and infant have been studied, including the effect of depression on:
- maternal well-being
- growth of the infant
- spontaneous abortion
- preterm delivery
- neonatal physiologic and neurobehavioral measures
- long-term considerations for the developing infant and child.
Within these categories of risk, a diagnosis of depression during pregnancy has been associated (in some but not all studies) with a higher risk, or rate, of:
- postpartum depression
- preterm birth
- lower maternal weight gain
- maternal tobacco, alcohol, and other substance use
- lower infant gestational age at birth
- small-for-gestational age infant birth.7-10
In terms of long-term impact on offspring, studies differ in their estimation of risk; however, children exposed to untreated, maternal depression at 18 weeks’ and 32 weeks’ gestation did show a greater degree of developmental delay at 18 months than children who were born to a mother who was not depressed during pregnancy.11
You discuss these risks with Megan. She asks: “What treatment do you recommend for me?” You turn to the 2009 guidelines published jointly by the American Psychiatric Association (APA) and ACOG.
These guidelines recommend that you consider 1) the severity of her current depression, 2) her history of depression severity, and 3) her preference for treatment.12 For mild depression during pregnancy, when there is no history of severe depression, or for a history of depression that responded well to psychotherapy in the past, a trial of psychotherapy without medications is recommended.
But Megan’s history of depression falls into the “severe” category, and a prior episode of depression did not respond well to psychotherapy. Your recommendation to her, therefore, is that she should continue taking an antidepressant—unless she feels strongly that she should discontinue it.

Megan considers what you’ve discussed about her high risk of developing recurrent depression during pregnancy. She decides that she wants to continue taking her antidepressant during pregnancy, but she has concerns—based on what she has been reading on the Internet.
Megan hands you a detailed printout downloaded from a Web site unfamiliar to you and asks about risks to the baby of such medications as paroxetine.
What should you tell Megan about SRIs in pregnancy—paroxetine, specifically?
You preface your remarks to her by noting that the data physicians work with are imperfect—because randomized, controlled clinical trials pose an ethical dilemma as a method of study in pregnant women. You then discuss with her current scientific understanding of potential risks to her fetus.
The difference in the rates of structural malformation among SRI-exposed and SRI-unexposed groups has been studied; most studies have found no increased rate of major or specific cardiac malformations.12 However, first-trimester paroxetine appeared, in some studies, to be associated with an increased rate of cardiac malformations. That led to a category-“D” pregnancy classification in 2005 and an FDA “Public Health Advisory.”
Other large cohort studies have not uncovered such an association. It has been hypothesized that the methodology of data collection may have influenced this finding.13
Other malformations have been implicated in some studies but not others, and have included associations between specific SRIs and cardiac ventricular outflow defects, craniosynostosis, and omphalocele. The absolute risk of these defects remains extremely low, however, and close to the background rate seen in the general population.14
Megan asks: “With that risk-category ‘D’ for paroxetine, do you recommend I continue taking it or should I switch to another medication while I’m pregnant?”
You review again with Megan that, although some studies have linked first-trimester paroxetine to an increased risk of cardiac malformation, that finding has not been replicated in several large cohort studies. You explain that, if she had a history of recurrent depression that had failed to respond to many antidepressants and only paroxetine worked, an attempt at switching the SRI would not be recommended because of the potential for relapse.
Megan tells you that she would feel safer not taking a category-“D” drug. You agree and propose a judicious approach: Because she has come to see you before she became pregnant, with enough time to complete a slow crossover to an alternative SRI, and because she has not had any earlier trials of other SRIs, a slow taper of paroxetine, coupled with a crossover to an alternative SRI, is a reasonable option—with the caution that substitution always carries a risk of relapse.
Problems in newborns
Megan considers the risks you’ve discussed so far. She remembers a recent article in a magazine for pregnant women that described severe “respiratory” and “withdrawal” symptoms in infants who were born to mothers taking an SRI antidepressant. She wonders if she should consider discontinuing her SRI in the third trimester to try to mitigate those risks.
Megan is asking you about an SRI exposure risk that has been fairly consistent across studies, called neonatal abstinence syndrome (NAS) or poor neonatal adaptation.
NAS is a cluster of symptoms that occurs in 15% to 30% of newborns who have been exposed to an SRI during the third trimester of pregnancy.15 Signs include irritability, weak cry, tachypnea, temperature instability, and hypoglycemia—all of which are transient, peak during the first 48 hours after delivery, and resolve in less than 2 weeks.
Multiple hypotheses have been put forward to account for NAS, including the possibilities that it reflects a withdrawal syndrome, pharmacotoxicity, or an underlying gene–SRI interaction. The physiology behind NAS remains unknown, however.12
Megan next asks you about persistent pulmonary hypertension of the newborn (PPHN). You explain that PPHN is of recent concern in women who have been taking an SRI in the latter half of their pregnancy.
The rate of PPHN in the general population is 0.5 to 2 newborns for every 1,000. Associated mortality is approximately 10% to 20%.16-18 This rate is thought to rise to approximately 6 of every 1,000 newborns among those who have been exposed to an SRI in utero—with some evidence of increased risk conferred through SRI exposure during later pregnancy (studies define this as the second half of the pregnancy).15 Although the relative risk of PPHN is increased threefold to sixfold when an SRI is used in pregnancy, absolute risk remains extremely low.
Concerns have been raised over research methodology in the few studies that have looked into SRI exposure and PPHN. Not all such studies found a change in relative risk or absolute risk of PPHN in SRI- exposed infants, compared to what was found in non-SRI–exposed infants.15,19,20
Megan presses you, however, with the understandable question of whether she should taper her SRI during the last trimester (which the Web site she has found recommends). With the above information in mind, you explain that, given current understanding of the low absolute risk of PPHN, and given her illness history and severity of prior depression, you would not recommend that she taper the antidepressant in the third trimester.
Furthermore, the same counsel applies in regard to NAS: Given the risk of psychiatric morbidity caused by discontinuing an SRI during the third trimester, you do not recommend that she taper an SRI during that period to avoid NAS.
You explain that, instead, physicians now counsel women who take an SRI about the signs of NAS so that they can be prepared if they observe any of them in their infant.
Megan has one more question: “Will I be able to breastfeed while I’m taking an antidepressant?”
Given the inherent difficulties and risks of relapse associated with a crossover to an alternative antidepressant postpartum, it makes sense, when possible, for a woman to take an antidepressant during pregnancy that can safely be continued while she is breastfeeding.
You tell Megan that, even though the quality of the data in this area is also thin, SRIs that have a low maternal serum profile are considered safest in breastfeeding.
To date, two SRIs—sertraline and paroxetine—have not been detectable in the breast milk of women taking either of them.21
CASE Appointment concluded, overflowing with information,
advice, and optimism
Megan says that, taking into account all that you and she have talked about, and even though she wants to return with her husband, she would like to switch to sertraline before she becomes pregnant—while she gauges its effectiveness at keeping her disorder in remission.
A good outcome requires you to prevail over obstacles
Because a diagnosis of depression spans a continuum of severity and, often, is not perceived as an acutely life-threatening illness, evaluating the risks and benefits of treatment is a murky undertaking.
Our role as physicians is to, first, educate ourselves and our patients about these variables and, second, support our patients in the decisions that they make. Physicians who care for pregnant women must be aware of the benefits and limitations of treatments as reported in the most current literature if they are going to assist women with decisions about treatment in the best possible way.
Social stigma. There remains the impact of stigma. Depressive and anxiety disorders are often perceived to be either under the control of an affected person’s “free will” or not as serious as other forms of “medical” disease. Consequently, the role that cultural and social pressures play in the risk–benefit analysis conducted by pregnant women and their physicians can’t be discounted.
Customized decision-making. As more data emerge about the treatment of depression in pregnancy, it has become clear: Treatment algorithms meant to simplify our decisions must always be individualized and extended into the postpartum period.
Treatment selection. Management of mild depression during pregnancy does not always require medication. Multiple variables—the list is long, and includes a patient’s psychiatric history, family psychiatric history, response to prior treatment, severity of depression, severity of prior depression, degree of social support, and personal desires—must be considered in determining what treatment is appropriate before, during, and after a pregnancy.
For a woman who suffers mild or moderate depression, with few antenatal depression risk factors, a trial of psychotherapy is recommended as first-line treatment. For a woman suffering from severe depression, or one who has a history of severe depression that has not responded well to psychotherapy alone, continuation or initiation of an SRI antidepressant is the current recommendation.
We want to hear from you! Tell us what you think.
1. Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
2. Cooper W, Willy M, Pont S, Ray W. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-e5.
3. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
4. Kramer MS, Lydon J, Séguin L, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169(11):1319-1326.
5. Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50(3):232-241.
6. Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5-14.
7. Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206-1213.
8. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact no pregnancy and neonatal outcomes.” Am J Psychiatry. 2009;166(5):557-566.
9. Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146-153.
10. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;150(5Pt 1):1107-1111.
11. Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
12. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703-713.
13. Gentile S, Bellantuono C. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of fetal major malformations: focus on paroxetine. J Clin Psychiatry. 2009;70(3):414-422.
14. Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. New Engl J Med. 2007;356(26):2675-2683.
15. Chambers CD, Hernandez-Diaz S, Marter LJV, et al. Selective seroteonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New Engl J Med. 2006;354(6):579-587.
16. Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. New Engl J Med. 1996;335(14):1010-1015.
17. Hageman JR, Adams MA, Gardner TH. Persistent pulmonary hypertension of the newborn. Trends in incidence, diagnosis and management. Am J Dis Child. 1984;137(6):592-595.
18. Fricker J. Nitric oxide may reduce need for extracorporeal membrane oxygenation. Lancet. 1996;347(9012):1397.-
19. Kallen B, Olausson P. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2008;17(8):801-806.
20. Andrade S, McPhillips H, Loren D, et al. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. Lanza di Scalea T, Wisner K. Antidepressant medication use during breastfeeding. Clin Obstet Gynecol. 2009;52(3):483-497.
1. Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105.
2. Cooper W, Willy M, Pont S, Ray W. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-e5.
3. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
4. Kramer MS, Lydon J, Séguin L, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169(11):1319-1326.
5. Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50(3):232-241.
6. Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5-14.
7. Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206-1213.
8. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact no pregnancy and neonatal outcomes.” Am J Psychiatry. 2009;166(5):557-566.
9. Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146-153.
10. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;150(5Pt 1):1107-1111.
11. Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
12. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703-713.
13. Gentile S, Bellantuono C. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of fetal major malformations: focus on paroxetine. J Clin Psychiatry. 2009;70(3):414-422.
14. Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. New Engl J Med. 2007;356(26):2675-2683.
15. Chambers CD, Hernandez-Diaz S, Marter LJV, et al. Selective seroteonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New Engl J Med. 2006;354(6):579-587.
16. Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. New Engl J Med. 1996;335(14):1010-1015.
17. Hageman JR, Adams MA, Gardner TH. Persistent pulmonary hypertension of the newborn. Trends in incidence, diagnosis and management. Am J Dis Child. 1984;137(6):592-595.
18. Fricker J. Nitric oxide may reduce need for extracorporeal membrane oxygenation. Lancet. 1996;347(9012):1397.-
19. Kallen B, Olausson P. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2008;17(8):801-806.
20. Andrade S, McPhillips H, Loren D, et al. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009;18(3):246-252.
21. Lanza di Scalea T, Wisner K. Antidepressant medication use during breastfeeding. Clin Obstet Gynecol. 2009;52(3):483-497.
Treating anxiety during pregnancy: Just how safe are SSRIs?
Ms. K, age 25, is 6 weeks pregnant and is taking medications for generalized anxiety disorder (GAD). When she was diagnosed with GAD at age 19, her symptoms included 6 months of excessive anxiety—insomnia, fatigue, difficulty with concentration, and psychomotor agitation—without mood symptoms. These symptoms interfered greatly with her schoolwork and other daily activities.
For 6 years Ms. K has been taking the selective serotonin reuptake inhibitor (SSRI) paroxetine, 15 mg/d, and the benzodiazepine clonazepam, 0.5 mg as needed, with good symptom control. Now that she is pregnant and her primary care doctor has refused to continue these medications, she is seeking treatment and advice.
Not enough is known about how to safely treat anxiety disorders during pregnancy, and physicians are not sure what to do with patients such as Ms. K. Without evidence-based guidelines, we feel anxious about potential risks to mother and fetus as we try to provide appropriate drug therapy.
To help you and your patients weigh the risks and benefits of perinatal treatments for anxiety disorders, this article briefly summarizes the evidence on:
- anxiety disorders’ natural history during pregnancy
- how untreated maternal anxiety affects the fetus
- nonpharmacologic therapies for anxiety disorders
- a plan to manage fetal risks by staggering SSRI and benzodiazepine use during the first and third trimesters.
Anxiety during pregnancy
Nearly one-third of women experience an anxiety disorder during their lives, with peak onset during childbearing years.1,2 Compared with research on perinatal depression, far fewer studies have examined anxiety disorders’ onset, presentation, prevalence, and treatment.1
The literature includes no studies of the course of preexisting GAD or posttraumatic stress disorder (PTSD) and no evidence that symptoms of preexisting obsessive-compulsive disorder (OCD) change during pregnancy. Some studies of panic disorder show symptoms improving during pregnancy, whereas others do not (Table 1).1
One small study done in late pregnancy found a significant association between the prevalence of an anxiety disorder, maternal primiparity, and comorbid medical conditions. Thus, a woman in her first pregnancy may be at increased risk to develop an anxiety disorder if she has a comorbid medical condition.3 As in the case of Ms. K, however, continuation of preexisting anxiety appears more likely than onset of a new anxiety disorder during pregnancy.
Table 1
How pregnancy affects the course of 4 anxiety disorders
| Disorder | Prevalence | Effect |
|---|---|---|
| Generalized anxiety disorder (GAD) | 8.5% of women experience GAD during the third trimester, compared with a 5% prevalence in the general population | No studies have reported on the course of GAD in pregnant women with preexisting disorder |
| Obsessive-compulsive disorder (OCD) | 2% to 12% of OCD outpatients of childbearing age report onset during pregnancy | Preexisting OCD usually shows no change during pregnancy but may worsen postpartum |
| Panic disorder (PD) | 1.3% to 2% in pregnant women, compared with 1.5% to 3.5% in the general population | Panic symptoms in women with preexisting PD may improve during pregnancy and worsen postpartum |
| Posttraumatic stress disorder (PTSD) | 2.3% to 7.7% in pregnant women and 0% to 6.9% postpartum, compared with 1% to 14% in the community | No studies have reported on the course of PTSD in pregnant women with preexisting disorder |
| Source: References 1,2 | ||
Fetal risks from maternal anxiety
Fetal risk from severe maternal anxiety is not zero. Offspring born to high-anxiety mothers exhibit neurobehavioral differences compared with offspring of calmer mothers. Changes in high-anxiety mothers’ offspring include:
- altered EEG activation and vagal tone
- increased time in deep sleep and less time in active alert states
- lower performance on the Brazelton Neonatal Behavior Assessment Scale.4
Exposure to maternal high anxiety has been associated with mental developmental delays in infants and increased risk for behavioral and emotional problems in young children.7-10 Anxiety may not directly cause intrauterine growth retardation and preterm delivery, but it is significantly associated with prenatal tobacco, alcohol, and narcotics use—which predicts these and other negative neonatal outcomes.11
7,13
CASE CONTINUED: ‘Stay the course’
Ms. K worries that she could not tolerate recurrence of her anxiety symptoms and wishes to continue both medications. Her husband concurs, but they want to minimize potential risks to their baby. You discuss the options for treating anxiety symptoms during pregnancy, including medications, psychotherapy, and behavioral treatments.
Treatment decisions
Ideally you’ll begin treating anxiety disorders in women of childbearing age with preconception psychoeducation. Explaining the risks of medications if she were to become pregnant and asking about the contraception she is using are de rigueur. Psychotherapy is low risk to the fetus and is considered first choice for treating mild to moderate anxiety in women of childbearing age who plan to become pregnant (Box).1,14-17
No studies directly address the efficacy or outcome of any psychotherapy for anxiety in pregnancy. Even so:
- For mild to moderate anxiety, psychotherapy is the first-line treatment for pregnant women.
- Interpersonal psychotherapy (IPT) without medications can reduce depressive symptoms in pregnant women with depression.14
- Cognitive-behavioral therapy (CBT) without medications has shown efficacy for anxiety disorders in psychiatric populations.15,16
Because no evidence suggests that pregnant women require different psychotherapeutic recommendations than other psychiatric patients, consider a course of CBT that targets anxiety symptoms or IPT for a pregnant patient with an anxiety disorder.
Relaxation therapy also has shown efficacy in treating anxiety disorders. In a randomized controlled trial of 110 pregnant women with high-level anxiety, 7 weeks of applied relaxation training sessions was associated with significant reductions in low-weight births, cesarean sections, and instrumental extractions.16,17
Because poor marital relationships are consistent psychosocial predictors of anxiety during pregnancy and postpartum depression,1 recommend family or marital therapy when appropriate.
Because Ms. K wishes to continue taking paroxetine and clonazepam, what can you tell her about the risks and benefits of SSRIs and benzodiazepines during pregnancy?
SSRIs in pregnancy
Teratogenicity. Compared with benzodiazepines, SSRIs have been considered agents of choice for use during pregnancy because of a lower risk of teratogenic effects.15 Paroxetine, however, appears to pose a greater risk for teratogenicity than other SSRIs.
The overall rate of fetal malformations from SSRIs appears to be low, although most studies have examined only fluoxetine or paroxetine. Some studies have reported various malformations with fluoxetine or sertraline, but others have not. In Finland, a population-based study found no increase in rate of major congenital malformations in offspring of 1,782 women who filled prescriptions for SSRIs during pregnancy, compared with the general population rate of 1% to 3%.21
Neurobehavioral effects. SSRI exposure during fetal life has shown no long-term neurobehavioral effects. A blinded prospective study by Nulman et al22 found no differences in global IQ scores, language development, or behavioral development among children age ≤5 who were exposed in utero to fluoxetine (n=40) or a tricyclic antidepressant (n=46), compared with unexposed children of nondepressed mothers (n=36). Similarly, using reports from teachers and clinical measures of internalizing behaviors, Misri et al10 found no increase in depression, anxiety, or withdrawal in 4-year-olds with prenatal exposure to SSRIs (n=22), compared with nonexposed children (n=14).
Pulmonary hypertension. SSRI exposure in later pregnancy may increase the rate of persistent pulmonary hypertension of the newborn (PPHN), which occurs in 1 to 2 infants per 1,000 live births. PPHN showed a statistically significant association with late prenatal SSRI exposure (OR 6.1) in a study that controlled for maternal smoking, body mass index, and diabetes.23 PPHN occurred in approximately 1% of infants exposed to SSRIs in late pregnancy. PPHN rates were not affected by maternal depression/anxiety, non-SSRI antidepressant exposure throughout pregnancy, or SSRI exposure during early pregnancy only.
Toxicity and withdrawal syndromes. Infants of women who continue to take SSRIs just before delivery can develop toxicity or withdrawal syndromes. Occurrence of either syndrome depends on SSRI half-life, serum concentration, and the pharmacodynamics of other medications given during pregnancy and labor.24
Discontinuation syndromes can occur in SSRI-exposed neonates within a few hours or days after birth and last up to 1 month after delivery, depending on the infant’s susceptibility.25 Nearly two-thirds of suspected SSRI-induced neonatal withdrawal syndromes have been associated with paroxetine, although all SSRIs appear be associated with some risk.26 Several trials, including a recent prospective study, found prenatal antidepressant use associated with lower gestational age at birth and increased risk of preterm birth.27
- greater tremulousness
- less flexible and dampened state regulation
- more time in uninterrupted REM sleep
- more frequent startles or sudden arousals
- greater generalized motor activity
- greater autonomic dysregulation.28
- tremor (37/60)
- GI disturbances (34/60)—including exaggerated sucking, poor feeding, regurgitation, vomiting, and loose stools
- sleep disturbance (21/60).
Recommendations. The perception that SSRIs have low fetal toxicity has guided prescribing practices in recent years. Newer evidence shows, however, that fetal exposure to SSRIs may have some adverse effects, including lower birth weight and early delivery. First-trimester paroxetine use has been associated with increased risk for fetal ventricular and/or atrial septal defects.
Discuss these risks with the patient when you consider starting or continuing SSRI use during pregnancy.24 If you prescribe an SSRI, use the minimum effective dosage and avoid paroxetine during pregnancy.18
To reduce the risk for PPHN, early delivery, and neonatal withdrawal syndromes, taper and discontinue the SSRI during the third trimester. Restarting the SSRI soon after delivery is the most effective way to prevent recurrence of anxiety symptoms or postpartum depression.
Benzodiazepines
Teratogenicity. Like SSRIs, benzodiazepines cross the placenta to the fetus.29 Benzodiazepine teratogenicity remains controversial.8 Some—but not all—data show a small but significant increased risk for major malformations/oral cleft malformations with first-trimester benzodiazepine exposure.
A Medline literature search from 1966 to 2000 found not enough information to determine whether potential benefits of benzodiazepines to the mother outweigh risks to the fetus.29 An ambitious meta-analysis of >1,400 studies by Dolovich et al30 found a small association between fetal exposure to benzodiazepines and major malformations/cleft palate, but only in pooled data from case-controlled studies. No association was found between fetal exposure to benzodiazepines and malformations/cleft palate in pooled data from cohort studies.
A 32-month, hospital-based surveillance program of 28,565 births found no increase in the rate of major malformations in 43 infants exposed to clonazepam monotherapy—33 (77%) in the first trimester.31 Thus, the risk of major malformations/cleft palate with the use of benzodiazepines in the first trimester appears to be low.
- Neonatal toxicity (“floppy infant syndrome”)—characterized by hypothermia, lethargy, poor respiratory effort, and feeding difficulties—occurs after maternal benzodiazepine use just before delivery.8
- Neonatal withdrawal may be caused by very late, third trimester exposure to benzodiazepines. Symptoms—which can persist ≤3 months after delivery—include restlessness, irritability, abnormal sleep patterns, suckling difficulties, growth retardation, hypertonia, hyperreflexia, tremulousness, apnea, diarrhea, and vomiting.8,29
To minimize neonatal withdrawal, gradually taper the mother’s benzodiazepine before delivery.29 Because the baby’s due date is calculated to be ±2 weeks before delivery, begin this taper 3 to 4 weeks before the due date and discontinue at least 1 week before delivery. Breastfeeding while taking benzodiazepines is not recommended because of the risk of over-sedating the infant.
A rational approach
Both benzodiazepines and SSRIs are associated with low but demonstrated risks to the fetus when used during pregnancy (Table 2).19,20,23,25,30,33 Use these medications to manage a patient’s anxiety only if the clinical benefit to the mother justifies the potential risks to the fetus.29
A staggered combination of SSRIs during the first 2 trimesters and benzodiazepines during the last 2 trimesters can help balance the risks and benefits of pharmacotherapy of anxiety disorders during pregnancy (Table 3).
Frankly discuss with your patient the risks and benefits in the context of her perceived need for symptom control to sustain her level of functioning. You could document this discussion in the progress note as “R, B, A, and pt C,” signifying that risks, benefits, and alternatives were discussed, and the patient consented. If possible, include the patient’s husband, partner, or parent in this discussion.
Table 2
Risks of SSRIs vs benzodiazepines during pregnancy stages
| Pregnancy stage when given | Fetal risk | SSRIs | Benzodiazepines |
|---|---|---|---|
| First trimester* | Teratogenicity | Paroxetine use associated with 2-fold increased risk of major congenital anomalies and 3-fold increased risk of major cardiac anomalies;19 meta-analysis calculated significant risk of cardiac malformations (odds ratio 1.72; population prevalence = 13.4/1,000 births)20,33 | Meta-analysis of case control studies showed increased risk of major malformations/cleft palate (odds ratio 3.01; population prevalence = 10 to 20/1,000 births); no association seen in cohort studies30 |
| Third trimester | PPHN | Case control study showed 3.7% of infants with PPHN were exposed to SSRIs vs 0.7% of controls; adjusted odds ratio 6.1, absolute risk to exposed population = 6 to 12/1,000 births)23 | |
| Perinatal and long-term effects | Toxicity/withdrawal syndromes | Cohort study of 60 infants concluded prevalence of discontinuation syndromes is 30% in neonates with third trimester SSRI exposure25 | Neonatal toxicity (“floppy infant syndrome”) and neonatal withdrawal reported with maternal benzodiazepine use in late third trimester; prevalence unknown† |
| Preterm birth, serotonin withdrawal syndromes, CNS effects, long-term neurobehavioral effects | Unknown† | Unknown† | |
| PPHN: persistent pulmonary hypertension of the newborn; SSRIs: selective serotonin reuptake inhibitors | |||
| * Available data indicate that first-trimester exposure to SSRIs (other than paroxetine) and benzodiazepines may increase the relative risk for congenital anomalies, but the absolute risk of having a child with an anomaly is small. | |||
| † Some case reports, but published literature is insufficient to determine prevalence or magnitude of risk. | |||
Staggered, combination therapy for anxiety disorders during pregnancy
| Pregnancy stage | Recommended to manage risks to mother and fetus |
|---|---|
| First trimester |
|
| Second trimester |
|
| Third trimester |
|
| SSRI: selective serotonin reuptake inhibitor | |
| * Nondrug therapies can include prenatal exercise, sleep hygiene, relaxation, and psychotherapy (cognitive-behavioral therapy, interpersonal therapy, supportive therapy, family/couples therapy) | |
CASE CONTINUED: CBT plus medication
Ms. K and her husband are open to adding weekly cognitive-behavioral therapy (CBT) for anxiety as long as she can continue her medications. You discuss the evidence regarding potential neonatal risks with paroxetine and clonazepam treatment. Because Ms. K is 6 weeks pregnant, you outline a plan for a rapid cross-taper off paroxetine and onto fluoxetine, 10 to 30 mg/d, explaining that paroxetine might pose a greater first-trimester risk of major congenital malformations and cardiac malformations. You discuss possible side effects of fluoxetine and explain a plan to taper off fluoxetine during the third trimester to reduce the risk of PPHN, early delivery, and withdrawal in the newborn.
Because Ms. K has been taking clonazepam at only 0.5 mg 1 to 2 times per week, you instruct her to stop taking the benzodiazepine for the next 6 weeks until she is through her first trimester. You also reassure her that she can use clonazepam after the first trimester, if necessary, as long as she agrees to taper off completely 1 to 2 weeks before to her due date.
You refer her to a CBT therapist and emphasize the importance of CBT, relaxation, and sleep hygiene—as well as support from her husband, family, and friends—to reduce her stress and facilitate the medication taper during her third trimester. You plan to see her monthly and co-manage her care with the CBT therapist and Ob/Gyn. You document this discussion in her medical record as evidence of informed consent.
Related resources
- Baby Center. Managing stress and anxiety during pregnancy. Patient information. www.babycenter.com/0_managing-stress-and-anxiety-during-pregnancy_1683.bc.
- Organization of Teratology Information Specialists (OTIS). www.otispregnancy.org.
Drug brand names
- Clonazepam • Klonopin
- Paroxetine • Paxil
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry 2006;67(8):1285-98.
2. Labad J, Menchon JM, Alonso P, et al. Female reproductive cycle and obsessive-compulsive disorder. J Clin Psychiatry 2005;66(4):428-35.
3. Adewuya AO, Ola BA, Aloba OO, Mapayi BM. Anxiety disorders among Nigerian women in late pregnancy: a controlled study. Arch Womens Ment Health 2006;9(6):325-8.
4. Field T, Hernandez-Reif M, Diego M, et al. Stability of mood states and biochemistry across pregnancy. Infant Behav Dev 2006;29(2):262-7.
5. Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318(7177):153-7.
6. Monk C, Myers MM, Sloan RP, et al. Effects of women’s stress-elicited physiological activity and chronic anxiety on fetal heart rate. J Dev Behav Pediatr 2003;24(1):32-8.
7. Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology 2007;32(1):1-13.
8. Levey L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22(4):863-93.
9. Oberlander TF, Reebye P, Misri S, et al. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007;161(1):22-9.
10. Misri S, Reebye P, Kendrick K, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006;163(6):1026-32.
11. Copper RL, Goldenberg RL, Das A, et al. The Preterm Prediction Study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. Am J Obstet Gynecol 1996;175(5):1286-92.
12. Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. Eur Psychiatry 2004;19(8):459-63.
13. Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med 2006;68(6):931-7.
14. Weissman MM. Recent non-medication trials of interpersonal psychotherapy for depression. Int J Neuropsychopharmacology 2007;10(1):117-22.
15. Ward RK, Zamorski MA. Benefits and risks of psychiatric medications during pregnancy. Am Fam Physician 2002;66(4):629-36.
16. Bastani F, Hidarnia A, Montgomery KS, et al. Does relaxation education in anxious primigravid Iranian women influence adverse pregnancy outcomes? A randomized controlled trial. J Perinat Neonatal Nurs 2006;20(2):138-46.
17. Fricchione G. Generalized anxiety disorder. N Engl J Med 2004;351(7):675-82.
18. Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol 2007;79(4):301-8.
19. Berard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 2007;80(1):18-27.
20. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther 2005;29(5):918-26.
21. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 2005;106(6):1289-96.
22. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159(11):1889-95.
23. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
24. Haddad PM, Pal BR, Clarke P, et al. Neonatal symptoms following maternal paroxetine treatment: serotonin toxicity or paroxetine discontinuation syndrome? J Psychopharmacology 2005;19(5):554-7.
25. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med 2006;160(2):173-6.
26. Sanz EJ, De-las-Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005;365(9458):482-7.
27. Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007;164(8):1206-13.
28. Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113(2):368-75.
29. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv 2002;53(1):39-49.
30. Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998;317(7162):839-43.
31. McElhatton PR. The effects of benzodiazepine use during pregnancy. Reprod Toxicol 1994;8(6):461-75.
32. Lin AE, Peller AJ, Westgate MN, et al. Clonazepam use in pregnancy and the risk of malformations. Birth Defects Res A Clin Mol Teratol 2004;70(8):534-6.
33. Levy M, James MS, Erickson JD, McClearn AB. Prevalence of birth defects. Birth outcomes Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/reproductivehealth/Products&Pubs/DatatoAction/pdf/birout4.pdf. Accessed January 9, 2008.
Ms. K, age 25, is 6 weeks pregnant and is taking medications for generalized anxiety disorder (GAD). When she was diagnosed with GAD at age 19, her symptoms included 6 months of excessive anxiety—insomnia, fatigue, difficulty with concentration, and psychomotor agitation—without mood symptoms. These symptoms interfered greatly with her schoolwork and other daily activities.
For 6 years Ms. K has been taking the selective serotonin reuptake inhibitor (SSRI) paroxetine, 15 mg/d, and the benzodiazepine clonazepam, 0.5 mg as needed, with good symptom control. Now that she is pregnant and her primary care doctor has refused to continue these medications, she is seeking treatment and advice.
Not enough is known about how to safely treat anxiety disorders during pregnancy, and physicians are not sure what to do with patients such as Ms. K. Without evidence-based guidelines, we feel anxious about potential risks to mother and fetus as we try to provide appropriate drug therapy.
To help you and your patients weigh the risks and benefits of perinatal treatments for anxiety disorders, this article briefly summarizes the evidence on:
- anxiety disorders’ natural history during pregnancy
- how untreated maternal anxiety affects the fetus
- nonpharmacologic therapies for anxiety disorders
- a plan to manage fetal risks by staggering SSRI and benzodiazepine use during the first and third trimesters.
Anxiety during pregnancy
Nearly one-third of women experience an anxiety disorder during their lives, with peak onset during childbearing years.1,2 Compared with research on perinatal depression, far fewer studies have examined anxiety disorders’ onset, presentation, prevalence, and treatment.1
The literature includes no studies of the course of preexisting GAD or posttraumatic stress disorder (PTSD) and no evidence that symptoms of preexisting obsessive-compulsive disorder (OCD) change during pregnancy. Some studies of panic disorder show symptoms improving during pregnancy, whereas others do not (Table 1).1
One small study done in late pregnancy found a significant association between the prevalence of an anxiety disorder, maternal primiparity, and comorbid medical conditions. Thus, a woman in her first pregnancy may be at increased risk to develop an anxiety disorder if she has a comorbid medical condition.3 As in the case of Ms. K, however, continuation of preexisting anxiety appears more likely than onset of a new anxiety disorder during pregnancy.
Table 1
How pregnancy affects the course of 4 anxiety disorders
| Disorder | Prevalence | Effect |
|---|---|---|
| Generalized anxiety disorder (GAD) | 8.5% of women experience GAD during the third trimester, compared with a 5% prevalence in the general population | No studies have reported on the course of GAD in pregnant women with preexisting disorder |
| Obsessive-compulsive disorder (OCD) | 2% to 12% of OCD outpatients of childbearing age report onset during pregnancy | Preexisting OCD usually shows no change during pregnancy but may worsen postpartum |
| Panic disorder (PD) | 1.3% to 2% in pregnant women, compared with 1.5% to 3.5% in the general population | Panic symptoms in women with preexisting PD may improve during pregnancy and worsen postpartum |
| Posttraumatic stress disorder (PTSD) | 2.3% to 7.7% in pregnant women and 0% to 6.9% postpartum, compared with 1% to 14% in the community | No studies have reported on the course of PTSD in pregnant women with preexisting disorder |
| Source: References 1,2 | ||
Fetal risks from maternal anxiety
Fetal risk from severe maternal anxiety is not zero. Offspring born to high-anxiety mothers exhibit neurobehavioral differences compared with offspring of calmer mothers. Changes in high-anxiety mothers’ offspring include:
- altered EEG activation and vagal tone
- increased time in deep sleep and less time in active alert states
- lower performance on the Brazelton Neonatal Behavior Assessment Scale.4
Exposure to maternal high anxiety has been associated with mental developmental delays in infants and increased risk for behavioral and emotional problems in young children.7-10 Anxiety may not directly cause intrauterine growth retardation and preterm delivery, but it is significantly associated with prenatal tobacco, alcohol, and narcotics use—which predicts these and other negative neonatal outcomes.11
7,13
CASE CONTINUED: ‘Stay the course’
Ms. K worries that she could not tolerate recurrence of her anxiety symptoms and wishes to continue both medications. Her husband concurs, but they want to minimize potential risks to their baby. You discuss the options for treating anxiety symptoms during pregnancy, including medications, psychotherapy, and behavioral treatments.
Treatment decisions
Ideally you’ll begin treating anxiety disorders in women of childbearing age with preconception psychoeducation. Explaining the risks of medications if she were to become pregnant and asking about the contraception she is using are de rigueur. Psychotherapy is low risk to the fetus and is considered first choice for treating mild to moderate anxiety in women of childbearing age who plan to become pregnant (Box).1,14-17
No studies directly address the efficacy or outcome of any psychotherapy for anxiety in pregnancy. Even so:
- For mild to moderate anxiety, psychotherapy is the first-line treatment for pregnant women.
- Interpersonal psychotherapy (IPT) without medications can reduce depressive symptoms in pregnant women with depression.14
- Cognitive-behavioral therapy (CBT) without medications has shown efficacy for anxiety disorders in psychiatric populations.15,16
Because no evidence suggests that pregnant women require different psychotherapeutic recommendations than other psychiatric patients, consider a course of CBT that targets anxiety symptoms or IPT for a pregnant patient with an anxiety disorder.
Relaxation therapy also has shown efficacy in treating anxiety disorders. In a randomized controlled trial of 110 pregnant women with high-level anxiety, 7 weeks of applied relaxation training sessions was associated with significant reductions in low-weight births, cesarean sections, and instrumental extractions.16,17
Because poor marital relationships are consistent psychosocial predictors of anxiety during pregnancy and postpartum depression,1 recommend family or marital therapy when appropriate.
Because Ms. K wishes to continue taking paroxetine and clonazepam, what can you tell her about the risks and benefits of SSRIs and benzodiazepines during pregnancy?
SSRIs in pregnancy
Teratogenicity. Compared with benzodiazepines, SSRIs have been considered agents of choice for use during pregnancy because of a lower risk of teratogenic effects.15 Paroxetine, however, appears to pose a greater risk for teratogenicity than other SSRIs.
The overall rate of fetal malformations from SSRIs appears to be low, although most studies have examined only fluoxetine or paroxetine. Some studies have reported various malformations with fluoxetine or sertraline, but others have not. In Finland, a population-based study found no increase in rate of major congenital malformations in offspring of 1,782 women who filled prescriptions for SSRIs during pregnancy, compared with the general population rate of 1% to 3%.21
Neurobehavioral effects. SSRI exposure during fetal life has shown no long-term neurobehavioral effects. A blinded prospective study by Nulman et al22 found no differences in global IQ scores, language development, or behavioral development among children age ≤5 who were exposed in utero to fluoxetine (n=40) or a tricyclic antidepressant (n=46), compared with unexposed children of nondepressed mothers (n=36). Similarly, using reports from teachers and clinical measures of internalizing behaviors, Misri et al10 found no increase in depression, anxiety, or withdrawal in 4-year-olds with prenatal exposure to SSRIs (n=22), compared with nonexposed children (n=14).
Pulmonary hypertension. SSRI exposure in later pregnancy may increase the rate of persistent pulmonary hypertension of the newborn (PPHN), which occurs in 1 to 2 infants per 1,000 live births. PPHN showed a statistically significant association with late prenatal SSRI exposure (OR 6.1) in a study that controlled for maternal smoking, body mass index, and diabetes.23 PPHN occurred in approximately 1% of infants exposed to SSRIs in late pregnancy. PPHN rates were not affected by maternal depression/anxiety, non-SSRI antidepressant exposure throughout pregnancy, or SSRI exposure during early pregnancy only.
Toxicity and withdrawal syndromes. Infants of women who continue to take SSRIs just before delivery can develop toxicity or withdrawal syndromes. Occurrence of either syndrome depends on SSRI half-life, serum concentration, and the pharmacodynamics of other medications given during pregnancy and labor.24
Discontinuation syndromes can occur in SSRI-exposed neonates within a few hours or days after birth and last up to 1 month after delivery, depending on the infant’s susceptibility.25 Nearly two-thirds of suspected SSRI-induced neonatal withdrawal syndromes have been associated with paroxetine, although all SSRIs appear be associated with some risk.26 Several trials, including a recent prospective study, found prenatal antidepressant use associated with lower gestational age at birth and increased risk of preterm birth.27
- greater tremulousness
- less flexible and dampened state regulation
- more time in uninterrupted REM sleep
- more frequent startles or sudden arousals
- greater generalized motor activity
- greater autonomic dysregulation.28
- tremor (37/60)
- GI disturbances (34/60)—including exaggerated sucking, poor feeding, regurgitation, vomiting, and loose stools
- sleep disturbance (21/60).
Recommendations. The perception that SSRIs have low fetal toxicity has guided prescribing practices in recent years. Newer evidence shows, however, that fetal exposure to SSRIs may have some adverse effects, including lower birth weight and early delivery. First-trimester paroxetine use has been associated with increased risk for fetal ventricular and/or atrial septal defects.
Discuss these risks with the patient when you consider starting or continuing SSRI use during pregnancy.24 If you prescribe an SSRI, use the minimum effective dosage and avoid paroxetine during pregnancy.18
To reduce the risk for PPHN, early delivery, and neonatal withdrawal syndromes, taper and discontinue the SSRI during the third trimester. Restarting the SSRI soon after delivery is the most effective way to prevent recurrence of anxiety symptoms or postpartum depression.
Benzodiazepines
Teratogenicity. Like SSRIs, benzodiazepines cross the placenta to the fetus.29 Benzodiazepine teratogenicity remains controversial.8 Some—but not all—data show a small but significant increased risk for major malformations/oral cleft malformations with first-trimester benzodiazepine exposure.
A Medline literature search from 1966 to 2000 found not enough information to determine whether potential benefits of benzodiazepines to the mother outweigh risks to the fetus.29 An ambitious meta-analysis of >1,400 studies by Dolovich et al30 found a small association between fetal exposure to benzodiazepines and major malformations/cleft palate, but only in pooled data from case-controlled studies. No association was found between fetal exposure to benzodiazepines and malformations/cleft palate in pooled data from cohort studies.
A 32-month, hospital-based surveillance program of 28,565 births found no increase in the rate of major malformations in 43 infants exposed to clonazepam monotherapy—33 (77%) in the first trimester.31 Thus, the risk of major malformations/cleft palate with the use of benzodiazepines in the first trimester appears to be low.
- Neonatal toxicity (“floppy infant syndrome”)—characterized by hypothermia, lethargy, poor respiratory effort, and feeding difficulties—occurs after maternal benzodiazepine use just before delivery.8
- Neonatal withdrawal may be caused by very late, third trimester exposure to benzodiazepines. Symptoms—which can persist ≤3 months after delivery—include restlessness, irritability, abnormal sleep patterns, suckling difficulties, growth retardation, hypertonia, hyperreflexia, tremulousness, apnea, diarrhea, and vomiting.8,29
To minimize neonatal withdrawal, gradually taper the mother’s benzodiazepine before delivery.29 Because the baby’s due date is calculated to be ±2 weeks before delivery, begin this taper 3 to 4 weeks before the due date and discontinue at least 1 week before delivery. Breastfeeding while taking benzodiazepines is not recommended because of the risk of over-sedating the infant.
A rational approach
Both benzodiazepines and SSRIs are associated with low but demonstrated risks to the fetus when used during pregnancy (Table 2).19,20,23,25,30,33 Use these medications to manage a patient’s anxiety only if the clinical benefit to the mother justifies the potential risks to the fetus.29
A staggered combination of SSRIs during the first 2 trimesters and benzodiazepines during the last 2 trimesters can help balance the risks and benefits of pharmacotherapy of anxiety disorders during pregnancy (Table 3).
Frankly discuss with your patient the risks and benefits in the context of her perceived need for symptom control to sustain her level of functioning. You could document this discussion in the progress note as “R, B, A, and pt C,” signifying that risks, benefits, and alternatives were discussed, and the patient consented. If possible, include the patient’s husband, partner, or parent in this discussion.
Table 2
Risks of SSRIs vs benzodiazepines during pregnancy stages
| Pregnancy stage when given | Fetal risk | SSRIs | Benzodiazepines |
|---|---|---|---|
| First trimester* | Teratogenicity | Paroxetine use associated with 2-fold increased risk of major congenital anomalies and 3-fold increased risk of major cardiac anomalies;19 meta-analysis calculated significant risk of cardiac malformations (odds ratio 1.72; population prevalence = 13.4/1,000 births)20,33 | Meta-analysis of case control studies showed increased risk of major malformations/cleft palate (odds ratio 3.01; population prevalence = 10 to 20/1,000 births); no association seen in cohort studies30 |
| Third trimester | PPHN | Case control study showed 3.7% of infants with PPHN were exposed to SSRIs vs 0.7% of controls; adjusted odds ratio 6.1, absolute risk to exposed population = 6 to 12/1,000 births)23 | |
| Perinatal and long-term effects | Toxicity/withdrawal syndromes | Cohort study of 60 infants concluded prevalence of discontinuation syndromes is 30% in neonates with third trimester SSRI exposure25 | Neonatal toxicity (“floppy infant syndrome”) and neonatal withdrawal reported with maternal benzodiazepine use in late third trimester; prevalence unknown† |
| Preterm birth, serotonin withdrawal syndromes, CNS effects, long-term neurobehavioral effects | Unknown† | Unknown† | |
| PPHN: persistent pulmonary hypertension of the newborn; SSRIs: selective serotonin reuptake inhibitors | |||
| * Available data indicate that first-trimester exposure to SSRIs (other than paroxetine) and benzodiazepines may increase the relative risk for congenital anomalies, but the absolute risk of having a child with an anomaly is small. | |||
| † Some case reports, but published literature is insufficient to determine prevalence or magnitude of risk. | |||
Staggered, combination therapy for anxiety disorders during pregnancy
| Pregnancy stage | Recommended to manage risks to mother and fetus |
|---|---|
| First trimester |
|
| Second trimester |
|
| Third trimester |
|
| SSRI: selective serotonin reuptake inhibitor | |
| * Nondrug therapies can include prenatal exercise, sleep hygiene, relaxation, and psychotherapy (cognitive-behavioral therapy, interpersonal therapy, supportive therapy, family/couples therapy) | |
CASE CONTINUED: CBT plus medication
Ms. K and her husband are open to adding weekly cognitive-behavioral therapy (CBT) for anxiety as long as she can continue her medications. You discuss the evidence regarding potential neonatal risks with paroxetine and clonazepam treatment. Because Ms. K is 6 weeks pregnant, you outline a plan for a rapid cross-taper off paroxetine and onto fluoxetine, 10 to 30 mg/d, explaining that paroxetine might pose a greater first-trimester risk of major congenital malformations and cardiac malformations. You discuss possible side effects of fluoxetine and explain a plan to taper off fluoxetine during the third trimester to reduce the risk of PPHN, early delivery, and withdrawal in the newborn.
Because Ms. K has been taking clonazepam at only 0.5 mg 1 to 2 times per week, you instruct her to stop taking the benzodiazepine for the next 6 weeks until she is through her first trimester. You also reassure her that she can use clonazepam after the first trimester, if necessary, as long as she agrees to taper off completely 1 to 2 weeks before to her due date.
You refer her to a CBT therapist and emphasize the importance of CBT, relaxation, and sleep hygiene—as well as support from her husband, family, and friends—to reduce her stress and facilitate the medication taper during her third trimester. You plan to see her monthly and co-manage her care with the CBT therapist and Ob/Gyn. You document this discussion in her medical record as evidence of informed consent.
Related resources
- Baby Center. Managing stress and anxiety during pregnancy. Patient information. www.babycenter.com/0_managing-stress-and-anxiety-during-pregnancy_1683.bc.
- Organization of Teratology Information Specialists (OTIS). www.otispregnancy.org.
Drug brand names
- Clonazepam • Klonopin
- Paroxetine • Paxil
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, age 25, is 6 weeks pregnant and is taking medications for generalized anxiety disorder (GAD). When she was diagnosed with GAD at age 19, her symptoms included 6 months of excessive anxiety—insomnia, fatigue, difficulty with concentration, and psychomotor agitation—without mood symptoms. These symptoms interfered greatly with her schoolwork and other daily activities.
For 6 years Ms. K has been taking the selective serotonin reuptake inhibitor (SSRI) paroxetine, 15 mg/d, and the benzodiazepine clonazepam, 0.5 mg as needed, with good symptom control. Now that she is pregnant and her primary care doctor has refused to continue these medications, she is seeking treatment and advice.
Not enough is known about how to safely treat anxiety disorders during pregnancy, and physicians are not sure what to do with patients such as Ms. K. Without evidence-based guidelines, we feel anxious about potential risks to mother and fetus as we try to provide appropriate drug therapy.
To help you and your patients weigh the risks and benefits of perinatal treatments for anxiety disorders, this article briefly summarizes the evidence on:
- anxiety disorders’ natural history during pregnancy
- how untreated maternal anxiety affects the fetus
- nonpharmacologic therapies for anxiety disorders
- a plan to manage fetal risks by staggering SSRI and benzodiazepine use during the first and third trimesters.
Anxiety during pregnancy
Nearly one-third of women experience an anxiety disorder during their lives, with peak onset during childbearing years.1,2 Compared with research on perinatal depression, far fewer studies have examined anxiety disorders’ onset, presentation, prevalence, and treatment.1
The literature includes no studies of the course of preexisting GAD or posttraumatic stress disorder (PTSD) and no evidence that symptoms of preexisting obsessive-compulsive disorder (OCD) change during pregnancy. Some studies of panic disorder show symptoms improving during pregnancy, whereas others do not (Table 1).1
One small study done in late pregnancy found a significant association between the prevalence of an anxiety disorder, maternal primiparity, and comorbid medical conditions. Thus, a woman in her first pregnancy may be at increased risk to develop an anxiety disorder if she has a comorbid medical condition.3 As in the case of Ms. K, however, continuation of preexisting anxiety appears more likely than onset of a new anxiety disorder during pregnancy.
Table 1
How pregnancy affects the course of 4 anxiety disorders
| Disorder | Prevalence | Effect |
|---|---|---|
| Generalized anxiety disorder (GAD) | 8.5% of women experience GAD during the third trimester, compared with a 5% prevalence in the general population | No studies have reported on the course of GAD in pregnant women with preexisting disorder |
| Obsessive-compulsive disorder (OCD) | 2% to 12% of OCD outpatients of childbearing age report onset during pregnancy | Preexisting OCD usually shows no change during pregnancy but may worsen postpartum |
| Panic disorder (PD) | 1.3% to 2% in pregnant women, compared with 1.5% to 3.5% in the general population | Panic symptoms in women with preexisting PD may improve during pregnancy and worsen postpartum |
| Posttraumatic stress disorder (PTSD) | 2.3% to 7.7% in pregnant women and 0% to 6.9% postpartum, compared with 1% to 14% in the community | No studies have reported on the course of PTSD in pregnant women with preexisting disorder |
| Source: References 1,2 | ||
Fetal risks from maternal anxiety
Fetal risk from severe maternal anxiety is not zero. Offspring born to high-anxiety mothers exhibit neurobehavioral differences compared with offspring of calmer mothers. Changes in high-anxiety mothers’ offspring include:
- altered EEG activation and vagal tone
- increased time in deep sleep and less time in active alert states
- lower performance on the Brazelton Neonatal Behavior Assessment Scale.4
Exposure to maternal high anxiety has been associated with mental developmental delays in infants and increased risk for behavioral and emotional problems in young children.7-10 Anxiety may not directly cause intrauterine growth retardation and preterm delivery, but it is significantly associated with prenatal tobacco, alcohol, and narcotics use—which predicts these and other negative neonatal outcomes.11
7,13
CASE CONTINUED: ‘Stay the course’
Ms. K worries that she could not tolerate recurrence of her anxiety symptoms and wishes to continue both medications. Her husband concurs, but they want to minimize potential risks to their baby. You discuss the options for treating anxiety symptoms during pregnancy, including medications, psychotherapy, and behavioral treatments.
Treatment decisions
Ideally you’ll begin treating anxiety disorders in women of childbearing age with preconception psychoeducation. Explaining the risks of medications if she were to become pregnant and asking about the contraception she is using are de rigueur. Psychotherapy is low risk to the fetus and is considered first choice for treating mild to moderate anxiety in women of childbearing age who plan to become pregnant (Box).1,14-17
No studies directly address the efficacy or outcome of any psychotherapy for anxiety in pregnancy. Even so:
- For mild to moderate anxiety, psychotherapy is the first-line treatment for pregnant women.
- Interpersonal psychotherapy (IPT) without medications can reduce depressive symptoms in pregnant women with depression.14
- Cognitive-behavioral therapy (CBT) without medications has shown efficacy for anxiety disorders in psychiatric populations.15,16
Because no evidence suggests that pregnant women require different psychotherapeutic recommendations than other psychiatric patients, consider a course of CBT that targets anxiety symptoms or IPT for a pregnant patient with an anxiety disorder.
Relaxation therapy also has shown efficacy in treating anxiety disorders. In a randomized controlled trial of 110 pregnant women with high-level anxiety, 7 weeks of applied relaxation training sessions was associated with significant reductions in low-weight births, cesarean sections, and instrumental extractions.16,17
Because poor marital relationships are consistent psychosocial predictors of anxiety during pregnancy and postpartum depression,1 recommend family or marital therapy when appropriate.
Because Ms. K wishes to continue taking paroxetine and clonazepam, what can you tell her about the risks and benefits of SSRIs and benzodiazepines during pregnancy?
SSRIs in pregnancy
Teratogenicity. Compared with benzodiazepines, SSRIs have been considered agents of choice for use during pregnancy because of a lower risk of teratogenic effects.15 Paroxetine, however, appears to pose a greater risk for teratogenicity than other SSRIs.
The overall rate of fetal malformations from SSRIs appears to be low, although most studies have examined only fluoxetine or paroxetine. Some studies have reported various malformations with fluoxetine or sertraline, but others have not. In Finland, a population-based study found no increase in rate of major congenital malformations in offspring of 1,782 women who filled prescriptions for SSRIs during pregnancy, compared with the general population rate of 1% to 3%.21
Neurobehavioral effects. SSRI exposure during fetal life has shown no long-term neurobehavioral effects. A blinded prospective study by Nulman et al22 found no differences in global IQ scores, language development, or behavioral development among children age ≤5 who were exposed in utero to fluoxetine (n=40) or a tricyclic antidepressant (n=46), compared with unexposed children of nondepressed mothers (n=36). Similarly, using reports from teachers and clinical measures of internalizing behaviors, Misri et al10 found no increase in depression, anxiety, or withdrawal in 4-year-olds with prenatal exposure to SSRIs (n=22), compared with nonexposed children (n=14).
Pulmonary hypertension. SSRI exposure in later pregnancy may increase the rate of persistent pulmonary hypertension of the newborn (PPHN), which occurs in 1 to 2 infants per 1,000 live births. PPHN showed a statistically significant association with late prenatal SSRI exposure (OR 6.1) in a study that controlled for maternal smoking, body mass index, and diabetes.23 PPHN occurred in approximately 1% of infants exposed to SSRIs in late pregnancy. PPHN rates were not affected by maternal depression/anxiety, non-SSRI antidepressant exposure throughout pregnancy, or SSRI exposure during early pregnancy only.
Toxicity and withdrawal syndromes. Infants of women who continue to take SSRIs just before delivery can develop toxicity or withdrawal syndromes. Occurrence of either syndrome depends on SSRI half-life, serum concentration, and the pharmacodynamics of other medications given during pregnancy and labor.24
Discontinuation syndromes can occur in SSRI-exposed neonates within a few hours or days after birth and last up to 1 month after delivery, depending on the infant’s susceptibility.25 Nearly two-thirds of suspected SSRI-induced neonatal withdrawal syndromes have been associated with paroxetine, although all SSRIs appear be associated with some risk.26 Several trials, including a recent prospective study, found prenatal antidepressant use associated with lower gestational age at birth and increased risk of preterm birth.27
- greater tremulousness
- less flexible and dampened state regulation
- more time in uninterrupted REM sleep
- more frequent startles or sudden arousals
- greater generalized motor activity
- greater autonomic dysregulation.28
- tremor (37/60)
- GI disturbances (34/60)—including exaggerated sucking, poor feeding, regurgitation, vomiting, and loose stools
- sleep disturbance (21/60).
Recommendations. The perception that SSRIs have low fetal toxicity has guided prescribing practices in recent years. Newer evidence shows, however, that fetal exposure to SSRIs may have some adverse effects, including lower birth weight and early delivery. First-trimester paroxetine use has been associated with increased risk for fetal ventricular and/or atrial septal defects.
Discuss these risks with the patient when you consider starting or continuing SSRI use during pregnancy.24 If you prescribe an SSRI, use the minimum effective dosage and avoid paroxetine during pregnancy.18
To reduce the risk for PPHN, early delivery, and neonatal withdrawal syndromes, taper and discontinue the SSRI during the third trimester. Restarting the SSRI soon after delivery is the most effective way to prevent recurrence of anxiety symptoms or postpartum depression.
Benzodiazepines
Teratogenicity. Like SSRIs, benzodiazepines cross the placenta to the fetus.29 Benzodiazepine teratogenicity remains controversial.8 Some—but not all—data show a small but significant increased risk for major malformations/oral cleft malformations with first-trimester benzodiazepine exposure.
A Medline literature search from 1966 to 2000 found not enough information to determine whether potential benefits of benzodiazepines to the mother outweigh risks to the fetus.29 An ambitious meta-analysis of >1,400 studies by Dolovich et al30 found a small association between fetal exposure to benzodiazepines and major malformations/cleft palate, but only in pooled data from case-controlled studies. No association was found between fetal exposure to benzodiazepines and malformations/cleft palate in pooled data from cohort studies.
A 32-month, hospital-based surveillance program of 28,565 births found no increase in the rate of major malformations in 43 infants exposed to clonazepam monotherapy—33 (77%) in the first trimester.31 Thus, the risk of major malformations/cleft palate with the use of benzodiazepines in the first trimester appears to be low.
- Neonatal toxicity (“floppy infant syndrome”)—characterized by hypothermia, lethargy, poor respiratory effort, and feeding difficulties—occurs after maternal benzodiazepine use just before delivery.8
- Neonatal withdrawal may be caused by very late, third trimester exposure to benzodiazepines. Symptoms—which can persist ≤3 months after delivery—include restlessness, irritability, abnormal sleep patterns, suckling difficulties, growth retardation, hypertonia, hyperreflexia, tremulousness, apnea, diarrhea, and vomiting.8,29
To minimize neonatal withdrawal, gradually taper the mother’s benzodiazepine before delivery.29 Because the baby’s due date is calculated to be ±2 weeks before delivery, begin this taper 3 to 4 weeks before the due date and discontinue at least 1 week before delivery. Breastfeeding while taking benzodiazepines is not recommended because of the risk of over-sedating the infant.
A rational approach
Both benzodiazepines and SSRIs are associated with low but demonstrated risks to the fetus when used during pregnancy (Table 2).19,20,23,25,30,33 Use these medications to manage a patient’s anxiety only if the clinical benefit to the mother justifies the potential risks to the fetus.29
A staggered combination of SSRIs during the first 2 trimesters and benzodiazepines during the last 2 trimesters can help balance the risks and benefits of pharmacotherapy of anxiety disorders during pregnancy (Table 3).
Frankly discuss with your patient the risks and benefits in the context of her perceived need for symptom control to sustain her level of functioning. You could document this discussion in the progress note as “R, B, A, and pt C,” signifying that risks, benefits, and alternatives were discussed, and the patient consented. If possible, include the patient’s husband, partner, or parent in this discussion.
Table 2
Risks of SSRIs vs benzodiazepines during pregnancy stages
| Pregnancy stage when given | Fetal risk | SSRIs | Benzodiazepines |
|---|---|---|---|
| First trimester* | Teratogenicity | Paroxetine use associated with 2-fold increased risk of major congenital anomalies and 3-fold increased risk of major cardiac anomalies;19 meta-analysis calculated significant risk of cardiac malformations (odds ratio 1.72; population prevalence = 13.4/1,000 births)20,33 | Meta-analysis of case control studies showed increased risk of major malformations/cleft palate (odds ratio 3.01; population prevalence = 10 to 20/1,000 births); no association seen in cohort studies30 |
| Third trimester | PPHN | Case control study showed 3.7% of infants with PPHN were exposed to SSRIs vs 0.7% of controls; adjusted odds ratio 6.1, absolute risk to exposed population = 6 to 12/1,000 births)23 | |
| Perinatal and long-term effects | Toxicity/withdrawal syndromes | Cohort study of 60 infants concluded prevalence of discontinuation syndromes is 30% in neonates with third trimester SSRI exposure25 | Neonatal toxicity (“floppy infant syndrome”) and neonatal withdrawal reported with maternal benzodiazepine use in late third trimester; prevalence unknown† |
| Preterm birth, serotonin withdrawal syndromes, CNS effects, long-term neurobehavioral effects | Unknown† | Unknown† | |
| PPHN: persistent pulmonary hypertension of the newborn; SSRIs: selective serotonin reuptake inhibitors | |||
| * Available data indicate that first-trimester exposure to SSRIs (other than paroxetine) and benzodiazepines may increase the relative risk for congenital anomalies, but the absolute risk of having a child with an anomaly is small. | |||
| † Some case reports, but published literature is insufficient to determine prevalence or magnitude of risk. | |||
Staggered, combination therapy for anxiety disorders during pregnancy
| Pregnancy stage | Recommended to manage risks to mother and fetus |
|---|---|
| First trimester |
|
| Second trimester |
|
| Third trimester |
|
| SSRI: selective serotonin reuptake inhibitor | |
| * Nondrug therapies can include prenatal exercise, sleep hygiene, relaxation, and psychotherapy (cognitive-behavioral therapy, interpersonal therapy, supportive therapy, family/couples therapy) | |
CASE CONTINUED: CBT plus medication
Ms. K and her husband are open to adding weekly cognitive-behavioral therapy (CBT) for anxiety as long as she can continue her medications. You discuss the evidence regarding potential neonatal risks with paroxetine and clonazepam treatment. Because Ms. K is 6 weeks pregnant, you outline a plan for a rapid cross-taper off paroxetine and onto fluoxetine, 10 to 30 mg/d, explaining that paroxetine might pose a greater first-trimester risk of major congenital malformations and cardiac malformations. You discuss possible side effects of fluoxetine and explain a plan to taper off fluoxetine during the third trimester to reduce the risk of PPHN, early delivery, and withdrawal in the newborn.
Because Ms. K has been taking clonazepam at only 0.5 mg 1 to 2 times per week, you instruct her to stop taking the benzodiazepine for the next 6 weeks until she is through her first trimester. You also reassure her that she can use clonazepam after the first trimester, if necessary, as long as she agrees to taper off completely 1 to 2 weeks before to her due date.
You refer her to a CBT therapist and emphasize the importance of CBT, relaxation, and sleep hygiene—as well as support from her husband, family, and friends—to reduce her stress and facilitate the medication taper during her third trimester. You plan to see her monthly and co-manage her care with the CBT therapist and Ob/Gyn. You document this discussion in her medical record as evidence of informed consent.
Related resources
- Baby Center. Managing stress and anxiety during pregnancy. Patient information. www.babycenter.com/0_managing-stress-and-anxiety-during-pregnancy_1683.bc.
- Organization of Teratology Information Specialists (OTIS). www.otispregnancy.org.
Drug brand names
- Clonazepam • Klonopin
- Paroxetine • Paxil
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry 2006;67(8):1285-98.
2. Labad J, Menchon JM, Alonso P, et al. Female reproductive cycle and obsessive-compulsive disorder. J Clin Psychiatry 2005;66(4):428-35.
3. Adewuya AO, Ola BA, Aloba OO, Mapayi BM. Anxiety disorders among Nigerian women in late pregnancy: a controlled study. Arch Womens Ment Health 2006;9(6):325-8.
4. Field T, Hernandez-Reif M, Diego M, et al. Stability of mood states and biochemistry across pregnancy. Infant Behav Dev 2006;29(2):262-7.
5. Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318(7177):153-7.
6. Monk C, Myers MM, Sloan RP, et al. Effects of women’s stress-elicited physiological activity and chronic anxiety on fetal heart rate. J Dev Behav Pediatr 2003;24(1):32-8.
7. Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology 2007;32(1):1-13.
8. Levey L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22(4):863-93.
9. Oberlander TF, Reebye P, Misri S, et al. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007;161(1):22-9.
10. Misri S, Reebye P, Kendrick K, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006;163(6):1026-32.
11. Copper RL, Goldenberg RL, Das A, et al. The Preterm Prediction Study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. Am J Obstet Gynecol 1996;175(5):1286-92.
12. Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. Eur Psychiatry 2004;19(8):459-63.
13. Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med 2006;68(6):931-7.
14. Weissman MM. Recent non-medication trials of interpersonal psychotherapy for depression. Int J Neuropsychopharmacology 2007;10(1):117-22.
15. Ward RK, Zamorski MA. Benefits and risks of psychiatric medications during pregnancy. Am Fam Physician 2002;66(4):629-36.
16. Bastani F, Hidarnia A, Montgomery KS, et al. Does relaxation education in anxious primigravid Iranian women influence adverse pregnancy outcomes? A randomized controlled trial. J Perinat Neonatal Nurs 2006;20(2):138-46.
17. Fricchione G. Generalized anxiety disorder. N Engl J Med 2004;351(7):675-82.
18. Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol 2007;79(4):301-8.
19. Berard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 2007;80(1):18-27.
20. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther 2005;29(5):918-26.
21. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 2005;106(6):1289-96.
22. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159(11):1889-95.
23. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
24. Haddad PM, Pal BR, Clarke P, et al. Neonatal symptoms following maternal paroxetine treatment: serotonin toxicity or paroxetine discontinuation syndrome? J Psychopharmacology 2005;19(5):554-7.
25. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med 2006;160(2):173-6.
26. Sanz EJ, De-las-Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005;365(9458):482-7.
27. Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007;164(8):1206-13.
28. Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113(2):368-75.
29. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv 2002;53(1):39-49.
30. Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998;317(7162):839-43.
31. McElhatton PR. The effects of benzodiazepine use during pregnancy. Reprod Toxicol 1994;8(6):461-75.
32. Lin AE, Peller AJ, Westgate MN, et al. Clonazepam use in pregnancy and the risk of malformations. Birth Defects Res A Clin Mol Teratol 2004;70(8):534-6.
33. Levy M, James MS, Erickson JD, McClearn AB. Prevalence of birth defects. Birth outcomes Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/reproductivehealth/Products&Pubs/DatatoAction/pdf/birout4.pdf. Accessed January 9, 2008.
1. Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry 2006;67(8):1285-98.
2. Labad J, Menchon JM, Alonso P, et al. Female reproductive cycle and obsessive-compulsive disorder. J Clin Psychiatry 2005;66(4):428-35.
3. Adewuya AO, Ola BA, Aloba OO, Mapayi BM. Anxiety disorders among Nigerian women in late pregnancy: a controlled study. Arch Womens Ment Health 2006;9(6):325-8.
4. Field T, Hernandez-Reif M, Diego M, et al. Stability of mood states and biochemistry across pregnancy. Infant Behav Dev 2006;29(2):262-7.
5. Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318(7177):153-7.
6. Monk C, Myers MM, Sloan RP, et al. Effects of women’s stress-elicited physiological activity and chronic anxiety on fetal heart rate. J Dev Behav Pediatr 2003;24(1):32-8.
7. Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology 2007;32(1):1-13.
8. Levey L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22(4):863-93.
9. Oberlander TF, Reebye P, Misri S, et al. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007;161(1):22-9.
10. Misri S, Reebye P, Kendrick K, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006;163(6):1026-32.
11. Copper RL, Goldenberg RL, Das A, et al. The Preterm Prediction Study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. Am J Obstet Gynecol 1996;175(5):1286-92.
12. Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. Eur Psychiatry 2004;19(8):459-63.
13. Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med 2006;68(6):931-7.
14. Weissman MM. Recent non-medication trials of interpersonal psychotherapy for depression. Int J Neuropsychopharmacology 2007;10(1):117-22.
15. Ward RK, Zamorski MA. Benefits and risks of psychiatric medications during pregnancy. Am Fam Physician 2002;66(4):629-36.
16. Bastani F, Hidarnia A, Montgomery KS, et al. Does relaxation education in anxious primigravid Iranian women influence adverse pregnancy outcomes? A randomized controlled trial. J Perinat Neonatal Nurs 2006;20(2):138-46.
17. Fricchione G. Generalized anxiety disorder. N Engl J Med 2004;351(7):675-82.
18. Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol 2007;79(4):301-8.
19. Berard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 2007;80(1):18-27.
20. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther 2005;29(5):918-26.
21. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 2005;106(6):1289-96.
22. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159(11):1889-95.
23. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
24. Haddad PM, Pal BR, Clarke P, et al. Neonatal symptoms following maternal paroxetine treatment: serotonin toxicity or paroxetine discontinuation syndrome? J Psychopharmacology 2005;19(5):554-7.
25. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med 2006;160(2):173-6.
26. Sanz EJ, De-las-Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005;365(9458):482-7.
27. Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007;164(8):1206-13.
28. Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113(2):368-75.
29. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv 2002;53(1):39-49.
30. Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998;317(7162):839-43.
31. McElhatton PR. The effects of benzodiazepine use during pregnancy. Reprod Toxicol 1994;8(6):461-75.
32. Lin AE, Peller AJ, Westgate MN, et al. Clonazepam use in pregnancy and the risk of malformations. Birth Defects Res A Clin Mol Teratol 2004;70(8):534-6.
33. Levy M, James MS, Erickson JD, McClearn AB. Prevalence of birth defects. Birth outcomes Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/reproductivehealth/Products&Pubs/DatatoAction/pdf/birout4.pdf. Accessed January 9, 2008.
Teen girl brain: High drama, high risk for depression
Kate, age 14, is referred for follow-up treatment of depression after she impulsively swallowed a bottle of acetaminophen. She says she is in academic trouble and has no friends. Kate describes her childhood as mostly happy except for her parents’ arguments. Her medical history indicates she began developing breasts at age 10 and had her first menstrual period at age 12.
Her father is largely absent, traveling and working long hours. Her mother developed postpartum depression and stopped working after Kate’s younger brother was born.
Girls and boys show similar depression risks during childhood, but girls are twice as likely as boys to become clinically depressed after puberty. The key to treating depression in teen girls is to recognize that brain development and fluctuating hormones can influence behavior in ways that confuse them and the people around them. Successfully treating teen girls’ depression may require a gender-specific approach.
3 stages of brain development
Fetal differentiation. All brains start out with female-type brain circuits. At 8 weeks of fetal life, however, tiny testicles in the male begin to produce large amounts of testosterone, which changes the brain and body to male. Thus, sex-specific genes and hormones guide aspects of the first phase of brain development.1
Table 1
Female hormonal development: Gestation to puberty
| Stage/age | Hormonal events | Effect on female brain |
|---|---|---|
| Gestation | Components of reproductive axis form in early embryonic development; at 8 weeks, testosterone from fetal testicles begins to change female-type brain areas to male | Unperturbed by testosterone, brain continues to develop along female lines |
| Birth to age 24 months | Hormone-secreting placenta detaches at birth, dramatically increasing GnRH and LH/FSH and driving infant gonads to produce estrogen in girls or testosterone in boys (“infantile puberty”) | Abundant ovarian estrogen secretion enhances development of brain circuits, such as those associated with reproduction, maternal behavior, and social relatedness |
| Age 24 months to prepuberty | “Brakes” put on GnRH and LH/FSH pulsatile brain cells | “Juvenile pause” begins, with constant low estrogen secretion in girls by 24 months (in boys, “brakes” are on by 12 months) |
| Puberty | “Brakes” released on GnRH and LH/FSH neurons, reactivating reproductive axis | Ovary resumes estrogen production (“adolescent puberty”); increase in estrogen, progesterone, and testosterone stimulates brain circuit development; unipolar depression rates increase to 2:1 (female to male) by age 15 |
| GnRH: gonadotropin-releasing hormone; LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Source: References 4,5 | ||
Infantile puberty and the second phase of brain development begin in early childhood, as the ovaries and testicles start to produce large amounts of estrogen and testosterone soon after birth.
Puberty launches the final brain development phase. Up to 2 years before menstruation begins, pulsatile gonadotropin-releasing hormone cells in the hypothalamus wake up and start stimulating the ovaries to produce estrogen, thrusting the girl brain into puberty (Figure). The teen girl brain begins to experience not only estrogen surges from the ovary but progesterone and testosterone surges as well.
Although brain size and basic circuitry are mostly set by age 5, puberty stimulates new brain cells and increases myelin production.2 Faster myelinated connections between emotionally impulsive limbic brain areas such as the amygdala and sensible, cognitive areas such as the prefrontal cortex are not finished until the early 20s.3
Hormonal changes at puberty
The female brain is remodeled during puberty, leading to sexually dimorphic brain activation and development that further differentiates it from the male brain.4
Estrogen surges are associated with increased production of neurohormones and neurochemicals, such as:
- oxytocin, which reinforces social bonding and intimacy
- dopamine, which stimulates motivation and pleasure circuits in the brain.
Hormonal changes and brain development alter gene expression and affect neurodevelopment. These events may trigger a first depression in pubertal girls with a family history of mood disorder (Table 1).4,5 Although menarche has begun at an average age of 12 in the United States for decades, the most recent National Health and Examination Survey (NHANES) shows puberty onset in girls is occurring earlier (Table 2).6-9
Tanner stage—a measure of pubertal status—is a more accurate predictor of depression in teen girls than age.10 Pubertal transition to Tanner stage 3 (development of pubic and axillary hair and breast buds) is associated with a sharp increase in depression rates. Girls at stage 3 and higher are approximately 3 times more likely to be depressed than girls at stages 1 or 2.11
Pubic hair, breast development, and menstruation are markers for underlying hormonal changes (Table 3).4,5 The onset of estrogen, progesterone, and testosterone surges closely correlates with the difference in depression rates between pre- and postpubertal girls.12 After estrogen and progesterone surges begin at puberty, negative emotions exert an increased activating effect on the female brain,13 and social stressors more deeply affect girls than they do boys. This may explain why girls are more susceptible to depression when a friendship fails.14
CASE CONTINUED: Boy troubles
Kate tells you that in 9th grade she and her best friend, Ellen, would talk about boys for hours after school and try on sexually provocative outfits. They both liked Matt, a 10th grader, so when he asked Kate out, Ellen stopped speaking to her. Kate and Matt began some heavy petting, and Kate said she felt selfish and guilty about hurting Ellen. But when girls at school began spreading rumors that Kate was a “slut,” Kate blamed Ellen and told her, “I hate you!”
Soon after, Matt broke up with Kate. Distraught, she dreaded going to school and cried in her room at night for several weeks. She became chronically tired and had difficulty concentrating in class. She ruminated about losing Matt and worried that she was too fat, too ugly, or too flat-chested. She missed Ellen and felt no one liked her.
Table 2
Puberty’s developmental milestones in U.S. girls (averages)
| Correlate | African Americans | Whites | School grade* |
|---|---|---|---|
| Breast bud development | Age 9 | Age 10 | 4th to 5th |
| Girls with puberty onset by age 8 | 32% | 11% | 3rd |
| Girls with puberty onset by age 10 | 76% | 53% | 5th |
| Menarche onset | Age 12.1 | Age 12.6 | 7th |
| Tanner stage 5† onset | Age 13.9 | Age 15.5 | 8th to 9th |
| * Approximate grade level for age groups | |||
| † Pubic hair and breast development reach adult stage | |||
| Source: Data from references 6-9, including the Pediatric Research in Office Settings network and Third National Health and Nutrition Examination Survey, 1988-1994. | |||
Figure Hypothalamic-pituitary-ovarian axis: Turned on at puberty in girls
Puberty onset stimulates depression in genetically vulnerable girls; more likely after Tanner stage 3 (development of pubic and axillary hair and breast buds).
Male vs female teen brains
Depression after a relationship failure in teen girls often begins with ruminative thoughts about her flaws, mistakes, or appearance. These negative thoughts may preoccupy her day and night. Teen girls often feel confused by contradictory social pressures to look and dress provocatively but resist having sex. A sexual encounter can trigger shame and fear.
Although clinical and developmental studies indicate that teen girls respond more dramatically to relationship troubles than boys, the brain and hormone differences responsible for these effects remain unclear. Male hormones hugely increase in boys at puberty—up to 25-fold between ages 9 and 15—but do not cycle. Male brains do not have the same capacity as female brains to respond to cyclical hormonal activity because exposure to androgens during fetal development eliminates this ability. The fetal testosterone surge causes the area associated with sexual pursuit to double in the male brain.
Outside of fertility considerations, Baron-Cohen et al15 suggest that male brain circuits have been formed by fetal testosterone to focus more on systematization—which emphasizes figuring out how things work and performing tasks—rather than empathy and bonding in relationships. This difference has been shown in neuroimaging studies comparing the genders’ attentional systems.16,17 In contrast to the systematizing male brain, female brains are more likely to activate the mirror neuron system—the area required for empathizing.18
Female brains, of course, respond to cyclical hormonal activity. However, the regular monthly waves of estrogen and progesterone do not affect all female brains the same. A subset of women who experience premenstrual dysphoric disorder appear to have brains that trigger depressed moods and irritability during the last 2 weeks of the menstrual cycle.19 A genetic difference in these women is suspected as the culprit; these genes may affect the way their brains metabolize progesterone.
CASE CONTINUED: An overdose of stress
Kate’s poor concentration lingered, and her grades continued to drop. She tells you her parents were having marital problems and she did not want to bother them with her difficulties. Two days before her period was due, she learned she had failed 2 classes. That night, as she got some acetaminophen for a headache, she impulsively took the rest of the bottle.
After swallowing the pills, Kate panicked. She forced herself to vomit and tearfully told her parents what she had done. They took her to the emergency room, where she was medically stabilized, evaluated by a psychiatrist, and referred to you for outpatient treatment.
Treatment recommendations
A combination of factors—genetic, hormonal, and neurodevelopmental—probably contributed to Kate’s acute depressed mood and overdose. Thus, to treat depression in adolescent girls, emerging evidence supports:
- stabilizing hormonal fluctuations such as rapidly falling progesterone just before the start of menstrual periods with an extended-cycle contraceptive (we would try an ethinyl estradiol/levonorgestrel combination such as Seasonale®)
- treating depressive symptoms with a selective serotonin reuptake inhibitor such as citalopram, 10 mg once daily, with careful monitoring for suicidal thoughts or behavior
- providing tools to manage stress and impulsive behavior through weekly psychotherapy (such as cognitive-behavioral therapy, dialectical behavioral therapy, or supportive therapy).
Genetic factors. Kate’s mother’s history of postpartum depression suggests genetic risk for Kate. Studies have found that the expression of particular genes—such as the serotonin transporter (5-HTT) gene—may be associated with depression. Staley et al20 found that depressed women show a significantly greater decrease in 5-HTT availability in the diencephalon (forebrain region containing the thalamus, hypothalamus, and part of the pituitary gland) when compared with healthy women and depressed men.
Table 3
3 stages of girls’ gonadal development
| Stage | Timing | Developmental events |
|---|---|---|
| Adrenarche | Onset around age 6, peaks by age 20 | Rise in weak androgens (DHEA and DHEAS) from adrenal gland results in pubic and axillary hair and increases likelihood of acne |
| Gonadarche | Usually ~2 years before menarche | Pulses of GnRH, LH/FSH lead to increased estrogen, which stimulates breast development, widening of hips, and increased subcutaneous fat deposition |
| Menarche | Relatively late in puberty (usually not before Tanner stage 4) | “Monthly” cycle established; ovarian estrogen pulses in response to GnRH and FSH, the LH surge, and ovulation; progesterone produced after ovulation |
| DHEA: dehydroepiandrosterone; DHEAS: dehydroepiandrosterone sulfate; GnRH: gonadotropin-releasing hormone; | ||
| LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Tanner stage 4: pubic hair and breast development typical of middle to late adolescence (ages 12 to 17) | ||
| Source: References 4,5 | ||
Although men and women have the same 5-HTT gene, women may possess a gender-specific factor—such as estrogen or progesterone—that differentially alters this and other genes’ expression in women with depression. Individuals who carry a short version of the gene may be at particular risk of becoming depressed when exposed to stressful life events.
Caspi et al21 found a polymorphism in the 5-HTT gene on chromosome 17 that can manifest differentially based on environmental factors. In this study, individuals with 2 copies of the long version of this gene were relatively resistant to stressful life events, whereas those with 1 or 2 copies of the short version were highly sensitive to stressful life events. The depression rate in short-gene individuals was:
- 9% in those who had not experienced stressful life events
- nearly 40% in those who had experienced ≥4 stressful life events.
Hormonal and stress factors. Stress responsiveness becomes sexually dimorphic at puberty. Compared with men, women are:
- at greater risk after puberty for heightened stress responsiveness, which is associated with major depressive disorder
- 3 times more likely to develop depression after a stressful life event.22
Women’s and men’s different biological responses to stress might be related to the gender-specific hormones that emerge during puberty. Kate could be at increased risk for depression—especially immediately before her period—if she inherited a stress-sensitive gene and now has increased stress sensitivity triggered by the hormones of puberty.23
Neurodevelopmental factors. Dorsolateral prefrontal cortex circuits associated with making good decisions and weighing the consequences of actions are immature in the adolescent and the last part of the brain to undergo myelination.24-26 Teens are well-known for erratic, emotionally driven behaviors.27,28 Kate’s impulsive overdose exemplifies the consequences of emotional reactivity without the benefit of inhibitory mature brain connections.
Related resources
- Brizendine L. Teen girl brain. In: The female brain. New York: Morgan Road Books; 2006:31-56. www.thefemalebrain.com.
- Strauch B. The primal teen: what discoveries about the teenage brain tell us about our kids. New York: Doubleday; 2003.
- Harter S. Self and identity development. In: Feldman S, Elliott G, eds. At the threshold: the developing adolescent. Cambridge, MA: Harvard University Press; 1990:352-87.
Drug brand names
- Ethinyl estradiol/levonorgestrel • Seasonale
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci 2004;5(9):701-8.
2. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6(4):551-60.
3. Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. In press.
4. Cameron J. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Ann NY Acad Sci 2004;1021:110-23.
5. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
6. Biro F, Huang B, Crawford P, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148(2):234-40.
7. Herman-Giddens M, Kaplowitz P, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics 2004;113(4):911-7.
8. Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997;99(4):505-12.
9. Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics 2002;110(4):752-7.
10. Rapkin A, Tsao J, Turk N, et al. Relationships among self-rated Tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol 2006;19:181-7.
11. Angold A, Costello E, Worthman C. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998;28:51-61.
12. Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med 1999;29:1043-53.
13. Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage 2006;32(2):854-62.
14. McClure EB, Parrish JM, Nelson EE, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. In press.
15. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358(1430):361-74.
16. Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 2005;28(3):618-26.
17. Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills 2004;99(2):371-91.
18. Cheng YW, Tzeng OJ, Decety J, et al. Gender differences in the human mirror system: a magnetoencephalography study. Neuroreport 2006;17(11):1115-9.
19. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and those without premenstrual syndrome. N Engl J Med 1998;338(4):209-16.
20. Staley J, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59:40-7.
21. Caspi A, Sugden K, Moffitt T, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9.
22. Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol Med 2001;31(4):593-604.
23. Seeman M. Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997;154(12):1641-7.
24. Kupfer D, Woodward H. Adolescent development and the regulation of behavior and emotion. Ann NY Acad Sci 2004;1021:320-2.
25. Kelley A, Schochet T, Landry C. Risk taking and novelty seeking in adolescence. Ann NY Acad Sci 2004;1021:27-32.
26. Ellis L, Rothbart M, Posner M. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Ann NY Acad Sci 2004;1021:337-40.
27. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
28. Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs 2003;5(4):243-65.
Kate, age 14, is referred for follow-up treatment of depression after she impulsively swallowed a bottle of acetaminophen. She says she is in academic trouble and has no friends. Kate describes her childhood as mostly happy except for her parents’ arguments. Her medical history indicates she began developing breasts at age 10 and had her first menstrual period at age 12.
Her father is largely absent, traveling and working long hours. Her mother developed postpartum depression and stopped working after Kate’s younger brother was born.
Girls and boys show similar depression risks during childhood, but girls are twice as likely as boys to become clinically depressed after puberty. The key to treating depression in teen girls is to recognize that brain development and fluctuating hormones can influence behavior in ways that confuse them and the people around them. Successfully treating teen girls’ depression may require a gender-specific approach.
3 stages of brain development
Fetal differentiation. All brains start out with female-type brain circuits. At 8 weeks of fetal life, however, tiny testicles in the male begin to produce large amounts of testosterone, which changes the brain and body to male. Thus, sex-specific genes and hormones guide aspects of the first phase of brain development.1
Table 1
Female hormonal development: Gestation to puberty
| Stage/age | Hormonal events | Effect on female brain |
|---|---|---|
| Gestation | Components of reproductive axis form in early embryonic development; at 8 weeks, testosterone from fetal testicles begins to change female-type brain areas to male | Unperturbed by testosterone, brain continues to develop along female lines |
| Birth to age 24 months | Hormone-secreting placenta detaches at birth, dramatically increasing GnRH and LH/FSH and driving infant gonads to produce estrogen in girls or testosterone in boys (“infantile puberty”) | Abundant ovarian estrogen secretion enhances development of brain circuits, such as those associated with reproduction, maternal behavior, and social relatedness |
| Age 24 months to prepuberty | “Brakes” put on GnRH and LH/FSH pulsatile brain cells | “Juvenile pause” begins, with constant low estrogen secretion in girls by 24 months (in boys, “brakes” are on by 12 months) |
| Puberty | “Brakes” released on GnRH and LH/FSH neurons, reactivating reproductive axis | Ovary resumes estrogen production (“adolescent puberty”); increase in estrogen, progesterone, and testosterone stimulates brain circuit development; unipolar depression rates increase to 2:1 (female to male) by age 15 |
| GnRH: gonadotropin-releasing hormone; LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Source: References 4,5 | ||
Infantile puberty and the second phase of brain development begin in early childhood, as the ovaries and testicles start to produce large amounts of estrogen and testosterone soon after birth.
Puberty launches the final brain development phase. Up to 2 years before menstruation begins, pulsatile gonadotropin-releasing hormone cells in the hypothalamus wake up and start stimulating the ovaries to produce estrogen, thrusting the girl brain into puberty (Figure). The teen girl brain begins to experience not only estrogen surges from the ovary but progesterone and testosterone surges as well.
Although brain size and basic circuitry are mostly set by age 5, puberty stimulates new brain cells and increases myelin production.2 Faster myelinated connections between emotionally impulsive limbic brain areas such as the amygdala and sensible, cognitive areas such as the prefrontal cortex are not finished until the early 20s.3
Hormonal changes at puberty
The female brain is remodeled during puberty, leading to sexually dimorphic brain activation and development that further differentiates it from the male brain.4
Estrogen surges are associated with increased production of neurohormones and neurochemicals, such as:
- oxytocin, which reinforces social bonding and intimacy
- dopamine, which stimulates motivation and pleasure circuits in the brain.
Hormonal changes and brain development alter gene expression and affect neurodevelopment. These events may trigger a first depression in pubertal girls with a family history of mood disorder (Table 1).4,5 Although menarche has begun at an average age of 12 in the United States for decades, the most recent National Health and Examination Survey (NHANES) shows puberty onset in girls is occurring earlier (Table 2).6-9
Tanner stage—a measure of pubertal status—is a more accurate predictor of depression in teen girls than age.10 Pubertal transition to Tanner stage 3 (development of pubic and axillary hair and breast buds) is associated with a sharp increase in depression rates. Girls at stage 3 and higher are approximately 3 times more likely to be depressed than girls at stages 1 or 2.11
Pubic hair, breast development, and menstruation are markers for underlying hormonal changes (Table 3).4,5 The onset of estrogen, progesterone, and testosterone surges closely correlates with the difference in depression rates between pre- and postpubertal girls.12 After estrogen and progesterone surges begin at puberty, negative emotions exert an increased activating effect on the female brain,13 and social stressors more deeply affect girls than they do boys. This may explain why girls are more susceptible to depression when a friendship fails.14
CASE CONTINUED: Boy troubles
Kate tells you that in 9th grade she and her best friend, Ellen, would talk about boys for hours after school and try on sexually provocative outfits. They both liked Matt, a 10th grader, so when he asked Kate out, Ellen stopped speaking to her. Kate and Matt began some heavy petting, and Kate said she felt selfish and guilty about hurting Ellen. But when girls at school began spreading rumors that Kate was a “slut,” Kate blamed Ellen and told her, “I hate you!”
Soon after, Matt broke up with Kate. Distraught, she dreaded going to school and cried in her room at night for several weeks. She became chronically tired and had difficulty concentrating in class. She ruminated about losing Matt and worried that she was too fat, too ugly, or too flat-chested. She missed Ellen and felt no one liked her.
Table 2
Puberty’s developmental milestones in U.S. girls (averages)
| Correlate | African Americans | Whites | School grade* |
|---|---|---|---|
| Breast bud development | Age 9 | Age 10 | 4th to 5th |
| Girls with puberty onset by age 8 | 32% | 11% | 3rd |
| Girls with puberty onset by age 10 | 76% | 53% | 5th |
| Menarche onset | Age 12.1 | Age 12.6 | 7th |
| Tanner stage 5† onset | Age 13.9 | Age 15.5 | 8th to 9th |
| * Approximate grade level for age groups | |||
| † Pubic hair and breast development reach adult stage | |||
| Source: Data from references 6-9, including the Pediatric Research in Office Settings network and Third National Health and Nutrition Examination Survey, 1988-1994. | |||
Figure Hypothalamic-pituitary-ovarian axis: Turned on at puberty in girls
Puberty onset stimulates depression in genetically vulnerable girls; more likely after Tanner stage 3 (development of pubic and axillary hair and breast buds).
Male vs female teen brains
Depression after a relationship failure in teen girls often begins with ruminative thoughts about her flaws, mistakes, or appearance. These negative thoughts may preoccupy her day and night. Teen girls often feel confused by contradictory social pressures to look and dress provocatively but resist having sex. A sexual encounter can trigger shame and fear.
Although clinical and developmental studies indicate that teen girls respond more dramatically to relationship troubles than boys, the brain and hormone differences responsible for these effects remain unclear. Male hormones hugely increase in boys at puberty—up to 25-fold between ages 9 and 15—but do not cycle. Male brains do not have the same capacity as female brains to respond to cyclical hormonal activity because exposure to androgens during fetal development eliminates this ability. The fetal testosterone surge causes the area associated with sexual pursuit to double in the male brain.
Outside of fertility considerations, Baron-Cohen et al15 suggest that male brain circuits have been formed by fetal testosterone to focus more on systematization—which emphasizes figuring out how things work and performing tasks—rather than empathy and bonding in relationships. This difference has been shown in neuroimaging studies comparing the genders’ attentional systems.16,17 In contrast to the systematizing male brain, female brains are more likely to activate the mirror neuron system—the area required for empathizing.18
Female brains, of course, respond to cyclical hormonal activity. However, the regular monthly waves of estrogen and progesterone do not affect all female brains the same. A subset of women who experience premenstrual dysphoric disorder appear to have brains that trigger depressed moods and irritability during the last 2 weeks of the menstrual cycle.19 A genetic difference in these women is suspected as the culprit; these genes may affect the way their brains metabolize progesterone.
CASE CONTINUED: An overdose of stress
Kate’s poor concentration lingered, and her grades continued to drop. She tells you her parents were having marital problems and she did not want to bother them with her difficulties. Two days before her period was due, she learned she had failed 2 classes. That night, as she got some acetaminophen for a headache, she impulsively took the rest of the bottle.
After swallowing the pills, Kate panicked. She forced herself to vomit and tearfully told her parents what she had done. They took her to the emergency room, where she was medically stabilized, evaluated by a psychiatrist, and referred to you for outpatient treatment.
Treatment recommendations
A combination of factors—genetic, hormonal, and neurodevelopmental—probably contributed to Kate’s acute depressed mood and overdose. Thus, to treat depression in adolescent girls, emerging evidence supports:
- stabilizing hormonal fluctuations such as rapidly falling progesterone just before the start of menstrual periods with an extended-cycle contraceptive (we would try an ethinyl estradiol/levonorgestrel combination such as Seasonale®)
- treating depressive symptoms with a selective serotonin reuptake inhibitor such as citalopram, 10 mg once daily, with careful monitoring for suicidal thoughts or behavior
- providing tools to manage stress and impulsive behavior through weekly psychotherapy (such as cognitive-behavioral therapy, dialectical behavioral therapy, or supportive therapy).
Genetic factors. Kate’s mother’s history of postpartum depression suggests genetic risk for Kate. Studies have found that the expression of particular genes—such as the serotonin transporter (5-HTT) gene—may be associated with depression. Staley et al20 found that depressed women show a significantly greater decrease in 5-HTT availability in the diencephalon (forebrain region containing the thalamus, hypothalamus, and part of the pituitary gland) when compared with healthy women and depressed men.
Table 3
3 stages of girls’ gonadal development
| Stage | Timing | Developmental events |
|---|---|---|
| Adrenarche | Onset around age 6, peaks by age 20 | Rise in weak androgens (DHEA and DHEAS) from adrenal gland results in pubic and axillary hair and increases likelihood of acne |
| Gonadarche | Usually ~2 years before menarche | Pulses of GnRH, LH/FSH lead to increased estrogen, which stimulates breast development, widening of hips, and increased subcutaneous fat deposition |
| Menarche | Relatively late in puberty (usually not before Tanner stage 4) | “Monthly” cycle established; ovarian estrogen pulses in response to GnRH and FSH, the LH surge, and ovulation; progesterone produced after ovulation |
| DHEA: dehydroepiandrosterone; DHEAS: dehydroepiandrosterone sulfate; GnRH: gonadotropin-releasing hormone; | ||
| LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Tanner stage 4: pubic hair and breast development typical of middle to late adolescence (ages 12 to 17) | ||
| Source: References 4,5 | ||
Although men and women have the same 5-HTT gene, women may possess a gender-specific factor—such as estrogen or progesterone—that differentially alters this and other genes’ expression in women with depression. Individuals who carry a short version of the gene may be at particular risk of becoming depressed when exposed to stressful life events.
Caspi et al21 found a polymorphism in the 5-HTT gene on chromosome 17 that can manifest differentially based on environmental factors. In this study, individuals with 2 copies of the long version of this gene were relatively resistant to stressful life events, whereas those with 1 or 2 copies of the short version were highly sensitive to stressful life events. The depression rate in short-gene individuals was:
- 9% in those who had not experienced stressful life events
- nearly 40% in those who had experienced ≥4 stressful life events.
Hormonal and stress factors. Stress responsiveness becomes sexually dimorphic at puberty. Compared with men, women are:
- at greater risk after puberty for heightened stress responsiveness, which is associated with major depressive disorder
- 3 times more likely to develop depression after a stressful life event.22
Women’s and men’s different biological responses to stress might be related to the gender-specific hormones that emerge during puberty. Kate could be at increased risk for depression—especially immediately before her period—if she inherited a stress-sensitive gene and now has increased stress sensitivity triggered by the hormones of puberty.23
Neurodevelopmental factors. Dorsolateral prefrontal cortex circuits associated with making good decisions and weighing the consequences of actions are immature in the adolescent and the last part of the brain to undergo myelination.24-26 Teens are well-known for erratic, emotionally driven behaviors.27,28 Kate’s impulsive overdose exemplifies the consequences of emotional reactivity without the benefit of inhibitory mature brain connections.
Related resources
- Brizendine L. Teen girl brain. In: The female brain. New York: Morgan Road Books; 2006:31-56. www.thefemalebrain.com.
- Strauch B. The primal teen: what discoveries about the teenage brain tell us about our kids. New York: Doubleday; 2003.
- Harter S. Self and identity development. In: Feldman S, Elliott G, eds. At the threshold: the developing adolescent. Cambridge, MA: Harvard University Press; 1990:352-87.
Drug brand names
- Ethinyl estradiol/levonorgestrel • Seasonale
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Kate, age 14, is referred for follow-up treatment of depression after she impulsively swallowed a bottle of acetaminophen. She says she is in academic trouble and has no friends. Kate describes her childhood as mostly happy except for her parents’ arguments. Her medical history indicates she began developing breasts at age 10 and had her first menstrual period at age 12.
Her father is largely absent, traveling and working long hours. Her mother developed postpartum depression and stopped working after Kate’s younger brother was born.
Girls and boys show similar depression risks during childhood, but girls are twice as likely as boys to become clinically depressed after puberty. The key to treating depression in teen girls is to recognize that brain development and fluctuating hormones can influence behavior in ways that confuse them and the people around them. Successfully treating teen girls’ depression may require a gender-specific approach.
3 stages of brain development
Fetal differentiation. All brains start out with female-type brain circuits. At 8 weeks of fetal life, however, tiny testicles in the male begin to produce large amounts of testosterone, which changes the brain and body to male. Thus, sex-specific genes and hormones guide aspects of the first phase of brain development.1
Table 1
Female hormonal development: Gestation to puberty
| Stage/age | Hormonal events | Effect on female brain |
|---|---|---|
| Gestation | Components of reproductive axis form in early embryonic development; at 8 weeks, testosterone from fetal testicles begins to change female-type brain areas to male | Unperturbed by testosterone, brain continues to develop along female lines |
| Birth to age 24 months | Hormone-secreting placenta detaches at birth, dramatically increasing GnRH and LH/FSH and driving infant gonads to produce estrogen in girls or testosterone in boys (“infantile puberty”) | Abundant ovarian estrogen secretion enhances development of brain circuits, such as those associated with reproduction, maternal behavior, and social relatedness |
| Age 24 months to prepuberty | “Brakes” put on GnRH and LH/FSH pulsatile brain cells | “Juvenile pause” begins, with constant low estrogen secretion in girls by 24 months (in boys, “brakes” are on by 12 months) |
| Puberty | “Brakes” released on GnRH and LH/FSH neurons, reactivating reproductive axis | Ovary resumes estrogen production (“adolescent puberty”); increase in estrogen, progesterone, and testosterone stimulates brain circuit development; unipolar depression rates increase to 2:1 (female to male) by age 15 |
| GnRH: gonadotropin-releasing hormone; LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Source: References 4,5 | ||
Infantile puberty and the second phase of brain development begin in early childhood, as the ovaries and testicles start to produce large amounts of estrogen and testosterone soon after birth.
Puberty launches the final brain development phase. Up to 2 years before menstruation begins, pulsatile gonadotropin-releasing hormone cells in the hypothalamus wake up and start stimulating the ovaries to produce estrogen, thrusting the girl brain into puberty (Figure). The teen girl brain begins to experience not only estrogen surges from the ovary but progesterone and testosterone surges as well.
Although brain size and basic circuitry are mostly set by age 5, puberty stimulates new brain cells and increases myelin production.2 Faster myelinated connections between emotionally impulsive limbic brain areas such as the amygdala and sensible, cognitive areas such as the prefrontal cortex are not finished until the early 20s.3
Hormonal changes at puberty
The female brain is remodeled during puberty, leading to sexually dimorphic brain activation and development that further differentiates it from the male brain.4
Estrogen surges are associated with increased production of neurohormones and neurochemicals, such as:
- oxytocin, which reinforces social bonding and intimacy
- dopamine, which stimulates motivation and pleasure circuits in the brain.
Hormonal changes and brain development alter gene expression and affect neurodevelopment. These events may trigger a first depression in pubertal girls with a family history of mood disorder (Table 1).4,5 Although menarche has begun at an average age of 12 in the United States for decades, the most recent National Health and Examination Survey (NHANES) shows puberty onset in girls is occurring earlier (Table 2).6-9
Tanner stage—a measure of pubertal status—is a more accurate predictor of depression in teen girls than age.10 Pubertal transition to Tanner stage 3 (development of pubic and axillary hair and breast buds) is associated with a sharp increase in depression rates. Girls at stage 3 and higher are approximately 3 times more likely to be depressed than girls at stages 1 or 2.11
Pubic hair, breast development, and menstruation are markers for underlying hormonal changes (Table 3).4,5 The onset of estrogen, progesterone, and testosterone surges closely correlates with the difference in depression rates between pre- and postpubertal girls.12 After estrogen and progesterone surges begin at puberty, negative emotions exert an increased activating effect on the female brain,13 and social stressors more deeply affect girls than they do boys. This may explain why girls are more susceptible to depression when a friendship fails.14
CASE CONTINUED: Boy troubles
Kate tells you that in 9th grade she and her best friend, Ellen, would talk about boys for hours after school and try on sexually provocative outfits. They both liked Matt, a 10th grader, so when he asked Kate out, Ellen stopped speaking to her. Kate and Matt began some heavy petting, and Kate said she felt selfish and guilty about hurting Ellen. But when girls at school began spreading rumors that Kate was a “slut,” Kate blamed Ellen and told her, “I hate you!”
Soon after, Matt broke up with Kate. Distraught, she dreaded going to school and cried in her room at night for several weeks. She became chronically tired and had difficulty concentrating in class. She ruminated about losing Matt and worried that she was too fat, too ugly, or too flat-chested. She missed Ellen and felt no one liked her.
Table 2
Puberty’s developmental milestones in U.S. girls (averages)
| Correlate | African Americans | Whites | School grade* |
|---|---|---|---|
| Breast bud development | Age 9 | Age 10 | 4th to 5th |
| Girls with puberty onset by age 8 | 32% | 11% | 3rd |
| Girls with puberty onset by age 10 | 76% | 53% | 5th |
| Menarche onset | Age 12.1 | Age 12.6 | 7th |
| Tanner stage 5† onset | Age 13.9 | Age 15.5 | 8th to 9th |
| * Approximate grade level for age groups | |||
| † Pubic hair and breast development reach adult stage | |||
| Source: Data from references 6-9, including the Pediatric Research in Office Settings network and Third National Health and Nutrition Examination Survey, 1988-1994. | |||
Figure Hypothalamic-pituitary-ovarian axis: Turned on at puberty in girls
Puberty onset stimulates depression in genetically vulnerable girls; more likely after Tanner stage 3 (development of pubic and axillary hair and breast buds).
Male vs female teen brains
Depression after a relationship failure in teen girls often begins with ruminative thoughts about her flaws, mistakes, or appearance. These negative thoughts may preoccupy her day and night. Teen girls often feel confused by contradictory social pressures to look and dress provocatively but resist having sex. A sexual encounter can trigger shame and fear.
Although clinical and developmental studies indicate that teen girls respond more dramatically to relationship troubles than boys, the brain and hormone differences responsible for these effects remain unclear. Male hormones hugely increase in boys at puberty—up to 25-fold between ages 9 and 15—but do not cycle. Male brains do not have the same capacity as female brains to respond to cyclical hormonal activity because exposure to androgens during fetal development eliminates this ability. The fetal testosterone surge causes the area associated with sexual pursuit to double in the male brain.
Outside of fertility considerations, Baron-Cohen et al15 suggest that male brain circuits have been formed by fetal testosterone to focus more on systematization—which emphasizes figuring out how things work and performing tasks—rather than empathy and bonding in relationships. This difference has been shown in neuroimaging studies comparing the genders’ attentional systems.16,17 In contrast to the systematizing male brain, female brains are more likely to activate the mirror neuron system—the area required for empathizing.18
Female brains, of course, respond to cyclical hormonal activity. However, the regular monthly waves of estrogen and progesterone do not affect all female brains the same. A subset of women who experience premenstrual dysphoric disorder appear to have brains that trigger depressed moods and irritability during the last 2 weeks of the menstrual cycle.19 A genetic difference in these women is suspected as the culprit; these genes may affect the way their brains metabolize progesterone.
CASE CONTINUED: An overdose of stress
Kate’s poor concentration lingered, and her grades continued to drop. She tells you her parents were having marital problems and she did not want to bother them with her difficulties. Two days before her period was due, she learned she had failed 2 classes. That night, as she got some acetaminophen for a headache, she impulsively took the rest of the bottle.
After swallowing the pills, Kate panicked. She forced herself to vomit and tearfully told her parents what she had done. They took her to the emergency room, where she was medically stabilized, evaluated by a psychiatrist, and referred to you for outpatient treatment.
Treatment recommendations
A combination of factors—genetic, hormonal, and neurodevelopmental—probably contributed to Kate’s acute depressed mood and overdose. Thus, to treat depression in adolescent girls, emerging evidence supports:
- stabilizing hormonal fluctuations such as rapidly falling progesterone just before the start of menstrual periods with an extended-cycle contraceptive (we would try an ethinyl estradiol/levonorgestrel combination such as Seasonale®)
- treating depressive symptoms with a selective serotonin reuptake inhibitor such as citalopram, 10 mg once daily, with careful monitoring for suicidal thoughts or behavior
- providing tools to manage stress and impulsive behavior through weekly psychotherapy (such as cognitive-behavioral therapy, dialectical behavioral therapy, or supportive therapy).
Genetic factors. Kate’s mother’s history of postpartum depression suggests genetic risk for Kate. Studies have found that the expression of particular genes—such as the serotonin transporter (5-HTT) gene—may be associated with depression. Staley et al20 found that depressed women show a significantly greater decrease in 5-HTT availability in the diencephalon (forebrain region containing the thalamus, hypothalamus, and part of the pituitary gland) when compared with healthy women and depressed men.
Table 3
3 stages of girls’ gonadal development
| Stage | Timing | Developmental events |
|---|---|---|
| Adrenarche | Onset around age 6, peaks by age 20 | Rise in weak androgens (DHEA and DHEAS) from adrenal gland results in pubic and axillary hair and increases likelihood of acne |
| Gonadarche | Usually ~2 years before menarche | Pulses of GnRH, LH/FSH lead to increased estrogen, which stimulates breast development, widening of hips, and increased subcutaneous fat deposition |
| Menarche | Relatively late in puberty (usually not before Tanner stage 4) | “Monthly” cycle established; ovarian estrogen pulses in response to GnRH and FSH, the LH surge, and ovulation; progesterone produced after ovulation |
| DHEA: dehydroepiandrosterone; DHEAS: dehydroepiandrosterone sulfate; GnRH: gonadotropin-releasing hormone; | ||
| LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Tanner stage 4: pubic hair and breast development typical of middle to late adolescence (ages 12 to 17) | ||
| Source: References 4,5 | ||
Although men and women have the same 5-HTT gene, women may possess a gender-specific factor—such as estrogen or progesterone—that differentially alters this and other genes’ expression in women with depression. Individuals who carry a short version of the gene may be at particular risk of becoming depressed when exposed to stressful life events.
Caspi et al21 found a polymorphism in the 5-HTT gene on chromosome 17 that can manifest differentially based on environmental factors. In this study, individuals with 2 copies of the long version of this gene were relatively resistant to stressful life events, whereas those with 1 or 2 copies of the short version were highly sensitive to stressful life events. The depression rate in short-gene individuals was:
- 9% in those who had not experienced stressful life events
- nearly 40% in those who had experienced ≥4 stressful life events.
Hormonal and stress factors. Stress responsiveness becomes sexually dimorphic at puberty. Compared with men, women are:
- at greater risk after puberty for heightened stress responsiveness, which is associated with major depressive disorder
- 3 times more likely to develop depression after a stressful life event.22
Women’s and men’s different biological responses to stress might be related to the gender-specific hormones that emerge during puberty. Kate could be at increased risk for depression—especially immediately before her period—if she inherited a stress-sensitive gene and now has increased stress sensitivity triggered by the hormones of puberty.23
Neurodevelopmental factors. Dorsolateral prefrontal cortex circuits associated with making good decisions and weighing the consequences of actions are immature in the adolescent and the last part of the brain to undergo myelination.24-26 Teens are well-known for erratic, emotionally driven behaviors.27,28 Kate’s impulsive overdose exemplifies the consequences of emotional reactivity without the benefit of inhibitory mature brain connections.
Related resources
- Brizendine L. Teen girl brain. In: The female brain. New York: Morgan Road Books; 2006:31-56. www.thefemalebrain.com.
- Strauch B. The primal teen: what discoveries about the teenage brain tell us about our kids. New York: Doubleday; 2003.
- Harter S. Self and identity development. In: Feldman S, Elliott G, eds. At the threshold: the developing adolescent. Cambridge, MA: Harvard University Press; 1990:352-87.
Drug brand names
- Ethinyl estradiol/levonorgestrel • Seasonale
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci 2004;5(9):701-8.
2. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6(4):551-60.
3. Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. In press.
4. Cameron J. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Ann NY Acad Sci 2004;1021:110-23.
5. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
6. Biro F, Huang B, Crawford P, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148(2):234-40.
7. Herman-Giddens M, Kaplowitz P, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics 2004;113(4):911-7.
8. Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997;99(4):505-12.
9. Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics 2002;110(4):752-7.
10. Rapkin A, Tsao J, Turk N, et al. Relationships among self-rated Tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol 2006;19:181-7.
11. Angold A, Costello E, Worthman C. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998;28:51-61.
12. Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med 1999;29:1043-53.
13. Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage 2006;32(2):854-62.
14. McClure EB, Parrish JM, Nelson EE, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. In press.
15. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358(1430):361-74.
16. Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 2005;28(3):618-26.
17. Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills 2004;99(2):371-91.
18. Cheng YW, Tzeng OJ, Decety J, et al. Gender differences in the human mirror system: a magnetoencephalography study. Neuroreport 2006;17(11):1115-9.
19. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and those without premenstrual syndrome. N Engl J Med 1998;338(4):209-16.
20. Staley J, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59:40-7.
21. Caspi A, Sugden K, Moffitt T, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9.
22. Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol Med 2001;31(4):593-604.
23. Seeman M. Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997;154(12):1641-7.
24. Kupfer D, Woodward H. Adolescent development and the regulation of behavior and emotion. Ann NY Acad Sci 2004;1021:320-2.
25. Kelley A, Schochet T, Landry C. Risk taking and novelty seeking in adolescence. Ann NY Acad Sci 2004;1021:27-32.
26. Ellis L, Rothbart M, Posner M. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Ann NY Acad Sci 2004;1021:337-40.
27. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
28. Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs 2003;5(4):243-65.
1. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci 2004;5(9):701-8.
2. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6(4):551-60.
3. Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. In press.
4. Cameron J. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Ann NY Acad Sci 2004;1021:110-23.
5. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
6. Biro F, Huang B, Crawford P, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148(2):234-40.
7. Herman-Giddens M, Kaplowitz P, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics 2004;113(4):911-7.
8. Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997;99(4):505-12.
9. Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics 2002;110(4):752-7.
10. Rapkin A, Tsao J, Turk N, et al. Relationships among self-rated Tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol 2006;19:181-7.
11. Angold A, Costello E, Worthman C. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998;28:51-61.
12. Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med 1999;29:1043-53.
13. Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage 2006;32(2):854-62.
14. McClure EB, Parrish JM, Nelson EE, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. In press.
15. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358(1430):361-74.
16. Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 2005;28(3):618-26.
17. Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills 2004;99(2):371-91.
18. Cheng YW, Tzeng OJ, Decety J, et al. Gender differences in the human mirror system: a magnetoencephalography study. Neuroreport 2006;17(11):1115-9.
19. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and those without premenstrual syndrome. N Engl J Med 1998;338(4):209-16.
20. Staley J, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59:40-7.
21. Caspi A, Sugden K, Moffitt T, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9.
22. Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol Med 2001;31(4):593-604.
23. Seeman M. Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997;154(12):1641-7.
24. Kupfer D, Woodward H. Adolescent development and the regulation of behavior and emotion. Ann NY Acad Sci 2004;1021:320-2.
25. Kelley A, Schochet T, Landry C. Risk taking and novelty seeking in adolescence. Ann NY Acad Sci 2004;1021:27-32.
26. Ellis L, Rothbart M, Posner M. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Ann NY Acad Sci 2004;1021:337-40.
27. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
28. Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs 2003;5(4):243-65.
How to treat depression, stress associated with infertility treatment
“I think it’s my fault we can’t get pregnant,” says Mrs. S, who has been referred by her gynecologist for evaluation of anxiety and depression. Mrs. S, age 33, and her husband have been trying to conceive their first child for 2 years.
The couple has undergone infertility workups, including a semen analysis and hysterosalpingography, and results have been within normal limits. The gynecologist recommended intercourse every other day, but Mr. S developed stress-related erectile dysfunction (which was treated with sildenafil).
Mrs. S has no personal or family history of depression. Her depression has worsened as she contemplates more invasive and expensive procedures, such as intrauterine insemination (IUI) and in vitro fertilization (IVF).
Her Beck Depression Inventory score of 22 indicates mild depression. She is not actively suicidal, but she sometimes doubts that life is worth living. She feels like a failure and wants to know if you think stress is contributing to her infertility.
Women with a 2- to 3-year history of infertility despite repeated treatments are at risk of stress, anxiety, and depression.1 Even if treatment eventually succeeds, anxiety often persists during pregnancy.2 Your knowledge of medical infertility treatments’ emotional toll will help you understand, educate, and support infertile women and their partners.
Infertility affects approximately 6 million U.S. women and their partners.3 As recently as the 1960s infertility was thought to be caused primarily by female psychological problems,4 such as neurotic, conflicted feelings about the transition to adulthood or about sex, pregnancy, labor, or motherhood.5,6
This belief changed as researchers identified organic causes of infertility, such as blocked fallopian tubes, sperm abnormalities, and anovulation. A definitive diagnosis can now be made in 85% to 90% of infertility cases, and two-thirds of couples can conceive after medical intervention.7
Age and fertility. Most experts recommend that women age >35 who wish to conceive seek gynecologic evaluation after 6 months of unsuccessful intercourse. Chances of becoming pregnant begin to decline at age 35 and drop sharply after age 40.8 Beyond age 43, the only infertility treatment likely to be successful is implanting an embryo created with an egg donated by a younger woman.
Stress and fertility
Infertility—failure to conceive after 1 year of regular unprotected intercourse—affects approximately 10% of the reproductive-age U.S. population (Box).3-8 Does stress affect a woman’s chance of becoming pregnant? Research into this question—voiced by Mrs. S—has produced conflicting results.5,9,10
Stress does not universally prevent pregnancy; women have conceived as a result of rape. However, chronic extreme stress—such as that imposed by war, imprisonment, or starvation—can change the menstrual cycle. Effects range from subtle luteal-phase deficiency to menses cessation.9 It may be that evolution favored females of species who could “turn off ” fertility during stressful times to conserve physical resources and “turn it back on” and bear offspring after the threat passed.
Neuroendocrine markers. Researchers examining the role of stress in infertility and its treatments have focused on the neuroendocrine system—particularly neurotransmitters such as prolactin, endorphin, norepinephrine, dopamine, and cortisol. Although chronic anxiety and depression have been linked in animal models to neuroendocrine mechanisms of infertility,4 findings in humans have been mixed (Table 1).11-15
Table 1
Does stress reduce fertility? Research results are mixed
| Study design (year of publication) | Results |
|---|---|
| Controlled prospective trial, 40 women undergoing IVF (1992)11 | IVF success rates were comparatively lower among women with high cortisol concentrations |
| Women with high prolactin concentrations had greater numbers of oocytes but lower fertilization rates | |
| Failure to conceive was associated with high depression symptom scores, maladaptive coping strategies, and avoidance behavior | |
| Controlled prospective trial, 330 infertile women (1993)12 | Depressed women had a lower pregnancy rate after a first IVF-ET, compared with nondepressed women |
| Uncontrolled prospective trial, 13 women without a history of infertility (1997)13 | Mean adrenaline, norepinephrine, and cortisol levels excreted in urine were not significantly different in menstrual cycles when women conceived, compared with nonconception cycles |
| Little relationship seen between psychological measures of mood state and excretion of adrenaline and cortisol | |
| Controlled, prospective trial, 49 infertile women (1997)14 | Patients who conceived with IVF-ET had lower systolic blood pressures and slower heart rates under stress-test conditions than did those who did not conceive |
| Controlled prospective trial, 40 women after successful IVF-ET (1998)15 | No difference in hormonal stress markers during first 27 days of pregnancy between women who later gave birth and those who experienced miscarriages |
| Physiologic stress hormone concentrations showed little association with psychological scores, and high anxiety and stress levels did not appear to prevent pregnancy | |
| IVF: In vitro fertilization | |
| IVF-ET: In vitro fertilization with embryo transplant | |
In one prospective, controlled, single-blind study, 184 women who had been trying to conceive for 1 to 2 years were randomly assigned to 10 sessions of group cognitive-behavioral therapy (CBT), a standard support group, or usual care. Sixty-four women withdrew before the study ended. After 1 year, women who received psychological interventions—47 in the CBT group and 48 in the standard support group—had statistically significant higher pregnancy rates, compared with 25 women who received usual care.16 Conversely, a literature review and evaluation of 25 studies found psychosocial interventions unlikely to improve pregnancy rates in infertile women.17
Methodologic problems. Most studies of stress’ influence on fertility are small, and many have methodologic problems.4 In some, researchers lumped together women whose infertility was caused by disparate diagnoses such as male-factor infertility, blocked fallopian tubes, and advanced age. Retrospective studies also must be interpreted with caution because:
- patients who did not become pregnant may have exaggerated the degree of their depression and its effects
- those with pre-existing medical problems would know they were unlikely to conceive and might have been more depressed before and during infertility treatments.18
Recommendation. When counseling patients about the role of stress in infertility and its treatment, we recommend emphasizing that:
- infertility can cause stress in many areas of life
- the effect of stress on fertility, if any, is likely to be minimal for most women.
Case continued: Strain and anger
You begin to see Mrs. S weekly for supportive therapy, using cognitive restructuring and relaxation techniques to alleviate her anxiety and depression. She decides not to start an antidepressant because she does not want to be on medication if she becomes pregnant.
During the next 2 months she finishes an unsuccessful IUI cycle and reports that her relationship with her husband has become strained. She avoids friends who have children and feels angry when she sees a pregnant woman. She dislikes going to family events because relatives sometimes ask, “When are you going to get pregnant?”
Her work as a manager is suffering because of her many visits to fertility specialists. Her Beck Depression Inventory score has increased to 33, indicating worsening depression.
Infertility’s psychological toll
Patients rarely accept infertility with equanimity, and their responses include shock, denial, anger, isolation, guilt, and grief.6 Some women say the experience of being infertile feels comparable to having cancer.20
The incidence of clinical major depression, poor self-esteem, and sexual dysfunction in women who undergo infertility evaluation does not differ significantly from that of their fertile peers.9 Even so, infertile women report a roller-coaster ride of emotions: hope as treatments are tried, despair when treatments fail.
Health care providers can add to the angst by telling women they have an “incompetent” cervix, “poor-quality” or “old” eggs, or “inadequate” mucus; these insensitive descriptions can lead women to blame themselves and feel ashamed, guilty, and depressed.4,5,18
Psychotherapy. Providing education and teaching skills such as relaxation training has been shown to reduce depressive symptoms more effectively than having patients discuss their thoughts and feelings about infertility.17 Helpful psychotherapies emphasize CBT and improved coping skills.
Negative coping strategies include escape/avoidance conduct or self-blame (such as, “I’m not getting pregnant because I work too hard”). Encourage patients to replace these with protective coping strategies, such as seeking social support and engaging in active problem-solving (“I reach out to friends who help comfort me, and I set limits with friends who make me feel bad about myself ”).21-23
Medication. Even though sadness and anxiety are normal responses to infertility, psychotropic medications might be appropriate after a thorough evaluation. Keep in mind, however, that selective serotonin reuptake inhibitors (SSRIs) can cause prolactinemia, which could interfere with ovulation.9 Miscarriage and stillbirth rates among women taking SSRIs are similar to those of the general population.24
Case continued: It takes two
Despite three IUI cycles over 12 months Mrs. S has not become pregnant. She considers IVF but is concerned about the cost and the less than 50% chance of success.
You encourage her to continue individual supportive and cognitive therapy and to consider couple’s therapy. She and her husband decide to attend a group for couples with infertility. She accepts your referral to RESOLVE, a national support program for infertile patients (see Related resources).
Problems facing infertile couples
Gender differences in coping style. Men and women experience infertility differently.
The women in infertile couples often are distressed, whereas the men tend to remain more confident that some kind of treatment will work. This imbalance can leave the woman feeling unsupported and the man feeling confused about why she is so upset about what he sees as just a medical problem to be solved.
When a couple’s infertility has been attributed to sperm abnormalities, however, the man’s stress level can equal the woman’s. Women tend to feel stress regardless of which partner is “at fault.”25
Grief reactions. The “loss” of a child never conceived generally goes unrecognized but has psychological consequences. Both partners can feel:
- low self-esteem
- sadness about being unable to experience parenting
- doubts about their femininity or masculinity
- regret over unfulfilled dreams.
Table 2
Fictions and facts about infertility
| Fiction | Fact |
|---|---|
| Infertility is a psychosomatic disorder | An organic cause is found in 85% to 90% of infertile couples7 |
| Infertility is a female problem | One-third of infertility cases are caused by female factors, one-third by male factors, and one-third by male and female factors or unknown causes26 |
| Infertility is epidemic | The number of patients seeking infertility treatment has increased dramatically in 20 years, but the infertility rate is stable3,5,18 |
| Infertility is rare | Approximately 10% of U.S. couples of childbearing age are infertile3 |
| If you adopt, you'll get pregnant | Conception rates are no higher following adoption than among childless couples7 |
Employment. Infertility treatments are time- and resource-intensive, and patients often miss work. Even while on the job, a woman distracted by infertility or treatment side effects might not perform as well as she could. Worries about job security add to her anxiety.
Finances. Infertility treatment is expensive and is not always covered by insurance. The American Society for Reproductive Medicine reports that the cost of an IVF cycle averages $12,400, and success rates are 26 (see Related resources).
To continue treatment, couples may take second jobs, acquire loans, deplete savings, or accumulate debt. Many couples—even with extraordinary effort—cannot afford to start or continue advanced infertility treatments.
Spirituality. Patients who believe that infertility is God’s punishment for past sins may experience a religious crisis. Those affiliated with religions that restrict assisted-reproductive technology may feel forced to choose between doctrinal dictates and their dreams of becoming parents.
Case continued: A new ‘RESOLVE’
Mrs. S enjoys her association with the online support of RESOLVE. Through message boards, she shares her concerns with other women undergoing infertility treatment. She also finds support from friends, although she continues to set limits such as declining invitations to baby showers. She practices relaxation techniques at home.
Since she and her husband have joined the group for infertile couples, their relationship has improved. Mrs. S feels that he better understands her fears after hearing other women in the group being “just as emotional.” He no longer tells her, “It’s just a medical problem.”
- National Infertility Association (RESOLVE). www.resolve.org.
- American Society for Reproductive Medicine. www.asrm.org.
- Sildenafil • Viagra
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Khademi A, Alleyassin A, Aghahosseini M, et al. Pretreatment Beck Depression Inventory score is an important predictor for post-treatment score in infertile patients: a before-after study. BMC Psychiatry 2005;5(1):25.-
2. Hjelmstedt A, Widstrom AM, Wramsby H, et al. Personality factors and emotional responses to pregnancy among IVF couples in early pregnancy: a comparative study. Acta Obstet Gynecol Scand 2003;82(2):152-61.
3. Abma J, Chandra A, Mosher W, et al. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and Health Statistics, Series 23, No. 19. National Center for Health Statistics, May 1997.
4. Wischmann TH. Psychogenic infertility-myths and facts. J Assist Reprod Genet 2003;20(12):485-94.
5. Burns LH, Covington SN, eds Infertility counseling: a comprehensive handbook for clinicians.. Pearl River, NY: Parthenon; 1999:122-35.
6. Stanton AL, Lobel M, Sears S, DeLuca RS. Psychosocial aspects of selected issues in women’s reproductive health: current status and future directions. J Consult Clin Psychol 2002;70(3):751-70.
7. National Infertility Association (RESOLVE) www.resolve.org. Accessed August 30, 2006.
8. Kasper DL, Braunwald E, Fauci A, et al. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill Professional; 2004.
9. Cedars M (ed) Infertility: practical pathways in obstetrics and gynecology. New York: McGraw-Hill; 2005:88-133.
10. Greil AL. Infertility and psychological distress: a critical review of the literature. Soc Sci Med 1997;45(11):1679-704.
11. Demyttenaere K, Nijs P, Evers-Kiebooms G, Koninckx PR. Coping and the ineffectiveness of coping influence the outcome of in vitro fertilization through stress responses. Psychoneuroendocrinology 1992;17(6):655-65.
12. Thiering P, Beaurepaire J, Jones M, et al. Mood state as a predictor of treatment outcome after in vitro fertilization/embryo transfer technology (IVF/ET). J Psychosom Res 1993;37(5):481-91.
13. Sanders KA, Bruce NW. A prospective study of psychological stress and fertility in women. Hum Reprod 1997;12(10):2324-9.
14. Facchinetti F, Matteo ML, Artini GP, et al. An increased vulnerability to stress is associated with a poor outcome of in vitro fertilization-embryo transfer treatment. Fertil Steril 1997;67(2):309-14.
15. Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod 1998;13(8):2296-300.
16. Domar AD, Clapp D, Slawsby EA, et al. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril 2000;73(4):805-11.
17. Boivin J. A review to psychosocial interventions in infertility. Soc Sci Med 2003;57(12):2325-41.
18. Pasch LA. Confronting fertility problems: current research and future challenges. In: Baum A, Revenson TA, Singer JE (eds). Handbook of health psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2001:559-70.
19. Stewart DE, Boydell KM, McCarthy K, et al. A prospective study of the effectiveness of brief professionally-led support groups for infertility patients. Int J Psychiatry Med 1992;22(2):173-82.
20. Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993;14(suppl):45-52.
21. Litt MD, Tennen H, Affleck G, Klock S. Coping and cognitive factors in adaptation to in vitro fertilization failure. J Behav Med 1992;15(2):171-87.
22. Peterson BD, Newton CR, Rosen KH, Skaggs GE. The relationship between coping and depression in men and women referred for in vitro fertilization. Fertil Steril 2006;85(3):802-4.
23. Morrow KA, Thoreson RW, Penney LL. Predictors of psychological distress among infertility clinic patients. J Consult Clin Psychol 1995;63(1):163-7.
24. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy: do antidepressants’ benefits outweigh the risks? Current Psychiatry 2006;5(4):31-40.
25. Nachtigall RD, Becker G, Wozny M. The effects of gender-specific diagnosis on men’s and women’s response to infertility. Fertil Steril 1992;57(1):113-21.
26. American Society for Reproductive Medicine Information for patients. Is infertility treatment expensive? Available at: http://www.asrm.org/Patients/faqs.html#Q6. Accessed September 12, 2006
“I think it’s my fault we can’t get pregnant,” says Mrs. S, who has been referred by her gynecologist for evaluation of anxiety and depression. Mrs. S, age 33, and her husband have been trying to conceive their first child for 2 years.
The couple has undergone infertility workups, including a semen analysis and hysterosalpingography, and results have been within normal limits. The gynecologist recommended intercourse every other day, but Mr. S developed stress-related erectile dysfunction (which was treated with sildenafil).
Mrs. S has no personal or family history of depression. Her depression has worsened as she contemplates more invasive and expensive procedures, such as intrauterine insemination (IUI) and in vitro fertilization (IVF).
Her Beck Depression Inventory score of 22 indicates mild depression. She is not actively suicidal, but she sometimes doubts that life is worth living. She feels like a failure and wants to know if you think stress is contributing to her infertility.
Women with a 2- to 3-year history of infertility despite repeated treatments are at risk of stress, anxiety, and depression.1 Even if treatment eventually succeeds, anxiety often persists during pregnancy.2 Your knowledge of medical infertility treatments’ emotional toll will help you understand, educate, and support infertile women and their partners.
Infertility affects approximately 6 million U.S. women and their partners.3 As recently as the 1960s infertility was thought to be caused primarily by female psychological problems,4 such as neurotic, conflicted feelings about the transition to adulthood or about sex, pregnancy, labor, or motherhood.5,6
This belief changed as researchers identified organic causes of infertility, such as blocked fallopian tubes, sperm abnormalities, and anovulation. A definitive diagnosis can now be made in 85% to 90% of infertility cases, and two-thirds of couples can conceive after medical intervention.7
Age and fertility. Most experts recommend that women age >35 who wish to conceive seek gynecologic evaluation after 6 months of unsuccessful intercourse. Chances of becoming pregnant begin to decline at age 35 and drop sharply after age 40.8 Beyond age 43, the only infertility treatment likely to be successful is implanting an embryo created with an egg donated by a younger woman.
Stress and fertility
Infertility—failure to conceive after 1 year of regular unprotected intercourse—affects approximately 10% of the reproductive-age U.S. population (Box).3-8 Does stress affect a woman’s chance of becoming pregnant? Research into this question—voiced by Mrs. S—has produced conflicting results.5,9,10
Stress does not universally prevent pregnancy; women have conceived as a result of rape. However, chronic extreme stress—such as that imposed by war, imprisonment, or starvation—can change the menstrual cycle. Effects range from subtle luteal-phase deficiency to menses cessation.9 It may be that evolution favored females of species who could “turn off ” fertility during stressful times to conserve physical resources and “turn it back on” and bear offspring after the threat passed.
Neuroendocrine markers. Researchers examining the role of stress in infertility and its treatments have focused on the neuroendocrine system—particularly neurotransmitters such as prolactin, endorphin, norepinephrine, dopamine, and cortisol. Although chronic anxiety and depression have been linked in animal models to neuroendocrine mechanisms of infertility,4 findings in humans have been mixed (Table 1).11-15
Table 1
Does stress reduce fertility? Research results are mixed
| Study design (year of publication) | Results |
|---|---|
| Controlled prospective trial, 40 women undergoing IVF (1992)11 | IVF success rates were comparatively lower among women with high cortisol concentrations |
| Women with high prolactin concentrations had greater numbers of oocytes but lower fertilization rates | |
| Failure to conceive was associated with high depression symptom scores, maladaptive coping strategies, and avoidance behavior | |
| Controlled prospective trial, 330 infertile women (1993)12 | Depressed women had a lower pregnancy rate after a first IVF-ET, compared with nondepressed women |
| Uncontrolled prospective trial, 13 women without a history of infertility (1997)13 | Mean adrenaline, norepinephrine, and cortisol levels excreted in urine were not significantly different in menstrual cycles when women conceived, compared with nonconception cycles |
| Little relationship seen between psychological measures of mood state and excretion of adrenaline and cortisol | |
| Controlled, prospective trial, 49 infertile women (1997)14 | Patients who conceived with IVF-ET had lower systolic blood pressures and slower heart rates under stress-test conditions than did those who did not conceive |
| Controlled prospective trial, 40 women after successful IVF-ET (1998)15 | No difference in hormonal stress markers during first 27 days of pregnancy between women who later gave birth and those who experienced miscarriages |
| Physiologic stress hormone concentrations showed little association with psychological scores, and high anxiety and stress levels did not appear to prevent pregnancy | |
| IVF: In vitro fertilization | |
| IVF-ET: In vitro fertilization with embryo transplant | |
In one prospective, controlled, single-blind study, 184 women who had been trying to conceive for 1 to 2 years were randomly assigned to 10 sessions of group cognitive-behavioral therapy (CBT), a standard support group, or usual care. Sixty-four women withdrew before the study ended. After 1 year, women who received psychological interventions—47 in the CBT group and 48 in the standard support group—had statistically significant higher pregnancy rates, compared with 25 women who received usual care.16 Conversely, a literature review and evaluation of 25 studies found psychosocial interventions unlikely to improve pregnancy rates in infertile women.17
Methodologic problems. Most studies of stress’ influence on fertility are small, and many have methodologic problems.4 In some, researchers lumped together women whose infertility was caused by disparate diagnoses such as male-factor infertility, blocked fallopian tubes, and advanced age. Retrospective studies also must be interpreted with caution because:
- patients who did not become pregnant may have exaggerated the degree of their depression and its effects
- those with pre-existing medical problems would know they were unlikely to conceive and might have been more depressed before and during infertility treatments.18
Recommendation. When counseling patients about the role of stress in infertility and its treatment, we recommend emphasizing that:
- infertility can cause stress in many areas of life
- the effect of stress on fertility, if any, is likely to be minimal for most women.
Case continued: Strain and anger
You begin to see Mrs. S weekly for supportive therapy, using cognitive restructuring and relaxation techniques to alleviate her anxiety and depression. She decides not to start an antidepressant because she does not want to be on medication if she becomes pregnant.
During the next 2 months she finishes an unsuccessful IUI cycle and reports that her relationship with her husband has become strained. She avoids friends who have children and feels angry when she sees a pregnant woman. She dislikes going to family events because relatives sometimes ask, “When are you going to get pregnant?”
Her work as a manager is suffering because of her many visits to fertility specialists. Her Beck Depression Inventory score has increased to 33, indicating worsening depression.
Infertility’s psychological toll
Patients rarely accept infertility with equanimity, and their responses include shock, denial, anger, isolation, guilt, and grief.6 Some women say the experience of being infertile feels comparable to having cancer.20
The incidence of clinical major depression, poor self-esteem, and sexual dysfunction in women who undergo infertility evaluation does not differ significantly from that of their fertile peers.9 Even so, infertile women report a roller-coaster ride of emotions: hope as treatments are tried, despair when treatments fail.
Health care providers can add to the angst by telling women they have an “incompetent” cervix, “poor-quality” or “old” eggs, or “inadequate” mucus; these insensitive descriptions can lead women to blame themselves and feel ashamed, guilty, and depressed.4,5,18
Psychotherapy. Providing education and teaching skills such as relaxation training has been shown to reduce depressive symptoms more effectively than having patients discuss their thoughts and feelings about infertility.17 Helpful psychotherapies emphasize CBT and improved coping skills.
Negative coping strategies include escape/avoidance conduct or self-blame (such as, “I’m not getting pregnant because I work too hard”). Encourage patients to replace these with protective coping strategies, such as seeking social support and engaging in active problem-solving (“I reach out to friends who help comfort me, and I set limits with friends who make me feel bad about myself ”).21-23
Medication. Even though sadness and anxiety are normal responses to infertility, psychotropic medications might be appropriate after a thorough evaluation. Keep in mind, however, that selective serotonin reuptake inhibitors (SSRIs) can cause prolactinemia, which could interfere with ovulation.9 Miscarriage and stillbirth rates among women taking SSRIs are similar to those of the general population.24
Case continued: It takes two
Despite three IUI cycles over 12 months Mrs. S has not become pregnant. She considers IVF but is concerned about the cost and the less than 50% chance of success.
You encourage her to continue individual supportive and cognitive therapy and to consider couple’s therapy. She and her husband decide to attend a group for couples with infertility. She accepts your referral to RESOLVE, a national support program for infertile patients (see Related resources).
Problems facing infertile couples
Gender differences in coping style. Men and women experience infertility differently.
The women in infertile couples often are distressed, whereas the men tend to remain more confident that some kind of treatment will work. This imbalance can leave the woman feeling unsupported and the man feeling confused about why she is so upset about what he sees as just a medical problem to be solved.
When a couple’s infertility has been attributed to sperm abnormalities, however, the man’s stress level can equal the woman’s. Women tend to feel stress regardless of which partner is “at fault.”25
Grief reactions. The “loss” of a child never conceived generally goes unrecognized but has psychological consequences. Both partners can feel:
- low self-esteem
- sadness about being unable to experience parenting
- doubts about their femininity or masculinity
- regret over unfulfilled dreams.
Table 2
Fictions and facts about infertility
| Fiction | Fact |
|---|---|
| Infertility is a psychosomatic disorder | An organic cause is found in 85% to 90% of infertile couples7 |
| Infertility is a female problem | One-third of infertility cases are caused by female factors, one-third by male factors, and one-third by male and female factors or unknown causes26 |
| Infertility is epidemic | The number of patients seeking infertility treatment has increased dramatically in 20 years, but the infertility rate is stable3,5,18 |
| Infertility is rare | Approximately 10% of U.S. couples of childbearing age are infertile3 |
| If you adopt, you'll get pregnant | Conception rates are no higher following adoption than among childless couples7 |
Employment. Infertility treatments are time- and resource-intensive, and patients often miss work. Even while on the job, a woman distracted by infertility or treatment side effects might not perform as well as she could. Worries about job security add to her anxiety.
Finances. Infertility treatment is expensive and is not always covered by insurance. The American Society for Reproductive Medicine reports that the cost of an IVF cycle averages $12,400, and success rates are 26 (see Related resources).
To continue treatment, couples may take second jobs, acquire loans, deplete savings, or accumulate debt. Many couples—even with extraordinary effort—cannot afford to start or continue advanced infertility treatments.
Spirituality. Patients who believe that infertility is God’s punishment for past sins may experience a religious crisis. Those affiliated with religions that restrict assisted-reproductive technology may feel forced to choose between doctrinal dictates and their dreams of becoming parents.
Case continued: A new ‘RESOLVE’
Mrs. S enjoys her association with the online support of RESOLVE. Through message boards, she shares her concerns with other women undergoing infertility treatment. She also finds support from friends, although she continues to set limits such as declining invitations to baby showers. She practices relaxation techniques at home.
Since she and her husband have joined the group for infertile couples, their relationship has improved. Mrs. S feels that he better understands her fears after hearing other women in the group being “just as emotional.” He no longer tells her, “It’s just a medical problem.”
- National Infertility Association (RESOLVE). www.resolve.org.
- American Society for Reproductive Medicine. www.asrm.org.
- Sildenafil • Viagra
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
“I think it’s my fault we can’t get pregnant,” says Mrs. S, who has been referred by her gynecologist for evaluation of anxiety and depression. Mrs. S, age 33, and her husband have been trying to conceive their first child for 2 years.
The couple has undergone infertility workups, including a semen analysis and hysterosalpingography, and results have been within normal limits. The gynecologist recommended intercourse every other day, but Mr. S developed stress-related erectile dysfunction (which was treated with sildenafil).
Mrs. S has no personal or family history of depression. Her depression has worsened as she contemplates more invasive and expensive procedures, such as intrauterine insemination (IUI) and in vitro fertilization (IVF).
Her Beck Depression Inventory score of 22 indicates mild depression. She is not actively suicidal, but she sometimes doubts that life is worth living. She feels like a failure and wants to know if you think stress is contributing to her infertility.
Women with a 2- to 3-year history of infertility despite repeated treatments are at risk of stress, anxiety, and depression.1 Even if treatment eventually succeeds, anxiety often persists during pregnancy.2 Your knowledge of medical infertility treatments’ emotional toll will help you understand, educate, and support infertile women and their partners.
Infertility affects approximately 6 million U.S. women and their partners.3 As recently as the 1960s infertility was thought to be caused primarily by female psychological problems,4 such as neurotic, conflicted feelings about the transition to adulthood or about sex, pregnancy, labor, or motherhood.5,6
This belief changed as researchers identified organic causes of infertility, such as blocked fallopian tubes, sperm abnormalities, and anovulation. A definitive diagnosis can now be made in 85% to 90% of infertility cases, and two-thirds of couples can conceive after medical intervention.7
Age and fertility. Most experts recommend that women age >35 who wish to conceive seek gynecologic evaluation after 6 months of unsuccessful intercourse. Chances of becoming pregnant begin to decline at age 35 and drop sharply after age 40.8 Beyond age 43, the only infertility treatment likely to be successful is implanting an embryo created with an egg donated by a younger woman.
Stress and fertility
Infertility—failure to conceive after 1 year of regular unprotected intercourse—affects approximately 10% of the reproductive-age U.S. population (Box).3-8 Does stress affect a woman’s chance of becoming pregnant? Research into this question—voiced by Mrs. S—has produced conflicting results.5,9,10
Stress does not universally prevent pregnancy; women have conceived as a result of rape. However, chronic extreme stress—such as that imposed by war, imprisonment, or starvation—can change the menstrual cycle. Effects range from subtle luteal-phase deficiency to menses cessation.9 It may be that evolution favored females of species who could “turn off ” fertility during stressful times to conserve physical resources and “turn it back on” and bear offspring after the threat passed.
Neuroendocrine markers. Researchers examining the role of stress in infertility and its treatments have focused on the neuroendocrine system—particularly neurotransmitters such as prolactin, endorphin, norepinephrine, dopamine, and cortisol. Although chronic anxiety and depression have been linked in animal models to neuroendocrine mechanisms of infertility,4 findings in humans have been mixed (Table 1).11-15
Table 1
Does stress reduce fertility? Research results are mixed
| Study design (year of publication) | Results |
|---|---|
| Controlled prospective trial, 40 women undergoing IVF (1992)11 | IVF success rates were comparatively lower among women with high cortisol concentrations |
| Women with high prolactin concentrations had greater numbers of oocytes but lower fertilization rates | |
| Failure to conceive was associated with high depression symptom scores, maladaptive coping strategies, and avoidance behavior | |
| Controlled prospective trial, 330 infertile women (1993)12 | Depressed women had a lower pregnancy rate after a first IVF-ET, compared with nondepressed women |
| Uncontrolled prospective trial, 13 women without a history of infertility (1997)13 | Mean adrenaline, norepinephrine, and cortisol levels excreted in urine were not significantly different in menstrual cycles when women conceived, compared with nonconception cycles |
| Little relationship seen between psychological measures of mood state and excretion of adrenaline and cortisol | |
| Controlled, prospective trial, 49 infertile women (1997)14 | Patients who conceived with IVF-ET had lower systolic blood pressures and slower heart rates under stress-test conditions than did those who did not conceive |
| Controlled prospective trial, 40 women after successful IVF-ET (1998)15 | No difference in hormonal stress markers during first 27 days of pregnancy between women who later gave birth and those who experienced miscarriages |
| Physiologic stress hormone concentrations showed little association with psychological scores, and high anxiety and stress levels did not appear to prevent pregnancy | |
| IVF: In vitro fertilization | |
| IVF-ET: In vitro fertilization with embryo transplant | |
In one prospective, controlled, single-blind study, 184 women who had been trying to conceive for 1 to 2 years were randomly assigned to 10 sessions of group cognitive-behavioral therapy (CBT), a standard support group, or usual care. Sixty-four women withdrew before the study ended. After 1 year, women who received psychological interventions—47 in the CBT group and 48 in the standard support group—had statistically significant higher pregnancy rates, compared with 25 women who received usual care.16 Conversely, a literature review and evaluation of 25 studies found psychosocial interventions unlikely to improve pregnancy rates in infertile women.17
Methodologic problems. Most studies of stress’ influence on fertility are small, and many have methodologic problems.4 In some, researchers lumped together women whose infertility was caused by disparate diagnoses such as male-factor infertility, blocked fallopian tubes, and advanced age. Retrospective studies also must be interpreted with caution because:
- patients who did not become pregnant may have exaggerated the degree of their depression and its effects
- those with pre-existing medical problems would know they were unlikely to conceive and might have been more depressed before and during infertility treatments.18
Recommendation. When counseling patients about the role of stress in infertility and its treatment, we recommend emphasizing that:
- infertility can cause stress in many areas of life
- the effect of stress on fertility, if any, is likely to be minimal for most women.
Case continued: Strain and anger
You begin to see Mrs. S weekly for supportive therapy, using cognitive restructuring and relaxation techniques to alleviate her anxiety and depression. She decides not to start an antidepressant because she does not want to be on medication if she becomes pregnant.
During the next 2 months she finishes an unsuccessful IUI cycle and reports that her relationship with her husband has become strained. She avoids friends who have children and feels angry when she sees a pregnant woman. She dislikes going to family events because relatives sometimes ask, “When are you going to get pregnant?”
Her work as a manager is suffering because of her many visits to fertility specialists. Her Beck Depression Inventory score has increased to 33, indicating worsening depression.
Infertility’s psychological toll
Patients rarely accept infertility with equanimity, and their responses include shock, denial, anger, isolation, guilt, and grief.6 Some women say the experience of being infertile feels comparable to having cancer.20
The incidence of clinical major depression, poor self-esteem, and sexual dysfunction in women who undergo infertility evaluation does not differ significantly from that of their fertile peers.9 Even so, infertile women report a roller-coaster ride of emotions: hope as treatments are tried, despair when treatments fail.
Health care providers can add to the angst by telling women they have an “incompetent” cervix, “poor-quality” or “old” eggs, or “inadequate” mucus; these insensitive descriptions can lead women to blame themselves and feel ashamed, guilty, and depressed.4,5,18
Psychotherapy. Providing education and teaching skills such as relaxation training has been shown to reduce depressive symptoms more effectively than having patients discuss their thoughts and feelings about infertility.17 Helpful psychotherapies emphasize CBT and improved coping skills.
Negative coping strategies include escape/avoidance conduct or self-blame (such as, “I’m not getting pregnant because I work too hard”). Encourage patients to replace these with protective coping strategies, such as seeking social support and engaging in active problem-solving (“I reach out to friends who help comfort me, and I set limits with friends who make me feel bad about myself ”).21-23
Medication. Even though sadness and anxiety are normal responses to infertility, psychotropic medications might be appropriate after a thorough evaluation. Keep in mind, however, that selective serotonin reuptake inhibitors (SSRIs) can cause prolactinemia, which could interfere with ovulation.9 Miscarriage and stillbirth rates among women taking SSRIs are similar to those of the general population.24
Case continued: It takes two
Despite three IUI cycles over 12 months Mrs. S has not become pregnant. She considers IVF but is concerned about the cost and the less than 50% chance of success.
You encourage her to continue individual supportive and cognitive therapy and to consider couple’s therapy. She and her husband decide to attend a group for couples with infertility. She accepts your referral to RESOLVE, a national support program for infertile patients (see Related resources).
Problems facing infertile couples
Gender differences in coping style. Men and women experience infertility differently.
The women in infertile couples often are distressed, whereas the men tend to remain more confident that some kind of treatment will work. This imbalance can leave the woman feeling unsupported and the man feeling confused about why she is so upset about what he sees as just a medical problem to be solved.
When a couple’s infertility has been attributed to sperm abnormalities, however, the man’s stress level can equal the woman’s. Women tend to feel stress regardless of which partner is “at fault.”25
Grief reactions. The “loss” of a child never conceived generally goes unrecognized but has psychological consequences. Both partners can feel:
- low self-esteem
- sadness about being unable to experience parenting
- doubts about their femininity or masculinity
- regret over unfulfilled dreams.
Table 2
Fictions and facts about infertility
| Fiction | Fact |
|---|---|
| Infertility is a psychosomatic disorder | An organic cause is found in 85% to 90% of infertile couples7 |
| Infertility is a female problem | One-third of infertility cases are caused by female factors, one-third by male factors, and one-third by male and female factors or unknown causes26 |
| Infertility is epidemic | The number of patients seeking infertility treatment has increased dramatically in 20 years, but the infertility rate is stable3,5,18 |
| Infertility is rare | Approximately 10% of U.S. couples of childbearing age are infertile3 |
| If you adopt, you'll get pregnant | Conception rates are no higher following adoption than among childless couples7 |
Employment. Infertility treatments are time- and resource-intensive, and patients often miss work. Even while on the job, a woman distracted by infertility or treatment side effects might not perform as well as she could. Worries about job security add to her anxiety.
Finances. Infertility treatment is expensive and is not always covered by insurance. The American Society for Reproductive Medicine reports that the cost of an IVF cycle averages $12,400, and success rates are 26 (see Related resources).
To continue treatment, couples may take second jobs, acquire loans, deplete savings, or accumulate debt. Many couples—even with extraordinary effort—cannot afford to start or continue advanced infertility treatments.
Spirituality. Patients who believe that infertility is God’s punishment for past sins may experience a religious crisis. Those affiliated with religions that restrict assisted-reproductive technology may feel forced to choose between doctrinal dictates and their dreams of becoming parents.
Case continued: A new ‘RESOLVE’
Mrs. S enjoys her association with the online support of RESOLVE. Through message boards, she shares her concerns with other women undergoing infertility treatment. She also finds support from friends, although she continues to set limits such as declining invitations to baby showers. She practices relaxation techniques at home.
Since she and her husband have joined the group for infertile couples, their relationship has improved. Mrs. S feels that he better understands her fears after hearing other women in the group being “just as emotional.” He no longer tells her, “It’s just a medical problem.”
- National Infertility Association (RESOLVE). www.resolve.org.
- American Society for Reproductive Medicine. www.asrm.org.
- Sildenafil • Viagra
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Khademi A, Alleyassin A, Aghahosseini M, et al. Pretreatment Beck Depression Inventory score is an important predictor for post-treatment score in infertile patients: a before-after study. BMC Psychiatry 2005;5(1):25.-
2. Hjelmstedt A, Widstrom AM, Wramsby H, et al. Personality factors and emotional responses to pregnancy among IVF couples in early pregnancy: a comparative study. Acta Obstet Gynecol Scand 2003;82(2):152-61.
3. Abma J, Chandra A, Mosher W, et al. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and Health Statistics, Series 23, No. 19. National Center for Health Statistics, May 1997.
4. Wischmann TH. Psychogenic infertility-myths and facts. J Assist Reprod Genet 2003;20(12):485-94.
5. Burns LH, Covington SN, eds Infertility counseling: a comprehensive handbook for clinicians.. Pearl River, NY: Parthenon; 1999:122-35.
6. Stanton AL, Lobel M, Sears S, DeLuca RS. Psychosocial aspects of selected issues in women’s reproductive health: current status and future directions. J Consult Clin Psychol 2002;70(3):751-70.
7. National Infertility Association (RESOLVE) www.resolve.org. Accessed August 30, 2006.
8. Kasper DL, Braunwald E, Fauci A, et al. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill Professional; 2004.
9. Cedars M (ed) Infertility: practical pathways in obstetrics and gynecology. New York: McGraw-Hill; 2005:88-133.
10. Greil AL. Infertility and psychological distress: a critical review of the literature. Soc Sci Med 1997;45(11):1679-704.
11. Demyttenaere K, Nijs P, Evers-Kiebooms G, Koninckx PR. Coping and the ineffectiveness of coping influence the outcome of in vitro fertilization through stress responses. Psychoneuroendocrinology 1992;17(6):655-65.
12. Thiering P, Beaurepaire J, Jones M, et al. Mood state as a predictor of treatment outcome after in vitro fertilization/embryo transfer technology (IVF/ET). J Psychosom Res 1993;37(5):481-91.
13. Sanders KA, Bruce NW. A prospective study of psychological stress and fertility in women. Hum Reprod 1997;12(10):2324-9.
14. Facchinetti F, Matteo ML, Artini GP, et al. An increased vulnerability to stress is associated with a poor outcome of in vitro fertilization-embryo transfer treatment. Fertil Steril 1997;67(2):309-14.
15. Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod 1998;13(8):2296-300.
16. Domar AD, Clapp D, Slawsby EA, et al. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril 2000;73(4):805-11.
17. Boivin J. A review to psychosocial interventions in infertility. Soc Sci Med 2003;57(12):2325-41.
18. Pasch LA. Confronting fertility problems: current research and future challenges. In: Baum A, Revenson TA, Singer JE (eds). Handbook of health psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2001:559-70.
19. Stewart DE, Boydell KM, McCarthy K, et al. A prospective study of the effectiveness of brief professionally-led support groups for infertility patients. Int J Psychiatry Med 1992;22(2):173-82.
20. Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993;14(suppl):45-52.
21. Litt MD, Tennen H, Affleck G, Klock S. Coping and cognitive factors in adaptation to in vitro fertilization failure. J Behav Med 1992;15(2):171-87.
22. Peterson BD, Newton CR, Rosen KH, Skaggs GE. The relationship between coping and depression in men and women referred for in vitro fertilization. Fertil Steril 2006;85(3):802-4.
23. Morrow KA, Thoreson RW, Penney LL. Predictors of psychological distress among infertility clinic patients. J Consult Clin Psychol 1995;63(1):163-7.
24. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy: do antidepressants’ benefits outweigh the risks? Current Psychiatry 2006;5(4):31-40.
25. Nachtigall RD, Becker G, Wozny M. The effects of gender-specific diagnosis on men’s and women’s response to infertility. Fertil Steril 1992;57(1):113-21.
26. American Society for Reproductive Medicine Information for patients. Is infertility treatment expensive? Available at: http://www.asrm.org/Patients/faqs.html#Q6. Accessed September 12, 2006
1. Khademi A, Alleyassin A, Aghahosseini M, et al. Pretreatment Beck Depression Inventory score is an important predictor for post-treatment score in infertile patients: a before-after study. BMC Psychiatry 2005;5(1):25.-
2. Hjelmstedt A, Widstrom AM, Wramsby H, et al. Personality factors and emotional responses to pregnancy among IVF couples in early pregnancy: a comparative study. Acta Obstet Gynecol Scand 2003;82(2):152-61.
3. Abma J, Chandra A, Mosher W, et al. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and Health Statistics, Series 23, No. 19. National Center for Health Statistics, May 1997.
4. Wischmann TH. Psychogenic infertility-myths and facts. J Assist Reprod Genet 2003;20(12):485-94.
5. Burns LH, Covington SN, eds Infertility counseling: a comprehensive handbook for clinicians.. Pearl River, NY: Parthenon; 1999:122-35.
6. Stanton AL, Lobel M, Sears S, DeLuca RS. Psychosocial aspects of selected issues in women’s reproductive health: current status and future directions. J Consult Clin Psychol 2002;70(3):751-70.
7. National Infertility Association (RESOLVE) www.resolve.org. Accessed August 30, 2006.
8. Kasper DL, Braunwald E, Fauci A, et al. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill Professional; 2004.
9. Cedars M (ed) Infertility: practical pathways in obstetrics and gynecology. New York: McGraw-Hill; 2005:88-133.
10. Greil AL. Infertility and psychological distress: a critical review of the literature. Soc Sci Med 1997;45(11):1679-704.
11. Demyttenaere K, Nijs P, Evers-Kiebooms G, Koninckx PR. Coping and the ineffectiveness of coping influence the outcome of in vitro fertilization through stress responses. Psychoneuroendocrinology 1992;17(6):655-65.
12. Thiering P, Beaurepaire J, Jones M, et al. Mood state as a predictor of treatment outcome after in vitro fertilization/embryo transfer technology (IVF/ET). J Psychosom Res 1993;37(5):481-91.
13. Sanders KA, Bruce NW. A prospective study of psychological stress and fertility in women. Hum Reprod 1997;12(10):2324-9.
14. Facchinetti F, Matteo ML, Artini GP, et al. An increased vulnerability to stress is associated with a poor outcome of in vitro fertilization-embryo transfer treatment. Fertil Steril 1997;67(2):309-14.
15. Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod 1998;13(8):2296-300.
16. Domar AD, Clapp D, Slawsby EA, et al. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril 2000;73(4):805-11.
17. Boivin J. A review to psychosocial interventions in infertility. Soc Sci Med 2003;57(12):2325-41.
18. Pasch LA. Confronting fertility problems: current research and future challenges. In: Baum A, Revenson TA, Singer JE (eds). Handbook of health psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2001:559-70.
19. Stewart DE, Boydell KM, McCarthy K, et al. A prospective study of the effectiveness of brief professionally-led support groups for infertility patients. Int J Psychiatry Med 1992;22(2):173-82.
20. Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993;14(suppl):45-52.
21. Litt MD, Tennen H, Affleck G, Klock S. Coping and cognitive factors in adaptation to in vitro fertilization failure. J Behav Med 1992;15(2):171-87.
22. Peterson BD, Newton CR, Rosen KH, Skaggs GE. The relationship between coping and depression in men and women referred for in vitro fertilization. Fertil Steril 2006;85(3):802-4.
23. Morrow KA, Thoreson RW, Penney LL. Predictors of psychological distress among infertility clinic patients. J Consult Clin Psychol 1995;63(1):163-7.
24. Hasser C, Brizendine L, Spielvogel A. SSRI use during pregnancy: do antidepressants’ benefits outweigh the risks? Current Psychiatry 2006;5(4):31-40.
25. Nachtigall RD, Becker G, Wozny M. The effects of gender-specific diagnosis on men’s and women’s response to infertility. Fertil Steril 1992;57(1):113-21.
26. American Society for Reproductive Medicine Information for patients. Is infertility treatment expensive? Available at: http://www.asrm.org/Patients/faqs.html#Q6. Accessed September 12, 2006
For women only: Hormones may prevent addiction relapse
Women become dependent more rapidly than men after initial cocaine, opioid, or alcohol use and may be more sensitive to drugs’ adverse health effects.1 And although men and women relapse to substance use at similar rates, ovulating women may be particularly vulnerable to relapse at certain times of the month.
Understanding the hormonal influences that increase women’s relapse risk can help you intervene more effectively. This article describes:
- how women’s relapse patterns differ from men’s
- why psychotherapy and hormone regulation may be preferred for relapse prevention in women with substance use disorders.
Case report: will she relapse again?
Ms. H, age 46, is in her third month of an alcohol and drug residential rehabilitation program. She has a 10-year history of alcohol and crack cocaine dependence and is battling cravings to use again. These feelings are usually triggered by being in places or with people associated with her drug use, but this time she is committed to staying sober.
She started smoking cigarettes in her teens and using drugs and alcohol in her mid 20s. She feels that her dependency has been out of control in the 10 years since her son was born.
She has tried to quit many times on her own but has managed no more than 1 month of abstinence. She often has relapsed in response to feeling anxious or depressed about being unemployed or after arguing with her partner.
Mechanisms of relapse
Dopamine release is essential for encoding learned associations. When a drug is used in the early dependency state, dopamine release produces pleasure that reinforces continued drug use. Once the behavior is learned, environmental stimuli can trigger dopamine and turn on the brain circuits for this familiar, highly rewarding behavior. Dopamine also is the primary cause of long-lasting brain changes that make it difficult for substance-dependent persons such as Ms. H to control desire for the drug.2
Early relapse—caused by dopamine’s and other neurotransmitters’ effects on various brain regions—is triggered by environmental stimuli such as:
- re-exposure to a small amount of the drug
- exposure to an environment or cues associated with past drug use
- exposure to stressful events.
Table 1
3 stages of relapse and their neurobiologic components
| Relapse stage | Key neurotransmitters | Brain regions involved |
|---|---|---|
| Early relapse triggered by: | ||
| • exposure to a small amount of the drug | Dopamine | Ventral tegmental area |
| Nucleus accumbens core | ||
| Prefrontal cortex | ||
| • environmental cues that re-trigger learned associations | Dopamine | Basolateral amygdala |
| Nucleus accumbens core | ||
| • stressful events and disappointments | Norepinephrine, corticotropin-releasing factor | Extended amygdala |
| Bed nucleus of the stria terminalis | ||
| Craving | Multiple, undetermined | Prefrontal cortex circuitry involving the anterior cingulate and orbitofrontal cortices |
| Relapse | Glutamate, dopamine | Prefrontal cortex |
| Nucleus accumbens core | ||
| Ventral pallidum | ||
| Source: References 2-4 | ||
Emotions, stress trigger relapse
Ms. H. reports increased irritability and impulsivity along with depressed mood—especially during the 3 to 4 days preceding her menstrual period. Her periods are regular, and these mood symptoms recur each month. She does not meet criteria for major depressive disorder.
Emotional reactions play a larger role in relapse for women than for men. Women report higher levels of craving and depressed mood during abstinence and experience stronger urges to drink and smoke when depressed. Women also are more likely to report substance use relapse in response to specific stressful events, disappointments, or depressed mood.1 This is consistent with evidence that women have heightened physiologic responses to social rejection and social stressors.5
A lower density of brain serotonin transporter has been associated with a higher risk of depression in women. Because estrogen and progesterone affect expression of the serotonin transporter, changes in these hormone levels might alter the risk of depression.6 Thus, ovarian hormones’ effect on the serotonin system may contribute to the higher rate of emotionally triggered relapse in women versus men.
Menstrual cycle phases. How men and women respond to stress may contribute to differences in their relapse behaviors (Table 2).
During the first 2 weeks of the menstrual cycle—the follicular phase—women show lower physiologic reactivity (as seen in blood pressure and catecholamine measurements) and lower cortisol responsiveness than men do in response to psychosocial stress. Estrogen contributes to this effect by attenuating sympathoadrenal responsiveness.5
During the latter 2 weeks of the menstrual cycle—the luteal phase—the ovulating woman’s hypothalamic-pituitary-adrenal (HPA) axis response increases and increases her sensitivity to stress. In this phase, progesterone’s presence reverses estrogen’s effect and makes the brain more reactive to emotions and stressors.
Higher stress responsiveness is associated with increased cocaine craving.7,8 A 20-year literature review of the role of substance abuse in depression indicates that HPA axis responsiveness of depressed women exceeds that of depressed men.9
Summary. Women may be more susceptible than men to emotionally triggered relapse, especially during the menstrual cycle’s luteal phase. Women may also be more susceptible than men to relapse in response to nicotine and cocaine cues.
Table 2
Substance use relapse patterns: Women versus men
| Emotions and mood state play a greater role in driving relapse in women |
| Craving. Women have greater craving than men in response to nicotine and cocaine drug cues |
| Nicotine dependency. Women are more likely to relapse to cigarette use |
| Abstinence. Women have shorter abstinence periods after cocaine treatment |
| Residential treatment. Women have a better prognosis than men 6 months after residential cocaine treatment |
| Premenstrual hormone changes increase women’s relapse risk |
Menstruation and relapse patterns
Research into a correlation between menstrual cycle phases and dependency behavior is in its infancy. Early nicotine, alcohol, and stimulant addiction studies have shown inconsistent results.
Nicotine. A naturalistic study of women smokers ages 20 to 39 showed that they smoked more cigarettes per day during the late luteal phase, but their nicotine boost and mood states were similar throughout the menstrual cycle.10
Some—but not all—studies suggest that nicotine withdrawal symptoms increase during the luteal phase.11,12 In an outpatient study designed to assess hormonal effects on nicotine response, 30 female smokers acutely abstinent of nicotine were randomly assigned by menstrual cycle phase to receive transdermal nicotine or a placebo patch. Both premenstrual and nicotine withdrawal symptoms intensified in the women’s late luteal phase, compared with the follicular phase.12
Conversely, the same researchers found that menstrual cycle phase did not affect withdrawal symptoms in 21 nicotine-dependent female inpatients, even though premenstrual changes occurred in the late luteal phase.
As the authors observed, drawing conclusions can be difficult when menstrual cycle hormone withdrawal and nicotine withdrawal symptoms overlap.12-16
Alcohol. Premenstrual syndrome (PMS) increases a woman’s risk of alcohol abuse. Alcohol and allopregnenolone—progesterone’s neuroactive metabolite—both facilitate gamma-aminobutyric acid ionotropic type A (GABAA) receptor activity. Thus, women with PMS and alcohol dependence may have a genetically more-sensitive GABAA system.17 Unfortunately, aside from one study that shows increased alcohol intake during the luteal phase, no studies have examined alcohol withdrawal, craving, and relapse across the menstrual cycle.18
Stimulants. Stimulant craving and relapse have not been examined in women at different menstrual cycle phases. Some authors speculate that women may have a higher subjective response to stimulants during the early follicular phase—when estrogen levels are higher and progesterone levels are lower—compared with the luteal phase.19
Sex hormones and relapse
Estrogen. Preclinical studies suggest that estrogen facilitates substance dependence by enhancing dopaminergic activity (Table 3).1,20-26 Because reinstatement (the animal model of relapse) is driven partially by dopaminergic activity in the striatum, one could hypothesize that:
- estrogen’s dopamine-enhancing effects facilitate dependence
- women with stimulant dependence are at higher risk of relapse in the follicular phase—when estrogen levels are higher—than in the luteal phase.
Table 3
Preclinical findings: How hormones may influence addictive behavior
| Hormone | Neurobiologic effect | Mechanism | Behavioral effect |
|---|---|---|---|
| Estrogen | Facilitates dopamine | Decreases inhibitory GABAB activity, regulates D2 autoreceptor expression, alters dopamine reuptake, modulates glutamate activity | Enhances reward, self-administration, sensitization,* and stimulant dependence; facilitates reinstatement† |
| Progesterone | Facilitates dopamine when given intermittently; might facilitate or inhibit estrogen’s effects on dopamine | Unclear | Attenuates response to stress, anxiety, pain, aggressiveness |
| Allopregnenolone‡ | Facilitates GABAA | GABAA-positive allosteric modulator, such as ethanol | Enhances ethanol consumption, promotes ethanol reinstatement |
| GABAA/GABAB: gamma-aminobutyric acid, ionotropic types A and B | |||
| * Sensitization: Repeated exposure to psychostimulants results in drug-seeking response to subsequent exposure, which plays an important role in addiction and craving. | |||
| † Reinstatement is the animal model of relapse. | |||
| ‡ Progesterone’s neuroactive metabolite | |||
| Source: References 1, 20-26 | |||
Treatment implications
Psychotherapy. Evidence suggests that emotions and mood states may play a larger role in triggering substance abuse relapse in women than in men. Psychotherapy and residential treatment therefore are particularly important components of women’s treatment.
In a 6-month follow-up outpatient study of cocaine dependence, women responded better than men did to behavioral treatment, even though the women had more-severe disorders at entry.27,28
A more-recent inpatient study followed 64 men and 37 women hospitalized for treatment of cocaine dependence. Researchers compared the patients’ drug use histories, psychiatric diagnoses, and Addiction Severity Index (ASI) scores during hospitalization and their cocaine use and ASI scores 6 months later. In initial evaluations, women had significantly more-severe family and social problems. At follow-up, however, significantly more women than men were abstinent from cocaine use, and their family/social problems had diminshed.28
Notably, a recent functional MRI study comparing 17 male and 10 female abstinent cocaine-dependent subjects indicated that the women more often used verbal coping strategies to decrease cocaine craving.29 This finding supports the potential benefit of psychotherapy to prevent relapse in women with a history of substance dependence.
Hormone regulation. For many women, continuous oral contraceptives (OCPs) can improve affect variability across the menstrual cycle and diminish negative mood. Others, however, experience negative changes in mood or affect while taking OCPs. Risk factors for a negative response include:
- history of depression or other psychological distress symptoms
- dysmenorrhea
- PMS
- history of pregnancy-related mood symptoms
- family history of OCP-related mood complaints
- being in the postpartum
- age 30
Continuous OCPs can be given so that women have only two to three menstrual periods per year. Formulations with ethinyl estradiol and norethindrone—such as Necon 0.5/35 or 1.0/35—may stabilize mood more effectively than others.
Give a 2-month trial, then re-evaluate progress. Because of the increased risk of clotting, only nonsmokers and women without a history of blood clots should take OCPs.
Case report: Fighting the cravings
Eight months ago, when Ms. H was still using cocaine, her primary care physician prescribed fluoxetine, 20 mg/d, for depressive symptoms. Her mood has not improved, nor has her menstrual cycle-related depression or irritability. She asks if anything else might stop her premenstrual cravings.
Because of Ms. H’s reported PMS, we counsel her to be especially vigilant for alcohol cravings around the luteal and late luteal phases of her menstrual cycle (Table 4). We discuss with her:
- the need to watch for signs of relapse
- the importance of aggressive treatment, including psychotherapy, group therapy, and residential treatment, as needed.
She agrees to a 2-month trial, and we schedule a follow-up appointment to re-evaluate her progress.
Table 4
Interventions to prevent relapse in women with addiction disorders
| Track craving and mood symptoms in relation to the patient’s menstrual cycle |
| Educate her about triggers for relapse |
| Provide psychotherapy to bolster her coping strategies for stressful life events |
| Screen for comorbid mood or psychiatric disorders and treat them aggressively |
| Treat premenstrual mood symptoms with a selective serotonin reuptake inhibitor and/or by regulating hormone levels with a continuous oral contraceptive |
- Carroll ME, Lynch WJ, Roth ME, et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci 2004;25(5):273-9.
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 2004;28(6):533-46.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8(11):1481-9.
- Fluoxetine • Prozac
- Ethinyl estradiol and norethindrone oral contraceptive • Necon, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121-37.
2. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403-13.
3. Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44-56.
4. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex decision-making and drug addiction. Trends Neurosci 2006;29(2):116-24.
5. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31(2):151-78.
6. Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59(1):40-7.
7. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 2006;63(3):324-31.
8. Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 2005;29(7):1351-5.
9. Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry 2002;63(7):616-27.
10. Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25(7):677-91.
11. O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 1989;14(6):595-600.
12. Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res 2000;2(3):231-41.
13. Allen SS, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res 1999;1(2):129-42.
14. Franklin TR, Napier K, Ehrman R, et al. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res 2004;6(1):171-5.
15. Pomerleau CS, Mehringer AM, Marks JL, et al. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav 2000;25(4):483-97.
16. Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relapse and withdrawal symptoms. In: Keefe FJ (ed). Thirteenth annual proceedings for the Society of Behavioral Medicine. Rockville, MD, 1992:107.
17. Backstrom T, Andersson A, Andree L, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 2003;1007:42-53.
18. Harvey SM, Beckman LJ. Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 1985;9(5):465-7.
19. White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73(4):729-41.
20. Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 2004;29(1):81-5.
21. Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 2004;46(5):672-87.
22. Lynch WJ, Roth ME, Mickelberg JL, Carroll M. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001;68(4):641-6.
23. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med 2004;71(Suppl 2):S4-10.
24. Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004;19(6):344-53.
25. Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 2003;30(1):1-7.
26. Nie H, Janak, PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1-2):222-8.
27. Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63-6.
28. Weiss RD, Martinez-Raga J, Griffin ML, et al. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 1997;44(1):35-40.
29. Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:487-94.
30. Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord 2002;70(3):229-40.
31. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am 2004;27(4):627-48.
Women become dependent more rapidly than men after initial cocaine, opioid, or alcohol use and may be more sensitive to drugs’ adverse health effects.1 And although men and women relapse to substance use at similar rates, ovulating women may be particularly vulnerable to relapse at certain times of the month.
Understanding the hormonal influences that increase women’s relapse risk can help you intervene more effectively. This article describes:
- how women’s relapse patterns differ from men’s
- why psychotherapy and hormone regulation may be preferred for relapse prevention in women with substance use disorders.
Case report: will she relapse again?
Ms. H, age 46, is in her third month of an alcohol and drug residential rehabilitation program. She has a 10-year history of alcohol and crack cocaine dependence and is battling cravings to use again. These feelings are usually triggered by being in places or with people associated with her drug use, but this time she is committed to staying sober.
She started smoking cigarettes in her teens and using drugs and alcohol in her mid 20s. She feels that her dependency has been out of control in the 10 years since her son was born.
She has tried to quit many times on her own but has managed no more than 1 month of abstinence. She often has relapsed in response to feeling anxious or depressed about being unemployed or after arguing with her partner.
Mechanisms of relapse
Dopamine release is essential for encoding learned associations. When a drug is used in the early dependency state, dopamine release produces pleasure that reinforces continued drug use. Once the behavior is learned, environmental stimuli can trigger dopamine and turn on the brain circuits for this familiar, highly rewarding behavior. Dopamine also is the primary cause of long-lasting brain changes that make it difficult for substance-dependent persons such as Ms. H to control desire for the drug.2
Early relapse—caused by dopamine’s and other neurotransmitters’ effects on various brain regions—is triggered by environmental stimuli such as:
- re-exposure to a small amount of the drug
- exposure to an environment or cues associated with past drug use
- exposure to stressful events.
Table 1
3 stages of relapse and their neurobiologic components
| Relapse stage | Key neurotransmitters | Brain regions involved |
|---|---|---|
| Early relapse triggered by: | ||
| • exposure to a small amount of the drug | Dopamine | Ventral tegmental area |
| Nucleus accumbens core | ||
| Prefrontal cortex | ||
| • environmental cues that re-trigger learned associations | Dopamine | Basolateral amygdala |
| Nucleus accumbens core | ||
| • stressful events and disappointments | Norepinephrine, corticotropin-releasing factor | Extended amygdala |
| Bed nucleus of the stria terminalis | ||
| Craving | Multiple, undetermined | Prefrontal cortex circuitry involving the anterior cingulate and orbitofrontal cortices |
| Relapse | Glutamate, dopamine | Prefrontal cortex |
| Nucleus accumbens core | ||
| Ventral pallidum | ||
| Source: References 2-4 | ||
Emotions, stress trigger relapse
Ms. H. reports increased irritability and impulsivity along with depressed mood—especially during the 3 to 4 days preceding her menstrual period. Her periods are regular, and these mood symptoms recur each month. She does not meet criteria for major depressive disorder.
Emotional reactions play a larger role in relapse for women than for men. Women report higher levels of craving and depressed mood during abstinence and experience stronger urges to drink and smoke when depressed. Women also are more likely to report substance use relapse in response to specific stressful events, disappointments, or depressed mood.1 This is consistent with evidence that women have heightened physiologic responses to social rejection and social stressors.5
A lower density of brain serotonin transporter has been associated with a higher risk of depression in women. Because estrogen and progesterone affect expression of the serotonin transporter, changes in these hormone levels might alter the risk of depression.6 Thus, ovarian hormones’ effect on the serotonin system may contribute to the higher rate of emotionally triggered relapse in women versus men.
Menstrual cycle phases. How men and women respond to stress may contribute to differences in their relapse behaviors (Table 2).
During the first 2 weeks of the menstrual cycle—the follicular phase—women show lower physiologic reactivity (as seen in blood pressure and catecholamine measurements) and lower cortisol responsiveness than men do in response to psychosocial stress. Estrogen contributes to this effect by attenuating sympathoadrenal responsiveness.5
During the latter 2 weeks of the menstrual cycle—the luteal phase—the ovulating woman’s hypothalamic-pituitary-adrenal (HPA) axis response increases and increases her sensitivity to stress. In this phase, progesterone’s presence reverses estrogen’s effect and makes the brain more reactive to emotions and stressors.
Higher stress responsiveness is associated with increased cocaine craving.7,8 A 20-year literature review of the role of substance abuse in depression indicates that HPA axis responsiveness of depressed women exceeds that of depressed men.9
Summary. Women may be more susceptible than men to emotionally triggered relapse, especially during the menstrual cycle’s luteal phase. Women may also be more susceptible than men to relapse in response to nicotine and cocaine cues.
Table 2
Substance use relapse patterns: Women versus men
| Emotions and mood state play a greater role in driving relapse in women |
| Craving. Women have greater craving than men in response to nicotine and cocaine drug cues |
| Nicotine dependency. Women are more likely to relapse to cigarette use |
| Abstinence. Women have shorter abstinence periods after cocaine treatment |
| Residential treatment. Women have a better prognosis than men 6 months after residential cocaine treatment |
| Premenstrual hormone changes increase women’s relapse risk |
Menstruation and relapse patterns
Research into a correlation between menstrual cycle phases and dependency behavior is in its infancy. Early nicotine, alcohol, and stimulant addiction studies have shown inconsistent results.
Nicotine. A naturalistic study of women smokers ages 20 to 39 showed that they smoked more cigarettes per day during the late luteal phase, but their nicotine boost and mood states were similar throughout the menstrual cycle.10
Some—but not all—studies suggest that nicotine withdrawal symptoms increase during the luteal phase.11,12 In an outpatient study designed to assess hormonal effects on nicotine response, 30 female smokers acutely abstinent of nicotine were randomly assigned by menstrual cycle phase to receive transdermal nicotine or a placebo patch. Both premenstrual and nicotine withdrawal symptoms intensified in the women’s late luteal phase, compared with the follicular phase.12
Conversely, the same researchers found that menstrual cycle phase did not affect withdrawal symptoms in 21 nicotine-dependent female inpatients, even though premenstrual changes occurred in the late luteal phase.
As the authors observed, drawing conclusions can be difficult when menstrual cycle hormone withdrawal and nicotine withdrawal symptoms overlap.12-16
Alcohol. Premenstrual syndrome (PMS) increases a woman’s risk of alcohol abuse. Alcohol and allopregnenolone—progesterone’s neuroactive metabolite—both facilitate gamma-aminobutyric acid ionotropic type A (GABAA) receptor activity. Thus, women with PMS and alcohol dependence may have a genetically more-sensitive GABAA system.17 Unfortunately, aside from one study that shows increased alcohol intake during the luteal phase, no studies have examined alcohol withdrawal, craving, and relapse across the menstrual cycle.18
Stimulants. Stimulant craving and relapse have not been examined in women at different menstrual cycle phases. Some authors speculate that women may have a higher subjective response to stimulants during the early follicular phase—when estrogen levels are higher and progesterone levels are lower—compared with the luteal phase.19
Sex hormones and relapse
Estrogen. Preclinical studies suggest that estrogen facilitates substance dependence by enhancing dopaminergic activity (Table 3).1,20-26 Because reinstatement (the animal model of relapse) is driven partially by dopaminergic activity in the striatum, one could hypothesize that:
- estrogen’s dopamine-enhancing effects facilitate dependence
- women with stimulant dependence are at higher risk of relapse in the follicular phase—when estrogen levels are higher—than in the luteal phase.
Table 3
Preclinical findings: How hormones may influence addictive behavior
| Hormone | Neurobiologic effect | Mechanism | Behavioral effect |
|---|---|---|---|
| Estrogen | Facilitates dopamine | Decreases inhibitory GABAB activity, regulates D2 autoreceptor expression, alters dopamine reuptake, modulates glutamate activity | Enhances reward, self-administration, sensitization,* and stimulant dependence; facilitates reinstatement† |
| Progesterone | Facilitates dopamine when given intermittently; might facilitate or inhibit estrogen’s effects on dopamine | Unclear | Attenuates response to stress, anxiety, pain, aggressiveness |
| Allopregnenolone‡ | Facilitates GABAA | GABAA-positive allosteric modulator, such as ethanol | Enhances ethanol consumption, promotes ethanol reinstatement |
| GABAA/GABAB: gamma-aminobutyric acid, ionotropic types A and B | |||
| * Sensitization: Repeated exposure to psychostimulants results in drug-seeking response to subsequent exposure, which plays an important role in addiction and craving. | |||
| † Reinstatement is the animal model of relapse. | |||
| ‡ Progesterone’s neuroactive metabolite | |||
| Source: References 1, 20-26 | |||
Treatment implications
Psychotherapy. Evidence suggests that emotions and mood states may play a larger role in triggering substance abuse relapse in women than in men. Psychotherapy and residential treatment therefore are particularly important components of women’s treatment.
In a 6-month follow-up outpatient study of cocaine dependence, women responded better than men did to behavioral treatment, even though the women had more-severe disorders at entry.27,28
A more-recent inpatient study followed 64 men and 37 women hospitalized for treatment of cocaine dependence. Researchers compared the patients’ drug use histories, psychiatric diagnoses, and Addiction Severity Index (ASI) scores during hospitalization and their cocaine use and ASI scores 6 months later. In initial evaluations, women had significantly more-severe family and social problems. At follow-up, however, significantly more women than men were abstinent from cocaine use, and their family/social problems had diminshed.28
Notably, a recent functional MRI study comparing 17 male and 10 female abstinent cocaine-dependent subjects indicated that the women more often used verbal coping strategies to decrease cocaine craving.29 This finding supports the potential benefit of psychotherapy to prevent relapse in women with a history of substance dependence.
Hormone regulation. For many women, continuous oral contraceptives (OCPs) can improve affect variability across the menstrual cycle and diminish negative mood. Others, however, experience negative changes in mood or affect while taking OCPs. Risk factors for a negative response include:
- history of depression or other psychological distress symptoms
- dysmenorrhea
- PMS
- history of pregnancy-related mood symptoms
- family history of OCP-related mood complaints
- being in the postpartum
- age 30
Continuous OCPs can be given so that women have only two to three menstrual periods per year. Formulations with ethinyl estradiol and norethindrone—such as Necon 0.5/35 or 1.0/35—may stabilize mood more effectively than others.
Give a 2-month trial, then re-evaluate progress. Because of the increased risk of clotting, only nonsmokers and women without a history of blood clots should take OCPs.
Case report: Fighting the cravings
Eight months ago, when Ms. H was still using cocaine, her primary care physician prescribed fluoxetine, 20 mg/d, for depressive symptoms. Her mood has not improved, nor has her menstrual cycle-related depression or irritability. She asks if anything else might stop her premenstrual cravings.
Because of Ms. H’s reported PMS, we counsel her to be especially vigilant for alcohol cravings around the luteal and late luteal phases of her menstrual cycle (Table 4). We discuss with her:
- the need to watch for signs of relapse
- the importance of aggressive treatment, including psychotherapy, group therapy, and residential treatment, as needed.
She agrees to a 2-month trial, and we schedule a follow-up appointment to re-evaluate her progress.
Table 4
Interventions to prevent relapse in women with addiction disorders
| Track craving and mood symptoms in relation to the patient’s menstrual cycle |
| Educate her about triggers for relapse |
| Provide psychotherapy to bolster her coping strategies for stressful life events |
| Screen for comorbid mood or psychiatric disorders and treat them aggressively |
| Treat premenstrual mood symptoms with a selective serotonin reuptake inhibitor and/or by regulating hormone levels with a continuous oral contraceptive |
- Carroll ME, Lynch WJ, Roth ME, et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci 2004;25(5):273-9.
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 2004;28(6):533-46.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8(11):1481-9.
- Fluoxetine • Prozac
- Ethinyl estradiol and norethindrone oral contraceptive • Necon, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Women become dependent more rapidly than men after initial cocaine, opioid, or alcohol use and may be more sensitive to drugs’ adverse health effects.1 And although men and women relapse to substance use at similar rates, ovulating women may be particularly vulnerable to relapse at certain times of the month.
Understanding the hormonal influences that increase women’s relapse risk can help you intervene more effectively. This article describes:
- how women’s relapse patterns differ from men’s
- why psychotherapy and hormone regulation may be preferred for relapse prevention in women with substance use disorders.
Case report: will she relapse again?
Ms. H, age 46, is in her third month of an alcohol and drug residential rehabilitation program. She has a 10-year history of alcohol and crack cocaine dependence and is battling cravings to use again. These feelings are usually triggered by being in places or with people associated with her drug use, but this time she is committed to staying sober.
She started smoking cigarettes in her teens and using drugs and alcohol in her mid 20s. She feels that her dependency has been out of control in the 10 years since her son was born.
She has tried to quit many times on her own but has managed no more than 1 month of abstinence. She often has relapsed in response to feeling anxious or depressed about being unemployed or after arguing with her partner.
Mechanisms of relapse
Dopamine release is essential for encoding learned associations. When a drug is used in the early dependency state, dopamine release produces pleasure that reinforces continued drug use. Once the behavior is learned, environmental stimuli can trigger dopamine and turn on the brain circuits for this familiar, highly rewarding behavior. Dopamine also is the primary cause of long-lasting brain changes that make it difficult for substance-dependent persons such as Ms. H to control desire for the drug.2
Early relapse—caused by dopamine’s and other neurotransmitters’ effects on various brain regions—is triggered by environmental stimuli such as:
- re-exposure to a small amount of the drug
- exposure to an environment or cues associated with past drug use
- exposure to stressful events.
Table 1
3 stages of relapse and their neurobiologic components
| Relapse stage | Key neurotransmitters | Brain regions involved |
|---|---|---|
| Early relapse triggered by: | ||
| • exposure to a small amount of the drug | Dopamine | Ventral tegmental area |
| Nucleus accumbens core | ||
| Prefrontal cortex | ||
| • environmental cues that re-trigger learned associations | Dopamine | Basolateral amygdala |
| Nucleus accumbens core | ||
| • stressful events and disappointments | Norepinephrine, corticotropin-releasing factor | Extended amygdala |
| Bed nucleus of the stria terminalis | ||
| Craving | Multiple, undetermined | Prefrontal cortex circuitry involving the anterior cingulate and orbitofrontal cortices |
| Relapse | Glutamate, dopamine | Prefrontal cortex |
| Nucleus accumbens core | ||
| Ventral pallidum | ||
| Source: References 2-4 | ||
Emotions, stress trigger relapse
Ms. H. reports increased irritability and impulsivity along with depressed mood—especially during the 3 to 4 days preceding her menstrual period. Her periods are regular, and these mood symptoms recur each month. She does not meet criteria for major depressive disorder.
Emotional reactions play a larger role in relapse for women than for men. Women report higher levels of craving and depressed mood during abstinence and experience stronger urges to drink and smoke when depressed. Women also are more likely to report substance use relapse in response to specific stressful events, disappointments, or depressed mood.1 This is consistent with evidence that women have heightened physiologic responses to social rejection and social stressors.5
A lower density of brain serotonin transporter has been associated with a higher risk of depression in women. Because estrogen and progesterone affect expression of the serotonin transporter, changes in these hormone levels might alter the risk of depression.6 Thus, ovarian hormones’ effect on the serotonin system may contribute to the higher rate of emotionally triggered relapse in women versus men.
Menstrual cycle phases. How men and women respond to stress may contribute to differences in their relapse behaviors (Table 2).
During the first 2 weeks of the menstrual cycle—the follicular phase—women show lower physiologic reactivity (as seen in blood pressure and catecholamine measurements) and lower cortisol responsiveness than men do in response to psychosocial stress. Estrogen contributes to this effect by attenuating sympathoadrenal responsiveness.5
During the latter 2 weeks of the menstrual cycle—the luteal phase—the ovulating woman’s hypothalamic-pituitary-adrenal (HPA) axis response increases and increases her sensitivity to stress. In this phase, progesterone’s presence reverses estrogen’s effect and makes the brain more reactive to emotions and stressors.
Higher stress responsiveness is associated with increased cocaine craving.7,8 A 20-year literature review of the role of substance abuse in depression indicates that HPA axis responsiveness of depressed women exceeds that of depressed men.9
Summary. Women may be more susceptible than men to emotionally triggered relapse, especially during the menstrual cycle’s luteal phase. Women may also be more susceptible than men to relapse in response to nicotine and cocaine cues.
Table 2
Substance use relapse patterns: Women versus men
| Emotions and mood state play a greater role in driving relapse in women |
| Craving. Women have greater craving than men in response to nicotine and cocaine drug cues |
| Nicotine dependency. Women are more likely to relapse to cigarette use |
| Abstinence. Women have shorter abstinence periods after cocaine treatment |
| Residential treatment. Women have a better prognosis than men 6 months after residential cocaine treatment |
| Premenstrual hormone changes increase women’s relapse risk |
Menstruation and relapse patterns
Research into a correlation between menstrual cycle phases and dependency behavior is in its infancy. Early nicotine, alcohol, and stimulant addiction studies have shown inconsistent results.
Nicotine. A naturalistic study of women smokers ages 20 to 39 showed that they smoked more cigarettes per day during the late luteal phase, but their nicotine boost and mood states were similar throughout the menstrual cycle.10
Some—but not all—studies suggest that nicotine withdrawal symptoms increase during the luteal phase.11,12 In an outpatient study designed to assess hormonal effects on nicotine response, 30 female smokers acutely abstinent of nicotine were randomly assigned by menstrual cycle phase to receive transdermal nicotine or a placebo patch. Both premenstrual and nicotine withdrawal symptoms intensified in the women’s late luteal phase, compared with the follicular phase.12
Conversely, the same researchers found that menstrual cycle phase did not affect withdrawal symptoms in 21 nicotine-dependent female inpatients, even though premenstrual changes occurred in the late luteal phase.
As the authors observed, drawing conclusions can be difficult when menstrual cycle hormone withdrawal and nicotine withdrawal symptoms overlap.12-16
Alcohol. Premenstrual syndrome (PMS) increases a woman’s risk of alcohol abuse. Alcohol and allopregnenolone—progesterone’s neuroactive metabolite—both facilitate gamma-aminobutyric acid ionotropic type A (GABAA) receptor activity. Thus, women with PMS and alcohol dependence may have a genetically more-sensitive GABAA system.17 Unfortunately, aside from one study that shows increased alcohol intake during the luteal phase, no studies have examined alcohol withdrawal, craving, and relapse across the menstrual cycle.18
Stimulants. Stimulant craving and relapse have not been examined in women at different menstrual cycle phases. Some authors speculate that women may have a higher subjective response to stimulants during the early follicular phase—when estrogen levels are higher and progesterone levels are lower—compared with the luteal phase.19
Sex hormones and relapse
Estrogen. Preclinical studies suggest that estrogen facilitates substance dependence by enhancing dopaminergic activity (Table 3).1,20-26 Because reinstatement (the animal model of relapse) is driven partially by dopaminergic activity in the striatum, one could hypothesize that:
- estrogen’s dopamine-enhancing effects facilitate dependence
- women with stimulant dependence are at higher risk of relapse in the follicular phase—when estrogen levels are higher—than in the luteal phase.
Table 3
Preclinical findings: How hormones may influence addictive behavior
| Hormone | Neurobiologic effect | Mechanism | Behavioral effect |
|---|---|---|---|
| Estrogen | Facilitates dopamine | Decreases inhibitory GABAB activity, regulates D2 autoreceptor expression, alters dopamine reuptake, modulates glutamate activity | Enhances reward, self-administration, sensitization,* and stimulant dependence; facilitates reinstatement† |
| Progesterone | Facilitates dopamine when given intermittently; might facilitate or inhibit estrogen’s effects on dopamine | Unclear | Attenuates response to stress, anxiety, pain, aggressiveness |
| Allopregnenolone‡ | Facilitates GABAA | GABAA-positive allosteric modulator, such as ethanol | Enhances ethanol consumption, promotes ethanol reinstatement |
| GABAA/GABAB: gamma-aminobutyric acid, ionotropic types A and B | |||
| * Sensitization: Repeated exposure to psychostimulants results in drug-seeking response to subsequent exposure, which plays an important role in addiction and craving. | |||
| † Reinstatement is the animal model of relapse. | |||
| ‡ Progesterone’s neuroactive metabolite | |||
| Source: References 1, 20-26 | |||
Treatment implications
Psychotherapy. Evidence suggests that emotions and mood states may play a larger role in triggering substance abuse relapse in women than in men. Psychotherapy and residential treatment therefore are particularly important components of women’s treatment.
In a 6-month follow-up outpatient study of cocaine dependence, women responded better than men did to behavioral treatment, even though the women had more-severe disorders at entry.27,28
A more-recent inpatient study followed 64 men and 37 women hospitalized for treatment of cocaine dependence. Researchers compared the patients’ drug use histories, psychiatric diagnoses, and Addiction Severity Index (ASI) scores during hospitalization and their cocaine use and ASI scores 6 months later. In initial evaluations, women had significantly more-severe family and social problems. At follow-up, however, significantly more women than men were abstinent from cocaine use, and their family/social problems had diminshed.28
Notably, a recent functional MRI study comparing 17 male and 10 female abstinent cocaine-dependent subjects indicated that the women more often used verbal coping strategies to decrease cocaine craving.29 This finding supports the potential benefit of psychotherapy to prevent relapse in women with a history of substance dependence.
Hormone regulation. For many women, continuous oral contraceptives (OCPs) can improve affect variability across the menstrual cycle and diminish negative mood. Others, however, experience negative changes in mood or affect while taking OCPs. Risk factors for a negative response include:
- history of depression or other psychological distress symptoms
- dysmenorrhea
- PMS
- history of pregnancy-related mood symptoms
- family history of OCP-related mood complaints
- being in the postpartum
- age 30
Continuous OCPs can be given so that women have only two to three menstrual periods per year. Formulations with ethinyl estradiol and norethindrone—such as Necon 0.5/35 or 1.0/35—may stabilize mood more effectively than others.
Give a 2-month trial, then re-evaluate progress. Because of the increased risk of clotting, only nonsmokers and women without a history of blood clots should take OCPs.
Case report: Fighting the cravings
Eight months ago, when Ms. H was still using cocaine, her primary care physician prescribed fluoxetine, 20 mg/d, for depressive symptoms. Her mood has not improved, nor has her menstrual cycle-related depression or irritability. She asks if anything else might stop her premenstrual cravings.
Because of Ms. H’s reported PMS, we counsel her to be especially vigilant for alcohol cravings around the luteal and late luteal phases of her menstrual cycle (Table 4). We discuss with her:
- the need to watch for signs of relapse
- the importance of aggressive treatment, including psychotherapy, group therapy, and residential treatment, as needed.
She agrees to a 2-month trial, and we schedule a follow-up appointment to re-evaluate her progress.
Table 4
Interventions to prevent relapse in women with addiction disorders
| Track craving and mood symptoms in relation to the patient’s menstrual cycle |
| Educate her about triggers for relapse |
| Provide psychotherapy to bolster her coping strategies for stressful life events |
| Screen for comorbid mood or psychiatric disorders and treat them aggressively |
| Treat premenstrual mood symptoms with a selective serotonin reuptake inhibitor and/or by regulating hormone levels with a continuous oral contraceptive |
- Carroll ME, Lynch WJ, Roth ME, et al. Sex and estrogen influence drug abuse. Trends Pharmacol Sci 2004;25(5):273-9.
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 2004;28(6):533-46.
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8(11):1481-9.
- Fluoxetine • Prozac
- Ethinyl estradiol and norethindrone oral contraceptive • Necon, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121-37.
2. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403-13.
3. Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44-56.
4. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex decision-making and drug addiction. Trends Neurosci 2006;29(2):116-24.
5. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31(2):151-78.
6. Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59(1):40-7.
7. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 2006;63(3):324-31.
8. Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 2005;29(7):1351-5.
9. Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry 2002;63(7):616-27.
10. Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25(7):677-91.
11. O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 1989;14(6):595-600.
12. Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res 2000;2(3):231-41.
13. Allen SS, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res 1999;1(2):129-42.
14. Franklin TR, Napier K, Ehrman R, et al. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res 2004;6(1):171-5.
15. Pomerleau CS, Mehringer AM, Marks JL, et al. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav 2000;25(4):483-97.
16. Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relapse and withdrawal symptoms. In: Keefe FJ (ed). Thirteenth annual proceedings for the Society of Behavioral Medicine. Rockville, MD, 1992:107.
17. Backstrom T, Andersson A, Andree L, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 2003;1007:42-53.
18. Harvey SM, Beckman LJ. Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 1985;9(5):465-7.
19. White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73(4):729-41.
20. Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 2004;29(1):81-5.
21. Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 2004;46(5):672-87.
22. Lynch WJ, Roth ME, Mickelberg JL, Carroll M. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001;68(4):641-6.
23. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med 2004;71(Suppl 2):S4-10.
24. Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004;19(6):344-53.
25. Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 2003;30(1):1-7.
26. Nie H, Janak, PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1-2):222-8.
27. Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63-6.
28. Weiss RD, Martinez-Raga J, Griffin ML, et al. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 1997;44(1):35-40.
29. Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:487-94.
30. Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord 2002;70(3):229-40.
31. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am 2004;27(4):627-48.
1. Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121-37.
2. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403-13.
3. Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44-56.
4. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex decision-making and drug addiction. Trends Neurosci 2006;29(2):116-24.
5. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31(2):151-78.
6. Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59(1):40-7.
7. Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 2006;63(3):324-31.
8. Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res 2005;29(7):1351-5.
9. Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry 2002;63(7):616-27.
10. Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25(7):677-91.
11. O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 1989;14(6):595-600.
12. Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res 2000;2(3):231-41.
13. Allen SS, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res 1999;1(2):129-42.
14. Franklin TR, Napier K, Ehrman R, et al. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res 2004;6(1):171-5.
15. Pomerleau CS, Mehringer AM, Marks JL, et al. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav 2000;25(4):483-97.
16. Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relapse and withdrawal symptoms. In: Keefe FJ (ed). Thirteenth annual proceedings for the Society of Behavioral Medicine. Rockville, MD, 1992:107.
17. Backstrom T, Andersson A, Andree L, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 2003;1007:42-53.
18. Harvey SM, Beckman LJ. Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 1985;9(5):465-7.
19. White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73(4):729-41.
20. Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 2004;29(1):81-5.
21. Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 2004;46(5):672-87.
22. Lynch WJ, Roth ME, Mickelberg JL, Carroll M. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001;68(4):641-6.
23. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med 2004;71(Suppl 2):S4-10.
24. Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004;19(6):344-53.
25. Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 2003;30(1):1-7.
26. Nie H, Janak, PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168(1-2):222-8.
27. Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63-6.
28. Weiss RD, Martinez-Raga J, Griffin ML, et al. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend 1997;44(1):35-40.
29. Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:487-94.
30. Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord 2002;70(3):229-40.
31. Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am 2004;27(4):627-48.
SSRI use during pregnancy
Untreated depression can have serious consequences, but many pregnant women resist taking antidepressants because they overestimate the risk of birth defects.Paroxetine in pregnancy”). Further study is needed to define the risks of teratogenesis with paroxetine compared with other antidepressants.
Third-trimester exposure
In a recent meta-analysis, infants exposed to SSRIs in utero showed an increased risk for prematurity (OR; 2.03) and low birth weight (OR; 2.37).15 Other studies, however, showed no differences in these risks in SSRI-exposed infants or attributed the results to untreated maternal depression or smoking.16
A Medline search across the last 20 years17 found 26 case reports, three prospective controlled cohort studies, and other records of >400 women who received fluoxetine, sertraline, or paroxetine in the third trimester. The authors found the evidence “ambiguous” as to the cause of adverse events and concluded that the risk of not treating major depression with adequate SSRI therapy at that stage of pregnancy “most likely” outweighs the risk of harm to infants.
Transient neonatal complications. Thirty percent of neonates exposed to SSRIs in the third trimester experience transient adaptation problems, which peak 48 hours after birth18 (Table 3). Symptoms may include initial lack of crying, increased muscle tonus, flush, irritability, jitteriness, hypothermia, abnormal breathing, and disrupted sleep and motor activity.2,19,20
Transient neonatal symptoms from SSRI exposure are thought to be a serotonin withdrawal syndrome or serotonin overstimulation.21 The syndrome is usually mild, self-limited, and requires only supportive treatments. All antidepressants’ labels warn of these effects.
Table 3
Neonatal SSRI withdrawal: Symptoms, causes, and treatment
| Symptoms | Initial lack of crying |
| Increased muscle tonus | |
| Irritability, jitteriness | |
| Abnormal breathing pattern | |
| Disrupted sleep and motor activity | |
| Hypotheses of cause | Serotonin overstimulation or withdrawal |
| Treatment | Close observation |
| Supportive measures |
Recommendation. Some authors have recommended tapering antidepressants in the third trimester, but the risk of postpartum depression appears to outweigh any potential benefit from discontinuation. Because birth timing is unpredictable, some women whose antidepressants are tapered off could be without medication for a long time.
Thus, we recommend:
- continuing SSRIs during late pregnancy
- monitoring the newborn for 48 hours for transient neonatal adaptation symptoms or PPHN.2,17,18
Long-term effects of SSRI exposure
Do SSRIs during pregnancy have long-term effects on infants’ neurodevelopment? Study results are mixed. For example:
- A prospective, controlled, cohort trial found no adverse effects on IQ, language, or behavioral development in children ages 15 months to 6 years whose mothers took tricyclic antidepressants (N=46) or fluoxetine (N=40) during pregnancy, compared with 36 unexposed controls.23
- Another prospective study showed lower Bayley Psychomotor Developmental Index scores in 31 SSRI-exposed infants compared with 13 infants born to depressed mothers not on antidepressants. Reduced body control, coordination, and fine motor skills might suggest possible subtle effects of SSRIs on motor development in exposed infants, the authors concluded.24
Case continued: A healthy delivery
Ms. P’s depression improves a few weeks after she restarts an SSRI. She delivers a healthy term baby with Apgar score of 7. The baby initially does not cry, awakens easily, and shows mild irritability. His mother’s SSRI use, her severe depression during part of the pregnancy, or some other factor may have caused his mild neonatal complications.
Nursing staff carefully observe the infant for 2 days in the newborn nursery, and his irritability fades away. Ms. P decides to continue taking antidepressants to care for herself and the baby.
Weighing treatment options
For each woman with a history of depression who is pregnant or intends to conceive, we recommend a risk-benefit analysis of her depression severity and need for an antidepressant:
Moderate to severe depression (history of recurrent depressive episodes, hospitalization, or suicidality). Strongly consider medication. If your patient is taking an SSRI, counsel her about:
- the 70% risk of depression relapse if she stops the medication, even for the first trimester
- risks of untreated depression during pregnancy (poor self-care, preterm labor, birth complications, and increased risk for poor stress adaptations in children).
Choosing an SSRI. No one SSRI is the safest choice for all women, especially when data on breast-feeding come into play.
- Fluoxetine has been studied more than other SSRIs during pregnancy; most evidence is reassuring, except for transient neonatal complications. With its long half-life, fluoxetine is not recommended during breastfeeding because it may accumulate in infant sera.
- Sertraline has shown low umbilical cord to maternal serum ratios in small samples and has reassuring breast-feeding data.
- Citalopram, compared with sertraline, has been studied more in pregnancy but has a higher fetal-to-maternal serum ratio (as does escitalopram). These SSRIs are usually second-line for starting a new antidepressant during pregnancy but could be first-line if they have worked well for a patient or she has had adverse effects with fluoxetine or sertraline.
You may need to increase SSRI dosages as pregnancy progresses. Increased metabolism and weight gain during pregnancy can lower SSRI serum levels, allowing depressive symptoms to re-emerge in the third trimester. Counsel the patient to continue taking the antidepressant for at least 12 months postpartum, then re-evaluate the need for medication based on her history.
Paroxetine precautions. If your patient is taking paroxetine and wishes to become pregnant, consider switching to another SSRI (using a slow cross-taper) unless paroxetine has been the only effective medication (Table 4). When discussing risks of any SSRI, explain that the baseline risk for congenital malformations is 3%. Paroxetine might increase this risk by 1% and other SSRIs by less.
If a woman becomes pregnant while taking paroxetine, often the time when cardiac defects occur is passed or will be before you slowly taper the medication to avoid withdrawal. If the patient’s depression has been severe, the risk of shifting her to an untested SSRI is probably higher than the possible 1% increased risk of fetal malformation. If she has taken paroxetine during the first-trimester, refer for ultrasound to monitor for cardiac anomalies.
Table 4
Recommendations for managing paroxetine risk during pregnancy
| Patient status | Recommendation |
|---|---|
| Taking paroxetine and planning pregnancy | Advise of possible 1% increase in risk of fetal malformation |
| Switch to another SSRI unless paroxetine has been the only successful therapy for depression | |
| If stopping paroxetine, slowly taper to avoid withdrawal symptoms | |
| Taking paroxetine and is pregnant | Advise of possible 1% increase in risk of fetal malformation |
| Continue paroxetine; a slow taper probably could not be completed before the first-trimester period associated with increased risk of fetal cardiac defects | |
| If any paroxetine exposure in first trimester, order ultrasound to monitor for fetal malformations |
- California Teratogen Information Service (CTIS). Pediatric department, University of California San Diego Medical Center. www.otispregnancy.org/ctis.html
- MGH Center for Women’s Mental Health, Massachusetts General Hospital. Psychiatric disorders during pregnancy and postpartum. www.womensmentalhealth.com
- MOTHERISK Web site. Teratogen information and updates on reproductive risk research. The Hospital for Sick Children, University of Toronto. www.motherisk.org
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence-based counseling, and determinants of decision making. Arch Women Ment Health 2005;8:214-20.
2. Hallberg P, Joblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73.
3. Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol 2004;104(3):469-66.
4. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49(11):726-35.
5. Cohen L, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295(5):499-507.
6. Hendrick V, Altshuler L. Management of major depression during pregnancy. Am J Psychiatry 2002;159:1667-73.
7. Sandman CA, Glynn L, Wadhwa PD, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci 2003;25(1):41-9.
8. Huot RL, Brennan PA, Stowe ZN, et al. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann NY Acad Sci 2004;1032:234-6.
9. Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology 2005;20:541-9.
10. Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry 2003;5:993-6.
11. GlaxoSmithKline study EPIP083. GSK medicine: buproprion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available at: http://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf. Accessed March 13, 2006.
12. Ericson A, Kallen B, Wiholm BE. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999;55:503-8.
13. Kulin NA, Pastuszak A, Sage S, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. A prospective controlled multicenter study. JAMA 1998;279:609-10.
14. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol 2003;17:255-61.
15. Lattimore K, Donn S, Kaciroti N, et al. Selective serotonin reuptake inhibitor use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 2005;25:595-604.
16. Levy L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22:863-93.
17. Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf 2005;28(7):565-81.
18. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors to term infants. Arch Pediatr Adolesc Med 2006;160:173-6.
19. Oberlander TF, Misri S, Fitzgerald CE, et al. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65(2):230-7.
20. Zeskind PS, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113:368-75.
21. Moses-Kolko E, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA 2005;293:2372-83.
22. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
23. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95.
24. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003;4(142):402-8.
25. Spinelli M, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62.
26. Oren D, Wisner K, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9.
Untreated depression can have serious consequences, but many pregnant women resist taking antidepressants because they overestimate the risk of birth defects.Paroxetine in pregnancy”). Further study is needed to define the risks of teratogenesis with paroxetine compared with other antidepressants.
Third-trimester exposure
In a recent meta-analysis, infants exposed to SSRIs in utero showed an increased risk for prematurity (OR; 2.03) and low birth weight (OR; 2.37).15 Other studies, however, showed no differences in these risks in SSRI-exposed infants or attributed the results to untreated maternal depression or smoking.16
A Medline search across the last 20 years17 found 26 case reports, three prospective controlled cohort studies, and other records of >400 women who received fluoxetine, sertraline, or paroxetine in the third trimester. The authors found the evidence “ambiguous” as to the cause of adverse events and concluded that the risk of not treating major depression with adequate SSRI therapy at that stage of pregnancy “most likely” outweighs the risk of harm to infants.
Transient neonatal complications. Thirty percent of neonates exposed to SSRIs in the third trimester experience transient adaptation problems, which peak 48 hours after birth18 (Table 3). Symptoms may include initial lack of crying, increased muscle tonus, flush, irritability, jitteriness, hypothermia, abnormal breathing, and disrupted sleep and motor activity.2,19,20
Transient neonatal symptoms from SSRI exposure are thought to be a serotonin withdrawal syndrome or serotonin overstimulation.21 The syndrome is usually mild, self-limited, and requires only supportive treatments. All antidepressants’ labels warn of these effects.
Table 3
Neonatal SSRI withdrawal: Symptoms, causes, and treatment
| Symptoms | Initial lack of crying |
| Increased muscle tonus | |
| Irritability, jitteriness | |
| Abnormal breathing pattern | |
| Disrupted sleep and motor activity | |
| Hypotheses of cause | Serotonin overstimulation or withdrawal |
| Treatment | Close observation |
| Supportive measures |
Recommendation. Some authors have recommended tapering antidepressants in the third trimester, but the risk of postpartum depression appears to outweigh any potential benefit from discontinuation. Because birth timing is unpredictable, some women whose antidepressants are tapered off could be without medication for a long time.
Thus, we recommend:
- continuing SSRIs during late pregnancy
- monitoring the newborn for 48 hours for transient neonatal adaptation symptoms or PPHN.2,17,18
Long-term effects of SSRI exposure
Do SSRIs during pregnancy have long-term effects on infants’ neurodevelopment? Study results are mixed. For example:
- A prospective, controlled, cohort trial found no adverse effects on IQ, language, or behavioral development in children ages 15 months to 6 years whose mothers took tricyclic antidepressants (N=46) or fluoxetine (N=40) during pregnancy, compared with 36 unexposed controls.23
- Another prospective study showed lower Bayley Psychomotor Developmental Index scores in 31 SSRI-exposed infants compared with 13 infants born to depressed mothers not on antidepressants. Reduced body control, coordination, and fine motor skills might suggest possible subtle effects of SSRIs on motor development in exposed infants, the authors concluded.24
Case continued: A healthy delivery
Ms. P’s depression improves a few weeks after she restarts an SSRI. She delivers a healthy term baby with Apgar score of 7. The baby initially does not cry, awakens easily, and shows mild irritability. His mother’s SSRI use, her severe depression during part of the pregnancy, or some other factor may have caused his mild neonatal complications.
Nursing staff carefully observe the infant for 2 days in the newborn nursery, and his irritability fades away. Ms. P decides to continue taking antidepressants to care for herself and the baby.
Weighing treatment options
For each woman with a history of depression who is pregnant or intends to conceive, we recommend a risk-benefit analysis of her depression severity and need for an antidepressant:
Moderate to severe depression (history of recurrent depressive episodes, hospitalization, or suicidality). Strongly consider medication. If your patient is taking an SSRI, counsel her about:
- the 70% risk of depression relapse if she stops the medication, even for the first trimester
- risks of untreated depression during pregnancy (poor self-care, preterm labor, birth complications, and increased risk for poor stress adaptations in children).
Choosing an SSRI. No one SSRI is the safest choice for all women, especially when data on breast-feeding come into play.
- Fluoxetine has been studied more than other SSRIs during pregnancy; most evidence is reassuring, except for transient neonatal complications. With its long half-life, fluoxetine is not recommended during breastfeeding because it may accumulate in infant sera.
- Sertraline has shown low umbilical cord to maternal serum ratios in small samples and has reassuring breast-feeding data.
- Citalopram, compared with sertraline, has been studied more in pregnancy but has a higher fetal-to-maternal serum ratio (as does escitalopram). These SSRIs are usually second-line for starting a new antidepressant during pregnancy but could be first-line if they have worked well for a patient or she has had adverse effects with fluoxetine or sertraline.
You may need to increase SSRI dosages as pregnancy progresses. Increased metabolism and weight gain during pregnancy can lower SSRI serum levels, allowing depressive symptoms to re-emerge in the third trimester. Counsel the patient to continue taking the antidepressant for at least 12 months postpartum, then re-evaluate the need for medication based on her history.
Paroxetine precautions. If your patient is taking paroxetine and wishes to become pregnant, consider switching to another SSRI (using a slow cross-taper) unless paroxetine has been the only effective medication (Table 4). When discussing risks of any SSRI, explain that the baseline risk for congenital malformations is 3%. Paroxetine might increase this risk by 1% and other SSRIs by less.
If a woman becomes pregnant while taking paroxetine, often the time when cardiac defects occur is passed or will be before you slowly taper the medication to avoid withdrawal. If the patient’s depression has been severe, the risk of shifting her to an untested SSRI is probably higher than the possible 1% increased risk of fetal malformation. If she has taken paroxetine during the first-trimester, refer for ultrasound to monitor for cardiac anomalies.
Table 4
Recommendations for managing paroxetine risk during pregnancy
| Patient status | Recommendation |
|---|---|
| Taking paroxetine and planning pregnancy | Advise of possible 1% increase in risk of fetal malformation |
| Switch to another SSRI unless paroxetine has been the only successful therapy for depression | |
| If stopping paroxetine, slowly taper to avoid withdrawal symptoms | |
| Taking paroxetine and is pregnant | Advise of possible 1% increase in risk of fetal malformation |
| Continue paroxetine; a slow taper probably could not be completed before the first-trimester period associated with increased risk of fetal cardiac defects | |
| If any paroxetine exposure in first trimester, order ultrasound to monitor for fetal malformations |
- California Teratogen Information Service (CTIS). Pediatric department, University of California San Diego Medical Center. www.otispregnancy.org/ctis.html
- MGH Center for Women’s Mental Health, Massachusetts General Hospital. Psychiatric disorders during pregnancy and postpartum. www.womensmentalhealth.com
- MOTHERISK Web site. Teratogen information and updates on reproductive risk research. The Hospital for Sick Children, University of Toronto. www.motherisk.org
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Untreated depression can have serious consequences, but many pregnant women resist taking antidepressants because they overestimate the risk of birth defects.Paroxetine in pregnancy”). Further study is needed to define the risks of teratogenesis with paroxetine compared with other antidepressants.
Third-trimester exposure
In a recent meta-analysis, infants exposed to SSRIs in utero showed an increased risk for prematurity (OR; 2.03) and low birth weight (OR; 2.37).15 Other studies, however, showed no differences in these risks in SSRI-exposed infants or attributed the results to untreated maternal depression or smoking.16
A Medline search across the last 20 years17 found 26 case reports, three prospective controlled cohort studies, and other records of >400 women who received fluoxetine, sertraline, or paroxetine in the third trimester. The authors found the evidence “ambiguous” as to the cause of adverse events and concluded that the risk of not treating major depression with adequate SSRI therapy at that stage of pregnancy “most likely” outweighs the risk of harm to infants.
Transient neonatal complications. Thirty percent of neonates exposed to SSRIs in the third trimester experience transient adaptation problems, which peak 48 hours after birth18 (Table 3). Symptoms may include initial lack of crying, increased muscle tonus, flush, irritability, jitteriness, hypothermia, abnormal breathing, and disrupted sleep and motor activity.2,19,20
Transient neonatal symptoms from SSRI exposure are thought to be a serotonin withdrawal syndrome or serotonin overstimulation.21 The syndrome is usually mild, self-limited, and requires only supportive treatments. All antidepressants’ labels warn of these effects.
Table 3
Neonatal SSRI withdrawal: Symptoms, causes, and treatment
| Symptoms | Initial lack of crying |
| Increased muscle tonus | |
| Irritability, jitteriness | |
| Abnormal breathing pattern | |
| Disrupted sleep and motor activity | |
| Hypotheses of cause | Serotonin overstimulation or withdrawal |
| Treatment | Close observation |
| Supportive measures |
Recommendation. Some authors have recommended tapering antidepressants in the third trimester, but the risk of postpartum depression appears to outweigh any potential benefit from discontinuation. Because birth timing is unpredictable, some women whose antidepressants are tapered off could be without medication for a long time.
Thus, we recommend:
- continuing SSRIs during late pregnancy
- monitoring the newborn for 48 hours for transient neonatal adaptation symptoms or PPHN.2,17,18
Long-term effects of SSRI exposure
Do SSRIs during pregnancy have long-term effects on infants’ neurodevelopment? Study results are mixed. For example:
- A prospective, controlled, cohort trial found no adverse effects on IQ, language, or behavioral development in children ages 15 months to 6 years whose mothers took tricyclic antidepressants (N=46) or fluoxetine (N=40) during pregnancy, compared with 36 unexposed controls.23
- Another prospective study showed lower Bayley Psychomotor Developmental Index scores in 31 SSRI-exposed infants compared with 13 infants born to depressed mothers not on antidepressants. Reduced body control, coordination, and fine motor skills might suggest possible subtle effects of SSRIs on motor development in exposed infants, the authors concluded.24
Case continued: A healthy delivery
Ms. P’s depression improves a few weeks after she restarts an SSRI. She delivers a healthy term baby with Apgar score of 7. The baby initially does not cry, awakens easily, and shows mild irritability. His mother’s SSRI use, her severe depression during part of the pregnancy, or some other factor may have caused his mild neonatal complications.
Nursing staff carefully observe the infant for 2 days in the newborn nursery, and his irritability fades away. Ms. P decides to continue taking antidepressants to care for herself and the baby.
Weighing treatment options
For each woman with a history of depression who is pregnant or intends to conceive, we recommend a risk-benefit analysis of her depression severity and need for an antidepressant:
Moderate to severe depression (history of recurrent depressive episodes, hospitalization, or suicidality). Strongly consider medication. If your patient is taking an SSRI, counsel her about:
- the 70% risk of depression relapse if she stops the medication, even for the first trimester
- risks of untreated depression during pregnancy (poor self-care, preterm labor, birth complications, and increased risk for poor stress adaptations in children).
Choosing an SSRI. No one SSRI is the safest choice for all women, especially when data on breast-feeding come into play.
- Fluoxetine has been studied more than other SSRIs during pregnancy; most evidence is reassuring, except for transient neonatal complications. With its long half-life, fluoxetine is not recommended during breastfeeding because it may accumulate in infant sera.
- Sertraline has shown low umbilical cord to maternal serum ratios in small samples and has reassuring breast-feeding data.
- Citalopram, compared with sertraline, has been studied more in pregnancy but has a higher fetal-to-maternal serum ratio (as does escitalopram). These SSRIs are usually second-line for starting a new antidepressant during pregnancy but could be first-line if they have worked well for a patient or she has had adverse effects with fluoxetine or sertraline.
You may need to increase SSRI dosages as pregnancy progresses. Increased metabolism and weight gain during pregnancy can lower SSRI serum levels, allowing depressive symptoms to re-emerge in the third trimester. Counsel the patient to continue taking the antidepressant for at least 12 months postpartum, then re-evaluate the need for medication based on her history.
Paroxetine precautions. If your patient is taking paroxetine and wishes to become pregnant, consider switching to another SSRI (using a slow cross-taper) unless paroxetine has been the only effective medication (Table 4). When discussing risks of any SSRI, explain that the baseline risk for congenital malformations is 3%. Paroxetine might increase this risk by 1% and other SSRIs by less.
If a woman becomes pregnant while taking paroxetine, often the time when cardiac defects occur is passed or will be before you slowly taper the medication to avoid withdrawal. If the patient’s depression has been severe, the risk of shifting her to an untested SSRI is probably higher than the possible 1% increased risk of fetal malformation. If she has taken paroxetine during the first-trimester, refer for ultrasound to monitor for cardiac anomalies.
Table 4
Recommendations for managing paroxetine risk during pregnancy
| Patient status | Recommendation |
|---|---|
| Taking paroxetine and planning pregnancy | Advise of possible 1% increase in risk of fetal malformation |
| Switch to another SSRI unless paroxetine has been the only successful therapy for depression | |
| If stopping paroxetine, slowly taper to avoid withdrawal symptoms | |
| Taking paroxetine and is pregnant | Advise of possible 1% increase in risk of fetal malformation |
| Continue paroxetine; a slow taper probably could not be completed before the first-trimester period associated with increased risk of fetal cardiac defects | |
| If any paroxetine exposure in first trimester, order ultrasound to monitor for fetal malformations |
- California Teratogen Information Service (CTIS). Pediatric department, University of California San Diego Medical Center. www.otispregnancy.org/ctis.html
- MGH Center for Women’s Mental Health, Massachusetts General Hospital. Psychiatric disorders during pregnancy and postpartum. www.womensmentalhealth.com
- MOTHERISK Web site. Teratogen information and updates on reproductive risk research. The Hospital for Sick Children, University of Toronto. www.motherisk.org
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence-based counseling, and determinants of decision making. Arch Women Ment Health 2005;8:214-20.
2. Hallberg P, Joblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73.
3. Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol 2004;104(3):469-66.
4. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49(11):726-35.
5. Cohen L, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295(5):499-507.
6. Hendrick V, Altshuler L. Management of major depression during pregnancy. Am J Psychiatry 2002;159:1667-73.
7. Sandman CA, Glynn L, Wadhwa PD, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci 2003;25(1):41-9.
8. Huot RL, Brennan PA, Stowe ZN, et al. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann NY Acad Sci 2004;1032:234-6.
9. Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology 2005;20:541-9.
10. Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry 2003;5:993-6.
11. GlaxoSmithKline study EPIP083. GSK medicine: buproprion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available at: http://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf. Accessed March 13, 2006.
12. Ericson A, Kallen B, Wiholm BE. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999;55:503-8.
13. Kulin NA, Pastuszak A, Sage S, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. A prospective controlled multicenter study. JAMA 1998;279:609-10.
14. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol 2003;17:255-61.
15. Lattimore K, Donn S, Kaciroti N, et al. Selective serotonin reuptake inhibitor use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 2005;25:595-604.
16. Levy L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22:863-93.
17. Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf 2005;28(7):565-81.
18. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors to term infants. Arch Pediatr Adolesc Med 2006;160:173-6.
19. Oberlander TF, Misri S, Fitzgerald CE, et al. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65(2):230-7.
20. Zeskind PS, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113:368-75.
21. Moses-Kolko E, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA 2005;293:2372-83.
22. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
23. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95.
24. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003;4(142):402-8.
25. Spinelli M, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62.
26. Oren D, Wisner K, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9.
1. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence-based counseling, and determinants of decision making. Arch Women Ment Health 2005;8:214-20.
2. Hallberg P, Joblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73.
3. Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol 2004;104(3):469-66.
4. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49(11):726-35.
5. Cohen L, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295(5):499-507.
6. Hendrick V, Altshuler L. Management of major depression during pregnancy. Am J Psychiatry 2002;159:1667-73.
7. Sandman CA, Glynn L, Wadhwa PD, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci 2003;25(1):41-9.
8. Huot RL, Brennan PA, Stowe ZN, et al. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann NY Acad Sci 2004;1032:234-6.
9. Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology 2005;20:541-9.
10. Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry 2003;5:993-6.
11. GlaxoSmithKline study EPIP083. GSK medicine: buproprion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available at: http://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf. Accessed March 13, 2006.
12. Ericson A, Kallen B, Wiholm BE. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999;55:503-8.
13. Kulin NA, Pastuszak A, Sage S, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. A prospective controlled multicenter study. JAMA 1998;279:609-10.
14. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol 2003;17:255-61.
15. Lattimore K, Donn S, Kaciroti N, et al. Selective serotonin reuptake inhibitor use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 2005;25:595-604.
16. Levy L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22:863-93.
17. Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf 2005;28(7):565-81.
18. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors to term infants. Arch Pediatr Adolesc Med 2006;160:173-6.
19. Oberlander TF, Misri S, Fitzgerald CE, et al. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65(2):230-7.
20. Zeskind PS, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113:368-75.
21. Moses-Kolko E, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA 2005;293:2372-83.
22. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
23. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95.
24. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003;4(142):402-8.
25. Spinelli M, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62.
26. Oren D, Wisner K, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9.
Managing menopause-related depression and low libido
- Depression is more likely when perimenopause exceeds 27 months and hot flashes are moderate to severe.
- All serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors have sexual side effects, including anorgasmia and loss of libido. Gabapentin is the only psychotropic that improves hot flashes and mood without interfering with sexual function.
- If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.
- Women with androgen deficiency symptoms and low testosterone should at least be considered for testosterone replacement.
Among psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
ANNE’S CASE
Mood improves, but still no libido
You and Anne decide to try the SNRI venlafaxine, 75 mg daily, to treat her hot flashes and depression. Four weeks later, she is having only half as many hot flashes and her mood has improved (Beck Depression Inventory score of 10). She feels much better and wishes to continue the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
Treating low interest in sex
Being angry with one’s partner is the number one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.16 Other agents the FDA is reviewing for erectile dysfunction may help.
Consideration of how hormones affect female sexual desire may suggest what advice to give Anne and how to coordinate her care with a psychiatrist, if necessary. For example, the psychiatrist might treat her sexual complaints and relationship problems while the Ob/Gyn manages gynecologic symptoms.
How androgens affect sexual desire
Testosterone is the hormone of sexual desire in men and women. Other female androgens are androstenedione, androstenediol, 5α-dihydrotestosterone, dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S).
Premenopausal women produce testosterone in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%); DHEA and DHEA-S are produced in the adrenal glands (95%).
Get the cardiologist’s clearance before giving testosterone to a woman with heart disease or an HDL below 45 mg/dL.
Average daily serum testosterone concentrations decline in women between ages 20 and 50. When values in women aged 20 to 29 were compared with those in women 40 to 49, they were 195.6 g/dL and 140.4 g/dL, respectively, for DHEA-S; 51.5 ng/dL and 33.7 ng/dL, respectively, for serum testosterone; and 1.51 pg/mL and 1.03 pg/mL, respectively, for free testosterone.17
Lower levels also are seen with estrogen replacement therapy, oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Total hysterectomy with bilateral oophorectomy induces a sudden 50% loss of testosterone and an 80% decline in estradiol.18
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—compared with what they had when in their 30s.19
The percentage of women reporting low libido increases with age until menopause: 30% at age 30 to 50% at age 50. The rate declines to 27% in women aged 50 to 59.20
Female androgen deficiency syndrome. After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire. Oral estrogen reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in menopausal women.21 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.22
- Symptoms. Diagnosis of female androgen deficiency syndrome23 requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
Value of testosterone replacement
Replacing testosterone can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.25 Muscle and bone stimulation and decreased hot flashes also are reported.26
Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Potential disadvantages. Patients should be informed that testosterone may lower levels of beneficial high-density lipoprotein (HDL) cholesterol. Get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level below 45 mg/dL.
A meta-analysis of 8 clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg daily of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone doses.27
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Monitoring progress. Depending on the patient’s progress through menopause, after 12 to 24 months, it may be possible to reduce the testosterone dose or to give it only 2 to 4 times per week. As estrogen levels drop off through menopause, free testosterone may rise and the increased LH drive to the ovary may increase production of ovarian testosterone.
Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone is tricky.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle third of the cycle—on cycle days 10 to 16, counting the first day of menstrual bleeding as day 1.28
- Oral contraceptives decrease androgen production by the ovary and can result in low libido in some women.29
New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.30
Restoring bioactive testosterone to the normal free androgen index range may improve low libido.
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEA-S. Measuring SHBG helps determine the free, biologically active testosterone level and helps in calculating the free androgen index in women (TABLE 2).31
TABLE 2
Free androgen index values in women, by age
| TO CALCULATE THE FREE ANDROGEN INDEX: | |
|---|---|
| Total testosterone in nmol/L (total testosterone in ng/mL x 0.0347 x 100), divided by sex hormone-binding globulin in nmol/L | |
| AGE | NORMAL RANGE |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Data from Guay et al31 | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 nmol/L (normal range, 1.78 to 2.86).
You and Anne decide to start testosterone replacement therapy. You prescribe Androgel, starting at one seventh of a 2.5-mg foil packet (about 0.35 mg daily of testosterone) and instruct her to rate her sexual energy daily, using a Sexual Energy Scale (see).
The Beck Depression Inventory-II
The questionnaire assesses level of depression by having the patient rate 21 psychological attributes. She chooses 1 of 4 graded statements for each attribute, which are assigned 0 to 3 points. The points are tallied at the end of the test for an overall view of the patient’s depression—or lack thereof.
For details, see: http://marketplace.psychcorp.com/PsychCorp.com/Cultures/en-US/default.htm
The Sexual Energy Scale
This 1-10 scale is designed to identify and follow-up patients with sexual dysfunction due to a general medical condition or substance-induced sexual dysfunction. It can be given at every visit, including at baseline, to evaluate a patient’s sexual energy level and response to therapy. In this assessment, the term “sexual energy” includes ease of arousal, sexual pleasure, orgasms, interest in sex, sexual fantasies, and sexual fulfillment.
Instructions to the patient are: “On a scale of 1 to 10, with 1 being the lowest sexual energy level you have experienced in your adult life, and 10 being the highest sexual energy level you have experienced in your adult life, rate your current energy level.”
Testosterone choices for women
Restoring a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options that I use clinically include:
Methyl testosterone sublingual pills, 0.5 mg daily, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels unless you order the very expensive test that is specific for methyl testosterone.
Two percent vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption.
Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg daily or one seventh of the 2.5-mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations, including a testosterone patch for women and a gel in female-sized doses.
Research is warranted to evaluate the benefits and safety of longer-term interventions with these therapies in women because of the large numbers of women experiencing diminished sexual interest and declining general wellbeing during their late reproductive years.32
Using the Sexual Energy Scale. At every visit, monitor therapeutic response with the Sexual Energy Scale—a scale numbered 1 to 10.33,34 Instruct her to define “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Conversely, “1” would be when she felt the worst sexually and had the least desire.
Supplemental estrogen, progestin. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS (0.625 mg esterified estrogens and 1.25 mg of methyl testosterone). Add a progestin if the patient is postmenopausal with an intact uterus.
A vaginal lubricant is not enough to defeat age-related vaginal atrophy, which can make intercourse difficult or impossible. Women with vaginal dryness need estrogen that can be applied vaginally.
ANNE’S CASE
Libido improves somewhat
Anne returns in 4 weeks with gradually improving sex drive (Sexual Energy Scale score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low.
You increase the Androgel dosage to one fifth of a 2.5-mg packet (0.5 mg daily) and continue to monitor her Sexual Energy Scale ratings at monthly visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Dr. Brizendine is a speaker for Pfizer, Lilly, and Wyeth.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric. 2003;6(Suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med. 2003;348:645-650.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392-398.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279-294.
6. Seppa N. Hormone therapy falls out of favor. Science News. 2002;162:61.-
7. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
8. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
9. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798-811.
10. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839-1854.
11. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum. 2002;29:33-40.
12. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827-2834.
13. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry. 2003;64:473-479.
14. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
15. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
16. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623-628.
17. Guay AR, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):112-120.
18. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357-365.
19. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363-366.
20. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
21. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
22. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995-998.
23. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
24. Guay AJ, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B. Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):121-129.
25. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:165-175.
26. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol. 2002;20:106-110.
27. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause. 1999;6:216-224.
28. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums. March 2003;8:3.-
29. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363-369.
30. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77(Suppl 4):S83-S88.
31. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(Suppl 1):129-142.
32. Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
33. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull. 1997;33:761-765.
34. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction. Poster presented at: North American Society for Psychosocial Obstetrics and Gynecology; 2001; Waikoloa, Hawaii.
- Depression is more likely when perimenopause exceeds 27 months and hot flashes are moderate to severe.
- All serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors have sexual side effects, including anorgasmia and loss of libido. Gabapentin is the only psychotropic that improves hot flashes and mood without interfering with sexual function.
- If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.
- Women with androgen deficiency symptoms and low testosterone should at least be considered for testosterone replacement.
Among psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
ANNE’S CASE
Mood improves, but still no libido
You and Anne decide to try the SNRI venlafaxine, 75 mg daily, to treat her hot flashes and depression. Four weeks later, she is having only half as many hot flashes and her mood has improved (Beck Depression Inventory score of 10). She feels much better and wishes to continue the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
Treating low interest in sex
Being angry with one’s partner is the number one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.16 Other agents the FDA is reviewing for erectile dysfunction may help.
Consideration of how hormones affect female sexual desire may suggest what advice to give Anne and how to coordinate her care with a psychiatrist, if necessary. For example, the psychiatrist might treat her sexual complaints and relationship problems while the Ob/Gyn manages gynecologic symptoms.
How androgens affect sexual desire
Testosterone is the hormone of sexual desire in men and women. Other female androgens are androstenedione, androstenediol, 5α-dihydrotestosterone, dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S).
Premenopausal women produce testosterone in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%); DHEA and DHEA-S are produced in the adrenal glands (95%).
Get the cardiologist’s clearance before giving testosterone to a woman with heart disease or an HDL below 45 mg/dL.
Average daily serum testosterone concentrations decline in women between ages 20 and 50. When values in women aged 20 to 29 were compared with those in women 40 to 49, they were 195.6 g/dL and 140.4 g/dL, respectively, for DHEA-S; 51.5 ng/dL and 33.7 ng/dL, respectively, for serum testosterone; and 1.51 pg/mL and 1.03 pg/mL, respectively, for free testosterone.17
Lower levels also are seen with estrogen replacement therapy, oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Total hysterectomy with bilateral oophorectomy induces a sudden 50% loss of testosterone and an 80% decline in estradiol.18
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—compared with what they had when in their 30s.19
The percentage of women reporting low libido increases with age until menopause: 30% at age 30 to 50% at age 50. The rate declines to 27% in women aged 50 to 59.20
Female androgen deficiency syndrome. After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire. Oral estrogen reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in menopausal women.21 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.22
- Symptoms. Diagnosis of female androgen deficiency syndrome23 requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
Value of testosterone replacement
Replacing testosterone can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.25 Muscle and bone stimulation and decreased hot flashes also are reported.26
Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Potential disadvantages. Patients should be informed that testosterone may lower levels of beneficial high-density lipoprotein (HDL) cholesterol. Get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level below 45 mg/dL.
A meta-analysis of 8 clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg daily of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone doses.27
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Monitoring progress. Depending on the patient’s progress through menopause, after 12 to 24 months, it may be possible to reduce the testosterone dose or to give it only 2 to 4 times per week. As estrogen levels drop off through menopause, free testosterone may rise and the increased LH drive to the ovary may increase production of ovarian testosterone.
Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone is tricky.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle third of the cycle—on cycle days 10 to 16, counting the first day of menstrual bleeding as day 1.28
- Oral contraceptives decrease androgen production by the ovary and can result in low libido in some women.29
New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.30
Restoring bioactive testosterone to the normal free androgen index range may improve low libido.
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEA-S. Measuring SHBG helps determine the free, biologically active testosterone level and helps in calculating the free androgen index in women (TABLE 2).31
TABLE 2
Free androgen index values in women, by age
| TO CALCULATE THE FREE ANDROGEN INDEX: | |
|---|---|
| Total testosterone in nmol/L (total testosterone in ng/mL x 0.0347 x 100), divided by sex hormone-binding globulin in nmol/L | |
| AGE | NORMAL RANGE |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Data from Guay et al31 | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 nmol/L (normal range, 1.78 to 2.86).
You and Anne decide to start testosterone replacement therapy. You prescribe Androgel, starting at one seventh of a 2.5-mg foil packet (about 0.35 mg daily of testosterone) and instruct her to rate her sexual energy daily, using a Sexual Energy Scale (see).
The Beck Depression Inventory-II
The questionnaire assesses level of depression by having the patient rate 21 psychological attributes. She chooses 1 of 4 graded statements for each attribute, which are assigned 0 to 3 points. The points are tallied at the end of the test for an overall view of the patient’s depression—or lack thereof.
For details, see: http://marketplace.psychcorp.com/PsychCorp.com/Cultures/en-US/default.htm
The Sexual Energy Scale
This 1-10 scale is designed to identify and follow-up patients with sexual dysfunction due to a general medical condition or substance-induced sexual dysfunction. It can be given at every visit, including at baseline, to evaluate a patient’s sexual energy level and response to therapy. In this assessment, the term “sexual energy” includes ease of arousal, sexual pleasure, orgasms, interest in sex, sexual fantasies, and sexual fulfillment.
Instructions to the patient are: “On a scale of 1 to 10, with 1 being the lowest sexual energy level you have experienced in your adult life, and 10 being the highest sexual energy level you have experienced in your adult life, rate your current energy level.”
Testosterone choices for women
Restoring a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options that I use clinically include:
Methyl testosterone sublingual pills, 0.5 mg daily, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels unless you order the very expensive test that is specific for methyl testosterone.
Two percent vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption.
Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg daily or one seventh of the 2.5-mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations, including a testosterone patch for women and a gel in female-sized doses.
Research is warranted to evaluate the benefits and safety of longer-term interventions with these therapies in women because of the large numbers of women experiencing diminished sexual interest and declining general wellbeing during their late reproductive years.32
Using the Sexual Energy Scale. At every visit, monitor therapeutic response with the Sexual Energy Scale—a scale numbered 1 to 10.33,34 Instruct her to define “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Conversely, “1” would be when she felt the worst sexually and had the least desire.
Supplemental estrogen, progestin. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS (0.625 mg esterified estrogens and 1.25 mg of methyl testosterone). Add a progestin if the patient is postmenopausal with an intact uterus.
A vaginal lubricant is not enough to defeat age-related vaginal atrophy, which can make intercourse difficult or impossible. Women with vaginal dryness need estrogen that can be applied vaginally.
ANNE’S CASE
Libido improves somewhat
Anne returns in 4 weeks with gradually improving sex drive (Sexual Energy Scale score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low.
You increase the Androgel dosage to one fifth of a 2.5-mg packet (0.5 mg daily) and continue to monitor her Sexual Energy Scale ratings at monthly visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Dr. Brizendine is a speaker for Pfizer, Lilly, and Wyeth.
- Depression is more likely when perimenopause exceeds 27 months and hot flashes are moderate to severe.
- All serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors have sexual side effects, including anorgasmia and loss of libido. Gabapentin is the only psychotropic that improves hot flashes and mood without interfering with sexual function.
- If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.
- Women with androgen deficiency symptoms and low testosterone should at least be considered for testosterone replacement.
Among psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
ANNE’S CASE
Mood improves, but still no libido
You and Anne decide to try the SNRI venlafaxine, 75 mg daily, to treat her hot flashes and depression. Four weeks later, she is having only half as many hot flashes and her mood has improved (Beck Depression Inventory score of 10). She feels much better and wishes to continue the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
Treating low interest in sex
Being angry with one’s partner is the number one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.16 Other agents the FDA is reviewing for erectile dysfunction may help.
Consideration of how hormones affect female sexual desire may suggest what advice to give Anne and how to coordinate her care with a psychiatrist, if necessary. For example, the psychiatrist might treat her sexual complaints and relationship problems while the Ob/Gyn manages gynecologic symptoms.
How androgens affect sexual desire
Testosterone is the hormone of sexual desire in men and women. Other female androgens are androstenedione, androstenediol, 5α-dihydrotestosterone, dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S).
Premenopausal women produce testosterone in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%); DHEA and DHEA-S are produced in the adrenal glands (95%).
Get the cardiologist’s clearance before giving testosterone to a woman with heart disease or an HDL below 45 mg/dL.
Average daily serum testosterone concentrations decline in women between ages 20 and 50. When values in women aged 20 to 29 were compared with those in women 40 to 49, they were 195.6 g/dL and 140.4 g/dL, respectively, for DHEA-S; 51.5 ng/dL and 33.7 ng/dL, respectively, for serum testosterone; and 1.51 pg/mL and 1.03 pg/mL, respectively, for free testosterone.17
Lower levels also are seen with estrogen replacement therapy, oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Total hysterectomy with bilateral oophorectomy induces a sudden 50% loss of testosterone and an 80% decline in estradiol.18
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—compared with what they had when in their 30s.19
The percentage of women reporting low libido increases with age until menopause: 30% at age 30 to 50% at age 50. The rate declines to 27% in women aged 50 to 59.20
Female androgen deficiency syndrome. After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire. Oral estrogen reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in menopausal women.21 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.22
- Symptoms. Diagnosis of female androgen deficiency syndrome23 requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
Value of testosterone replacement
Replacing testosterone can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.25 Muscle and bone stimulation and decreased hot flashes also are reported.26
Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Potential disadvantages. Patients should be informed that testosterone may lower levels of beneficial high-density lipoprotein (HDL) cholesterol. Get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level below 45 mg/dL.
A meta-analysis of 8 clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg daily of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone doses.27
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Monitoring progress. Depending on the patient’s progress through menopause, after 12 to 24 months, it may be possible to reduce the testosterone dose or to give it only 2 to 4 times per week. As estrogen levels drop off through menopause, free testosterone may rise and the increased LH drive to the ovary may increase production of ovarian testosterone.
Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone is tricky.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle third of the cycle—on cycle days 10 to 16, counting the first day of menstrual bleeding as day 1.28
- Oral contraceptives decrease androgen production by the ovary and can result in low libido in some women.29
New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.30
Restoring bioactive testosterone to the normal free androgen index range may improve low libido.
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEA-S. Measuring SHBG helps determine the free, biologically active testosterone level and helps in calculating the free androgen index in women (TABLE 2).31
TABLE 2
Free androgen index values in women, by age
| TO CALCULATE THE FREE ANDROGEN INDEX: | |
|---|---|
| Total testosterone in nmol/L (total testosterone in ng/mL x 0.0347 x 100), divided by sex hormone-binding globulin in nmol/L | |
| AGE | NORMAL RANGE |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Data from Guay et al31 | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 nmol/L (normal range, 1.78 to 2.86).
You and Anne decide to start testosterone replacement therapy. You prescribe Androgel, starting at one seventh of a 2.5-mg foil packet (about 0.35 mg daily of testosterone) and instruct her to rate her sexual energy daily, using a Sexual Energy Scale (see).
The Beck Depression Inventory-II
The questionnaire assesses level of depression by having the patient rate 21 psychological attributes. She chooses 1 of 4 graded statements for each attribute, which are assigned 0 to 3 points. The points are tallied at the end of the test for an overall view of the patient’s depression—or lack thereof.
For details, see: http://marketplace.psychcorp.com/PsychCorp.com/Cultures/en-US/default.htm
The Sexual Energy Scale
This 1-10 scale is designed to identify and follow-up patients with sexual dysfunction due to a general medical condition or substance-induced sexual dysfunction. It can be given at every visit, including at baseline, to evaluate a patient’s sexual energy level and response to therapy. In this assessment, the term “sexual energy” includes ease of arousal, sexual pleasure, orgasms, interest in sex, sexual fantasies, and sexual fulfillment.
Instructions to the patient are: “On a scale of 1 to 10, with 1 being the lowest sexual energy level you have experienced in your adult life, and 10 being the highest sexual energy level you have experienced in your adult life, rate your current energy level.”
Testosterone choices for women
Restoring a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options that I use clinically include:
Methyl testosterone sublingual pills, 0.5 mg daily, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels unless you order the very expensive test that is specific for methyl testosterone.
Two percent vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption.
Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg daily or one seventh of the 2.5-mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations, including a testosterone patch for women and a gel in female-sized doses.
Research is warranted to evaluate the benefits and safety of longer-term interventions with these therapies in women because of the large numbers of women experiencing diminished sexual interest and declining general wellbeing during their late reproductive years.32
Using the Sexual Energy Scale. At every visit, monitor therapeutic response with the Sexual Energy Scale—a scale numbered 1 to 10.33,34 Instruct her to define “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Conversely, “1” would be when she felt the worst sexually and had the least desire.
Supplemental estrogen, progestin. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS (0.625 mg esterified estrogens and 1.25 mg of methyl testosterone). Add a progestin if the patient is postmenopausal with an intact uterus.
A vaginal lubricant is not enough to defeat age-related vaginal atrophy, which can make intercourse difficult or impossible. Women with vaginal dryness need estrogen that can be applied vaginally.
ANNE’S CASE
Libido improves somewhat
Anne returns in 4 weeks with gradually improving sex drive (Sexual Energy Scale score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low.
You increase the Androgel dosage to one fifth of a 2.5-mg packet (0.5 mg daily) and continue to monitor her Sexual Energy Scale ratings at monthly visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Dr. Brizendine is a speaker for Pfizer, Lilly, and Wyeth.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric. 2003;6(Suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med. 2003;348:645-650.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392-398.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279-294.
6. Seppa N. Hormone therapy falls out of favor. Science News. 2002;162:61.-
7. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
8. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
9. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798-811.
10. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839-1854.
11. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum. 2002;29:33-40.
12. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827-2834.
13. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry. 2003;64:473-479.
14. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
15. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
16. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623-628.
17. Guay AR, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):112-120.
18. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357-365.
19. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363-366.
20. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
21. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
22. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995-998.
23. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
24. Guay AJ, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B. Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):121-129.
25. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:165-175.
26. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol. 2002;20:106-110.
27. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause. 1999;6:216-224.
28. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums. March 2003;8:3.-
29. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363-369.
30. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77(Suppl 4):S83-S88.
31. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(Suppl 1):129-142.
32. Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
33. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull. 1997;33:761-765.
34. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction. Poster presented at: North American Society for Psychosocial Obstetrics and Gynecology; 2001; Waikoloa, Hawaii.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric. 2003;6(Suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med. 2003;348:645-650.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392-398.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279-294.
6. Seppa N. Hormone therapy falls out of favor. Science News. 2002;162:61.-
7. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
8. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
9. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798-811.
10. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839-1854.
11. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum. 2002;29:33-40.
12. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827-2834.
13. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry. 2003;64:473-479.
14. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
15. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
16. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623-628.
17. Guay AR, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):112-120.
18. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357-365.
19. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363-366.
20. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
21. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
22. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995-998.
23. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
24. Guay AJ, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B. Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):121-129.
25. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:165-175.
26. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol. 2002;20:106-110.
27. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause. 1999;6:216-224.
28. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums. March 2003;8:3.-
29. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363-369.
30. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77(Suppl 4):S83-S88.
31. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(Suppl 1):129-142.
32. Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
33. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull. 1997;33:761-765.
34. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction. Poster presented at: North American Society for Psychosocial Obstetrics and Gynecology; 2001; Waikoloa, Hawaii.
Minding menopause: Psychotropics vs. estrogen? What you need to know now
Psychiatrists are suddenly viewed as experts in treating menopause-related mood problems because of our expertise with using psychotropics. Practically overnight, the Women’s Health Initiative studies1,2 have made women and their doctors think twice about using estrogen. Instead, many are turning to psychiatric medications that have been shown to improve both mood and hot flashes—without estrogen’s potential risks.
Chances are good that after an Ob/Gyn has tried one or two psychotropics without success or with too many side effects, he or she will ask a psychiatrist to consult for certain patients. How well-prepared are you to assume this role?
If your recall of female reproductive physiology from medical school is incomplete, read on about one approach to a perimenopausal patient with depressed mood. This review can help you:
- discuss menopause knowledgeably when other physicians refer their patients to you
- provide effective, up-to-date treatments for menopause-related mood and sexual problems, using psychotropics or hormones, alone or in combination.
Irritable, with no interest in sex
Anne, age 51, has been referred to you for complaints of depressed mood and low libido. She says she has become irritable and snaps easily at her two children and her husband. She has no interest in sex, no urge to masturbate, and has had no sexual intercourse for 6 months.
Table 1
Why mood problems may occur during menopause
| Hypothesis | Explanation |
|---|---|
| Psychodynamic | Onset of menopause is a critical life event and a readjustment of self-concept |
| Sociologic | Mood changes are caused by changing life circumstances at menopause (‘empty nest,’ aging parents, health changes) |
| Domino | Depressed mood is caused by hot flashes due to declining estrogen levels, which cause chronic sleep deprivation with subsequent irritability and memory and mood changes |
| Biochemical | Decreasing estrogen leads to neurochemical changes in the brain (serotonin, dopamine, cholinergic, GABA, norepinephrine) |
Anne also complains of fatigue, dry hair and skin, warm flushes, and painful joints. She has no personal or family history of depression. She is not suicidal but states that she really doesn’t want to live anymore if “this is it.”
HOT FLASHES: A SPARK FOR DEPRESSION
Women who experience their first depression after age 50 do not fit the usual DSM-IV diagnostic criteria for depression. The Massachusetts Women’s Health Study3 found that 52% of women who experience depressed mood in the perimenopause have never had a depression before. This study also found a correlation between a longer perimenopause (>27 months) and increased risk of depressed mood. At the same time, women who have had a prior depression are 4 to 9 times more likely to experience depressive symptoms during perimenopause than those who have never had a depression before.4
The increased mood symptoms may be related to psychodynamic, sociologic, or biochemical factors, or they may result from a domino effect triggered by declining estrogen levels (Table 1). Women who experience vasomotor symptoms such as hot flashes are at 4.6 times greater risk for depression than those who are hot flash-free.5
Hot flashes begin on average at age 51, which is also the average age when natural menopause begins. During menopause, most women (82%) experience hot flashes (suddenly feeling hot and sweating during the day), warm flushes (a sensation of warmth or heat spreading over the skin), and night sweats (Table 2). All women who undergo surgical menopause experience hot flashes.
Hot flashes are moderate to severe for 40% of women who experience them and persist for 5 to 15 years. By definition, moderate to severe hot flashes occur 6 to 10 or more times daily, last 6 to 10 minutes each, and are often preceded by anxiety, palpitations, irritability, nervousness, or panic.
A marriage under stress
Anne says that her husband is angry about the lack of sexual intercourse, and she feels the stress in their marriage. She also is worrying about her children leaving for college and about her mother’s ill health.
She scores 20 on the Beck Depression Inventory, which indicates that she has mild to moderate depression. Her menstrual periods remain regular, but her cycle has shortened from 29 to 24 days. She reports experiencing some hot flashes that wake her at night and says she hasn’t had a good night’s sleep in months.
Laboratory tests show FSH of 25 mIU/mL and inhibin B <45 pg/mL. Her estradiol is 80 pg/mL, which is not yet in the menopausal range of 10 to 20 pg/mL. Her thyroid stimulating hormone (TSH) is normal. Her endocrinologic and reproductive diagnosis is perimenopause.
Table 2
Symptoms of menopause related to decreased estrogen
| Brain | Irritability, mood swings, depressed mood, forgetfulness, low sex interest, sleep problems, decreased well-being |
| Body | Hot flashes, vaginal dryness, painful intercourse, fatigue, joint pain, pain with orgasm, bladder dysfunction |
TREATING HOT FLASHES IMPROVES MOOD
Until July 2002, estrogen was standard treatment for controlling hot flashes in patients such as Anne. Then the Women’s Health Initiative trial reported that estrogen’s health risks—heart attack, stroke, breast cancer, and blood clots—exceeded potential benefits during 5 years of therapy. As a result, fewer women want to take estrogen,6 and many Ob/Gyns are advising patients to get through menopause without hormones if they can.
For mild hot flashes—one to three per day—patients may only need vitamin E, 800 mg/d, and deep relaxation breathing to “rev down” the sympathetic nervous system when a hot flash occurs.
For moderate to severe hot flashes—four to 10 or more per day—estrogen replacement is the most effective therapy. Estradiol, 1 mg/d, reduces hot flashes by approximately 80 to 90%.7 Many small studies have shown that patients’ mood often improves as estrogen reduces their hot flashes.8 The recent Women’s Health Initiative Quality-of-Life study, however, reported that estrogen plus progestin did not improve mood in women ages 50 to 54 with moderate-to-severe vasomotor symptoms, even though hot flashes were reduced and sleep may have improved.9
New drugs of choice. Because of estrogen’s effectiveness in controlling hot flashes, some women and their doctors may choose to use it briefly (18 to 24 months). For others, psychotropics are becoming the drugs of choice for mood disorders with moderate to severe hot flashes.
The serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine, 75 or 150 mg/d, has been shown to reduce hot flashes by 60 to 70%.10 A new trial is investigating whether duloxetine—an SNRI awaiting FDA approval—also reduces hot flashes. Other useful agents that have been shown to reduce hot flashes by 50% or more include:
- selective serotonin reuptake inhibitors (SSRIs) paroxetine CR, 12.5 mg/d to 25 mg/d,11 citalopram, 20 to 60 mg/d,12 and fluoxetine, 20 mg/d13
- gabapentin, 900 mg/d.14
For hot flashes and moderate to major depression, try an SNRI or SSRI first (see Algorithm), but consider the possible effects on sexual function. All SNRIs and SSRIs have sexual side effects, including anorgasmia and loss of libido in women and men. Among the psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
Mood improves, but still no libido
You and Ann decide on a trial of the SNRI venlafaxine, 75 mg/d, to treat her hot flashes and depressed mood. Four weeks later, her hot flashes are reduced by 50% in frequency and her mood has improved (Beck Depression Inventory score is now 10). She is feeling much better and wishes to continue taking the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
TREATING LOW INTEREST IN SEX
Being angry with one’s partner is the number-one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.15 Other agents the FDA is reviewing for erectile dysfunction—such as tadalafil and vardenafil—may also help arousal problems in women.
Understanding how hormones affect female sexual desire also may help you decide what advice to give Anne and how you and her Ob/Gyn coordinate her care. For example, you might treat her sexual complaints and relationship problems while the Ob/Gyn manages symptoms of the vagina, uterus, and breast.
HOW TESTOSTERONE AFFECTS SEXUAL DESIRE
Testosterone is the hormone of sexual desire in men and women. Other female androgens include androstenedione, androstenediol, 5 α-dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), and its sulfate (DHEA-S). Premenopausal women produce these androgens in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%).
Average daily serum testosterone concentrations decline in women between ages 20 and 50. Lower levels are also seen with estrogen replacement therapy or oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Women who undergo total hysterectomy with bilateral oophorectomy experience a sudden 50% loss of testosterone and an 80% decline in estradiol.16
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—than they had when they were in their 30s.17 The percentage of women reporting low libido increases with age until menopause, from 30% at age 30 to about 50% at age 50. Then the rate declines to 27% in women age 50 to 59.18 After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire.
Oral estrogen replacement therapy reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in a menopausal woman.19 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.20
Female androgen deficiency. A number of papers have been published on female androgen deficiency syndrome (FADS).21 Its diagnosis requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
TREATING TESTOSTERONE DEFICIENCY
Benefits of replacement therapy. Replacing testosterone in women with FADS can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.22 Muscle and bone stimulation and decreased hot flashes are also reported.23 Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Risks of replacement therapy. Androgen replacement therapy does carry some risks, which need to be discussed with the patient. Testosterone may lower levels of beneficial HDL cholesterol, so get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level <45 mg/dL.
Algorithm Managing mood and libido problems during perimenopause
A meta-analysis of eight clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg/d of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone dosages.24
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle one-third of the cycle—on days 10 to 16, counting the first day of menstrual bleeding as day 1.25
- Oral contraceptives also decrease androgen production by the ovary and can result in low libido in some women.26
Tests developed to measure testosterone levels in men are not sensitive enough to accurately measure women’s naturally lower serum concentrations, let alone the even lower levels characteristic of female androgen or testosterone deficiency. New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.27
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEAS. Measuring SHBG will help you determine the free, biologically active testosterone level and calculate the Free Androgen Index (FAI) for women (Table 3).28
Table 3
Free androgen index (FAI) values in women, by age
| Replacing a woman’s bioactive testosterone to the normal free androgen index range for her age may improve low libido. | |
| How to calculate FAI | |
| Total testosterone in nmol/L (total testosterone in ng/ml X 0.0347 X 100), divided by sex hormone-binding globulin (SHBG) in nmol/L. | |
| Age | Normal range |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Source: Guay et al, reference 28. | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 (normal range, 1.78 to 2.86).
In collaboration with her Ob/Gyn, you and Anne decide to start her on testosterone replacement therapy. You prescribe Androgel, starting at 1/7th of a 2.5-mg foil packet (0.35 mg/d of testosterone), and instruct her to rate her sexual energy daily, using a Sexual Energy Scale.
TESTOSTERONE CHOICES FOR WOMEN
Replacing a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options include:
- methyl testosterone sublingual pills, 0.5 mg/d, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels—unless you order the very expensive test that is specific for methyl testosterone.
- 2% vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption
- Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg/d or one-seventh of the 2.5 mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations—including a testosterone patch for women and a gel in female-sized doses.
Using the Sexual Energy Scale. To monitor for a therapeutic response, ask the patient to use the Sexual Energy Scale (Figure 1).29,30 Instruct her to define her “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Her “1” would be when she felt the worst sexually and had the least desire.
Giving supplemental estrogen. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS, which contains 0.625 mg esterified estrogens and 1.25 mg of methyl testosterone. Add a progestin if the patient is postmenopausal and has not had a hysterectomy, to protect the uterus from endometrial hyperplasia.
Women with vaginal dryness also need supplemental estrogen, which can be applied vaginally (such as Premarin cream or Estrace cream). A vaginal lubricant is not sufficient to avoid age-related vaginal atrophy, which may make intercourse difficult or impossible.
Figure 1 How to use the Sexual Energy Scale to monitor response to therapy
Libido improves modestly
Ann returns in 4 weeks with gradually improving sex drive (Sexual Energy Score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but also did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low. You increase the Androgel dosage to 1/5th of a 2.5 mg packet (0.5 mg/day) and continue to monitor Anne’s Sexual Energy Scale ratings at monthly follow-up visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Louann Brizendine, MD
Medicine’s understanding of menopause’s physiologic and psychological consequences is changing, just as the “baby-boom” generation is navigating this passage called the change of life. Many midlife women are unaware that the menopause transition does not begin around age 50 but spans 30 years—from ages 35 to 65. Natural menopause begins 15 years before and ends 15 years after menstruation ceases—as the brain and tissues adjust to first fluctuating then decreased estrogen levels. It occurs in three phases—early menopause, perimenopause, and late menopause—that reflect a progression of hormone changes.
EARLY MENOPAUSE: OVULATION ACCELERATES
At approximately age 35, the ovaries start producing lower levels of inhibin—a glycoprotein that inhibits pituitary production of follicle-stimulating hormone (FSH) (Figure 2). Less inhibin means less negative feedback to the pituitary and an increase in pituitary FSH production. More FSH means more activin—an ovarian glycoprotein that stimulates ripening of eggs—and so ovulation begins to accelerate at approximately age 36. Activin stimulates more and more eggs in the ovary to develop faster and faster.
By age 37, the ovarian egg reserve starts to decline, and—because FSH has increased—the follicle is driven to produce greater amounts of estrogen. Estrogen serum levels in fertile women average 100 pg/mL. During perimenopause, estrogen levels sometimes soar to 300, 400, or even 500 pg/mL, then may crash down to 50 to 80 pg/mL. These wild fluctuations are thought to trigger headaches, sleep disturbance, mood swings, and sexual complaints in some women.
Hysterectomy and mood symptoms. Women in their early 40s are exposed to high levels of estrogen in some menstrual cycles and low levels in others. Excess estrogen thickens the endometrium—causing heavier bleeding—and stimulates fibroid growth, which is the leading reason for hysterectomies.
One in four American women undergoes surgical menopause. The average age of the 700,000 U.S. women who undergo hysterectomy each year is 40 to 44. Women who have had a hysterectomy and have mood symptoms and sexual adjustment problems are likely to see psychiatrists earlier than women who undergo a more gradual natural menopause.
PERIMENOPAUSE: HOT FLASHES, DRY VAGINA
At ages 45 to 55, most women (90%) who have not had a hysterectomy start to cycle irregularly, tending at first toward shorter cycles and then skipping periods. Some periods are heavier and some lighter than usual. The remaining 10% of women continue to cycle regularly until their menstrual periods stop abruptly.
Many women notice temperature dysregulation during perimenopause. When they exercise, their cool-down times may double. Menopausal symptoms such as hot flashes occur when estrogen levels drop below the point that some researchers call a woman’s “estrogen set point.”
Screening for estrogen decline. When you see a patient in your office, you can often determine whether her affective symptoms—irritability, mood swings, depressed mood, and forgetfulness—might be related to estrogen decline by asking two screening questions:
- Are you having any warm flushes or hot flashes?
- Do you have vaginal dryness?
Figure 2 Normal female reproductive cycle: The rhythm of the hypothalamic-pituitary-ovarian axis
LATE MENOPAUSE: ESTROGEN LOW, MOOD UP
During late menopause, approximately age 55 and older, women commonly complain of vaginal dryness, hot flashes, night sweats, sleep problems, and fatigue. Sexual interest may decrease, and a decline in sexual activity can become a problem for some couples. Asking “How is your sex life?” often will open a discussion of the couple’s sexual and emotional relationship.
Other physiologic changes caused by estrogen and androgen deficiency include thinning body hair—including pubic, auxiliary, and leg hair—decreased body odor, thinning skin, wrinkling skin, and decreasing bone density.
Table 4
Three phases of menopause: A 30-year process
| Early Ages 35 to 45 | Middle (perimenopause) Ages 46 to 55 | Late Ages 56 to 65+ |
|---|---|---|
| Physiologic changes | ||
| Ovary starts producing less inhibin 15 years before menses stop | Irregular menstrual cycles, with shorter cycles, skipping periods for 90% of women | Depletion of eggs and follicle |
| Decreased inhibin increases FSH and stimulates follicle to produce more estrogen | Some periods heavier, some lighter than usual | |
| Increased estrogen thickens endometrium and leads to heavier menstrual bleeding and increased risk of fibroids | ||
| Increased FSH produces more activin, which makes eggs develop faster and accelerates egg depletion | ||
| Lab values | ||
| Menses: Normal | Cycle shorter (24-26 days) | None for >12 months |
| FSH day 3: 10-25 mIU/mL | 20-30 mIU/mL | 50-90 mIU/mL |
| Estradiol day 3: 40-200 pg/mL | 40-200 pg/mL | 10-20 pg/mL |
| Inhibin B day 3: Varies | <45 pg/mL | 0 |
| Symptoms | ||
| Headaches, sleep disturbances, mood swings, urinary problems, sexual complaints | Warm flushes, hot flashes, night sweats in 82% of women (moderate to severe in 40%) | Vaginal dryness, hot flashes, night sweats, sleep problems, fatigue, sexual interest changes, thinning body hair (pubic, legs, axillary), decreased body odor, thinning skin, wrinkling skin, decreasing bone density, BUT mood starts to stabilize |
| FSH: follicle-stimulating hormone | ||
Urinary symptoms. Bladder problems and urinary symptoms are persistent symptoms of menopause for 75% of women. Although we psychiatrists don’t review the urinary system, it is important to remember that embarrassment because of urinary incontinence during sex may have a lot to do with a woman’s “loss of interest” in sexual intercourse.
Despite sometimes-difficult physiologic changes, the good news for many women is that mood symptoms start to stabilize after perimenopause (Table 4). Women whose moods are very responsive to hormonal fluctuations—such as those with severe premenstrual syndrome or premenstrual dysphoric disorder—sometimes get much better after menopause.
Related resources
- The Women’s Health Site. Duke Academic Program in Women’s Health. www.thewomenshealthsite.org
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Paroxetine • Paxil
- Sildenafil • Viagra
- Venlafaxine • Effexor
Disclosure
Dr. Brizendine reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric 2003;6(suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med 2003;348:645-50.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9(6):392-8.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am 1997;26(2):279-94.
6. Seppa N. Hormone therapy falls out of favor. Science News 2002;162:61.-
7. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am 2003;32(2):325-36.
8. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44(9):798-811.
9. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1839-54.
10. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002;29(1):33-40.
11. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289(21):2827-34.
12. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64(4):473-9.
13. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20(6):1578-83.
14. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101(2):337-45.
15. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108(6):623-8.
16. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric 2002;5(4):357-65.
17. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol 2003;85(2-5):363-6.
18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
19. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril 2003;79(6):1341-52.
20. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol 1997;90(6):995-8.
21. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77(4):660-5.
22. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab 2003;17(1):165-75.
23. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol 2002;20(2):106-10.
24. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause 1999;6(3):216-24.
25. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums 2003;8(March):3.-
26. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995;52(6):363-9.
27. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril 2002;77(suppl 4):S83-8.
28. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28(suppl 1):129-42.
29. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull 1997;33(4):761-5.
30. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction (poster presentation). Waikoloa, HI: North American Society for Psychosocial Obstetrics and Gynecology, 2001.
Psychiatrists are suddenly viewed as experts in treating menopause-related mood problems because of our expertise with using psychotropics. Practically overnight, the Women’s Health Initiative studies1,2 have made women and their doctors think twice about using estrogen. Instead, many are turning to psychiatric medications that have been shown to improve both mood and hot flashes—without estrogen’s potential risks.
Chances are good that after an Ob/Gyn has tried one or two psychotropics without success or with too many side effects, he or she will ask a psychiatrist to consult for certain patients. How well-prepared are you to assume this role?
If your recall of female reproductive physiology from medical school is incomplete, read on about one approach to a perimenopausal patient with depressed mood. This review can help you:
- discuss menopause knowledgeably when other physicians refer their patients to you
- provide effective, up-to-date treatments for menopause-related mood and sexual problems, using psychotropics or hormones, alone or in combination.
Irritable, with no interest in sex
Anne, age 51, has been referred to you for complaints of depressed mood and low libido. She says she has become irritable and snaps easily at her two children and her husband. She has no interest in sex, no urge to masturbate, and has had no sexual intercourse for 6 months.
Table 1
Why mood problems may occur during menopause
| Hypothesis | Explanation |
|---|---|
| Psychodynamic | Onset of menopause is a critical life event and a readjustment of self-concept |
| Sociologic | Mood changes are caused by changing life circumstances at menopause (‘empty nest,’ aging parents, health changes) |
| Domino | Depressed mood is caused by hot flashes due to declining estrogen levels, which cause chronic sleep deprivation with subsequent irritability and memory and mood changes |
| Biochemical | Decreasing estrogen leads to neurochemical changes in the brain (serotonin, dopamine, cholinergic, GABA, norepinephrine) |
Anne also complains of fatigue, dry hair and skin, warm flushes, and painful joints. She has no personal or family history of depression. She is not suicidal but states that she really doesn’t want to live anymore if “this is it.”
HOT FLASHES: A SPARK FOR DEPRESSION
Women who experience their first depression after age 50 do not fit the usual DSM-IV diagnostic criteria for depression. The Massachusetts Women’s Health Study3 found that 52% of women who experience depressed mood in the perimenopause have never had a depression before. This study also found a correlation between a longer perimenopause (>27 months) and increased risk of depressed mood. At the same time, women who have had a prior depression are 4 to 9 times more likely to experience depressive symptoms during perimenopause than those who have never had a depression before.4
The increased mood symptoms may be related to psychodynamic, sociologic, or biochemical factors, or they may result from a domino effect triggered by declining estrogen levels (Table 1). Women who experience vasomotor symptoms such as hot flashes are at 4.6 times greater risk for depression than those who are hot flash-free.5
Hot flashes begin on average at age 51, which is also the average age when natural menopause begins. During menopause, most women (82%) experience hot flashes (suddenly feeling hot and sweating during the day), warm flushes (a sensation of warmth or heat spreading over the skin), and night sweats (Table 2). All women who undergo surgical menopause experience hot flashes.
Hot flashes are moderate to severe for 40% of women who experience them and persist for 5 to 15 years. By definition, moderate to severe hot flashes occur 6 to 10 or more times daily, last 6 to 10 minutes each, and are often preceded by anxiety, palpitations, irritability, nervousness, or panic.
A marriage under stress
Anne says that her husband is angry about the lack of sexual intercourse, and she feels the stress in their marriage. She also is worrying about her children leaving for college and about her mother’s ill health.
She scores 20 on the Beck Depression Inventory, which indicates that she has mild to moderate depression. Her menstrual periods remain regular, but her cycle has shortened from 29 to 24 days. She reports experiencing some hot flashes that wake her at night and says she hasn’t had a good night’s sleep in months.
Laboratory tests show FSH of 25 mIU/mL and inhibin B <45 pg/mL. Her estradiol is 80 pg/mL, which is not yet in the menopausal range of 10 to 20 pg/mL. Her thyroid stimulating hormone (TSH) is normal. Her endocrinologic and reproductive diagnosis is perimenopause.
Table 2
Symptoms of menopause related to decreased estrogen
| Brain | Irritability, mood swings, depressed mood, forgetfulness, low sex interest, sleep problems, decreased well-being |
| Body | Hot flashes, vaginal dryness, painful intercourse, fatigue, joint pain, pain with orgasm, bladder dysfunction |
TREATING HOT FLASHES IMPROVES MOOD
Until July 2002, estrogen was standard treatment for controlling hot flashes in patients such as Anne. Then the Women’s Health Initiative trial reported that estrogen’s health risks—heart attack, stroke, breast cancer, and blood clots—exceeded potential benefits during 5 years of therapy. As a result, fewer women want to take estrogen,6 and many Ob/Gyns are advising patients to get through menopause without hormones if they can.
For mild hot flashes—one to three per day—patients may only need vitamin E, 800 mg/d, and deep relaxation breathing to “rev down” the sympathetic nervous system when a hot flash occurs.
For moderate to severe hot flashes—four to 10 or more per day—estrogen replacement is the most effective therapy. Estradiol, 1 mg/d, reduces hot flashes by approximately 80 to 90%.7 Many small studies have shown that patients’ mood often improves as estrogen reduces their hot flashes.8 The recent Women’s Health Initiative Quality-of-Life study, however, reported that estrogen plus progestin did not improve mood in women ages 50 to 54 with moderate-to-severe vasomotor symptoms, even though hot flashes were reduced and sleep may have improved.9
New drugs of choice. Because of estrogen’s effectiveness in controlling hot flashes, some women and their doctors may choose to use it briefly (18 to 24 months). For others, psychotropics are becoming the drugs of choice for mood disorders with moderate to severe hot flashes.
The serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine, 75 or 150 mg/d, has been shown to reduce hot flashes by 60 to 70%.10 A new trial is investigating whether duloxetine—an SNRI awaiting FDA approval—also reduces hot flashes. Other useful agents that have been shown to reduce hot flashes by 50% or more include:
- selective serotonin reuptake inhibitors (SSRIs) paroxetine CR, 12.5 mg/d to 25 mg/d,11 citalopram, 20 to 60 mg/d,12 and fluoxetine, 20 mg/d13
- gabapentin, 900 mg/d.14
For hot flashes and moderate to major depression, try an SNRI or SSRI first (see Algorithm), but consider the possible effects on sexual function. All SNRIs and SSRIs have sexual side effects, including anorgasmia and loss of libido in women and men. Among the psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
Mood improves, but still no libido
You and Ann decide on a trial of the SNRI venlafaxine, 75 mg/d, to treat her hot flashes and depressed mood. Four weeks later, her hot flashes are reduced by 50% in frequency and her mood has improved (Beck Depression Inventory score is now 10). She is feeling much better and wishes to continue taking the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
TREATING LOW INTEREST IN SEX
Being angry with one’s partner is the number-one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.15 Other agents the FDA is reviewing for erectile dysfunction—such as tadalafil and vardenafil—may also help arousal problems in women.
Understanding how hormones affect female sexual desire also may help you decide what advice to give Anne and how you and her Ob/Gyn coordinate her care. For example, you might treat her sexual complaints and relationship problems while the Ob/Gyn manages symptoms of the vagina, uterus, and breast.
HOW TESTOSTERONE AFFECTS SEXUAL DESIRE
Testosterone is the hormone of sexual desire in men and women. Other female androgens include androstenedione, androstenediol, 5 α-dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), and its sulfate (DHEA-S). Premenopausal women produce these androgens in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%).
Average daily serum testosterone concentrations decline in women between ages 20 and 50. Lower levels are also seen with estrogen replacement therapy or oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Women who undergo total hysterectomy with bilateral oophorectomy experience a sudden 50% loss of testosterone and an 80% decline in estradiol.16
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—than they had when they were in their 30s.17 The percentage of women reporting low libido increases with age until menopause, from 30% at age 30 to about 50% at age 50. Then the rate declines to 27% in women age 50 to 59.18 After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire.
Oral estrogen replacement therapy reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in a menopausal woman.19 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.20
Female androgen deficiency. A number of papers have been published on female androgen deficiency syndrome (FADS).21 Its diagnosis requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
TREATING TESTOSTERONE DEFICIENCY
Benefits of replacement therapy. Replacing testosterone in women with FADS can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.22 Muscle and bone stimulation and decreased hot flashes are also reported.23 Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Risks of replacement therapy. Androgen replacement therapy does carry some risks, which need to be discussed with the patient. Testosterone may lower levels of beneficial HDL cholesterol, so get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level <45 mg/dL.
Algorithm Managing mood and libido problems during perimenopause
A meta-analysis of eight clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg/d of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone dosages.24
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle one-third of the cycle—on days 10 to 16, counting the first day of menstrual bleeding as day 1.25
- Oral contraceptives also decrease androgen production by the ovary and can result in low libido in some women.26
Tests developed to measure testosterone levels in men are not sensitive enough to accurately measure women’s naturally lower serum concentrations, let alone the even lower levels characteristic of female androgen or testosterone deficiency. New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.27
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEAS. Measuring SHBG will help you determine the free, biologically active testosterone level and calculate the Free Androgen Index (FAI) for women (Table 3).28
Table 3
Free androgen index (FAI) values in women, by age
| Replacing a woman’s bioactive testosterone to the normal free androgen index range for her age may improve low libido. | |
| How to calculate FAI | |
| Total testosterone in nmol/L (total testosterone in ng/ml X 0.0347 X 100), divided by sex hormone-binding globulin (SHBG) in nmol/L. | |
| Age | Normal range |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Source: Guay et al, reference 28. | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 (normal range, 1.78 to 2.86).
In collaboration with her Ob/Gyn, you and Anne decide to start her on testosterone replacement therapy. You prescribe Androgel, starting at 1/7th of a 2.5-mg foil packet (0.35 mg/d of testosterone), and instruct her to rate her sexual energy daily, using a Sexual Energy Scale.
TESTOSTERONE CHOICES FOR WOMEN
Replacing a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options include:
- methyl testosterone sublingual pills, 0.5 mg/d, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels—unless you order the very expensive test that is specific for methyl testosterone.
- 2% vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption
- Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg/d or one-seventh of the 2.5 mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations—including a testosterone patch for women and a gel in female-sized doses.
Using the Sexual Energy Scale. To monitor for a therapeutic response, ask the patient to use the Sexual Energy Scale (Figure 1).29,30 Instruct her to define her “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Her “1” would be when she felt the worst sexually and had the least desire.
Giving supplemental estrogen. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS, which contains 0.625 mg esterified estrogens and 1.25 mg of methyl testosterone. Add a progestin if the patient is postmenopausal and has not had a hysterectomy, to protect the uterus from endometrial hyperplasia.
Women with vaginal dryness also need supplemental estrogen, which can be applied vaginally (such as Premarin cream or Estrace cream). A vaginal lubricant is not sufficient to avoid age-related vaginal atrophy, which may make intercourse difficult or impossible.
Figure 1 How to use the Sexual Energy Scale to monitor response to therapy
Libido improves modestly
Ann returns in 4 weeks with gradually improving sex drive (Sexual Energy Score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but also did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low. You increase the Androgel dosage to 1/5th of a 2.5 mg packet (0.5 mg/day) and continue to monitor Anne’s Sexual Energy Scale ratings at monthly follow-up visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Louann Brizendine, MD
Medicine’s understanding of menopause’s physiologic and psychological consequences is changing, just as the “baby-boom” generation is navigating this passage called the change of life. Many midlife women are unaware that the menopause transition does not begin around age 50 but spans 30 years—from ages 35 to 65. Natural menopause begins 15 years before and ends 15 years after menstruation ceases—as the brain and tissues adjust to first fluctuating then decreased estrogen levels. It occurs in three phases—early menopause, perimenopause, and late menopause—that reflect a progression of hormone changes.
EARLY MENOPAUSE: OVULATION ACCELERATES
At approximately age 35, the ovaries start producing lower levels of inhibin—a glycoprotein that inhibits pituitary production of follicle-stimulating hormone (FSH) (Figure 2). Less inhibin means less negative feedback to the pituitary and an increase in pituitary FSH production. More FSH means more activin—an ovarian glycoprotein that stimulates ripening of eggs—and so ovulation begins to accelerate at approximately age 36. Activin stimulates more and more eggs in the ovary to develop faster and faster.
By age 37, the ovarian egg reserve starts to decline, and—because FSH has increased—the follicle is driven to produce greater amounts of estrogen. Estrogen serum levels in fertile women average 100 pg/mL. During perimenopause, estrogen levels sometimes soar to 300, 400, or even 500 pg/mL, then may crash down to 50 to 80 pg/mL. These wild fluctuations are thought to trigger headaches, sleep disturbance, mood swings, and sexual complaints in some women.
Hysterectomy and mood symptoms. Women in their early 40s are exposed to high levels of estrogen in some menstrual cycles and low levels in others. Excess estrogen thickens the endometrium—causing heavier bleeding—and stimulates fibroid growth, which is the leading reason for hysterectomies.
One in four American women undergoes surgical menopause. The average age of the 700,000 U.S. women who undergo hysterectomy each year is 40 to 44. Women who have had a hysterectomy and have mood symptoms and sexual adjustment problems are likely to see psychiatrists earlier than women who undergo a more gradual natural menopause.
PERIMENOPAUSE: HOT FLASHES, DRY VAGINA
At ages 45 to 55, most women (90%) who have not had a hysterectomy start to cycle irregularly, tending at first toward shorter cycles and then skipping periods. Some periods are heavier and some lighter than usual. The remaining 10% of women continue to cycle regularly until their menstrual periods stop abruptly.
Many women notice temperature dysregulation during perimenopause. When they exercise, their cool-down times may double. Menopausal symptoms such as hot flashes occur when estrogen levels drop below the point that some researchers call a woman’s “estrogen set point.”
Screening for estrogen decline. When you see a patient in your office, you can often determine whether her affective symptoms—irritability, mood swings, depressed mood, and forgetfulness—might be related to estrogen decline by asking two screening questions:
- Are you having any warm flushes or hot flashes?
- Do you have vaginal dryness?
Figure 2 Normal female reproductive cycle: The rhythm of the hypothalamic-pituitary-ovarian axis
LATE MENOPAUSE: ESTROGEN LOW, MOOD UP
During late menopause, approximately age 55 and older, women commonly complain of vaginal dryness, hot flashes, night sweats, sleep problems, and fatigue. Sexual interest may decrease, and a decline in sexual activity can become a problem for some couples. Asking “How is your sex life?” often will open a discussion of the couple’s sexual and emotional relationship.
Other physiologic changes caused by estrogen and androgen deficiency include thinning body hair—including pubic, auxiliary, and leg hair—decreased body odor, thinning skin, wrinkling skin, and decreasing bone density.
Table 4
Three phases of menopause: A 30-year process
| Early Ages 35 to 45 | Middle (perimenopause) Ages 46 to 55 | Late Ages 56 to 65+ |
|---|---|---|
| Physiologic changes | ||
| Ovary starts producing less inhibin 15 years before menses stop | Irregular menstrual cycles, with shorter cycles, skipping periods for 90% of women | Depletion of eggs and follicle |
| Decreased inhibin increases FSH and stimulates follicle to produce more estrogen | Some periods heavier, some lighter than usual | |
| Increased estrogen thickens endometrium and leads to heavier menstrual bleeding and increased risk of fibroids | ||
| Increased FSH produces more activin, which makes eggs develop faster and accelerates egg depletion | ||
| Lab values | ||
| Menses: Normal | Cycle shorter (24-26 days) | None for >12 months |
| FSH day 3: 10-25 mIU/mL | 20-30 mIU/mL | 50-90 mIU/mL |
| Estradiol day 3: 40-200 pg/mL | 40-200 pg/mL | 10-20 pg/mL |
| Inhibin B day 3: Varies | <45 pg/mL | 0 |
| Symptoms | ||
| Headaches, sleep disturbances, mood swings, urinary problems, sexual complaints | Warm flushes, hot flashes, night sweats in 82% of women (moderate to severe in 40%) | Vaginal dryness, hot flashes, night sweats, sleep problems, fatigue, sexual interest changes, thinning body hair (pubic, legs, axillary), decreased body odor, thinning skin, wrinkling skin, decreasing bone density, BUT mood starts to stabilize |
| FSH: follicle-stimulating hormone | ||
Urinary symptoms. Bladder problems and urinary symptoms are persistent symptoms of menopause for 75% of women. Although we psychiatrists don’t review the urinary system, it is important to remember that embarrassment because of urinary incontinence during sex may have a lot to do with a woman’s “loss of interest” in sexual intercourse.
Despite sometimes-difficult physiologic changes, the good news for many women is that mood symptoms start to stabilize after perimenopause (Table 4). Women whose moods are very responsive to hormonal fluctuations—such as those with severe premenstrual syndrome or premenstrual dysphoric disorder—sometimes get much better after menopause.
Related resources
- The Women’s Health Site. Duke Academic Program in Women’s Health. www.thewomenshealthsite.org
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Paroxetine • Paxil
- Sildenafil • Viagra
- Venlafaxine • Effexor
Disclosure
Dr. Brizendine reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatrists are suddenly viewed as experts in treating menopause-related mood problems because of our expertise with using psychotropics. Practically overnight, the Women’s Health Initiative studies1,2 have made women and their doctors think twice about using estrogen. Instead, many are turning to psychiatric medications that have been shown to improve both mood and hot flashes—without estrogen’s potential risks.
Chances are good that after an Ob/Gyn has tried one or two psychotropics without success or with too many side effects, he or she will ask a psychiatrist to consult for certain patients. How well-prepared are you to assume this role?
If your recall of female reproductive physiology from medical school is incomplete, read on about one approach to a perimenopausal patient with depressed mood. This review can help you:
- discuss menopause knowledgeably when other physicians refer their patients to you
- provide effective, up-to-date treatments for menopause-related mood and sexual problems, using psychotropics or hormones, alone or in combination.
Irritable, with no interest in sex
Anne, age 51, has been referred to you for complaints of depressed mood and low libido. She says she has become irritable and snaps easily at her two children and her husband. She has no interest in sex, no urge to masturbate, and has had no sexual intercourse for 6 months.
Table 1
Why mood problems may occur during menopause
| Hypothesis | Explanation |
|---|---|
| Psychodynamic | Onset of menopause is a critical life event and a readjustment of self-concept |
| Sociologic | Mood changes are caused by changing life circumstances at menopause (‘empty nest,’ aging parents, health changes) |
| Domino | Depressed mood is caused by hot flashes due to declining estrogen levels, which cause chronic sleep deprivation with subsequent irritability and memory and mood changes |
| Biochemical | Decreasing estrogen leads to neurochemical changes in the brain (serotonin, dopamine, cholinergic, GABA, norepinephrine) |
Anne also complains of fatigue, dry hair and skin, warm flushes, and painful joints. She has no personal or family history of depression. She is not suicidal but states that she really doesn’t want to live anymore if “this is it.”
HOT FLASHES: A SPARK FOR DEPRESSION
Women who experience their first depression after age 50 do not fit the usual DSM-IV diagnostic criteria for depression. The Massachusetts Women’s Health Study3 found that 52% of women who experience depressed mood in the perimenopause have never had a depression before. This study also found a correlation between a longer perimenopause (>27 months) and increased risk of depressed mood. At the same time, women who have had a prior depression are 4 to 9 times more likely to experience depressive symptoms during perimenopause than those who have never had a depression before.4
The increased mood symptoms may be related to psychodynamic, sociologic, or biochemical factors, or they may result from a domino effect triggered by declining estrogen levels (Table 1). Women who experience vasomotor symptoms such as hot flashes are at 4.6 times greater risk for depression than those who are hot flash-free.5
Hot flashes begin on average at age 51, which is also the average age when natural menopause begins. During menopause, most women (82%) experience hot flashes (suddenly feeling hot and sweating during the day), warm flushes (a sensation of warmth or heat spreading over the skin), and night sweats (Table 2). All women who undergo surgical menopause experience hot flashes.
Hot flashes are moderate to severe for 40% of women who experience them and persist for 5 to 15 years. By definition, moderate to severe hot flashes occur 6 to 10 or more times daily, last 6 to 10 minutes each, and are often preceded by anxiety, palpitations, irritability, nervousness, or panic.
A marriage under stress
Anne says that her husband is angry about the lack of sexual intercourse, and she feels the stress in their marriage. She also is worrying about her children leaving for college and about her mother’s ill health.
She scores 20 on the Beck Depression Inventory, which indicates that she has mild to moderate depression. Her menstrual periods remain regular, but her cycle has shortened from 29 to 24 days. She reports experiencing some hot flashes that wake her at night and says she hasn’t had a good night’s sleep in months.
Laboratory tests show FSH of 25 mIU/mL and inhibin B <45 pg/mL. Her estradiol is 80 pg/mL, which is not yet in the menopausal range of 10 to 20 pg/mL. Her thyroid stimulating hormone (TSH) is normal. Her endocrinologic and reproductive diagnosis is perimenopause.
Table 2
Symptoms of menopause related to decreased estrogen
| Brain | Irritability, mood swings, depressed mood, forgetfulness, low sex interest, sleep problems, decreased well-being |
| Body | Hot flashes, vaginal dryness, painful intercourse, fatigue, joint pain, pain with orgasm, bladder dysfunction |
TREATING HOT FLASHES IMPROVES MOOD
Until July 2002, estrogen was standard treatment for controlling hot flashes in patients such as Anne. Then the Women’s Health Initiative trial reported that estrogen’s health risks—heart attack, stroke, breast cancer, and blood clots—exceeded potential benefits during 5 years of therapy. As a result, fewer women want to take estrogen,6 and many Ob/Gyns are advising patients to get through menopause without hormones if they can.
For mild hot flashes—one to three per day—patients may only need vitamin E, 800 mg/d, and deep relaxation breathing to “rev down” the sympathetic nervous system when a hot flash occurs.
For moderate to severe hot flashes—four to 10 or more per day—estrogen replacement is the most effective therapy. Estradiol, 1 mg/d, reduces hot flashes by approximately 80 to 90%.7 Many small studies have shown that patients’ mood often improves as estrogen reduces their hot flashes.8 The recent Women’s Health Initiative Quality-of-Life study, however, reported that estrogen plus progestin did not improve mood in women ages 50 to 54 with moderate-to-severe vasomotor symptoms, even though hot flashes were reduced and sleep may have improved.9
New drugs of choice. Because of estrogen’s effectiveness in controlling hot flashes, some women and their doctors may choose to use it briefly (18 to 24 months). For others, psychotropics are becoming the drugs of choice for mood disorders with moderate to severe hot flashes.
The serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine, 75 or 150 mg/d, has been shown to reduce hot flashes by 60 to 70%.10 A new trial is investigating whether duloxetine—an SNRI awaiting FDA approval—also reduces hot flashes. Other useful agents that have been shown to reduce hot flashes by 50% or more include:
- selective serotonin reuptake inhibitors (SSRIs) paroxetine CR, 12.5 mg/d to 25 mg/d,11 citalopram, 20 to 60 mg/d,12 and fluoxetine, 20 mg/d13
- gabapentin, 900 mg/d.14
For hot flashes and moderate to major depression, try an SNRI or SSRI first (see Algorithm), but consider the possible effects on sexual function. All SNRIs and SSRIs have sexual side effects, including anorgasmia and loss of libido in women and men. Among the psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
Mood improves, but still no libido
You and Ann decide on a trial of the SNRI venlafaxine, 75 mg/d, to treat her hot flashes and depressed mood. Four weeks later, her hot flashes are reduced by 50% in frequency and her mood has improved (Beck Depression Inventory score is now 10). She is feeling much better and wishes to continue taking the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
TREATING LOW INTEREST IN SEX
Being angry with one’s partner is the number-one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.15 Other agents the FDA is reviewing for erectile dysfunction—such as tadalafil and vardenafil—may also help arousal problems in women.
Understanding how hormones affect female sexual desire also may help you decide what advice to give Anne and how you and her Ob/Gyn coordinate her care. For example, you might treat her sexual complaints and relationship problems while the Ob/Gyn manages symptoms of the vagina, uterus, and breast.
HOW TESTOSTERONE AFFECTS SEXUAL DESIRE
Testosterone is the hormone of sexual desire in men and women. Other female androgens include androstenedione, androstenediol, 5 α-dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), and its sulfate (DHEA-S). Premenopausal women produce these androgens in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%).
Average daily serum testosterone concentrations decline in women between ages 20 and 50. Lower levels are also seen with estrogen replacement therapy or oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Women who undergo total hysterectomy with bilateral oophorectomy experience a sudden 50% loss of testosterone and an 80% decline in estradiol.16
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—than they had when they were in their 30s.17 The percentage of women reporting low libido increases with age until menopause, from 30% at age 30 to about 50% at age 50. Then the rate declines to 27% in women age 50 to 59.18 After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire.
Oral estrogen replacement therapy reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in a menopausal woman.19 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.20
Female androgen deficiency. A number of papers have been published on female androgen deficiency syndrome (FADS).21 Its diagnosis requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
TREATING TESTOSTERONE DEFICIENCY
Benefits of replacement therapy. Replacing testosterone in women with FADS can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.22 Muscle and bone stimulation and decreased hot flashes are also reported.23 Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Risks of replacement therapy. Androgen replacement therapy does carry some risks, which need to be discussed with the patient. Testosterone may lower levels of beneficial HDL cholesterol, so get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level <45 mg/dL.
Algorithm Managing mood and libido problems during perimenopause
A meta-analysis of eight clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg/d of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone dosages.24
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle one-third of the cycle—on days 10 to 16, counting the first day of menstrual bleeding as day 1.25
- Oral contraceptives also decrease androgen production by the ovary and can result in low libido in some women.26
Tests developed to measure testosterone levels in men are not sensitive enough to accurately measure women’s naturally lower serum concentrations, let alone the even lower levels characteristic of female androgen or testosterone deficiency. New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.27
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEAS. Measuring SHBG will help you determine the free, biologically active testosterone level and calculate the Free Androgen Index (FAI) for women (Table 3).28
Table 3
Free androgen index (FAI) values in women, by age
| Replacing a woman’s bioactive testosterone to the normal free androgen index range for her age may improve low libido. | |
| How to calculate FAI | |
| Total testosterone in nmol/L (total testosterone in ng/ml X 0.0347 X 100), divided by sex hormone-binding globulin (SHBG) in nmol/L. | |
| Age | Normal range |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Source: Guay et al, reference 28. | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 (normal range, 1.78 to 2.86).
In collaboration with her Ob/Gyn, you and Anne decide to start her on testosterone replacement therapy. You prescribe Androgel, starting at 1/7th of a 2.5-mg foil packet (0.35 mg/d of testosterone), and instruct her to rate her sexual energy daily, using a Sexual Energy Scale.
TESTOSTERONE CHOICES FOR WOMEN
Replacing a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options include:
- methyl testosterone sublingual pills, 0.5 mg/d, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels—unless you order the very expensive test that is specific for methyl testosterone.
- 2% vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption
- Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg/d or one-seventh of the 2.5 mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations—including a testosterone patch for women and a gel in female-sized doses.
Using the Sexual Energy Scale. To monitor for a therapeutic response, ask the patient to use the Sexual Energy Scale (Figure 1).29,30 Instruct her to define her “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Her “1” would be when she felt the worst sexually and had the least desire.
Giving supplemental estrogen. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS, which contains 0.625 mg esterified estrogens and 1.25 mg of methyl testosterone. Add a progestin if the patient is postmenopausal and has not had a hysterectomy, to protect the uterus from endometrial hyperplasia.
Women with vaginal dryness also need supplemental estrogen, which can be applied vaginally (such as Premarin cream or Estrace cream). A vaginal lubricant is not sufficient to avoid age-related vaginal atrophy, which may make intercourse difficult or impossible.
Figure 1 How to use the Sexual Energy Scale to monitor response to therapy
Libido improves modestly
Ann returns in 4 weeks with gradually improving sex drive (Sexual Energy Score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but also did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low. You increase the Androgel dosage to 1/5th of a 2.5 mg packet (0.5 mg/day) and continue to monitor Anne’s Sexual Energy Scale ratings at monthly follow-up visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Louann Brizendine, MD
Medicine’s understanding of menopause’s physiologic and psychological consequences is changing, just as the “baby-boom” generation is navigating this passage called the change of life. Many midlife women are unaware that the menopause transition does not begin around age 50 but spans 30 years—from ages 35 to 65. Natural menopause begins 15 years before and ends 15 years after menstruation ceases—as the brain and tissues adjust to first fluctuating then decreased estrogen levels. It occurs in three phases—early menopause, perimenopause, and late menopause—that reflect a progression of hormone changes.
EARLY MENOPAUSE: OVULATION ACCELERATES
At approximately age 35, the ovaries start producing lower levels of inhibin—a glycoprotein that inhibits pituitary production of follicle-stimulating hormone (FSH) (Figure 2). Less inhibin means less negative feedback to the pituitary and an increase in pituitary FSH production. More FSH means more activin—an ovarian glycoprotein that stimulates ripening of eggs—and so ovulation begins to accelerate at approximately age 36. Activin stimulates more and more eggs in the ovary to develop faster and faster.
By age 37, the ovarian egg reserve starts to decline, and—because FSH has increased—the follicle is driven to produce greater amounts of estrogen. Estrogen serum levels in fertile women average 100 pg/mL. During perimenopause, estrogen levels sometimes soar to 300, 400, or even 500 pg/mL, then may crash down to 50 to 80 pg/mL. These wild fluctuations are thought to trigger headaches, sleep disturbance, mood swings, and sexual complaints in some women.
Hysterectomy and mood symptoms. Women in their early 40s are exposed to high levels of estrogen in some menstrual cycles and low levels in others. Excess estrogen thickens the endometrium—causing heavier bleeding—and stimulates fibroid growth, which is the leading reason for hysterectomies.
One in four American women undergoes surgical menopause. The average age of the 700,000 U.S. women who undergo hysterectomy each year is 40 to 44. Women who have had a hysterectomy and have mood symptoms and sexual adjustment problems are likely to see psychiatrists earlier than women who undergo a more gradual natural menopause.
PERIMENOPAUSE: HOT FLASHES, DRY VAGINA
At ages 45 to 55, most women (90%) who have not had a hysterectomy start to cycle irregularly, tending at first toward shorter cycles and then skipping periods. Some periods are heavier and some lighter than usual. The remaining 10% of women continue to cycle regularly until their menstrual periods stop abruptly.
Many women notice temperature dysregulation during perimenopause. When they exercise, their cool-down times may double. Menopausal symptoms such as hot flashes occur when estrogen levels drop below the point that some researchers call a woman’s “estrogen set point.”
Screening for estrogen decline. When you see a patient in your office, you can often determine whether her affective symptoms—irritability, mood swings, depressed mood, and forgetfulness—might be related to estrogen decline by asking two screening questions:
- Are you having any warm flushes or hot flashes?
- Do you have vaginal dryness?
Figure 2 Normal female reproductive cycle: The rhythm of the hypothalamic-pituitary-ovarian axis
LATE MENOPAUSE: ESTROGEN LOW, MOOD UP
During late menopause, approximately age 55 and older, women commonly complain of vaginal dryness, hot flashes, night sweats, sleep problems, and fatigue. Sexual interest may decrease, and a decline in sexual activity can become a problem for some couples. Asking “How is your sex life?” often will open a discussion of the couple’s sexual and emotional relationship.
Other physiologic changes caused by estrogen and androgen deficiency include thinning body hair—including pubic, auxiliary, and leg hair—decreased body odor, thinning skin, wrinkling skin, and decreasing bone density.
Table 4
Three phases of menopause: A 30-year process
| Early Ages 35 to 45 | Middle (perimenopause) Ages 46 to 55 | Late Ages 56 to 65+ |
|---|---|---|
| Physiologic changes | ||
| Ovary starts producing less inhibin 15 years before menses stop | Irregular menstrual cycles, with shorter cycles, skipping periods for 90% of women | Depletion of eggs and follicle |
| Decreased inhibin increases FSH and stimulates follicle to produce more estrogen | Some periods heavier, some lighter than usual | |
| Increased estrogen thickens endometrium and leads to heavier menstrual bleeding and increased risk of fibroids | ||
| Increased FSH produces more activin, which makes eggs develop faster and accelerates egg depletion | ||
| Lab values | ||
| Menses: Normal | Cycle shorter (24-26 days) | None for >12 months |
| FSH day 3: 10-25 mIU/mL | 20-30 mIU/mL | 50-90 mIU/mL |
| Estradiol day 3: 40-200 pg/mL | 40-200 pg/mL | 10-20 pg/mL |
| Inhibin B day 3: Varies | <45 pg/mL | 0 |
| Symptoms | ||
| Headaches, sleep disturbances, mood swings, urinary problems, sexual complaints | Warm flushes, hot flashes, night sweats in 82% of women (moderate to severe in 40%) | Vaginal dryness, hot flashes, night sweats, sleep problems, fatigue, sexual interest changes, thinning body hair (pubic, legs, axillary), decreased body odor, thinning skin, wrinkling skin, decreasing bone density, BUT mood starts to stabilize |
| FSH: follicle-stimulating hormone | ||
Urinary symptoms. Bladder problems and urinary symptoms are persistent symptoms of menopause for 75% of women. Although we psychiatrists don’t review the urinary system, it is important to remember that embarrassment because of urinary incontinence during sex may have a lot to do with a woman’s “loss of interest” in sexual intercourse.
Despite sometimes-difficult physiologic changes, the good news for many women is that mood symptoms start to stabilize after perimenopause (Table 4). Women whose moods are very responsive to hormonal fluctuations—such as those with severe premenstrual syndrome or premenstrual dysphoric disorder—sometimes get much better after menopause.
Related resources
- The Women’s Health Site. Duke Academic Program in Women’s Health. www.thewomenshealthsite.org
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Paroxetine • Paxil
- Sildenafil • Viagra
- Venlafaxine • Effexor
Disclosure
Dr. Brizendine reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric 2003;6(suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med 2003;348:645-50.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9(6):392-8.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am 1997;26(2):279-94.
6. Seppa N. Hormone therapy falls out of favor. Science News 2002;162:61.-
7. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am 2003;32(2):325-36.
8. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44(9):798-811.
9. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1839-54.
10. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002;29(1):33-40.
11. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289(21):2827-34.
12. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64(4):473-9.
13. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20(6):1578-83.
14. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101(2):337-45.
15. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108(6):623-8.
16. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric 2002;5(4):357-65.
17. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol 2003;85(2-5):363-6.
18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
19. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril 2003;79(6):1341-52.
20. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol 1997;90(6):995-8.
21. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77(4):660-5.
22. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab 2003;17(1):165-75.
23. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol 2002;20(2):106-10.
24. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause 1999;6(3):216-24.
25. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums 2003;8(March):3.-
26. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995;52(6):363-9.
27. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril 2002;77(suppl 4):S83-8.
28. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28(suppl 1):129-42.
29. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull 1997;33(4):761-5.
30. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction (poster presentation). Waikoloa, HI: North American Society for Psychosocial Obstetrics and Gynecology, 2001.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric 2003;6(suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med 2003;348:645-50.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9(6):392-8.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am 1997;26(2):279-94.
6. Seppa N. Hormone therapy falls out of favor. Science News 2002;162:61.-
7. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am 2003;32(2):325-36.
8. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44(9):798-811.
9. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1839-54.
10. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002;29(1):33-40.
11. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289(21):2827-34.
12. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64(4):473-9.
13. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20(6):1578-83.
14. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101(2):337-45.
15. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108(6):623-8.
16. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric 2002;5(4):357-65.
17. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol 2003;85(2-5):363-6.
18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
19. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril 2003;79(6):1341-52.
20. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol 1997;90(6):995-8.
21. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77(4):660-5.
22. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab 2003;17(1):165-75.
23. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol 2002;20(2):106-10.
24. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause 1999;6(3):216-24.
25. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums 2003;8(March):3.-
26. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995;52(6):363-9.
27. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril 2002;77(suppl 4):S83-8.
28. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28(suppl 1):129-42.
29. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull 1997;33(4):761-5.
30. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction (poster presentation). Waikoloa, HI: North American Society for Psychosocial Obstetrics and Gynecology, 2001.