User login
What are the indications for evaluating a patient with cough for pertussis?
Pertussis should be considered in infants with apnea or severe coughing illnesses of any duration, and in older children or adults with prolonged cough (eg, longer than 2 weeks), especially if accompanied by inspiratory whoop or household exposure to a prolonged cough illness (strength of recommendation [SOR]: B, based on consecutive cohort studies with poor reference standards). Coughing paroxysms, posttussive vomiting, and absence of fever, while typical of pertussis, are of little help in distinguishing it from other causes of prolonged coughing illnesses (SOR: B, based on consecutive cohort studies with poor reference standards).
Evidence summary
Pertussis is an important cause of cough in all age groups. Ten prevalence studies of adolescents and adults seeking medical attention for a prolonged cough (defined variously as >1–4 weeks) found acute pertussis in 12% to 32%.1
While cough longer than 2 weeks, inspiratory whoop, posttussive vomiting, coughing paroxysms, and absence of fever are commonly associated with pertussis, relatively few studies have assessed the sensitivities and specificities of these symptoms. The TABLE summarizes results from 5 cohort series of children and adults with laboratory-confirmed pertussis. Comparison groups were variously defined by negative pertussis cultures, negative pertussis serology, or serologic confirmation of other respiratory infections. Likelihood ratios (LR) were calculated from the data presented in each paper.
The magnitude and variability of these likelihood ratios suggest that individual symptoms may be of limited help in distinguishing pertussis from other causes of prolonged cough. Combinations of symptoms may be slightly more helpful. In a study comparing 10 patients with culture-confirmed pertussis with 10 patients with serologically confirmed mycoplasma pneumonia, the combination of cough >14 days and whoop had a sensitivity of 80%, a positive LR (LR+) of 8 and a negative LR (LR–) of 0.22.2 A cohort series of children aged <5 years with suspected pertussis compared 33 with positive cultures to 55 with negative cultures. The constellation of spasmodic cough and lymphocytosis (>10,000) had a sensitivity of 83%, a LR+ of 2.5, and a LR– of 0.25. Cough >14 days with whoop and vomiting had a sensitivity of 67%, a LR+ of 3.2, and LR– of 0.42.3
Infants aged <6 months with pertussis are at particular risk for atypical presentations and serious complications. In a US series of 18,500 infants with pertussis, apnea was seen in 64% of infants under 1 month and in 44% between 6 and 11 months. Forty percent of the 6- to 11-month-olds had received at least 3 doses of pertussis vaccine.4 A British study of 126 infants aged <5 months admitted to the pediatric intensive care unit with apnea, bradycardia, or respiratory failure found that 20% had pertussis. Apnea as a predictor of pertussis had a sensitivity of 68% and a specificity of 60%.5
Pertussis should be considered early in the evaluation of young infants with cough. In a case-control study comparing 15 fatal cases of pertussis with 32 who survived (infants aged <6 months), the mean number of days from symptom onset to hospital admission were 5.3 (fatal) and 8.6 (survivors). Rates of apnea on admission were 40% and 52%.6 A case series of 9 infants aged <7 weeks requiring admission to an intensive care unit for pertussis found that 8 had been sick for less than 4 days at the time of admission. All 9 presented with poor feeding and cough, and 5 had experienced apnea.7
TABLE
Clinical features of pertussis
| History | Sensitivity | Specificity | LR+ | LR– |
|---|---|---|---|---|
| Cough >2 weeks3,12 | 84%–100% | 35%–36% | 1.3–1.5 | 0–0.44 |
| Cough >3 weeks3,12 | 75%–97% | 51%–59% | 1.8–2.0 | 0.06–0.42 |
| Whoop3,10-12 | 37%–90% | 49%–96% | 1.6–9.2 | 0.18–0.66 |
| Posttussivevomiting3,10,11,13 | 28%–84% | 45%–84% | 0.9–2.2 | 0.36–1.0 |
| Paroxysms3,12,13 | 68%–94% | 15%–45% | 1.1–1.4 | 0.29–0.71 |
| Householdexposure11,13 | 20%–50% | 73%–91% | 1.9–2.2 | 0.68–0.88 |
| Afebrile(temp <38°C)11-13 | 62%–96% | 12%–54% | 0.8–1.1 | 0–1.7 |
| Lymphocytosis3 | 88% | 57% | 2.0 | 0.21 |
| LR+ = positive likelihood ratio: sensitivity/(1–specificity); † LR– = negative likelihood ratio: (1–sensitivity)/specificity. | ||||
Recommendations from others
The Centers for Disease Control and Prevention and the World Health Organization describe the clinical case definition for pertussis as a cough illness lasting at least 2 weeks with at least 1 of the following: paroxysms of coughing, inspiratory whoop, or posttussive vomiting, without other apparent cause. Laboratory criteria for diagnosis include a positive Bordetella pertussis. culture or a positive polymerase chain reaction (PCR) for B pertussis.8,9
Infants may have complications; evaluate if there is apnea or significant cough
Michael Ohl, MD
University of Missouri–Columbia
Immunity to pertussis wanes following vaccination, leaving many adolescents and adults susceptible to infection. In older children and adults, there is often little in the clinical presentation that distinguishes chronic cough due to pertussis from that associated with other causes. Clinicians should consider evaluating for pertussis in older children and adults with chronic cough (>2 weeks) if there is reason to suspect they have been exposed, if the cough is associated with inspiratory whoop, or if the individual has household or frequent contact with infants.
Infants may suffer severe complications from pertussis, and should receive evaluation when presenting with apnea or significant cough of any duration. In current practice, evaluation usually includes obtaining a nasopharyngeal swab for culture and PCR, though these tests may be insensitive, especially in later phases of illness. The usefulness of single, quantitative immunoglobulin G titers with comparison to popimpact the epidemiology of pertussis in the US.
1. von König CHW, Halperin S, Riffelman M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis 2002;2:744-750.
2. Davis SF, Sutter RW, Strebel PM, Orton C, Alexander V, Sanden GN, et al. Concurrent outbreaks of pertussis and Mycoplasma pneumoniae infection: clinical and epidemiological characteristics of illnesses manifested by cough. Clin Infect Dis 1995;20:621-628.
3. Strebel PM, Cochi SL, Farizo KM, Payne BJ, Hanauer SD, Baughman AL. Pertussis in Missouri: evaluation of nasopharyngeal culture, direct fluorescent antibody testing, and clinical case definitions in the diagnosis of pertussis. Clin Infect Dis 1993;16:276-285.
4. Tanaka M, Vitck CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003;290:2968-2975.
5. Crowcroft NS, Booy R, Harrison T, Spicer L, Britto J, Mok Q, et al. Severe and unrecognised: pertussis in UK infants. Arch Dis Child 2003;88:802-806.
6. Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr 2003;143:575-581.
7. Smith C, Vyas H. Early infantile pertussis; increasingly prevalent and potentially fatal. Eur J Pediatr 2000;159:898-900.
8. Pertussis (Bordetella pertussis) (Whooping cough). Epidemiology Program Office, Centers for Disease Control and Prevention. Last updated April 7, 2004. Available at: www.cdc.gov/epo/dphsi/casedef/pertussis_current.htm. Accessed on December 8, 2004.
9. VaccinesImmunizations and Biologicals: Pertussis. Geneva, Switzerland: World Health Organization; 2003. Available at: www.who.int/vaccines-surveillance/deseasedesc/ RSS_pertus.htm. Accessed on December 8, 2004.
10. Wirsing von Konig CH, Rott H, Bogaerts H, Schmitt HJ. A serologic study of organisms possibly associated with pertussis-like coughing. Pediatr Infect Dis J 1998;17:645-649.
11. Granström G, Wretlind B, Granström M. Diagnostic value of clinical and bacteriological findings in pertussis. J Infect 1991;22:17-26.
12. Heininger U, Cherry JD, Eckhardt T, Lorenz C, Christenson P, Stehr K. Clinical and laboratory diagnosis of pertussis in the regions of a large vaccine efficacy trial in Germany. Pediatr Infect Dis J 1993;12:504-509.
13. Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA 1995;273:1044-1046.
Pertussis should be considered in infants with apnea or severe coughing illnesses of any duration, and in older children or adults with prolonged cough (eg, longer than 2 weeks), especially if accompanied by inspiratory whoop or household exposure to a prolonged cough illness (strength of recommendation [SOR]: B, based on consecutive cohort studies with poor reference standards). Coughing paroxysms, posttussive vomiting, and absence of fever, while typical of pertussis, are of little help in distinguishing it from other causes of prolonged coughing illnesses (SOR: B, based on consecutive cohort studies with poor reference standards).
Evidence summary
Pertussis is an important cause of cough in all age groups. Ten prevalence studies of adolescents and adults seeking medical attention for a prolonged cough (defined variously as >1–4 weeks) found acute pertussis in 12% to 32%.1
While cough longer than 2 weeks, inspiratory whoop, posttussive vomiting, coughing paroxysms, and absence of fever are commonly associated with pertussis, relatively few studies have assessed the sensitivities and specificities of these symptoms. The TABLE summarizes results from 5 cohort series of children and adults with laboratory-confirmed pertussis. Comparison groups were variously defined by negative pertussis cultures, negative pertussis serology, or serologic confirmation of other respiratory infections. Likelihood ratios (LR) were calculated from the data presented in each paper.
The magnitude and variability of these likelihood ratios suggest that individual symptoms may be of limited help in distinguishing pertussis from other causes of prolonged cough. Combinations of symptoms may be slightly more helpful. In a study comparing 10 patients with culture-confirmed pertussis with 10 patients with serologically confirmed mycoplasma pneumonia, the combination of cough >14 days and whoop had a sensitivity of 80%, a positive LR (LR+) of 8 and a negative LR (LR–) of 0.22.2 A cohort series of children aged <5 years with suspected pertussis compared 33 with positive cultures to 55 with negative cultures. The constellation of spasmodic cough and lymphocytosis (>10,000) had a sensitivity of 83%, a LR+ of 2.5, and a LR– of 0.25. Cough >14 days with whoop and vomiting had a sensitivity of 67%, a LR+ of 3.2, and LR– of 0.42.3
Infants aged <6 months with pertussis are at particular risk for atypical presentations and serious complications. In a US series of 18,500 infants with pertussis, apnea was seen in 64% of infants under 1 month and in 44% between 6 and 11 months. Forty percent of the 6- to 11-month-olds had received at least 3 doses of pertussis vaccine.4 A British study of 126 infants aged <5 months admitted to the pediatric intensive care unit with apnea, bradycardia, or respiratory failure found that 20% had pertussis. Apnea as a predictor of pertussis had a sensitivity of 68% and a specificity of 60%.5
Pertussis should be considered early in the evaluation of young infants with cough. In a case-control study comparing 15 fatal cases of pertussis with 32 who survived (infants aged <6 months), the mean number of days from symptom onset to hospital admission were 5.3 (fatal) and 8.6 (survivors). Rates of apnea on admission were 40% and 52%.6 A case series of 9 infants aged <7 weeks requiring admission to an intensive care unit for pertussis found that 8 had been sick for less than 4 days at the time of admission. All 9 presented with poor feeding and cough, and 5 had experienced apnea.7
TABLE
Clinical features of pertussis
| History | Sensitivity | Specificity | LR+ | LR– |
|---|---|---|---|---|
| Cough >2 weeks3,12 | 84%–100% | 35%–36% | 1.3–1.5 | 0–0.44 |
| Cough >3 weeks3,12 | 75%–97% | 51%–59% | 1.8–2.0 | 0.06–0.42 |
| Whoop3,10-12 | 37%–90% | 49%–96% | 1.6–9.2 | 0.18–0.66 |
| Posttussivevomiting3,10,11,13 | 28%–84% | 45%–84% | 0.9–2.2 | 0.36–1.0 |
| Paroxysms3,12,13 | 68%–94% | 15%–45% | 1.1–1.4 | 0.29–0.71 |
| Householdexposure11,13 | 20%–50% | 73%–91% | 1.9–2.2 | 0.68–0.88 |
| Afebrile(temp <38°C)11-13 | 62%–96% | 12%–54% | 0.8–1.1 | 0–1.7 |
| Lymphocytosis3 | 88% | 57% | 2.0 | 0.21 |
| LR+ = positive likelihood ratio: sensitivity/(1–specificity); † LR– = negative likelihood ratio: (1–sensitivity)/specificity. | ||||
Recommendations from others
The Centers for Disease Control and Prevention and the World Health Organization describe the clinical case definition for pertussis as a cough illness lasting at least 2 weeks with at least 1 of the following: paroxysms of coughing, inspiratory whoop, or posttussive vomiting, without other apparent cause. Laboratory criteria for diagnosis include a positive Bordetella pertussis. culture or a positive polymerase chain reaction (PCR) for B pertussis.8,9
Infants may have complications; evaluate if there is apnea or significant cough
Michael Ohl, MD
University of Missouri–Columbia
Immunity to pertussis wanes following vaccination, leaving many adolescents and adults susceptible to infection. In older children and adults, there is often little in the clinical presentation that distinguishes chronic cough due to pertussis from that associated with other causes. Clinicians should consider evaluating for pertussis in older children and adults with chronic cough (>2 weeks) if there is reason to suspect they have been exposed, if the cough is associated with inspiratory whoop, or if the individual has household or frequent contact with infants.
Infants may suffer severe complications from pertussis, and should receive evaluation when presenting with apnea or significant cough of any duration. In current practice, evaluation usually includes obtaining a nasopharyngeal swab for culture and PCR, though these tests may be insensitive, especially in later phases of illness. The usefulness of single, quantitative immunoglobulin G titers with comparison to popimpact the epidemiology of pertussis in the US.
Pertussis should be considered in infants with apnea or severe coughing illnesses of any duration, and in older children or adults with prolonged cough (eg, longer than 2 weeks), especially if accompanied by inspiratory whoop or household exposure to a prolonged cough illness (strength of recommendation [SOR]: B, based on consecutive cohort studies with poor reference standards). Coughing paroxysms, posttussive vomiting, and absence of fever, while typical of pertussis, are of little help in distinguishing it from other causes of prolonged coughing illnesses (SOR: B, based on consecutive cohort studies with poor reference standards).
Evidence summary
Pertussis is an important cause of cough in all age groups. Ten prevalence studies of adolescents and adults seeking medical attention for a prolonged cough (defined variously as >1–4 weeks) found acute pertussis in 12% to 32%.1
While cough longer than 2 weeks, inspiratory whoop, posttussive vomiting, coughing paroxysms, and absence of fever are commonly associated with pertussis, relatively few studies have assessed the sensitivities and specificities of these symptoms. The TABLE summarizes results from 5 cohort series of children and adults with laboratory-confirmed pertussis. Comparison groups were variously defined by negative pertussis cultures, negative pertussis serology, or serologic confirmation of other respiratory infections. Likelihood ratios (LR) were calculated from the data presented in each paper.
The magnitude and variability of these likelihood ratios suggest that individual symptoms may be of limited help in distinguishing pertussis from other causes of prolonged cough. Combinations of symptoms may be slightly more helpful. In a study comparing 10 patients with culture-confirmed pertussis with 10 patients with serologically confirmed mycoplasma pneumonia, the combination of cough >14 days and whoop had a sensitivity of 80%, a positive LR (LR+) of 8 and a negative LR (LR–) of 0.22.2 A cohort series of children aged <5 years with suspected pertussis compared 33 with positive cultures to 55 with negative cultures. The constellation of spasmodic cough and lymphocytosis (>10,000) had a sensitivity of 83%, a LR+ of 2.5, and a LR– of 0.25. Cough >14 days with whoop and vomiting had a sensitivity of 67%, a LR+ of 3.2, and LR– of 0.42.3
Infants aged <6 months with pertussis are at particular risk for atypical presentations and serious complications. In a US series of 18,500 infants with pertussis, apnea was seen in 64% of infants under 1 month and in 44% between 6 and 11 months. Forty percent of the 6- to 11-month-olds had received at least 3 doses of pertussis vaccine.4 A British study of 126 infants aged <5 months admitted to the pediatric intensive care unit with apnea, bradycardia, or respiratory failure found that 20% had pertussis. Apnea as a predictor of pertussis had a sensitivity of 68% and a specificity of 60%.5
Pertussis should be considered early in the evaluation of young infants with cough. In a case-control study comparing 15 fatal cases of pertussis with 32 who survived (infants aged <6 months), the mean number of days from symptom onset to hospital admission were 5.3 (fatal) and 8.6 (survivors). Rates of apnea on admission were 40% and 52%.6 A case series of 9 infants aged <7 weeks requiring admission to an intensive care unit for pertussis found that 8 had been sick for less than 4 days at the time of admission. All 9 presented with poor feeding and cough, and 5 had experienced apnea.7
TABLE
Clinical features of pertussis
| History | Sensitivity | Specificity | LR+ | LR– |
|---|---|---|---|---|
| Cough >2 weeks3,12 | 84%–100% | 35%–36% | 1.3–1.5 | 0–0.44 |
| Cough >3 weeks3,12 | 75%–97% | 51%–59% | 1.8–2.0 | 0.06–0.42 |
| Whoop3,10-12 | 37%–90% | 49%–96% | 1.6–9.2 | 0.18–0.66 |
| Posttussivevomiting3,10,11,13 | 28%–84% | 45%–84% | 0.9–2.2 | 0.36–1.0 |
| Paroxysms3,12,13 | 68%–94% | 15%–45% | 1.1–1.4 | 0.29–0.71 |
| Householdexposure11,13 | 20%–50% | 73%–91% | 1.9–2.2 | 0.68–0.88 |
| Afebrile(temp <38°C)11-13 | 62%–96% | 12%–54% | 0.8–1.1 | 0–1.7 |
| Lymphocytosis3 | 88% | 57% | 2.0 | 0.21 |
| LR+ = positive likelihood ratio: sensitivity/(1–specificity); † LR– = negative likelihood ratio: (1–sensitivity)/specificity. | ||||
Recommendations from others
The Centers for Disease Control and Prevention and the World Health Organization describe the clinical case definition for pertussis as a cough illness lasting at least 2 weeks with at least 1 of the following: paroxysms of coughing, inspiratory whoop, or posttussive vomiting, without other apparent cause. Laboratory criteria for diagnosis include a positive Bordetella pertussis. culture or a positive polymerase chain reaction (PCR) for B pertussis.8,9
Infants may have complications; evaluate if there is apnea or significant cough
Michael Ohl, MD
University of Missouri–Columbia
Immunity to pertussis wanes following vaccination, leaving many adolescents and adults susceptible to infection. In older children and adults, there is often little in the clinical presentation that distinguishes chronic cough due to pertussis from that associated with other causes. Clinicians should consider evaluating for pertussis in older children and adults with chronic cough (>2 weeks) if there is reason to suspect they have been exposed, if the cough is associated with inspiratory whoop, or if the individual has household or frequent contact with infants.
Infants may suffer severe complications from pertussis, and should receive evaluation when presenting with apnea or significant cough of any duration. In current practice, evaluation usually includes obtaining a nasopharyngeal swab for culture and PCR, though these tests may be insensitive, especially in later phases of illness. The usefulness of single, quantitative immunoglobulin G titers with comparison to popimpact the epidemiology of pertussis in the US.
1. von König CHW, Halperin S, Riffelman M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis 2002;2:744-750.
2. Davis SF, Sutter RW, Strebel PM, Orton C, Alexander V, Sanden GN, et al. Concurrent outbreaks of pertussis and Mycoplasma pneumoniae infection: clinical and epidemiological characteristics of illnesses manifested by cough. Clin Infect Dis 1995;20:621-628.
3. Strebel PM, Cochi SL, Farizo KM, Payne BJ, Hanauer SD, Baughman AL. Pertussis in Missouri: evaluation of nasopharyngeal culture, direct fluorescent antibody testing, and clinical case definitions in the diagnosis of pertussis. Clin Infect Dis 1993;16:276-285.
4. Tanaka M, Vitck CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003;290:2968-2975.
5. Crowcroft NS, Booy R, Harrison T, Spicer L, Britto J, Mok Q, et al. Severe and unrecognised: pertussis in UK infants. Arch Dis Child 2003;88:802-806.
6. Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr 2003;143:575-581.
7. Smith C, Vyas H. Early infantile pertussis; increasingly prevalent and potentially fatal. Eur J Pediatr 2000;159:898-900.
8. Pertussis (Bordetella pertussis) (Whooping cough). Epidemiology Program Office, Centers for Disease Control and Prevention. Last updated April 7, 2004. Available at: www.cdc.gov/epo/dphsi/casedef/pertussis_current.htm. Accessed on December 8, 2004.
9. VaccinesImmunizations and Biologicals: Pertussis. Geneva, Switzerland: World Health Organization; 2003. Available at: www.who.int/vaccines-surveillance/deseasedesc/ RSS_pertus.htm. Accessed on December 8, 2004.
10. Wirsing von Konig CH, Rott H, Bogaerts H, Schmitt HJ. A serologic study of organisms possibly associated with pertussis-like coughing. Pediatr Infect Dis J 1998;17:645-649.
11. Granström G, Wretlind B, Granström M. Diagnostic value of clinical and bacteriological findings in pertussis. J Infect 1991;22:17-26.
12. Heininger U, Cherry JD, Eckhardt T, Lorenz C, Christenson P, Stehr K. Clinical and laboratory diagnosis of pertussis in the regions of a large vaccine efficacy trial in Germany. Pediatr Infect Dis J 1993;12:504-509.
13. Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA 1995;273:1044-1046.
1. von König CHW, Halperin S, Riffelman M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis 2002;2:744-750.
2. Davis SF, Sutter RW, Strebel PM, Orton C, Alexander V, Sanden GN, et al. Concurrent outbreaks of pertussis and Mycoplasma pneumoniae infection: clinical and epidemiological characteristics of illnesses manifested by cough. Clin Infect Dis 1995;20:621-628.
3. Strebel PM, Cochi SL, Farizo KM, Payne BJ, Hanauer SD, Baughman AL. Pertussis in Missouri: evaluation of nasopharyngeal culture, direct fluorescent antibody testing, and clinical case definitions in the diagnosis of pertussis. Clin Infect Dis 1993;16:276-285.
4. Tanaka M, Vitck CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003;290:2968-2975.
5. Crowcroft NS, Booy R, Harrison T, Spicer L, Britto J, Mok Q, et al. Severe and unrecognised: pertussis in UK infants. Arch Dis Child 2003;88:802-806.
6. Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr 2003;143:575-581.
7. Smith C, Vyas H. Early infantile pertussis; increasingly prevalent and potentially fatal. Eur J Pediatr 2000;159:898-900.
8. Pertussis (Bordetella pertussis) (Whooping cough). Epidemiology Program Office, Centers for Disease Control and Prevention. Last updated April 7, 2004. Available at: www.cdc.gov/epo/dphsi/casedef/pertussis_current.htm. Accessed on December 8, 2004.
9. VaccinesImmunizations and Biologicals: Pertussis. Geneva, Switzerland: World Health Organization; 2003. Available at: www.who.int/vaccines-surveillance/deseasedesc/ RSS_pertus.htm. Accessed on December 8, 2004.
10. Wirsing von Konig CH, Rott H, Bogaerts H, Schmitt HJ. A serologic study of organisms possibly associated with pertussis-like coughing. Pediatr Infect Dis J 1998;17:645-649.
11. Granström G, Wretlind B, Granström M. Diagnostic value of clinical and bacteriological findings in pertussis. J Infect 1991;22:17-26.
12. Heininger U, Cherry JD, Eckhardt T, Lorenz C, Christenson P, Stehr K. Clinical and laboratory diagnosis of pertussis in the regions of a large vaccine efficacy trial in Germany. Pediatr Infect Dis J 1993;12:504-509.
13. Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA 1995;273:1044-1046.
Evidence-based answers from the Family Physicians Inquiries Network
How effective are leukotriene inhibitors for asthma in children?
Evidence on the use of leukotriene inhibitors in children is insufficient to permit conclusions regarding efficacy. Given the proven efficacy of inhaled corticosteroids in asthma management, leukotriene inhibitors should not replace inhaled corticosteroids for maintenance of asthma in children (strength of recommendation: B).
Current guidelines that list leukotriene inhibitors as a potential addition or alternative to corticosteroid therapy in children with asthma appear to be based on scant studies and extrapolation from adult research.
Evidence summary
Asthma is characterized by inflammation of the bronchial airways. Leukotrienes are potent mediators of inflammation and are believed to contribute significantly to the inflammatory pathophysiology of asthma. Leukotriene inhibitors interfere with leukotriene production or leukotriene receptors and thus inhibit inflammation.1
Leukotriene inhibitors are administered orally, a significant advantage over inhalation in the pediatric population. For children, the theoretical corticosteroid-sparing effect of leukotriene inhibitors is appealing but has not been demonstrated.
In January 2002, Cochrane reviewers identified 3 studies of leukotriene inhibitor use in children that met their quality criteria for meta-analysis. Unfortunately, recent changes in asthma classification terminology make it difficult to precisely translate past studies into current practice. Based on these studies, the Cochrane reviewers concluded there is insufficient evidence to support the use of leukotriene inhibitors in children as monotherapy or as an addition to corticosteroids.1,2
One randomized, double-blind crossover study of 279 children with corticosteroid-dependent (persistent) asthma compared montelukast 5 mg (Singulair) once a day plus inhaled budesonide 200 μg (Pulmicort) twice a day with placebo plus budesonide (Rhinocort). Each study period lasted only 4 weeks, starting after a 4-week run-in period. Montelukast modestly improved asthma control over placebo. Compared with the placebo period, montelukast decreased the average use of beta-agonists by 1 puff per day. Asthma exacerbation days decreased by about 1 per month during montelukast treatment. The effects of montelukast and placebo on forced expiratory volume in 1 second (FEV1), quality of life, and adverse events did not differ significantly.3
One randomized, open-label crossover study of 124 children with “mild” asthma found that montelukast provided equivalent control and superior patient and parent satisfaction when compared with inhaled corticosteroids. Outcomes assessed were FEV1, school and work loss, medical resource utilization, safety, and patient and parent satisfaction. Children entering this study were self-selected to extend participation from a previous larger study that did not meet Cochrane quality criteria for inclusion in meta-analysis. The authors acknowledge the potential for selection bias.4
A randomized, double-blind, placebo-controlled study of 338 patients aged 12 years to adult compared zafirlukast (Accolate) with fluticasone propionate (Flovent) for control of persistent asthma. This study concluded that fluticasone was superior for all clinical outcomes measured including symptom scores, albuterol use, nighttime awakenings pulmonary function, and number of exacerbations requiring oral corticosteroids. Pooling of adult and adolescent cases in this study limits generalized application of these results to pediatric practice.5
Recommendations from others
The National Asthma Education and Prevention Program6 and the Global Initiative for Asthma7 guidelines conclude that inhaled corticosteroid, at the lowest effective dose, is the preferred therapy for children of all ages with persistent asthma whether mild, moderate, or severe.
Both guidelines list leukotriene inhibitors as a potential adjunct to corticosteroids for moderate persistent asthma, as an alternative to corticosteroids plus long-acting beta2-agonist. The guidelines also list leukotriene inhibitors as an alternative treatment to inhaled corticosteroids for mild persistent asthma in patients aged >5 years. Montelukast (Singulair) is approved for use in children aged ≥12 months, zafirlukast (Accolate) is approved for children aged≥5 years, and zileuton (Zyflo) is approved only for children aged >12 years.
An inhaled corticosteroid controller should be the first step
Lawrence S. Slotnick, MD
Moses Cone Health System, Greensboro, NC
Until evidence supports a different conclusion, I think we should continue to follow current national and global guidelines. The most important concept in both is that once a child is diagnosed with persistent asthma, starting an inhaled corticosteroid controller should be the first step.
Leukotriene inhibitors should be considered as second or third choice as a controller. The main indications for using a leukotriene inhibitor are aspirin-sensitive, exerciseinduced, and nocturnal asthma. I would use a leukotriene inhibitor as a controller only if a patient could not comply with inhaled corticosteroids.
1. Ducharme F, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteriods for chronic asthma. Cochrane Database Syst Rev 2002;(1):CD003133.-
2. Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled coritcosteriods in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD002314-
3. Simons FE, Villa JR, Lee BW G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001;138:694-698.
4. Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclamethasone in 6- to 11- year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction and adherence with therapy. Curr Med Res Opin. 2001;17:96-104.
5. Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001;50:595-602.
6. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. 1997 (rev 2002). Available at: www.nhlbi.nih.gov/guidelines/asthma/. Accessed on March 5, 2004.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Rockville, Md: National Heart, Lung, and Blood Institute. 1995 (revised 2002). Available at: www.ginasthma.com/wr.html. Accessed on March 5, 2004.
Evidence on the use of leukotriene inhibitors in children is insufficient to permit conclusions regarding efficacy. Given the proven efficacy of inhaled corticosteroids in asthma management, leukotriene inhibitors should not replace inhaled corticosteroids for maintenance of asthma in children (strength of recommendation: B).
Current guidelines that list leukotriene inhibitors as a potential addition or alternative to corticosteroid therapy in children with asthma appear to be based on scant studies and extrapolation from adult research.
Evidence summary
Asthma is characterized by inflammation of the bronchial airways. Leukotrienes are potent mediators of inflammation and are believed to contribute significantly to the inflammatory pathophysiology of asthma. Leukotriene inhibitors interfere with leukotriene production or leukotriene receptors and thus inhibit inflammation.1
Leukotriene inhibitors are administered orally, a significant advantage over inhalation in the pediatric population. For children, the theoretical corticosteroid-sparing effect of leukotriene inhibitors is appealing but has not been demonstrated.
In January 2002, Cochrane reviewers identified 3 studies of leukotriene inhibitor use in children that met their quality criteria for meta-analysis. Unfortunately, recent changes in asthma classification terminology make it difficult to precisely translate past studies into current practice. Based on these studies, the Cochrane reviewers concluded there is insufficient evidence to support the use of leukotriene inhibitors in children as monotherapy or as an addition to corticosteroids.1,2
One randomized, double-blind crossover study of 279 children with corticosteroid-dependent (persistent) asthma compared montelukast 5 mg (Singulair) once a day plus inhaled budesonide 200 μg (Pulmicort) twice a day with placebo plus budesonide (Rhinocort). Each study period lasted only 4 weeks, starting after a 4-week run-in period. Montelukast modestly improved asthma control over placebo. Compared with the placebo period, montelukast decreased the average use of beta-agonists by 1 puff per day. Asthma exacerbation days decreased by about 1 per month during montelukast treatment. The effects of montelukast and placebo on forced expiratory volume in 1 second (FEV1), quality of life, and adverse events did not differ significantly.3
One randomized, open-label crossover study of 124 children with “mild” asthma found that montelukast provided equivalent control and superior patient and parent satisfaction when compared with inhaled corticosteroids. Outcomes assessed were FEV1, school and work loss, medical resource utilization, safety, and patient and parent satisfaction. Children entering this study were self-selected to extend participation from a previous larger study that did not meet Cochrane quality criteria for inclusion in meta-analysis. The authors acknowledge the potential for selection bias.4
A randomized, double-blind, placebo-controlled study of 338 patients aged 12 years to adult compared zafirlukast (Accolate) with fluticasone propionate (Flovent) for control of persistent asthma. This study concluded that fluticasone was superior for all clinical outcomes measured including symptom scores, albuterol use, nighttime awakenings pulmonary function, and number of exacerbations requiring oral corticosteroids. Pooling of adult and adolescent cases in this study limits generalized application of these results to pediatric practice.5
Recommendations from others
The National Asthma Education and Prevention Program6 and the Global Initiative for Asthma7 guidelines conclude that inhaled corticosteroid, at the lowest effective dose, is the preferred therapy for children of all ages with persistent asthma whether mild, moderate, or severe.
Both guidelines list leukotriene inhibitors as a potential adjunct to corticosteroids for moderate persistent asthma, as an alternative to corticosteroids plus long-acting beta2-agonist. The guidelines also list leukotriene inhibitors as an alternative treatment to inhaled corticosteroids for mild persistent asthma in patients aged >5 years. Montelukast (Singulair) is approved for use in children aged ≥12 months, zafirlukast (Accolate) is approved for children aged≥5 years, and zileuton (Zyflo) is approved only for children aged >12 years.
An inhaled corticosteroid controller should be the first step
Lawrence S. Slotnick, MD
Moses Cone Health System, Greensboro, NC
Until evidence supports a different conclusion, I think we should continue to follow current national and global guidelines. The most important concept in both is that once a child is diagnosed with persistent asthma, starting an inhaled corticosteroid controller should be the first step.
Leukotriene inhibitors should be considered as second or third choice as a controller. The main indications for using a leukotriene inhibitor are aspirin-sensitive, exerciseinduced, and nocturnal asthma. I would use a leukotriene inhibitor as a controller only if a patient could not comply with inhaled corticosteroids.
Evidence on the use of leukotriene inhibitors in children is insufficient to permit conclusions regarding efficacy. Given the proven efficacy of inhaled corticosteroids in asthma management, leukotriene inhibitors should not replace inhaled corticosteroids for maintenance of asthma in children (strength of recommendation: B).
Current guidelines that list leukotriene inhibitors as a potential addition or alternative to corticosteroid therapy in children with asthma appear to be based on scant studies and extrapolation from adult research.
Evidence summary
Asthma is characterized by inflammation of the bronchial airways. Leukotrienes are potent mediators of inflammation and are believed to contribute significantly to the inflammatory pathophysiology of asthma. Leukotriene inhibitors interfere with leukotriene production or leukotriene receptors and thus inhibit inflammation.1
Leukotriene inhibitors are administered orally, a significant advantage over inhalation in the pediatric population. For children, the theoretical corticosteroid-sparing effect of leukotriene inhibitors is appealing but has not been demonstrated.
In January 2002, Cochrane reviewers identified 3 studies of leukotriene inhibitor use in children that met their quality criteria for meta-analysis. Unfortunately, recent changes in asthma classification terminology make it difficult to precisely translate past studies into current practice. Based on these studies, the Cochrane reviewers concluded there is insufficient evidence to support the use of leukotriene inhibitors in children as monotherapy or as an addition to corticosteroids.1,2
One randomized, double-blind crossover study of 279 children with corticosteroid-dependent (persistent) asthma compared montelukast 5 mg (Singulair) once a day plus inhaled budesonide 200 μg (Pulmicort) twice a day with placebo plus budesonide (Rhinocort). Each study period lasted only 4 weeks, starting after a 4-week run-in period. Montelukast modestly improved asthma control over placebo. Compared with the placebo period, montelukast decreased the average use of beta-agonists by 1 puff per day. Asthma exacerbation days decreased by about 1 per month during montelukast treatment. The effects of montelukast and placebo on forced expiratory volume in 1 second (FEV1), quality of life, and adverse events did not differ significantly.3
One randomized, open-label crossover study of 124 children with “mild” asthma found that montelukast provided equivalent control and superior patient and parent satisfaction when compared with inhaled corticosteroids. Outcomes assessed were FEV1, school and work loss, medical resource utilization, safety, and patient and parent satisfaction. Children entering this study were self-selected to extend participation from a previous larger study that did not meet Cochrane quality criteria for inclusion in meta-analysis. The authors acknowledge the potential for selection bias.4
A randomized, double-blind, placebo-controlled study of 338 patients aged 12 years to adult compared zafirlukast (Accolate) with fluticasone propionate (Flovent) for control of persistent asthma. This study concluded that fluticasone was superior for all clinical outcomes measured including symptom scores, albuterol use, nighttime awakenings pulmonary function, and number of exacerbations requiring oral corticosteroids. Pooling of adult and adolescent cases in this study limits generalized application of these results to pediatric practice.5
Recommendations from others
The National Asthma Education and Prevention Program6 and the Global Initiative for Asthma7 guidelines conclude that inhaled corticosteroid, at the lowest effective dose, is the preferred therapy for children of all ages with persistent asthma whether mild, moderate, or severe.
Both guidelines list leukotriene inhibitors as a potential adjunct to corticosteroids for moderate persistent asthma, as an alternative to corticosteroids plus long-acting beta2-agonist. The guidelines also list leukotriene inhibitors as an alternative treatment to inhaled corticosteroids for mild persistent asthma in patients aged >5 years. Montelukast (Singulair) is approved for use in children aged ≥12 months, zafirlukast (Accolate) is approved for children aged≥5 years, and zileuton (Zyflo) is approved only for children aged >12 years.
An inhaled corticosteroid controller should be the first step
Lawrence S. Slotnick, MD
Moses Cone Health System, Greensboro, NC
Until evidence supports a different conclusion, I think we should continue to follow current national and global guidelines. The most important concept in both is that once a child is diagnosed with persistent asthma, starting an inhaled corticosteroid controller should be the first step.
Leukotriene inhibitors should be considered as second or third choice as a controller. The main indications for using a leukotriene inhibitor are aspirin-sensitive, exerciseinduced, and nocturnal asthma. I would use a leukotriene inhibitor as a controller only if a patient could not comply with inhaled corticosteroids.
1. Ducharme F, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteriods for chronic asthma. Cochrane Database Syst Rev 2002;(1):CD003133.-
2. Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled coritcosteriods in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD002314-
3. Simons FE, Villa JR, Lee BW G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001;138:694-698.
4. Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclamethasone in 6- to 11- year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction and adherence with therapy. Curr Med Res Opin. 2001;17:96-104.
5. Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001;50:595-602.
6. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. 1997 (rev 2002). Available at: www.nhlbi.nih.gov/guidelines/asthma/. Accessed on March 5, 2004.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Rockville, Md: National Heart, Lung, and Blood Institute. 1995 (revised 2002). Available at: www.ginasthma.com/wr.html. Accessed on March 5, 2004.
1. Ducharme F, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteriods for chronic asthma. Cochrane Database Syst Rev 2002;(1):CD003133.-
2. Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled coritcosteriods in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2002;(3):CD002314-
3. Simons FE, Villa JR, Lee BW G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001;138:694-698.
4. Maspero JF, Duenas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclamethasone in 6- to 11- year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction and adherence with therapy. Curr Med Res Opin. 2001;17:96-104.
5. Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001;50:595-602.
6. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. 1997 (rev 2002). Available at: www.nhlbi.nih.gov/guidelines/asthma/. Accessed on March 5, 2004.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Rockville, Md: National Heart, Lung, and Blood Institute. 1995 (revised 2002). Available at: www.ginasthma.com/wr.html. Accessed on March 5, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Are nasal steroid sprays effective for otitis media with effusion?
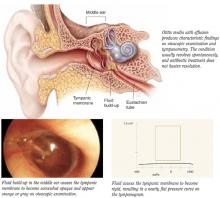
Treatment of otitis media with effusion (OME) with nasal steroids is not recommended (strength of recommendation [SOR]=A, based on systematic review).
Limited evidence exists that shows nasal steroids may increase the rate of resolution of OME in the short term, alone or in combination with antibiotics (SOR: A, based on randomized controlled trials). However, within 3 to 12 weeks, resolution of OME with nasal steroids is no better than placebo. No evidence exists that treatment with nasal steroids has any effect on decreasing potential complications of OME, such as hearing loss and delayed language development.
Evidence summary
OME is diagnosed by visualization of an effusion on otoscopy, by limited tympanic membrane movement on insufflation, or by abnormal tympanometry, all in the absence of acute inflammation. OME is defined as chronic when the effusion has been present for at least 3 months.
The natural course of OME was observed in a longitudinal cohort study of 1439 children aged 2 years in the Netherlands. Single or recurrent flat screening tympanograms were noted in 20% and remitted spontaneously at a rate of 50% every 3 months.1 This prevalence and spontaneous resolution rate is consistent with other studies.
Three randomized controlled trials published in English tested intranasal steroids for OME (Table).
The Lilholdt study enrolled children through a private ear, nose, and throat clinic over autumn, winter, and spring with a primary or new bout of OME.
The Shapiro study enrolled children who had documented allergic rhinitis and OME with failure to respond to 4 weeks of oral antihistamine and decongestant therapy at time of entry. This was the only study with short-term follow-up comparing intranasal steroids with control. The odds ratio for OME persisting after 3 weeks was 2.12 (95% confidence interval [CI], 0.65-6.90).3
The Tracy study enrolled children with chronic OME referred to a chronic ear clinic from October to June. Inclusion criteria included 3 episodes of acute otitis media in the prior 6 months or 4 episodes in the prior 12 months. This was a randomized comparison study with 3 treatment arms: an active nasal spray group and 2 control groups. The odds ratio for OME persisting after short-term follow-up was 0.79 (95% CI, 0.20-3.19); after intermediate follow-up the odds ratio was 0.72 (95% CI, 0.21-2.44).
This study, which included a symptom score after 3 months, favored treatment, with a weighted mean difference of -4.5, but with wide 95% CI of -10.28 to 1.28. An effect was demonstrated on clearing effusions in the short term, but the advantage appeared to vanish for the most part by 3 months. The study did not evaluate improvements in hearing.4
No adverse effects of intranasal steroid treatment were seen except for transient drops in cortisol levels in the Shapiro study, which tested dexamethasone. Approximately 8 randomized controlled trials using oral steroids with and without antibiotics for OME and chronic OME mirror a trend for short-term benefit of treatment, spontaneous resolution, and frequent recurrence.
In summary, limited evidence exists for short-term improvement of OME with intranasal steroids plus antibiotics, and no evidence exists for lasting beneficial effect on effusion or OME associated hearing loss.
TABLE
Clinical trials: Intranasal steroids for otitis media with effusion
| Study | Subjects | Groups | Duration | Outcome |
|---|---|---|---|---|
| Lilholdt 1982 | n=70 (aged 4-14 yrs with OME) | Beclomethasone vs placebo | 2 mo | No benefit at end of treatment month or after second month with no treatment by otoscopy, tympanometry, or audiometry. |
| Spontaneous improvement in 25% and resolution in 25%.1 | ||||
| Shapiro 1982 | n=45 | Dexamethasone vs placebo | 3 wk (aged 2-12 yrs with OME >1 mo) | Normalization of ear pressure and middle ear gradient at 1 and 2 weeks of treatment group over placebo (P<.05). |
| No significant differences by third week.2 | ||||
| Tracy 1998 | n=61 (aged 3-11 yrs with chronic OME) | Beclomethasone + amoxicillin vs placebo + amoxicillin vs amoxicillin alone | 12 wk | Beclomethasone group showed a significantly greater frequency of resolution of chronic effusion at 4 and 8 weeks (P<.05) but not at 12 weeks, with improved middle ear pressures: left (P=.004) and right (P=.010) over the 12 weeks.3 |
| OME, otitis media with effusion | ||||
Recommendations from others
The Canadian Task Force on Preventative Health Care found insufficient evidence to recommend screening for OME to prevent delayed language development.5
The Cochrane Ear, Nose and Throat Disorders Group concludes that both oral and topical intranasal steroids alone or in combination with an antibiotic lead to a quicker resolution of OME in the short term, but no long-term benefit is seen from treating OME effusions or associated hearing loss with topical intranasal steroids.6 They separately reviewed antibiotic treatment for OME, noting the short-term benefit above, but cited several drawbacks including cost and increased antibacterial resistance.7
The American Academy of Family Physicians Clinical Recommendation on Otitis Media with Effusion in Young Children does not recommend steroid medications for treatment of OME in a child of any age.8
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado, Denver
Management of OME can be challenging and expensive—annual costs are estimated at $5 billion. Antibiotics are often inappropriately prescribed for OME, which may promote bacterial resistance. Commonly, clinicians augment OME treatment with antihistamines, decongestants, and steroids. Yet studies such as those cited above confirm that these treatments offer limited or no benefit. We must avoid the kitchen-sink treatment of OME. Furthermore, randomized controlled trials have shown that 80% to 90% of cases of acute otitis media and OME resolve without any therapy.
However, children with chronic OME, especially those with bilateral disease or possible hearing loss, may benefit from tympanostomy tube placement and adenoidectomy. If the OME doesn’t clear within 3 months, refer to an ear, nose, and throat specialist.
Prevention efforts are valuable. Immunization of infants with pneumococcal conjugate vaccine reduced tympanostomy tube placement by 20% to 39%.9,10 Since increased incidence of OME and recurrent acute otitis media are associated with secondhand smoke exposure, motivating parents to quit smoking may further reduce chronic OME.
ACKNOWLEDGMENTS
Thanks to Marianne Broers, MD for her translation of reference 1 from Dutch.
1. Zielhuis GA, Schilder A, van den Broek P. The spontaneous course of otitis media with effusion in young children [in Dutch]. Ned Tijdschr Geneeskd 1991;135:1754-1757.
2. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion—a double-blind study. Int J Pediatr Otorhinolaryngol 1982;4:133-137.
3. Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy 1982;49:81-85.
4. Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann Allergy Asthma Immunol 1998;80:198-206.
5. Butler CC, MacMillan HL. Early detection of OME in the first four years of life to prevent delayed language development. Systematic Review & Recommendations. CTF-PHC Technical Report #01-3. September 2000. London, Ont: Canadian Task Force; 2000. Available at: http://www.ctfphc.org, or by request from the task force office ctf@ctfphc.org. Accessed on July 10, 2003.
6. Butler CC, Van Der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2002;(4):CD001935.-
7. Van Balen FAM, Canekin II LJ, Williamson IG. Antibiotic treatment for otitis media with effusion in children aged 6 months-12 years (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
8. American Academy of Family Physicians. Otitis Media with Effusion in Young Children. AAFP Clinical Recommendations, Part II—Clinical Policies. Leawood, Kansas: American Academy of Family Physicians; 1994 (reaffirmed 2000, 2001). Available at: http://www.aafp.org/x1596.xml. Accessed on June 30, 2003.
9. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
10. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
Treatment of otitis media with effusion (OME) with nasal steroids is not recommended (strength of recommendation [SOR]=A, based on systematic review).
Limited evidence exists that shows nasal steroids may increase the rate of resolution of OME in the short term, alone or in combination with antibiotics (SOR: A, based on randomized controlled trials). However, within 3 to 12 weeks, resolution of OME with nasal steroids is no better than placebo. No evidence exists that treatment with nasal steroids has any effect on decreasing potential complications of OME, such as hearing loss and delayed language development.
Evidence summary
OME is diagnosed by visualization of an effusion on otoscopy, by limited tympanic membrane movement on insufflation, or by abnormal tympanometry, all in the absence of acute inflammation. OME is defined as chronic when the effusion has been present for at least 3 months.
The natural course of OME was observed in a longitudinal cohort study of 1439 children aged 2 years in the Netherlands. Single or recurrent flat screening tympanograms were noted in 20% and remitted spontaneously at a rate of 50% every 3 months.1 This prevalence and spontaneous resolution rate is consistent with other studies.
Three randomized controlled trials published in English tested intranasal steroids for OME (Table).
The Lilholdt study enrolled children through a private ear, nose, and throat clinic over autumn, winter, and spring with a primary or new bout of OME.
The Shapiro study enrolled children who had documented allergic rhinitis and OME with failure to respond to 4 weeks of oral antihistamine and decongestant therapy at time of entry. This was the only study with short-term follow-up comparing intranasal steroids with control. The odds ratio for OME persisting after 3 weeks was 2.12 (95% confidence interval [CI], 0.65-6.90).3
The Tracy study enrolled children with chronic OME referred to a chronic ear clinic from October to June. Inclusion criteria included 3 episodes of acute otitis media in the prior 6 months or 4 episodes in the prior 12 months. This was a randomized comparison study with 3 treatment arms: an active nasal spray group and 2 control groups. The odds ratio for OME persisting after short-term follow-up was 0.79 (95% CI, 0.20-3.19); after intermediate follow-up the odds ratio was 0.72 (95% CI, 0.21-2.44).
This study, which included a symptom score after 3 months, favored treatment, with a weighted mean difference of -4.5, but with wide 95% CI of -10.28 to 1.28. An effect was demonstrated on clearing effusions in the short term, but the advantage appeared to vanish for the most part by 3 months. The study did not evaluate improvements in hearing.4
No adverse effects of intranasal steroid treatment were seen except for transient drops in cortisol levels in the Shapiro study, which tested dexamethasone. Approximately 8 randomized controlled trials using oral steroids with and without antibiotics for OME and chronic OME mirror a trend for short-term benefit of treatment, spontaneous resolution, and frequent recurrence.
In summary, limited evidence exists for short-term improvement of OME with intranasal steroids plus antibiotics, and no evidence exists for lasting beneficial effect on effusion or OME associated hearing loss.
TABLE
Clinical trials: Intranasal steroids for otitis media with effusion
| Study | Subjects | Groups | Duration | Outcome |
|---|---|---|---|---|
| Lilholdt 1982 | n=70 (aged 4-14 yrs with OME) | Beclomethasone vs placebo | 2 mo | No benefit at end of treatment month or after second month with no treatment by otoscopy, tympanometry, or audiometry. |
| Spontaneous improvement in 25% and resolution in 25%.1 | ||||
| Shapiro 1982 | n=45 | Dexamethasone vs placebo | 3 wk (aged 2-12 yrs with OME >1 mo) | Normalization of ear pressure and middle ear gradient at 1 and 2 weeks of treatment group over placebo (P<.05). |
| No significant differences by third week.2 | ||||
| Tracy 1998 | n=61 (aged 3-11 yrs with chronic OME) | Beclomethasone + amoxicillin vs placebo + amoxicillin vs amoxicillin alone | 12 wk | Beclomethasone group showed a significantly greater frequency of resolution of chronic effusion at 4 and 8 weeks (P<.05) but not at 12 weeks, with improved middle ear pressures: left (P=.004) and right (P=.010) over the 12 weeks.3 |
| OME, otitis media with effusion | ||||
Recommendations from others
The Canadian Task Force on Preventative Health Care found insufficient evidence to recommend screening for OME to prevent delayed language development.5
The Cochrane Ear, Nose and Throat Disorders Group concludes that both oral and topical intranasal steroids alone or in combination with an antibiotic lead to a quicker resolution of OME in the short term, but no long-term benefit is seen from treating OME effusions or associated hearing loss with topical intranasal steroids.6 They separately reviewed antibiotic treatment for OME, noting the short-term benefit above, but cited several drawbacks including cost and increased antibacterial resistance.7
The American Academy of Family Physicians Clinical Recommendation on Otitis Media with Effusion in Young Children does not recommend steroid medications for treatment of OME in a child of any age.8
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado, Denver
Management of OME can be challenging and expensive—annual costs are estimated at $5 billion. Antibiotics are often inappropriately prescribed for OME, which may promote bacterial resistance. Commonly, clinicians augment OME treatment with antihistamines, decongestants, and steroids. Yet studies such as those cited above confirm that these treatments offer limited or no benefit. We must avoid the kitchen-sink treatment of OME. Furthermore, randomized controlled trials have shown that 80% to 90% of cases of acute otitis media and OME resolve without any therapy.
However, children with chronic OME, especially those with bilateral disease or possible hearing loss, may benefit from tympanostomy tube placement and adenoidectomy. If the OME doesn’t clear within 3 months, refer to an ear, nose, and throat specialist.
Prevention efforts are valuable. Immunization of infants with pneumococcal conjugate vaccine reduced tympanostomy tube placement by 20% to 39%.9,10 Since increased incidence of OME and recurrent acute otitis media are associated with secondhand smoke exposure, motivating parents to quit smoking may further reduce chronic OME.
ACKNOWLEDGMENTS
Thanks to Marianne Broers, MD for her translation of reference 1 from Dutch.
Treatment of otitis media with effusion (OME) with nasal steroids is not recommended (strength of recommendation [SOR]=A, based on systematic review).
Limited evidence exists that shows nasal steroids may increase the rate of resolution of OME in the short term, alone or in combination with antibiotics (SOR: A, based on randomized controlled trials). However, within 3 to 12 weeks, resolution of OME with nasal steroids is no better than placebo. No evidence exists that treatment with nasal steroids has any effect on decreasing potential complications of OME, such as hearing loss and delayed language development.
Evidence summary
OME is diagnosed by visualization of an effusion on otoscopy, by limited tympanic membrane movement on insufflation, or by abnormal tympanometry, all in the absence of acute inflammation. OME is defined as chronic when the effusion has been present for at least 3 months.
The natural course of OME was observed in a longitudinal cohort study of 1439 children aged 2 years in the Netherlands. Single or recurrent flat screening tympanograms were noted in 20% and remitted spontaneously at a rate of 50% every 3 months.1 This prevalence and spontaneous resolution rate is consistent with other studies.
Three randomized controlled trials published in English tested intranasal steroids for OME (Table).
The Lilholdt study enrolled children through a private ear, nose, and throat clinic over autumn, winter, and spring with a primary or new bout of OME.
The Shapiro study enrolled children who had documented allergic rhinitis and OME with failure to respond to 4 weeks of oral antihistamine and decongestant therapy at time of entry. This was the only study with short-term follow-up comparing intranasal steroids with control. The odds ratio for OME persisting after 3 weeks was 2.12 (95% confidence interval [CI], 0.65-6.90).3
The Tracy study enrolled children with chronic OME referred to a chronic ear clinic from October to June. Inclusion criteria included 3 episodes of acute otitis media in the prior 6 months or 4 episodes in the prior 12 months. This was a randomized comparison study with 3 treatment arms: an active nasal spray group and 2 control groups. The odds ratio for OME persisting after short-term follow-up was 0.79 (95% CI, 0.20-3.19); after intermediate follow-up the odds ratio was 0.72 (95% CI, 0.21-2.44).
This study, which included a symptom score after 3 months, favored treatment, with a weighted mean difference of -4.5, but with wide 95% CI of -10.28 to 1.28. An effect was demonstrated on clearing effusions in the short term, but the advantage appeared to vanish for the most part by 3 months. The study did not evaluate improvements in hearing.4
No adverse effects of intranasal steroid treatment were seen except for transient drops in cortisol levels in the Shapiro study, which tested dexamethasone. Approximately 8 randomized controlled trials using oral steroids with and without antibiotics for OME and chronic OME mirror a trend for short-term benefit of treatment, spontaneous resolution, and frequent recurrence.
In summary, limited evidence exists for short-term improvement of OME with intranasal steroids plus antibiotics, and no evidence exists for lasting beneficial effect on effusion or OME associated hearing loss.
TABLE
Clinical trials: Intranasal steroids for otitis media with effusion
| Study | Subjects | Groups | Duration | Outcome |
|---|---|---|---|---|
| Lilholdt 1982 | n=70 (aged 4-14 yrs with OME) | Beclomethasone vs placebo | 2 mo | No benefit at end of treatment month or after second month with no treatment by otoscopy, tympanometry, or audiometry. |
| Spontaneous improvement in 25% and resolution in 25%.1 | ||||
| Shapiro 1982 | n=45 | Dexamethasone vs placebo | 3 wk (aged 2-12 yrs with OME >1 mo) | Normalization of ear pressure and middle ear gradient at 1 and 2 weeks of treatment group over placebo (P<.05). |
| No significant differences by third week.2 | ||||
| Tracy 1998 | n=61 (aged 3-11 yrs with chronic OME) | Beclomethasone + amoxicillin vs placebo + amoxicillin vs amoxicillin alone | 12 wk | Beclomethasone group showed a significantly greater frequency of resolution of chronic effusion at 4 and 8 weeks (P<.05) but not at 12 weeks, with improved middle ear pressures: left (P=.004) and right (P=.010) over the 12 weeks.3 |
| OME, otitis media with effusion | ||||
Recommendations from others
The Canadian Task Force on Preventative Health Care found insufficient evidence to recommend screening for OME to prevent delayed language development.5
The Cochrane Ear, Nose and Throat Disorders Group concludes that both oral and topical intranasal steroids alone or in combination with an antibiotic lead to a quicker resolution of OME in the short term, but no long-term benefit is seen from treating OME effusions or associated hearing loss with topical intranasal steroids.6 They separately reviewed antibiotic treatment for OME, noting the short-term benefit above, but cited several drawbacks including cost and increased antibacterial resistance.7
The American Academy of Family Physicians Clinical Recommendation on Otitis Media with Effusion in Young Children does not recommend steroid medications for treatment of OME in a child of any age.8
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado, Denver
Management of OME can be challenging and expensive—annual costs are estimated at $5 billion. Antibiotics are often inappropriately prescribed for OME, which may promote bacterial resistance. Commonly, clinicians augment OME treatment with antihistamines, decongestants, and steroids. Yet studies such as those cited above confirm that these treatments offer limited or no benefit. We must avoid the kitchen-sink treatment of OME. Furthermore, randomized controlled trials have shown that 80% to 90% of cases of acute otitis media and OME resolve without any therapy.
However, children with chronic OME, especially those with bilateral disease or possible hearing loss, may benefit from tympanostomy tube placement and adenoidectomy. If the OME doesn’t clear within 3 months, refer to an ear, nose, and throat specialist.
Prevention efforts are valuable. Immunization of infants with pneumococcal conjugate vaccine reduced tympanostomy tube placement by 20% to 39%.9,10 Since increased incidence of OME and recurrent acute otitis media are associated with secondhand smoke exposure, motivating parents to quit smoking may further reduce chronic OME.
ACKNOWLEDGMENTS
Thanks to Marianne Broers, MD for her translation of reference 1 from Dutch.
1. Zielhuis GA, Schilder A, van den Broek P. The spontaneous course of otitis media with effusion in young children [in Dutch]. Ned Tijdschr Geneeskd 1991;135:1754-1757.
2. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion—a double-blind study. Int J Pediatr Otorhinolaryngol 1982;4:133-137.
3. Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy 1982;49:81-85.
4. Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann Allergy Asthma Immunol 1998;80:198-206.
5. Butler CC, MacMillan HL. Early detection of OME in the first four years of life to prevent delayed language development. Systematic Review & Recommendations. CTF-PHC Technical Report #01-3. September 2000. London, Ont: Canadian Task Force; 2000. Available at: http://www.ctfphc.org, or by request from the task force office ctf@ctfphc.org. Accessed on July 10, 2003.
6. Butler CC, Van Der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2002;(4):CD001935.-
7. Van Balen FAM, Canekin II LJ, Williamson IG. Antibiotic treatment for otitis media with effusion in children aged 6 months-12 years (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
8. American Academy of Family Physicians. Otitis Media with Effusion in Young Children. AAFP Clinical Recommendations, Part II—Clinical Policies. Leawood, Kansas: American Academy of Family Physicians; 1994 (reaffirmed 2000, 2001). Available at: http://www.aafp.org/x1596.xml. Accessed on June 30, 2003.
9. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
10. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
1. Zielhuis GA, Schilder A, van den Broek P. The spontaneous course of otitis media with effusion in young children [in Dutch]. Ned Tijdschr Geneeskd 1991;135:1754-1757.
2. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion—a double-blind study. Int J Pediatr Otorhinolaryngol 1982;4:133-137.
3. Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy 1982;49:81-85.
4. Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann Allergy Asthma Immunol 1998;80:198-206.
5. Butler CC, MacMillan HL. Early detection of OME in the first four years of life to prevent delayed language development. Systematic Review & Recommendations. CTF-PHC Technical Report #01-3. September 2000. London, Ont: Canadian Task Force; 2000. Available at: http://www.ctfphc.org, or by request from the task force office ctf@ctfphc.org. Accessed on July 10, 2003.
6. Butler CC, Van Der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2002;(4):CD001935.-
7. Van Balen FAM, Canekin II LJ, Williamson IG. Antibiotic treatment for otitis media with effusion in children aged 6 months-12 years (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
8. American Academy of Family Physicians. Otitis Media with Effusion in Young Children. AAFP Clinical Recommendations, Part II—Clinical Policies. Leawood, Kansas: American Academy of Family Physicians; 1994 (reaffirmed 2000, 2001). Available at: http://www.aafp.org/x1596.xml. Accessed on June 30, 2003.
9. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
10. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
Evidence-based answers from the Family Physicians Inquiries Network
Heat or ice for acute ankle sprain?
For grade 3 and 4 ankle sprains, ice works better than heat to speed recovery (return to play) (strength of recommendation [SOR]: B, based on a single retrospective cohort study). No studies support faster return to play with the application of heat at any time after injury (SOR: B, based on head-to-head randomized trials). Ice therapy also reduces edema, but the clinical significance of this finding is unclear.
Evidence summary
Studies of ankle sprain use variable diagnostic criteria for sprain and definition of recovery (return to play). They often report indirect outcomes such as edema. The effect of decreased edema on recovery time is not addressed.
Only 1 study has directly compared heat vs ice therapy and recovery time for ankle sprains. A retrospective cohort study of 32 patients in a sports medicine clinic demonstrated that early cryotherapy (within 36 hours of injury) for grades 3 and 4 ankle sprains, when compared with early heat therapy, resulted in earlier return to activity, as defined by ability to walk, climb stairs, run, and jump without pain.1 Grade 3 sprains treated with ice recovered in 11.0 days vs 14.8 days with heat. Grade 4 sprains treated with ice recovered in 13.2 days vs 30.4 days with heat. This study also showed that early application of ice (within 36 hours) decreased time to recovery compared with late application of ice.
However, evidence is heterogeneous about the effect of ice on return to play. In 2 of 3 randomized controlled trials, early application of ice vs placebo did not significantly speed return to play.
One randomized controlled trial compared ice therapy (in the form of a cooling anklet applied upon presentation) with placebo in 143 patients presenting within 24 hours of injury to a university emergency department in England.2 All patients received high-dose nonsteroidal anti-inflammatory agents. Though a trend was found in favor of ice therapy, no statistically significant difference was found in recovery time, as defined by pain relief and ability to bear weight. The grade of sprain was not specifically accounted for in this study.
Another randomized controlled trial compared ice with placebo in 30 patients with grade 3 and 4 sprains referred to a physiotherapy department within 2 days of ankle injury. No statistical difference was found in recovery time, defined as ability to bear weight with only mild to moderate pain.3
However, a randomized controlled trial of 60 patients with acute ankle sprains of all grades presenting to an emergency department compared cryogel plus bandaging with bandaging alone (cooling vs no cooling). This study found the mean time to recovery—defined as decreased pain—was reduced from 14.8 days to 9.7 days with constant cooling for the first 48 hours.4
The application of ice—but not heat—within 24 to 48 hours of acute ankle sprain also reduced edema. Several studies looked at reduction of edema with cooling. One study measured edema in 30 patients with grade 1 and 2 sprains treated with cold, heat, or contrast baths during the third, fourth, and fifth days.5 Only ice therapy alone significantly reduced edema.
Recommendations from others
The American Academy of Orthopaedic Surgeons recommends initial treatment of stable ankle sprains with rest, ice, gentle compression, and elevation (RICE).6 These guidelines are echoed by the American Academy of Family Physicians. In addition, the Institute for Clinical Systems Improvement and the National Guidelines Clearinghouse recommend PRICE, where protecting the ankle is explicitly added to RICE therapy.7
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes; Department of Family Medicine, University of Colorado
Ice should be the first choice for all acute ankle sprains. The immediate goals of treating an ankle sprain are reducing edema, stabilizing the ankle, and enabling early weight-bearing. Applying heat may increase swelling and subsequently slow recovery. Once the initial phase of recovery is achieved through cryotherapy, compression, and elevation, the injured patient may initiate work to increase strength, flexibility, and range of motion of the injured ankle. As a result, icing an ankle sprain facilitates an earlier return to full activity and sports participation by speeding the first phase of recovery.
1. Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med 1989;6:1-6.
2. Laba E, Roestenburg M. Clinical evaluation of ice therapy for acute ankle sprain injuries. N Z J Physiother 1989;17:7-9.
3. Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner 1976;216:708-711.
4. Hocutt JE, Jr, Jaffee R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med 1982;10:316-319.
5. Cote DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther 1988;68:1072-1076.
6. American Academy of Orthopaedic Surgeons. Clinical Guideline on Ankle Injury. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1997:7.
7. Institute for Clinical Systems Improvement (ICSI). Ankle Sprain. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002:24.
For grade 3 and 4 ankle sprains, ice works better than heat to speed recovery (return to play) (strength of recommendation [SOR]: B, based on a single retrospective cohort study). No studies support faster return to play with the application of heat at any time after injury (SOR: B, based on head-to-head randomized trials). Ice therapy also reduces edema, but the clinical significance of this finding is unclear.
Evidence summary
Studies of ankle sprain use variable diagnostic criteria for sprain and definition of recovery (return to play). They often report indirect outcomes such as edema. The effect of decreased edema on recovery time is not addressed.
Only 1 study has directly compared heat vs ice therapy and recovery time for ankle sprains. A retrospective cohort study of 32 patients in a sports medicine clinic demonstrated that early cryotherapy (within 36 hours of injury) for grades 3 and 4 ankle sprains, when compared with early heat therapy, resulted in earlier return to activity, as defined by ability to walk, climb stairs, run, and jump without pain.1 Grade 3 sprains treated with ice recovered in 11.0 days vs 14.8 days with heat. Grade 4 sprains treated with ice recovered in 13.2 days vs 30.4 days with heat. This study also showed that early application of ice (within 36 hours) decreased time to recovery compared with late application of ice.
However, evidence is heterogeneous about the effect of ice on return to play. In 2 of 3 randomized controlled trials, early application of ice vs placebo did not significantly speed return to play.
One randomized controlled trial compared ice therapy (in the form of a cooling anklet applied upon presentation) with placebo in 143 patients presenting within 24 hours of injury to a university emergency department in England.2 All patients received high-dose nonsteroidal anti-inflammatory agents. Though a trend was found in favor of ice therapy, no statistically significant difference was found in recovery time, as defined by pain relief and ability to bear weight. The grade of sprain was not specifically accounted for in this study.
Another randomized controlled trial compared ice with placebo in 30 patients with grade 3 and 4 sprains referred to a physiotherapy department within 2 days of ankle injury. No statistical difference was found in recovery time, defined as ability to bear weight with only mild to moderate pain.3
However, a randomized controlled trial of 60 patients with acute ankle sprains of all grades presenting to an emergency department compared cryogel plus bandaging with bandaging alone (cooling vs no cooling). This study found the mean time to recovery—defined as decreased pain—was reduced from 14.8 days to 9.7 days with constant cooling for the first 48 hours.4
The application of ice—but not heat—within 24 to 48 hours of acute ankle sprain also reduced edema. Several studies looked at reduction of edema with cooling. One study measured edema in 30 patients with grade 1 and 2 sprains treated with cold, heat, or contrast baths during the third, fourth, and fifth days.5 Only ice therapy alone significantly reduced edema.
Recommendations from others
The American Academy of Orthopaedic Surgeons recommends initial treatment of stable ankle sprains with rest, ice, gentle compression, and elevation (RICE).6 These guidelines are echoed by the American Academy of Family Physicians. In addition, the Institute for Clinical Systems Improvement and the National Guidelines Clearinghouse recommend PRICE, where protecting the ankle is explicitly added to RICE therapy.7
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes; Department of Family Medicine, University of Colorado
Ice should be the first choice for all acute ankle sprains. The immediate goals of treating an ankle sprain are reducing edema, stabilizing the ankle, and enabling early weight-bearing. Applying heat may increase swelling and subsequently slow recovery. Once the initial phase of recovery is achieved through cryotherapy, compression, and elevation, the injured patient may initiate work to increase strength, flexibility, and range of motion of the injured ankle. As a result, icing an ankle sprain facilitates an earlier return to full activity and sports participation by speeding the first phase of recovery.
For grade 3 and 4 ankle sprains, ice works better than heat to speed recovery (return to play) (strength of recommendation [SOR]: B, based on a single retrospective cohort study). No studies support faster return to play with the application of heat at any time after injury (SOR: B, based on head-to-head randomized trials). Ice therapy also reduces edema, but the clinical significance of this finding is unclear.
Evidence summary
Studies of ankle sprain use variable diagnostic criteria for sprain and definition of recovery (return to play). They often report indirect outcomes such as edema. The effect of decreased edema on recovery time is not addressed.
Only 1 study has directly compared heat vs ice therapy and recovery time for ankle sprains. A retrospective cohort study of 32 patients in a sports medicine clinic demonstrated that early cryotherapy (within 36 hours of injury) for grades 3 and 4 ankle sprains, when compared with early heat therapy, resulted in earlier return to activity, as defined by ability to walk, climb stairs, run, and jump without pain.1 Grade 3 sprains treated with ice recovered in 11.0 days vs 14.8 days with heat. Grade 4 sprains treated with ice recovered in 13.2 days vs 30.4 days with heat. This study also showed that early application of ice (within 36 hours) decreased time to recovery compared with late application of ice.
However, evidence is heterogeneous about the effect of ice on return to play. In 2 of 3 randomized controlled trials, early application of ice vs placebo did not significantly speed return to play.
One randomized controlled trial compared ice therapy (in the form of a cooling anklet applied upon presentation) with placebo in 143 patients presenting within 24 hours of injury to a university emergency department in England.2 All patients received high-dose nonsteroidal anti-inflammatory agents. Though a trend was found in favor of ice therapy, no statistically significant difference was found in recovery time, as defined by pain relief and ability to bear weight. The grade of sprain was not specifically accounted for in this study.
Another randomized controlled trial compared ice with placebo in 30 patients with grade 3 and 4 sprains referred to a physiotherapy department within 2 days of ankle injury. No statistical difference was found in recovery time, defined as ability to bear weight with only mild to moderate pain.3
However, a randomized controlled trial of 60 patients with acute ankle sprains of all grades presenting to an emergency department compared cryogel plus bandaging with bandaging alone (cooling vs no cooling). This study found the mean time to recovery—defined as decreased pain—was reduced from 14.8 days to 9.7 days with constant cooling for the first 48 hours.4
The application of ice—but not heat—within 24 to 48 hours of acute ankle sprain also reduced edema. Several studies looked at reduction of edema with cooling. One study measured edema in 30 patients with grade 1 and 2 sprains treated with cold, heat, or contrast baths during the third, fourth, and fifth days.5 Only ice therapy alone significantly reduced edema.
Recommendations from others
The American Academy of Orthopaedic Surgeons recommends initial treatment of stable ankle sprains with rest, ice, gentle compression, and elevation (RICE).6 These guidelines are echoed by the American Academy of Family Physicians. In addition, the Institute for Clinical Systems Improvement and the National Guidelines Clearinghouse recommend PRICE, where protecting the ankle is explicitly added to RICE therapy.7
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes; Department of Family Medicine, University of Colorado
Ice should be the first choice for all acute ankle sprains. The immediate goals of treating an ankle sprain are reducing edema, stabilizing the ankle, and enabling early weight-bearing. Applying heat may increase swelling and subsequently slow recovery. Once the initial phase of recovery is achieved through cryotherapy, compression, and elevation, the injured patient may initiate work to increase strength, flexibility, and range of motion of the injured ankle. As a result, icing an ankle sprain facilitates an earlier return to full activity and sports participation by speeding the first phase of recovery.
1. Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med 1989;6:1-6.
2. Laba E, Roestenburg M. Clinical evaluation of ice therapy for acute ankle sprain injuries. N Z J Physiother 1989;17:7-9.
3. Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner 1976;216:708-711.
4. Hocutt JE, Jr, Jaffee R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med 1982;10:316-319.
5. Cote DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther 1988;68:1072-1076.
6. American Academy of Orthopaedic Surgeons. Clinical Guideline on Ankle Injury. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1997:7.
7. Institute for Clinical Systems Improvement (ICSI). Ankle Sprain. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002:24.
1. Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med 1989;6:1-6.
2. Laba E, Roestenburg M. Clinical evaluation of ice therapy for acute ankle sprain injuries. N Z J Physiother 1989;17:7-9.
3. Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner 1976;216:708-711.
4. Hocutt JE, Jr, Jaffee R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med 1982;10:316-319.
5. Cote DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther 1988;68:1072-1076.
6. American Academy of Orthopaedic Surgeons. Clinical Guideline on Ankle Injury. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1997:7.
7. Institute for Clinical Systems Improvement (ICSI). Ankle Sprain. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002:24.
Evidence-based answers from the Family Physicians Inquiries Network
Are antibiotics effective for otitis media with effusion?
Antibiotics provide little or no long-term benefit for children with otitis media with effusion (OME), defined as fluid in the middle ear without signs or symptoms of infection.
Most meta-analyses show a modest, short-term reduction in effusion rates. However, the most rigorous meta-analysis shows no benefit (strength of recommendation [SOR]: D, based on conflicting meta-analyses). No significant effect was noted on longer-term (>1 month) outcomes after treatment (SOR: A, based on a meta-analysis of 8 trials). In addition, there is no reliable evidence regarding patient-oriented outcomes (hearing loss, speech delay).
Evidence summary
Longitudinal studies show spontaneous resolution in more than half of children within 3 months of the development of the effusion. After 3 months, the rate of spontaneous resolution remains constant, so that only a small percentage of children have OME a year or longer. There is a theoretical basis for the use of antibiotics for OME, since between 27%–50% of middle-ear aspirates of patients with OME contain bacteria.1
In the last 10 years, 4 meta-analyses reported mild short-term improvement in OME with antibiotic treatment (effusion clearance rates of 23%,2 16%,3 14%,1 and 4%,4 respectively—see Table). The last study was the only meta-analysis that restricted inclusion to only randomized, blinded, placebo-controlled trials. The small difference reported (4%) was not significant. None of the studies that assessed outcomes beyond a month showed a significant difference in the persistence of OME.
The meta-analyses vary significantly in methodology, inclusion/exclusion criteria, and interpretation, making a definitive conclusion on treatment results difficult. The included trials varied in antibiotics chosen, use of placebo, duration of therapy, time to measurement of OME resolution, and method of diagnosis (tympanography, otoscopy, audiometry).
The reviews commented on potential harms of antibiotic therapy, including medication cost and the development of antibiotic resistance. Nausea, vomiting, and diarrhea were reported in 2%–30% of children on antibiotic therapy.1 The reviews did not address the treatment of OME in the nonpediatric population or such long-term patient-oriented outcomes as hearing loss or speech delay.
Recommendations from others
The American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology– Head and Neck Surgery participated in the meta-analysis by Stool et al,1 under contract with the Agency for Health Care Policy and Research. The resulting clinical practice guideline has been adopted by the AAP, AAFP, and the Centers for Disease Control and Prevention. The guideline stresses that observation or antibiotics are treatment options for children with OME present less than 4 to 6 months. Antibiotic therapy is never considered a required treatment for OME of any duration. All published guidelines are applicable to the pediatric population only.
Conflicting evidence indicates short-term or no benefit for antibiotics, and complications such as nausea, vomiting, diarrhea, and rash have been reported in 2%–32% of children. Long-term antibiotics lead to poor adherence, more office visits, and antibiotic resistance.5
Lynda Montgomery, MD
Case Western University School of Medicine Cleveland, Ohio
Conflicting meta-analyses and a guideline that hedges leaves the clinician who practices evidence-based medicine in the uncomfortable position of saying “maybe” when asked whether antibiotics are helpful. In the majority of cases of OME, I would seek to avoid the possible complications of antibiotics, given that there is no clear benefit. I await more data on speech and hearing outcomes in OME, as these studies will provide the most helpful evidence to primary care physicians.
TABLE
Meta-analyses of otitis media with effusion
| Meta-analysis | # of trials | Number of subjects | Description | Rate difference (95% CI) |
|---|---|---|---|---|
| Cantekin et al4 | 8 | 775 children | Includes only non-placebo-controlled RCTs. Variable timing of outcome measure | 32 (25.8–38.8) |
| Rosenfeld et al2 | 10 | 1325 children | Includes some nonblinded and nonplacebo-controlled RCTs. Variable timing | 22.8 (10.5–35.1) |
| Williams et al3 | 12 | 1697 children | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on bilateral resolution of OME within 1 month of starting therapy | 16 (3–29) |
| Williams et al3 | 8 | 2052 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on unilateral resolution of OME within 1 month of starting therapy | 25 (10–40) |
| Williams et al3 | 8 | 1313 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Long-term outcomes measured more than 1 month after treatment was completed | 6 (-3–14) |
| Stool et al1 | 10 | 1041 children | All blinded RCTs. Not all placebocontrolled. Variable timing | 14.0 (3.6–24.2) |
| Cantekin et al4 | 8 | 1292 children | Includes only blinded, placebo-controlled RCTs. Variable timing | 4.3 (-0.1–8.6) |
| RCT, randomized clinical trial; CI, confidence interval; OME, otitis media with effusion | ||||
Otitis media with effusion
1. Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical Practice Guideline No. 12. AHCPR Publication 94-0622. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, July 1994.
2. Rosenfeld RM, Post JC. Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 1992;106:378-386.
3. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve to brouhaha. JAMA 1993;270:1344-351.
4. Cantekin EI, McGuire TW. Antibiotics are not effective for otitis media with effusion: reanalysis of meta-analyses. Otorhinolaryngol Nova 1998;8:214-222.
5. Williamson I. Otitis media with effusion. Clinical Evidence [online]. Available at: http://www.clinicalevidence.com. Accessed on February 24, 2003.
Antibiotics provide little or no long-term benefit for children with otitis media with effusion (OME), defined as fluid in the middle ear without signs or symptoms of infection.
Most meta-analyses show a modest, short-term reduction in effusion rates. However, the most rigorous meta-analysis shows no benefit (strength of recommendation [SOR]: D, based on conflicting meta-analyses). No significant effect was noted on longer-term (>1 month) outcomes after treatment (SOR: A, based on a meta-analysis of 8 trials). In addition, there is no reliable evidence regarding patient-oriented outcomes (hearing loss, speech delay).
Evidence summary
Longitudinal studies show spontaneous resolution in more than half of children within 3 months of the development of the effusion. After 3 months, the rate of spontaneous resolution remains constant, so that only a small percentage of children have OME a year or longer. There is a theoretical basis for the use of antibiotics for OME, since between 27%–50% of middle-ear aspirates of patients with OME contain bacteria.1
In the last 10 years, 4 meta-analyses reported mild short-term improvement in OME with antibiotic treatment (effusion clearance rates of 23%,2 16%,3 14%,1 and 4%,4 respectively—see Table). The last study was the only meta-analysis that restricted inclusion to only randomized, blinded, placebo-controlled trials. The small difference reported (4%) was not significant. None of the studies that assessed outcomes beyond a month showed a significant difference in the persistence of OME.
The meta-analyses vary significantly in methodology, inclusion/exclusion criteria, and interpretation, making a definitive conclusion on treatment results difficult. The included trials varied in antibiotics chosen, use of placebo, duration of therapy, time to measurement of OME resolution, and method of diagnosis (tympanography, otoscopy, audiometry).
The reviews commented on potential harms of antibiotic therapy, including medication cost and the development of antibiotic resistance. Nausea, vomiting, and diarrhea were reported in 2%–30% of children on antibiotic therapy.1 The reviews did not address the treatment of OME in the nonpediatric population or such long-term patient-oriented outcomes as hearing loss or speech delay.
Recommendations from others
The American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology– Head and Neck Surgery participated in the meta-analysis by Stool et al,1 under contract with the Agency for Health Care Policy and Research. The resulting clinical practice guideline has been adopted by the AAP, AAFP, and the Centers for Disease Control and Prevention. The guideline stresses that observation or antibiotics are treatment options for children with OME present less than 4 to 6 months. Antibiotic therapy is never considered a required treatment for OME of any duration. All published guidelines are applicable to the pediatric population only.
Conflicting evidence indicates short-term or no benefit for antibiotics, and complications such as nausea, vomiting, diarrhea, and rash have been reported in 2%–32% of children. Long-term antibiotics lead to poor adherence, more office visits, and antibiotic resistance.5
Lynda Montgomery, MD
Case Western University School of Medicine Cleveland, Ohio
Conflicting meta-analyses and a guideline that hedges leaves the clinician who practices evidence-based medicine in the uncomfortable position of saying “maybe” when asked whether antibiotics are helpful. In the majority of cases of OME, I would seek to avoid the possible complications of antibiotics, given that there is no clear benefit. I await more data on speech and hearing outcomes in OME, as these studies will provide the most helpful evidence to primary care physicians.
TABLE
Meta-analyses of otitis media with effusion
| Meta-analysis | # of trials | Number of subjects | Description | Rate difference (95% CI) |
|---|---|---|---|---|
| Cantekin et al4 | 8 | 775 children | Includes only non-placebo-controlled RCTs. Variable timing of outcome measure | 32 (25.8–38.8) |
| Rosenfeld et al2 | 10 | 1325 children | Includes some nonblinded and nonplacebo-controlled RCTs. Variable timing | 22.8 (10.5–35.1) |
| Williams et al3 | 12 | 1697 children | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on bilateral resolution of OME within 1 month of starting therapy | 16 (3–29) |
| Williams et al3 | 8 | 2052 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on unilateral resolution of OME within 1 month of starting therapy | 25 (10–40) |
| Williams et al3 | 8 | 1313 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Long-term outcomes measured more than 1 month after treatment was completed | 6 (-3–14) |
| Stool et al1 | 10 | 1041 children | All blinded RCTs. Not all placebocontrolled. Variable timing | 14.0 (3.6–24.2) |
| Cantekin et al4 | 8 | 1292 children | Includes only blinded, placebo-controlled RCTs. Variable timing | 4.3 (-0.1–8.6) |
| RCT, randomized clinical trial; CI, confidence interval; OME, otitis media with effusion | ||||
Otitis media with effusion
Antibiotics provide little or no long-term benefit for children with otitis media with effusion (OME), defined as fluid in the middle ear without signs or symptoms of infection.
Most meta-analyses show a modest, short-term reduction in effusion rates. However, the most rigorous meta-analysis shows no benefit (strength of recommendation [SOR]: D, based on conflicting meta-analyses). No significant effect was noted on longer-term (>1 month) outcomes after treatment (SOR: A, based on a meta-analysis of 8 trials). In addition, there is no reliable evidence regarding patient-oriented outcomes (hearing loss, speech delay).
Evidence summary
Longitudinal studies show spontaneous resolution in more than half of children within 3 months of the development of the effusion. After 3 months, the rate of spontaneous resolution remains constant, so that only a small percentage of children have OME a year or longer. There is a theoretical basis for the use of antibiotics for OME, since between 27%–50% of middle-ear aspirates of patients with OME contain bacteria.1
In the last 10 years, 4 meta-analyses reported mild short-term improvement in OME with antibiotic treatment (effusion clearance rates of 23%,2 16%,3 14%,1 and 4%,4 respectively—see Table). The last study was the only meta-analysis that restricted inclusion to only randomized, blinded, placebo-controlled trials. The small difference reported (4%) was not significant. None of the studies that assessed outcomes beyond a month showed a significant difference in the persistence of OME.
The meta-analyses vary significantly in methodology, inclusion/exclusion criteria, and interpretation, making a definitive conclusion on treatment results difficult. The included trials varied in antibiotics chosen, use of placebo, duration of therapy, time to measurement of OME resolution, and method of diagnosis (tympanography, otoscopy, audiometry).
The reviews commented on potential harms of antibiotic therapy, including medication cost and the development of antibiotic resistance. Nausea, vomiting, and diarrhea were reported in 2%–30% of children on antibiotic therapy.1 The reviews did not address the treatment of OME in the nonpediatric population or such long-term patient-oriented outcomes as hearing loss or speech delay.
Recommendations from others
The American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology– Head and Neck Surgery participated in the meta-analysis by Stool et al,1 under contract with the Agency for Health Care Policy and Research. The resulting clinical practice guideline has been adopted by the AAP, AAFP, and the Centers for Disease Control and Prevention. The guideline stresses that observation or antibiotics are treatment options for children with OME present less than 4 to 6 months. Antibiotic therapy is never considered a required treatment for OME of any duration. All published guidelines are applicable to the pediatric population only.
Conflicting evidence indicates short-term or no benefit for antibiotics, and complications such as nausea, vomiting, diarrhea, and rash have been reported in 2%–32% of children. Long-term antibiotics lead to poor adherence, more office visits, and antibiotic resistance.5
Lynda Montgomery, MD
Case Western University School of Medicine Cleveland, Ohio
Conflicting meta-analyses and a guideline that hedges leaves the clinician who practices evidence-based medicine in the uncomfortable position of saying “maybe” when asked whether antibiotics are helpful. In the majority of cases of OME, I would seek to avoid the possible complications of antibiotics, given that there is no clear benefit. I await more data on speech and hearing outcomes in OME, as these studies will provide the most helpful evidence to primary care physicians.
TABLE
Meta-analyses of otitis media with effusion
| Meta-analysis | # of trials | Number of subjects | Description | Rate difference (95% CI) |
|---|---|---|---|---|
| Cantekin et al4 | 8 | 775 children | Includes only non-placebo-controlled RCTs. Variable timing of outcome measure | 32 (25.8–38.8) |
| Rosenfeld et al2 | 10 | 1325 children | Includes some nonblinded and nonplacebo-controlled RCTs. Variable timing | 22.8 (10.5–35.1) |
| Williams et al3 | 12 | 1697 children | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on bilateral resolution of OME within 1 month of starting therapy | 16 (3–29) |
| Williams et al3 | 8 | 2052 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on unilateral resolution of OME within 1 month of starting therapy | 25 (10–40) |
| Williams et al3 | 8 | 1313 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Long-term outcomes measured more than 1 month after treatment was completed | 6 (-3–14) |
| Stool et al1 | 10 | 1041 children | All blinded RCTs. Not all placebocontrolled. Variable timing | 14.0 (3.6–24.2) |
| Cantekin et al4 | 8 | 1292 children | Includes only blinded, placebo-controlled RCTs. Variable timing | 4.3 (-0.1–8.6) |
| RCT, randomized clinical trial; CI, confidence interval; OME, otitis media with effusion | ||||
Otitis media with effusion
1. Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical Practice Guideline No. 12. AHCPR Publication 94-0622. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, July 1994.
2. Rosenfeld RM, Post JC. Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 1992;106:378-386.
3. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve to brouhaha. JAMA 1993;270:1344-351.
4. Cantekin EI, McGuire TW. Antibiotics are not effective for otitis media with effusion: reanalysis of meta-analyses. Otorhinolaryngol Nova 1998;8:214-222.
5. Williamson I. Otitis media with effusion. Clinical Evidence [online]. Available at: http://www.clinicalevidence.com. Accessed on February 24, 2003.
1. Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical Practice Guideline No. 12. AHCPR Publication 94-0622. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, July 1994.
2. Rosenfeld RM, Post JC. Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 1992;106:378-386.
3. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve to brouhaha. JAMA 1993;270:1344-351.
4. Cantekin EI, McGuire TW. Antibiotics are not effective for otitis media with effusion: reanalysis of meta-analyses. Otorhinolaryngol Nova 1998;8:214-222.
5. Williamson I. Otitis media with effusion. Clinical Evidence [online]. Available at: http://www.clinicalevidence.com. Accessed on February 24, 2003.
Evidence-based answers from the Family Physicians Inquiries Network