User login
Atopic Dermatitis: Evolution and Revolution in Therapy
Atopic dermatitis (AD) is an incredibly common chronic skin disease, affecting up to 25% of children and 7% of adults in the United States.1,2 Despite the prevalence of this disease and its impact on patient quality of life, research and scholarly work in AD has been limited until recent years. A PubMed search of articles indexed for MEDLINE using the term atopic dermatitis showed that there were fewer than 500 articles published in 2000 and 965 in 2010; with our more recent acceleration in research, there were 2168 articles published in 2020 and more than 1300 published in just the first half of 2021 (through June). This new research includes insights into the pathogenesis of AD and study of the disease impact and comorbidities as well as an extensive amount of drug development and clinical trial work for new topical and systemic therapies.
New Agents to Treat AD
The 2016 approval of crisaborole,3 a phosphodiesterase 4 inhibitor, followed by the approval of dupilumab, an IL-4 and IL-13 pathway inhibitor and the first biologic agent approved for AD,4 ushered in a new age of therapy. We currently are awaiting the incorporation of a new set of topical nonsteroidal agents, oral Janus kinase (JAK) inhibitors, and new biologic agents for AD, several of which have completed phase 3 trials and extended safety evaluations. How these new drugs will impact our standard treatment across the spectrum of care for AD is not yet known.
The emergence of new systemic therapies is timely, as the most used systemic medications previously were oral corticosteroids, despite their use being advised against in standard practice guidelines. Other agents such as methotrexate, cyclosporine, azathioprine, and mycophenolate are discussed in the literature and AD treatment guidelines as being potentially useful, though absence of US Food and Drug Administration (FDA) approval and the need for frequent laboratory monitoring, as well as drug-specific side effects and an increased risk of infection, limit their use in the United States, especially in pediatric and adolescent populations.5
The approval of dupilumab as a systemic therapy—initially for adults and subsequently for teenagers (12–17 years of age) and then children (6–11 years of age)—has markedly influenced the standard of care for moderate to severe AD. This agent has been shown to have a considerable impact on disease severity and quality of life, with a good safety profile and the added benefit of not requiring continuous (or any) laboratory monitoring.6-8 Ongoing studies of dupilumab in children (ClinicalTrials.gov identifiers NCT02612454, NCT03346434), including those younger than 1 year,9 raise the question of how commonly this medication might be incorporated into care across the entire age spectrum of patients with AD. What standards will there be for assessment of severity, disease impact, and persistence to warrant use in younger ages? Will early treatment with novel systemic agents change the overall course of the disease and minimize the development of comorbidities? The answers to these questions remain to be seen.
JAK Inhibitors for AD
Additional novel therapeutics currently are undergoing studies for treatment of AD, most notably the oral JAK inhibitors upadacitinib,10 baricitinib,11 and abrocitinib.12 Each of these agents has completed phase 3 trials for AD. Two of these agents—upadacitinib and baricitinib—have prior FDA approval for use in other disease states. Of note, baricitinib is already approved for treatment of moderate to severe AD in adults in more than 40 countries13; however, the use of these agents in other diseases brings about concerns of malignancy, severe infection, and thrombosis. In the clinical trials for AD, many of these events have not been seen, but the number of patients treated is limited, and longer-term safety assessment is important.10,11
How will the oral JAK inhibitors be incorporated into care compared to biologic agents such as dupilumab? Tolerance and more serious potential adverse events are concerns, with nausea, headaches, and acneform eruptions being associated with some of the medications, in addition to potential issues with herpes simplex and zoster infections. However, oral JAK inhibitors have the benefit of not requiring injections, something that many patients may prefer, and data show that these drugs generally are associated with a rapid reduction in pruritus and, depending on the drug, very quick and profound effects on objective signs of AD.10-12 Two head-to-head studies have been completed comparing dupilumab to oral JAK inhibitors in adults: the JADE COMPARE trial examining dupilumab vs abrocitinib12 and the Heads UP trial comparing dupilumab vs upadacitinib.14 Compared to dupilumab, higher-dose abrocitinib showed more rapid responses, superiority in itch response, and similarity or superiority in other outcomes depending on the time point of the evaluation. Adverse event profiles differed; for example, abrocitinib was associated with more nausea, acneform eruptions, and herpes zoster, while dupilumab had higher rates of conjunctivitis.12 Upadacitinib, which was only studied at higher dosing (30 mg daily), showed superiority to dupilumab in itch response and in improvement in AD severity in multiple outcome measures; however, there were increases in serious infections, eczema herpeticum, herpes zoster, and laboratory-related adverse events.14 Dupilumab has the advantage of studies of extended use along with real-world experience, generally with excellent safety and tolerance other than injection-site reactions and conjunctivitis.8 Biologics targeting IL-13—tralokinumab and lebrikizumab—also are to be added to our armamentarium.15,16 The addition of these agents and JAK inhibitors as new systemic treatment options points to the quickly evolving future of AD treatment for patients with extensive disease.
New topical therapies in development provide even more treatment options. New nonsteroidal topicals include topical JAK inhibitors such as ruxolitinib17; tapinarof,18 an aryl hydrocarbon receptor modulator; and phosphodiesterase 4 inhibitors. These agents may be useful either as monotherapy, as studied, potentially without the regional limitations associated with stronger topical corticosteroids, but also should be useful in clinical practice as part of therapeutic regimens with other topical steroid and nonsteroidal agents.
The Microbiome and AD
In addition, research looking at topical microbes as specific interventions that may mediate the microbiome and inflammation of AD are intriguing. A recent phase 1 trial from the University of California San Diego19 indicated that topical bacteriotherapy directed at decreasing Staphylococcus aureus may provide an impact in AD. Observations by Kong et al20 showed that gram-negative microbiome differences are seen in AD patients compared to unaffected individuals, which has fueled studies showing that Roseomonas mucosa, a gram-negative skin commensal, when applied as a topical live biotherapeutic agent has improved disease severity in children and adults with AD.21 Although further studies are underway, these initial data suggest a role for microbiome-modifying therapies as AD treatment.
Chronic Hand Eczema
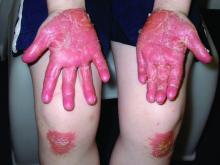
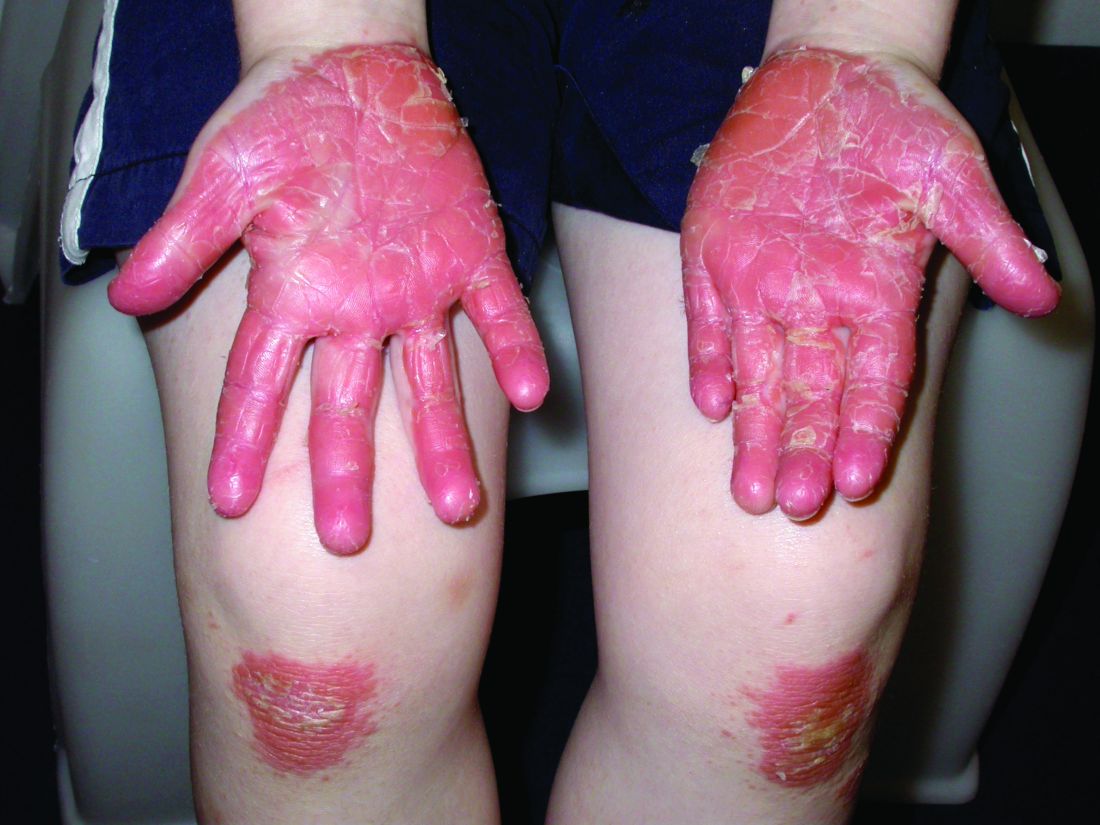
Chronic hand eczema (CHE), which has considerable overlap with AD in many patients, especially children and adolescents,22-24 is another area of interesting research. This high-prevalence condition is associated with allergic and irritant contact dermatitis24-26—conditions that are both considered alternative diagnoses for and exacerbators of AD27—and is a disease process currently being targeted for new therapies. Delgocitinib (NCT04872101, NCT04871711), the novel JAK inhibitor ARQ-252 (NCT04378569), among other topical agents, as well as systemic therapeutics such as gusacitinib (NCT03728504), are in active trials for CHE. Given CHE’s impact on quality of life28 and its overlap with AD, investigation into this disorder can help drive future AD research as well as lead to better knowledge and treatment of CHE.
Final Thoughts
Despite the promising results of these myriad new therapies in AD, there are many factors that influence how and when we use these drugs, including their approval status, FDA labeling, and the ability of patients to access and afford treatment. Additionally, continued study is needed to evaluate the long-term safety and extended efficacy of newer drugs, such as the oral JAK inhibitors. Despite these hurdles, the current landscape of research and development is rapidly evolving. Compared to the many years when only one main group of therapies was a reasonable option for patients, the future of AD treatment looks bright.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- FDA approves Eucrisa for eczema. News release. US Food and Drug Administration; December 14, 2016. Accessed August 16, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293. doi:10.1016/j.jaad.2020.06.054
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-388. doi:10.1016/j.jaad.2019.07.074
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. doi: 10.1111/jdv.16928
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70. doi:10.1016/j.jaad.2021.02.028
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112. doi:10.1056/NEJMoa2019380
- Lilly and Incyte provide update on supplemental New Drug Application for baricitinib for the treatment of moderate to severe atopic dermatitis. News release. Eli Lilly and Company; July 16, 2021. Accessed August 16, 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-provide-update-supplemental new-drug
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial [published online August 4, 2021]. JAMA Dermatol. doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre,placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-463. doi:10.1111/bjd.19573
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies [published online May 4, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji, T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial [published online February 22, 2021]. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-859. doi:10.1101/gr.131029.111
- Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12:eaaz8631. doi:10.1126/scitranslmed.aaz8631
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523-532. doi:10.1046/j.1365-2133.2001.04078.x
- Grönhagen C, Lidén C, Wahlgren CF, et al. Hand eczema and atopic dermatitis in adolescents: a prospective cohort study from the BAMSE project. Br J Dermatol. 2015;173:1175-1182. doi:10.1111/bjd.14019
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Contact allergy and allergic contact dermatitis in adolescents: prevalence measures and associations. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol. 2002;82:352-358. doi:10.1080/000155502320624087
- Isaksson M, Olhardt S, Rådehed J, et al. Children with atopic dermatitis should always be patch-tested if they have hand or foot dermatitis. Acta Derm Venereol. 2015;95:583-586. doi:10.2340/00015555-1995
- Silverberg JI, Warshaw EM, Maibach HI, et al. Hand eczema in children referred for patch testing: North American Contact Dermatitis Group Data, 2000-2016. Br J Dermatol. 2021;185:185-194. doi:10.1111/bjd.19818
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(suppl 1):4-12. doi:10.1111/jdv.16061
- Cazzaniga S, Ballmer-Weber BK, Gräni N, et al. Medical, psychological and socio-economic implications of chronic hand eczema: a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:628-637. doi:10.1111/jdv.13479
Atopic dermatitis (AD) is an incredibly common chronic skin disease, affecting up to 25% of children and 7% of adults in the United States.1,2 Despite the prevalence of this disease and its impact on patient quality of life, research and scholarly work in AD has been limited until recent years. A PubMed search of articles indexed for MEDLINE using the term atopic dermatitis showed that there were fewer than 500 articles published in 2000 and 965 in 2010; with our more recent acceleration in research, there were 2168 articles published in 2020 and more than 1300 published in just the first half of 2021 (through June). This new research includes insights into the pathogenesis of AD and study of the disease impact and comorbidities as well as an extensive amount of drug development and clinical trial work for new topical and systemic therapies.
New Agents to Treat AD
The 2016 approval of crisaborole,3 a phosphodiesterase 4 inhibitor, followed by the approval of dupilumab, an IL-4 and IL-13 pathway inhibitor and the first biologic agent approved for AD,4 ushered in a new age of therapy. We currently are awaiting the incorporation of a new set of topical nonsteroidal agents, oral Janus kinase (JAK) inhibitors, and new biologic agents for AD, several of which have completed phase 3 trials and extended safety evaluations. How these new drugs will impact our standard treatment across the spectrum of care for AD is not yet known.
The emergence of new systemic therapies is timely, as the most used systemic medications previously were oral corticosteroids, despite their use being advised against in standard practice guidelines. Other agents such as methotrexate, cyclosporine, azathioprine, and mycophenolate are discussed in the literature and AD treatment guidelines as being potentially useful, though absence of US Food and Drug Administration (FDA) approval and the need for frequent laboratory monitoring, as well as drug-specific side effects and an increased risk of infection, limit their use in the United States, especially in pediatric and adolescent populations.5
The approval of dupilumab as a systemic therapy—initially for adults and subsequently for teenagers (12–17 years of age) and then children (6–11 years of age)—has markedly influenced the standard of care for moderate to severe AD. This agent has been shown to have a considerable impact on disease severity and quality of life, with a good safety profile and the added benefit of not requiring continuous (or any) laboratory monitoring.6-8 Ongoing studies of dupilumab in children (ClinicalTrials.gov identifiers NCT02612454, NCT03346434), including those younger than 1 year,9 raise the question of how commonly this medication might be incorporated into care across the entire age spectrum of patients with AD. What standards will there be for assessment of severity, disease impact, and persistence to warrant use in younger ages? Will early treatment with novel systemic agents change the overall course of the disease and minimize the development of comorbidities? The answers to these questions remain to be seen.
JAK Inhibitors for AD
Additional novel therapeutics currently are undergoing studies for treatment of AD, most notably the oral JAK inhibitors upadacitinib,10 baricitinib,11 and abrocitinib.12 Each of these agents has completed phase 3 trials for AD. Two of these agents—upadacitinib and baricitinib—have prior FDA approval for use in other disease states. Of note, baricitinib is already approved for treatment of moderate to severe AD in adults in more than 40 countries13; however, the use of these agents in other diseases brings about concerns of malignancy, severe infection, and thrombosis. In the clinical trials for AD, many of these events have not been seen, but the number of patients treated is limited, and longer-term safety assessment is important.10,11
How will the oral JAK inhibitors be incorporated into care compared to biologic agents such as dupilumab? Tolerance and more serious potential adverse events are concerns, with nausea, headaches, and acneform eruptions being associated with some of the medications, in addition to potential issues with herpes simplex and zoster infections. However, oral JAK inhibitors have the benefit of not requiring injections, something that many patients may prefer, and data show that these drugs generally are associated with a rapid reduction in pruritus and, depending on the drug, very quick and profound effects on objective signs of AD.10-12 Two head-to-head studies have been completed comparing dupilumab to oral JAK inhibitors in adults: the JADE COMPARE trial examining dupilumab vs abrocitinib12 and the Heads UP trial comparing dupilumab vs upadacitinib.14 Compared to dupilumab, higher-dose abrocitinib showed more rapid responses, superiority in itch response, and similarity or superiority in other outcomes depending on the time point of the evaluation. Adverse event profiles differed; for example, abrocitinib was associated with more nausea, acneform eruptions, and herpes zoster, while dupilumab had higher rates of conjunctivitis.12 Upadacitinib, which was only studied at higher dosing (30 mg daily), showed superiority to dupilumab in itch response and in improvement in AD severity in multiple outcome measures; however, there were increases in serious infections, eczema herpeticum, herpes zoster, and laboratory-related adverse events.14 Dupilumab has the advantage of studies of extended use along with real-world experience, generally with excellent safety and tolerance other than injection-site reactions and conjunctivitis.8 Biologics targeting IL-13—tralokinumab and lebrikizumab—also are to be added to our armamentarium.15,16 The addition of these agents and JAK inhibitors as new systemic treatment options points to the quickly evolving future of AD treatment for patients with extensive disease.
New topical therapies in development provide even more treatment options. New nonsteroidal topicals include topical JAK inhibitors such as ruxolitinib17; tapinarof,18 an aryl hydrocarbon receptor modulator; and phosphodiesterase 4 inhibitors. These agents may be useful either as monotherapy, as studied, potentially without the regional limitations associated with stronger topical corticosteroids, but also should be useful in clinical practice as part of therapeutic regimens with other topical steroid and nonsteroidal agents.
The Microbiome and AD
In addition, research looking at topical microbes as specific interventions that may mediate the microbiome and inflammation of AD are intriguing. A recent phase 1 trial from the University of California San Diego19 indicated that topical bacteriotherapy directed at decreasing Staphylococcus aureus may provide an impact in AD. Observations by Kong et al20 showed that gram-negative microbiome differences are seen in AD patients compared to unaffected individuals, which has fueled studies showing that Roseomonas mucosa, a gram-negative skin commensal, when applied as a topical live biotherapeutic agent has improved disease severity in children and adults with AD.21 Although further studies are underway, these initial data suggest a role for microbiome-modifying therapies as AD treatment.
Chronic Hand Eczema
Chronic hand eczema (CHE), which has considerable overlap with AD in many patients, especially children and adolescents,22-24 is another area of interesting research. This high-prevalence condition is associated with allergic and irritant contact dermatitis24-26—conditions that are both considered alternative diagnoses for and exacerbators of AD27—and is a disease process currently being targeted for new therapies. Delgocitinib (NCT04872101, NCT04871711), the novel JAK inhibitor ARQ-252 (NCT04378569), among other topical agents, as well as systemic therapeutics such as gusacitinib (NCT03728504), are in active trials for CHE. Given CHE’s impact on quality of life28 and its overlap with AD, investigation into this disorder can help drive future AD research as well as lead to better knowledge and treatment of CHE.
Final Thoughts
Despite the promising results of these myriad new therapies in AD, there are many factors that influence how and when we use these drugs, including their approval status, FDA labeling, and the ability of patients to access and afford treatment. Additionally, continued study is needed to evaluate the long-term safety and extended efficacy of newer drugs, such as the oral JAK inhibitors. Despite these hurdles, the current landscape of research and development is rapidly evolving. Compared to the many years when only one main group of therapies was a reasonable option for patients, the future of AD treatment looks bright.
Atopic dermatitis (AD) is an incredibly common chronic skin disease, affecting up to 25% of children and 7% of adults in the United States.1,2 Despite the prevalence of this disease and its impact on patient quality of life, research and scholarly work in AD has been limited until recent years. A PubMed search of articles indexed for MEDLINE using the term atopic dermatitis showed that there were fewer than 500 articles published in 2000 and 965 in 2010; with our more recent acceleration in research, there were 2168 articles published in 2020 and more than 1300 published in just the first half of 2021 (through June). This new research includes insights into the pathogenesis of AD and study of the disease impact and comorbidities as well as an extensive amount of drug development and clinical trial work for new topical and systemic therapies.
New Agents to Treat AD
The 2016 approval of crisaborole,3 a phosphodiesterase 4 inhibitor, followed by the approval of dupilumab, an IL-4 and IL-13 pathway inhibitor and the first biologic agent approved for AD,4 ushered in a new age of therapy. We currently are awaiting the incorporation of a new set of topical nonsteroidal agents, oral Janus kinase (JAK) inhibitors, and new biologic agents for AD, several of which have completed phase 3 trials and extended safety evaluations. How these new drugs will impact our standard treatment across the spectrum of care for AD is not yet known.
The emergence of new systemic therapies is timely, as the most used systemic medications previously were oral corticosteroids, despite their use being advised against in standard practice guidelines. Other agents such as methotrexate, cyclosporine, azathioprine, and mycophenolate are discussed in the literature and AD treatment guidelines as being potentially useful, though absence of US Food and Drug Administration (FDA) approval and the need for frequent laboratory monitoring, as well as drug-specific side effects and an increased risk of infection, limit their use in the United States, especially in pediatric and adolescent populations.5
The approval of dupilumab as a systemic therapy—initially for adults and subsequently for teenagers (12–17 years of age) and then children (6–11 years of age)—has markedly influenced the standard of care for moderate to severe AD. This agent has been shown to have a considerable impact on disease severity and quality of life, with a good safety profile and the added benefit of not requiring continuous (or any) laboratory monitoring.6-8 Ongoing studies of dupilumab in children (ClinicalTrials.gov identifiers NCT02612454, NCT03346434), including those younger than 1 year,9 raise the question of how commonly this medication might be incorporated into care across the entire age spectrum of patients with AD. What standards will there be for assessment of severity, disease impact, and persistence to warrant use in younger ages? Will early treatment with novel systemic agents change the overall course of the disease and minimize the development of comorbidities? The answers to these questions remain to be seen.
JAK Inhibitors for AD
Additional novel therapeutics currently are undergoing studies for treatment of AD, most notably the oral JAK inhibitors upadacitinib,10 baricitinib,11 and abrocitinib.12 Each of these agents has completed phase 3 trials for AD. Two of these agents—upadacitinib and baricitinib—have prior FDA approval for use in other disease states. Of note, baricitinib is already approved for treatment of moderate to severe AD in adults in more than 40 countries13; however, the use of these agents in other diseases brings about concerns of malignancy, severe infection, and thrombosis. In the clinical trials for AD, many of these events have not been seen, but the number of patients treated is limited, and longer-term safety assessment is important.10,11
How will the oral JAK inhibitors be incorporated into care compared to biologic agents such as dupilumab? Tolerance and more serious potential adverse events are concerns, with nausea, headaches, and acneform eruptions being associated with some of the medications, in addition to potential issues with herpes simplex and zoster infections. However, oral JAK inhibitors have the benefit of not requiring injections, something that many patients may prefer, and data show that these drugs generally are associated with a rapid reduction in pruritus and, depending on the drug, very quick and profound effects on objective signs of AD.10-12 Two head-to-head studies have been completed comparing dupilumab to oral JAK inhibitors in adults: the JADE COMPARE trial examining dupilumab vs abrocitinib12 and the Heads UP trial comparing dupilumab vs upadacitinib.14 Compared to dupilumab, higher-dose abrocitinib showed more rapid responses, superiority in itch response, and similarity or superiority in other outcomes depending on the time point of the evaluation. Adverse event profiles differed; for example, abrocitinib was associated with more nausea, acneform eruptions, and herpes zoster, while dupilumab had higher rates of conjunctivitis.12 Upadacitinib, which was only studied at higher dosing (30 mg daily), showed superiority to dupilumab in itch response and in improvement in AD severity in multiple outcome measures; however, there were increases in serious infections, eczema herpeticum, herpes zoster, and laboratory-related adverse events.14 Dupilumab has the advantage of studies of extended use along with real-world experience, generally with excellent safety and tolerance other than injection-site reactions and conjunctivitis.8 Biologics targeting IL-13—tralokinumab and lebrikizumab—also are to be added to our armamentarium.15,16 The addition of these agents and JAK inhibitors as new systemic treatment options points to the quickly evolving future of AD treatment for patients with extensive disease.
New topical therapies in development provide even more treatment options. New nonsteroidal topicals include topical JAK inhibitors such as ruxolitinib17; tapinarof,18 an aryl hydrocarbon receptor modulator; and phosphodiesterase 4 inhibitors. These agents may be useful either as monotherapy, as studied, potentially without the regional limitations associated with stronger topical corticosteroids, but also should be useful in clinical practice as part of therapeutic regimens with other topical steroid and nonsteroidal agents.
The Microbiome and AD
In addition, research looking at topical microbes as specific interventions that may mediate the microbiome and inflammation of AD are intriguing. A recent phase 1 trial from the University of California San Diego19 indicated that topical bacteriotherapy directed at decreasing Staphylococcus aureus may provide an impact in AD. Observations by Kong et al20 showed that gram-negative microbiome differences are seen in AD patients compared to unaffected individuals, which has fueled studies showing that Roseomonas mucosa, a gram-negative skin commensal, when applied as a topical live biotherapeutic agent has improved disease severity in children and adults with AD.21 Although further studies are underway, these initial data suggest a role for microbiome-modifying therapies as AD treatment.
Chronic Hand Eczema
Chronic hand eczema (CHE), which has considerable overlap with AD in many patients, especially children and adolescents,22-24 is another area of interesting research. This high-prevalence condition is associated with allergic and irritant contact dermatitis24-26—conditions that are both considered alternative diagnoses for and exacerbators of AD27—and is a disease process currently being targeted for new therapies. Delgocitinib (NCT04872101, NCT04871711), the novel JAK inhibitor ARQ-252 (NCT04378569), among other topical agents, as well as systemic therapeutics such as gusacitinib (NCT03728504), are in active trials for CHE. Given CHE’s impact on quality of life28 and its overlap with AD, investigation into this disorder can help drive future AD research as well as lead to better knowledge and treatment of CHE.
Final Thoughts
Despite the promising results of these myriad new therapies in AD, there are many factors that influence how and when we use these drugs, including their approval status, FDA labeling, and the ability of patients to access and afford treatment. Additionally, continued study is needed to evaluate the long-term safety and extended efficacy of newer drugs, such as the oral JAK inhibitors. Despite these hurdles, the current landscape of research and development is rapidly evolving. Compared to the many years when only one main group of therapies was a reasonable option for patients, the future of AD treatment looks bright.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- FDA approves Eucrisa for eczema. News release. US Food and Drug Administration; December 14, 2016. Accessed August 16, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293. doi:10.1016/j.jaad.2020.06.054
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-388. doi:10.1016/j.jaad.2019.07.074
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. doi: 10.1111/jdv.16928
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70. doi:10.1016/j.jaad.2021.02.028
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112. doi:10.1056/NEJMoa2019380
- Lilly and Incyte provide update on supplemental New Drug Application for baricitinib for the treatment of moderate to severe atopic dermatitis. News release. Eli Lilly and Company; July 16, 2021. Accessed August 16, 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-provide-update-supplemental new-drug
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial [published online August 4, 2021]. JAMA Dermatol. doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre,placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-463. doi:10.1111/bjd.19573
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies [published online May 4, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji, T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial [published online February 22, 2021]. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-859. doi:10.1101/gr.131029.111
- Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12:eaaz8631. doi:10.1126/scitranslmed.aaz8631
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523-532. doi:10.1046/j.1365-2133.2001.04078.x
- Grönhagen C, Lidén C, Wahlgren CF, et al. Hand eczema and atopic dermatitis in adolescents: a prospective cohort study from the BAMSE project. Br J Dermatol. 2015;173:1175-1182. doi:10.1111/bjd.14019
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Contact allergy and allergic contact dermatitis in adolescents: prevalence measures and associations. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol. 2002;82:352-358. doi:10.1080/000155502320624087
- Isaksson M, Olhardt S, Rådehed J, et al. Children with atopic dermatitis should always be patch-tested if they have hand or foot dermatitis. Acta Derm Venereol. 2015;95:583-586. doi:10.2340/00015555-1995
- Silverberg JI, Warshaw EM, Maibach HI, et al. Hand eczema in children referred for patch testing: North American Contact Dermatitis Group Data, 2000-2016. Br J Dermatol. 2021;185:185-194. doi:10.1111/bjd.19818
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(suppl 1):4-12. doi:10.1111/jdv.16061
- Cazzaniga S, Ballmer-Weber BK, Gräni N, et al. Medical, psychological and socio-economic implications of chronic hand eczema: a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:628-637. doi:10.1111/jdv.13479
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- FDA approves Eucrisa for eczema. News release. US Food and Drug Administration; December 14, 2016. Accessed August 16, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293. doi:10.1016/j.jaad.2020.06.054
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-388. doi:10.1016/j.jaad.2019.07.074
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. doi: 10.1111/jdv.16928
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70. doi:10.1016/j.jaad.2021.02.028
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112. doi:10.1056/NEJMoa2019380
- Lilly and Incyte provide update on supplemental New Drug Application for baricitinib for the treatment of moderate to severe atopic dermatitis. News release. Eli Lilly and Company; July 16, 2021. Accessed August 16, 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-provide-update-supplemental new-drug
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial [published online August 4, 2021]. JAMA Dermatol. doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre,placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-463. doi:10.1111/bjd.19573
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies [published online May 4, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji, T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial [published online February 22, 2021]. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-859. doi:10.1101/gr.131029.111
- Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12:eaaz8631. doi:10.1126/scitranslmed.aaz8631
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523-532. doi:10.1046/j.1365-2133.2001.04078.x
- Grönhagen C, Lidén C, Wahlgren CF, et al. Hand eczema and atopic dermatitis in adolescents: a prospective cohort study from the BAMSE project. Br J Dermatol. 2015;173:1175-1182. doi:10.1111/bjd.14019
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Contact allergy and allergic contact dermatitis in adolescents: prevalence measures and associations. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol. 2002;82:352-358. doi:10.1080/000155502320624087
- Isaksson M, Olhardt S, Rådehed J, et al. Children with atopic dermatitis should always be patch-tested if they have hand or foot dermatitis. Acta Derm Venereol. 2015;95:583-586. doi:10.2340/00015555-1995
- Silverberg JI, Warshaw EM, Maibach HI, et al. Hand eczema in children referred for patch testing: North American Contact Dermatitis Group Data, 2000-2016. Br J Dermatol. 2021;185:185-194. doi:10.1111/bjd.19818
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(suppl 1):4-12. doi:10.1111/jdv.16061
- Cazzaniga S, Ballmer-Weber BK, Gräni N, et al. Medical, psychological and socio-economic implications of chronic hand eczema: a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:628-637. doi:10.1111/jdv.13479
Treatment Options for Atopic Dermatitis in Children
Until recently, atopic dermatitis was considered a childhood disease that was self-limited over a few years. Emerging studies have shown that the burden of atopic dermatitis includes potential cardiac disease in adulthood, comorbidities including allergy and psychological disorders, and possible superinfection complications.
Dr Lawrence F. Eichenfield, chief of the department of pediatric and adolescent dermatology at Rady Children's Hospital, reports on biological, systemic, and topical treatments either currently in use or being studied for children suffering from atopic dermatitis. These studies include both steroid and steroid-sparing topical agents, a novel AhR modulating agent, as well as JAK inhibitors that are under active investigation.
--
Lawrence F. Eichenfield, MD, Distinguished Professor; Vice Chair, Department of Dermatology and Pediatrics, University of California, San Diego; Chief, Department of Pediatric and Adolescent Dermatology, Rady Children's Hospital, San Diego, California.
Lawrence F. Eichenfield, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Dermavant; Dermira; Forte Biosciences; Galderma Laboratories; Incyte; Leo Pharma; Eli Lilly and Company; Otsuka; Novartis; Pfizer. Serve(d) as a speaker or a member of a speakers bureau for: Regeneron; Sanofi-Genzyme; Pfizer. Received research grant from: AbbVie; Regeneron; Sanofi Genzyme; Ortho Dermatology. Serve(d) on the data safety monitoring board for: Asana; Glenmark/Ichnos.
Until recently, atopic dermatitis was considered a childhood disease that was self-limited over a few years. Emerging studies have shown that the burden of atopic dermatitis includes potential cardiac disease in adulthood, comorbidities including allergy and psychological disorders, and possible superinfection complications.
Dr Lawrence F. Eichenfield, chief of the department of pediatric and adolescent dermatology at Rady Children's Hospital, reports on biological, systemic, and topical treatments either currently in use or being studied for children suffering from atopic dermatitis. These studies include both steroid and steroid-sparing topical agents, a novel AhR modulating agent, as well as JAK inhibitors that are under active investigation.
--
Lawrence F. Eichenfield, MD, Distinguished Professor; Vice Chair, Department of Dermatology and Pediatrics, University of California, San Diego; Chief, Department of Pediatric and Adolescent Dermatology, Rady Children's Hospital, San Diego, California.
Lawrence F. Eichenfield, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Dermavant; Dermira; Forte Biosciences; Galderma Laboratories; Incyte; Leo Pharma; Eli Lilly and Company; Otsuka; Novartis; Pfizer. Serve(d) as a speaker or a member of a speakers bureau for: Regeneron; Sanofi-Genzyme; Pfizer. Received research grant from: AbbVie; Regeneron; Sanofi Genzyme; Ortho Dermatology. Serve(d) on the data safety monitoring board for: Asana; Glenmark/Ichnos.
Until recently, atopic dermatitis was considered a childhood disease that was self-limited over a few years. Emerging studies have shown that the burden of atopic dermatitis includes potential cardiac disease in adulthood, comorbidities including allergy and psychological disorders, and possible superinfection complications.
Dr Lawrence F. Eichenfield, chief of the department of pediatric and adolescent dermatology at Rady Children's Hospital, reports on biological, systemic, and topical treatments either currently in use or being studied for children suffering from atopic dermatitis. These studies include both steroid and steroid-sparing topical agents, a novel AhR modulating agent, as well as JAK inhibitors that are under active investigation.
--
Lawrence F. Eichenfield, MD, Distinguished Professor; Vice Chair, Department of Dermatology and Pediatrics, University of California, San Diego; Chief, Department of Pediatric and Adolescent Dermatology, Rady Children's Hospital, San Diego, California.
Lawrence F. Eichenfield, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Dermavant; Dermira; Forte Biosciences; Galderma Laboratories; Incyte; Leo Pharma; Eli Lilly and Company; Otsuka; Novartis; Pfizer. Serve(d) as a speaker or a member of a speakers bureau for: Regeneron; Sanofi-Genzyme; Pfizer. Received research grant from: AbbVie; Regeneron; Sanofi Genzyme; Ortho Dermatology. Serve(d) on the data safety monitoring board for: Asana; Glenmark/Ichnos.

What's the diagnosis?
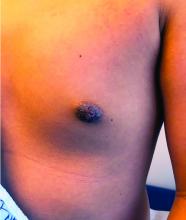
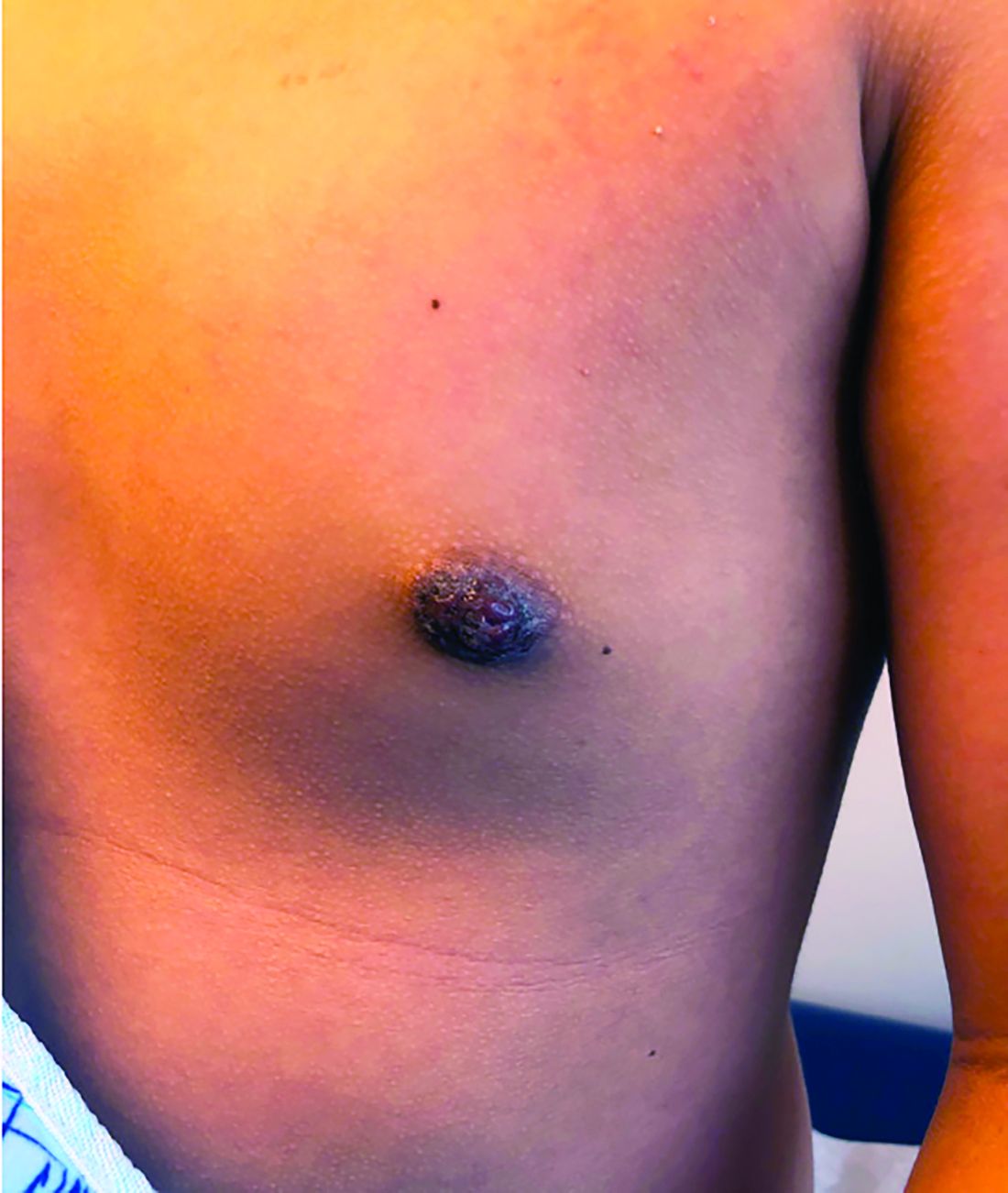
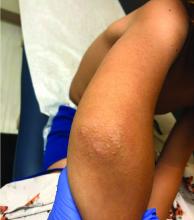
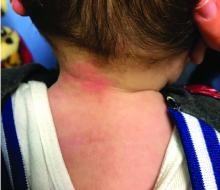
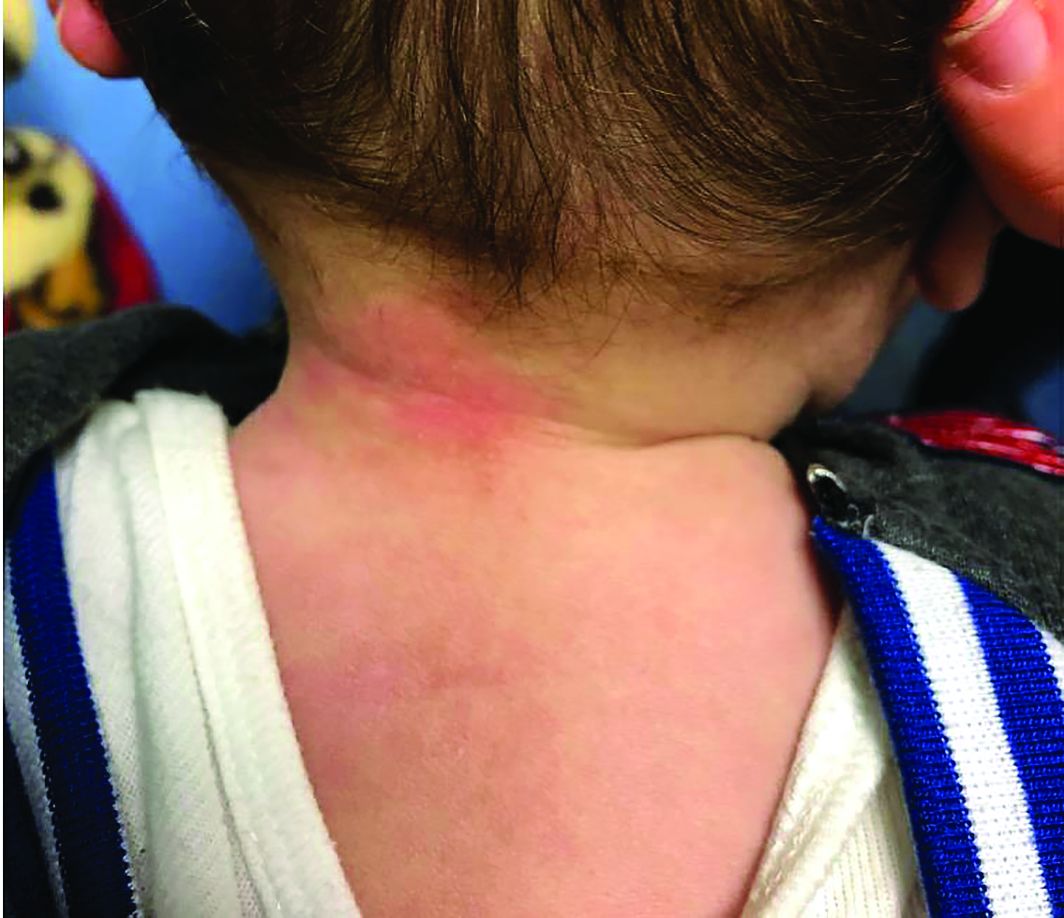
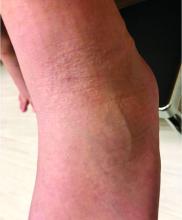
Nipple eczema is a dermatitis of the nipple and areola with clinical features such as erythema, fissures, scaling, pruritus, and crusting.1,2 It is classically associated with atopic dermatitis (AD), though it may occur as an isolated condition less commonly. While it may affect female adolescents, nipple eczema has also been reported in boys and breastfeeding women.3,4 The overall risk of incidence of nipple dermatitis has also been shown to increase with age.5 Nipple eczema is considered a cutaneous finding of AD, and is listed as a minor diagnostic criteria for AD in the Hanifin-Rajka criteria.6 The patient had not related his history of AD, which was elicited after finding typical antecubital eczematous dermatitis, and he had not been actively treating it.
Diagnosis and differential
Nipple eczema may be a challenging diagnosis for various reasons. For example, a unilateral presentation and the changes in the eczematous lesions overlying the nipple and areola’s varying textures and colors can make it difficult for clinicians to identify.3 Many children and adolescents, including our patient, are initially diagnosed as having impetigo and treated with antibiotics. The diagnosis of nipple eczema is made clinically, and management straightforward (see below). However, additional testing may be appropriate including patch testing for allergic contact dermatitis or bacterial cultures if bacterial infection or superinfection is considered.7,8 The differential diagnosis for nipple eczema includes impetigo, gynecomastia, scabies, and allergic contact dermatitis.
Impetigo typically presents with honey-colored crusts or pustules caused by infection with Staphylococcus aureus or Streptococcus. Patients with AD have higher rates of colonization with S. aureus and impetiginized eczema in common. Impetigo of the nipple and areola is more common in breastfeeding women as skin cracking from lactation can lead to exposure to bacteria from the infant’s mouth.9 Treatments involve topical or oral antibiotics.
Gynecomastia is the development of male breast tissue with most cases hypothesized to be caused by an imbalance between androgens and estrogens.10 Some other causes include direct skin contact with topical estrogen sprays and recreational use of marijuana and heroin.11 It is usually a benign exam finding in adolescent boys. However, clinical findings such as overlying skin changes, rapidly enlarging masses, and constitutional symptoms are concerning in the setting of gynecomastia and warrant further evaluation.
Scabies, which is caused by the infestation of scabies mites, is a common infectious skin disease. The classic presentation includes a rash that is intensely itchy, especially at night. Crusted scabies of the nipples may be difficult to distinguish from nipple eczema. Areas of frequent involvement of scabies include palms, between fingers, armpits, groin, between toes, and feet. Treatments include treating all household members with permethrin cream and washing all clothes and bedding in contact with a scabies-infected patient in high heat, or oral ivermectin in certain circumstances.12
Allergic contact dermatitis is a common cause of breast and nipple dermatitis and should be considered within the differential diagnosis of nipple eczema with atopic dermatitis, or as an exacerbator.7,9 Patients in particular who present with bilateral involvement extending to the periareolar skin, or unusual bilateral focal patterns suggestive for contact allergy should be considered for allergic contact dermatitis evaluation with patch tests. A common causative agent for allergic contact dermatitis of the breast and nipple includes Cl+Me-isothiazolinone, commonly found in detergents and fabric softeners.7 Primary treatment includes avoidance of the offending agents.
Treatment
Topical corticosteroids are first-line treatment for treating nipple eczema. Low-potency topical steroids can be used for maintenance and mild eczema while more potent steroids are useful for more severe cases. In addition to topical medication therapy, frequent emollient use to protect the skin barrier and the elimination of any irritants are essential to a successful treatment course. Unilateral nipple eczema can also be secondary to inadequate treatment of AD, demonstrating the importance of addressing the underlying AD with therapy.3
Our patient was diagnosed with nipple eczema based on clinical presentation of an eczematous left nipple in the setting of active atopic dermatitis and minimal improvement on topical antibiotic. He was started on a 3-week course of fluocinonide 0.05% topical ointment (a potent topical corticosteroid) twice daily for 2 weeks with plans to transition to triamcinolone 0.1% topical ointment several times a week.
Ms. Park is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Neither Ms. Park nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Pediatr Dermatol. 2005;22(1):64-6.
2. Am J Dermatopathol. 2015;37(4):284-8.
3. Pediatr Dermatol. 2015;32(5):718-22.
4. J Cutan Med Surg. 2004;8(2):126-30.
5. Pediatr Dermatol. 2012;29(5):580-3.
6. Dermatologica. 1988;177(6):360-4.
7. Ann Dermatol. 2014;26(3):413-4.
8. BMJ Case Rep. 2020;13(8).
9. J Am Acad Dermatol. 2019;80(6):1483-94.
10. Pediatr Endocrinol Rev. 2017;14(4):371-7.
11. JAMA. 2010;304(9):953.
12. JAMA. 2018;320(6):612.
Nipple eczema is a dermatitis of the nipple and areola with clinical features such as erythema, fissures, scaling, pruritus, and crusting.1,2 It is classically associated with atopic dermatitis (AD), though it may occur as an isolated condition less commonly. While it may affect female adolescents, nipple eczema has also been reported in boys and breastfeeding women.3,4 The overall risk of incidence of nipple dermatitis has also been shown to increase with age.5 Nipple eczema is considered a cutaneous finding of AD, and is listed as a minor diagnostic criteria for AD in the Hanifin-Rajka criteria.6 The patient had not related his history of AD, which was elicited after finding typical antecubital eczematous dermatitis, and he had not been actively treating it.
Diagnosis and differential
Nipple eczema may be a challenging diagnosis for various reasons. For example, a unilateral presentation and the changes in the eczematous lesions overlying the nipple and areola’s varying textures and colors can make it difficult for clinicians to identify.3 Many children and adolescents, including our patient, are initially diagnosed as having impetigo and treated with antibiotics. The diagnosis of nipple eczema is made clinically, and management straightforward (see below). However, additional testing may be appropriate including patch testing for allergic contact dermatitis or bacterial cultures if bacterial infection or superinfection is considered.7,8 The differential diagnosis for nipple eczema includes impetigo, gynecomastia, scabies, and allergic contact dermatitis.
Impetigo typically presents with honey-colored crusts or pustules caused by infection with Staphylococcus aureus or Streptococcus. Patients with AD have higher rates of colonization with S. aureus and impetiginized eczema in common. Impetigo of the nipple and areola is more common in breastfeeding women as skin cracking from lactation can lead to exposure to bacteria from the infant’s mouth.9 Treatments involve topical or oral antibiotics.
Gynecomastia is the development of male breast tissue with most cases hypothesized to be caused by an imbalance between androgens and estrogens.10 Some other causes include direct skin contact with topical estrogen sprays and recreational use of marijuana and heroin.11 It is usually a benign exam finding in adolescent boys. However, clinical findings such as overlying skin changes, rapidly enlarging masses, and constitutional symptoms are concerning in the setting of gynecomastia and warrant further evaluation.
Scabies, which is caused by the infestation of scabies mites, is a common infectious skin disease. The classic presentation includes a rash that is intensely itchy, especially at night. Crusted scabies of the nipples may be difficult to distinguish from nipple eczema. Areas of frequent involvement of scabies include palms, between fingers, armpits, groin, between toes, and feet. Treatments include treating all household members with permethrin cream and washing all clothes and bedding in contact with a scabies-infected patient in high heat, or oral ivermectin in certain circumstances.12
Allergic contact dermatitis is a common cause of breast and nipple dermatitis and should be considered within the differential diagnosis of nipple eczema with atopic dermatitis, or as an exacerbator.7,9 Patients in particular who present with bilateral involvement extending to the periareolar skin, or unusual bilateral focal patterns suggestive for contact allergy should be considered for allergic contact dermatitis evaluation with patch tests. A common causative agent for allergic contact dermatitis of the breast and nipple includes Cl+Me-isothiazolinone, commonly found in detergents and fabric softeners.7 Primary treatment includes avoidance of the offending agents.
Treatment
Topical corticosteroids are first-line treatment for treating nipple eczema. Low-potency topical steroids can be used for maintenance and mild eczema while more potent steroids are useful for more severe cases. In addition to topical medication therapy, frequent emollient use to protect the skin barrier and the elimination of any irritants are essential to a successful treatment course. Unilateral nipple eczema can also be secondary to inadequate treatment of AD, demonstrating the importance of addressing the underlying AD with therapy.3
Our patient was diagnosed with nipple eczema based on clinical presentation of an eczematous left nipple in the setting of active atopic dermatitis and minimal improvement on topical antibiotic. He was started on a 3-week course of fluocinonide 0.05% topical ointment (a potent topical corticosteroid) twice daily for 2 weeks with plans to transition to triamcinolone 0.1% topical ointment several times a week.
Ms. Park is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Neither Ms. Park nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Pediatr Dermatol. 2005;22(1):64-6.
2. Am J Dermatopathol. 2015;37(4):284-8.
3. Pediatr Dermatol. 2015;32(5):718-22.
4. J Cutan Med Surg. 2004;8(2):126-30.
5. Pediatr Dermatol. 2012;29(5):580-3.
6. Dermatologica. 1988;177(6):360-4.
7. Ann Dermatol. 2014;26(3):413-4.
8. BMJ Case Rep. 2020;13(8).
9. J Am Acad Dermatol. 2019;80(6):1483-94.
10. Pediatr Endocrinol Rev. 2017;14(4):371-7.
11. JAMA. 2010;304(9):953.
12. JAMA. 2018;320(6):612.
Nipple eczema is a dermatitis of the nipple and areola with clinical features such as erythema, fissures, scaling, pruritus, and crusting.1,2 It is classically associated with atopic dermatitis (AD), though it may occur as an isolated condition less commonly. While it may affect female adolescents, nipple eczema has also been reported in boys and breastfeeding women.3,4 The overall risk of incidence of nipple dermatitis has also been shown to increase with age.5 Nipple eczema is considered a cutaneous finding of AD, and is listed as a minor diagnostic criteria for AD in the Hanifin-Rajka criteria.6 The patient had not related his history of AD, which was elicited after finding typical antecubital eczematous dermatitis, and he had not been actively treating it.
Diagnosis and differential
Nipple eczema may be a challenging diagnosis for various reasons. For example, a unilateral presentation and the changes in the eczematous lesions overlying the nipple and areola’s varying textures and colors can make it difficult for clinicians to identify.3 Many children and adolescents, including our patient, are initially diagnosed as having impetigo and treated with antibiotics. The diagnosis of nipple eczema is made clinically, and management straightforward (see below). However, additional testing may be appropriate including patch testing for allergic contact dermatitis or bacterial cultures if bacterial infection or superinfection is considered.7,8 The differential diagnosis for nipple eczema includes impetigo, gynecomastia, scabies, and allergic contact dermatitis.
Impetigo typically presents with honey-colored crusts or pustules caused by infection with Staphylococcus aureus or Streptococcus. Patients with AD have higher rates of colonization with S. aureus and impetiginized eczema in common. Impetigo of the nipple and areola is more common in breastfeeding women as skin cracking from lactation can lead to exposure to bacteria from the infant’s mouth.9 Treatments involve topical or oral antibiotics.
Gynecomastia is the development of male breast tissue with most cases hypothesized to be caused by an imbalance between androgens and estrogens.10 Some other causes include direct skin contact with topical estrogen sprays and recreational use of marijuana and heroin.11 It is usually a benign exam finding in adolescent boys. However, clinical findings such as overlying skin changes, rapidly enlarging masses, and constitutional symptoms are concerning in the setting of gynecomastia and warrant further evaluation.
Scabies, which is caused by the infestation of scabies mites, is a common infectious skin disease. The classic presentation includes a rash that is intensely itchy, especially at night. Crusted scabies of the nipples may be difficult to distinguish from nipple eczema. Areas of frequent involvement of scabies include palms, between fingers, armpits, groin, between toes, and feet. Treatments include treating all household members with permethrin cream and washing all clothes and bedding in contact with a scabies-infected patient in high heat, or oral ivermectin in certain circumstances.12
Allergic contact dermatitis is a common cause of breast and nipple dermatitis and should be considered within the differential diagnosis of nipple eczema with atopic dermatitis, or as an exacerbator.7,9 Patients in particular who present with bilateral involvement extending to the periareolar skin, or unusual bilateral focal patterns suggestive for contact allergy should be considered for allergic contact dermatitis evaluation with patch tests. A common causative agent for allergic contact dermatitis of the breast and nipple includes Cl+Me-isothiazolinone, commonly found in detergents and fabric softeners.7 Primary treatment includes avoidance of the offending agents.
Treatment
Topical corticosteroids are first-line treatment for treating nipple eczema. Low-potency topical steroids can be used for maintenance and mild eczema while more potent steroids are useful for more severe cases. In addition to topical medication therapy, frequent emollient use to protect the skin barrier and the elimination of any irritants are essential to a successful treatment course. Unilateral nipple eczema can also be secondary to inadequate treatment of AD, demonstrating the importance of addressing the underlying AD with therapy.3
Our patient was diagnosed with nipple eczema based on clinical presentation of an eczematous left nipple in the setting of active atopic dermatitis and minimal improvement on topical antibiotic. He was started on a 3-week course of fluocinonide 0.05% topical ointment (a potent topical corticosteroid) twice daily for 2 weeks with plans to transition to triamcinolone 0.1% topical ointment several times a week.
Ms. Park is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Neither Ms. Park nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Pediatr Dermatol. 2005;22(1):64-6.
2. Am J Dermatopathol. 2015;37(4):284-8.
3. Pediatr Dermatol. 2015;32(5):718-22.
4. J Cutan Med Surg. 2004;8(2):126-30.
5. Pediatr Dermatol. 2012;29(5):580-3.
6. Dermatologica. 1988;177(6):360-4.
7. Ann Dermatol. 2014;26(3):413-4.
8. BMJ Case Rep. 2020;13(8).
9. J Am Acad Dermatol. 2019;80(6):1483-94.
10. Pediatr Endocrinol Rev. 2017;14(4):371-7.
11. JAMA. 2010;304(9):953.
12. JAMA. 2018;320(6):612.
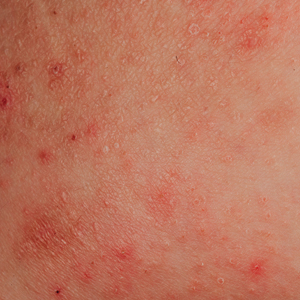
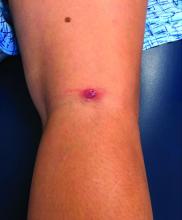
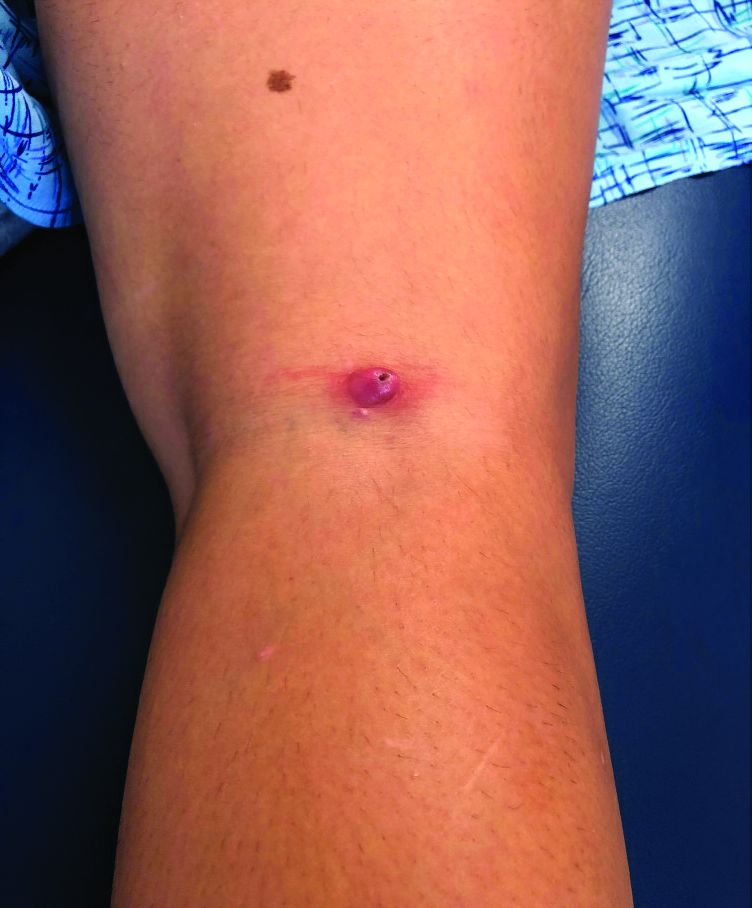
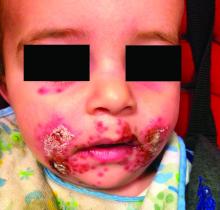
A 12-year-old boy presents to the dermatology clinic with a 1-month history of crusting and watery sticky drainage from the left nipple. Given concern for a possible skin infection, the patient was initially treated with mupirocin ointment for several weeks but without improvement. The affected area is sometimes itchy but not painful. He reports no prior history of similar problems.
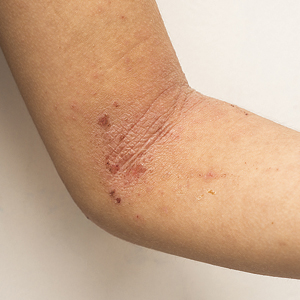
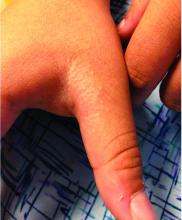
On physical exam, he is noted to have an eczematous left nipple with edema, xerosis, and scaling overlying the entire areola. There is no evidence of visible discharge, pustules, or honey-colored crusts in the area. The extensor surfaces of his arms bilaterally have skin-colored follicular papules, and his antecubital fossa display erythematous scaling plaques with mild lichenification and excoriations.
Pediatric dermatology: Reflecting on 50 years
As part of the 50th anniversary of Dermatology News, it is intriguing to think about where a time machine journey 5 decades back would find the field of pediatric dermatology, and to assess the changes in the specialty during the time that Dermatology News (operating then as “Skin & Allergy News”) has been reporting on innovations and changes in the practice of dermatology.
So, starting . It was not until 3 years later, in October 1973 in Mexico City, that the first international symposium on Pediatric Dermatology was held, and the International Society for Pediatric Dermatology was founded. I reached out to Andrew Margileth, MD, 100 years old this past July, and still active voluntary faculty in pediatric dermatology at the University of Miami, to help me “reach back” to those days. Dr. Margileth commented on how the first symposium was “brilliantly orchestrated by Ramon Ruiz-Maldonado,” from the National Institute of Paediatrics in Mexico, and that it was his “Aha moment for future practice!” That meeting spurred discussions on the development of the Society for Pediatric Dermatology the next year, with Alvin Jacobs, MD; Samuel Weinberg, MD; Nancy Esterly, MD; Sidney Hurwitz, MD; William Weston, MD; and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers,” and the society was officially established in 1975.
The field of pediatric dermatology was fairly “infantile” 50 years ago, with few practitioners. But the early leaders in the field recognized that up to 30% of pediatric primary care visits included skin problems, and that there was limited training for dermatologists, as well as pediatricians, about skin diseases in children. There were clearly clinical and educational needs to establish a subspecialty of pediatric dermatology, and over the next 1-2 decades, the field expanded. The journal Pediatric Dermatology was established (in 1982), the Section on Dermatology was established by the American Academy of Pediatrics (in 1986), and fellowship programs were launched at select academic centers. And it was 30 years into our timeline before the formal subspecialty of pediatric dermatology was established through the American Board of Dermatology (2000).
The field of pediatric dermatology has evolved and matured rapidly. Standard reference textbooks have been developed in the United States and around the world (and of course, online). Pediatric dermatology is an essential part of the core curriculum for dermatologist trainees. Organizations promoting pediatric research have developed to influence basic, translational, and clinical research in conditions in neonates through adolescents, such as the Pediatric Dermatology Research Alliance (PeDRA). And meetings throughout the world now feature pediatric dermatology sessions and help to spread the advances in the diagnosis and management of pediatric skin disorders.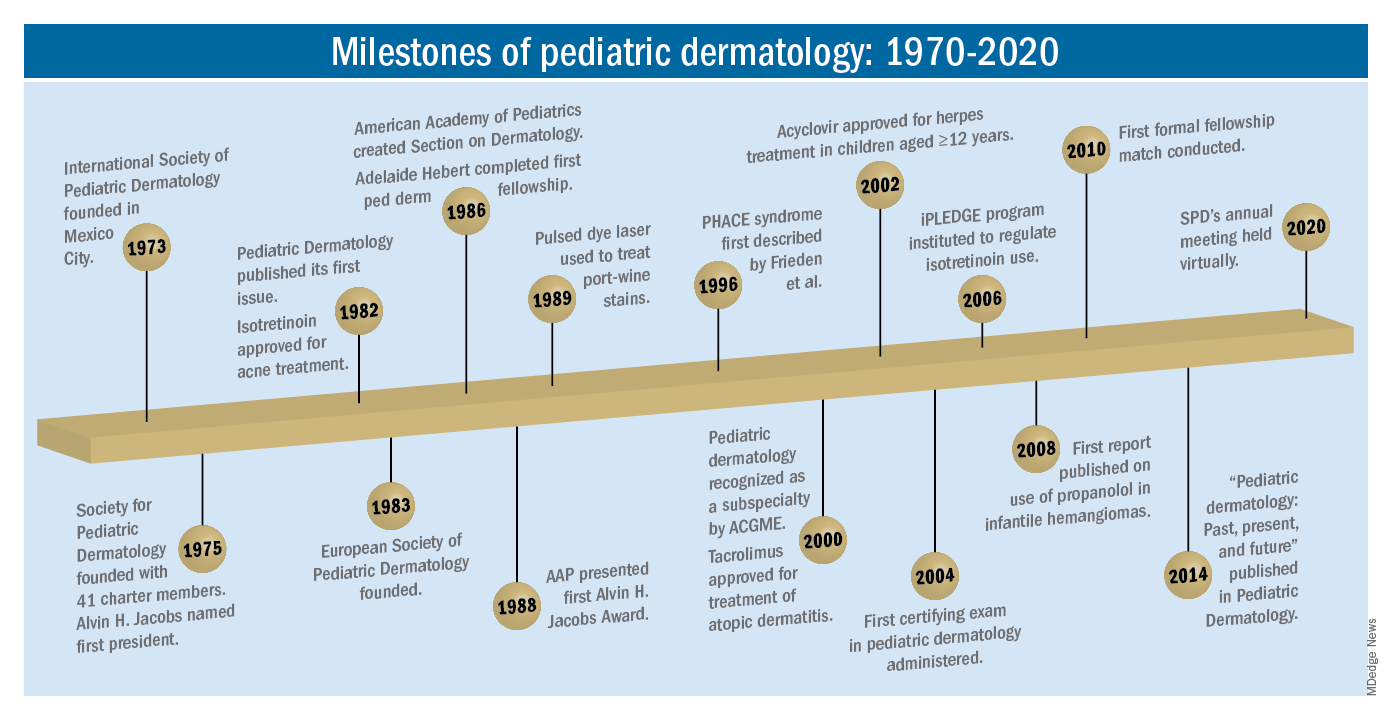
The practice of pediatric dermatology: How has it changed?
It is beyond the scope of this article to try to comprehensively review all of the changes in pediatric dermatology practice. But review of the evolution of a few disease states (choices influenced by my discussions with my 100-year old history guide, Dr. Margileth) displays examples of where we have been, and where we are going in our next 5, 10, or 50 years.
Hemangiomas and vascular malformations
Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that standard cutaneous hemangiomas had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of interest, the hallmark article’s first author was Dr. Margileth, published in 1965 in JAMA!.This was still at a time when the identification of hemangiomas of infancy (or “HOI” as we say in the trade) was confused with vascular malformations, and no one had recognized the distinct variant tumors such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, or hemangioendotheliomas. PHACE syndrome was not yet described (that was done in 1996 by Ilona Frieden, MD, and colleagues). And for a time, hemangiomas were treated with x-rays, before the negative impact of such radiation was acknowledged. It seems that, as a consequence of the use of x-ray therapy and as a backlash from the radiation therapy side effects and potential toxicities, even deforming and functionally significant lesions were “followed clinically” for natural involution, with a sensibility that doing nothing might be better than doing the wrong thing.
Over the next 15 years, the recognition of functionally significant hemangiomas, deformation associated with their proliferation, and the recognition of PHACE syndrome made hemangiomas of infancy an area of concern, with systemic steroids and occasionally chemotherapeutic agents (such as vincristine) being used for problematic lesions.
It has now been 12 years since the work of Christine Léauté-Labrèze, MD, et al., from the University of Bordeaux (France), led to the breakthrough of propranolol for hemangioma treatment, profoundly changing hemangioma management to an incredibly effective medical therapy extensively studied, tested in formal clinical trials, and approved by regulatory authorities. And how intriguing that this was pursued after the chance (but skilled) observation that a child who developed hypertension as a side effect of systemic steroids for nasal hemangioma treatment was prescribed propranolol for the hypertension and had his nasal hemangioma rapidly shrink, with a response superior and much quicker than that to corticosteroids.
The evolution of management of hemangiomas has another story within it, that of collaborative research. The Hemangioma Investigator Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development, and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas. Our knowledge of these lesions is now evidence based and broad, and the impact on care tremendous! The HIG has also influenced the practice of pediatricians and other specialists, including otorhinolaryngologists, hematologist/oncologists, and surgeons, is partnering with advocacy groups to support patients and families, and is helping guide patients and families to contribute to ongoing research.
Vascular malformations (VM) reflect an incredible change in our understanding of the developmental pathways and pathophysiology of blood vessel tumors, and, in fact, birthmarks other than vascular lesions! First, important work separated out hemangiomas of infancy and hemangiomalike tumors from vascular malformations, with the thought being that hemangiomas had a rapid growth phase, often arising from lesions that were minimally evident or not evident at birth, unlike malformations, which were “programing errors,” all present at birth and expected to be fairly static with proportionate growth over a lifetime. Approaches to vascular malformations were limited to sclerotherapy, laser, and/or surgery. While this general schema of classification is still useful, our sense of the “why and how” of vascular malformations is remarkably different. Vascular malformations – still usefully subdivided into capillary, lymphatic, venous arteriovenous, or mixed malformations – are mostly associated with inherited or somatic mutations. Mutations are most commonly found in two signal pathways: RAS/MAPK/ERK and PI3K/AKT/mTOR pathways, with specific sets of mutations seen in both localized and multifocal lesions, with or without overgrowth or other systemic anomalies. The discovery of specific mutations has led to the possibility of small-molecule inhibitors, many already existing as anticancer drugs, being utilized as targeted therapies for VM.
And similar advances in understanding of other birthmarks, with or without syndromic features, are being made steadily. The mutations in congenital melanocytic nevi, epidermal nevi, acquired tumors (pilomatricomas), and other lesions, along with steady epidemiologic, translational, and clinical work, evolves our knowledge and potential therapies.
Inflammatory skin disorders: Acne, psoriasis, and atopic dermatitis
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris is now recognized as much more common under age 12 than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later, with expert recommendations formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013. Over the past few years, there has been a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance. There are unanswered questions as we evolve our care: How will the new topical antiandrogens be used? Will spironolactone become part of hormonal therapy under age 18? Will the insights on certain strains of Cutibacterium acnes being associated with worse acne translate to microbiome or vaccine-based strategies?
Pediatric psoriasis has suffered, being “behind in the revolution” of biologic agents because of delayed approval of any biologic agent for treatment of pediatric psoriasis in the United States until just a few years ago, and lags behind Europe and elsewhere in the world by almost a decade. Only this year have we expanded beyond one biologic agent approved for under age 12 and two for ages 12 and older, with other approvals expected including interleukin (IL)-17 and IL-23 agents. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, with new recommendations on how to screen children with psoriasis, supplied first by PeDRA and then in the new American Academy of Dermatology-National Psoriasis Foundation pediatric psoriasis guidelines .
Pediatric atopic dermatitis (AD) is in its early years of revolution. In the 50-year period of our thought experiment, AD has increased in prevalence from 5% or less of the pediatric population to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, and management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their use of these useful agents. But newer studies are markedly reassuring about their safe use in children.
Steroid phobia, as well as concerns about potential side effects of the TCIs, has resulted in undertreatment of childhood AD. It is quite common to see multiple children during pediatric dermatology office hours with poorly controlled eczema, high body-surface areas of eczema, compromised sleep, secondary infections, and anxiety and depression, especially in our moderate to severe adolescents. The field is “hot” with new topical and systemic agents, including our few years’ experience with topical crisaborole, a phosphodiesterase (PDE)-4 inhibitor; and dupilumab, an IL-4-alpha blocker – the first biologic agent approved for AD and the first systemic agent (other than oral corticosteroids), just extended from 12 years to 6 years of age! As dupilumab gets studied for younger children, other biologics (including IL-13 and IL-31 blockers) are undertaking pediatric and/or adolescent trials, oral and topical JAK inhibitors are including adolescents in core clinical trials, and other novel topical agents are under study, including an aryl-hydrocarbon receptor–modulating agent and other PDE-4 inhibitors.
Procedural pediatric dermatology: From liquid nitrogen to laser, surgery, and multimodal skin care
The first generation of pediatric dermatologists were considered medical dermatologist specialists. The care of the conditions discussed above, as well as genodermatoses, diagnostic dilemmas, and management of dermatologic manifestations of systemic disease and other conditions, was the “bread and butter” of pediatric dermatology care. When I was in training, my mentor Paul Honig, MD, at the Children’s Hospital of Philadelphia had a procedure half-day each week, where he would care for a few patients who needed liquid nitrogen therapy for warts, or who needed biopsies. It was uncommon to have a large procedural/surgical part of pediatric dermatology practice. But this is now a routine part of many specialists in the field. How did this change occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser for treatment of port-wine birthmarks in children with minimal scarring, and a seminal article published in the New England Journal of Medicine in 1989. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists developed the exposure and skill to do this work. An added advantage was having the knowledge of how to handle children and adolescents in an age-appropriate manner, with consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota, for example), hair laser, and combinations of lasers, including fractionated CO2 technology, to treat hypertrophic, constrictive and/or deforming scars.
The future
What will pediatric dermatology be like in 10, 20, or 50 years?
I have not yet discussed some of the most challenging diseases in our field, including epidermolysis bullosa, ichthyosis, and neurocutaneous disorders and other genetic skin disorders that have an incredible impact on the lives of affected children and their families, with incredible morbidity and with many conditions that shorten lifespans. But these are the conditions where “the future is happening now,” and we are looking forward to our new gene therapy techniques helping to transform our care.
And other aspects of practice? Will we be doing a large percentage of practice over the phone (or whatever devices we have then – remember, the first iPhone was only released 13 years ago)?
Will our patients be using their own imaging systems to evaluate their nevi and skin growths, and perhaps to diagnose and manage their rashes?
Will we have prevented our inflammatory skin disorders, or “turned them off” in early life with aggressive therapy in infantile life?
I project only that all of us in dermatology will still be a resource to our pediatric patients, from neonate through young adult, through our work of preventing, caring, healing and minimizing disease impact, and hopefully enjoying the pleasures of seeing our patients healthfully develop and evolve! As will our field.
Dr. Eichenfield is professor of dermatology and pediatrics and vice-chair of the department of dermatology at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. Dr. Eichenfield reports financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including products for the diseases discussed here.
As part of the 50th anniversary of Dermatology News, it is intriguing to think about where a time machine journey 5 decades back would find the field of pediatric dermatology, and to assess the changes in the specialty during the time that Dermatology News (operating then as “Skin & Allergy News”) has been reporting on innovations and changes in the practice of dermatology.
So, starting . It was not until 3 years later, in October 1973 in Mexico City, that the first international symposium on Pediatric Dermatology was held, and the International Society for Pediatric Dermatology was founded. I reached out to Andrew Margileth, MD, 100 years old this past July, and still active voluntary faculty in pediatric dermatology at the University of Miami, to help me “reach back” to those days. Dr. Margileth commented on how the first symposium was “brilliantly orchestrated by Ramon Ruiz-Maldonado,” from the National Institute of Paediatrics in Mexico, and that it was his “Aha moment for future practice!” That meeting spurred discussions on the development of the Society for Pediatric Dermatology the next year, with Alvin Jacobs, MD; Samuel Weinberg, MD; Nancy Esterly, MD; Sidney Hurwitz, MD; William Weston, MD; and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers,” and the society was officially established in 1975.
The field of pediatric dermatology was fairly “infantile” 50 years ago, with few practitioners. But the early leaders in the field recognized that up to 30% of pediatric primary care visits included skin problems, and that there was limited training for dermatologists, as well as pediatricians, about skin diseases in children. There were clearly clinical and educational needs to establish a subspecialty of pediatric dermatology, and over the next 1-2 decades, the field expanded. The journal Pediatric Dermatology was established (in 1982), the Section on Dermatology was established by the American Academy of Pediatrics (in 1986), and fellowship programs were launched at select academic centers. And it was 30 years into our timeline before the formal subspecialty of pediatric dermatology was established through the American Board of Dermatology (2000).
The field of pediatric dermatology has evolved and matured rapidly. Standard reference textbooks have been developed in the United States and around the world (and of course, online). Pediatric dermatology is an essential part of the core curriculum for dermatologist trainees. Organizations promoting pediatric research have developed to influence basic, translational, and clinical research in conditions in neonates through adolescents, such as the Pediatric Dermatology Research Alliance (PeDRA). And meetings throughout the world now feature pediatric dermatology sessions and help to spread the advances in the diagnosis and management of pediatric skin disorders.
The practice of pediatric dermatology: How has it changed?
It is beyond the scope of this article to try to comprehensively review all of the changes in pediatric dermatology practice. But review of the evolution of a few disease states (choices influenced by my discussions with my 100-year old history guide, Dr. Margileth) displays examples of where we have been, and where we are going in our next 5, 10, or 50 years.
Hemangiomas and vascular malformations
Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that standard cutaneous hemangiomas had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of interest, the hallmark article’s first author was Dr. Margileth, published in 1965 in JAMA!.This was still at a time when the identification of hemangiomas of infancy (or “HOI” as we say in the trade) was confused with vascular malformations, and no one had recognized the distinct variant tumors such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, or hemangioendotheliomas. PHACE syndrome was not yet described (that was done in 1996 by Ilona Frieden, MD, and colleagues). And for a time, hemangiomas were treated with x-rays, before the negative impact of such radiation was acknowledged. It seems that, as a consequence of the use of x-ray therapy and as a backlash from the radiation therapy side effects and potential toxicities, even deforming and functionally significant lesions were “followed clinically” for natural involution, with a sensibility that doing nothing might be better than doing the wrong thing.
Over the next 15 years, the recognition of functionally significant hemangiomas, deformation associated with their proliferation, and the recognition of PHACE syndrome made hemangiomas of infancy an area of concern, with systemic steroids and occasionally chemotherapeutic agents (such as vincristine) being used for problematic lesions.
It has now been 12 years since the work of Christine Léauté-Labrèze, MD, et al., from the University of Bordeaux (France), led to the breakthrough of propranolol for hemangioma treatment, profoundly changing hemangioma management to an incredibly effective medical therapy extensively studied, tested in formal clinical trials, and approved by regulatory authorities. And how intriguing that this was pursued after the chance (but skilled) observation that a child who developed hypertension as a side effect of systemic steroids for nasal hemangioma treatment was prescribed propranolol for the hypertension and had his nasal hemangioma rapidly shrink, with a response superior and much quicker than that to corticosteroids.
The evolution of management of hemangiomas has another story within it, that of collaborative research. The Hemangioma Investigator Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development, and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas. Our knowledge of these lesions is now evidence based and broad, and the impact on care tremendous! The HIG has also influenced the practice of pediatricians and other specialists, including otorhinolaryngologists, hematologist/oncologists, and surgeons, is partnering with advocacy groups to support patients and families, and is helping guide patients and families to contribute to ongoing research.
Vascular malformations (VM) reflect an incredible change in our understanding of the developmental pathways and pathophysiology of blood vessel tumors, and, in fact, birthmarks other than vascular lesions! First, important work separated out hemangiomas of infancy and hemangiomalike tumors from vascular malformations, with the thought being that hemangiomas had a rapid growth phase, often arising from lesions that were minimally evident or not evident at birth, unlike malformations, which were “programing errors,” all present at birth and expected to be fairly static with proportionate growth over a lifetime. Approaches to vascular malformations were limited to sclerotherapy, laser, and/or surgery. While this general schema of classification is still useful, our sense of the “why and how” of vascular malformations is remarkably different. Vascular malformations – still usefully subdivided into capillary, lymphatic, venous arteriovenous, or mixed malformations – are mostly associated with inherited or somatic mutations. Mutations are most commonly found in two signal pathways: RAS/MAPK/ERK and PI3K/AKT/mTOR pathways, with specific sets of mutations seen in both localized and multifocal lesions, with or without overgrowth or other systemic anomalies. The discovery of specific mutations has led to the possibility of small-molecule inhibitors, many already existing as anticancer drugs, being utilized as targeted therapies for VM.
And similar advances in understanding of other birthmarks, with or without syndromic features, are being made steadily. The mutations in congenital melanocytic nevi, epidermal nevi, acquired tumors (pilomatricomas), and other lesions, along with steady epidemiologic, translational, and clinical work, evolves our knowledge and potential therapies.
Inflammatory skin disorders: Acne, psoriasis, and atopic dermatitis
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris is now recognized as much more common under age 12 than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later, with expert recommendations formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013. Over the past few years, there has been a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance. There are unanswered questions as we evolve our care: How will the new topical antiandrogens be used? Will spironolactone become part of hormonal therapy under age 18? Will the insights on certain strains of Cutibacterium acnes being associated with worse acne translate to microbiome or vaccine-based strategies?
Pediatric psoriasis has suffered, being “behind in the revolution” of biologic agents because of delayed approval of any biologic agent for treatment of pediatric psoriasis in the United States until just a few years ago, and lags behind Europe and elsewhere in the world by almost a decade. Only this year have we expanded beyond one biologic agent approved for under age 12 and two for ages 12 and older, with other approvals expected including interleukin (IL)-17 and IL-23 agents. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, with new recommendations on how to screen children with psoriasis, supplied first by PeDRA and then in the new American Academy of Dermatology-National Psoriasis Foundation pediatric psoriasis guidelines .
Pediatric atopic dermatitis (AD) is in its early years of revolution. In the 50-year period of our thought experiment, AD has increased in prevalence from 5% or less of the pediatric population to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, and management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their use of these useful agents. But newer studies are markedly reassuring about their safe use in children.
Steroid phobia, as well as concerns about potential side effects of the TCIs, has resulted in undertreatment of childhood AD. It is quite common to see multiple children during pediatric dermatology office hours with poorly controlled eczema, high body-surface areas of eczema, compromised sleep, secondary infections, and anxiety and depression, especially in our moderate to severe adolescents. The field is “hot” with new topical and systemic agents, including our few years’ experience with topical crisaborole, a phosphodiesterase (PDE)-4 inhibitor; and dupilumab, an IL-4-alpha blocker – the first biologic agent approved for AD and the first systemic agent (other than oral corticosteroids), just extended from 12 years to 6 years of age! As dupilumab gets studied for younger children, other biologics (including IL-13 and IL-31 blockers) are undertaking pediatric and/or adolescent trials, oral and topical JAK inhibitors are including adolescents in core clinical trials, and other novel topical agents are under study, including an aryl-hydrocarbon receptor–modulating agent and other PDE-4 inhibitors.
Procedural pediatric dermatology: From liquid nitrogen to laser, surgery, and multimodal skin care
The first generation of pediatric dermatologists were considered medical dermatologist specialists. The care of the conditions discussed above, as well as genodermatoses, diagnostic dilemmas, and management of dermatologic manifestations of systemic disease and other conditions, was the “bread and butter” of pediatric dermatology care. When I was in training, my mentor Paul Honig, MD, at the Children’s Hospital of Philadelphia had a procedure half-day each week, where he would care for a few patients who needed liquid nitrogen therapy for warts, or who needed biopsies. It was uncommon to have a large procedural/surgical part of pediatric dermatology practice. But this is now a routine part of many specialists in the field. How did this change occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser for treatment of port-wine birthmarks in children with minimal scarring, and a seminal article published in the New England Journal of Medicine in 1989. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists developed the exposure and skill to do this work. An added advantage was having the knowledge of how to handle children and adolescents in an age-appropriate manner, with consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota, for example), hair laser, and combinations of lasers, including fractionated CO2 technology, to treat hypertrophic, constrictive and/or deforming scars.
The future
What will pediatric dermatology be like in 10, 20, or 50 years?
I have not yet discussed some of the most challenging diseases in our field, including epidermolysis bullosa, ichthyosis, and neurocutaneous disorders and other genetic skin disorders that have an incredible impact on the lives of affected children and their families, with incredible morbidity and with many conditions that shorten lifespans. But these are the conditions where “the future is happening now,” and we are looking forward to our new gene therapy techniques helping to transform our care.
And other aspects of practice? Will we be doing a large percentage of practice over the phone (or whatever devices we have then – remember, the first iPhone was only released 13 years ago)?
Will our patients be using their own imaging systems to evaluate their nevi and skin growths, and perhaps to diagnose and manage their rashes?
Will we have prevented our inflammatory skin disorders, or “turned them off” in early life with aggressive therapy in infantile life?
I project only that all of us in dermatology will still be a resource to our pediatric patients, from neonate through young adult, through our work of preventing, caring, healing and minimizing disease impact, and hopefully enjoying the pleasures of seeing our patients healthfully develop and evolve! As will our field.
Dr. Eichenfield is professor of dermatology and pediatrics and vice-chair of the department of dermatology at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. Dr. Eichenfield reports financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including products for the diseases discussed here.
As part of the 50th anniversary of Dermatology News, it is intriguing to think about where a time machine journey 5 decades back would find the field of pediatric dermatology, and to assess the changes in the specialty during the time that Dermatology News (operating then as “Skin & Allergy News”) has been reporting on innovations and changes in the practice of dermatology.
So, starting . It was not until 3 years later, in October 1973 in Mexico City, that the first international symposium on Pediatric Dermatology was held, and the International Society for Pediatric Dermatology was founded. I reached out to Andrew Margileth, MD, 100 years old this past July, and still active voluntary faculty in pediatric dermatology at the University of Miami, to help me “reach back” to those days. Dr. Margileth commented on how the first symposium was “brilliantly orchestrated by Ramon Ruiz-Maldonado,” from the National Institute of Paediatrics in Mexico, and that it was his “Aha moment for future practice!” That meeting spurred discussions on the development of the Society for Pediatric Dermatology the next year, with Alvin Jacobs, MD; Samuel Weinberg, MD; Nancy Esterly, MD; Sidney Hurwitz, MD; William Weston, MD; and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers,” and the society was officially established in 1975.
The field of pediatric dermatology was fairly “infantile” 50 years ago, with few practitioners. But the early leaders in the field recognized that up to 30% of pediatric primary care visits included skin problems, and that there was limited training for dermatologists, as well as pediatricians, about skin diseases in children. There were clearly clinical and educational needs to establish a subspecialty of pediatric dermatology, and over the next 1-2 decades, the field expanded. The journal Pediatric Dermatology was established (in 1982), the Section on Dermatology was established by the American Academy of Pediatrics (in 1986), and fellowship programs were launched at select academic centers. And it was 30 years into our timeline before the formal subspecialty of pediatric dermatology was established through the American Board of Dermatology (2000).
The field of pediatric dermatology has evolved and matured rapidly. Standard reference textbooks have been developed in the United States and around the world (and of course, online). Pediatric dermatology is an essential part of the core curriculum for dermatologist trainees. Organizations promoting pediatric research have developed to influence basic, translational, and clinical research in conditions in neonates through adolescents, such as the Pediatric Dermatology Research Alliance (PeDRA). And meetings throughout the world now feature pediatric dermatology sessions and help to spread the advances in the diagnosis and management of pediatric skin disorders.
The practice of pediatric dermatology: How has it changed?
It is beyond the scope of this article to try to comprehensively review all of the changes in pediatric dermatology practice. But review of the evolution of a few disease states (choices influenced by my discussions with my 100-year old history guide, Dr. Margileth) displays examples of where we have been, and where we are going in our next 5, 10, or 50 years.
Hemangiomas and vascular malformations
Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that standard cutaneous hemangiomas had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of interest, the hallmark article’s first author was Dr. Margileth, published in 1965 in JAMA!.This was still at a time when the identification of hemangiomas of infancy (or “HOI” as we say in the trade) was confused with vascular malformations, and no one had recognized the distinct variant tumors such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, or hemangioendotheliomas. PHACE syndrome was not yet described (that was done in 1996 by Ilona Frieden, MD, and colleagues). And for a time, hemangiomas were treated with x-rays, before the negative impact of such radiation was acknowledged. It seems that, as a consequence of the use of x-ray therapy and as a backlash from the radiation therapy side effects and potential toxicities, even deforming and functionally significant lesions were “followed clinically” for natural involution, with a sensibility that doing nothing might be better than doing the wrong thing.
Over the next 15 years, the recognition of functionally significant hemangiomas, deformation associated with their proliferation, and the recognition of PHACE syndrome made hemangiomas of infancy an area of concern, with systemic steroids and occasionally chemotherapeutic agents (such as vincristine) being used for problematic lesions.
It has now been 12 years since the work of Christine Léauté-Labrèze, MD, et al., from the University of Bordeaux (France), led to the breakthrough of propranolol for hemangioma treatment, profoundly changing hemangioma management to an incredibly effective medical therapy extensively studied, tested in formal clinical trials, and approved by regulatory authorities. And how intriguing that this was pursued after the chance (but skilled) observation that a child who developed hypertension as a side effect of systemic steroids for nasal hemangioma treatment was prescribed propranolol for the hypertension and had his nasal hemangioma rapidly shrink, with a response superior and much quicker than that to corticosteroids.
The evolution of management of hemangiomas has another story within it, that of collaborative research. The Hemangioma Investigator Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development, and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas. Our knowledge of these lesions is now evidence based and broad, and the impact on care tremendous! The HIG has also influenced the practice of pediatricians and other specialists, including otorhinolaryngologists, hematologist/oncologists, and surgeons, is partnering with advocacy groups to support patients and families, and is helping guide patients and families to contribute to ongoing research.
Vascular malformations (VM) reflect an incredible change in our understanding of the developmental pathways and pathophysiology of blood vessel tumors, and, in fact, birthmarks other than vascular lesions! First, important work separated out hemangiomas of infancy and hemangiomalike tumors from vascular malformations, with the thought being that hemangiomas had a rapid growth phase, often arising from lesions that were minimally evident or not evident at birth, unlike malformations, which were “programing errors,” all present at birth and expected to be fairly static with proportionate growth over a lifetime. Approaches to vascular malformations were limited to sclerotherapy, laser, and/or surgery. While this general schema of classification is still useful, our sense of the “why and how” of vascular malformations is remarkably different. Vascular malformations – still usefully subdivided into capillary, lymphatic, venous arteriovenous, or mixed malformations – are mostly associated with inherited or somatic mutations. Mutations are most commonly found in two signal pathways: RAS/MAPK/ERK and PI3K/AKT/mTOR pathways, with specific sets of mutations seen in both localized and multifocal lesions, with or without overgrowth or other systemic anomalies. The discovery of specific mutations has led to the possibility of small-molecule inhibitors, many already existing as anticancer drugs, being utilized as targeted therapies for VM.
And similar advances in understanding of other birthmarks, with or without syndromic features, are being made steadily. The mutations in congenital melanocytic nevi, epidermal nevi, acquired tumors (pilomatricomas), and other lesions, along with steady epidemiologic, translational, and clinical work, evolves our knowledge and potential therapies.
Inflammatory skin disorders: Acne, psoriasis, and atopic dermatitis
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris is now recognized as much more common under age 12 than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later, with expert recommendations formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013. Over the past few years, there has been a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance. There are unanswered questions as we evolve our care: How will the new topical antiandrogens be used? Will spironolactone become part of hormonal therapy under age 18? Will the insights on certain strains of Cutibacterium acnes being associated with worse acne translate to microbiome or vaccine-based strategies?
Pediatric psoriasis has suffered, being “behind in the revolution” of biologic agents because of delayed approval of any biologic agent for treatment of pediatric psoriasis in the United States until just a few years ago, and lags behind Europe and elsewhere in the world by almost a decade. Only this year have we expanded beyond one biologic agent approved for under age 12 and two for ages 12 and older, with other approvals expected including interleukin (IL)-17 and IL-23 agents. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, with new recommendations on how to screen children with psoriasis, supplied first by PeDRA and then in the new American Academy of Dermatology-National Psoriasis Foundation pediatric psoriasis guidelines .
Pediatric atopic dermatitis (AD) is in its early years of revolution. In the 50-year period of our thought experiment, AD has increased in prevalence from 5% or less of the pediatric population to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, and management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their use of these useful agents. But newer studies are markedly reassuring about their safe use in children.
Steroid phobia, as well as concerns about potential side effects of the TCIs, has resulted in undertreatment of childhood AD. It is quite common to see multiple children during pediatric dermatology office hours with poorly controlled eczema, high body-surface areas of eczema, compromised sleep, secondary infections, and anxiety and depression, especially in our moderate to severe adolescents. The field is “hot” with new topical and systemic agents, including our few years’ experience with topical crisaborole, a phosphodiesterase (PDE)-4 inhibitor; and dupilumab, an IL-4-alpha blocker – the first biologic agent approved for AD and the first systemic agent (other than oral corticosteroids), just extended from 12 years to 6 years of age! As dupilumab gets studied for younger children, other biologics (including IL-13 and IL-31 blockers) are undertaking pediatric and/or adolescent trials, oral and topical JAK inhibitors are including adolescents in core clinical trials, and other novel topical agents are under study, including an aryl-hydrocarbon receptor–modulating agent and other PDE-4 inhibitors.
Procedural pediatric dermatology: From liquid nitrogen to laser, surgery, and multimodal skin care
The first generation of pediatric dermatologists were considered medical dermatologist specialists. The care of the conditions discussed above, as well as genodermatoses, diagnostic dilemmas, and management of dermatologic manifestations of systemic disease and other conditions, was the “bread and butter” of pediatric dermatology care. When I was in training, my mentor Paul Honig, MD, at the Children’s Hospital of Philadelphia had a procedure half-day each week, where he would care for a few patients who needed liquid nitrogen therapy for warts, or who needed biopsies. It was uncommon to have a large procedural/surgical part of pediatric dermatology practice. But this is now a routine part of many specialists in the field. How did this change occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser for treatment of port-wine birthmarks in children with minimal scarring, and a seminal article published in the New England Journal of Medicine in 1989. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists developed the exposure and skill to do this work. An added advantage was having the knowledge of how to handle children and adolescents in an age-appropriate manner, with consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota, for example), hair laser, and combinations of lasers, including fractionated CO2 technology, to treat hypertrophic, constrictive and/or deforming scars.
The future
What will pediatric dermatology be like in 10, 20, or 50 years?
I have not yet discussed some of the most challenging diseases in our field, including epidermolysis bullosa, ichthyosis, and neurocutaneous disorders and other genetic skin disorders that have an incredible impact on the lives of affected children and their families, with incredible morbidity and with many conditions that shorten lifespans. But these are the conditions where “the future is happening now,” and we are looking forward to our new gene therapy techniques helping to transform our care.
And other aspects of practice? Will we be doing a large percentage of practice over the phone (or whatever devices we have then – remember, the first iPhone was only released 13 years ago)?
Will our patients be using their own imaging systems to evaluate their nevi and skin growths, and perhaps to diagnose and manage their rashes?
Will we have prevented our inflammatory skin disorders, or “turned them off” in early life with aggressive therapy in infantile life?
I project only that all of us in dermatology will still be a resource to our pediatric patients, from neonate through young adult, through our work of preventing, caring, healing and minimizing disease impact, and hopefully enjoying the pleasures of seeing our patients healthfully develop and evolve! As will our field.
Dr. Eichenfield is professor of dermatology and pediatrics and vice-chair of the department of dermatology at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. Dr. Eichenfield reports financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including products for the diseases discussed here.
A teen girl presents with a pinkish-red bump on her right leg
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
Update on Pediatric Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Practice Points
- There has been tremendous growth in our understanding of atopic dermatitis, with further insight into epidemiology, the impact on quality of life of affected individuals and their families, best bathing practices, and expanding treatment options.
- There are several novel topical and systemic agents recently approved and in late-stage clinical development programs that are evolving therapeutic approaches to pediatric disease.
Four-year-old boy presents with itchy rash on face, extremities
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
A 4-year-old healthy male with no significant prior medical history presents for evaluation of "itchy bumps" on the face and extremities of 2 weeks' duration.
The child was well until around 2 and a half weeks ago when he presented for evaluation of two lesions on the lower eyelids, diagnosed as hordeolum (a stye). He was prescribed ofloxacin ophthalmic solution.
One week later he developed bilateral itchy red eyes with red, thickened areas on the upper lids, followed several days later by pruritic papules on the ears, wrists, elbows, knees, and ankles. His mother used Vaseline for the eyelids for 1 week with no improvement. Physical exam at the dermatologist's office showed mild erythema, induration, and lichenification of the upper eyelids, and bilateral periocular eczematous patches with overlying scale. Subtle papules were evident on the elbows and feet.
What's your diagnosis?
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
A 10-year-old, otherwise healthy female with no prior significant medical history is brought into clinic for evaluation of orange-red scaly papules and plaques that first started on the face, neck, and fingers and began spreading to the trunk, arms, and knees. The mother of the patient also had noticed thickening of the skin on her palms and soles. The rash has been present for 2 months. Patient does not appear to be itchy, and otherwise is in normal state without pain, fever, drainage from sites, or known exposures. She was initially treated with topical triamcinolone with minimal improvement.
On physical exam, she is noted to have reddish-orange hyperkeratotic scaling papules coalescing into large plaques with follicular prominence diffusely on the face, neck, trunk, and upper extremities with smaller islands of skin that are normal-appearing. There is diffuse fine scale throughout the scalp and thickening of the skin on the palms and soles with a yellowish waxy appearance.
A 7-month-old male presents with perioral rash and fever
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Rash on hands and feet
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
A 9-year-old healthy Kuwaiti male with no significant past medical history presents with a rash on his hands and feet that has been present for 3 years.
His mother reports that he has been seen by dermatologists in various countries and was last seen by a dermatologist in Kuwait 3 years ago. At that time, he was told that it was dryness and advised to not shower daily. Since then he has been taking showers three times weekly and using Cetaphil once weekly without improvement. He was seen by his pediatrician 6 months ago, diagnosed with xerosis, and was given hydrocortisone 2.5% to use twice daily, again without any improvement.
The rash is not itchy, and he has no oral lesions or nail involvement. Exam revealed lichenoid papules on bilateral dorsal hands and feet, bilateral upper arms, bilateral axilla, lower abdomen, and left upper chest.