User login
IPF Patient Registry will expand
The number of patients enrolled in the Idiopathic Pulmonary Fibrosis–Prospective Outcomes (IPF-PRO) Registry will be increased to 1,500, Boehringer Ingelheim Pharmaceuticals and the Duke Clinical Research Institute have announced.
The organizations plan to accomplish this goal by increasing the number of sites they use to gather IPF patient data, according to a statement; the patients enrolled in the registry will now come from 45 sites instead of 18 sites.
IPF-PRO, which was launched in June 2014, is the first multicenter longitudinal disease state registry in the United States focused specifically on IPF. It was designed for the purpose of studying the progression of IPF and the effectiveness of various treatment approaches for the disease. The registry includes a biorepository that stores blood samples that provide patient genetic material.
“In collecting data from a larger, more diverse group of patients ... this registry will allow us to better assess the impact of the disease over time on clinical and patient-centered outcomes,” said Scott M. Palmer, MD, director of pulmonary research at the Duke Clinical Research Institute, Durham, N.C., in the statement.
More information on the registry is available at clinicaltrials.gov/ct2/show/NCT01915511.
The number of patients enrolled in the Idiopathic Pulmonary Fibrosis–Prospective Outcomes (IPF-PRO) Registry will be increased to 1,500, Boehringer Ingelheim Pharmaceuticals and the Duke Clinical Research Institute have announced.
The organizations plan to accomplish this goal by increasing the number of sites they use to gather IPF patient data, according to a statement; the patients enrolled in the registry will now come from 45 sites instead of 18 sites.
IPF-PRO, which was launched in June 2014, is the first multicenter longitudinal disease state registry in the United States focused specifically on IPF. It was designed for the purpose of studying the progression of IPF and the effectiveness of various treatment approaches for the disease. The registry includes a biorepository that stores blood samples that provide patient genetic material.
“In collecting data from a larger, more diverse group of patients ... this registry will allow us to better assess the impact of the disease over time on clinical and patient-centered outcomes,” said Scott M. Palmer, MD, director of pulmonary research at the Duke Clinical Research Institute, Durham, N.C., in the statement.
More information on the registry is available at clinicaltrials.gov/ct2/show/NCT01915511.
The number of patients enrolled in the Idiopathic Pulmonary Fibrosis–Prospective Outcomes (IPF-PRO) Registry will be increased to 1,500, Boehringer Ingelheim Pharmaceuticals and the Duke Clinical Research Institute have announced.
The organizations plan to accomplish this goal by increasing the number of sites they use to gather IPF patient data, according to a statement; the patients enrolled in the registry will now come from 45 sites instead of 18 sites.
IPF-PRO, which was launched in June 2014, is the first multicenter longitudinal disease state registry in the United States focused specifically on IPF. It was designed for the purpose of studying the progression of IPF and the effectiveness of various treatment approaches for the disease. The registry includes a biorepository that stores blood samples that provide patient genetic material.
“In collecting data from a larger, more diverse group of patients ... this registry will allow us to better assess the impact of the disease over time on clinical and patient-centered outcomes,” said Scott M. Palmer, MD, director of pulmonary research at the Duke Clinical Research Institute, Durham, N.C., in the statement.
More information on the registry is available at clinicaltrials.gov/ct2/show/NCT01915511.
Teams boost confidence in IPF diagnoses
The accuracy of idiopathic pulmonary fibrosis (IPF) diagnoses is improving with the use of multidisciplinary team meetings and updated guidelines, based on the findings of a study that compared diagnostic agreement of individual clinicians and teams evaluating patients with interstitial lung disease.
Pulmonologists who participate in multidisciplinary team meetings said the findings validate the team approach.
“The [study’s] data confirm what we see in clinical practice ... it takes a multidisciplinary – and perhaps often multiple pulmonologists – to review these cases,” Marilyn K. Glassberg, MD,professor of medicine and surgery and director of the interstitial lung disease program at the University of Miami Health System, said in an interview.“This study demonstrates the importance of multiple perspectives when evaluating a patient and coming to a diagnosis at a time when reliable biomarkers are not available.”
The study, published in The Lancet Respiratory Medicine (2016;4[7]:557-65), is the first evaluation of multidisciplinary team agreement on diagnosis of interstitial lung disease since updated guidelines were published, according to Simon L. F. Walsh, MD, of Kings College Hospital NHS Foundation Trust, London, and his colleagues.
In 2015, the American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and Latin American Thoracic Association (ALTA) adopted joint guidelines for the treatment of IPF. In 2013, the ATS and ERS updated guidelines for the classification and terminology for idiopathic interstitial pneumonias.
“Our study shows ... in [IPF], MDTMs (multidisciplinary team meetings) have a higher level of agreement on diagnoses, assign diagnoses with higher confidence more frequently, and provide diagnoses that have non-significant greater prognostic separation than do clinicians or radiologists in most cases,” the researchers wrote.
Before MDTMs were initiated, the clinicians, radiologists, and pathologists who would be participating in them independently reviewed each patient’s case without consulting other specialists and provided up to five diagnoses with diagnostic likelihoods for each patient.
For the study, 70 patients were evaluated and the level of diagnostic agreement was assessed at seven international centers for the diagnosis of interstitial lung disease (diffuse parenchymal lung disease). Following independent reviews of the 70 cases, the clinician, radiologist, and pathologist from each center met as a multidisciplinary team to review the same cases together and give up to five diagnoses with diagnostic likelihoods.
All clinical information supplied in the first stage of the study, including pulmonary function test results, high-resolution CT at presentation, and digitalized surgical lung biopsy slides, were available to the multidisciplinary team. The patients’ outcomes were used to validate the diagnoses. The survival period for each patient was calculated based on the date of referral to the host institution to the minimum date of death, date patient was last known to be alive, or June 1, 2015 – the end of the study period.
The inter-MDTM agreement was better than interobserver agreement for all diagnoses (unweighted kappa value (K) = 0.50), and inter-MDTM agreement was highest for IPF (K = 0.60) and connective tissue disease-related interstitial lung disease (K = 0.64).
“We have shown an acceptable level [based on a K of greater than 0.40 being deemed clinically acceptable] of diagnostic agreement exists between multidisciplinary teams in the setting of diffuse parenchymal lung disease. Additionally, we showed that this agreement was validated by the nonsignificant increases toward greater prognostic separation of an IPF diagnosis made by multidisciplinary teams than by individual clinicians or radiologists,” the researchers wrote.
The weighted kappa (KW) values for estimation of diagnostic likelihood for diagnoses of IPF were 0.72 (0.67-0.76) for clinicians, 0.60 (0.46-0.66) for radiologists, 0.58 (0.45-0.66) for pathologists and 0.71 (0.64-0.77) for MDTMs.
For connective tissue disease–related interstitial lung diseases, the KW for estimation of diagnostic likelihood for diagnoses for MDTMs were 0.73 (0.68-0.78), compared with 0.76 (0.70-0.78) for clinicians, 0.17 (0.08-0.31) for radiologists, and 0.21 (0.06-0.36) for pathologists.
Krishna Thavarajah, MD,, who sees patients with interstitial lung disease within the Henry Ford Health System in Detroit, has been participating in MDTMs for nearly 6 years.
“The accuracies of diagnoses for patients with IPF are much better than even 10 years ago,” she said in an interview. “I think this is because of the improvement in consistency in diagnostic criteria based on the updated guidelines in IPF. Among the MDTMs that participated in the study, the agreement about diagnoses was highest for IPF. The interobserver agreement for clinicians was also pretty high for IPF.”
In her work within an academic center, Dr. Glassberg sees patients in an IPF clinic and in a separate autoimmune disorders clinic. For each clinic, there is a multidisciplinary team. In the IPF clinic, there are three pulmonologists and a radiologist, and when there is a biopsy, there are two pathologists. Dr. Glassberg’s IPF team also includes four pulmonary radiologists.
During her MDTMs, Dr. Thavarajah, a radiologist, and a pathologist will examine a patient’s chest imaging and pathology slides. They sit together until they become confident of their diagnosis in the absence of a biopsy.
There are times when the team tells a patient the probable diagnosis and acknowledges the small chance of an alternative diagnosis. “It was comforting to me that, in the Lancet study, there was a good level of agreement in diagnosis of IPF among multidisciplinary teams, whether the patients had undergone a biopsy or not,” said Dr. Thavarajah. “The mortality of patients given a diagnosis of IPF was worse than those given a diagnosis of non-IPF to validate the IPF diagnosis.”
Establishing and implementing MDTMs is challenging, though, said Dr. Glassberg.
“[We] need to address how multidisciplinary teams could work for doctors who are in smaller cities or who are not in academic centers. We need to utilize existing channels to create new avenues for these colleagues to present their cases – particularly challenging ones or patients who need to be referred – to be evaluated by an interdisciplinary team. The Internet may offer these opportunities for networking and decision making, said Dr. Glassberg.
The study was funded by the National Institute of Health Research, Imperial College London. Several of the study’s authors declared receiving personal fees, grants, or research support from a variety of sources, but had no financial disclosures relevant to this study.
Daniel R. Ouellette, MD, FCCP, comments: “Recommendations have been that multidisciplinary teams add to the accuracy of the diagnosis of IPF. The value of this study is that it provides objective data that this is so.”
Daniel R. Ouellette, MD, FCCP, comments: “Recommendations have been that multidisciplinary teams add to the accuracy of the diagnosis of IPF. The value of this study is that it provides objective data that this is so.”
Daniel R. Ouellette, MD, FCCP, comments: “Recommendations have been that multidisciplinary teams add to the accuracy of the diagnosis of IPF. The value of this study is that it provides objective data that this is so.”
The accuracy of idiopathic pulmonary fibrosis (IPF) diagnoses is improving with the use of multidisciplinary team meetings and updated guidelines, based on the findings of a study that compared diagnostic agreement of individual clinicians and teams evaluating patients with interstitial lung disease.
Pulmonologists who participate in multidisciplinary team meetings said the findings validate the team approach.
“The [study’s] data confirm what we see in clinical practice ... it takes a multidisciplinary – and perhaps often multiple pulmonologists – to review these cases,” Marilyn K. Glassberg, MD,professor of medicine and surgery and director of the interstitial lung disease program at the University of Miami Health System, said in an interview.“This study demonstrates the importance of multiple perspectives when evaluating a patient and coming to a diagnosis at a time when reliable biomarkers are not available.”
The study, published in The Lancet Respiratory Medicine (2016;4[7]:557-65), is the first evaluation of multidisciplinary team agreement on diagnosis of interstitial lung disease since updated guidelines were published, according to Simon L. F. Walsh, MD, of Kings College Hospital NHS Foundation Trust, London, and his colleagues.
In 2015, the American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and Latin American Thoracic Association (ALTA) adopted joint guidelines for the treatment of IPF. In 2013, the ATS and ERS updated guidelines for the classification and terminology for idiopathic interstitial pneumonias.
“Our study shows ... in [IPF], MDTMs (multidisciplinary team meetings) have a higher level of agreement on diagnoses, assign diagnoses with higher confidence more frequently, and provide diagnoses that have non-significant greater prognostic separation than do clinicians or radiologists in most cases,” the researchers wrote.
Before MDTMs were initiated, the clinicians, radiologists, and pathologists who would be participating in them independently reviewed each patient’s case without consulting other specialists and provided up to five diagnoses with diagnostic likelihoods for each patient.
For the study, 70 patients were evaluated and the level of diagnostic agreement was assessed at seven international centers for the diagnosis of interstitial lung disease (diffuse parenchymal lung disease). Following independent reviews of the 70 cases, the clinician, radiologist, and pathologist from each center met as a multidisciplinary team to review the same cases together and give up to five diagnoses with diagnostic likelihoods.
All clinical information supplied in the first stage of the study, including pulmonary function test results, high-resolution CT at presentation, and digitalized surgical lung biopsy slides, were available to the multidisciplinary team. The patients’ outcomes were used to validate the diagnoses. The survival period for each patient was calculated based on the date of referral to the host institution to the minimum date of death, date patient was last known to be alive, or June 1, 2015 – the end of the study period.
The inter-MDTM agreement was better than interobserver agreement for all diagnoses (unweighted kappa value (K) = 0.50), and inter-MDTM agreement was highest for IPF (K = 0.60) and connective tissue disease-related interstitial lung disease (K = 0.64).
“We have shown an acceptable level [based on a K of greater than 0.40 being deemed clinically acceptable] of diagnostic agreement exists between multidisciplinary teams in the setting of diffuse parenchymal lung disease. Additionally, we showed that this agreement was validated by the nonsignificant increases toward greater prognostic separation of an IPF diagnosis made by multidisciplinary teams than by individual clinicians or radiologists,” the researchers wrote.
The weighted kappa (KW) values for estimation of diagnostic likelihood for diagnoses of IPF were 0.72 (0.67-0.76) for clinicians, 0.60 (0.46-0.66) for radiologists, 0.58 (0.45-0.66) for pathologists and 0.71 (0.64-0.77) for MDTMs.
For connective tissue disease–related interstitial lung diseases, the KW for estimation of diagnostic likelihood for diagnoses for MDTMs were 0.73 (0.68-0.78), compared with 0.76 (0.70-0.78) for clinicians, 0.17 (0.08-0.31) for radiologists, and 0.21 (0.06-0.36) for pathologists.
Krishna Thavarajah, MD,, who sees patients with interstitial lung disease within the Henry Ford Health System in Detroit, has been participating in MDTMs for nearly 6 years.
“The accuracies of diagnoses for patients with IPF are much better than even 10 years ago,” she said in an interview. “I think this is because of the improvement in consistency in diagnostic criteria based on the updated guidelines in IPF. Among the MDTMs that participated in the study, the agreement about diagnoses was highest for IPF. The interobserver agreement for clinicians was also pretty high for IPF.”
In her work within an academic center, Dr. Glassberg sees patients in an IPF clinic and in a separate autoimmune disorders clinic. For each clinic, there is a multidisciplinary team. In the IPF clinic, there are three pulmonologists and a radiologist, and when there is a biopsy, there are two pathologists. Dr. Glassberg’s IPF team also includes four pulmonary radiologists.
During her MDTMs, Dr. Thavarajah, a radiologist, and a pathologist will examine a patient’s chest imaging and pathology slides. They sit together until they become confident of their diagnosis in the absence of a biopsy.
There are times when the team tells a patient the probable diagnosis and acknowledges the small chance of an alternative diagnosis. “It was comforting to me that, in the Lancet study, there was a good level of agreement in diagnosis of IPF among multidisciplinary teams, whether the patients had undergone a biopsy or not,” said Dr. Thavarajah. “The mortality of patients given a diagnosis of IPF was worse than those given a diagnosis of non-IPF to validate the IPF diagnosis.”
Establishing and implementing MDTMs is challenging, though, said Dr. Glassberg.
“[We] need to address how multidisciplinary teams could work for doctors who are in smaller cities or who are not in academic centers. We need to utilize existing channels to create new avenues for these colleagues to present their cases – particularly challenging ones or patients who need to be referred – to be evaluated by an interdisciplinary team. The Internet may offer these opportunities for networking and decision making, said Dr. Glassberg.
The study was funded by the National Institute of Health Research, Imperial College London. Several of the study’s authors declared receiving personal fees, grants, or research support from a variety of sources, but had no financial disclosures relevant to this study.
The accuracy of idiopathic pulmonary fibrosis (IPF) diagnoses is improving with the use of multidisciplinary team meetings and updated guidelines, based on the findings of a study that compared diagnostic agreement of individual clinicians and teams evaluating patients with interstitial lung disease.
Pulmonologists who participate in multidisciplinary team meetings said the findings validate the team approach.
“The [study’s] data confirm what we see in clinical practice ... it takes a multidisciplinary – and perhaps often multiple pulmonologists – to review these cases,” Marilyn K. Glassberg, MD,professor of medicine and surgery and director of the interstitial lung disease program at the University of Miami Health System, said in an interview.“This study demonstrates the importance of multiple perspectives when evaluating a patient and coming to a diagnosis at a time when reliable biomarkers are not available.”
The study, published in The Lancet Respiratory Medicine (2016;4[7]:557-65), is the first evaluation of multidisciplinary team agreement on diagnosis of interstitial lung disease since updated guidelines were published, according to Simon L. F. Walsh, MD, of Kings College Hospital NHS Foundation Trust, London, and his colleagues.
In 2015, the American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and Latin American Thoracic Association (ALTA) adopted joint guidelines for the treatment of IPF. In 2013, the ATS and ERS updated guidelines for the classification and terminology for idiopathic interstitial pneumonias.
“Our study shows ... in [IPF], MDTMs (multidisciplinary team meetings) have a higher level of agreement on diagnoses, assign diagnoses with higher confidence more frequently, and provide diagnoses that have non-significant greater prognostic separation than do clinicians or radiologists in most cases,” the researchers wrote.
Before MDTMs were initiated, the clinicians, radiologists, and pathologists who would be participating in them independently reviewed each patient’s case without consulting other specialists and provided up to five diagnoses with diagnostic likelihoods for each patient.
For the study, 70 patients were evaluated and the level of diagnostic agreement was assessed at seven international centers for the diagnosis of interstitial lung disease (diffuse parenchymal lung disease). Following independent reviews of the 70 cases, the clinician, radiologist, and pathologist from each center met as a multidisciplinary team to review the same cases together and give up to five diagnoses with diagnostic likelihoods.
All clinical information supplied in the first stage of the study, including pulmonary function test results, high-resolution CT at presentation, and digitalized surgical lung biopsy slides, were available to the multidisciplinary team. The patients’ outcomes were used to validate the diagnoses. The survival period for each patient was calculated based on the date of referral to the host institution to the minimum date of death, date patient was last known to be alive, or June 1, 2015 – the end of the study period.
The inter-MDTM agreement was better than interobserver agreement for all diagnoses (unweighted kappa value (K) = 0.50), and inter-MDTM agreement was highest for IPF (K = 0.60) and connective tissue disease-related interstitial lung disease (K = 0.64).
“We have shown an acceptable level [based on a K of greater than 0.40 being deemed clinically acceptable] of diagnostic agreement exists between multidisciplinary teams in the setting of diffuse parenchymal lung disease. Additionally, we showed that this agreement was validated by the nonsignificant increases toward greater prognostic separation of an IPF diagnosis made by multidisciplinary teams than by individual clinicians or radiologists,” the researchers wrote.
The weighted kappa (KW) values for estimation of diagnostic likelihood for diagnoses of IPF were 0.72 (0.67-0.76) for clinicians, 0.60 (0.46-0.66) for radiologists, 0.58 (0.45-0.66) for pathologists and 0.71 (0.64-0.77) for MDTMs.
For connective tissue disease–related interstitial lung diseases, the KW for estimation of diagnostic likelihood for diagnoses for MDTMs were 0.73 (0.68-0.78), compared with 0.76 (0.70-0.78) for clinicians, 0.17 (0.08-0.31) for radiologists, and 0.21 (0.06-0.36) for pathologists.
Krishna Thavarajah, MD,, who sees patients with interstitial lung disease within the Henry Ford Health System in Detroit, has been participating in MDTMs for nearly 6 years.
“The accuracies of diagnoses for patients with IPF are much better than even 10 years ago,” she said in an interview. “I think this is because of the improvement in consistency in diagnostic criteria based on the updated guidelines in IPF. Among the MDTMs that participated in the study, the agreement about diagnoses was highest for IPF. The interobserver agreement for clinicians was also pretty high for IPF.”
In her work within an academic center, Dr. Glassberg sees patients in an IPF clinic and in a separate autoimmune disorders clinic. For each clinic, there is a multidisciplinary team. In the IPF clinic, there are three pulmonologists and a radiologist, and when there is a biopsy, there are two pathologists. Dr. Glassberg’s IPF team also includes four pulmonary radiologists.
During her MDTMs, Dr. Thavarajah, a radiologist, and a pathologist will examine a patient’s chest imaging and pathology slides. They sit together until they become confident of their diagnosis in the absence of a biopsy.
There are times when the team tells a patient the probable diagnosis and acknowledges the small chance of an alternative diagnosis. “It was comforting to me that, in the Lancet study, there was a good level of agreement in diagnosis of IPF among multidisciplinary teams, whether the patients had undergone a biopsy or not,” said Dr. Thavarajah. “The mortality of patients given a diagnosis of IPF was worse than those given a diagnosis of non-IPF to validate the IPF diagnosis.”
Establishing and implementing MDTMs is challenging, though, said Dr. Glassberg.
“[We] need to address how multidisciplinary teams could work for doctors who are in smaller cities or who are not in academic centers. We need to utilize existing channels to create new avenues for these colleagues to present their cases – particularly challenging ones or patients who need to be referred – to be evaluated by an interdisciplinary team. The Internet may offer these opportunities for networking and decision making, said Dr. Glassberg.
The study was funded by the National Institute of Health Research, Imperial College London. Several of the study’s authors declared receiving personal fees, grants, or research support from a variety of sources, but had no financial disclosures relevant to this study.
USPSTF opposes screening for obstructive sleep apnea in draft recommendation
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
 |
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.
 |
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.
 |
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
Diffuse alveolar damage ups death risk in acute respiratory distress syndrome
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
FROM CHEST
Key clinical point: Among patients with ARDS, those who are also diagnosed with DAD faced nearly twice as high a mortality risk as did those without DAD.
Major finding: The pooled odds ratio for mortality in patients who had acute respiratory distress syndrome with diffuse alveolar damage, compared with patients with ARDs who did not have DAD was 1.81.
Data source: A systematic review and meta-analysis of eight studies, including 350 patients, published between 1967 and 2015.
Disclosures: No financial or nonfinancial disclosures were declared.
Respiratory exacerbations rate high in patients not meeting COPD criteria
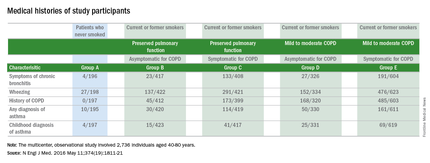
The clinical definition of chronic obstructive pulmonary disease (COPD) may need to be revised, based on results from a multicenter observational study of 2,736 individuals.
Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD. The 425 study participants who were symptomatic for COPD but were considered to have preserved pulmonary function had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).
Using spirometry to define who should receive a diagnosis of COPD does not address all people with symptomatic smoking-related lung disease. This large population needs to be studied to better define appropriate treatment strategies, Dr. Prescott G. Woodruff, a professor of medicine at the University of California, San Francisco, and his associates noted (N Engl J Med. 2016 May 11;[19]374:1811-21.).
Study participants were classified as not having COPD if the ratios of their forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was 0.70 or more after bronchodilator use and if their FVC was above the lower limit of the normal range. During a stable phase of disease, which was defined as greater than six weeks after a respiratory exacerbation, patients participated in the COPD Assessment Test (CAT), an eight-question health status instrument. Patients with a CAT score of greater than or equal to 10 were considered to be symptomatic for COPD and those with CAT scores of less than 10 were considered to be asymptomatic for COPD.
While 963 of the 1,812 study participants who were current or former smokers were classified as having Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 or 2 COPD, half of the current or former smokers who were not classified as having COPD were still symptomatic for the disease. Additionally, among the 199 study participants who had never smoked, 16% had COPD symptoms.
Current or former smokers classified as not having COPD, who were symptomatic for the disease, had elevations in all components of the CAT score, were younger, were more likely to be nonwhite or Hispanic, and had a higher body mass index than those with a CAT score of less than 10. These patients with preserved pulmonary function despite being symptomatic for COPD also were more likely to be current smokers, report symptoms of chronic bronchitis, report a history of wheezing and asthma, and report a previous diagnosis of COPD, compared with the asymptomatic patients with preserved pulmonary function.
From 2010 through 2015, 2,736 individuals, aged 40-80 years, were enrolled in the study. The study participants had either never smoked (defined as having smoked less than or equal to 1 pack-year of tobacco use) or were current or former smokers with a smoking history of more than 20 pack-years. Spirometry was used to determine if study participants had COPD. This was performed before and after four inhalations each of albuterol, at a dose of 90 mcg per inhalation, and ipratropium, at a dose of 18 mcg per inhalation.
Among the other data obtained by the researchers were the patients’ 6-minute walk distances. The average 6-minute walk distance was shorter for patients with symptoms of COPD, compared with those with CAT scores of less than 10.
This study shows that the CAT can identify smokers at risk for exacerbations and provides evidence that individuals with COPD symptoms may have airway disease, according to the researchers.
“Clinical trials are needed to determine whether maintenance therapy with bronchodilators or inhaled glucocorticoids will alleviate symptoms and reduce the rate of respiratory exacerbations in [current or former smokers with elevated respiratory exacerbation rates who do not meet the standard criteria for having COPD],” the researchers said.
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
It is often symptoms that bring patients to seek hospital care. This study points out a split between smoking-related respiratory symptoms and the preserved spirometry that was found in a portion of the study group. Further research may better characterize this population of symptomatic current and former smokers. With identification of effective treatments and improved symptom management, we may be able to promote better respiratory health, thereby reducing hospital utilization.
Dr. Vera De Palo is Editor in Chief of CHEST Physician. She is the Chief Medical Officer at Signature Healthcare in Brockton, Massachusetts.
It is often symptoms that bring patients to seek hospital care. This study points out a split between smoking-related respiratory symptoms and the preserved spirometry that was found in a portion of the study group. Further research may better characterize this population of symptomatic current and former smokers. With identification of effective treatments and improved symptom management, we may be able to promote better respiratory health, thereby reducing hospital utilization.
Dr. Vera De Palo is Editor in Chief of CHEST Physician. She is the Chief Medical Officer at Signature Healthcare in Brockton, Massachusetts.
It is often symptoms that bring patients to seek hospital care. This study points out a split between smoking-related respiratory symptoms and the preserved spirometry that was found in a portion of the study group. Further research may better characterize this population of symptomatic current and former smokers. With identification of effective treatments and improved symptom management, we may be able to promote better respiratory health, thereby reducing hospital utilization.
Dr. Vera De Palo is Editor in Chief of CHEST Physician. She is the Chief Medical Officer at Signature Healthcare in Brockton, Massachusetts.
The clinical definition of chronic obstructive pulmonary disease (COPD) may need to be revised, based on results from a multicenter observational study of 2,736 individuals.
Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD. The 425 study participants who were symptomatic for COPD but were considered to have preserved pulmonary function had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).

Using spirometry to define who should receive a diagnosis of COPD does not address all people with symptomatic smoking-related lung disease. This large population needs to be studied to better define appropriate treatment strategies, Dr. Prescott G. Woodruff, a professor of medicine at the University of California, San Francisco, and his associates noted (N Engl J Med. 2016 May 11;[19]374:1811-21.).
Study participants were classified as not having COPD if the ratios of their forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was 0.70 or more after bronchodilator use and if their FVC was above the lower limit of the normal range. During a stable phase of disease, which was defined as greater than six weeks after a respiratory exacerbation, patients participated in the COPD Assessment Test (CAT), an eight-question health status instrument. Patients with a CAT score of greater than or equal to 10 were considered to be symptomatic for COPD and those with CAT scores of less than 10 were considered to be asymptomatic for COPD.
While 963 of the 1,812 study participants who were current or former smokers were classified as having Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 or 2 COPD, half of the current or former smokers who were not classified as having COPD were still symptomatic for the disease. Additionally, among the 199 study participants who had never smoked, 16% had COPD symptoms.
Current or former smokers classified as not having COPD, who were symptomatic for the disease, had elevations in all components of the CAT score, were younger, were more likely to be nonwhite or Hispanic, and had a higher body mass index than those with a CAT score of less than 10. These patients with preserved pulmonary function despite being symptomatic for COPD also were more likely to be current smokers, report symptoms of chronic bronchitis, report a history of wheezing and asthma, and report a previous diagnosis of COPD, compared with the asymptomatic patients with preserved pulmonary function.
From 2010 through 2015, 2,736 individuals, aged 40-80 years, were enrolled in the study. The study participants had either never smoked (defined as having smoked less than or equal to 1 pack-year of tobacco use) or were current or former smokers with a smoking history of more than 20 pack-years. Spirometry was used to determine if study participants had COPD. This was performed before and after four inhalations each of albuterol, at a dose of 90 mcg per inhalation, and ipratropium, at a dose of 18 mcg per inhalation.
Among the other data obtained by the researchers were the patients’ 6-minute walk distances. The average 6-minute walk distance was shorter for patients with symptoms of COPD, compared with those with CAT scores of less than 10.
This study shows that the CAT can identify smokers at risk for exacerbations and provides evidence that individuals with COPD symptoms may have airway disease, according to the researchers.
“Clinical trials are needed to determine whether maintenance therapy with bronchodilators or inhaled glucocorticoids will alleviate symptoms and reduce the rate of respiratory exacerbations in [current or former smokers with elevated respiratory exacerbation rates who do not meet the standard criteria for having COPD],” the researchers said.
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
The clinical definition of chronic obstructive pulmonary disease (COPD) may need to be revised, based on results from a multicenter observational study of 2,736 individuals.
Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD. The 425 study participants who were symptomatic for COPD but were considered to have preserved pulmonary function had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).

Using spirometry to define who should receive a diagnosis of COPD does not address all people with symptomatic smoking-related lung disease. This large population needs to be studied to better define appropriate treatment strategies, Dr. Prescott G. Woodruff, a professor of medicine at the University of California, San Francisco, and his associates noted (N Engl J Med. 2016 May 11;[19]374:1811-21.).
Study participants were classified as not having COPD if the ratios of their forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was 0.70 or more after bronchodilator use and if their FVC was above the lower limit of the normal range. During a stable phase of disease, which was defined as greater than six weeks after a respiratory exacerbation, patients participated in the COPD Assessment Test (CAT), an eight-question health status instrument. Patients with a CAT score of greater than or equal to 10 were considered to be symptomatic for COPD and those with CAT scores of less than 10 were considered to be asymptomatic for COPD.
While 963 of the 1,812 study participants who were current or former smokers were classified as having Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 or 2 COPD, half of the current or former smokers who were not classified as having COPD were still symptomatic for the disease. Additionally, among the 199 study participants who had never smoked, 16% had COPD symptoms.
Current or former smokers classified as not having COPD, who were symptomatic for the disease, had elevations in all components of the CAT score, were younger, were more likely to be nonwhite or Hispanic, and had a higher body mass index than those with a CAT score of less than 10. These patients with preserved pulmonary function despite being symptomatic for COPD also were more likely to be current smokers, report symptoms of chronic bronchitis, report a history of wheezing and asthma, and report a previous diagnosis of COPD, compared with the asymptomatic patients with preserved pulmonary function.
From 2010 through 2015, 2,736 individuals, aged 40-80 years, were enrolled in the study. The study participants had either never smoked (defined as having smoked less than or equal to 1 pack-year of tobacco use) or were current or former smokers with a smoking history of more than 20 pack-years. Spirometry was used to determine if study participants had COPD. This was performed before and after four inhalations each of albuterol, at a dose of 90 mcg per inhalation, and ipratropium, at a dose of 18 mcg per inhalation.
Among the other data obtained by the researchers were the patients’ 6-minute walk distances. The average 6-minute walk distance was shorter for patients with symptoms of COPD, compared with those with CAT scores of less than 10.
This study shows that the CAT can identify smokers at risk for exacerbations and provides evidence that individuals with COPD symptoms may have airway disease, according to the researchers.
“Clinical trials are needed to determine whether maintenance therapy with bronchodilators or inhaled glucocorticoids will alleviate symptoms and reduce the rate of respiratory exacerbations in [current or former smokers with elevated respiratory exacerbation rates who do not meet the standard criteria for having COPD],” the researchers said.
This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Respiratory symptoms of COPD were present in 425 of 849 current or former smokers who did not meet the standard spirometry criteria for diagnosing COPD.
Major finding: The 425 study participants who were symptomatic for COPD but did not meet the standard spirometry criteria for diagnosing COPD had a significantly higher rate of respiratory exacerbations, compared with that of the 424 current or former smokers who were asymptomatic for COPD and were not classified as having the disease (0.27 +/- 0.67 events vs. 0.08 +/- 0.31 events; P less than .001).
Data source: Results are from a multicenter observational study that enrolled 2,736 individuals from 2010 to 2015.
Disclosures: This study was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by the Foundation for the National Institutes of Health through contributions made to an external advisory board composed of members from several pharmaceutical companies. The authors’ disclosures are available at NEJM.org.
Yoga Improves Asthmatics’ Quality of Life, Data Review Suggests
Yoga seems to improve the quality of life and symptoms of people with asthma, suggests a review of 15 randomized controlled trials comprising 1,048 patients with varying degrees of asthma severity.
The studies generally compared the outcomes for asthma patients participating in at least 2 weeks of yoga with the outcomes for those who were treated with usual care for asthma, a sham intervention, or no intervention.
Average improvements in the Asthma Quality of Life Questionnaire scores of 0.57 units per item on a 7-point scale were found through an analysis of responses from 375 individuals, with each person having participated in one of the five randomized controlled trials (RCTs). While the average increase exceeded the minimal clinically important difference (MCID) for this questionnaire, outcomes of two of the trials raise questions about whether the reported improvements in patients’ quality of life can be attributed to yoga. In those two trials, which included a placebo or sham intervention for some of the participants, no differences in these questionnaire scores were found following the interventions.
For 243 asthma patients who participated in three of the RCTs, on average, yoga improved their symptoms by 0.37 standard deviation units of the disease severity scores used.
“Our findings are preliminary and suggestive, rather than conclusive, and therefore should be interpreted cautiously. Yoga probably improves quality of life and symptoms in people with asthma to some extent. However, whether or not the improvements in symptoms exceed the MCID is uncertain due to lack of an established MCID for the severity scores used in the included studies,” noted Zu-Yao Yang of the Chinese University of Hong Kong, and colleagues.
They used various methods to collect data, including searching the Cochrane Airways Group Register of Trials, which is derived from systematic searches of bibliographic databases, and hand-searching respiratory journals and meeting abstracts. They searched all databases from their inception to July 22, 2015, and placed no restriction on language of publication. All studies were parallel-group trials, except for one cross-over trial.
While two of the studies reported adverse events, four of the studies reported having investigated the occurrences of such types of incidents. One of the studies said three participants in its control group required oral steroids because of acute exacerbations of their asthma, but that these adverse events could not be counted as having been caused by yoga. Another study showed that one participant in its yoga group, who used the Pink City Lung Exerciser (a medical device used to mimic the typical patterns of yoga breathing), reported mild dyspnea during the exercise.
“[As] the included studies were mostly small in sample size and at high risk of bias, high-quality RCTs with large sample sizes are needed to confirm the effects of yoga,” the researchers said.
They reported that they had no known declarations of interest. The project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group.
The full review is available in the Cochrane Database of Systematic Reviews (doi: 10.1002/14651858.CD010346.pub2).
Yoga seems to improve the quality of life and symptoms of people with asthma, suggests a review of 15 randomized controlled trials comprising 1,048 patients with varying degrees of asthma severity.
The studies generally compared the outcomes for asthma patients participating in at least 2 weeks of yoga with the outcomes for those who were treated with usual care for asthma, a sham intervention, or no intervention.
Average improvements in the Asthma Quality of Life Questionnaire scores of 0.57 units per item on a 7-point scale were found through an analysis of responses from 375 individuals, with each person having participated in one of the five randomized controlled trials (RCTs). While the average increase exceeded the minimal clinically important difference (MCID) for this questionnaire, outcomes of two of the trials raise questions about whether the reported improvements in patients’ quality of life can be attributed to yoga. In those two trials, which included a placebo or sham intervention for some of the participants, no differences in these questionnaire scores were found following the interventions.
For 243 asthma patients who participated in three of the RCTs, on average, yoga improved their symptoms by 0.37 standard deviation units of the disease severity scores used.
“Our findings are preliminary and suggestive, rather than conclusive, and therefore should be interpreted cautiously. Yoga probably improves quality of life and symptoms in people with asthma to some extent. However, whether or not the improvements in symptoms exceed the MCID is uncertain due to lack of an established MCID for the severity scores used in the included studies,” noted Zu-Yao Yang of the Chinese University of Hong Kong, and colleagues.
They used various methods to collect data, including searching the Cochrane Airways Group Register of Trials, which is derived from systematic searches of bibliographic databases, and hand-searching respiratory journals and meeting abstracts. They searched all databases from their inception to July 22, 2015, and placed no restriction on language of publication. All studies were parallel-group trials, except for one cross-over trial.
While two of the studies reported adverse events, four of the studies reported having investigated the occurrences of such types of incidents. One of the studies said three participants in its control group required oral steroids because of acute exacerbations of their asthma, but that these adverse events could not be counted as having been caused by yoga. Another study showed that one participant in its yoga group, who used the Pink City Lung Exerciser (a medical device used to mimic the typical patterns of yoga breathing), reported mild dyspnea during the exercise.
“[As] the included studies were mostly small in sample size and at high risk of bias, high-quality RCTs with large sample sizes are needed to confirm the effects of yoga,” the researchers said.
They reported that they had no known declarations of interest. The project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group.
The full review is available in the Cochrane Database of Systematic Reviews (doi: 10.1002/14651858.CD010346.pub2).
Yoga seems to improve the quality of life and symptoms of people with asthma, suggests a review of 15 randomized controlled trials comprising 1,048 patients with varying degrees of asthma severity.
The studies generally compared the outcomes for asthma patients participating in at least 2 weeks of yoga with the outcomes for those who were treated with usual care for asthma, a sham intervention, or no intervention.
Average improvements in the Asthma Quality of Life Questionnaire scores of 0.57 units per item on a 7-point scale were found through an analysis of responses from 375 individuals, with each person having participated in one of the five randomized controlled trials (RCTs). While the average increase exceeded the minimal clinically important difference (MCID) for this questionnaire, outcomes of two of the trials raise questions about whether the reported improvements in patients’ quality of life can be attributed to yoga. In those two trials, which included a placebo or sham intervention for some of the participants, no differences in these questionnaire scores were found following the interventions.
For 243 asthma patients who participated in three of the RCTs, on average, yoga improved their symptoms by 0.37 standard deviation units of the disease severity scores used.
“Our findings are preliminary and suggestive, rather than conclusive, and therefore should be interpreted cautiously. Yoga probably improves quality of life and symptoms in people with asthma to some extent. However, whether or not the improvements in symptoms exceed the MCID is uncertain due to lack of an established MCID for the severity scores used in the included studies,” noted Zu-Yao Yang of the Chinese University of Hong Kong, and colleagues.
They used various methods to collect data, including searching the Cochrane Airways Group Register of Trials, which is derived from systematic searches of bibliographic databases, and hand-searching respiratory journals and meeting abstracts. They searched all databases from their inception to July 22, 2015, and placed no restriction on language of publication. All studies were parallel-group trials, except for one cross-over trial.
While two of the studies reported adverse events, four of the studies reported having investigated the occurrences of such types of incidents. One of the studies said three participants in its control group required oral steroids because of acute exacerbations of their asthma, but that these adverse events could not be counted as having been caused by yoga. Another study showed that one participant in its yoga group, who used the Pink City Lung Exerciser (a medical device used to mimic the typical patterns of yoga breathing), reported mild dyspnea during the exercise.
“[As] the included studies were mostly small in sample size and at high risk of bias, high-quality RCTs with large sample sizes are needed to confirm the effects of yoga,” the researchers said.
They reported that they had no known declarations of interest. The project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group.
The full review is available in the Cochrane Database of Systematic Reviews (doi: 10.1002/14651858.CD010346.pub2).
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Yoga improves asthmatics’ quality of life, data review suggests
Yoga seems to improve the quality of life and symptoms of people with asthma, suggests a review of 15 randomized controlled trials comprising 1,048 patients with varying degrees of asthma severity.
The studies generally compared the outcomes for asthma patients participating in at least 2 weeks of yoga with the outcomes for those who were treated with usual care for asthma, a sham intervention, or no intervention.
Average improvements in the Asthma Quality of Life Questionnaire scores of 0.57 units per item on a 7-point scale were found through an analysis of responses from 375 individuals, with each person having participated in one of the five randomized controlled trials (RCTs). While the average increase exceeded the minimal clinically important difference (MCID) for this questionnaire, outcomes of two of the trials raise questions about whether the reported improvements in patients’ quality of life can be attributed to yoga. In those two trials, which included a placebo or sham intervention for some of the participants, no differences in these questionnaire scores were found following the interventions.
For 243 asthma patients who participated in three of the RCTs, on average, yoga improved their symptoms by 0.37 standard deviation units of the disease severity scores used.
“Our findings are preliminary and suggestive, rather than conclusive, and therefore should be interpreted cautiously. Yoga probably improves quality of life and symptoms in people with asthma to some extent. However, whether or not the improvements in symptoms exceed the MCID is uncertain due to lack of an established MCID for the severity scores used in the included studies,” noted Zu-Yao Yang of the Chinese University of Hong Kong, and colleagues.
They used various methods to collect data, including searching the Cochrane Airways Group Register of Trials, which is derived from systematic searches of bibliographic databases, and hand-searching respiratory journals and meeting abstracts. They searched all databases from their inception to July 22, 2015, and placed no restriction on language of publication. All studies were parallel-group trials, except for one cross-over trial.
While two of the studies reported adverse events, four of the studies reported having investigated the occurrences of such types of incidents. One of the studies said three participants in its control group required oral steroids because of acute exacerbations of their asthma, but that these adverse events could not be counted as having been caused by yoga. Another study showed that one participant in its yoga group, who used the Pink City Lung Exerciser (a medical device used to mimic the typical patterns of yoga breathing), reported mild dyspnea during the exercise.
“[As] the included studies were mostly small in sample size and at high risk of bias, high-quality RCTs with large sample sizes are needed to confirm the effects of yoga,” the researchers said.
They reported that they had no known declarations of interest. The project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group.
The full review is available in the Cochrane Database of Systematic Reviews (doi: 10.1002/14651858.CD010346.pub2).
Yoga seems to improve the quality of life and symptoms of people with asthma, suggests a review of 15 randomized controlled trials comprising 1,048 patients with varying degrees of asthma severity.
The studies generally compared the outcomes for asthma patients participating in at least 2 weeks of yoga with the outcomes for those who were treated with usual care for asthma, a sham intervention, or no intervention.
Average improvements in the Asthma Quality of Life Questionnaire scores of 0.57 units per item on a 7-point scale were found through an analysis of responses from 375 individuals, with each person having participated in one of the five randomized controlled trials (RCTs). While the average increase exceeded the minimal clinically important difference (MCID) for this questionnaire, outcomes of two of the trials raise questions about whether the reported improvements in patients’ quality of life can be attributed to yoga. In those two trials, which included a placebo or sham intervention for some of the participants, no differences in these questionnaire scores were found following the interventions.
For 243 asthma patients who participated in three of the RCTs, on average, yoga improved their symptoms by 0.37 standard deviation units of the disease severity scores used.
“Our findings are preliminary and suggestive, rather than conclusive, and therefore should be interpreted cautiously. Yoga probably improves quality of life and symptoms in people with asthma to some extent. However, whether or not the improvements in symptoms exceed the MCID is uncertain due to lack of an established MCID for the severity scores used in the included studies,” noted Zu-Yao Yang of the Chinese University of Hong Kong, and colleagues.
They used various methods to collect data, including searching the Cochrane Airways Group Register of Trials, which is derived from systematic searches of bibliographic databases, and hand-searching respiratory journals and meeting abstracts. They searched all databases from their inception to July 22, 2015, and placed no restriction on language of publication. All studies were parallel-group trials, except for one cross-over trial.
While two of the studies reported adverse events, four of the studies reported having investigated the occurrences of such types of incidents. One of the studies said three participants in its control group required oral steroids because of acute exacerbations of their asthma, but that these adverse events could not be counted as having been caused by yoga. Another study showed that one participant in its yoga group, who used the Pink City Lung Exerciser (a medical device used to mimic the typical patterns of yoga breathing), reported mild dyspnea during the exercise.
“[As] the included studies were mostly small in sample size and at high risk of bias, high-quality RCTs with large sample sizes are needed to confirm the effects of yoga,” the researchers said.
They reported that they had no known declarations of interest. The project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group.
The full review is available in the Cochrane Database of Systematic Reviews (doi: 10.1002/14651858.CD010346.pub2).
Yoga seems to improve the quality of life and symptoms of people with asthma, suggests a review of 15 randomized controlled trials comprising 1,048 patients with varying degrees of asthma severity.
The studies generally compared the outcomes for asthma patients participating in at least 2 weeks of yoga with the outcomes for those who were treated with usual care for asthma, a sham intervention, or no intervention.
Average improvements in the Asthma Quality of Life Questionnaire scores of 0.57 units per item on a 7-point scale were found through an analysis of responses from 375 individuals, with each person having participated in one of the five randomized controlled trials (RCTs). While the average increase exceeded the minimal clinically important difference (MCID) for this questionnaire, outcomes of two of the trials raise questions about whether the reported improvements in patients’ quality of life can be attributed to yoga. In those two trials, which included a placebo or sham intervention for some of the participants, no differences in these questionnaire scores were found following the interventions.
For 243 asthma patients who participated in three of the RCTs, on average, yoga improved their symptoms by 0.37 standard deviation units of the disease severity scores used.
“Our findings are preliminary and suggestive, rather than conclusive, and therefore should be interpreted cautiously. Yoga probably improves quality of life and symptoms in people with asthma to some extent. However, whether or not the improvements in symptoms exceed the MCID is uncertain due to lack of an established MCID for the severity scores used in the included studies,” noted Zu-Yao Yang of the Chinese University of Hong Kong, and colleagues.
They used various methods to collect data, including searching the Cochrane Airways Group Register of Trials, which is derived from systematic searches of bibliographic databases, and hand-searching respiratory journals and meeting abstracts. They searched all databases from their inception to July 22, 2015, and placed no restriction on language of publication. All studies were parallel-group trials, except for one cross-over trial.
While two of the studies reported adverse events, four of the studies reported having investigated the occurrences of such types of incidents. One of the studies said three participants in its control group required oral steroids because of acute exacerbations of their asthma, but that these adverse events could not be counted as having been caused by yoga. Another study showed that one participant in its yoga group, who used the Pink City Lung Exerciser (a medical device used to mimic the typical patterns of yoga breathing), reported mild dyspnea during the exercise.
“[As] the included studies were mostly small in sample size and at high risk of bias, high-quality RCTs with large sample sizes are needed to confirm the effects of yoga,” the researchers said.
They reported that they had no known declarations of interest. The project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group.
The full review is available in the Cochrane Database of Systematic Reviews (doi: 10.1002/14651858.CD010346.pub2).
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
More connective tissue disease–associated PAH seen in older patients
Patient age contributes to significant differences in the characteristics and etiology of pulmonary arterial hypertension seen in randomized, controlled trials, including more frequent connective tissue disease–associated disease in older patients, according to a post-hoc analysis of trials.
Additionally, older age was associated with worse baseline functional status, worse outcomes in the 6-minute walk distance, and an overall reduced response to treatment, while hemodynamic severity was higher in younger patients.
“Although registry data have shown that idiopathic PAH [pulmonary arterial hypertension] is increasingly recognized in older populations, our analysis shows that idiopathic etiology was less frequent in the older group. In contrast, CTD [connective tissue disease]–associated PAH accounted for a higher proportion of PAH etiology in the oldest age group,” wrote Jonathan A. Rose of Case Western Reserve University, Cleveland, and his colleagues (Chest. 2016;149[5]:1234-44. doi: 10.1016/j.chest.2015.11.008).
The researchers analyzed seven multicenter, randomized, double-blind, placebo-controlled treatment trials for PAH conducted by United Therapeutics and one open-label extension study that involved following patients being treated with subcutaneous treprostinil for 4 additional years. Data from the open-label extension trial and one of the trials were combined and reported as one trial. All trials excluded patients with a history of left-sided heart disease, pulmonary artery wedge pressure (PAWP) greater than 15 mmHg, or pulmonary vascular resistance (PVR) less than 3 Woods units. The researchers categorized the 2,627 patients included in the trials in the following three age groups: 50 years or younger, 51-64 years, and 65 years or older.
Between 53% and 74% of patients in all trials across all age groups had idiopathic PAH, but older patients comprised a significantly smaller proportion of the patients with idiopathic PAH in three of the trials (P = .004) and a significantly higher percentage of the patients with CTD-associated PAH in all of the trials (P less than .001). Across the trials, CTD-associated PAH occurred in 15%-21% of patients 50 years or younger, 25%-40% of those aged 51-64 years, and 27%-49% of those aged 65 years or older.
From baseline to the end in three of the studies, a smaller change in the 6-minute walk distance was seen in older patients and a higher proportion of older patients had an overall decrease in total 6-minute walk distance. Older age remained associated with this outcome measure when only data from treatment-naive patients or the subgroup of patients with CTD-associated PAH were included.
The association between age and lower baseline functional status was observed in four of the trials; A lower proportion of patients in the oldest age group were classified as being of the World Health Organization functional classes I and II, with 9%-32% of patients aged 65 years or older, 10%-33% in those aged 51-64 years, and 16%-43% of those aged 50 years or younger.
Hemodynamic severity was among the areas in which older patients performed better than younger patients, with the oldest age group having lower baseline mean pulmonary artery pressure (mPAP) and PVR in the two trials that measured hemodynamics.
“The difference in the clinical function and hemodynamics in older patients may reflect different mechanisms of disease. Older patients may have diminished cardiopulmonary reserve, and similar degrees of hemodynamic severity manifest with a larger functional impairment. Alternatively, additional comorbidities in older patients may contribute to a larger extent, and clinical function may not be as directly related to hemodynamic severity,” according to the researchers.
Mortality was generally small in these studies and not significantly different among age groups, except for in the open-label extension study of Subcutaneous Infusion of Treprostinil in Patients with PAH (SC-TRE) and the Study of Intravenous Remodulin in Patients in India with PAH (TRUST) trials, which were combined and reported on as one trial (P = .0004).
Nausea was the only adverse effect found to be associated with age in more than one trial, and the relationship between it and age in two trials were inconsistent. While a higher incidence of nausea was found in the oldest age group in the Oral Treprostinil as Monotherapy for the Treatment of Pulmonary Arterial Hypertension (FREEDOM-M) trial (P = .03), a lower incidence of nausea was found in the oldest age group in the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) trial (P = .02).
The analysis was supported by a grant from the National Institutes of Health. Two of the authors, Jody M. Cleveland and Youlan Rao, Ph.D., reported being employees of United Therapeutics, while Dr. Omar A. Minai, another author of the report, serves on the scientific advisory board of United Therapeutics. The other authors declared no conflicts.
Patient age contributes to significant differences in the characteristics and etiology of pulmonary arterial hypertension seen in randomized, controlled trials, including more frequent connective tissue disease–associated disease in older patients, according to a post-hoc analysis of trials.
Additionally, older age was associated with worse baseline functional status, worse outcomes in the 6-minute walk distance, and an overall reduced response to treatment, while hemodynamic severity was higher in younger patients.
“Although registry data have shown that idiopathic PAH [pulmonary arterial hypertension] is increasingly recognized in older populations, our analysis shows that idiopathic etiology was less frequent in the older group. In contrast, CTD [connective tissue disease]–associated PAH accounted for a higher proportion of PAH etiology in the oldest age group,” wrote Jonathan A. Rose of Case Western Reserve University, Cleveland, and his colleagues (Chest. 2016;149[5]:1234-44. doi: 10.1016/j.chest.2015.11.008).
The researchers analyzed seven multicenter, randomized, double-blind, placebo-controlled treatment trials for PAH conducted by United Therapeutics and one open-label extension study that involved following patients being treated with subcutaneous treprostinil for 4 additional years. Data from the open-label extension trial and one of the trials were combined and reported as one trial. All trials excluded patients with a history of left-sided heart disease, pulmonary artery wedge pressure (PAWP) greater than 15 mmHg, or pulmonary vascular resistance (PVR) less than 3 Woods units. The researchers categorized the 2,627 patients included in the trials in the following three age groups: 50 years or younger, 51-64 years, and 65 years or older.
Between 53% and 74% of patients in all trials across all age groups had idiopathic PAH, but older patients comprised a significantly smaller proportion of the patients with idiopathic PAH in three of the trials (P = .004) and a significantly higher percentage of the patients with CTD-associated PAH in all of the trials (P less than .001). Across the trials, CTD-associated PAH occurred in 15%-21% of patients 50 years or younger, 25%-40% of those aged 51-64 years, and 27%-49% of those aged 65 years or older.
From baseline to the end in three of the studies, a smaller change in the 6-minute walk distance was seen in older patients and a higher proportion of older patients had an overall decrease in total 6-minute walk distance. Older age remained associated with this outcome measure when only data from treatment-naive patients or the subgroup of patients with CTD-associated PAH were included.
The association between age and lower baseline functional status was observed in four of the trials; A lower proportion of patients in the oldest age group were classified as being of the World Health Organization functional classes I and II, with 9%-32% of patients aged 65 years or older, 10%-33% in those aged 51-64 years, and 16%-43% of those aged 50 years or younger.
Hemodynamic severity was among the areas in which older patients performed better than younger patients, with the oldest age group having lower baseline mean pulmonary artery pressure (mPAP) and PVR in the two trials that measured hemodynamics.
“The difference in the clinical function and hemodynamics in older patients may reflect different mechanisms of disease. Older patients may have diminished cardiopulmonary reserve, and similar degrees of hemodynamic severity manifest with a larger functional impairment. Alternatively, additional comorbidities in older patients may contribute to a larger extent, and clinical function may not be as directly related to hemodynamic severity,” according to the researchers.
Mortality was generally small in these studies and not significantly different among age groups, except for in the open-label extension study of Subcutaneous Infusion of Treprostinil in Patients with PAH (SC-TRE) and the Study of Intravenous Remodulin in Patients in India with PAH (TRUST) trials, which were combined and reported on as one trial (P = .0004).
Nausea was the only adverse effect found to be associated with age in more than one trial, and the relationship between it and age in two trials were inconsistent. While a higher incidence of nausea was found in the oldest age group in the Oral Treprostinil as Monotherapy for the Treatment of Pulmonary Arterial Hypertension (FREEDOM-M) trial (P = .03), a lower incidence of nausea was found in the oldest age group in the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) trial (P = .02).
The analysis was supported by a grant from the National Institutes of Health. Two of the authors, Jody M. Cleveland and Youlan Rao, Ph.D., reported being employees of United Therapeutics, while Dr. Omar A. Minai, another author of the report, serves on the scientific advisory board of United Therapeutics. The other authors declared no conflicts.
Patient age contributes to significant differences in the characteristics and etiology of pulmonary arterial hypertension seen in randomized, controlled trials, including more frequent connective tissue disease–associated disease in older patients, according to a post-hoc analysis of trials.
Additionally, older age was associated with worse baseline functional status, worse outcomes in the 6-minute walk distance, and an overall reduced response to treatment, while hemodynamic severity was higher in younger patients.
“Although registry data have shown that idiopathic PAH [pulmonary arterial hypertension] is increasingly recognized in older populations, our analysis shows that idiopathic etiology was less frequent in the older group. In contrast, CTD [connective tissue disease]–associated PAH accounted for a higher proportion of PAH etiology in the oldest age group,” wrote Jonathan A. Rose of Case Western Reserve University, Cleveland, and his colleagues (Chest. 2016;149[5]:1234-44. doi: 10.1016/j.chest.2015.11.008).
The researchers analyzed seven multicenter, randomized, double-blind, placebo-controlled treatment trials for PAH conducted by United Therapeutics and one open-label extension study that involved following patients being treated with subcutaneous treprostinil for 4 additional years. Data from the open-label extension trial and one of the trials were combined and reported as one trial. All trials excluded patients with a history of left-sided heart disease, pulmonary artery wedge pressure (PAWP) greater than 15 mmHg, or pulmonary vascular resistance (PVR) less than 3 Woods units. The researchers categorized the 2,627 patients included in the trials in the following three age groups: 50 years or younger, 51-64 years, and 65 years or older.
Between 53% and 74% of patients in all trials across all age groups had idiopathic PAH, but older patients comprised a significantly smaller proportion of the patients with idiopathic PAH in three of the trials (P = .004) and a significantly higher percentage of the patients with CTD-associated PAH in all of the trials (P less than .001). Across the trials, CTD-associated PAH occurred in 15%-21% of patients 50 years or younger, 25%-40% of those aged 51-64 years, and 27%-49% of those aged 65 years or older.
From baseline to the end in three of the studies, a smaller change in the 6-minute walk distance was seen in older patients and a higher proportion of older patients had an overall decrease in total 6-minute walk distance. Older age remained associated with this outcome measure when only data from treatment-naive patients or the subgroup of patients with CTD-associated PAH were included.
The association between age and lower baseline functional status was observed in four of the trials; A lower proportion of patients in the oldest age group were classified as being of the World Health Organization functional classes I and II, with 9%-32% of patients aged 65 years or older, 10%-33% in those aged 51-64 years, and 16%-43% of those aged 50 years or younger.
Hemodynamic severity was among the areas in which older patients performed better than younger patients, with the oldest age group having lower baseline mean pulmonary artery pressure (mPAP) and PVR in the two trials that measured hemodynamics.
“The difference in the clinical function and hemodynamics in older patients may reflect different mechanisms of disease. Older patients may have diminished cardiopulmonary reserve, and similar degrees of hemodynamic severity manifest with a larger functional impairment. Alternatively, additional comorbidities in older patients may contribute to a larger extent, and clinical function may not be as directly related to hemodynamic severity,” according to the researchers.
Mortality was generally small in these studies and not significantly different among age groups, except for in the open-label extension study of Subcutaneous Infusion of Treprostinil in Patients with PAH (SC-TRE) and the Study of Intravenous Remodulin in Patients in India with PAH (TRUST) trials, which were combined and reported on as one trial (P = .0004).
Nausea was the only adverse effect found to be associated with age in more than one trial, and the relationship between it and age in two trials were inconsistent. While a higher incidence of nausea was found in the oldest age group in the Oral Treprostinil as Monotherapy for the Treatment of Pulmonary Arterial Hypertension (FREEDOM-M) trial (P = .03), a lower incidence of nausea was found in the oldest age group in the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) trial (P = .02).
The analysis was supported by a grant from the National Institutes of Health. Two of the authors, Jody M. Cleveland and Youlan Rao, Ph.D., reported being employees of United Therapeutics, while Dr. Omar A. Minai, another author of the report, serves on the scientific advisory board of United Therapeutics. The other authors declared no conflicts.
FROM CHEST
FDA okays glycopyrrolate/formoterol combo for COPD
The Food and Drug Administration has approved glycopyrrolate and formoterol fumarate inhalation aerosol for long-term maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), according to a statement from AstraZeneca.
AstraZeneca is marketing the bronchodilator as Bevespi Aerosphere. The product is a fixed-dose dual bronchodilator delivered through a pressurized, metered-dose inhaler to be used twice daily. Glycopyrrolate, a long-acting muscarinic antagonist, is available as monotherapy. The FDA also has granted the long-acting beta-2 agonist formoterol fumarate tentative approval for use as monotherapy.
The approval of glycopyrrolate/formoterol fumarate 9 mcg/4.8 mcg is based on the PINNACLE trials, which demonstrated the product achieved improvement in morning predose forced expiratory volume in 1 second at 24 weeks (P less than .001), compared with its monotherapy components and placebo, according to AstraZeneca’s statement.
The most common adverse reactions were urinary tract infection and cough. The drug is not indicated for the treatment of asthma or for the relief of acute bronchospasm.
The Food and Drug Administration has approved glycopyrrolate and formoterol fumarate inhalation aerosol for long-term maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), according to a statement from AstraZeneca.
AstraZeneca is marketing the bronchodilator as Bevespi Aerosphere. The product is a fixed-dose dual bronchodilator delivered through a pressurized, metered-dose inhaler to be used twice daily. Glycopyrrolate, a long-acting muscarinic antagonist, is available as monotherapy. The FDA also has granted the long-acting beta-2 agonist formoterol fumarate tentative approval for use as monotherapy.
The approval of glycopyrrolate/formoterol fumarate 9 mcg/4.8 mcg is based on the PINNACLE trials, which demonstrated the product achieved improvement in morning predose forced expiratory volume in 1 second at 24 weeks (P less than .001), compared with its monotherapy components and placebo, according to AstraZeneca’s statement.
The most common adverse reactions were urinary tract infection and cough. The drug is not indicated for the treatment of asthma or for the relief of acute bronchospasm.
The Food and Drug Administration has approved glycopyrrolate and formoterol fumarate inhalation aerosol for long-term maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), according to a statement from AstraZeneca.
AstraZeneca is marketing the bronchodilator as Bevespi Aerosphere. The product is a fixed-dose dual bronchodilator delivered through a pressurized, metered-dose inhaler to be used twice daily. Glycopyrrolate, a long-acting muscarinic antagonist, is available as monotherapy. The FDA also has granted the long-acting beta-2 agonist formoterol fumarate tentative approval for use as monotherapy.
The approval of glycopyrrolate/formoterol fumarate 9 mcg/4.8 mcg is based on the PINNACLE trials, which demonstrated the product achieved improvement in morning predose forced expiratory volume in 1 second at 24 weeks (P less than .001), compared with its monotherapy components and placebo, according to AstraZeneca’s statement.
The most common adverse reactions were urinary tract infection and cough. The drug is not indicated for the treatment of asthma or for the relief of acute bronchospasm.
Reintubation avoided by majority of patients on noninvasive ventilation therapy, high-flow oxygen
Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen had a lower risk of reintubation, compared with extubated patients who received some form of standard oxygen therapy, according to the results of two multicenter, randomized clinical trials published online in JAMA.
Participants in one of the studies, which included abdominal surgery patients diagnosed with respiratory failure within 7 days following surgery, either received NIV or standard oxygen therapy for 30 days or until ICU discharge, whichever came first. While NIV has been effectively used to treat nonsurgical patients with acute exacerbations of chronic obstructive pulmonary disease and cardiogenic pulmonary edema, there is no evidence to support the use of NIV in surgical patients with hypoxemic acute respiratory failure after abdominal surgery, according to Dr. Samir Jaber of the Saint Eloi University Hospital and Montpellier School of Medicine, both in Montpellier, France, and his colleagues (JAMA. 2016 Apr 5;315[13]:1345-53).
The second study included adult patients who had received mechanical ventilation for more than 12 hours and who met criteria for being considered at low risk for reintubation. Patients were administered either high-flow oxygen therapy through nasal cannula immediately after extubation or continuous conventional oxygen therapy through nasal cannula or nonrebreather facemask; the patients were observed for 72 hours. High-flow therapy has been shown to improve oxygenation and survival in clinical studies of critically ill patients in the acute phase of respiratory failure. “[A study by S.M. Maggiore and his colleagues (Am J Respir Crit Care Med. 2014;190(3):282-8)] suggested that high-flow therapy after planned extubation decreased the reintubation rate in a general population of critical patients, but the benefits might be mainly attributable to improvements in high-risk patients,” said Dr. Gonzalo Hernandez, of the Hospital Virgen de la Salud, Toledo, Spain, and his colleagues (JAMA. 2016 Apr 5;315[13]:1354-61).
In the first study, 148 patients received NIV and 145 patients received standard oxygen therapy only. NIV was administered through a facemask connected to an ICU- or a NIV-dedicated ventilator, using either a heated humidifier or heat and moisture exchanger to warm and humidify inspired gases. Patients were encouraged to use NIV for 6 hours during the first 24 hours of the study and received standard oxygen therapy at a rate of up to 15 L/minute to maintain an arterial oxygen saturation estimate (SpO2) of at least 94% in between NIV sessions. NIV was started at an inspiratory positive airway pressure of 5 cm H2O, increasing to a maximum inspiratory pressure of 15 cm H2O, aiming to achieve an expiratory tidal volume between 6 and 8 mL/kg of predicted body weight and a respiratory rate lower than 25/min. The patients in this study’s control group only received the standard oxygen therapy.
In the other study, 263 patients received conventional therapy, with the oxygen flow having been adjusted to maintain an arterial oxygen saturation estimate of greater than 92%. This study’s other 264 patients received high-flow oxygen therapy, with the flow having been initially set at 10 L/min and titrated upward in 5-L/min steps until patients experienced discomfort. The high-flow therapy was stopped after 24 hours and was followed by conventional oxygen therapy, when needed.
The primary outcome measure in the study involving NIV was cause for reintubation within 7 days of randomization.
Secondary outcome measures included gas exchange, healthcare-associated infection rate within 30 days, number of ventilator-free days between days 1 and 30, antibiotic use duration, ICU and in-hospital length of stay, and 30- and 90-day mortality.
Reintubation occurred in 49 patients in the NIV group and 66 patients in the standard oxygen therapy group, a significant difference (P = .03). Among the reintubated patients, those who had received NIV spent less time under invasive mechanical ventilation as did the patients given standard oxygen therapy. The interquartile ranges of days of invasive mechanical ventilation were 0-3 for patients in the NIV group and 0-5 for patients in the standard oxygen therapy group (P = .05). At 30 days, NIV was associated with significantly more ventilator-free days than standard oxygen therapy (25.4 vs. 23.2; P = .04). At 90 days, 22 patients in the NIV group and 31 patients in the standard oxygen therapy group had died (P = .15).
“Recent high-impact trials have demonstrated the benefits in nonsurgical hypoxemic respiratory failure or equivalence of high-flow nasal cannula compared with NIV in patients after cardiothoracic surgery with moderate to severe hypoxemia. Future studies comparing use of high-flow oxygen cannula vs standard oxygen therapy and NIV for patients after abdominal surgery as preventive (prophylactic) or curative applications are needed,” according to Dr. Jaber and his colleagues.
The primary outcome measure for the study of patients receiving high-flow oxygen therapy was reintubation within 72 hours after extubation; this occurred in fewer patients in the high-flow oxygen group than in the conventional therapy group (13 or 4.9% vs. 32 or 12.2%.) This statistically significant difference was mainly attributable to a lower incidence of respiratory-related reintubation in the high-flow group, compared with the conventional therapy group (1.5% vs. 8.7%), said Dr. Hernandez and his colleagues.
Secondary outcome measures included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, ICU and hospital length of stay and mortality, time to reintubation, and adverse effects. Postintubation respiratory failure was less common in the high-flow therapy group than in the conventional therapy group (22 patients or 8.3% vs. 38 or 14.4%). Differences between the two groups in other secondary outcomes were not statistically significant.
“The main finding of this study was that high-flow oxygen significantly reduced the reintubation rate in critically ill patients at low risk for extubation failure ... High-flow therapy improves oxygenation, and the lower rate of reintubation secondary to hypoxia in the high-flow group corroborates this finding. High-flow oxygen also seems to reduce other causes of respiratory failure such as increased work of breathing and respiratory muscle fatigue, which are frequently associated with reintubation secondary to hypoxia. Another way in which high-flow therapy improves extubation outcome is by conditioning the inspired gas,” said Dr. Hernandez and his colleagues.
No adverse events were reported in either study.
Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with their study’s sponsors, Montpellier (France) University Hospital and the APARD Foundation.
Dr. Eric Gartman, FCCP, comments: These two studies augment a growing body of literature supporting the use of adjunctive therapies immediately following extubation to prevent reintubation for respiratory failure.
It has been known for several years that the use of noninvasive ventilation (NIV) immediately after extubation in COPD patients prevents reintubation rates, and these new data demonstrate efficacy in an expanded population. Further, the use of high-flow humidified oxygen therapy in acute respiratory failure has been shown to prevent progression to initial intubation, and now these data expand potential use to prevent reintubation, as well.
While not studied, if high-flow oxygen therapy is found to be equivalent to NIV to prevent reintubation (similar to the previously-published prevention of intubation studies), that would be clinically important since there is a significant difference in tolerance to these two therapies. Across these trials, the very important point to remember is that these therapies were found to be effective if put on directly after extubation, and one cannot wait to apply them at the point where the patient shows signs of respiratory decline.
Dr. Eric Gartman, FCCP, comments: These two studies augment a growing body of literature supporting the use of adjunctive therapies immediately following extubation to prevent reintubation for respiratory failure.
It has been known for several years that the use of noninvasive ventilation (NIV) immediately after extubation in COPD patients prevents reintubation rates, and these new data demonstrate efficacy in an expanded population. Further, the use of high-flow humidified oxygen therapy in acute respiratory failure has been shown to prevent progression to initial intubation, and now these data expand potential use to prevent reintubation, as well.
While not studied, if high-flow oxygen therapy is found to be equivalent to NIV to prevent reintubation (similar to the previously-published prevention of intubation studies), that would be clinically important since there is a significant difference in tolerance to these two therapies. Across these trials, the very important point to remember is that these therapies were found to be effective if put on directly after extubation, and one cannot wait to apply them at the point where the patient shows signs of respiratory decline.
Dr. Eric Gartman, FCCP, comments: These two studies augment a growing body of literature supporting the use of adjunctive therapies immediately following extubation to prevent reintubation for respiratory failure.
It has been known for several years that the use of noninvasive ventilation (NIV) immediately after extubation in COPD patients prevents reintubation rates, and these new data demonstrate efficacy in an expanded population. Further, the use of high-flow humidified oxygen therapy in acute respiratory failure has been shown to prevent progression to initial intubation, and now these data expand potential use to prevent reintubation, as well.
While not studied, if high-flow oxygen therapy is found to be equivalent to NIV to prevent reintubation (similar to the previously-published prevention of intubation studies), that would be clinically important since there is a significant difference in tolerance to these two therapies. Across these trials, the very important point to remember is that these therapies were found to be effective if put on directly after extubation, and one cannot wait to apply them at the point where the patient shows signs of respiratory decline.
Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen had a lower risk of reintubation, compared with extubated patients who received some form of standard oxygen therapy, according to the results of two multicenter, randomized clinical trials published online in JAMA.
Participants in one of the studies, which included abdominal surgery patients diagnosed with respiratory failure within 7 days following surgery, either received NIV or standard oxygen therapy for 30 days or until ICU discharge, whichever came first. While NIV has been effectively used to treat nonsurgical patients with acute exacerbations of chronic obstructive pulmonary disease and cardiogenic pulmonary edema, there is no evidence to support the use of NIV in surgical patients with hypoxemic acute respiratory failure after abdominal surgery, according to Dr. Samir Jaber of the Saint Eloi University Hospital and Montpellier School of Medicine, both in Montpellier, France, and his colleagues (JAMA. 2016 Apr 5;315[13]:1345-53).
The second study included adult patients who had received mechanical ventilation for more than 12 hours and who met criteria for being considered at low risk for reintubation. Patients were administered either high-flow oxygen therapy through nasal cannula immediately after extubation or continuous conventional oxygen therapy through nasal cannula or nonrebreather facemask; the patients were observed for 72 hours. High-flow therapy has been shown to improve oxygenation and survival in clinical studies of critically ill patients in the acute phase of respiratory failure. “[A study by S.M. Maggiore and his colleagues (Am J Respir Crit Care Med. 2014;190(3):282-8)] suggested that high-flow therapy after planned extubation decreased the reintubation rate in a general population of critical patients, but the benefits might be mainly attributable to improvements in high-risk patients,” said Dr. Gonzalo Hernandez, of the Hospital Virgen de la Salud, Toledo, Spain, and his colleagues (JAMA. 2016 Apr 5;315[13]:1354-61).
In the first study, 148 patients received NIV and 145 patients received standard oxygen therapy only. NIV was administered through a facemask connected to an ICU- or a NIV-dedicated ventilator, using either a heated humidifier or heat and moisture exchanger to warm and humidify inspired gases. Patients were encouraged to use NIV for 6 hours during the first 24 hours of the study and received standard oxygen therapy at a rate of up to 15 L/minute to maintain an arterial oxygen saturation estimate (SpO2) of at least 94% in between NIV sessions. NIV was started at an inspiratory positive airway pressure of 5 cm H2O, increasing to a maximum inspiratory pressure of 15 cm H2O, aiming to achieve an expiratory tidal volume between 6 and 8 mL/kg of predicted body weight and a respiratory rate lower than 25/min. The patients in this study’s control group only received the standard oxygen therapy.
In the other study, 263 patients received conventional therapy, with the oxygen flow having been adjusted to maintain an arterial oxygen saturation estimate of greater than 92%. This study’s other 264 patients received high-flow oxygen therapy, with the flow having been initially set at 10 L/min and titrated upward in 5-L/min steps until patients experienced discomfort. The high-flow therapy was stopped after 24 hours and was followed by conventional oxygen therapy, when needed.
The primary outcome measure in the study involving NIV was cause for reintubation within 7 days of randomization.
Secondary outcome measures included gas exchange, healthcare-associated infection rate within 30 days, number of ventilator-free days between days 1 and 30, antibiotic use duration, ICU and in-hospital length of stay, and 30- and 90-day mortality.
Reintubation occurred in 49 patients in the NIV group and 66 patients in the standard oxygen therapy group, a significant difference (P = .03). Among the reintubated patients, those who had received NIV spent less time under invasive mechanical ventilation as did the patients given standard oxygen therapy. The interquartile ranges of days of invasive mechanical ventilation were 0-3 for patients in the NIV group and 0-5 for patients in the standard oxygen therapy group (P = .05). At 30 days, NIV was associated with significantly more ventilator-free days than standard oxygen therapy (25.4 vs. 23.2; P = .04). At 90 days, 22 patients in the NIV group and 31 patients in the standard oxygen therapy group had died (P = .15).
“Recent high-impact trials have demonstrated the benefits in nonsurgical hypoxemic respiratory failure or equivalence of high-flow nasal cannula compared with NIV in patients after cardiothoracic surgery with moderate to severe hypoxemia. Future studies comparing use of high-flow oxygen cannula vs standard oxygen therapy and NIV for patients after abdominal surgery as preventive (prophylactic) or curative applications are needed,” according to Dr. Jaber and his colleagues.
The primary outcome measure for the study of patients receiving high-flow oxygen therapy was reintubation within 72 hours after extubation; this occurred in fewer patients in the high-flow oxygen group than in the conventional therapy group (13 or 4.9% vs. 32 or 12.2%.) This statistically significant difference was mainly attributable to a lower incidence of respiratory-related reintubation in the high-flow group, compared with the conventional therapy group (1.5% vs. 8.7%), said Dr. Hernandez and his colleagues.
Secondary outcome measures included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, ICU and hospital length of stay and mortality, time to reintubation, and adverse effects. Postintubation respiratory failure was less common in the high-flow therapy group than in the conventional therapy group (22 patients or 8.3% vs. 38 or 14.4%). Differences between the two groups in other secondary outcomes were not statistically significant.
“The main finding of this study was that high-flow oxygen significantly reduced the reintubation rate in critically ill patients at low risk for extubation failure ... High-flow therapy improves oxygenation, and the lower rate of reintubation secondary to hypoxia in the high-flow group corroborates this finding. High-flow oxygen also seems to reduce other causes of respiratory failure such as increased work of breathing and respiratory muscle fatigue, which are frequently associated with reintubation secondary to hypoxia. Another way in which high-flow therapy improves extubation outcome is by conditioning the inspired gas,” said Dr. Hernandez and his colleagues.
No adverse events were reported in either study.
Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with their study’s sponsors, Montpellier (France) University Hospital and the APARD Foundation.
Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen had a lower risk of reintubation, compared with extubated patients who received some form of standard oxygen therapy, according to the results of two multicenter, randomized clinical trials published online in JAMA.
Participants in one of the studies, which included abdominal surgery patients diagnosed with respiratory failure within 7 days following surgery, either received NIV or standard oxygen therapy for 30 days or until ICU discharge, whichever came first. While NIV has been effectively used to treat nonsurgical patients with acute exacerbations of chronic obstructive pulmonary disease and cardiogenic pulmonary edema, there is no evidence to support the use of NIV in surgical patients with hypoxemic acute respiratory failure after abdominal surgery, according to Dr. Samir Jaber of the Saint Eloi University Hospital and Montpellier School of Medicine, both in Montpellier, France, and his colleagues (JAMA. 2016 Apr 5;315[13]:1345-53).
The second study included adult patients who had received mechanical ventilation for more than 12 hours and who met criteria for being considered at low risk for reintubation. Patients were administered either high-flow oxygen therapy through nasal cannula immediately after extubation or continuous conventional oxygen therapy through nasal cannula or nonrebreather facemask; the patients were observed for 72 hours. High-flow therapy has been shown to improve oxygenation and survival in clinical studies of critically ill patients in the acute phase of respiratory failure. “[A study by S.M. Maggiore and his colleagues (Am J Respir Crit Care Med. 2014;190(3):282-8)] suggested that high-flow therapy after planned extubation decreased the reintubation rate in a general population of critical patients, but the benefits might be mainly attributable to improvements in high-risk patients,” said Dr. Gonzalo Hernandez, of the Hospital Virgen de la Salud, Toledo, Spain, and his colleagues (JAMA. 2016 Apr 5;315[13]:1354-61).
In the first study, 148 patients received NIV and 145 patients received standard oxygen therapy only. NIV was administered through a facemask connected to an ICU- or a NIV-dedicated ventilator, using either a heated humidifier or heat and moisture exchanger to warm and humidify inspired gases. Patients were encouraged to use NIV for 6 hours during the first 24 hours of the study and received standard oxygen therapy at a rate of up to 15 L/minute to maintain an arterial oxygen saturation estimate (SpO2) of at least 94% in between NIV sessions. NIV was started at an inspiratory positive airway pressure of 5 cm H2O, increasing to a maximum inspiratory pressure of 15 cm H2O, aiming to achieve an expiratory tidal volume between 6 and 8 mL/kg of predicted body weight and a respiratory rate lower than 25/min. The patients in this study’s control group only received the standard oxygen therapy.
In the other study, 263 patients received conventional therapy, with the oxygen flow having been adjusted to maintain an arterial oxygen saturation estimate of greater than 92%. This study’s other 264 patients received high-flow oxygen therapy, with the flow having been initially set at 10 L/min and titrated upward in 5-L/min steps until patients experienced discomfort. The high-flow therapy was stopped after 24 hours and was followed by conventional oxygen therapy, when needed.
The primary outcome measure in the study involving NIV was cause for reintubation within 7 days of randomization.
Secondary outcome measures included gas exchange, healthcare-associated infection rate within 30 days, number of ventilator-free days between days 1 and 30, antibiotic use duration, ICU and in-hospital length of stay, and 30- and 90-day mortality.
Reintubation occurred in 49 patients in the NIV group and 66 patients in the standard oxygen therapy group, a significant difference (P = .03). Among the reintubated patients, those who had received NIV spent less time under invasive mechanical ventilation as did the patients given standard oxygen therapy. The interquartile ranges of days of invasive mechanical ventilation were 0-3 for patients in the NIV group and 0-5 for patients in the standard oxygen therapy group (P = .05). At 30 days, NIV was associated with significantly more ventilator-free days than standard oxygen therapy (25.4 vs. 23.2; P = .04). At 90 days, 22 patients in the NIV group and 31 patients in the standard oxygen therapy group had died (P = .15).
“Recent high-impact trials have demonstrated the benefits in nonsurgical hypoxemic respiratory failure or equivalence of high-flow nasal cannula compared with NIV in patients after cardiothoracic surgery with moderate to severe hypoxemia. Future studies comparing use of high-flow oxygen cannula vs standard oxygen therapy and NIV for patients after abdominal surgery as preventive (prophylactic) or curative applications are needed,” according to Dr. Jaber and his colleagues.
The primary outcome measure for the study of patients receiving high-flow oxygen therapy was reintubation within 72 hours after extubation; this occurred in fewer patients in the high-flow oxygen group than in the conventional therapy group (13 or 4.9% vs. 32 or 12.2%.) This statistically significant difference was mainly attributable to a lower incidence of respiratory-related reintubation in the high-flow group, compared with the conventional therapy group (1.5% vs. 8.7%), said Dr. Hernandez and his colleagues.
Secondary outcome measures included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, ICU and hospital length of stay and mortality, time to reintubation, and adverse effects. Postintubation respiratory failure was less common in the high-flow therapy group than in the conventional therapy group (22 patients or 8.3% vs. 38 or 14.4%). Differences between the two groups in other secondary outcomes were not statistically significant.
“The main finding of this study was that high-flow oxygen significantly reduced the reintubation rate in critically ill patients at low risk for extubation failure ... High-flow therapy improves oxygenation, and the lower rate of reintubation secondary to hypoxia in the high-flow group corroborates this finding. High-flow oxygen also seems to reduce other causes of respiratory failure such as increased work of breathing and respiratory muscle fatigue, which are frequently associated with reintubation secondary to hypoxia. Another way in which high-flow therapy improves extubation outcome is by conditioning the inspired gas,” said Dr. Hernandez and his colleagues.
No adverse events were reported in either study.
Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with their study’s sponsors, Montpellier (France) University Hospital and the APARD Foundation.
FROM JAMA
Key clinical point: Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen reduced their risk of reintubation, compared with patients who received some form of standard oxygen therapy.
Major finding: In one study, significantly fewer of the patients who received NIV needed to be reintubated than the patients who received standard oxygen therapy.
Data source: Two multicenter, randomized clinical trials published online in JAMA.
Disclosures: Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with Montpellier (France) University Hospital and the APARD Foundation, who funded their study.