User login
Primary Cutaneous Mycobacterium avium Complex Infection Following Squamous Cell Carcinoma Excision
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
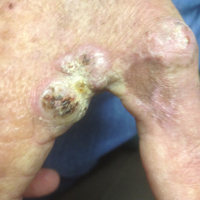
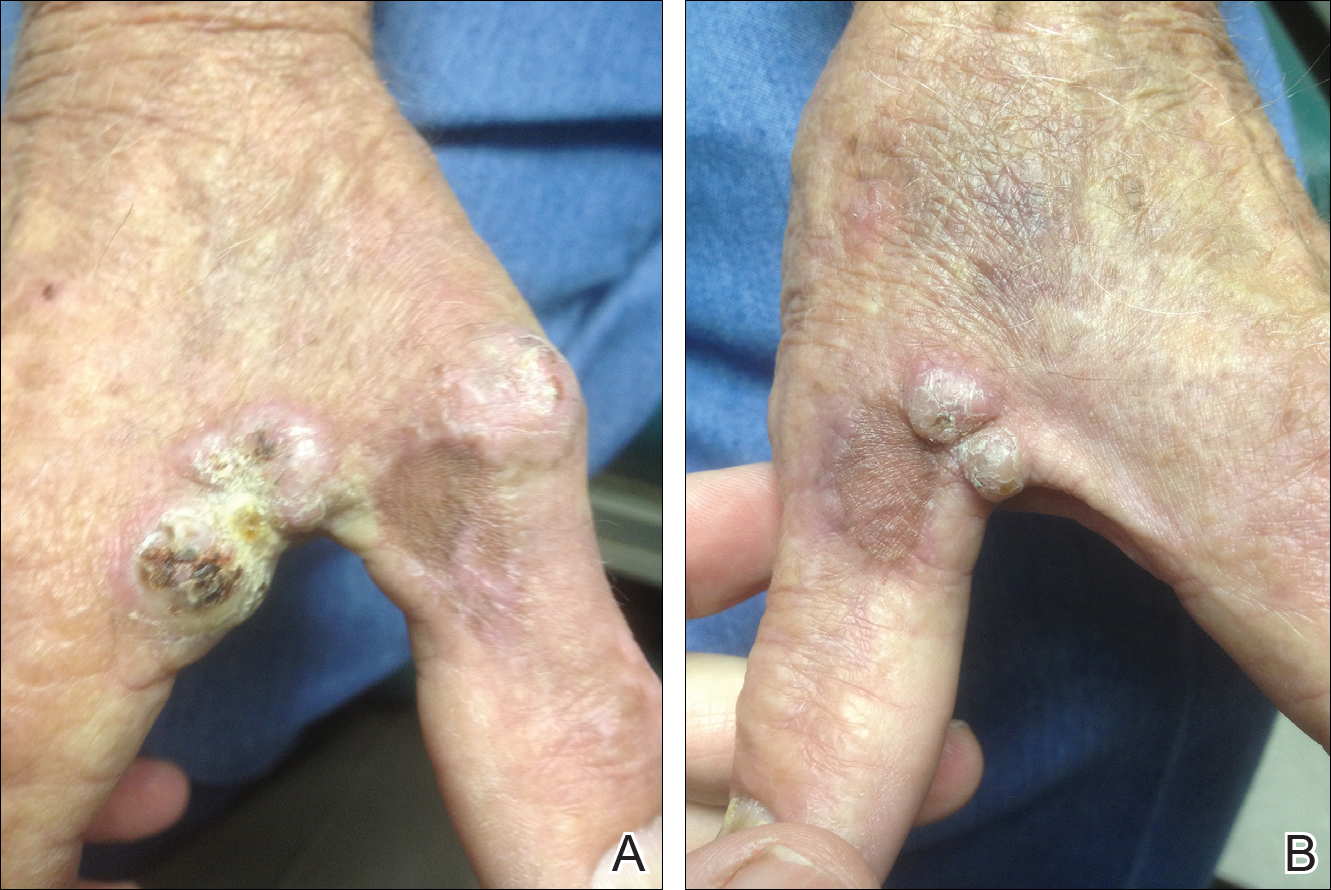
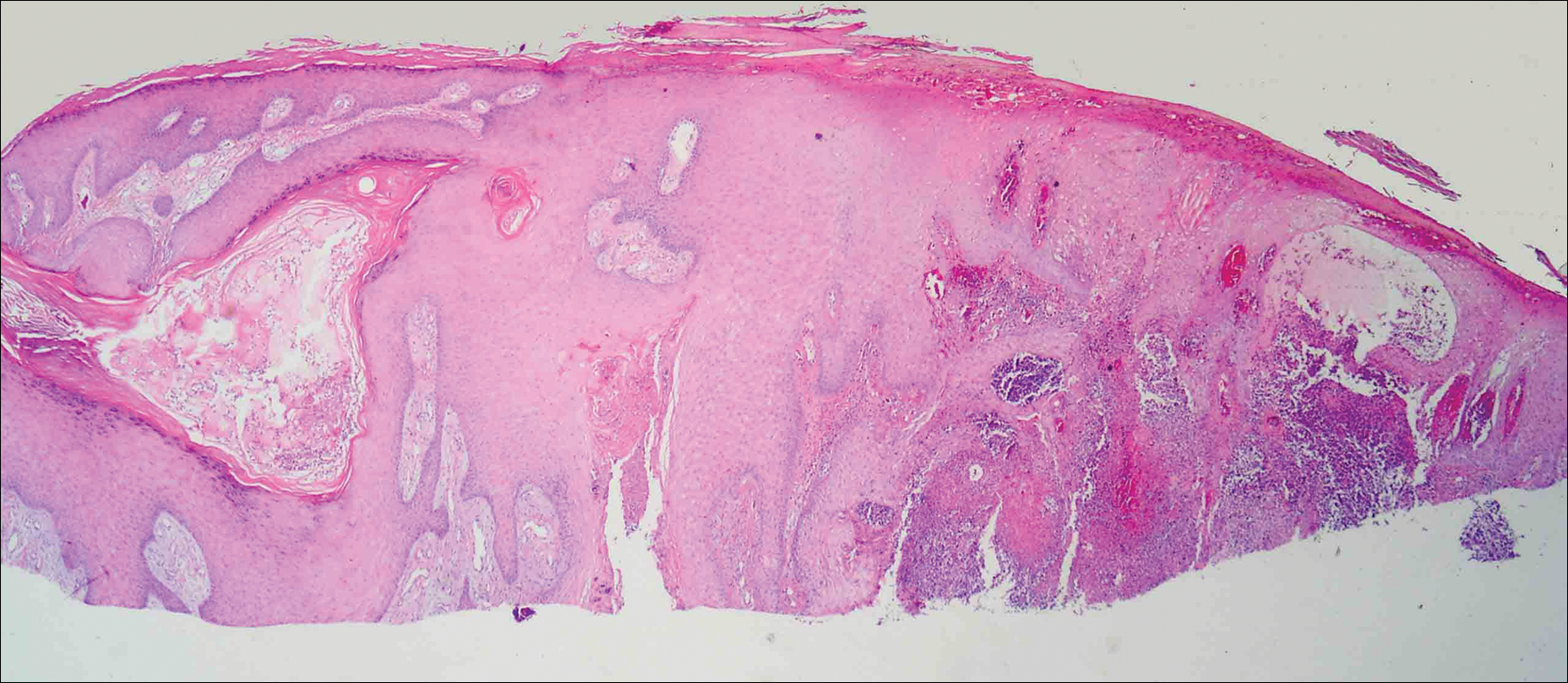
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.
Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Practice Points
- Mycobacterium avium complex (MAC) is a ubiquitous bacterium that commonly infects the lungs and less commonly infects the skin.
- Clinically, cutaneous MAC infection is polymorphous and may present as a nodule, plaque, or ulcer.
- Standard treatment of primary cutaneous MAC includes systemic antibiotics with or without surgical excision.