User login
Vitreous Hemorrhage in the Setting of a Vascular Loop
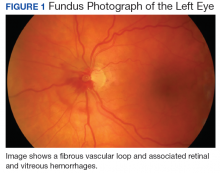
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
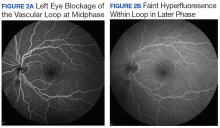
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Peripheral Exudative Hemorrhagic Chorioretinopathy in Patients With Nonexudative Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is a common condition that affects the elderly white population. About 6.5% of Americans have been diagnosed with AMD, and 0.8% have received an end-stage AMD diagnosis.1 Exudative AMD is typically more visually debilitating and comprises between 10% and 15% of all AMD cases, with conversion from dry to wet about 10%.1
A thorough examination of the posterior pole is of utmost importance in patients with dry AMD in order to ensure there is no conversion to the exudative form. However, it also is imperative to perform a peripheral evaluation in these patients due to the incidence of peripheral choroidal neovascular membrane (CNVM) and its potential visual significance.
Case Report 1
An 80-year-old white male with type 2 diabetes mellitus (DM) without retinopathy, dry AMD, and epiretinal membranes (ERM) in both eyes presented to the eye clinic for a 6-month follow-up. On examination, he had visual acuity (VA) of 20/25 in both eyes and reported no ocular problems. The intraocular pressures were 17 mm Hg in the right eye and 20 mm Hg in the left eye. Slit-lamp examination of the anterior segment of both eyes was significant for 2+ nuclear sclerotic cataracts.
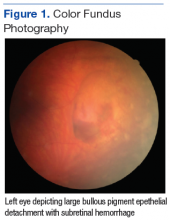
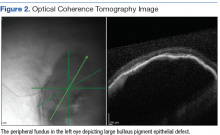
On dilated fundus exam, there were macular drusen and ERM in both eyes; peripherally in the right eye, there was cobblestone degeneration and pigmentary changes. Peripherally in the left eye, there was a large retinal pigment epithelial detachment (PED) with subretinal hemorrhage in the inferior temporal quadrant (Figure 1) along with cobblestone degeneration and pigmentary changes. Peripheral optical coherence tomography (OCT) in the left eye showed a large PED in the location of the hemorrhage (Figure 2).
Case Report 2
An 88-year-old white male presented to the eye clinic reporting blurred vision at distance and dry eyes. The patient’s medical history was remarkable for vascular and heart disease, treated with warfarin. The patient also had insulin controlled DM, with no prior history of retinopathy. His past ocular history included hard drusen in the macula, peripheral drusen, pavingstone degeneration, and a fibrotic scar temporally in the right eye.
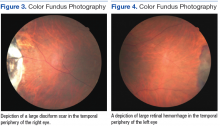
At his annual eye examination, the patient’s vision was correctable to 20/25 in both eyes. His anterior segment slit-lamp exam was remarkable for posterior chamber intraocular lenses, clear and centered in each eye. His posterior pole exam was remarkable for small hard drusen at the macula in both eyes. Peripherally in the right eye, there was a large disciform fibrotic scar temporally (Figure 3) as well as cobblestone degeneration and peripheral drusen. The left eye revealed a large disciform hemorrhage temporally (Figure 4) with cobblestone degeneration and peripheral drusen.
Both patients currently are being closely monitored for any encroachment of the peripheral lesions into the posterior poles.
Discussion
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR), also referred to in the literature as eccentric disciform CNVM, peripheral CNVM, and peripheral age-related degeneration, is a rare condition more prevalent in elderly white females.2-4 Mean age ranges from 70 to 82 years, with bilateral involvement ranging from 18% to 37%.2-4 The mid-periphery or periphery is the most common location for these lesions, more specifically, in the inferior temporal quadrant.2,3,5,6
Age-related macular degeneration is not pathognomonic for PEHCR. Mantel and colleagues reported that 68.9% of the patients in their study had AMD.3 Visual acuity ranges from 20/20 to light perception, dependent upon ocular comorbidities.2,3 As reported by Mantel and colleagues, patients with symptomatic PEHCR commonly experience visual loss, floaters, photopsias, metamorphopsia, and scotoma.3
Peripheral exudative hemorrhagic chorioretinopathy is a hemorrhagic or exudative process that can occur either as an isolated lesion or as multiple lesions that consist of a PED along with hemorrhage, subretinal fluid and/or fibrotic scarring.2-5 Peripheral exudative hemorrhagic chorioretinopathy is not visually significant unless a vitreous hemorrhage is evident or the blood and/or fluid extends to the macular region.2,5
The exact etiology of peripheral CNVM remains unknown; however, ischemia, mechanical forces, and defects in Bruch’s membrane all have been speculated as causative factors.2,3,6 Others have hypothesized that PEHCR is a form of polypoidal choroidal vasculopathy.3,7,8 A rupture in Bruch’s membrane with a vascular complex contributes to the pathophysiology and histology of this condition.3,6
Given the propensity for cardiovascular diseases, such as DM and hypertension, to lead to retinal ischemia, it is important to take a good case history.2,4,6 Additionally, anticoagulants have been shown to exacerbate bleeding.2,5 Due to PEHCR’s location in the periphery, as well as its appearance as an elevated dark mass, it is important to differentiate these lesions from a choroidal melanoma.2,6 Recognition of PEHCR can save the patient from unnecessary treatment with radiation or enucleation.
Peripheral exudative hemorrhagic chorioretinopathy is a self-limiting condition that generally requires close observation only. Long-term follow-up studies show resolution, regression, or stability of the peripheral lesions.4,5,8 If a hemorrhage is present, the blood will resolve and leave a disciform scar with pigmentary changes.2-4 In cases where vision is threatened, CNVM has been treated with photocoagulation, cryopexy, and more recently, intravitreal anti-VEGF injections.4,5,9,10 Given that VEGF is more prevalent in the presence of a choroidal neovascular complex, the goal of anti-VEGF therapy is to prevent the growth of and further damage from these abnormal blood vessels.5
Conclusion
The authors have described 2 cases of asymptomatic PEHCR in elderly white males who are both currently being observed closely. Peripheral exudative hemorrhagic chorioretinopathy is an uncommon finding; therefore, knowledge of this condition also may be rare. Through this article and these cases, the importance of routine peripheral fundus examination to detect PEHCR should be stressed. It also is important to include PEHCR as a differential diagnosis when evaluating a peripheral dark elevated lesion to distinguish from peripheral melanomas and avoid unnecessary treatments. If identified, these lesions often require close observation only, and a retina referral is warranted if there is macular involvement or a rapidly progressive lesion.5
1. Pron G. Optical coherence tomography monitoring strategies for A-VEGF–treated age-related macular degeneration: an evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(10):1–64.
2. Annesley WH Jr. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-364.
3. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical angiographic, and histologic study. Am J Ophthalmol. 2009;148(6):932-938.
4. Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27(1):111-112.
5. Seibel I, Hager A, Duncker T, et al. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):653-659.
6. Collaer N, James C. Peripheral exudative and hemorrhagic chorio-retinopathy…the peripheral form of age-related macular degeneration? Report on 2 cases. Bull Soc Belge Ophtalmol. 2007;(305):23-26.
7. Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: A report of 10 eyes. Retina. 2013;33(1):48-55.
8. Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013;8(3):264-267.
9. Takayama K, Enoki T, Kojima T, Ishikawa S, Takeuchi M. Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clin Ophthalmol. 2012;6:865-869.
10. Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011;21(4):506-508.
Age-related macular degeneration (AMD) is a common condition that affects the elderly white population. About 6.5% of Americans have been diagnosed with AMD, and 0.8% have received an end-stage AMD diagnosis.1 Exudative AMD is typically more visually debilitating and comprises between 10% and 15% of all AMD cases, with conversion from dry to wet about 10%.1
A thorough examination of the posterior pole is of utmost importance in patients with dry AMD in order to ensure there is no conversion to the exudative form. However, it also is imperative to perform a peripheral evaluation in these patients due to the incidence of peripheral choroidal neovascular membrane (CNVM) and its potential visual significance.
Case Report 1
An 80-year-old white male with type 2 diabetes mellitus (DM) without retinopathy, dry AMD, and epiretinal membranes (ERM) in both eyes presented to the eye clinic for a 6-month follow-up. On examination, he had visual acuity (VA) of 20/25 in both eyes and reported no ocular problems. The intraocular pressures were 17 mm Hg in the right eye and 20 mm Hg in the left eye. Slit-lamp examination of the anterior segment of both eyes was significant for 2+ nuclear sclerotic cataracts.
On dilated fundus exam, there were macular drusen and ERM in both eyes; peripherally in the right eye, there was cobblestone degeneration and pigmentary changes. Peripherally in the left eye, there was a large retinal pigment epithelial detachment (PED) with subretinal hemorrhage in the inferior temporal quadrant (Figure 1) along with cobblestone degeneration and pigmentary changes. Peripheral optical coherence tomography (OCT) in the left eye showed a large PED in the location of the hemorrhage (Figure 2).
Case Report 2
An 88-year-old white male presented to the eye clinic reporting blurred vision at distance and dry eyes. The patient’s medical history was remarkable for vascular and heart disease, treated with warfarin. The patient also had insulin controlled DM, with no prior history of retinopathy. His past ocular history included hard drusen in the macula, peripheral drusen, pavingstone degeneration, and a fibrotic scar temporally in the right eye.
At his annual eye examination, the patient’s vision was correctable to 20/25 in both eyes. His anterior segment slit-lamp exam was remarkable for posterior chamber intraocular lenses, clear and centered in each eye. His posterior pole exam was remarkable for small hard drusen at the macula in both eyes. Peripherally in the right eye, there was a large disciform fibrotic scar temporally (Figure 3) as well as cobblestone degeneration and peripheral drusen. The left eye revealed a large disciform hemorrhage temporally (Figure 4) with cobblestone degeneration and peripheral drusen.
Both patients currently are being closely monitored for any encroachment of the peripheral lesions into the posterior poles.
Discussion
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR), also referred to in the literature as eccentric disciform CNVM, peripheral CNVM, and peripheral age-related degeneration, is a rare condition more prevalent in elderly white females.2-4 Mean age ranges from 70 to 82 years, with bilateral involvement ranging from 18% to 37%.2-4 The mid-periphery or periphery is the most common location for these lesions, more specifically, in the inferior temporal quadrant.2,3,5,6
Age-related macular degeneration is not pathognomonic for PEHCR. Mantel and colleagues reported that 68.9% of the patients in their study had AMD.3 Visual acuity ranges from 20/20 to light perception, dependent upon ocular comorbidities.2,3 As reported by Mantel and colleagues, patients with symptomatic PEHCR commonly experience visual loss, floaters, photopsias, metamorphopsia, and scotoma.3
Peripheral exudative hemorrhagic chorioretinopathy is a hemorrhagic or exudative process that can occur either as an isolated lesion or as multiple lesions that consist of a PED along with hemorrhage, subretinal fluid and/or fibrotic scarring.2-5 Peripheral exudative hemorrhagic chorioretinopathy is not visually significant unless a vitreous hemorrhage is evident or the blood and/or fluid extends to the macular region.2,5
The exact etiology of peripheral CNVM remains unknown; however, ischemia, mechanical forces, and defects in Bruch’s membrane all have been speculated as causative factors.2,3,6 Others have hypothesized that PEHCR is a form of polypoidal choroidal vasculopathy.3,7,8 A rupture in Bruch’s membrane with a vascular complex contributes to the pathophysiology and histology of this condition.3,6
Given the propensity for cardiovascular diseases, such as DM and hypertension, to lead to retinal ischemia, it is important to take a good case history.2,4,6 Additionally, anticoagulants have been shown to exacerbate bleeding.2,5 Due to PEHCR’s location in the periphery, as well as its appearance as an elevated dark mass, it is important to differentiate these lesions from a choroidal melanoma.2,6 Recognition of PEHCR can save the patient from unnecessary treatment with radiation or enucleation.
Peripheral exudative hemorrhagic chorioretinopathy is a self-limiting condition that generally requires close observation only. Long-term follow-up studies show resolution, regression, or stability of the peripheral lesions.4,5,8 If a hemorrhage is present, the blood will resolve and leave a disciform scar with pigmentary changes.2-4 In cases where vision is threatened, CNVM has been treated with photocoagulation, cryopexy, and more recently, intravitreal anti-VEGF injections.4,5,9,10 Given that VEGF is more prevalent in the presence of a choroidal neovascular complex, the goal of anti-VEGF therapy is to prevent the growth of and further damage from these abnormal blood vessels.5
Conclusion
The authors have described 2 cases of asymptomatic PEHCR in elderly white males who are both currently being observed closely. Peripheral exudative hemorrhagic chorioretinopathy is an uncommon finding; therefore, knowledge of this condition also may be rare. Through this article and these cases, the importance of routine peripheral fundus examination to detect PEHCR should be stressed. It also is important to include PEHCR as a differential diagnosis when evaluating a peripheral dark elevated lesion to distinguish from peripheral melanomas and avoid unnecessary treatments. If identified, these lesions often require close observation only, and a retina referral is warranted if there is macular involvement or a rapidly progressive lesion.5
Age-related macular degeneration (AMD) is a common condition that affects the elderly white population. About 6.5% of Americans have been diagnosed with AMD, and 0.8% have received an end-stage AMD diagnosis.1 Exudative AMD is typically more visually debilitating and comprises between 10% and 15% of all AMD cases, with conversion from dry to wet about 10%.1
A thorough examination of the posterior pole is of utmost importance in patients with dry AMD in order to ensure there is no conversion to the exudative form. However, it also is imperative to perform a peripheral evaluation in these patients due to the incidence of peripheral choroidal neovascular membrane (CNVM) and its potential visual significance.
Case Report 1
An 80-year-old white male with type 2 diabetes mellitus (DM) without retinopathy, dry AMD, and epiretinal membranes (ERM) in both eyes presented to the eye clinic for a 6-month follow-up. On examination, he had visual acuity (VA) of 20/25 in both eyes and reported no ocular problems. The intraocular pressures were 17 mm Hg in the right eye and 20 mm Hg in the left eye. Slit-lamp examination of the anterior segment of both eyes was significant for 2+ nuclear sclerotic cataracts.
On dilated fundus exam, there were macular drusen and ERM in both eyes; peripherally in the right eye, there was cobblestone degeneration and pigmentary changes. Peripherally in the left eye, there was a large retinal pigment epithelial detachment (PED) with subretinal hemorrhage in the inferior temporal quadrant (Figure 1) along with cobblestone degeneration and pigmentary changes. Peripheral optical coherence tomography (OCT) in the left eye showed a large PED in the location of the hemorrhage (Figure 2).
Case Report 2
An 88-year-old white male presented to the eye clinic reporting blurred vision at distance and dry eyes. The patient’s medical history was remarkable for vascular and heart disease, treated with warfarin. The patient also had insulin controlled DM, with no prior history of retinopathy. His past ocular history included hard drusen in the macula, peripheral drusen, pavingstone degeneration, and a fibrotic scar temporally in the right eye.
At his annual eye examination, the patient’s vision was correctable to 20/25 in both eyes. His anterior segment slit-lamp exam was remarkable for posterior chamber intraocular lenses, clear and centered in each eye. His posterior pole exam was remarkable for small hard drusen at the macula in both eyes. Peripherally in the right eye, there was a large disciform fibrotic scar temporally (Figure 3) as well as cobblestone degeneration and peripheral drusen. The left eye revealed a large disciform hemorrhage temporally (Figure 4) with cobblestone degeneration and peripheral drusen.
Both patients currently are being closely monitored for any encroachment of the peripheral lesions into the posterior poles.
Discussion
Peripheral exudative hemorrhagic chorioretinopathy (PEHCR), also referred to in the literature as eccentric disciform CNVM, peripheral CNVM, and peripheral age-related degeneration, is a rare condition more prevalent in elderly white females.2-4 Mean age ranges from 70 to 82 years, with bilateral involvement ranging from 18% to 37%.2-4 The mid-periphery or periphery is the most common location for these lesions, more specifically, in the inferior temporal quadrant.2,3,5,6
Age-related macular degeneration is not pathognomonic for PEHCR. Mantel and colleagues reported that 68.9% of the patients in their study had AMD.3 Visual acuity ranges from 20/20 to light perception, dependent upon ocular comorbidities.2,3 As reported by Mantel and colleagues, patients with symptomatic PEHCR commonly experience visual loss, floaters, photopsias, metamorphopsia, and scotoma.3
Peripheral exudative hemorrhagic chorioretinopathy is a hemorrhagic or exudative process that can occur either as an isolated lesion or as multiple lesions that consist of a PED along with hemorrhage, subretinal fluid and/or fibrotic scarring.2-5 Peripheral exudative hemorrhagic chorioretinopathy is not visually significant unless a vitreous hemorrhage is evident or the blood and/or fluid extends to the macular region.2,5
The exact etiology of peripheral CNVM remains unknown; however, ischemia, mechanical forces, and defects in Bruch’s membrane all have been speculated as causative factors.2,3,6 Others have hypothesized that PEHCR is a form of polypoidal choroidal vasculopathy.3,7,8 A rupture in Bruch’s membrane with a vascular complex contributes to the pathophysiology and histology of this condition.3,6
Given the propensity for cardiovascular diseases, such as DM and hypertension, to lead to retinal ischemia, it is important to take a good case history.2,4,6 Additionally, anticoagulants have been shown to exacerbate bleeding.2,5 Due to PEHCR’s location in the periphery, as well as its appearance as an elevated dark mass, it is important to differentiate these lesions from a choroidal melanoma.2,6 Recognition of PEHCR can save the patient from unnecessary treatment with radiation or enucleation.
Peripheral exudative hemorrhagic chorioretinopathy is a self-limiting condition that generally requires close observation only. Long-term follow-up studies show resolution, regression, or stability of the peripheral lesions.4,5,8 If a hemorrhage is present, the blood will resolve and leave a disciform scar with pigmentary changes.2-4 In cases where vision is threatened, CNVM has been treated with photocoagulation, cryopexy, and more recently, intravitreal anti-VEGF injections.4,5,9,10 Given that VEGF is more prevalent in the presence of a choroidal neovascular complex, the goal of anti-VEGF therapy is to prevent the growth of and further damage from these abnormal blood vessels.5
Conclusion
The authors have described 2 cases of asymptomatic PEHCR in elderly white males who are both currently being observed closely. Peripheral exudative hemorrhagic chorioretinopathy is an uncommon finding; therefore, knowledge of this condition also may be rare. Through this article and these cases, the importance of routine peripheral fundus examination to detect PEHCR should be stressed. It also is important to include PEHCR as a differential diagnosis when evaluating a peripheral dark elevated lesion to distinguish from peripheral melanomas and avoid unnecessary treatments. If identified, these lesions often require close observation only, and a retina referral is warranted if there is macular involvement or a rapidly progressive lesion.5
1. Pron G. Optical coherence tomography monitoring strategies for A-VEGF–treated age-related macular degeneration: an evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(10):1–64.
2. Annesley WH Jr. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-364.
3. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical angiographic, and histologic study. Am J Ophthalmol. 2009;148(6):932-938.
4. Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27(1):111-112.
5. Seibel I, Hager A, Duncker T, et al. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):653-659.
6. Collaer N, James C. Peripheral exudative and hemorrhagic chorio-retinopathy…the peripheral form of age-related macular degeneration? Report on 2 cases. Bull Soc Belge Ophtalmol. 2007;(305):23-26.
7. Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: A report of 10 eyes. Retina. 2013;33(1):48-55.
8. Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013;8(3):264-267.
9. Takayama K, Enoki T, Kojima T, Ishikawa S, Takeuchi M. Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clin Ophthalmol. 2012;6:865-869.
10. Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011;21(4):506-508.
1. Pron G. Optical coherence tomography monitoring strategies for A-VEGF–treated age-related macular degeneration: an evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(10):1–64.
2. Annesley WH Jr. Peripheral exudative hemorrhagic chorioretinopathy. Trans Am Ophthalmol Soc. 1980;78:321-364.
3. Mantel I, Uffer S, Zografos L. Peripheral exudative hemorrhagic chorioretinopathy: a clinical angiographic, and histologic study. Am J Ophthalmol. 2009;148(6):932-938.
4. Pinarci EY, Kilic I, Bayar SA, Sizmaz S, Akkoyun I, Yilmaz G. Clinical characteristics of peripheral exudative hemorrhagic chorioretinopathy and its response to bevacizumab therapy. Eye (Lond). 2013;27(1):111-112.
5. Seibel I, Hager A, Duncker T, et al. Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):653-659.
6. Collaer N, James C. Peripheral exudative and hemorrhagic chorio-retinopathy…the peripheral form of age-related macular degeneration? Report on 2 cases. Bull Soc Belge Ophtalmol. 2007;(305):23-26.
7. Goldman DR, Freund KB, McCannel CA, Sarraf D. Peripheral polypoidal choroidal vasculopathy as a cause of peripheral exudative hemorrhagic chorioretinopathy: A report of 10 eyes. Retina. 2013;33(1):48-55.
8. Mashayekhi A, Shields CL, Shields JA. Peripheral exudative hemorrhagic chorioretinopathy: a variant of polypoidal choroidal vasculopathy? J Ophthalmic Vis Res. 2013;8(3):264-267.
9. Takayama K, Enoki T, Kojima T, Ishikawa S, Takeuchi M. Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clin Ophthalmol. 2012;6:865-869.
10. Barkmeier AJ, Kadikoy H, Holz ER, Carvounis PE. Regression of serous macular detachment due to peripheral exudative hemorrhagic chorioretinopathy following intravitreal bevacizumab. Eur J Ophthalmol. 2011;21(4):506-508.