User login
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
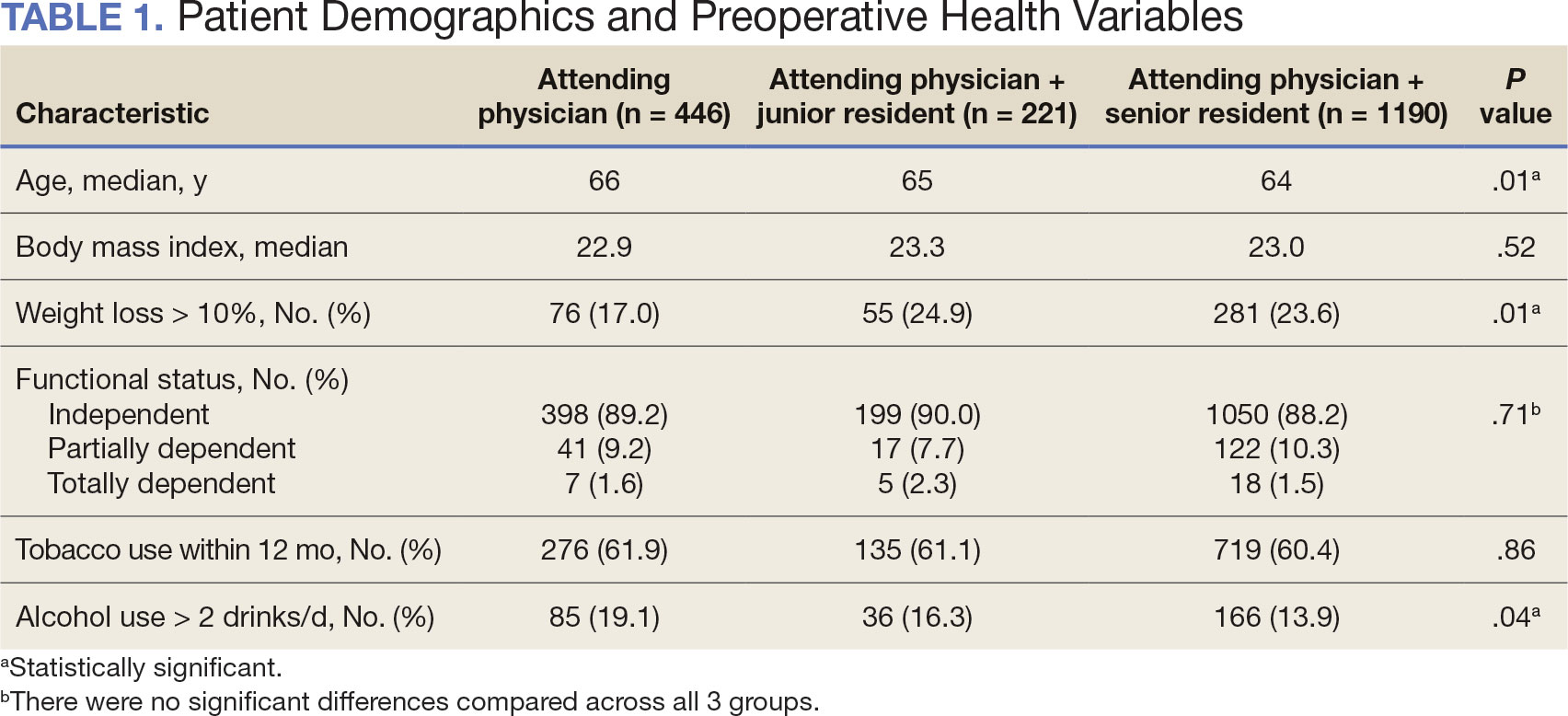
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).
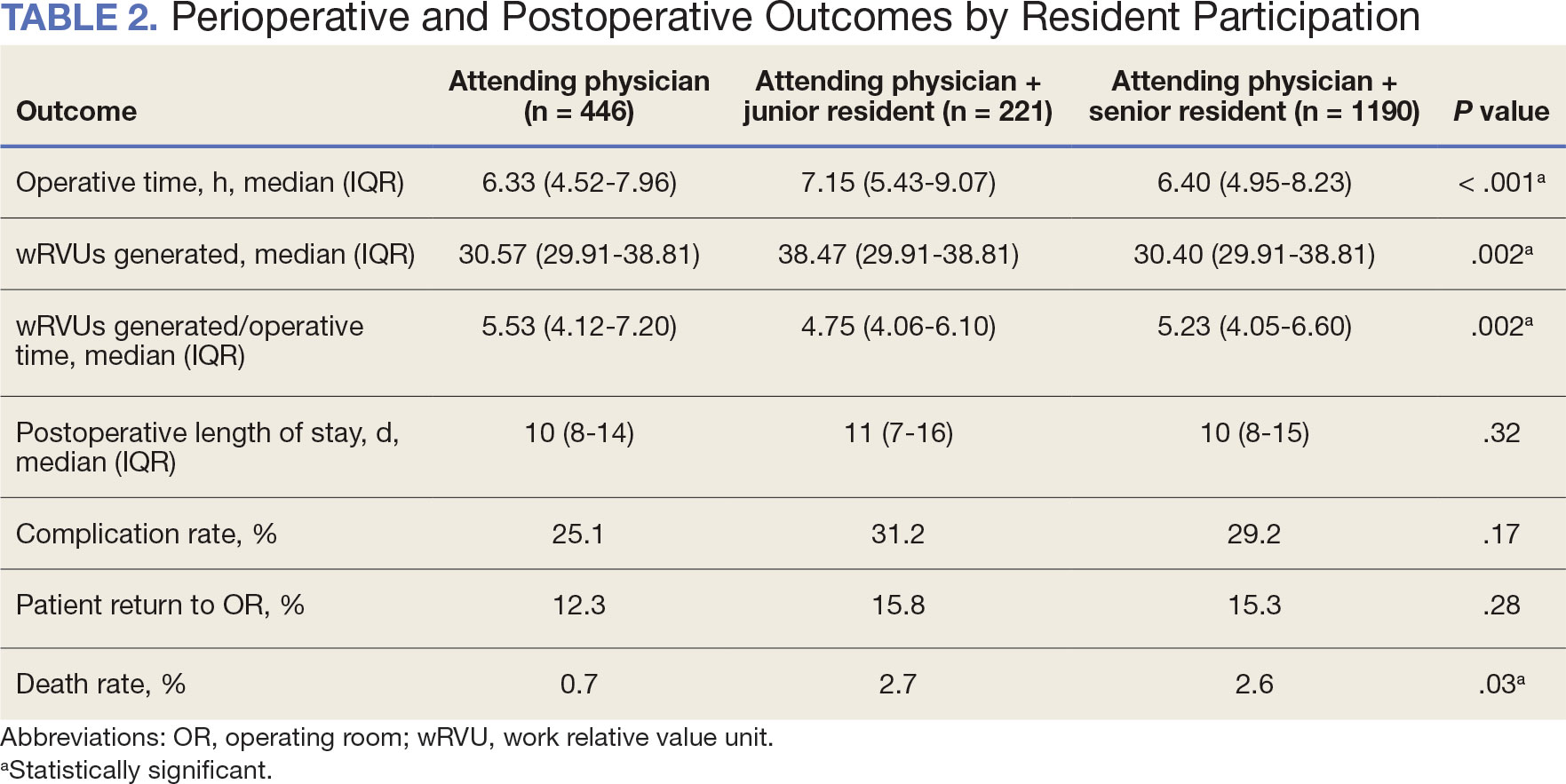
When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.
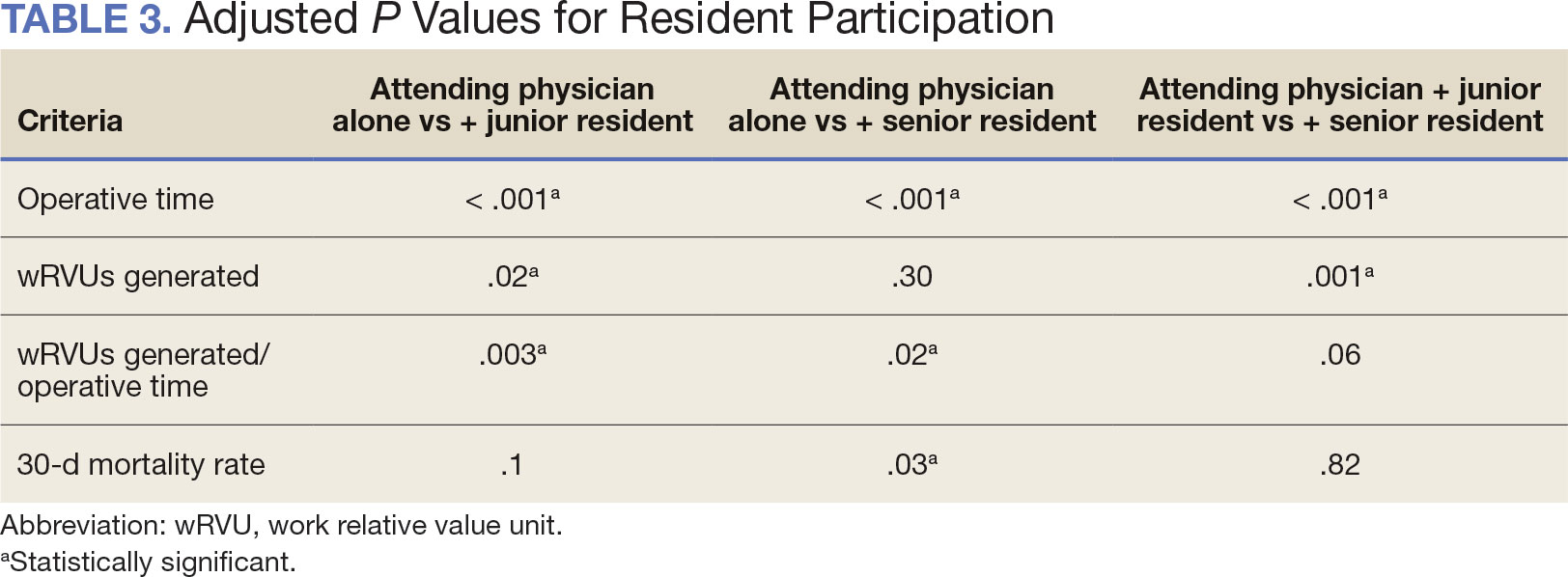
The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).
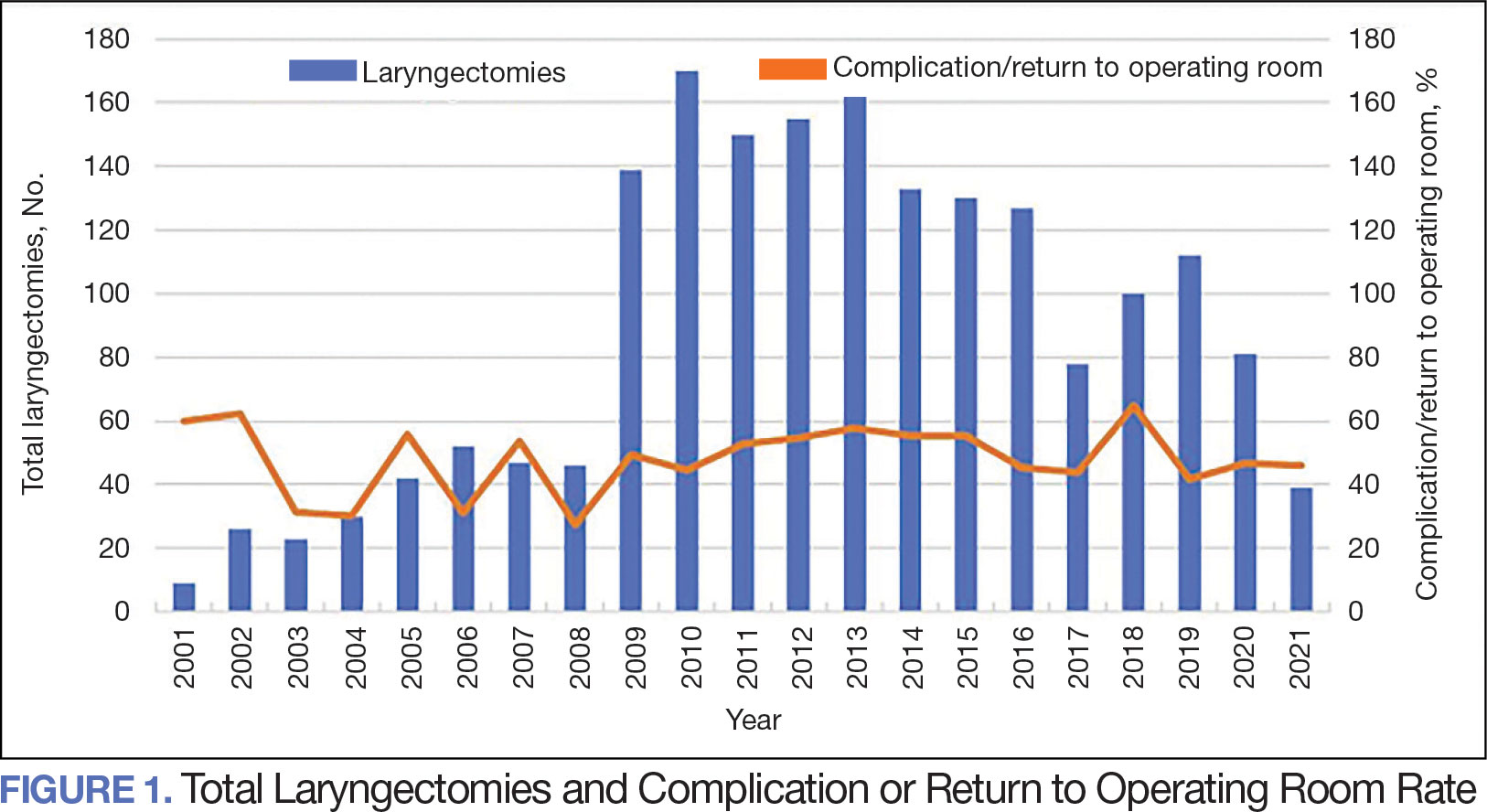
The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).
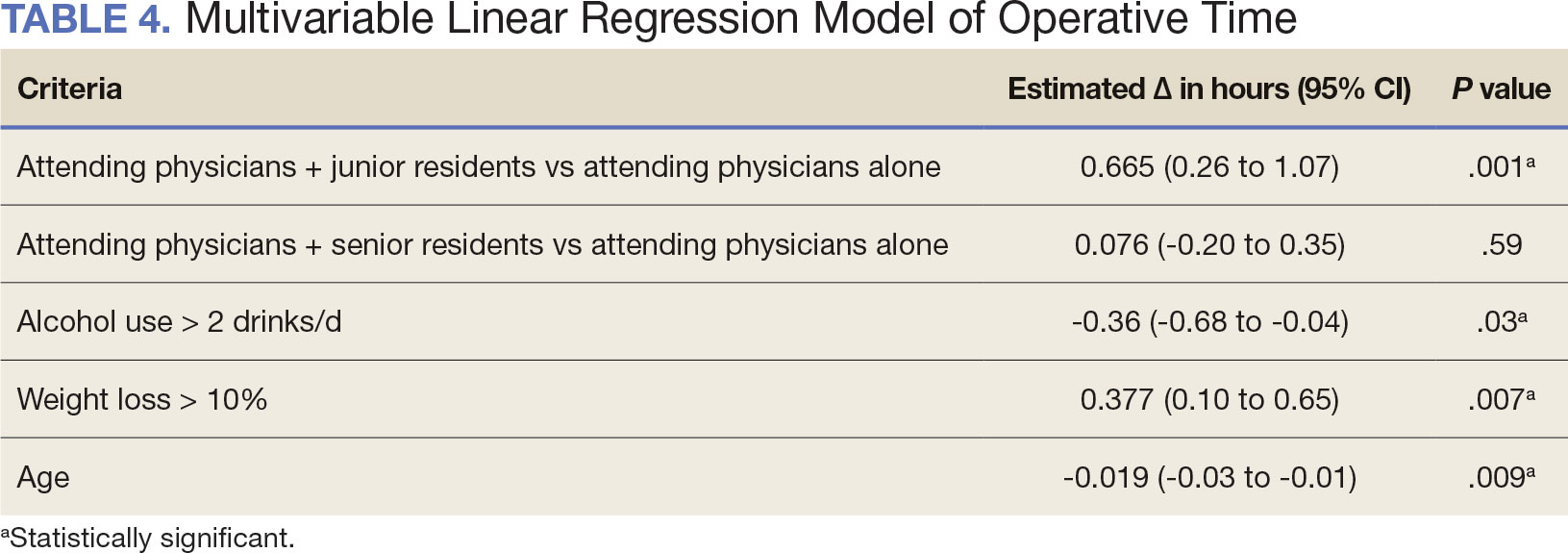
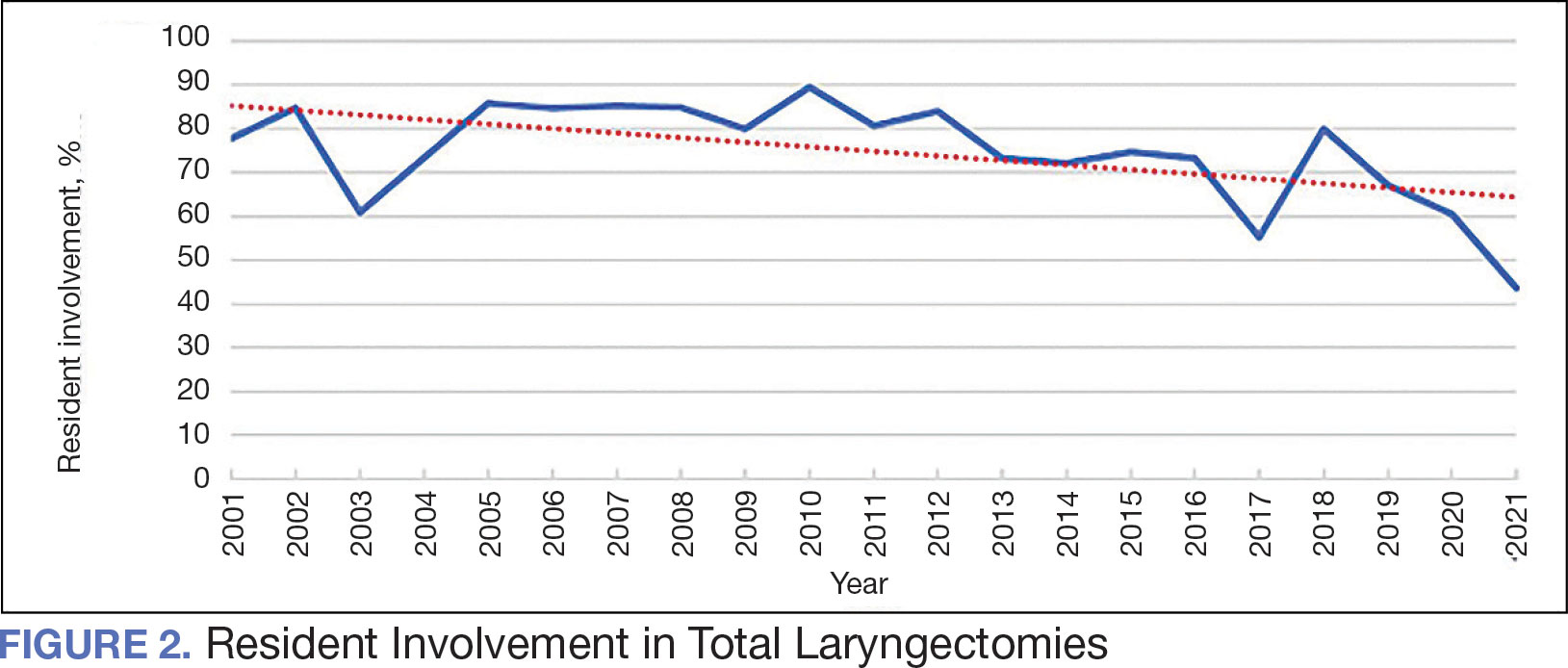
A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.
Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Skull Base Regeneration During Treatment With Chemoradiation for Nasopharyngeal Carcinoma: A Case Report
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
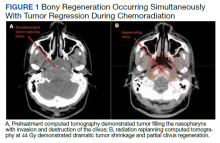
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
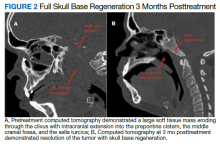
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.