User login
Once-weekly exenatide can be especially useful to treat diabetes in schizophrenia
The incidence of diabetes in people with schizophrenia is 2- to 3-fold that of the general population—which has been attributed to several variables, including adverse effects of antipsychotic drugs, susceptibility related to mental illness, lifestyle, and social health determinants.1 Controlling diabetes is important because cardiovascular consequences of the disease contribute to the shortened life expectancy seen in patients with schizophrenia.2
The dosing frequency of a newer formulation of exenatide, a glucose-lowering drug that has been available for almost a decade, can help manage type 2 diabetes mellitus in your patient with schizophrenia.
What is the new formulation and why is it appealing?
Exenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2
diabetes mellitus. GLP-1 agonists lower the blood glucose level by enhancing glucose-dependent insulin secretion, suppressing glucagon secretion, slowing gastric emptying, and enhancing satiety. Exenatide was approved by the FDA
in 2005 as a twice-daily subcutaneous injection (brand name, Byetta); the once-weekly formulation, also for subcutaneous injection (brand name, Bydureon), was approved in 2012.
Practical use for psychiatric patients
Because psychiatric patients face medication adherence challenges, the once-
weekly formulation of exenatide is appealing. The patient or a member of his (her) care team can administer the once-weekly injection.
Practitioners might be concerned that patients with comorbid psychiatric illness and diabetes will overreact to an elevated blood glucose reading by overusing medications such as oral hypoglycemics and insulin. The fixed-dosage of weekly exenatide minimizes the risk that a patient will react to a single elevated blood glucose reading.
Exenatide can produce weight loss, which might benefit patients who suffer from the metabolic adverse effects of an atypical antipsychotic, including an elevated blood glucose level and weight gain.
Real-world application
We have used once-weekly exenatide successfully in a female patient with schizophrenia who was taking quetiapine and haloperidol, and had uncontrolled diabetes resulting from medication nonadherence and lack of insight into diabetes.
The patient’s hemoglobin A1c level remained at 8.8% (target A1c, <7%, as set by the American Diabetes Association), despite taking 3 oral diabetes medications at maximum dosage.
The care team determined that daily insulin injections were too risky, given the patient’s compulsive behavior; she had a history of medication overuse in response to significantly elevated blood glucose.
Once-weekly exenatide, however, was a feasible alternative. Three months after she was started on once-weekly exenatide, and with additional lifestyle modifications, her hemoglobin A1c level had fallen to 6.4%, without any hypoglycemic episodes.
Select patients carefully
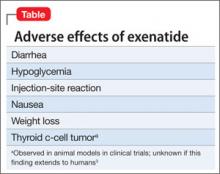
Exenatide is not a first-line therapy because of its potential side effects (Table), route of administration, and cost. Consider the once-weekly formulation of the drug on a patient-by-patient basis for patients with schizophrenia whose diabetes otherwise cannot be controlled.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health. 2006;2:14.
2. Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83-88.
3. Bydureon [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2012.
The incidence of diabetes in people with schizophrenia is 2- to 3-fold that of the general population—which has been attributed to several variables, including adverse effects of antipsychotic drugs, susceptibility related to mental illness, lifestyle, and social health determinants.1 Controlling diabetes is important because cardiovascular consequences of the disease contribute to the shortened life expectancy seen in patients with schizophrenia.2
The dosing frequency of a newer formulation of exenatide, a glucose-lowering drug that has been available for almost a decade, can help manage type 2 diabetes mellitus in your patient with schizophrenia.
What is the new formulation and why is it appealing?
Exenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2
diabetes mellitus. GLP-1 agonists lower the blood glucose level by enhancing glucose-dependent insulin secretion, suppressing glucagon secretion, slowing gastric emptying, and enhancing satiety. Exenatide was approved by the FDA
in 2005 as a twice-daily subcutaneous injection (brand name, Byetta); the once-weekly formulation, also for subcutaneous injection (brand name, Bydureon), was approved in 2012.
Practical use for psychiatric patients
Because psychiatric patients face medication adherence challenges, the once-
weekly formulation of exenatide is appealing. The patient or a member of his (her) care team can administer the once-weekly injection.
Practitioners might be concerned that patients with comorbid psychiatric illness and diabetes will overreact to an elevated blood glucose reading by overusing medications such as oral hypoglycemics and insulin. The fixed-dosage of weekly exenatide minimizes the risk that a patient will react to a single elevated blood glucose reading.
Exenatide can produce weight loss, which might benefit patients who suffer from the metabolic adverse effects of an atypical antipsychotic, including an elevated blood glucose level and weight gain.
Real-world application
We have used once-weekly exenatide successfully in a female patient with schizophrenia who was taking quetiapine and haloperidol, and had uncontrolled diabetes resulting from medication nonadherence and lack of insight into diabetes.
The patient’s hemoglobin A1c level remained at 8.8% (target A1c, <7%, as set by the American Diabetes Association), despite taking 3 oral diabetes medications at maximum dosage.
The care team determined that daily insulin injections were too risky, given the patient’s compulsive behavior; she had a history of medication overuse in response to significantly elevated blood glucose.
Once-weekly exenatide, however, was a feasible alternative. Three months after she was started on once-weekly exenatide, and with additional lifestyle modifications, her hemoglobin A1c level had fallen to 6.4%, without any hypoglycemic episodes.
Select patients carefully
Exenatide is not a first-line therapy because of its potential side effects (Table), route of administration, and cost. Consider the once-weekly formulation of the drug on a patient-by-patient basis for patients with schizophrenia whose diabetes otherwise cannot be controlled.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The incidence of diabetes in people with schizophrenia is 2- to 3-fold that of the general population—which has been attributed to several variables, including adverse effects of antipsychotic drugs, susceptibility related to mental illness, lifestyle, and social health determinants.1 Controlling diabetes is important because cardiovascular consequences of the disease contribute to the shortened life expectancy seen in patients with schizophrenia.2
The dosing frequency of a newer formulation of exenatide, a glucose-lowering drug that has been available for almost a decade, can help manage type 2 diabetes mellitus in your patient with schizophrenia.
What is the new formulation and why is it appealing?
Exenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2
diabetes mellitus. GLP-1 agonists lower the blood glucose level by enhancing glucose-dependent insulin secretion, suppressing glucagon secretion, slowing gastric emptying, and enhancing satiety. Exenatide was approved by the FDA
in 2005 as a twice-daily subcutaneous injection (brand name, Byetta); the once-weekly formulation, also for subcutaneous injection (brand name, Bydureon), was approved in 2012.
Practical use for psychiatric patients
Because psychiatric patients face medication adherence challenges, the once-
weekly formulation of exenatide is appealing. The patient or a member of his (her) care team can administer the once-weekly injection.
Practitioners might be concerned that patients with comorbid psychiatric illness and diabetes will overreact to an elevated blood glucose reading by overusing medications such as oral hypoglycemics and insulin. The fixed-dosage of weekly exenatide minimizes the risk that a patient will react to a single elevated blood glucose reading.
Exenatide can produce weight loss, which might benefit patients who suffer from the metabolic adverse effects of an atypical antipsychotic, including an elevated blood glucose level and weight gain.
Real-world application
We have used once-weekly exenatide successfully in a female patient with schizophrenia who was taking quetiapine and haloperidol, and had uncontrolled diabetes resulting from medication nonadherence and lack of insight into diabetes.
The patient’s hemoglobin A1c level remained at 8.8% (target A1c, <7%, as set by the American Diabetes Association), despite taking 3 oral diabetes medications at maximum dosage.
The care team determined that daily insulin injections were too risky, given the patient’s compulsive behavior; she had a history of medication overuse in response to significantly elevated blood glucose.
Once-weekly exenatide, however, was a feasible alternative. Three months after she was started on once-weekly exenatide, and with additional lifestyle modifications, her hemoglobin A1c level had fallen to 6.4%, without any hypoglycemic episodes.
Select patients carefully
Exenatide is not a first-line therapy because of its potential side effects (Table), route of administration, and cost. Consider the once-weekly formulation of the drug on a patient-by-patient basis for patients with schizophrenia whose diabetes otherwise cannot be controlled.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health. 2006;2:14.
2. Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83-88.
3. Bydureon [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2012.
1. De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health. 2006;2:14.
2. Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83-88.
3. Bydureon [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2012.