User login
RA Treatment Did Not Reduce Use of Sick Leave
Major Finding: Sick leave and disability pension in Sweden increased rapidly in patients with rheumatoid arthritis before the initiation of therapy, which halted but did not reverse this development.
Data Source: Data obtained from the Swedish Rheumatology Quality Register and the Social Insurance Office database.
Disclosures: The ARTIS study group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
Patients with newly diagnosed but still untreated rheumatoid arthritis increase their use of sick leave and disability pension benefits, judging from findings from a Swedish study. Initiation of standard treatment halted but did not reverse this development.
These findings suggest that the ability to work may be an important clinical measure when evaluating the needs of patients with RA, and may suggest the need for earlier intervention.
Lost productivity figures are among the indirect costs of rheumatoid arthritis. Yet, the only evidence of patients' ability to work with RA comes from studies that were small or included limited or no history of sick leave. Some limited data come in the form of secondary findings from trials of RA treatments.
To try to fill in the gaps, Martin Neovius, Ph.D., of the Karolinska Institute in Stockholm, and his colleagues in the Anti-Rheumatic Therapies in Sweden (ARTIS) study group investigated the number of days of sick leave and disability taken by patients with RA – both before and after initiation of standard therapies (Ann. Rheum. Dis. 2011;70:1407-14).
First, they identified patients aged 19-60 years who were diagnosed with RA between 1999 and 2007.
Data on when treatment was initiated came from the Swedish Rheumatology Quality Register. Next, they used each patient's personal identification number to retrieve data on sick leave and disability pension from the Social Insurance Office database.
The researchers divided the patients into four cohorts: 2,796 patients who received nonbiologic disease-modifying anti-rheumatic drug (DMARD) monotherapy; 973 patients who received nonbiologic DMARD combination therapy; 1,600 patients with RA for less than 5 years who received biologic therapy; and 4,787 patients who received biologic agents regardless of RA duration.
The prevalence of sick leave and disability pension was lowest before the start of DMARD therapy, the researchers found.
Specifically, 10% and 12% of patients received disability pension benefits during the year before starting DMARD monotherapy and combination therapy, respectively, and 43% of patients received benefits before starting biologic treatment.
The mean number of days of disability pension per year was 25 during the year before the start of DMARD monotherapy, 27 before the start of DMARD combination therapy, and 111 for biologic therapy. However, fewer days of sick leave were taken in the year before the start of biologic agents and DMARD monotherapy (mean 79 and 54 days, respectively) than before the start of DMARD combination therapy (105 days).
The mean number of monthly days of sick leave and disability pension increased in all cohorts in the year before treatment and peaked at 1 month after treatment began, the researchers said. After that first month following treatment, sick leave stabilized below the peak level, but disability pensions increased.
“We made a series of important observations,” the researchers said. “One, irrespective of treatment type, initiators had on average a long history of gradual deterioration in work ability, although, as expected, the level of days off work was higher among patients selected for biological therapy than those starting a first nonbiological DMARD monotherapy. Two, irrespective of treatment type, patients selected for these treatments were characterized by a breakpoint in the deteriorating work ability following treatment start.” The third lesson was that patients continued to use disability despite their increased ability to work, the authors reported.
Intensive treatment halted but did not reverse the deterioration of work ability, the researchers said. After initiation of treatment with biologic agents, the annual level of sick leave and disability pension was close to 200 days a year out of a maximum of 365, and more than 150 days for those who started on combination DMARD treatment.
“Given the increase observed already before treatment start, there is an obvious need to identify patients at risk of work ability deterioration much earlier than currently, and potentially break the development,” the researchers said.
Major Finding: Sick leave and disability pension in Sweden increased rapidly in patients with rheumatoid arthritis before the initiation of therapy, which halted but did not reverse this development.
Data Source: Data obtained from the Swedish Rheumatology Quality Register and the Social Insurance Office database.
Disclosures: The ARTIS study group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
Patients with newly diagnosed but still untreated rheumatoid arthritis increase their use of sick leave and disability pension benefits, judging from findings from a Swedish study. Initiation of standard treatment halted but did not reverse this development.
These findings suggest that the ability to work may be an important clinical measure when evaluating the needs of patients with RA, and may suggest the need for earlier intervention.
Lost productivity figures are among the indirect costs of rheumatoid arthritis. Yet, the only evidence of patients' ability to work with RA comes from studies that were small or included limited or no history of sick leave. Some limited data come in the form of secondary findings from trials of RA treatments.
To try to fill in the gaps, Martin Neovius, Ph.D., of the Karolinska Institute in Stockholm, and his colleagues in the Anti-Rheumatic Therapies in Sweden (ARTIS) study group investigated the number of days of sick leave and disability taken by patients with RA – both before and after initiation of standard therapies (Ann. Rheum. Dis. 2011;70:1407-14).
First, they identified patients aged 19-60 years who were diagnosed with RA between 1999 and 2007.
Data on when treatment was initiated came from the Swedish Rheumatology Quality Register. Next, they used each patient's personal identification number to retrieve data on sick leave and disability pension from the Social Insurance Office database.
The researchers divided the patients into four cohorts: 2,796 patients who received nonbiologic disease-modifying anti-rheumatic drug (DMARD) monotherapy; 973 patients who received nonbiologic DMARD combination therapy; 1,600 patients with RA for less than 5 years who received biologic therapy; and 4,787 patients who received biologic agents regardless of RA duration.
The prevalence of sick leave and disability pension was lowest before the start of DMARD therapy, the researchers found.
Specifically, 10% and 12% of patients received disability pension benefits during the year before starting DMARD monotherapy and combination therapy, respectively, and 43% of patients received benefits before starting biologic treatment.
The mean number of days of disability pension per year was 25 during the year before the start of DMARD monotherapy, 27 before the start of DMARD combination therapy, and 111 for biologic therapy. However, fewer days of sick leave were taken in the year before the start of biologic agents and DMARD monotherapy (mean 79 and 54 days, respectively) than before the start of DMARD combination therapy (105 days).
The mean number of monthly days of sick leave and disability pension increased in all cohorts in the year before treatment and peaked at 1 month after treatment began, the researchers said. After that first month following treatment, sick leave stabilized below the peak level, but disability pensions increased.
“We made a series of important observations,” the researchers said. “One, irrespective of treatment type, initiators had on average a long history of gradual deterioration in work ability, although, as expected, the level of days off work was higher among patients selected for biological therapy than those starting a first nonbiological DMARD monotherapy. Two, irrespective of treatment type, patients selected for these treatments were characterized by a breakpoint in the deteriorating work ability following treatment start.” The third lesson was that patients continued to use disability despite their increased ability to work, the authors reported.
Intensive treatment halted but did not reverse the deterioration of work ability, the researchers said. After initiation of treatment with biologic agents, the annual level of sick leave and disability pension was close to 200 days a year out of a maximum of 365, and more than 150 days for those who started on combination DMARD treatment.
“Given the increase observed already before treatment start, there is an obvious need to identify patients at risk of work ability deterioration much earlier than currently, and potentially break the development,” the researchers said.
Major Finding: Sick leave and disability pension in Sweden increased rapidly in patients with rheumatoid arthritis before the initiation of therapy, which halted but did not reverse this development.
Data Source: Data obtained from the Swedish Rheumatology Quality Register and the Social Insurance Office database.
Disclosures: The ARTIS study group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
Patients with newly diagnosed but still untreated rheumatoid arthritis increase their use of sick leave and disability pension benefits, judging from findings from a Swedish study. Initiation of standard treatment halted but did not reverse this development.
These findings suggest that the ability to work may be an important clinical measure when evaluating the needs of patients with RA, and may suggest the need for earlier intervention.
Lost productivity figures are among the indirect costs of rheumatoid arthritis. Yet, the only evidence of patients' ability to work with RA comes from studies that were small or included limited or no history of sick leave. Some limited data come in the form of secondary findings from trials of RA treatments.
To try to fill in the gaps, Martin Neovius, Ph.D., of the Karolinska Institute in Stockholm, and his colleagues in the Anti-Rheumatic Therapies in Sweden (ARTIS) study group investigated the number of days of sick leave and disability taken by patients with RA – both before and after initiation of standard therapies (Ann. Rheum. Dis. 2011;70:1407-14).
First, they identified patients aged 19-60 years who were diagnosed with RA between 1999 and 2007.
Data on when treatment was initiated came from the Swedish Rheumatology Quality Register. Next, they used each patient's personal identification number to retrieve data on sick leave and disability pension from the Social Insurance Office database.
The researchers divided the patients into four cohorts: 2,796 patients who received nonbiologic disease-modifying anti-rheumatic drug (DMARD) monotherapy; 973 patients who received nonbiologic DMARD combination therapy; 1,600 patients with RA for less than 5 years who received biologic therapy; and 4,787 patients who received biologic agents regardless of RA duration.
The prevalence of sick leave and disability pension was lowest before the start of DMARD therapy, the researchers found.
Specifically, 10% and 12% of patients received disability pension benefits during the year before starting DMARD monotherapy and combination therapy, respectively, and 43% of patients received benefits before starting biologic treatment.
The mean number of days of disability pension per year was 25 during the year before the start of DMARD monotherapy, 27 before the start of DMARD combination therapy, and 111 for biologic therapy. However, fewer days of sick leave were taken in the year before the start of biologic agents and DMARD monotherapy (mean 79 and 54 days, respectively) than before the start of DMARD combination therapy (105 days).
The mean number of monthly days of sick leave and disability pension increased in all cohorts in the year before treatment and peaked at 1 month after treatment began, the researchers said. After that first month following treatment, sick leave stabilized below the peak level, but disability pensions increased.
“We made a series of important observations,” the researchers said. “One, irrespective of treatment type, initiators had on average a long history of gradual deterioration in work ability, although, as expected, the level of days off work was higher among patients selected for biological therapy than those starting a first nonbiological DMARD monotherapy. Two, irrespective of treatment type, patients selected for these treatments were characterized by a breakpoint in the deteriorating work ability following treatment start.” The third lesson was that patients continued to use disability despite their increased ability to work, the authors reported.
Intensive treatment halted but did not reverse the deterioration of work ability, the researchers said. After initiation of treatment with biologic agents, the annual level of sick leave and disability pension was close to 200 days a year out of a maximum of 365, and more than 150 days for those who started on combination DMARD treatment.
“Given the increase observed already before treatment start, there is an obvious need to identify patients at risk of work ability deterioration much earlier than currently, and potentially break the development,” the researchers said.
Joint Distraction May Delay Need for Knee Replacement
Joint distraction can induce tissue structure modification in knee osteoarthritis, possibly reversing structural damage to cartilage tissue and delaying the need for knee replacement surgery. This is according to results of an open 1-year pilot study reported in the August issue of Annals of the Rheumatic Diseases.
Endoprosthesis currently is the accepted method for treating pain caused by end-stage knee OA. However, the growing number of procedures carries a high price tag, and there is a higher risk of failure in patients aged younger than 65 years.
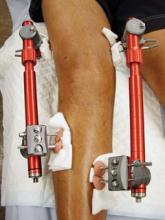
With that in mind, Dr. Femke Intema of the University Medical Center Utrecht (the Netherlands) and colleagues wanted to determine whether joint distraction could halt and possibly reverse joint degeneration in knee OA (Ann. Rheum. Dis. 2011;70:1441-6).
The study included 11 men and 9 women who had knee OA and in whom knee replacement surgery was indicated in 2006-2008. Patients were an average of 48 years old; 18 of them had predominant OA in the medial compartment; the remaining two had OA in the lateral compartment. Patients had a score of 60 mm or higher on the Visual Analogue Scale (VAS) of pain, as well as radiographic signs of joint damage, and primarily tibiofemoral OA.
Joint distraction was applied for 2 months via an external fixation frame. At the 1-year follow-up, researchers evaluated tissue structure modification according to the following:
• Radiographic analysis. This showed that the mean joint space width (JSW) of the most affected compartment increased from 2.7 mm to 3.6 mm between baseline and 12 months, whereas the minimum JSW increased from 1 mm to 1.9 mm.
• Quantitative MRI analysis. At 1 year, this showed an increase in the mean thickness of cartilage over total area of bone (ThCtAB) from 2.4 mm to 3 mm in the most affected compartment, and a decrease in mean percentage area of denuded bone (dABp) from 22% to 5%. The thickness of cartilage over area of bone covered with cartilage (ThCcAB), a secondary structural outcome parameter, showed a borderline increase from 2.9 mm to 3.1 mm.
• Biomarker analysis on serum and urine samples. These showed an 11% decrease of CTXII (a collagen type II breakdown marker), and a 103% increase in PILANP/CTXII (a collage type II synthesis marker), between 6 and 12 months. These findings suggest a net increase in collagen synthesis, the researchers said.
The primary outcome parameter of this study was the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index questionnaire, which decreased from 55 points at baseline to 23 points at 1 year. In all, 18 of the 20 patients showed a greater-than-10% improvement, and 16 showed a greater-than-25% improvement. There were significant improvements in the individual components of the WOMAC index, namely pain, stiffness, and function.
One secondary measure, the VAS pain score, decreased from 73 mm at baseline to 31 mm at 1 year. Physical examination of the joint, which assessed crepitus, pain on palpation, pain with flexion, and joint effusion, showed improvement from 46% to 75%.
"This study is the first to demonstrate intrinsic tissue structure repair in OA," the researchers said. "Historically, the regenerative capacity of cartilage has been questioned owing to the slow turnover rate of cartilage matrix, especially of collagen. However, this study shows that a significant amount of cartilage tissue is formed within 1 year after the distraction, demonstrating that under certain conditions, cartilage has regenerative capacity."
There is uncertainty as to the underlying mechanism of the structural repair that was seen in this study. One possibility is that temporary distraction prevents mechanical stress on the cartilage, eliminates further wear and tear, and allows tissue repair to begin, the researchers said.
For now, the researchers are unsure which patients may benefit from this procedure, as the study included only those patients who were younger than 50 years, had severe OA, and were likely candidates for joint replacement surgery. Referrals from peripheral hospitals may have led to selection bias, the researchers say.
Safety concerns exist as well. Two patients developed lung emboli and required hospitalization and anticoagulative treatment. Also, 17 patients developed single or multiple pin-tract infections, all of which were successfully treated with antibiotics.
Larger and longer trials in a variety of OA populations are needed to optimize treatment, to determine which patients would benefit the most and for the longest period of time, and to pay attention to reducing the number of complications, the researchers said.
The authors had no relationships to disclose. The Dutch Arthritis Foundation provided financial support for this study.
Joint distraction can induce tissue structure modification in knee osteoarthritis, possibly reversing structural damage to cartilage tissue and delaying the need for knee replacement surgery. This is according to results of an open 1-year pilot study reported in the August issue of Annals of the Rheumatic Diseases.
Endoprosthesis currently is the accepted method for treating pain caused by end-stage knee OA. However, the growing number of procedures carries a high price tag, and there is a higher risk of failure in patients aged younger than 65 years.
With that in mind, Dr. Femke Intema of the University Medical Center Utrecht (the Netherlands) and colleagues wanted to determine whether joint distraction could halt and possibly reverse joint degeneration in knee OA (Ann. Rheum. Dis. 2011;70:1441-6).
The study included 11 men and 9 women who had knee OA and in whom knee replacement surgery was indicated in 2006-2008. Patients were an average of 48 years old; 18 of them had predominant OA in the medial compartment; the remaining two had OA in the lateral compartment. Patients had a score of 60 mm or higher on the Visual Analogue Scale (VAS) of pain, as well as radiographic signs of joint damage, and primarily tibiofemoral OA.
Joint distraction was applied for 2 months via an external fixation frame. At the 1-year follow-up, researchers evaluated tissue structure modification according to the following:
• Radiographic analysis. This showed that the mean joint space width (JSW) of the most affected compartment increased from 2.7 mm to 3.6 mm between baseline and 12 months, whereas the minimum JSW increased from 1 mm to 1.9 mm.
• Quantitative MRI analysis. At 1 year, this showed an increase in the mean thickness of cartilage over total area of bone (ThCtAB) from 2.4 mm to 3 mm in the most affected compartment, and a decrease in mean percentage area of denuded bone (dABp) from 22% to 5%. The thickness of cartilage over area of bone covered with cartilage (ThCcAB), a secondary structural outcome parameter, showed a borderline increase from 2.9 mm to 3.1 mm.
• Biomarker analysis on serum and urine samples. These showed an 11% decrease of CTXII (a collagen type II breakdown marker), and a 103% increase in PILANP/CTXII (a collage type II synthesis marker), between 6 and 12 months. These findings suggest a net increase in collagen synthesis, the researchers said.
The primary outcome parameter of this study was the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index questionnaire, which decreased from 55 points at baseline to 23 points at 1 year. In all, 18 of the 20 patients showed a greater-than-10% improvement, and 16 showed a greater-than-25% improvement. There were significant improvements in the individual components of the WOMAC index, namely pain, stiffness, and function.
One secondary measure, the VAS pain score, decreased from 73 mm at baseline to 31 mm at 1 year. Physical examination of the joint, which assessed crepitus, pain on palpation, pain with flexion, and joint effusion, showed improvement from 46% to 75%.
"This study is the first to demonstrate intrinsic tissue structure repair in OA," the researchers said. "Historically, the regenerative capacity of cartilage has been questioned owing to the slow turnover rate of cartilage matrix, especially of collagen. However, this study shows that a significant amount of cartilage tissue is formed within 1 year after the distraction, demonstrating that under certain conditions, cartilage has regenerative capacity."
There is uncertainty as to the underlying mechanism of the structural repair that was seen in this study. One possibility is that temporary distraction prevents mechanical stress on the cartilage, eliminates further wear and tear, and allows tissue repair to begin, the researchers said.
For now, the researchers are unsure which patients may benefit from this procedure, as the study included only those patients who were younger than 50 years, had severe OA, and were likely candidates for joint replacement surgery. Referrals from peripheral hospitals may have led to selection bias, the researchers say.
Safety concerns exist as well. Two patients developed lung emboli and required hospitalization and anticoagulative treatment. Also, 17 patients developed single or multiple pin-tract infections, all of which were successfully treated with antibiotics.
Larger and longer trials in a variety of OA populations are needed to optimize treatment, to determine which patients would benefit the most and for the longest period of time, and to pay attention to reducing the number of complications, the researchers said.
The authors had no relationships to disclose. The Dutch Arthritis Foundation provided financial support for this study.
Joint distraction can induce tissue structure modification in knee osteoarthritis, possibly reversing structural damage to cartilage tissue and delaying the need for knee replacement surgery. This is according to results of an open 1-year pilot study reported in the August issue of Annals of the Rheumatic Diseases.
Endoprosthesis currently is the accepted method for treating pain caused by end-stage knee OA. However, the growing number of procedures carries a high price tag, and there is a higher risk of failure in patients aged younger than 65 years.
With that in mind, Dr. Femke Intema of the University Medical Center Utrecht (the Netherlands) and colleagues wanted to determine whether joint distraction could halt and possibly reverse joint degeneration in knee OA (Ann. Rheum. Dis. 2011;70:1441-6).
The study included 11 men and 9 women who had knee OA and in whom knee replacement surgery was indicated in 2006-2008. Patients were an average of 48 years old; 18 of them had predominant OA in the medial compartment; the remaining two had OA in the lateral compartment. Patients had a score of 60 mm or higher on the Visual Analogue Scale (VAS) of pain, as well as radiographic signs of joint damage, and primarily tibiofemoral OA.
Joint distraction was applied for 2 months via an external fixation frame. At the 1-year follow-up, researchers evaluated tissue structure modification according to the following:
• Radiographic analysis. This showed that the mean joint space width (JSW) of the most affected compartment increased from 2.7 mm to 3.6 mm between baseline and 12 months, whereas the minimum JSW increased from 1 mm to 1.9 mm.
• Quantitative MRI analysis. At 1 year, this showed an increase in the mean thickness of cartilage over total area of bone (ThCtAB) from 2.4 mm to 3 mm in the most affected compartment, and a decrease in mean percentage area of denuded bone (dABp) from 22% to 5%. The thickness of cartilage over area of bone covered with cartilage (ThCcAB), a secondary structural outcome parameter, showed a borderline increase from 2.9 mm to 3.1 mm.
• Biomarker analysis on serum and urine samples. These showed an 11% decrease of CTXII (a collagen type II breakdown marker), and a 103% increase in PILANP/CTXII (a collage type II synthesis marker), between 6 and 12 months. These findings suggest a net increase in collagen synthesis, the researchers said.
The primary outcome parameter of this study was the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index questionnaire, which decreased from 55 points at baseline to 23 points at 1 year. In all, 18 of the 20 patients showed a greater-than-10% improvement, and 16 showed a greater-than-25% improvement. There were significant improvements in the individual components of the WOMAC index, namely pain, stiffness, and function.
One secondary measure, the VAS pain score, decreased from 73 mm at baseline to 31 mm at 1 year. Physical examination of the joint, which assessed crepitus, pain on palpation, pain with flexion, and joint effusion, showed improvement from 46% to 75%.
"This study is the first to demonstrate intrinsic tissue structure repair in OA," the researchers said. "Historically, the regenerative capacity of cartilage has been questioned owing to the slow turnover rate of cartilage matrix, especially of collagen. However, this study shows that a significant amount of cartilage tissue is formed within 1 year after the distraction, demonstrating that under certain conditions, cartilage has regenerative capacity."
There is uncertainty as to the underlying mechanism of the structural repair that was seen in this study. One possibility is that temporary distraction prevents mechanical stress on the cartilage, eliminates further wear and tear, and allows tissue repair to begin, the researchers said.
For now, the researchers are unsure which patients may benefit from this procedure, as the study included only those patients who were younger than 50 years, had severe OA, and were likely candidates for joint replacement surgery. Referrals from peripheral hospitals may have led to selection bias, the researchers say.
Safety concerns exist as well. Two patients developed lung emboli and required hospitalization and anticoagulative treatment. Also, 17 patients developed single or multiple pin-tract infections, all of which were successfully treated with antibiotics.
Larger and longer trials in a variety of OA populations are needed to optimize treatment, to determine which patients would benefit the most and for the longest period of time, and to pay attention to reducing the number of complications, the researchers said.
The authors had no relationships to disclose. The Dutch Arthritis Foundation provided financial support for this study.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major Finding: Joint distraction can induce tissue structure modification in knee osteoarthritis, as shown on radiography, MRI, and blood work, possibly delaying the need for endoprosthesis.
Data Source: An open, 1-year pilot study of 20 patients with tibiofemoral osteoarthritis who were treated surgically with joint distraction.
Disclosures: The authors had no relationships to disclose. The Dutch Arthritis Foundation provided financial support for this study.
Lost Productivity Chief Among Indirect Costs of Rheumatoid Arthritis
Patients with newly diagnosed but still untreated rheumatoid arthritis increase their use of sick leave and disability pension benefits, judging from findings from a Swedish study. Initiation of standard treatment halted but did not reverse this development.
These findings, reported in the August issue of Annals of the Rheumatic Diseases, suggest that the ability to work may be an important clinical measure when evaluating the needs of patients with RA, and may indicate the need for earlier intervention.
Lost productivity figures large among the indirect costs of rheumatoid arthritis. Yet, the only evidence of patients’ ability to work with RA comes from studies that were small or included limited or no history of sick leave. Some limited data come in the form of secondary findings from trials of RA treatments.
To try to fill in the gaps, Martin Neovius, Ph.D., of the Karolinska Institute in Stockholm, and his colleagues in the Anti-Rheumatic Therapies in Sweden (ARTIS) Study Group investigated the number of days of sick leave and disability taken by patients with RA – both before and after initiation of standard therapies (Ann. Rheum. Dis. 2011;70:1407-14).
First, they identified patients aged 19-60 years who were diagnosed with RA between 1999 and 2007. Data on when treatment was initiated came from the Swedish Rheumatology Quality Register. Next, they used each patient’s personal identification number to retrieve data on sick leave and disability pension from the Social Insurance Office database.
The researchers divided the patients into four cohorts: 2,796 patients who received nonbiologic disease-modifying anti-rheumatic drug (DMARD) monotherapy; 973 patients who received nonbiologic DMARD combination therapy; 1,600 patients with RA for less than 5 years who received biologic therapy; and 4,787 patients who received biologic agents regardless of RA duration.
The prevalence of sick leave and disability pension was lowest before the start of DMARD therapy, the researchers found. Specifically, 10% and 12% of patients received disability pension benefits during the year before starting DMARD monotherapy and combination therapy, respectively, and 43% of patients received benefits before starting biologic treatment.
The mean number of days of disability pension per year was 25 during the year before the start of DMARD monotherapy, 27 before the start of DMARD combination therapy, and 111 for biologic therapy. However, fewer days of sick leave were taken in the year before the start of biologic agents and DMARD monotherapy (mean 79 and 54 days, respectively) than before the start of DMARD combination therapy (105 days).
The mean number of monthly days of sick leave and disability pension increased in all cohorts in the year before treatment and peaked at 1 month after treatment began, the researchers said. After that first month following treatment, sick leave stabilized below the peak level, but disability pensions increased.
"We made a series of important observations," the researchers said. "One, irrespective of treatment type, initiators had on average a long history of gradual deterioration in work ability, although, as expected, the level of days off work was higher among patients selected for biological therapy than those starting a first nonbiological DMARD monotherapy. Two, irrespective of treatment type, patients selected for these treatments were characterized by a breakpoint in the deteriorating work ability following treatment start." The third lesson was that patients continued to use disability despite their increased ability to work, the authors reported.
Intensive treatment halted but did not reverse the deterioration of work ability, the researchers said. After initiation of treatment with biologic agents, the annual level of sick leave and disability pension was close to 200 days a year out of a maximum of 365, and more than 150 days for those who started on combination DMARD treatment.
"Given the increase observed already before treatment start, there is an obvious need to identify patients at risk of work ability deterioration much earlier than currently, and potentially break the development," the researchers said.
The ARTIS Study Group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
Patients with newly diagnosed but still untreated rheumatoid arthritis increase their use of sick leave and disability pension benefits, judging from findings from a Swedish study. Initiation of standard treatment halted but did not reverse this development.
These findings, reported in the August issue of Annals of the Rheumatic Diseases, suggest that the ability to work may be an important clinical measure when evaluating the needs of patients with RA, and may indicate the need for earlier intervention.
Lost productivity figures large among the indirect costs of rheumatoid arthritis. Yet, the only evidence of patients’ ability to work with RA comes from studies that were small or included limited or no history of sick leave. Some limited data come in the form of secondary findings from trials of RA treatments.
To try to fill in the gaps, Martin Neovius, Ph.D., of the Karolinska Institute in Stockholm, and his colleagues in the Anti-Rheumatic Therapies in Sweden (ARTIS) Study Group investigated the number of days of sick leave and disability taken by patients with RA – both before and after initiation of standard therapies (Ann. Rheum. Dis. 2011;70:1407-14).
First, they identified patients aged 19-60 years who were diagnosed with RA between 1999 and 2007. Data on when treatment was initiated came from the Swedish Rheumatology Quality Register. Next, they used each patient’s personal identification number to retrieve data on sick leave and disability pension from the Social Insurance Office database.
The researchers divided the patients into four cohorts: 2,796 patients who received nonbiologic disease-modifying anti-rheumatic drug (DMARD) monotherapy; 973 patients who received nonbiologic DMARD combination therapy; 1,600 patients with RA for less than 5 years who received biologic therapy; and 4,787 patients who received biologic agents regardless of RA duration.
The prevalence of sick leave and disability pension was lowest before the start of DMARD therapy, the researchers found. Specifically, 10% and 12% of patients received disability pension benefits during the year before starting DMARD monotherapy and combination therapy, respectively, and 43% of patients received benefits before starting biologic treatment.
The mean number of days of disability pension per year was 25 during the year before the start of DMARD monotherapy, 27 before the start of DMARD combination therapy, and 111 for biologic therapy. However, fewer days of sick leave were taken in the year before the start of biologic agents and DMARD monotherapy (mean 79 and 54 days, respectively) than before the start of DMARD combination therapy (105 days).
The mean number of monthly days of sick leave and disability pension increased in all cohorts in the year before treatment and peaked at 1 month after treatment began, the researchers said. After that first month following treatment, sick leave stabilized below the peak level, but disability pensions increased.
"We made a series of important observations," the researchers said. "One, irrespective of treatment type, initiators had on average a long history of gradual deterioration in work ability, although, as expected, the level of days off work was higher among patients selected for biological therapy than those starting a first nonbiological DMARD monotherapy. Two, irrespective of treatment type, patients selected for these treatments were characterized by a breakpoint in the deteriorating work ability following treatment start." The third lesson was that patients continued to use disability despite their increased ability to work, the authors reported.
Intensive treatment halted but did not reverse the deterioration of work ability, the researchers said. After initiation of treatment with biologic agents, the annual level of sick leave and disability pension was close to 200 days a year out of a maximum of 365, and more than 150 days for those who started on combination DMARD treatment.
"Given the increase observed already before treatment start, there is an obvious need to identify patients at risk of work ability deterioration much earlier than currently, and potentially break the development," the researchers said.
The ARTIS Study Group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
Patients with newly diagnosed but still untreated rheumatoid arthritis increase their use of sick leave and disability pension benefits, judging from findings from a Swedish study. Initiation of standard treatment halted but did not reverse this development.
These findings, reported in the August issue of Annals of the Rheumatic Diseases, suggest that the ability to work may be an important clinical measure when evaluating the needs of patients with RA, and may indicate the need for earlier intervention.
Lost productivity figures large among the indirect costs of rheumatoid arthritis. Yet, the only evidence of patients’ ability to work with RA comes from studies that were small or included limited or no history of sick leave. Some limited data come in the form of secondary findings from trials of RA treatments.
To try to fill in the gaps, Martin Neovius, Ph.D., of the Karolinska Institute in Stockholm, and his colleagues in the Anti-Rheumatic Therapies in Sweden (ARTIS) Study Group investigated the number of days of sick leave and disability taken by patients with RA – both before and after initiation of standard therapies (Ann. Rheum. Dis. 2011;70:1407-14).
First, they identified patients aged 19-60 years who were diagnosed with RA between 1999 and 2007. Data on when treatment was initiated came from the Swedish Rheumatology Quality Register. Next, they used each patient’s personal identification number to retrieve data on sick leave and disability pension from the Social Insurance Office database.
The researchers divided the patients into four cohorts: 2,796 patients who received nonbiologic disease-modifying anti-rheumatic drug (DMARD) monotherapy; 973 patients who received nonbiologic DMARD combination therapy; 1,600 patients with RA for less than 5 years who received biologic therapy; and 4,787 patients who received biologic agents regardless of RA duration.
The prevalence of sick leave and disability pension was lowest before the start of DMARD therapy, the researchers found. Specifically, 10% and 12% of patients received disability pension benefits during the year before starting DMARD monotherapy and combination therapy, respectively, and 43% of patients received benefits before starting biologic treatment.
The mean number of days of disability pension per year was 25 during the year before the start of DMARD monotherapy, 27 before the start of DMARD combination therapy, and 111 for biologic therapy. However, fewer days of sick leave were taken in the year before the start of biologic agents and DMARD monotherapy (mean 79 and 54 days, respectively) than before the start of DMARD combination therapy (105 days).
The mean number of monthly days of sick leave and disability pension increased in all cohorts in the year before treatment and peaked at 1 month after treatment began, the researchers said. After that first month following treatment, sick leave stabilized below the peak level, but disability pensions increased.
"We made a series of important observations," the researchers said. "One, irrespective of treatment type, initiators had on average a long history of gradual deterioration in work ability, although, as expected, the level of days off work was higher among patients selected for biological therapy than those starting a first nonbiological DMARD monotherapy. Two, irrespective of treatment type, patients selected for these treatments were characterized by a breakpoint in the deteriorating work ability following treatment start." The third lesson was that patients continued to use disability despite their increased ability to work, the authors reported.
Intensive treatment halted but did not reverse the deterioration of work ability, the researchers said. After initiation of treatment with biologic agents, the annual level of sick leave and disability pension was close to 200 days a year out of a maximum of 365, and more than 150 days for those who started on combination DMARD treatment.
"Given the increase observed already before treatment start, there is an obvious need to identify patients at risk of work ability deterioration much earlier than currently, and potentially break the development," the researchers said.
The ARTIS Study Group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major Finding: Sick leave and disability pension in Sweden increased rapidly in patients with rheumatoid arthritis before the initiation of therapy, which halted but did not reverse this development.
Data Source: Data obtained from the Swedish Rheumatology Quality Register and the Social Insurance Office database.
Disclosures: The ARTIS Study Group conducts scientific analyses using data from the Swedish Biologics Register ARTIS run by the Swedish Society for Rheumatology. For the maintenance of this register, the Swedish Society for Rheumatology has received funding, independent of the conduct of these scientific analyses, from Abbott Laboratories, BMS, Roche, Schering-Plough, UCB, and Wyeth.
Pulmonary Embolism Treatment Often Lags
Contrary to previous studies, only 1 in 100 patients with pulmonary embolism dies in emergency departments, but more standardized treatment, including earlier use of anticoagulants, is needed, according to the initial report of the EMPEROR study.
Of the 20 patients in this study whose death was due to PE, only 3 received anticoagulant treatment before a diagnosis was confirmed, even though previous studies have shown that delays in starting anticoagulants can be fatal.
Only another 3 of the 20 patients received fibrinolytic therapy in the emergency department, the Emergency Medicine Pulmonary Embolism in the Real World Registry (EMPEROR) researchers found.
Dr. Charles V. Pollack of Pennsylvania Hospital, Philadelphia, and colleagues created the national, prospective, multicenter, observational registry to establish more definitive signs and symptoms of PE, compare treatment strategies, and measure use of risk stratification methods, frequency of empiric anticoagulation use, and rates of major hemorrhage and death.
Earlier registries of patients with PE have not provided specific data about race or ethnicity, risk stratification methods, or the frequency of anticoagulant use in the emergency department.
Between Jan. 1, 2005, and Dec. 29, 2008, the researchers enrolled 2,408 patients from 22 emergency departments, 1,880 of whom had confirmed PE (J. Am. Coll. Cardiol. 2011;57:700-6).
Systemic non–vitamin K–dependent anticoagulation was initiated in the ED in 1,593 (84%) patients, but only 173 (9%) received heparin of any type before the results of diagnostic imaging were available and 33 (1.7%) received a fibrinolytic agent.
While other registries show a mortality of 10%-15%, only 20 of the 1,880 patients (1%) in this study died as a direct result of the embolism, with 0.2% mortality from hemorrhage.
At 30 days, patients whose acute PE was diagnosed in emergency departments had an all-cause mortality of 5.4%.
The lower mortality in this report may reflect the fact that the registry included only outpatients who experienced PE and that this population was younger and less ill than those in other registries, the researchers said.
Of the 1,880 patients with confirmed PE, 1,654 (88%) were diagnosed based on CT pulmonary angiogram, 91 (5%) on formal pulmonary angiography, 82 (4%) on ventilation-perfusion scan, 51 (3%) on deep vein thrombosis with appropriate PE symptoms, and 2 (0.1%) on pulmonary MRI.
The mean age was 57 years, with one-third past age 65; 53% of the patients were women, and 68% were white. The racial and ethnic distribution of patients with PE closely parallels that of all patients who present to emergency departments.
The most common presenting signs and symptoms of PE were dyspnea at rest (50%), pleuritic chest pain (39%), dyspnea with exertion (27%), extremity swelling suggestive of deep vein thrombosis (24%), and syncope (5%).
The most common comorbidities that could represent potential risk factors for PE were hypertension (46%), obesity (27%), recent hospitalization (24%), and active malignancy (22%). Based on the absence of any predefined risk factors for PE, 312 (16.6%) of the 1,880 patients were considered to have idiopathic PE.
Most of the patients (79%) diagnosed with PE in the ED lived independently, while 11.6% reported generalized immobility.
The low rate of early treatment found by the study "suggested that empiric anticoagulation in patients with suspected PE should be instituted more often in the ED and that timely, therapeutic anticoagulation should be administered after the diagnosis is confirmed," the researchers wrote.
"Future treatment studies of PE conducted in U.S. EDs should focus on accelerating the time frame of administration of systemic anticoagulation and fibrinolysis to patients with evidence of severe PE."
Several coauthors disclosed consulting relationships with and/or funding from a wide range of pharmaceutical companies that make anticoagulant agents.
This registry data probably underestimate the occurrence of fatal PE as it includes only outputs and has a younger patient population presumably with fewer comorbidities. Timely recognition with rapid achievement of therapeutic anti coagulation is critical. Hemodynamically unstable patients should be considered for pulmonary artery thrombectomy. PE should be included in the differential diagnosis of all hemodynamically unstable patients who present to the ED.
Joann M. Lohr, M.D., R.V.T., is an associate program director of the Good Samaritan Hospital Vascular Surgery Residency Program in Cincinnati, Ohio, and the director of the John J. Cranley Vascular Laboratory at Good Samaritan Hospital in Cincinnati.
This registry data probably underestimate the occurrence of fatal PE as it includes only outputs and has a younger patient population presumably with fewer comorbidities. Timely recognition with rapid achievement of therapeutic anti coagulation is critical. Hemodynamically unstable patients should be considered for pulmonary artery thrombectomy. PE should be included in the differential diagnosis of all hemodynamically unstable patients who present to the ED.
Joann M. Lohr, M.D., R.V.T., is an associate program director of the Good Samaritan Hospital Vascular Surgery Residency Program in Cincinnati, Ohio, and the director of the John J. Cranley Vascular Laboratory at Good Samaritan Hospital in Cincinnati.
This registry data probably underestimate the occurrence of fatal PE as it includes only outputs and has a younger patient population presumably with fewer comorbidities. Timely recognition with rapid achievement of therapeutic anti coagulation is critical. Hemodynamically unstable patients should be considered for pulmonary artery thrombectomy. PE should be included in the differential diagnosis of all hemodynamically unstable patients who present to the ED.
Joann M. Lohr, M.D., R.V.T., is an associate program director of the Good Samaritan Hospital Vascular Surgery Residency Program in Cincinnati, Ohio, and the director of the John J. Cranley Vascular Laboratory at Good Samaritan Hospital in Cincinnati.
Contrary to previous studies, only 1 in 100 patients with pulmonary embolism dies in emergency departments, but more standardized treatment, including earlier use of anticoagulants, is needed, according to the initial report of the EMPEROR study.
Of the 20 patients in this study whose death was due to PE, only 3 received anticoagulant treatment before a diagnosis was confirmed, even though previous studies have shown that delays in starting anticoagulants can be fatal.
Only another 3 of the 20 patients received fibrinolytic therapy in the emergency department, the Emergency Medicine Pulmonary Embolism in the Real World Registry (EMPEROR) researchers found.
Dr. Charles V. Pollack of Pennsylvania Hospital, Philadelphia, and colleagues created the national, prospective, multicenter, observational registry to establish more definitive signs and symptoms of PE, compare treatment strategies, and measure use of risk stratification methods, frequency of empiric anticoagulation use, and rates of major hemorrhage and death.
Earlier registries of patients with PE have not provided specific data about race or ethnicity, risk stratification methods, or the frequency of anticoagulant use in the emergency department.
Between Jan. 1, 2005, and Dec. 29, 2008, the researchers enrolled 2,408 patients from 22 emergency departments, 1,880 of whom had confirmed PE (J. Am. Coll. Cardiol. 2011;57:700-6).
Systemic non–vitamin K–dependent anticoagulation was initiated in the ED in 1,593 (84%) patients, but only 173 (9%) received heparin of any type before the results of diagnostic imaging were available and 33 (1.7%) received a fibrinolytic agent.
While other registries show a mortality of 10%-15%, only 20 of the 1,880 patients (1%) in this study died as a direct result of the embolism, with 0.2% mortality from hemorrhage.
At 30 days, patients whose acute PE was diagnosed in emergency departments had an all-cause mortality of 5.4%.
The lower mortality in this report may reflect the fact that the registry included only outpatients who experienced PE and that this population was younger and less ill than those in other registries, the researchers said.
Of the 1,880 patients with confirmed PE, 1,654 (88%) were diagnosed based on CT pulmonary angiogram, 91 (5%) on formal pulmonary angiography, 82 (4%) on ventilation-perfusion scan, 51 (3%) on deep vein thrombosis with appropriate PE symptoms, and 2 (0.1%) on pulmonary MRI.
The mean age was 57 years, with one-third past age 65; 53% of the patients were women, and 68% were white. The racial and ethnic distribution of patients with PE closely parallels that of all patients who present to emergency departments.
The most common presenting signs and symptoms of PE were dyspnea at rest (50%), pleuritic chest pain (39%), dyspnea with exertion (27%), extremity swelling suggestive of deep vein thrombosis (24%), and syncope (5%).
The most common comorbidities that could represent potential risk factors for PE were hypertension (46%), obesity (27%), recent hospitalization (24%), and active malignancy (22%). Based on the absence of any predefined risk factors for PE, 312 (16.6%) of the 1,880 patients were considered to have idiopathic PE.
Most of the patients (79%) diagnosed with PE in the ED lived independently, while 11.6% reported generalized immobility.
The low rate of early treatment found by the study "suggested that empiric anticoagulation in patients with suspected PE should be instituted more often in the ED and that timely, therapeutic anticoagulation should be administered after the diagnosis is confirmed," the researchers wrote.
"Future treatment studies of PE conducted in U.S. EDs should focus on accelerating the time frame of administration of systemic anticoagulation and fibrinolysis to patients with evidence of severe PE."
Several coauthors disclosed consulting relationships with and/or funding from a wide range of pharmaceutical companies that make anticoagulant agents.
Contrary to previous studies, only 1 in 100 patients with pulmonary embolism dies in emergency departments, but more standardized treatment, including earlier use of anticoagulants, is needed, according to the initial report of the EMPEROR study.
Of the 20 patients in this study whose death was due to PE, only 3 received anticoagulant treatment before a diagnosis was confirmed, even though previous studies have shown that delays in starting anticoagulants can be fatal.
Only another 3 of the 20 patients received fibrinolytic therapy in the emergency department, the Emergency Medicine Pulmonary Embolism in the Real World Registry (EMPEROR) researchers found.
Dr. Charles V. Pollack of Pennsylvania Hospital, Philadelphia, and colleagues created the national, prospective, multicenter, observational registry to establish more definitive signs and symptoms of PE, compare treatment strategies, and measure use of risk stratification methods, frequency of empiric anticoagulation use, and rates of major hemorrhage and death.
Earlier registries of patients with PE have not provided specific data about race or ethnicity, risk stratification methods, or the frequency of anticoagulant use in the emergency department.
Between Jan. 1, 2005, and Dec. 29, 2008, the researchers enrolled 2,408 patients from 22 emergency departments, 1,880 of whom had confirmed PE (J. Am. Coll. Cardiol. 2011;57:700-6).
Systemic non–vitamin K–dependent anticoagulation was initiated in the ED in 1,593 (84%) patients, but only 173 (9%) received heparin of any type before the results of diagnostic imaging were available and 33 (1.7%) received a fibrinolytic agent.
While other registries show a mortality of 10%-15%, only 20 of the 1,880 patients (1%) in this study died as a direct result of the embolism, with 0.2% mortality from hemorrhage.
At 30 days, patients whose acute PE was diagnosed in emergency departments had an all-cause mortality of 5.4%.
The lower mortality in this report may reflect the fact that the registry included only outpatients who experienced PE and that this population was younger and less ill than those in other registries, the researchers said.
Of the 1,880 patients with confirmed PE, 1,654 (88%) were diagnosed based on CT pulmonary angiogram, 91 (5%) on formal pulmonary angiography, 82 (4%) on ventilation-perfusion scan, 51 (3%) on deep vein thrombosis with appropriate PE symptoms, and 2 (0.1%) on pulmonary MRI.
The mean age was 57 years, with one-third past age 65; 53% of the patients were women, and 68% were white. The racial and ethnic distribution of patients with PE closely parallels that of all patients who present to emergency departments.
The most common presenting signs and symptoms of PE were dyspnea at rest (50%), pleuritic chest pain (39%), dyspnea with exertion (27%), extremity swelling suggestive of deep vein thrombosis (24%), and syncope (5%).
The most common comorbidities that could represent potential risk factors for PE were hypertension (46%), obesity (27%), recent hospitalization (24%), and active malignancy (22%). Based on the absence of any predefined risk factors for PE, 312 (16.6%) of the 1,880 patients were considered to have idiopathic PE.
Most of the patients (79%) diagnosed with PE in the ED lived independently, while 11.6% reported generalized immobility.
The low rate of early treatment found by the study "suggested that empiric anticoagulation in patients with suspected PE should be instituted more often in the ED and that timely, therapeutic anticoagulation should be administered after the diagnosis is confirmed," the researchers wrote.
"Future treatment studies of PE conducted in U.S. EDs should focus on accelerating the time frame of administration of systemic anticoagulation and fibrinolysis to patients with evidence of severe PE."
Several coauthors disclosed consulting relationships with and/or funding from a wide range of pharmaceutical companies that make anticoagulant agents.
Amblyopia Treatment Yields Best Response in Younger Children
Amblyopia treatment can lead to improved visual acuity in children from ages 3 to less than 13 years, but the average treatment response is smaller in children aged 7 and older, according to a meta-analysis of individual subject data from four randomized amblyopia treatment trials published online July 11 in Archives of Ophthalmology.
Studies have shown that amblyopia treatment is effective in some older children, but one question remains: Is there a relationship between the child’s age and the magnitude of treatment response.
To find out, Jonathan M. Holmes of the department of ophthalmology at the Mayo Clinic, Rochester, Minn., and his colleagues conducted a meta-analysis of four previous randomized multicenter clinical trials for the treatment of amblyopia conducted by the Pediatric Eye Disease Investigator Group, or PEDIG (Arch. Ophthalmol. 2011 July [doi:10.1001/archophthalmol.2011.179]). They used data from 996 children, each involved in one of four possible protocols:
• Protocol 1: Patching for 2 hours daily with near or distance activities (aged 3 to less than 7 years).
• Protocol 2: Treatment with atropine with or without a plano lens (aged 3 to less than 7 years).
• Protocol 3: Treatment with atropine or patching 2 hours daily (aged 7 to less than 13 years).
• Protocol 4: Use of Bangerter filter or patching 2 hours per day (aged 3 to less than 10 years).
Treatment response significantly decreased as children got older, especially among children whose amblyopia was more severe, the analysis showed. After researchers adjusted for covariates, children aged 3 to less than 5 with severe amblyopia showed a mean 4.16 lines of improved visual acuity. Children with severe amblyopia showed a mean 3.60 lines of improved visual acuity from ages 5 to less than 7 and 1.99 lines from ages 7 to less than 13.
Although the treatment response is significantly lower in older children versus younger children, the researchers pointed out that there was still an improvement – dramatic in some cases – in mean visual acuity among all age groups.
The analysis also found almost no difference in treatment response between children aged 3 to less than 5 years and children aged 5 to less than 7 years who had moderate amblyopia. The adjusted mean visual acuity was 2.29 lines for those aged 3 to less than 5 years, and 2.41 among those aged 5 to less than 7 years. For those children aged 7 to less than 13 years with moderate amblyopia, the adjusted mean improvement in visual acuity was 1.65 lines – a statistically significant difference.
"There are at least two possible reasons for reduced response to amblyopia treatment in older children," Mr. Holmes and his associates said. "It is widely believed that there is declining plasticity of the central nervous system as children age, although recent data on the treatment of amblyopia suggest that the plasticity of the nervous system remains throughout adolescence. Second, there may be poorer compliance when treating older children."
The strength of the study is that the meta-analysis used four randomized trials of individual subject data with similar entry criteria, length of follow-up, and use of a masked and standardized outcome assessment, the researchers said.
However, they added, the current study has several limitations. "Our ability to separate protocol effects from age effects is dependent on a single protocol (e.g., protocol 4), which was the only protocol that had overlap in age with all other protocols," the researchers said. "Also, protocol 4 did not include any children with severe amblyopia. Thus, separating an age effect from a protocol effect in severe amblyopia required that we assume the same protocol effects that were seen in moderate amblyopia ... (assuming that the lesser improvement seen in children 7 to less than 13 years of age was due to age and not to protocol)."
Additional limitations, the researchers said, include using arbitrary age categories to model age effects and using different visual acuity testing materials for children who were younger than age 7 years than for those aged 7 or older.
The researchers said they had no relevant financial disclosures. This study was supported by grants from the National Institutes of Health and Research to Prevent Blindness.
Amblyopia treatment can lead to improved visual acuity in children from ages 3 to less than 13 years, but the average treatment response is smaller in children aged 7 and older, according to a meta-analysis of individual subject data from four randomized amblyopia treatment trials published online July 11 in Archives of Ophthalmology.
Studies have shown that amblyopia treatment is effective in some older children, but one question remains: Is there a relationship between the child’s age and the magnitude of treatment response.
To find out, Jonathan M. Holmes of the department of ophthalmology at the Mayo Clinic, Rochester, Minn., and his colleagues conducted a meta-analysis of four previous randomized multicenter clinical trials for the treatment of amblyopia conducted by the Pediatric Eye Disease Investigator Group, or PEDIG (Arch. Ophthalmol. 2011 July [doi:10.1001/archophthalmol.2011.179]). They used data from 996 children, each involved in one of four possible protocols:
• Protocol 1: Patching for 2 hours daily with near or distance activities (aged 3 to less than 7 years).
• Protocol 2: Treatment with atropine with or without a plano lens (aged 3 to less than 7 years).
• Protocol 3: Treatment with atropine or patching 2 hours daily (aged 7 to less than 13 years).
• Protocol 4: Use of Bangerter filter or patching 2 hours per day (aged 3 to less than 10 years).
Treatment response significantly decreased as children got older, especially among children whose amblyopia was more severe, the analysis showed. After researchers adjusted for covariates, children aged 3 to less than 5 with severe amblyopia showed a mean 4.16 lines of improved visual acuity. Children with severe amblyopia showed a mean 3.60 lines of improved visual acuity from ages 5 to less than 7 and 1.99 lines from ages 7 to less than 13.
Although the treatment response is significantly lower in older children versus younger children, the researchers pointed out that there was still an improvement – dramatic in some cases – in mean visual acuity among all age groups.
The analysis also found almost no difference in treatment response between children aged 3 to less than 5 years and children aged 5 to less than 7 years who had moderate amblyopia. The adjusted mean visual acuity was 2.29 lines for those aged 3 to less than 5 years, and 2.41 among those aged 5 to less than 7 years. For those children aged 7 to less than 13 years with moderate amblyopia, the adjusted mean improvement in visual acuity was 1.65 lines – a statistically significant difference.
"There are at least two possible reasons for reduced response to amblyopia treatment in older children," Mr. Holmes and his associates said. "It is widely believed that there is declining plasticity of the central nervous system as children age, although recent data on the treatment of amblyopia suggest that the plasticity of the nervous system remains throughout adolescence. Second, there may be poorer compliance when treating older children."
The strength of the study is that the meta-analysis used four randomized trials of individual subject data with similar entry criteria, length of follow-up, and use of a masked and standardized outcome assessment, the researchers said.
However, they added, the current study has several limitations. "Our ability to separate protocol effects from age effects is dependent on a single protocol (e.g., protocol 4), which was the only protocol that had overlap in age with all other protocols," the researchers said. "Also, protocol 4 did not include any children with severe amblyopia. Thus, separating an age effect from a protocol effect in severe amblyopia required that we assume the same protocol effects that were seen in moderate amblyopia ... (assuming that the lesser improvement seen in children 7 to less than 13 years of age was due to age and not to protocol)."
Additional limitations, the researchers said, include using arbitrary age categories to model age effects and using different visual acuity testing materials for children who were younger than age 7 years than for those aged 7 or older.
The researchers said they had no relevant financial disclosures. This study was supported by grants from the National Institutes of Health and Research to Prevent Blindness.
Amblyopia treatment can lead to improved visual acuity in children from ages 3 to less than 13 years, but the average treatment response is smaller in children aged 7 and older, according to a meta-analysis of individual subject data from four randomized amblyopia treatment trials published online July 11 in Archives of Ophthalmology.
Studies have shown that amblyopia treatment is effective in some older children, but one question remains: Is there a relationship between the child’s age and the magnitude of treatment response.
To find out, Jonathan M. Holmes of the department of ophthalmology at the Mayo Clinic, Rochester, Minn., and his colleagues conducted a meta-analysis of four previous randomized multicenter clinical trials for the treatment of amblyopia conducted by the Pediatric Eye Disease Investigator Group, or PEDIG (Arch. Ophthalmol. 2011 July [doi:10.1001/archophthalmol.2011.179]). They used data from 996 children, each involved in one of four possible protocols:
• Protocol 1: Patching for 2 hours daily with near or distance activities (aged 3 to less than 7 years).
• Protocol 2: Treatment with atropine with or without a plano lens (aged 3 to less than 7 years).
• Protocol 3: Treatment with atropine or patching 2 hours daily (aged 7 to less than 13 years).
• Protocol 4: Use of Bangerter filter or patching 2 hours per day (aged 3 to less than 10 years).
Treatment response significantly decreased as children got older, especially among children whose amblyopia was more severe, the analysis showed. After researchers adjusted for covariates, children aged 3 to less than 5 with severe amblyopia showed a mean 4.16 lines of improved visual acuity. Children with severe amblyopia showed a mean 3.60 lines of improved visual acuity from ages 5 to less than 7 and 1.99 lines from ages 7 to less than 13.
Although the treatment response is significantly lower in older children versus younger children, the researchers pointed out that there was still an improvement – dramatic in some cases – in mean visual acuity among all age groups.
The analysis also found almost no difference in treatment response between children aged 3 to less than 5 years and children aged 5 to less than 7 years who had moderate amblyopia. The adjusted mean visual acuity was 2.29 lines for those aged 3 to less than 5 years, and 2.41 among those aged 5 to less than 7 years. For those children aged 7 to less than 13 years with moderate amblyopia, the adjusted mean improvement in visual acuity was 1.65 lines – a statistically significant difference.
"There are at least two possible reasons for reduced response to amblyopia treatment in older children," Mr. Holmes and his associates said. "It is widely believed that there is declining plasticity of the central nervous system as children age, although recent data on the treatment of amblyopia suggest that the plasticity of the nervous system remains throughout adolescence. Second, there may be poorer compliance when treating older children."
The strength of the study is that the meta-analysis used four randomized trials of individual subject data with similar entry criteria, length of follow-up, and use of a masked and standardized outcome assessment, the researchers said.
However, they added, the current study has several limitations. "Our ability to separate protocol effects from age effects is dependent on a single protocol (e.g., protocol 4), which was the only protocol that had overlap in age with all other protocols," the researchers said. "Also, protocol 4 did not include any children with severe amblyopia. Thus, separating an age effect from a protocol effect in severe amblyopia required that we assume the same protocol effects that were seen in moderate amblyopia ... (assuming that the lesser improvement seen in children 7 to less than 13 years of age was due to age and not to protocol)."
Additional limitations, the researchers said, include using arbitrary age categories to model age effects and using different visual acuity testing materials for children who were younger than age 7 years than for those aged 7 or older.
The researchers said they had no relevant financial disclosures. This study was supported by grants from the National Institutes of Health and Research to Prevent Blindness.
FROM ARCHIVES OF OPHTHALMOLOGY
Major Finding: Younger children (aged 3 to less than 7 years) generally respond better to amblyopia treatment than do older children (aged 7-13 years), although some older children show a marked response to treatment.
Data Source: A meta-analysis of individual subject data from four recently completed randomized amblyopia treatment trials involving 996 children.
Disclosures: The researchers said they had no relevant financial disclosures. This study was supported by grants from the National Institutes of Health and Research to Prevent Blindness.
Ranibizumab Could Dramatically Reduce Legal Blindness, Study Shows
Monthly treatments with ranibizumab in patients with neovascular age-related macular degeneration could reduce the incidence of legal blindness and vision impairment by 72% and 37%, respectively, in 2 years, according to a study in the June 2011 issue of Archives of Ophthlamology.
Before ranibizumab (Lucentis) became available in 2006, neovascular age-related macular degeneration (AMD) was considered to be the leading cause of blindness in individuals aged 50 and older in the United States, with its prevalence estimated to be 1.6 million cases by 2050. Studies have shown that monthly injections of ranibizumab, a vascular endothelial growth factor (VEGF) inhibitor, not only reduce the risk of substantial vision loss, but may lead substantial improvement in visual acuity.
With this in mind, Dr. Neil M. Bressler of the Wilmer Eye Institute at Johns Hopkins University in Baltimore and his colleagues attempted to estimate how many individuals in the United States who have neovascular AMD might avoid legal blindness or visual impairment (defined as a best-corrected visual acuity of 20/40 or less in both eyes) by receiving monthly injections of ranibizumab (Arch. Ophthalmol. 2011;129:709-17).
In research sponsored by Genentech, Dr. Bressler’s team designed a computer modeling study that estimated the incidence of neovascular AMD among non-Hispanic whites, as well as the number of patients in whom ranibizumab would be indicated and accessible, and the anticipated visual acuity outcomes. The researchers did not include racial subgroups because there is limited information regarding the incidence of choroidal neovascularization (CNV) in other populations.
First, the authors multiplied estimates of the population of non-Hispanic whites older than 50 years in the United States, based on 2008 data from the U.S. Census Bureau, and then multiplied that number by the incidence of neovascular AMD in the Beaver Dam Eye Study. The Beaver Dam Eye Study estimated a 15-year cumulative incidence of AMD.
The researchers also classified lesion characteristics and based anticipated visual acuity outcomes on three studies: AREDS (Age-Related Eye Disease Study) Report No. 11, the ANCHOR (Antivascular Endothelial Growth Factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) study, and the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration).
The model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Based on data from AREDS, ANCHOR, and MARINA, the model predicted that 51,000 (33.7%) of these individuals who already had AMD in one eye also already had CNV in the opposite eye, making them ineligible for the model used in this study. The researchers also estimated that 103,582 individuals (68.4%) in whom monthly ranibizumab was indicated would have access to ranibizumab, making them eligible for inclusion in the study’s modeling criteria.
According to the model, if none of these eligible individuals received treatment, 16,268 (15.7%) would become legally blind in 2 years, and another 34,702 (33.5%) would become visually impaired. If all these individuals received monthly injections, however, 4,484 individuals (4.3%) would become legally blind, and 21,919 individuals would become visually impaired. This represents a 72% and 37% reduction in the incidence of legal blindness and visual impairment, respectively.
The researchers did report limitations in the study. "This model has several assumptions and weaknesses that require one to interpret the numbers put forth in this study as only an approximation," the researchers said.
For example, the incidence rates of CNV were based only on the Beaver Dam Eye Study, the model did not consider other racial subgroups, the estimates in this model assumed measurement of best-corrected visual acuity on high-contrast charts, and the results assumed access to monthly ranibizumab for 2 years, even though many clinicians discontinue treatment before then.
Still, the authors believe that monthly ranibizumab, when indicated and accessible, would dramatically reduce the cases of legal blindness resulting from neovascular AMD in individuals aged 50 years and older.
Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Lucentis, AMD, blindness, vascular endothelial growth factor, VEGF inhibitor, vision loss, visual acuity, Dr. Neil M. Bressler,
Monthly treatments with ranibizumab in patients with neovascular age-related macular degeneration could reduce the incidence of legal blindness and vision impairment by 72% and 37%, respectively, in 2 years, according to a study in the June 2011 issue of Archives of Ophthlamology.
Before ranibizumab (Lucentis) became available in 2006, neovascular age-related macular degeneration (AMD) was considered to be the leading cause of blindness in individuals aged 50 and older in the United States, with its prevalence estimated to be 1.6 million cases by 2050. Studies have shown that monthly injections of ranibizumab, a vascular endothelial growth factor (VEGF) inhibitor, not only reduce the risk of substantial vision loss, but may lead substantial improvement in visual acuity.
With this in mind, Dr. Neil M. Bressler of the Wilmer Eye Institute at Johns Hopkins University in Baltimore and his colleagues attempted to estimate how many individuals in the United States who have neovascular AMD might avoid legal blindness or visual impairment (defined as a best-corrected visual acuity of 20/40 or less in both eyes) by receiving monthly injections of ranibizumab (Arch. Ophthalmol. 2011;129:709-17).
In research sponsored by Genentech, Dr. Bressler’s team designed a computer modeling study that estimated the incidence of neovascular AMD among non-Hispanic whites, as well as the number of patients in whom ranibizumab would be indicated and accessible, and the anticipated visual acuity outcomes. The researchers did not include racial subgroups because there is limited information regarding the incidence of choroidal neovascularization (CNV) in other populations.
First, the authors multiplied estimates of the population of non-Hispanic whites older than 50 years in the United States, based on 2008 data from the U.S. Census Bureau, and then multiplied that number by the incidence of neovascular AMD in the Beaver Dam Eye Study. The Beaver Dam Eye Study estimated a 15-year cumulative incidence of AMD.
The researchers also classified lesion characteristics and based anticipated visual acuity outcomes on three studies: AREDS (Age-Related Eye Disease Study) Report No. 11, the ANCHOR (Antivascular Endothelial Growth Factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) study, and the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration).
The model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Based on data from AREDS, ANCHOR, and MARINA, the model predicted that 51,000 (33.7%) of these individuals who already had AMD in one eye also already had CNV in the opposite eye, making them ineligible for the model used in this study. The researchers also estimated that 103,582 individuals (68.4%) in whom monthly ranibizumab was indicated would have access to ranibizumab, making them eligible for inclusion in the study’s modeling criteria.
According to the model, if none of these eligible individuals received treatment, 16,268 (15.7%) would become legally blind in 2 years, and another 34,702 (33.5%) would become visually impaired. If all these individuals received monthly injections, however, 4,484 individuals (4.3%) would become legally blind, and 21,919 individuals would become visually impaired. This represents a 72% and 37% reduction in the incidence of legal blindness and visual impairment, respectively.
The researchers did report limitations in the study. "This model has several assumptions and weaknesses that require one to interpret the numbers put forth in this study as only an approximation," the researchers said.
For example, the incidence rates of CNV were based only on the Beaver Dam Eye Study, the model did not consider other racial subgroups, the estimates in this model assumed measurement of best-corrected visual acuity on high-contrast charts, and the results assumed access to monthly ranibizumab for 2 years, even though many clinicians discontinue treatment before then.
Still, the authors believe that monthly ranibizumab, when indicated and accessible, would dramatically reduce the cases of legal blindness resulting from neovascular AMD in individuals aged 50 years and older.
Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Monthly treatments with ranibizumab in patients with neovascular age-related macular degeneration could reduce the incidence of legal blindness and vision impairment by 72% and 37%, respectively, in 2 years, according to a study in the June 2011 issue of Archives of Ophthlamology.
Before ranibizumab (Lucentis) became available in 2006, neovascular age-related macular degeneration (AMD) was considered to be the leading cause of blindness in individuals aged 50 and older in the United States, with its prevalence estimated to be 1.6 million cases by 2050. Studies have shown that monthly injections of ranibizumab, a vascular endothelial growth factor (VEGF) inhibitor, not only reduce the risk of substantial vision loss, but may lead substantial improvement in visual acuity.
With this in mind, Dr. Neil M. Bressler of the Wilmer Eye Institute at Johns Hopkins University in Baltimore and his colleagues attempted to estimate how many individuals in the United States who have neovascular AMD might avoid legal blindness or visual impairment (defined as a best-corrected visual acuity of 20/40 or less in both eyes) by receiving monthly injections of ranibizumab (Arch. Ophthalmol. 2011;129:709-17).
In research sponsored by Genentech, Dr. Bressler’s team designed a computer modeling study that estimated the incidence of neovascular AMD among non-Hispanic whites, as well as the number of patients in whom ranibizumab would be indicated and accessible, and the anticipated visual acuity outcomes. The researchers did not include racial subgroups because there is limited information regarding the incidence of choroidal neovascularization (CNV) in other populations.
First, the authors multiplied estimates of the population of non-Hispanic whites older than 50 years in the United States, based on 2008 data from the U.S. Census Bureau, and then multiplied that number by the incidence of neovascular AMD in the Beaver Dam Eye Study. The Beaver Dam Eye Study estimated a 15-year cumulative incidence of AMD.
The researchers also classified lesion characteristics and based anticipated visual acuity outcomes on three studies: AREDS (Age-Related Eye Disease Study) Report No. 11, the ANCHOR (Antivascular Endothelial Growth Factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) study, and the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration).
The model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Based on data from AREDS, ANCHOR, and MARINA, the model predicted that 51,000 (33.7%) of these individuals who already had AMD in one eye also already had CNV in the opposite eye, making them ineligible for the model used in this study. The researchers also estimated that 103,582 individuals (68.4%) in whom monthly ranibizumab was indicated would have access to ranibizumab, making them eligible for inclusion in the study’s modeling criteria.
According to the model, if none of these eligible individuals received treatment, 16,268 (15.7%) would become legally blind in 2 years, and another 34,702 (33.5%) would become visually impaired. If all these individuals received monthly injections, however, 4,484 individuals (4.3%) would become legally blind, and 21,919 individuals would become visually impaired. This represents a 72% and 37% reduction in the incidence of legal blindness and visual impairment, respectively.
The researchers did report limitations in the study. "This model has several assumptions and weaknesses that require one to interpret the numbers put forth in this study as only an approximation," the researchers said.
For example, the incidence rates of CNV were based only on the Beaver Dam Eye Study, the model did not consider other racial subgroups, the estimates in this model assumed measurement of best-corrected visual acuity on high-contrast charts, and the results assumed access to monthly ranibizumab for 2 years, even though many clinicians discontinue treatment before then.
Still, the authors believe that monthly ranibizumab, when indicated and accessible, would dramatically reduce the cases of legal blindness resulting from neovascular AMD in individuals aged 50 years and older.
Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Lucentis, AMD, blindness, vascular endothelial growth factor, VEGF inhibitor, vision loss, visual acuity, Dr. Neil M. Bressler,
Lucentis, AMD, blindness, vascular endothelial growth factor, VEGF inhibitor, vision loss, visual acuity, Dr. Neil M. Bressler,
FROM ARCHIVES OF OPHTHALMOLOGY
Major Finding: Ranibizumab could have a substantial effect on reducing the incidence of legal blindness (by 72%) and visual impairment (by 37%) within 2 years of diagnosis of neovascular age-related macular degeneration among non-Hispanic whites.
Data Source: A computer-modeling study using data from the U.S. Census Bureau, the Beaver Dam Eye Study, and the Age-Related Eye Disease Study.
Disclosures: Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Ranibizumab Could Dramatically Reduce Legal Blindness, Study Shows
Monthly treatments with ranibizumab in patients with neovascular age-related macular degeneration could reduce the incidence of legal blindness and vision impairment by 72% and 37%, respectively, in 2 years, according to a study in the June 2011 issue of Archives of Ophthlamology.
Before ranibizumab (Lucentis) became available in 2006, neovascular age-related macular degeneration (AMD) was considered to be the leading cause of blindness in individuals aged 50 and older in the United States, with its prevalence estimated to be 1.6 million cases by 2050. Studies have shown that monthly injections of ranibizumab, a vascular endothelial growth factor (VEGF) inhibitor, not only reduce the risk of substantial vision loss, but may lead substantial improvement in visual acuity.
With this in mind, Dr. Neil M. Bressler of the Wilmer Eye Institute at Johns Hopkins University in Baltimore and his colleagues attempted to estimate how many individuals in the United States who have neovascular AMD might avoid legal blindness or visual impairment (defined as a best-corrected visual acuity of 20/40 or less in both eyes) by receiving monthly injections of ranibizumab (Arch. Ophthalmol. 2011;129:709-17).
In research sponsored by Genentech, Dr. Bressler’s team designed a computer modeling study that estimated the incidence of neovascular AMD among non-Hispanic whites, as well as the number of patients in whom ranibizumab would be indicated and accessible, and the anticipated visual acuity outcomes. The researchers did not include racial subgroups because there is limited information regarding the incidence of choroidal neovascularization (CNV) in other populations.
First, the authors multiplied estimates of the population of non-Hispanic whites older than 50 years in the United States, based on 2008 data from the U.S. Census Bureau, and then multiplied that number by the incidence of neovascular AMD in the Beaver Dam Eye Study. The Beaver Dam Eye Study estimated a 15-year cumulative incidence of AMD.
The researchers also classified lesion characteristics and based anticipated visual acuity outcomes on three studies: AREDS (Age-Related Eye Disease Study) Report No. 11, the ANCHOR (Antivascular Endothelial Growth Factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) study, and the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration).
The model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Based on data from AREDS, ANCHOR, and MARINA, the model predicted that 51,000 (33.7%) of these individuals who already had AMD in one eye also already had CNV in the opposite eye, making them ineligible for the model used in this study. The researchers also estimated that 103,582 individuals (68.4%) in whom monthly ranibizumab was indicated would have access to ranibizumab, making them eligible for inclusion in the study’s modeling criteria.
According to the model, if none of these eligible individuals received treatment, 16,268 (15.7%) would become legally blind in 2 years, and another 34,702 (33.5%) would become visually impaired. If all these individuals received monthly injections, however, 4,484 individuals (4.3%) would become legally blind, and 21,919 individuals would become visually impaired. This represents a 72% and 37% reduction in the incidence of legal blindness and visual impairment, respectively.
The researchers did report limitations in the study. "This model has several assumptions and weaknesses that require one to interpret the numbers put forth in this study as only an approximation," the researchers said.
For example, the incidence rates of CNV were based only on the Beaver Dam Eye Study, the model did not consider other racial subgroups, the estimates in this model assumed measurement of best-corrected visual acuity on high-contrast charts, and the results assumed access to monthly ranibizumab for 2 years, even though many clinicians discontinue treatment before then.
Still, the authors believe that monthly ranibizumab, when indicated and accessible, would dramatically reduce the cases of legal blindness resulting from neovascular AMD in individuals aged 50 years and older.
Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Lucentis, AMD, blindness, vascular endothelial growth factor, VEGF inhibitor, vision loss, visual acuity, Dr. Neil M. Bressler,
Monthly treatments with ranibizumab in patients with neovascular age-related macular degeneration could reduce the incidence of legal blindness and vision impairment by 72% and 37%, respectively, in 2 years, according to a study in the June 2011 issue of Archives of Ophthlamology.
Before ranibizumab (Lucentis) became available in 2006, neovascular age-related macular degeneration (AMD) was considered to be the leading cause of blindness in individuals aged 50 and older in the United States, with its prevalence estimated to be 1.6 million cases by 2050. Studies have shown that monthly injections of ranibizumab, a vascular endothelial growth factor (VEGF) inhibitor, not only reduce the risk of substantial vision loss, but may lead substantial improvement in visual acuity.
With this in mind, Dr. Neil M. Bressler of the Wilmer Eye Institute at Johns Hopkins University in Baltimore and his colleagues attempted to estimate how many individuals in the United States who have neovascular AMD might avoid legal blindness or visual impairment (defined as a best-corrected visual acuity of 20/40 or less in both eyes) by receiving monthly injections of ranibizumab (Arch. Ophthalmol. 2011;129:709-17).
In research sponsored by Genentech, Dr. Bressler’s team designed a computer modeling study that estimated the incidence of neovascular AMD among non-Hispanic whites, as well as the number of patients in whom ranibizumab would be indicated and accessible, and the anticipated visual acuity outcomes. The researchers did not include racial subgroups because there is limited information regarding the incidence of choroidal neovascularization (CNV) in other populations.
First, the authors multiplied estimates of the population of non-Hispanic whites older than 50 years in the United States, based on 2008 data from the U.S. Census Bureau, and then multiplied that number by the incidence of neovascular AMD in the Beaver Dam Eye Study. The Beaver Dam Eye Study estimated a 15-year cumulative incidence of AMD.
The researchers also classified lesion characteristics and based anticipated visual acuity outcomes on three studies: AREDS (Age-Related Eye Disease Study) Report No. 11, the ANCHOR (Antivascular Endothelial Growth Factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) study, and the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration).
The model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Based on data from AREDS, ANCHOR, and MARINA, the model predicted that 51,000 (33.7%) of these individuals who already had AMD in one eye also already had CNV in the opposite eye, making them ineligible for the model used in this study. The researchers also estimated that 103,582 individuals (68.4%) in whom monthly ranibizumab was indicated would have access to ranibizumab, making them eligible for inclusion in the study’s modeling criteria.
According to the model, if none of these eligible individuals received treatment, 16,268 (15.7%) would become legally blind in 2 years, and another 34,702 (33.5%) would become visually impaired. If all these individuals received monthly injections, however, 4,484 individuals (4.3%) would become legally blind, and 21,919 individuals would become visually impaired. This represents a 72% and 37% reduction in the incidence of legal blindness and visual impairment, respectively.
The researchers did report limitations in the study. "This model has several assumptions and weaknesses that require one to interpret the numbers put forth in this study as only an approximation," the researchers said.
For example, the incidence rates of CNV were based only on the Beaver Dam Eye Study, the model did not consider other racial subgroups, the estimates in this model assumed measurement of best-corrected visual acuity on high-contrast charts, and the results assumed access to monthly ranibizumab for 2 years, even though many clinicians discontinue treatment before then.
Still, the authors believe that monthly ranibizumab, when indicated and accessible, would dramatically reduce the cases of legal blindness resulting from neovascular AMD in individuals aged 50 years and older.
Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Monthly treatments with ranibizumab in patients with neovascular age-related macular degeneration could reduce the incidence of legal blindness and vision impairment by 72% and 37%, respectively, in 2 years, according to a study in the June 2011 issue of Archives of Ophthlamology.
Before ranibizumab (Lucentis) became available in 2006, neovascular age-related macular degeneration (AMD) was considered to be the leading cause of blindness in individuals aged 50 and older in the United States, with its prevalence estimated to be 1.6 million cases by 2050. Studies have shown that monthly injections of ranibizumab, a vascular endothelial growth factor (VEGF) inhibitor, not only reduce the risk of substantial vision loss, but may lead substantial improvement in visual acuity.
With this in mind, Dr. Neil M. Bressler of the Wilmer Eye Institute at Johns Hopkins University in Baltimore and his colleagues attempted to estimate how many individuals in the United States who have neovascular AMD might avoid legal blindness or visual impairment (defined as a best-corrected visual acuity of 20/40 or less in both eyes) by receiving monthly injections of ranibizumab (Arch. Ophthalmol. 2011;129:709-17).
In research sponsored by Genentech, Dr. Bressler’s team designed a computer modeling study that estimated the incidence of neovascular AMD among non-Hispanic whites, as well as the number of patients in whom ranibizumab would be indicated and accessible, and the anticipated visual acuity outcomes. The researchers did not include racial subgroups because there is limited information regarding the incidence of choroidal neovascularization (CNV) in other populations.
First, the authors multiplied estimates of the population of non-Hispanic whites older than 50 years in the United States, based on 2008 data from the U.S. Census Bureau, and then multiplied that number by the incidence of neovascular AMD in the Beaver Dam Eye Study. The Beaver Dam Eye Study estimated a 15-year cumulative incidence of AMD.
The researchers also classified lesion characteristics and based anticipated visual acuity outcomes on three studies: AREDS (Age-Related Eye Disease Study) Report No. 11, the ANCHOR (Antivascular Endothelial Growth Factor Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) study, and the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration).
The model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Based on data from AREDS, ANCHOR, and MARINA, the model predicted that 51,000 (33.7%) of these individuals who already had AMD in one eye also already had CNV in the opposite eye, making them ineligible for the model used in this study. The researchers also estimated that 103,582 individuals (68.4%) in whom monthly ranibizumab was indicated would have access to ranibizumab, making them eligible for inclusion in the study’s modeling criteria.
According to the model, if none of these eligible individuals received treatment, 16,268 (15.7%) would become legally blind in 2 years, and another 34,702 (33.5%) would become visually impaired. If all these individuals received monthly injections, however, 4,484 individuals (4.3%) would become legally blind, and 21,919 individuals would become visually impaired. This represents a 72% and 37% reduction in the incidence of legal blindness and visual impairment, respectively.
The researchers did report limitations in the study. "This model has several assumptions and weaknesses that require one to interpret the numbers put forth in this study as only an approximation," the researchers said.
For example, the incidence rates of CNV were based only on the Beaver Dam Eye Study, the model did not consider other racial subgroups, the estimates in this model assumed measurement of best-corrected visual acuity on high-contrast charts, and the results assumed access to monthly ranibizumab for 2 years, even though many clinicians discontinue treatment before then.
Still, the authors believe that monthly ranibizumab, when indicated and accessible, would dramatically reduce the cases of legal blindness resulting from neovascular AMD in individuals aged 50 years and older.
Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Lucentis, AMD, blindness, vascular endothelial growth factor, VEGF inhibitor, vision loss, visual acuity, Dr. Neil M. Bressler,
Lucentis, AMD, blindness, vascular endothelial growth factor, VEGF inhibitor, vision loss, visual acuity, Dr. Neil M. Bressler,
FROM ARCHIVES OF OPHTHALMOLOGY
Major Finding: Ranibizumab could have a substantial effect on reducing the incidence of legal blindness (by 72%) and visual impairment (by 37%) within 2 years of diagnosis of neovascular age-related macular degeneration among non-Hispanic whites.
Data Source: A computer-modeling study using data from the U.S. Census Bureau, the Beaver Dam Eye Study, and the Age-Related Eye Disease Study.
Disclosures: Genentech funded the study and participated in the design and conduct of the study; in the distribution of the raw data; in the analysis and interpretation of the data; in the preparation of the manuscript, and in review of the manuscript before submission. Several of the coauthors are consultants for Genentech.
Higher BMI Protects Women From Glaucoma
Among lifestyle and socioeconomic factors only obesity appears to increase the risk of developing elevated intraocular pressure, and it might also protect women against developing open-angle glaucoma, according to new results from the Rotterdam Study published June 13 in Archives of Ophthalmology.
Given that individuals can modify several lifestyle and socioeconomic factors, Dr. Wishal D. Ramdas of Erasmus Medical Centre, Rotterdam, the Netherlands, and colleagues wanted to further investigate their association with glaucoma. To do so, they used data from the Rotterdam Study, a prospective, population- based cohort study of chronic diseases, including ocular disease, in residents aged 55 years and older living in a suburb of Rotterdam (Arch. Ophthalmol. 2011;129:767-72).
Patients underwent comprehensive eye examinations at baseline (1991 to 1993) and follow-up (1997-1999 and 2002-2006). Trained research assistants used questionnaires to determine patients’ income, education level, smoking habits, and alcohol intake. Researchers determined patients’ body mass index (BMI) and waist-to-hip ratio.
In this study, the researchers used data from participants who did not have open-angle glaucoma (OAG) at baseline and who completed at least one follow-up examination. They considered patients to have incident open-angle glaucoma (OAG) if neither eye had glaucomatous visual field loss at baseline and at least one eye did at follow-up.
During a mean follow-up of nearly 10 years, 108 of 3,939 participants (2.7%) developed OAG. Participants who developed OAG were significantly older, often had high myopia, and were more often male, the researchers found.
On regression analysis, the researchers found that BMI was associated with elevated intraocular pressure (IOP), yet seemed to reduce the risk of developing OAG. Further analysis, however, showed that this occurred only in women, who had a 7% decreased risk of developing OAG for each unit increase in BMI. There was no significant effect in men.
One possible explanation for the contrary findings, according to Dr. Ramdas and colleagues, is that excess fat tissue increases orbital and episcleral venous pressure. Also, obesity also may increase blood viscosity, resulting in outflow resistance of the episcleral veins.
Also, "with Goldmann applanation tonometry, the thorax and abdomen are pushed against the slit lamp table while breath holding works like a Valsalva maneuver," the researchers wrote. "This is especially relevant for obese women."
The apparent protective effect of obesity against OAG might really be a protective effect of high estrogen levels and hormone therapy. Obesity may be positively related to postmenopausal plasma estrogen levels.
There was no significant association between income, education, smoking, or alcohol intake with elevated IOP or OAG. Still, the researchers suggested caution in interpreting these results.
"Although these findings are in line with those from earlier studies, our findings are based on a relatively low number of OAG cases, and as a consequence, small effects of these lifestyle-related risk factors cannot be ruled out because of power limitations."
The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
Among lifestyle and socioeconomic factors only obesity appears to increase the risk of developing elevated intraocular pressure, and it might also protect women against developing open-angle glaucoma, according to new results from the Rotterdam Study published June 13 in Archives of Ophthalmology.
Given that individuals can modify several lifestyle and socioeconomic factors, Dr. Wishal D. Ramdas of Erasmus Medical Centre, Rotterdam, the Netherlands, and colleagues wanted to further investigate their association with glaucoma. To do so, they used data from the Rotterdam Study, a prospective, population- based cohort study of chronic diseases, including ocular disease, in residents aged 55 years and older living in a suburb of Rotterdam (Arch. Ophthalmol. 2011;129:767-72).
Patients underwent comprehensive eye examinations at baseline (1991 to 1993) and follow-up (1997-1999 and 2002-2006). Trained research assistants used questionnaires to determine patients’ income, education level, smoking habits, and alcohol intake. Researchers determined patients’ body mass index (BMI) and waist-to-hip ratio.
In this study, the researchers used data from participants who did not have open-angle glaucoma (OAG) at baseline and who completed at least one follow-up examination. They considered patients to have incident open-angle glaucoma (OAG) if neither eye had glaucomatous visual field loss at baseline and at least one eye did at follow-up.
During a mean follow-up of nearly 10 years, 108 of 3,939 participants (2.7%) developed OAG. Participants who developed OAG were significantly older, often had high myopia, and were more often male, the researchers found.
On regression analysis, the researchers found that BMI was associated with elevated intraocular pressure (IOP), yet seemed to reduce the risk of developing OAG. Further analysis, however, showed that this occurred only in women, who had a 7% decreased risk of developing OAG for each unit increase in BMI. There was no significant effect in men.
One possible explanation for the contrary findings, according to Dr. Ramdas and colleagues, is that excess fat tissue increases orbital and episcleral venous pressure. Also, obesity also may increase blood viscosity, resulting in outflow resistance of the episcleral veins.
Also, "with Goldmann applanation tonometry, the thorax and abdomen are pushed against the slit lamp table while breath holding works like a Valsalva maneuver," the researchers wrote. "This is especially relevant for obese women."
The apparent protective effect of obesity against OAG might really be a protective effect of high estrogen levels and hormone therapy. Obesity may be positively related to postmenopausal plasma estrogen levels.
There was no significant association between income, education, smoking, or alcohol intake with elevated IOP or OAG. Still, the researchers suggested caution in interpreting these results.
"Although these findings are in line with those from earlier studies, our findings are based on a relatively low number of OAG cases, and as a consequence, small effects of these lifestyle-related risk factors cannot be ruled out because of power limitations."
The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
Among lifestyle and socioeconomic factors only obesity appears to increase the risk of developing elevated intraocular pressure, and it might also protect women against developing open-angle glaucoma, according to new results from the Rotterdam Study published June 13 in Archives of Ophthalmology.
Given that individuals can modify several lifestyle and socioeconomic factors, Dr. Wishal D. Ramdas of Erasmus Medical Centre, Rotterdam, the Netherlands, and colleagues wanted to further investigate their association with glaucoma. To do so, they used data from the Rotterdam Study, a prospective, population- based cohort study of chronic diseases, including ocular disease, in residents aged 55 years and older living in a suburb of Rotterdam (Arch. Ophthalmol. 2011;129:767-72).
Patients underwent comprehensive eye examinations at baseline (1991 to 1993) and follow-up (1997-1999 and 2002-2006). Trained research assistants used questionnaires to determine patients’ income, education level, smoking habits, and alcohol intake. Researchers determined patients’ body mass index (BMI) and waist-to-hip ratio.
In this study, the researchers used data from participants who did not have open-angle glaucoma (OAG) at baseline and who completed at least one follow-up examination. They considered patients to have incident open-angle glaucoma (OAG) if neither eye had glaucomatous visual field loss at baseline and at least one eye did at follow-up.
During a mean follow-up of nearly 10 years, 108 of 3,939 participants (2.7%) developed OAG. Participants who developed OAG were significantly older, often had high myopia, and were more often male, the researchers found.
On regression analysis, the researchers found that BMI was associated with elevated intraocular pressure (IOP), yet seemed to reduce the risk of developing OAG. Further analysis, however, showed that this occurred only in women, who had a 7% decreased risk of developing OAG for each unit increase in BMI. There was no significant effect in men.
One possible explanation for the contrary findings, according to Dr. Ramdas and colleagues, is that excess fat tissue increases orbital and episcleral venous pressure. Also, obesity also may increase blood viscosity, resulting in outflow resistance of the episcleral veins.
Also, "with Goldmann applanation tonometry, the thorax and abdomen are pushed against the slit lamp table while breath holding works like a Valsalva maneuver," the researchers wrote. "This is especially relevant for obese women."
The apparent protective effect of obesity against OAG might really be a protective effect of high estrogen levels and hormone therapy. Obesity may be positively related to postmenopausal plasma estrogen levels.
There was no significant association between income, education, smoking, or alcohol intake with elevated IOP or OAG. Still, the researchers suggested caution in interpreting these results.
"Although these findings are in line with those from earlier studies, our findings are based on a relatively low number of OAG cases, and as a consequence, small effects of these lifestyle-related risk factors cannot be ruled out because of power limitations."
The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
FROM ARCHIVES OF OPHTHALMOLOGY
Major Finding: Body mass index was associated with a higher intraocular pressure but was protective against open-angle glaucoma in women. Socioeconomic status and lifestyle-related factors such as smoking and alcohol consumption were not associated with open-angle glaucoma.
Data Source: A subset of 3,939 patients from the Rotterdam Study, a prospective population-based study investigating chronic diseases in subjects age 55 and older.
Disclosures: The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
Higher BMI Protects Women From Glaucoma
Among lifestyle and socioeconomic factors only obesity appears to increase the risk of developing elevated intraocular pressure, and it might also protect women against developing open-angle glaucoma, according to new results from the Rotterdam Study published June 13 in Archives of Ophthalmology.
Given that individuals can modify several lifestyle and socioeconomic factors, Dr. Wishal D. Ramdas of Erasmus Medical Centre, Rotterdam, the Netherlands, and colleagues wanted to further investigate their association with glaucoma. To do so, they used data from the Rotterdam Study, a prospective, population- based cohort study of chronic diseases, including ocular disease, in residents aged 55 years and older living in a suburb of Rotterdam (Arch. Ophthalmol. 2011;129:767-72).
Patients underwent comprehensive eye examinations at baseline (1991 to 1993) and follow-up (1997-1999 and 2002-2006). Trained research assistants used questionnaires to determine patients’ income, education level, smoking habits, and alcohol intake. Researchers determined patients’ body mass index (BMI) and waist-to-hip ratio.
In this study, the researchers used data from participants who did not have open-angle glaucoma (OAG) at baseline and who completed at least one follow-up examination. They considered patients to have incident open-angle glaucoma (OAG) if neither eye had glaucomatous visual field loss at baseline and at least one eye did at follow-up.
During a mean follow-up of nearly 10 years, 108 of 3,939 participants (2.7%) developed OAG. Participants who developed OAG were significantly older, often had high myopia, and were more often male, the researchers found.
On regression analysis, the researchers found that BMI was associated with elevated intraocular pressure (IOP), yet seemed to reduce the risk of developing OAG. Further analysis, however, showed that this occurred only in women, who had a 7% decreased risk of developing OAG for each unit increase in BMI. There was no significant effect in men.
One possible explanation for the contrary findings, according to Dr. Ramdas and colleagues, is that excess fat tissue increases orbital and episcleral venous pressure. Also, obesity also may increase blood viscosity, resulting in outflow resistance of the episcleral veins.
Also, "with Goldmann applanation tonometry, the thorax and abdomen are pushed against the slit lamp table while breath holding works like a Valsalva maneuver," the researchers wrote. "This is especially relevant for obese women."
The apparent protective effect of obesity against OAG might really be a protective effect of high estrogen levels and hormone therapy. Obesity may be positively related to postmenopausal plasma estrogen levels.
There was no significant association between income, education, smoking, or alcohol intake with elevated IOP or OAG. Still, the researchers suggested caution in interpreting these results.
"Although these findings are in line with those from earlier studies, our findings are based on a relatively low number of OAG cases, and as a consequence, small effects of these lifestyle-related risk factors cannot be ruled out because of power limitations."
The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
Among lifestyle and socioeconomic factors only obesity appears to increase the risk of developing elevated intraocular pressure, and it might also protect women against developing open-angle glaucoma, according to new results from the Rotterdam Study published June 13 in Archives of Ophthalmology.
Given that individuals can modify several lifestyle and socioeconomic factors, Dr. Wishal D. Ramdas of Erasmus Medical Centre, Rotterdam, the Netherlands, and colleagues wanted to further investigate their association with glaucoma. To do so, they used data from the Rotterdam Study, a prospective, population- based cohort study of chronic diseases, including ocular disease, in residents aged 55 years and older living in a suburb of Rotterdam (Arch. Ophthalmol. 2011;129:767-72).
Patients underwent comprehensive eye examinations at baseline (1991 to 1993) and follow-up (1997-1999 and 2002-2006). Trained research assistants used questionnaires to determine patients’ income, education level, smoking habits, and alcohol intake. Researchers determined patients’ body mass index (BMI) and waist-to-hip ratio.
In this study, the researchers used data from participants who did not have open-angle glaucoma (OAG) at baseline and who completed at least one follow-up examination. They considered patients to have incident open-angle glaucoma (OAG) if neither eye had glaucomatous visual field loss at baseline and at least one eye did at follow-up.
During a mean follow-up of nearly 10 years, 108 of 3,939 participants (2.7%) developed OAG. Participants who developed OAG were significantly older, often had high myopia, and were more often male, the researchers found.
On regression analysis, the researchers found that BMI was associated with elevated intraocular pressure (IOP), yet seemed to reduce the risk of developing OAG. Further analysis, however, showed that this occurred only in women, who had a 7% decreased risk of developing OAG for each unit increase in BMI. There was no significant effect in men.
One possible explanation for the contrary findings, according to Dr. Ramdas and colleagues, is that excess fat tissue increases orbital and episcleral venous pressure. Also, obesity also may increase blood viscosity, resulting in outflow resistance of the episcleral veins.
Also, "with Goldmann applanation tonometry, the thorax and abdomen are pushed against the slit lamp table while breath holding works like a Valsalva maneuver," the researchers wrote. "This is especially relevant for obese women."
The apparent protective effect of obesity against OAG might really be a protective effect of high estrogen levels and hormone therapy. Obesity may be positively related to postmenopausal plasma estrogen levels.
There was no significant association between income, education, smoking, or alcohol intake with elevated IOP or OAG. Still, the researchers suggested caution in interpreting these results.
"Although these findings are in line with those from earlier studies, our findings are based on a relatively low number of OAG cases, and as a consequence, small effects of these lifestyle-related risk factors cannot be ruled out because of power limitations."
The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
Among lifestyle and socioeconomic factors only obesity appears to increase the risk of developing elevated intraocular pressure, and it might also protect women against developing open-angle glaucoma, according to new results from the Rotterdam Study published June 13 in Archives of Ophthalmology.
Given that individuals can modify several lifestyle and socioeconomic factors, Dr. Wishal D. Ramdas of Erasmus Medical Centre, Rotterdam, the Netherlands, and colleagues wanted to further investigate their association with glaucoma. To do so, they used data from the Rotterdam Study, a prospective, population- based cohort study of chronic diseases, including ocular disease, in residents aged 55 years and older living in a suburb of Rotterdam (Arch. Ophthalmol. 2011;129:767-72).
Patients underwent comprehensive eye examinations at baseline (1991 to 1993) and follow-up (1997-1999 and 2002-2006). Trained research assistants used questionnaires to determine patients’ income, education level, smoking habits, and alcohol intake. Researchers determined patients’ body mass index (BMI) and waist-to-hip ratio.
In this study, the researchers used data from participants who did not have open-angle glaucoma (OAG) at baseline and who completed at least one follow-up examination. They considered patients to have incident open-angle glaucoma (OAG) if neither eye had glaucomatous visual field loss at baseline and at least one eye did at follow-up.
During a mean follow-up of nearly 10 years, 108 of 3,939 participants (2.7%) developed OAG. Participants who developed OAG were significantly older, often had high myopia, and were more often male, the researchers found.
On regression analysis, the researchers found that BMI was associated with elevated intraocular pressure (IOP), yet seemed to reduce the risk of developing OAG. Further analysis, however, showed that this occurred only in women, who had a 7% decreased risk of developing OAG for each unit increase in BMI. There was no significant effect in men.
One possible explanation for the contrary findings, according to Dr. Ramdas and colleagues, is that excess fat tissue increases orbital and episcleral venous pressure. Also, obesity also may increase blood viscosity, resulting in outflow resistance of the episcleral veins.
Also, "with Goldmann applanation tonometry, the thorax and abdomen are pushed against the slit lamp table while breath holding works like a Valsalva maneuver," the researchers wrote. "This is especially relevant for obese women."
The apparent protective effect of obesity against OAG might really be a protective effect of high estrogen levels and hormone therapy. Obesity may be positively related to postmenopausal plasma estrogen levels.
There was no significant association between income, education, smoking, or alcohol intake with elevated IOP or OAG. Still, the researchers suggested caution in interpreting these results.
"Although these findings are in line with those from earlier studies, our findings are based on a relatively low number of OAG cases, and as a consequence, small effects of these lifestyle-related risk factors cannot be ruled out because of power limitations."
The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
FROM ARCHIVES OF OPHTHALMOLOGY
Major Finding: Body mass index was associated with a higher intraocular pressure but was protective against open-angle glaucoma in women. Socioeconomic status and lifestyle-related factors such as smoking and alcohol consumption were not associated with open-angle glaucoma.
Data Source: A subset of 3,939 patients from the Rotterdam Study, a prospective population-based study investigating chronic diseases in subjects age 55 and older.
Disclosures: The Rotterdam Study is supported by the municipality of Capelle aan de IJsell and two Netherlands-based ophthalmologic devices companies. Additional funding was provided by Netherlands-based vision advocacy organizations.
Diet May Modify Risk of Developing Macular Degeneration
High dietary intake of antioxidants, zinc and omega-3 fatty acids may reduce the risk of early age-related macular degeneration in patients with the high genetic risk, according to the findings of a prospective, population-based study in the June issue of Archives of Ophthalmology.
Studies have shown that some 80% of late age-related macular degeneration (AMD) cases are due to variants in the complement factor H (CFH) and LOC387715/HTRA1 genes. "To reduce the burden of this disease, it is therefore essential to find means to counteract these major gene effects," the researchers said. "The only protective factors for AMD known to date are nutrients."
With this in mind, Dr. Lintje Ho of Erasmus Medical Center, in Rotterdam, the Netherlands, and colleagues investigated whether antioxidant, zinc and omega-3 fatty acid intake from daily foods could reduce the risk of early AMD in patients with the various genotypes of CFH Y402H and LOC387715 A69S (Arch. Ophthalmol. 2011;129:758-66).
To do so, they analyzed a subset of patients from Rotterdam Study, a prospective, population-based cohort investigation of chronic diseases in residents aged 55 and older of a Rotterdam suburb. Subjects underwent a comprehensive eye examination at baseline (1990-1993) and at three follow-up visits (1993-1994, 1997-1999 and 2000-2004).
To be eligible, participants had no AMD during the entire study period or developed early AMD during follow-up. A total of 2,768 individuals were eligible for the study, and 2,167 individuals were included in the final analysis.
Of these subjects, 517 (24%) developed early AMD during a median follow-up period of 9 years. These patients were slightly older (mean age 68 years) than were those with no AMD (mean 66 years), fewer were diabetic (7% vs. 10%), and they had a higher frequency of CFH Y402H (62% vs. 55%) and LOC387715 A69S genotypes (41% vs. 33%).
Results showed significant interactions between the CFH Y402H genotype and intake of zinc, beta-carotene, lutein/zeaxanthin, and eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA). Specifically, dietary intake of zinc in the highest tertile reduced the hazard ratio for early AMD from 2.25 to 1.27. Hazard ratios were reduced from 2.54 to 1.47 for highest intakes of beta-carotene, 2.63 to 1.72 for lutein/zeaxanthin, and 1.97 to 1.30 for EPA/DHA.
Results also showed a significant interaction between LOC387715 A69S genotype and intake of zinc and EPA/DHA. Carriers with the highest intake of zinc reduced their risk from 1.70 to 1.17, while those with the highest intake of EPA/DHA reduced their risk from 1.59 to 0.95.
Dietary antioxidants may help counteract oxidative damage that can activate the complement system, which plays a role in the pathogenesis of AMD. For example, zinc may help reduce complement activation that is already underway. Also, omega-3 fatty acids help prevent inflammation in the retina, another factor in the pathogenesis of AMD, by lowering acute-phase proteins, including complement C4, immunoglobulin M (IgM), haptoglobin, C-reactive protein, and fibrinogen, the researchers say.
Individuals need only consume the recommended daily allowances rather than excessive amounts of these nutrients to benefit, the researchers said.
They recommend fortified cereals, meats, dairy products, nuts, and seeds as sources of zinc, dark green leafy vegetables and bright orange vegetables as sources of beta-carotene, and oily fish as a source of EPA and DHA.
The relatively low number of cases in the stratified analyses is a limitation, although the researchers said they still could identify clear trends of risk reduction.
The authors reported having no financial conflicts. This study was supported by the Netherlands Organization for Scientific Research, among other sources.
complement factor H, CFH, LOC387715/HTRA1 genes, Dr. Lintje Ho,
High dietary intake of antioxidants, zinc and omega-3 fatty acids may reduce the risk of early age-related macular degeneration in patients with the high genetic risk, according to the findings of a prospective, population-based study in the June issue of Archives of Ophthalmology.
Studies have shown that some 80% of late age-related macular degeneration (AMD) cases are due to variants in the complement factor H (CFH) and LOC387715/HTRA1 genes. "To reduce the burden of this disease, it is therefore essential to find means to counteract these major gene effects," the researchers said. "The only protective factors for AMD known to date are nutrients."
With this in mind, Dr. Lintje Ho of Erasmus Medical Center, in Rotterdam, the Netherlands, and colleagues investigated whether antioxidant, zinc and omega-3 fatty acid intake from daily foods could reduce the risk of early AMD in patients with the various genotypes of CFH Y402H and LOC387715 A69S (Arch. Ophthalmol. 2011;129:758-66).
To do so, they analyzed a subset of patients from Rotterdam Study, a prospective, population-based cohort investigation of chronic diseases in residents aged 55 and older of a Rotterdam suburb. Subjects underwent a comprehensive eye examination at baseline (1990-1993) and at three follow-up visits (1993-1994, 1997-1999 and 2000-2004).
To be eligible, participants had no AMD during the entire study period or developed early AMD during follow-up. A total of 2,768 individuals were eligible for the study, and 2,167 individuals were included in the final analysis.
Of these subjects, 517 (24%) developed early AMD during a median follow-up period of 9 years. These patients were slightly older (mean age 68 years) than were those with no AMD (mean 66 years), fewer were diabetic (7% vs. 10%), and they had a higher frequency of CFH Y402H (62% vs. 55%) and LOC387715 A69S genotypes (41% vs. 33%).
Results showed significant interactions between the CFH Y402H genotype and intake of zinc, beta-carotene, lutein/zeaxanthin, and eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA). Specifically, dietary intake of zinc in the highest tertile reduced the hazard ratio for early AMD from 2.25 to 1.27. Hazard ratios were reduced from 2.54 to 1.47 for highest intakes of beta-carotene, 2.63 to 1.72 for lutein/zeaxanthin, and 1.97 to 1.30 for EPA/DHA.
Results also showed a significant interaction between LOC387715 A69S genotype and intake of zinc and EPA/DHA. Carriers with the highest intake of zinc reduced their risk from 1.70 to 1.17, while those with the highest intake of EPA/DHA reduced their risk from 1.59 to 0.95.
Dietary antioxidants may help counteract oxidative damage that can activate the complement system, which plays a role in the pathogenesis of AMD. For example, zinc may help reduce complement activation that is already underway. Also, omega-3 fatty acids help prevent inflammation in the retina, another factor in the pathogenesis of AMD, by lowering acute-phase proteins, including complement C4, immunoglobulin M (IgM), haptoglobin, C-reactive protein, and fibrinogen, the researchers say.
Individuals need only consume the recommended daily allowances rather than excessive amounts of these nutrients to benefit, the researchers said.
They recommend fortified cereals, meats, dairy products, nuts, and seeds as sources of zinc, dark green leafy vegetables and bright orange vegetables as sources of beta-carotene, and oily fish as a source of EPA and DHA.
The relatively low number of cases in the stratified analyses is a limitation, although the researchers said they still could identify clear trends of risk reduction.
The authors reported having no financial conflicts. This study was supported by the Netherlands Organization for Scientific Research, among other sources.
High dietary intake of antioxidants, zinc and omega-3 fatty acids may reduce the risk of early age-related macular degeneration in patients with the high genetic risk, according to the findings of a prospective, population-based study in the June issue of Archives of Ophthalmology.
Studies have shown that some 80% of late age-related macular degeneration (AMD) cases are due to variants in the complement factor H (CFH) and LOC387715/HTRA1 genes. "To reduce the burden of this disease, it is therefore essential to find means to counteract these major gene effects," the researchers said. "The only protective factors for AMD known to date are nutrients."
With this in mind, Dr. Lintje Ho of Erasmus Medical Center, in Rotterdam, the Netherlands, and colleagues investigated whether antioxidant, zinc and omega-3 fatty acid intake from daily foods could reduce the risk of early AMD in patients with the various genotypes of CFH Y402H and LOC387715 A69S (Arch. Ophthalmol. 2011;129:758-66).
To do so, they analyzed a subset of patients from Rotterdam Study, a prospective, population-based cohort investigation of chronic diseases in residents aged 55 and older of a Rotterdam suburb. Subjects underwent a comprehensive eye examination at baseline (1990-1993) and at three follow-up visits (1993-1994, 1997-1999 and 2000-2004).
To be eligible, participants had no AMD during the entire study period or developed early AMD during follow-up. A total of 2,768 individuals were eligible for the study, and 2,167 individuals were included in the final analysis.
Of these subjects, 517 (24%) developed early AMD during a median follow-up period of 9 years. These patients were slightly older (mean age 68 years) than were those with no AMD (mean 66 years), fewer were diabetic (7% vs. 10%), and they had a higher frequency of CFH Y402H (62% vs. 55%) and LOC387715 A69S genotypes (41% vs. 33%).
Results showed significant interactions between the CFH Y402H genotype and intake of zinc, beta-carotene, lutein/zeaxanthin, and eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA). Specifically, dietary intake of zinc in the highest tertile reduced the hazard ratio for early AMD from 2.25 to 1.27. Hazard ratios were reduced from 2.54 to 1.47 for highest intakes of beta-carotene, 2.63 to 1.72 for lutein/zeaxanthin, and 1.97 to 1.30 for EPA/DHA.
Results also showed a significant interaction between LOC387715 A69S genotype and intake of zinc and EPA/DHA. Carriers with the highest intake of zinc reduced their risk from 1.70 to 1.17, while those with the highest intake of EPA/DHA reduced their risk from 1.59 to 0.95.
Dietary antioxidants may help counteract oxidative damage that can activate the complement system, which plays a role in the pathogenesis of AMD. For example, zinc may help reduce complement activation that is already underway. Also, omega-3 fatty acids help prevent inflammation in the retina, another factor in the pathogenesis of AMD, by lowering acute-phase proteins, including complement C4, immunoglobulin M (IgM), haptoglobin, C-reactive protein, and fibrinogen, the researchers say.
Individuals need only consume the recommended daily allowances rather than excessive amounts of these nutrients to benefit, the researchers said.
They recommend fortified cereals, meats, dairy products, nuts, and seeds as sources of zinc, dark green leafy vegetables and bright orange vegetables as sources of beta-carotene, and oily fish as a source of EPA and DHA.
The relatively low number of cases in the stratified analyses is a limitation, although the researchers said they still could identify clear trends of risk reduction.
The authors reported having no financial conflicts. This study was supported by the Netherlands Organization for Scientific Research, among other sources.
complement factor H, CFH, LOC387715/HTRA1 genes, Dr. Lintje Ho,
complement factor H, CFH, LOC387715/HTRA1 genes, Dr. Lintje Ho,
FROM ARCHIVES OF OPHTHALMOLOGY
Major Finding: In patients with the CFH Y402H genotype, dietary intake of zinc in the highest tertile reduced the hazard ratio for early AMD from 2.25 to 1.27. Hazard ratios were reduced from 2.54 to 1.47 for highest intakes of beta-carotene, 2.63 to 1.72 for lutein/zeaxanthin, and 1.97 to 1.30 for EPA/DHA.
Data Source: A subset analysis of 2,167 individuals from the Rotterdam Study, a prospective, population-based study investigating chronic diseases in subjects age 55 years and older.
Disclosures: The authors reported having no financial conflicts. This study was supported by the Netherlands Organization for Scientific Research, among other sources.