User login
Topical Natural Products in Managing Dermatologic Conditions: Observations and Recommendations
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
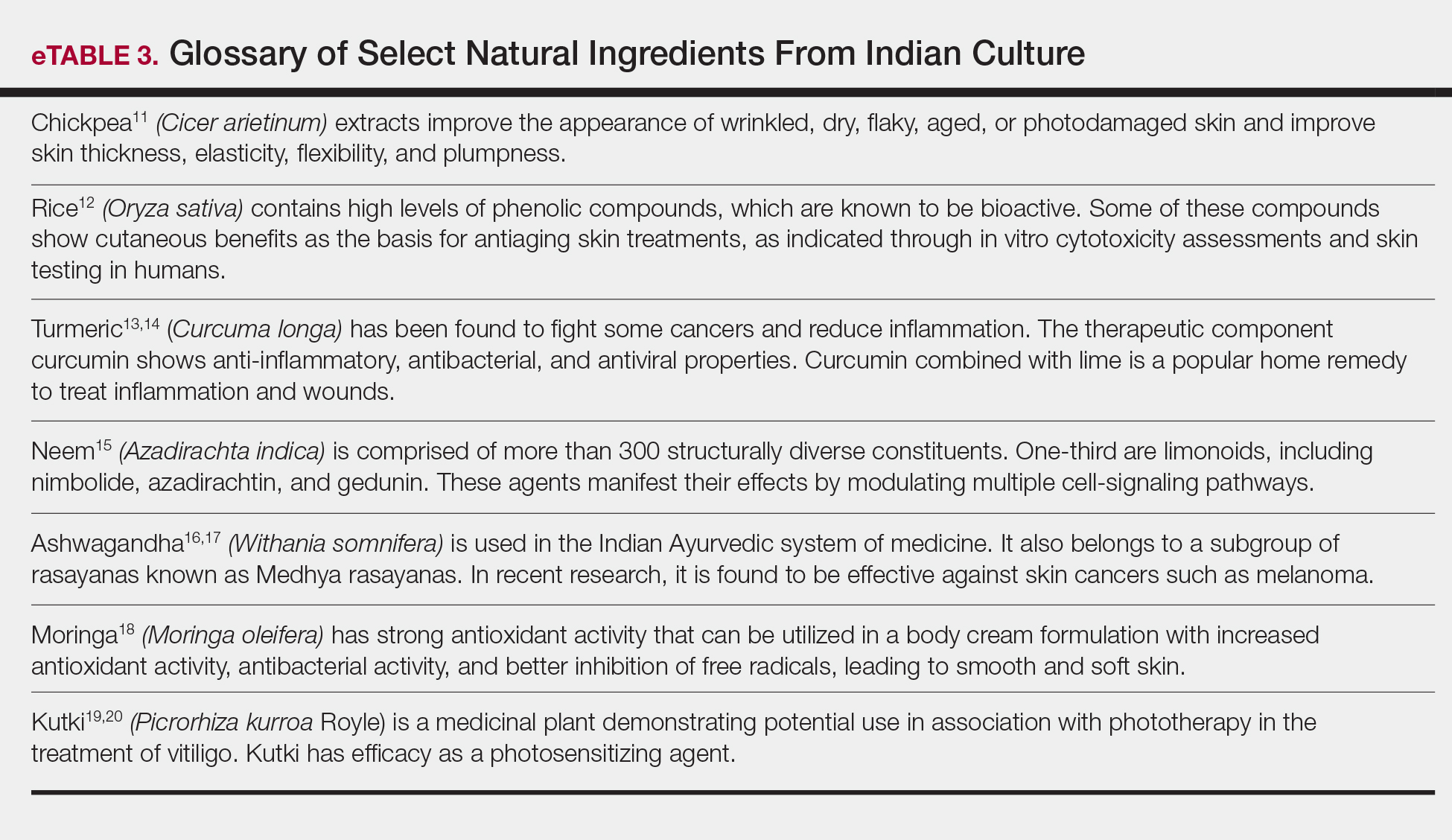
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.


For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.



For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.



For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Practice Points
- Patients are increasingly interested in and asking for nature-based products and formulations to manage dermatologic conditions.
- Physicians can satisfy patient interests with nature-based formulations that are as beneficial or more so than synthetic formulations because of the physiologic activity of the ingredients within these formulations.
- Physicians should have resources available to them that adequately educate on nature-based ingredients and how to recommend them.
