User login
Evaluating automated rules for rapid response system alarm triggers in medical and surgical patients
Patients typically show signs and symptoms of deterioration hours to days prior to cardiorespiratory arrest.1,2 The rate of inhospital cardiorespiratory arrest (CRA) requiring cardiopulmonary resuscitation is estimated to be 0.174 per bed per year in the United States.3 After CRA, survival to discharge is estimated to be as low as 18%.3,4 Efforts to predict and prevent arrest could prove beneficial.1,2
Rapid response systems (RRS) have been proposed as a means of identifying clinical deterioration and facilitating a timely response. These systems were designed to bring clinicians with critical care expertise to the bedside to prevent unnecessary deaths. They typically include an afferent limb (detects deteriorating patients), an efferent limb (responds to calls and acts to avoid further deterioration), and administrative and data analysis limbs.5,6 Automatic provision of recommendations and computer-based systems are desirable components of the afferent limb of the detection system.6 Both are independent predictors of improved clinical practices for clinical decision support systems.7 However, the existing early warning scores (EWS) may not be ready for automation due to low positive predictive values (PPV) and sensitivities.8
It is possible that the low discriminatory accuracy of the published EWS may be secondary to the use of aggregate patient populations for derivation of scores. We hypothesized that these EWS perform differently in medical and in surgical subpopulations. Also, the EWS need to be tested in a time-dependent manner to serve as a realistic clinical support tool for hospitalized patients.
STUDY AIM
The aim of this study was to evaluate the differential performance of widely used EWS in medical vs surgical patients.
METHODS
Site
The study was conducted in an academic center with 2 hospitals in Southeastern Minnesota totaling approximately 1500 general care nonintensive care unit (ICU) beds. The Mayo Clinic Institutional Review Board approved the research proposal.
Subjects
Our retrospective cohort was comprised of all adult inpatients discharged from 2 academic hospitals between January 1, 2011 and December 31, 2011 who spent any time in a general care (non-ICU) unit. We excluded patients younger than 18 years, psychiatric or rehabilitation inpatients, those without research authorization, and patients admitted for research purposes.
Study patients were divided into medical and surgical cohorts. Hospitalizations were considered surgical if patients had surgery at any time during their hospital stay according to billing data. A trigger was an instance in which a patient met the conditions of a specific rule (score/vital sign exceeded the published/defined threshold).
A resuscitation call was defined as a call for cardiopulmonary resuscitation when a patient has a CRA.
An event was an occurrence of 1 of the following in a general care setting: unplanned transfer to the ICU, resuscitation call, or RRS activation.
The RRS activation criteria consisted of an “acute and persistent change” in any 1 or more of the following: oxygen saturations less than 90%, heart rate less than 40 or greater than 130 beats/minute, systolic blood pressure less than 90 mm Hg, or respiratory rate less than 10 or greater than 28 breaths/minute. The RRS activation requires health provider action; they are not electronically generated. Nurses and physicians may also activate the RRS if they are concerned about a patient, even if calling criteria are not met. This is in contrast to the EWS analyzed, which are aggregate composites of multiple parameters. However, whether or not a derangement in vital signs is considered an “acute and persistent change” still involves clinical judgment. Any movement from a general care bed to an ICU bed, or from a general care bed to a procedure area, and from there to an ICU, was considered unplanned. Transfers to the ICU directly from the emergency department or operating room (OR) were not considered as an unplanned transfer and were not included in the analyses.
Coverage time was the period observed for events after a rule was triggered. In this analysis, a coverage time of 24 hours was considered, with a 1-hour look-back. A trigger was counted as a true positive if an event occurred during the following 24 hours. The 1-hour look-back was included to take into account the nursing clinical process of prioritizing a call to the RRS followed by documentation of the altered vital signs that prompted the call.
An episode was the continuous time on the general care floor within a hospitalization, excluding times when a patient was in the OR or ICU. For example, if a patient was admitted to a general bed on a surgery floor, subsequently went to the OR, and then returned to the surgery floor, the 2 episodes were considered separate: the time on the floor before surgery, and the time on the floor after surgery.
Assessment of implementation of RRS in our hospitals showed a significant drop in the failure-to-rescue rate (issues considered related to delay or failure to identify or intervene appropriately when a patient was deteriorating, as identified through mortality review) and a decrease in non-ICU mortality.9,10 This suggests that our current process captures many of the relevant episodes of acute deterioration when a rapid response team is needed and supports using RRS activation as outcomes.
Data Sources
We developed a time-stamped longitudinal database of patient data from the electronic health record, including vital signs, laboratory test results, demographics (age, sex), administrative data (including length of stay), comorbidities, resuscitation code status, location in hospital, and at the minute level throughout each patient’s hospital stay. Physiologically impossible values (eg, blood pressures of 1200 mm Hg) were considered entered in error and eliminated from the database. Time spent in the OR or ICU was excluded because RRS activation would not be applied in these already highly monitored areas. SAS Statistical software (SAS Institute Inc. Cary, North Carolina) was used for database creation.
We applied the current RRS calling criteria in our institution and calculated the Kirkland score,11 along with some of the most widely used early warning scores:12 Modified Early Warning System (MEWS),13 Standardized Early Warning Scoring System (SEWS),14 Global Modified Early Warning Score (GMEWS),15 Worthing physiologic scoring system,16 National Early Warning Score (NEWS),17 and VitaPAC Early Warning Score (ViEWS).18 Published thresholds for these scores were used to create rule triggers in the data. Once a trigger was created to calculate the number of false positives and true positives, all subsequent triggers were ignored until the end of the episode or until 24 hours elapsed. We calculated triggers in a rolling fashion throughout the episodes of care. The EWS score was updated every time a new parameter was entered into the analytical electronic health record, and the most recent value for each was used to calculate the score. SAS statistical software was used for calculation of scores and identification of outcomes.
For our analysis, events were treated as dependent variables, and triggers were independent variables. We calculated the score for each EWS to the minute level throughout our retrospective database. If the score for a specific EWS was higher than the published/recommended threshold for that EWS, an alert was considered to have been issued, and the patient was followed for 24 hours. If the patient had an event in the subsequent 24 hours, or 1 hour before (1-hour look-back), the alert was considered a true positive; if not, a false positive. Events that were not preceded by an alert were false negatives, and 24-hour intervals without either an alert or an event were considered true negatives. This simulation exercise was performed for each EWS in both subcohorts (medical and surgical). Clusters of RRS calls followed by transfers to the ICU within 3 hours were considered as a single adverse event (RRS calls, as it was the first event to occur) to avoid double counting. We have described how well this simulation methodology,8 correlates with results from prospective studies.19
Statistical Analysis
To calculate whether results were statistically significant for subgroups, a jackknife method of calculating variance20 was used. The jackknife method calculates variance by repeating the calculations of the statistic leaving out 1 sample at a time. In our case, we repeated the calculation of sensitivity and PPV leaving out 1 patient at a time. Once the simulation method had been run and the false/true positives/negatives had been assigned, calculation of each metric (PPV and sensitivity) was repeated for n subsamples, each leaving out 1 patient. The variance was calculated and 2 Student t tests were performed for each EWS: 1 for PPV and another for sensitivity. SAS statistical software v 9.3 was used for the simulation analysis; R statistical software v 3.0.2 (The R Foundation, Vienna, Austria) was used for the calculation of the statistical significance of results. A univariable analysis was also performed to assess the sensitivity and PPVs for the published thresholds of the most common variables in each EWS: respiratory rate, systolic blood pressure, heart rate, temperature, and mental status as measured by the modified Richmond Agitation Sedation Score.21
RESULTS
The initial cohort included 60,020 hospitalizations, of which the following were excluded: 2751 because of a lack of appropriate research authorization; 6433 because the patients were younger than 18 years; 2129 as psychiatric admissions; 284 as rehabilitation admissions; 872 as research purposes-only admissions; and 1185 because the patient was never in a general care bed (eg, they were either admitted directly to the ICU, or they were admitted for an outpatient surgical procedure and spent time in the postanesthesia care unit).
Table 1 summarizes patient and trigger characteristics, overall and by subgroup. The final cohort included 75,240 total episodes in 46,366 hospitalizations, from 34,898 unique patients, of which 48.7% were male. There were 23,831 medical and 22,535 surgical hospitalizations. Median length of episode was 2 days both for medical and surgical patients. Median length of stay was 3 days, both for medical and for surgical patients.
There were 3332 events in total, of which 1709 were RRS calls, 185 were resuscitation calls, and 1438 were unscheduled transfers to the ICU. The rate of events was 4.67 events per 100 episodes in the aggregate adult population. There were 3.93 events per 100 episodes for surgical hospitalizations, and 5.86 events per 100 episodes for medical hospitalizations (P < .001). The number of CRAs in our cohort was 0.27 per 100 episodes, 0.128 per hospital bed per year, or 4.37 per 1000 hospital admissions, similar to other reported numbers in the literature.3, 22,23
The total number of EWS triggers varied greatly between EWS rules, with the volume ranging during the study year from 1363 triggers with the GMEWS rule to 77,711 triggers with the ViEWS score.
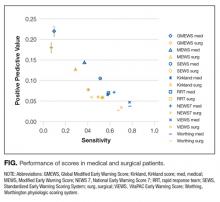
All scores had PPVs less than 25%. As seen in Table 2 and shown graphically in the Figure, all scores performed better on medical patients (blue) than on surgical patients (yellow). The P value was < .0001 for both PPV and sensitivity. The Worthing score had the highest sensitivity (0.78 for medical and 0.68 for surgical) but a very low PPV (0.04 for medical and 0.03 for surgical), while GMEWS was the opposite: low sensitivity (0.10 and 0.07) but the highest PPV (0.22 and 0.18).
The results of the univariable analysis can be seen in Table 3. Most of the criteria performed better (higher sensitivity and PPV) as predictors in the medical hospitalizations than in the surgical hospitalizations.
DISCUSSION
We hypothesized that EWS may perform differently when applied to medical rather than surgical patients. Studies had not analyzed this in a time-dependent manner,24-26 which limited the applicability of the results.8
All analyzed scores performed better in medical patients than in surgical patients (Figure). This could reflect a behavioral difference by the teams on surgical and medical floors in the decision to activate the RRS, or a bias of the clinicians who designed the scores (mostly nonsurgeons). The difference could also mean that physiological deteriorations are intrinsically different in patients who have undergone anesthesia and surgery. For example, in surgical patients, a bleeding episode is more likely to be the cause of their physiological deterioration, or the lingering effects of anesthesia could mask underlying deterioration. Such patients would benefit from scores where variables such as heart rate, blood pressure, or hemoglobin had more influence.
When comparing the different scores, it was much easier for a patient to meet the alerting score with the Worthing score than with GMEWS. In the Worthing score, a respiratory rate greater than 22 breaths per minute, or a systolic blood pressure less than 100 mm Hg, already meet alerting criteria. Similar vital signs result in 0 and 1 points (respectively) in GMEWS, far from its alerting score of 5. This reflects the intrinsic tradeoff of EWS: as the threshold for considering a patient “at risk” drops, not only does the number of true positives (and the sensitivity) increase, but also the number of false positives, thus lowering the PPV.
However, none of the scores analyzed were considered to perform well based on their PPV and sensitivity, particularly in the surgical subpopulation. Focusing on another metric, the area under the receiver operator curve can give misleadingly optimistic results.24,27 However, the extremely low prevalence of acute physiological deterioration can produce low PPVs even when specificity seems acceptable, which is why it is important to evaluate PPV directly.28
To use EWS effectively to activate RRS, they need to be combined with clinical judgment to avoid high levels of false alerts, particularly in surgical patients. It has been reported that RRS is activated only 30% of the time a patient meets RRS calling criteria.29 While there may be cultural characteristics inhibiting the decision to call,30 our study hints at another explanation: if RRS was activated every time a patient met calling criteria based on the scores analyzed, the number of RRS calls would be very high and difficult to manage. So health providers may be doing the right thing when “filtering” RRS calls and not applying the criteria strictly, but in conjunction with clinical judgment.
A limitation of any study like this is how to define “acute physiological deterioration.” We defined an event as recognized episodes of acute physiological deterioration that are signaled by escalations of care (eg, RRS, resuscitation calls, or transfers to an ICU) or unexpected death. By definition, our calculated PPV is affected by clinicians’ recognition of clinical deteriorations. This definition, common in the literature, has the limitation of potentially underestimating EWS’ performance by missing some events that are resolved by the primary care team without an escalation of care. However, we believe our interpretation is not unreasonable since the purpose of EWS is to trigger escalations of care in a timely fashion. Prospective studies could define an event in a way that is less affected by the clinicians’ judgment.
Regarding patient demographics, age was similar between the 2 groups (average, 58.2 years for medical vs 58.9 years for surgical), and there was only a small difference in gender ratios (45.1% male in the medical vs 51.4% in the surgical group). These differences are unlikely to have affected the results significantly, but unknown differences in demographics or other patient characteristics between groups may account for differences in score performance between surgical and medical patients.
Several of the EWS analyzed had overlapping trigger criteria with our own RRS activation criteria (although as single-parameter triggers and not as aggregate). To test how these potential biases could affect our results, we performed a post hoc sensitivity analysis eliminating calls to the RRS as an outcome (so using the alternative outcome of unexpected transfers to the ICU and resuscitation calls). The results are similar to those of our main analysis, with all analyzed scores having lower sensitivity and PPV in surgical hospitalizations when compared to medical hospitalizations.
Our study suggests that, to optimize detection of physiological deterioration events, EWS should try to take into account different patient types, with the most basic distinction being surgical vs medical. This tailoring will make EWS more complex, and less suited for paper-based calculation, but new electronic health records are increasingly able to incorporate decision support, and some EWS have been developed for electronic calculation only. Of particular interest in this regard is the score developed by Escobar et al,31 which groups patients into categories according to the reason for admission, and calculates a different subscore based on that category. While the score by Escobar et al. does not split patients based on medical or surgical status, a more general interpretation of our results suggests that a score may be more accurate if it classifies patients into subgroups with different subscores. This seems to be confirmed by the fact that the score by Escobar et al performs better than MEWS.28 Unfortunately, the paper describing it does not provide enough detail to use it in our database.
A recent systematic review showed increasing evidence that RRS may be effective in reducing CRAs occurring in a non-ICU setting and, more important, overall inhospital mortality.32 While differing implementation strategies (eg, different length of the educational effort, changes in the frequency of vital signs monitoring) can impact the success of such an initiative, it has been speculated that the afferent limb (which often includes an EWS) might be the most critical part of the system.33 Our results show that the most widely used EWS perform significantly worse on surgical patients, and suggest that a way to improve the accuracy of EWS would be to tailor the risk calculation to different patient subgroups (eg, medical and surgical patients). Plausible next steps would be to demonstrate that tailoring risk calculation to medical and surgical patients separately can improve risk predictions and accuracy of EWS.
Disclosure
The authors report no financial conflicts of interest.
1. Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999; 171(1):22-25. PubMed
2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. PubMed
3. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003; 58(3):297-308. PubMed
4. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57. PubMed
5. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463-2478. PubMed
6. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. PubMed
7. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
8. Romero-Brufau S, Huddleston JM, Naessens JM, Johnson MG, Hickman J, Morlan BW, et al. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549-552. PubMed
9. Huddleston JM, Diedrich DA, Kinsey GC, Enzler MJ, Manning DM. Learning from every death. J Patient Saf. 2014;10(1):6-12. PubMed
10. Moriarty JP, Schiebel NE, Johnson MG, Jensen JB, Caples SM, Morlan BW, et al. Evaluating implementation of a rapid response team: considering alternative outcome measures. Int J Qual Health Care. 2014;26(1):49-57. PubMed
11. Kirkland LL, Malinchoc M, O’Byrne M, Benson JT, Kashiwagi DT, Burton MC, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142. PubMed
12. Griffiths JR, Kidney EM. Current use of early warning scores in UK emergency departments. Emerg Med J. 2012;29(1):65-66. PubMed
13. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-526. PubMed
14. Paterson R, MacLeod DC, Thetford D, Beattie A, Graham C, Lam S, et al.. Prediction of in-hospital mortality and length of stay using an early warning scoring system: clinical audit. Clin Med (Lond). 2006;6(3):281-284. PubMed
15. Harrison GA, Jacques T, McLaws ML, Kilborn G. Combinations of early signs of critical illness predict in-hospital death–the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71(3):327-334. PubMed
16. Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007; 98(6):769-774. PubMed
17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-470. PubMed
18. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932-937. PubMed
19. Romero-Brufau S, Huddleston JM. Reply to letter: widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(10):e159. PubMed
20. Efron B, Stein C. The jackknife estimate of variance. Annals of Statistics. 1981;586-596.
21. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. PubMed
22. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. PubMed
23. Goncales PD, Polessi JA, Bass LM, Santos Gde P, Yokota PK, Laselva CR, et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442-448. PubMed
24. Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. PubMed
25. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88(6):571-575. PubMed
26. Stenhouse C, Coates S, Tivey M, Allsop P, Parker T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. British Journal of Anaesthesia. 2000;84(5):663-663.
27. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation. 2008;77(2):170-179. PubMed
28. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015; 19:285. PubMed
29. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. PubMed
30. Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569-575. PubMed
31. Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. PubMed
32. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417-425. PubMed
33. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-146. PubMed
Patients typically show signs and symptoms of deterioration hours to days prior to cardiorespiratory arrest.1,2 The rate of inhospital cardiorespiratory arrest (CRA) requiring cardiopulmonary resuscitation is estimated to be 0.174 per bed per year in the United States.3 After CRA, survival to discharge is estimated to be as low as 18%.3,4 Efforts to predict and prevent arrest could prove beneficial.1,2
Rapid response systems (RRS) have been proposed as a means of identifying clinical deterioration and facilitating a timely response. These systems were designed to bring clinicians with critical care expertise to the bedside to prevent unnecessary deaths. They typically include an afferent limb (detects deteriorating patients), an efferent limb (responds to calls and acts to avoid further deterioration), and administrative and data analysis limbs.5,6 Automatic provision of recommendations and computer-based systems are desirable components of the afferent limb of the detection system.6 Both are independent predictors of improved clinical practices for clinical decision support systems.7 However, the existing early warning scores (EWS) may not be ready for automation due to low positive predictive values (PPV) and sensitivities.8
It is possible that the low discriminatory accuracy of the published EWS may be secondary to the use of aggregate patient populations for derivation of scores. We hypothesized that these EWS perform differently in medical and in surgical subpopulations. Also, the EWS need to be tested in a time-dependent manner to serve as a realistic clinical support tool for hospitalized patients.
STUDY AIM
The aim of this study was to evaluate the differential performance of widely used EWS in medical vs surgical patients.
METHODS
Site
The study was conducted in an academic center with 2 hospitals in Southeastern Minnesota totaling approximately 1500 general care nonintensive care unit (ICU) beds. The Mayo Clinic Institutional Review Board approved the research proposal.
Subjects
Our retrospective cohort was comprised of all adult inpatients discharged from 2 academic hospitals between January 1, 2011 and December 31, 2011 who spent any time in a general care (non-ICU) unit. We excluded patients younger than 18 years, psychiatric or rehabilitation inpatients, those without research authorization, and patients admitted for research purposes.
Study patients were divided into medical and surgical cohorts. Hospitalizations were considered surgical if patients had surgery at any time during their hospital stay according to billing data. A trigger was an instance in which a patient met the conditions of a specific rule (score/vital sign exceeded the published/defined threshold).
A resuscitation call was defined as a call for cardiopulmonary resuscitation when a patient has a CRA.
An event was an occurrence of 1 of the following in a general care setting: unplanned transfer to the ICU, resuscitation call, or RRS activation.
The RRS activation criteria consisted of an “acute and persistent change” in any 1 or more of the following: oxygen saturations less than 90%, heart rate less than 40 or greater than 130 beats/minute, systolic blood pressure less than 90 mm Hg, or respiratory rate less than 10 or greater than 28 breaths/minute. The RRS activation requires health provider action; they are not electronically generated. Nurses and physicians may also activate the RRS if they are concerned about a patient, even if calling criteria are not met. This is in contrast to the EWS analyzed, which are aggregate composites of multiple parameters. However, whether or not a derangement in vital signs is considered an “acute and persistent change” still involves clinical judgment. Any movement from a general care bed to an ICU bed, or from a general care bed to a procedure area, and from there to an ICU, was considered unplanned. Transfers to the ICU directly from the emergency department or operating room (OR) were not considered as an unplanned transfer and were not included in the analyses.
Coverage time was the period observed for events after a rule was triggered. In this analysis, a coverage time of 24 hours was considered, with a 1-hour look-back. A trigger was counted as a true positive if an event occurred during the following 24 hours. The 1-hour look-back was included to take into account the nursing clinical process of prioritizing a call to the RRS followed by documentation of the altered vital signs that prompted the call.
An episode was the continuous time on the general care floor within a hospitalization, excluding times when a patient was in the OR or ICU. For example, if a patient was admitted to a general bed on a surgery floor, subsequently went to the OR, and then returned to the surgery floor, the 2 episodes were considered separate: the time on the floor before surgery, and the time on the floor after surgery.
Assessment of implementation of RRS in our hospitals showed a significant drop in the failure-to-rescue rate (issues considered related to delay or failure to identify or intervene appropriately when a patient was deteriorating, as identified through mortality review) and a decrease in non-ICU mortality.9,10 This suggests that our current process captures many of the relevant episodes of acute deterioration when a rapid response team is needed and supports using RRS activation as outcomes.
Data Sources
We developed a time-stamped longitudinal database of patient data from the electronic health record, including vital signs, laboratory test results, demographics (age, sex), administrative data (including length of stay), comorbidities, resuscitation code status, location in hospital, and at the minute level throughout each patient’s hospital stay. Physiologically impossible values (eg, blood pressures of 1200 mm Hg) were considered entered in error and eliminated from the database. Time spent in the OR or ICU was excluded because RRS activation would not be applied in these already highly monitored areas. SAS Statistical software (SAS Institute Inc. Cary, North Carolina) was used for database creation.
We applied the current RRS calling criteria in our institution and calculated the Kirkland score,11 along with some of the most widely used early warning scores:12 Modified Early Warning System (MEWS),13 Standardized Early Warning Scoring System (SEWS),14 Global Modified Early Warning Score (GMEWS),15 Worthing physiologic scoring system,16 National Early Warning Score (NEWS),17 and VitaPAC Early Warning Score (ViEWS).18 Published thresholds for these scores were used to create rule triggers in the data. Once a trigger was created to calculate the number of false positives and true positives, all subsequent triggers were ignored until the end of the episode or until 24 hours elapsed. We calculated triggers in a rolling fashion throughout the episodes of care. The EWS score was updated every time a new parameter was entered into the analytical electronic health record, and the most recent value for each was used to calculate the score. SAS statistical software was used for calculation of scores and identification of outcomes.
For our analysis, events were treated as dependent variables, and triggers were independent variables. We calculated the score for each EWS to the minute level throughout our retrospective database. If the score for a specific EWS was higher than the published/recommended threshold for that EWS, an alert was considered to have been issued, and the patient was followed for 24 hours. If the patient had an event in the subsequent 24 hours, or 1 hour before (1-hour look-back), the alert was considered a true positive; if not, a false positive. Events that were not preceded by an alert were false negatives, and 24-hour intervals without either an alert or an event were considered true negatives. This simulation exercise was performed for each EWS in both subcohorts (medical and surgical). Clusters of RRS calls followed by transfers to the ICU within 3 hours were considered as a single adverse event (RRS calls, as it was the first event to occur) to avoid double counting. We have described how well this simulation methodology,8 correlates with results from prospective studies.19
Statistical Analysis
To calculate whether results were statistically significant for subgroups, a jackknife method of calculating variance20 was used. The jackknife method calculates variance by repeating the calculations of the statistic leaving out 1 sample at a time. In our case, we repeated the calculation of sensitivity and PPV leaving out 1 patient at a time. Once the simulation method had been run and the false/true positives/negatives had been assigned, calculation of each metric (PPV and sensitivity) was repeated for n subsamples, each leaving out 1 patient. The variance was calculated and 2 Student t tests were performed for each EWS: 1 for PPV and another for sensitivity. SAS statistical software v 9.3 was used for the simulation analysis; R statistical software v 3.0.2 (The R Foundation, Vienna, Austria) was used for the calculation of the statistical significance of results. A univariable analysis was also performed to assess the sensitivity and PPVs for the published thresholds of the most common variables in each EWS: respiratory rate, systolic blood pressure, heart rate, temperature, and mental status as measured by the modified Richmond Agitation Sedation Score.21
RESULTS
The initial cohort included 60,020 hospitalizations, of which the following were excluded: 2751 because of a lack of appropriate research authorization; 6433 because the patients were younger than 18 years; 2129 as psychiatric admissions; 284 as rehabilitation admissions; 872 as research purposes-only admissions; and 1185 because the patient was never in a general care bed (eg, they were either admitted directly to the ICU, or they were admitted for an outpatient surgical procedure and spent time in the postanesthesia care unit).
Table 1 summarizes patient and trigger characteristics, overall and by subgroup. The final cohort included 75,240 total episodes in 46,366 hospitalizations, from 34,898 unique patients, of which 48.7% were male. There were 23,831 medical and 22,535 surgical hospitalizations. Median length of episode was 2 days both for medical and surgical patients. Median length of stay was 3 days, both for medical and for surgical patients.
There were 3332 events in total, of which 1709 were RRS calls, 185 were resuscitation calls, and 1438 were unscheduled transfers to the ICU. The rate of events was 4.67 events per 100 episodes in the aggregate adult population. There were 3.93 events per 100 episodes for surgical hospitalizations, and 5.86 events per 100 episodes for medical hospitalizations (P < .001). The number of CRAs in our cohort was 0.27 per 100 episodes, 0.128 per hospital bed per year, or 4.37 per 1000 hospital admissions, similar to other reported numbers in the literature.3, 22,23
The total number of EWS triggers varied greatly between EWS rules, with the volume ranging during the study year from 1363 triggers with the GMEWS rule to 77,711 triggers with the ViEWS score.
All scores had PPVs less than 25%. As seen in Table 2 and shown graphically in the Figure, all scores performed better on medical patients (blue) than on surgical patients (yellow). The P value was < .0001 for both PPV and sensitivity. The Worthing score had the highest sensitivity (0.78 for medical and 0.68 for surgical) but a very low PPV (0.04 for medical and 0.03 for surgical), while GMEWS was the opposite: low sensitivity (0.10 and 0.07) but the highest PPV (0.22 and 0.18).
The results of the univariable analysis can be seen in Table 3. Most of the criteria performed better (higher sensitivity and PPV) as predictors in the medical hospitalizations than in the surgical hospitalizations.
DISCUSSION
We hypothesized that EWS may perform differently when applied to medical rather than surgical patients. Studies had not analyzed this in a time-dependent manner,24-26 which limited the applicability of the results.8
All analyzed scores performed better in medical patients than in surgical patients (Figure). This could reflect a behavioral difference by the teams on surgical and medical floors in the decision to activate the RRS, or a bias of the clinicians who designed the scores (mostly nonsurgeons). The difference could also mean that physiological deteriorations are intrinsically different in patients who have undergone anesthesia and surgery. For example, in surgical patients, a bleeding episode is more likely to be the cause of their physiological deterioration, or the lingering effects of anesthesia could mask underlying deterioration. Such patients would benefit from scores where variables such as heart rate, blood pressure, or hemoglobin had more influence.
When comparing the different scores, it was much easier for a patient to meet the alerting score with the Worthing score than with GMEWS. In the Worthing score, a respiratory rate greater than 22 breaths per minute, or a systolic blood pressure less than 100 mm Hg, already meet alerting criteria. Similar vital signs result in 0 and 1 points (respectively) in GMEWS, far from its alerting score of 5. This reflects the intrinsic tradeoff of EWS: as the threshold for considering a patient “at risk” drops, not only does the number of true positives (and the sensitivity) increase, but also the number of false positives, thus lowering the PPV.
However, none of the scores analyzed were considered to perform well based on their PPV and sensitivity, particularly in the surgical subpopulation. Focusing on another metric, the area under the receiver operator curve can give misleadingly optimistic results.24,27 However, the extremely low prevalence of acute physiological deterioration can produce low PPVs even when specificity seems acceptable, which is why it is important to evaluate PPV directly.28
To use EWS effectively to activate RRS, they need to be combined with clinical judgment to avoid high levels of false alerts, particularly in surgical patients. It has been reported that RRS is activated only 30% of the time a patient meets RRS calling criteria.29 While there may be cultural characteristics inhibiting the decision to call,30 our study hints at another explanation: if RRS was activated every time a patient met calling criteria based on the scores analyzed, the number of RRS calls would be very high and difficult to manage. So health providers may be doing the right thing when “filtering” RRS calls and not applying the criteria strictly, but in conjunction with clinical judgment.
A limitation of any study like this is how to define “acute physiological deterioration.” We defined an event as recognized episodes of acute physiological deterioration that are signaled by escalations of care (eg, RRS, resuscitation calls, or transfers to an ICU) or unexpected death. By definition, our calculated PPV is affected by clinicians’ recognition of clinical deteriorations. This definition, common in the literature, has the limitation of potentially underestimating EWS’ performance by missing some events that are resolved by the primary care team without an escalation of care. However, we believe our interpretation is not unreasonable since the purpose of EWS is to trigger escalations of care in a timely fashion. Prospective studies could define an event in a way that is less affected by the clinicians’ judgment.
Regarding patient demographics, age was similar between the 2 groups (average, 58.2 years for medical vs 58.9 years for surgical), and there was only a small difference in gender ratios (45.1% male in the medical vs 51.4% in the surgical group). These differences are unlikely to have affected the results significantly, but unknown differences in demographics or other patient characteristics between groups may account for differences in score performance between surgical and medical patients.
Several of the EWS analyzed had overlapping trigger criteria with our own RRS activation criteria (although as single-parameter triggers and not as aggregate). To test how these potential biases could affect our results, we performed a post hoc sensitivity analysis eliminating calls to the RRS as an outcome (so using the alternative outcome of unexpected transfers to the ICU and resuscitation calls). The results are similar to those of our main analysis, with all analyzed scores having lower sensitivity and PPV in surgical hospitalizations when compared to medical hospitalizations.
Our study suggests that, to optimize detection of physiological deterioration events, EWS should try to take into account different patient types, with the most basic distinction being surgical vs medical. This tailoring will make EWS more complex, and less suited for paper-based calculation, but new electronic health records are increasingly able to incorporate decision support, and some EWS have been developed for electronic calculation only. Of particular interest in this regard is the score developed by Escobar et al,31 which groups patients into categories according to the reason for admission, and calculates a different subscore based on that category. While the score by Escobar et al. does not split patients based on medical or surgical status, a more general interpretation of our results suggests that a score may be more accurate if it classifies patients into subgroups with different subscores. This seems to be confirmed by the fact that the score by Escobar et al performs better than MEWS.28 Unfortunately, the paper describing it does not provide enough detail to use it in our database.
A recent systematic review showed increasing evidence that RRS may be effective in reducing CRAs occurring in a non-ICU setting and, more important, overall inhospital mortality.32 While differing implementation strategies (eg, different length of the educational effort, changes in the frequency of vital signs monitoring) can impact the success of such an initiative, it has been speculated that the afferent limb (which often includes an EWS) might be the most critical part of the system.33 Our results show that the most widely used EWS perform significantly worse on surgical patients, and suggest that a way to improve the accuracy of EWS would be to tailor the risk calculation to different patient subgroups (eg, medical and surgical patients). Plausible next steps would be to demonstrate that tailoring risk calculation to medical and surgical patients separately can improve risk predictions and accuracy of EWS.
Disclosure
The authors report no financial conflicts of interest.
Patients typically show signs and symptoms of deterioration hours to days prior to cardiorespiratory arrest.1,2 The rate of inhospital cardiorespiratory arrest (CRA) requiring cardiopulmonary resuscitation is estimated to be 0.174 per bed per year in the United States.3 After CRA, survival to discharge is estimated to be as low as 18%.3,4 Efforts to predict and prevent arrest could prove beneficial.1,2
Rapid response systems (RRS) have been proposed as a means of identifying clinical deterioration and facilitating a timely response. These systems were designed to bring clinicians with critical care expertise to the bedside to prevent unnecessary deaths. They typically include an afferent limb (detects deteriorating patients), an efferent limb (responds to calls and acts to avoid further deterioration), and administrative and data analysis limbs.5,6 Automatic provision of recommendations and computer-based systems are desirable components of the afferent limb of the detection system.6 Both are independent predictors of improved clinical practices for clinical decision support systems.7 However, the existing early warning scores (EWS) may not be ready for automation due to low positive predictive values (PPV) and sensitivities.8
It is possible that the low discriminatory accuracy of the published EWS may be secondary to the use of aggregate patient populations for derivation of scores. We hypothesized that these EWS perform differently in medical and in surgical subpopulations. Also, the EWS need to be tested in a time-dependent manner to serve as a realistic clinical support tool for hospitalized patients.
STUDY AIM
The aim of this study was to evaluate the differential performance of widely used EWS in medical vs surgical patients.
METHODS
Site
The study was conducted in an academic center with 2 hospitals in Southeastern Minnesota totaling approximately 1500 general care nonintensive care unit (ICU) beds. The Mayo Clinic Institutional Review Board approved the research proposal.
Subjects
Our retrospective cohort was comprised of all adult inpatients discharged from 2 academic hospitals between January 1, 2011 and December 31, 2011 who spent any time in a general care (non-ICU) unit. We excluded patients younger than 18 years, psychiatric or rehabilitation inpatients, those without research authorization, and patients admitted for research purposes.
Study patients were divided into medical and surgical cohorts. Hospitalizations were considered surgical if patients had surgery at any time during their hospital stay according to billing data. A trigger was an instance in which a patient met the conditions of a specific rule (score/vital sign exceeded the published/defined threshold).
A resuscitation call was defined as a call for cardiopulmonary resuscitation when a patient has a CRA.
An event was an occurrence of 1 of the following in a general care setting: unplanned transfer to the ICU, resuscitation call, or RRS activation.
The RRS activation criteria consisted of an “acute and persistent change” in any 1 or more of the following: oxygen saturations less than 90%, heart rate less than 40 or greater than 130 beats/minute, systolic blood pressure less than 90 mm Hg, or respiratory rate less than 10 or greater than 28 breaths/minute. The RRS activation requires health provider action; they are not electronically generated. Nurses and physicians may also activate the RRS if they are concerned about a patient, even if calling criteria are not met. This is in contrast to the EWS analyzed, which are aggregate composites of multiple parameters. However, whether or not a derangement in vital signs is considered an “acute and persistent change” still involves clinical judgment. Any movement from a general care bed to an ICU bed, or from a general care bed to a procedure area, and from there to an ICU, was considered unplanned. Transfers to the ICU directly from the emergency department or operating room (OR) were not considered as an unplanned transfer and were not included in the analyses.
Coverage time was the period observed for events after a rule was triggered. In this analysis, a coverage time of 24 hours was considered, with a 1-hour look-back. A trigger was counted as a true positive if an event occurred during the following 24 hours. The 1-hour look-back was included to take into account the nursing clinical process of prioritizing a call to the RRS followed by documentation of the altered vital signs that prompted the call.
An episode was the continuous time on the general care floor within a hospitalization, excluding times when a patient was in the OR or ICU. For example, if a patient was admitted to a general bed on a surgery floor, subsequently went to the OR, and then returned to the surgery floor, the 2 episodes were considered separate: the time on the floor before surgery, and the time on the floor after surgery.
Assessment of implementation of RRS in our hospitals showed a significant drop in the failure-to-rescue rate (issues considered related to delay or failure to identify or intervene appropriately when a patient was deteriorating, as identified through mortality review) and a decrease in non-ICU mortality.9,10 This suggests that our current process captures many of the relevant episodes of acute deterioration when a rapid response team is needed and supports using RRS activation as outcomes.
Data Sources
We developed a time-stamped longitudinal database of patient data from the electronic health record, including vital signs, laboratory test results, demographics (age, sex), administrative data (including length of stay), comorbidities, resuscitation code status, location in hospital, and at the minute level throughout each patient’s hospital stay. Physiologically impossible values (eg, blood pressures of 1200 mm Hg) were considered entered in error and eliminated from the database. Time spent in the OR or ICU was excluded because RRS activation would not be applied in these already highly monitored areas. SAS Statistical software (SAS Institute Inc. Cary, North Carolina) was used for database creation.
We applied the current RRS calling criteria in our institution and calculated the Kirkland score,11 along with some of the most widely used early warning scores:12 Modified Early Warning System (MEWS),13 Standardized Early Warning Scoring System (SEWS),14 Global Modified Early Warning Score (GMEWS),15 Worthing physiologic scoring system,16 National Early Warning Score (NEWS),17 and VitaPAC Early Warning Score (ViEWS).18 Published thresholds for these scores were used to create rule triggers in the data. Once a trigger was created to calculate the number of false positives and true positives, all subsequent triggers were ignored until the end of the episode or until 24 hours elapsed. We calculated triggers in a rolling fashion throughout the episodes of care. The EWS score was updated every time a new parameter was entered into the analytical electronic health record, and the most recent value for each was used to calculate the score. SAS statistical software was used for calculation of scores and identification of outcomes.
For our analysis, events were treated as dependent variables, and triggers were independent variables. We calculated the score for each EWS to the minute level throughout our retrospective database. If the score for a specific EWS was higher than the published/recommended threshold for that EWS, an alert was considered to have been issued, and the patient was followed for 24 hours. If the patient had an event in the subsequent 24 hours, or 1 hour before (1-hour look-back), the alert was considered a true positive; if not, a false positive. Events that were not preceded by an alert were false negatives, and 24-hour intervals without either an alert or an event were considered true negatives. This simulation exercise was performed for each EWS in both subcohorts (medical and surgical). Clusters of RRS calls followed by transfers to the ICU within 3 hours were considered as a single adverse event (RRS calls, as it was the first event to occur) to avoid double counting. We have described how well this simulation methodology,8 correlates with results from prospective studies.19
Statistical Analysis
To calculate whether results were statistically significant for subgroups, a jackknife method of calculating variance20 was used. The jackknife method calculates variance by repeating the calculations of the statistic leaving out 1 sample at a time. In our case, we repeated the calculation of sensitivity and PPV leaving out 1 patient at a time. Once the simulation method had been run and the false/true positives/negatives had been assigned, calculation of each metric (PPV and sensitivity) was repeated for n subsamples, each leaving out 1 patient. The variance was calculated and 2 Student t tests were performed for each EWS: 1 for PPV and another for sensitivity. SAS statistical software v 9.3 was used for the simulation analysis; R statistical software v 3.0.2 (The R Foundation, Vienna, Austria) was used for the calculation of the statistical significance of results. A univariable analysis was also performed to assess the sensitivity and PPVs for the published thresholds of the most common variables in each EWS: respiratory rate, systolic blood pressure, heart rate, temperature, and mental status as measured by the modified Richmond Agitation Sedation Score.21
RESULTS
The initial cohort included 60,020 hospitalizations, of which the following were excluded: 2751 because of a lack of appropriate research authorization; 6433 because the patients were younger than 18 years; 2129 as psychiatric admissions; 284 as rehabilitation admissions; 872 as research purposes-only admissions; and 1185 because the patient was never in a general care bed (eg, they were either admitted directly to the ICU, or they were admitted for an outpatient surgical procedure and spent time in the postanesthesia care unit).
Table 1 summarizes patient and trigger characteristics, overall and by subgroup. The final cohort included 75,240 total episodes in 46,366 hospitalizations, from 34,898 unique patients, of which 48.7% were male. There were 23,831 medical and 22,535 surgical hospitalizations. Median length of episode was 2 days both for medical and surgical patients. Median length of stay was 3 days, both for medical and for surgical patients.
There were 3332 events in total, of which 1709 were RRS calls, 185 were resuscitation calls, and 1438 were unscheduled transfers to the ICU. The rate of events was 4.67 events per 100 episodes in the aggregate adult population. There were 3.93 events per 100 episodes for surgical hospitalizations, and 5.86 events per 100 episodes for medical hospitalizations (P < .001). The number of CRAs in our cohort was 0.27 per 100 episodes, 0.128 per hospital bed per year, or 4.37 per 1000 hospital admissions, similar to other reported numbers in the literature.3, 22,23
The total number of EWS triggers varied greatly between EWS rules, with the volume ranging during the study year from 1363 triggers with the GMEWS rule to 77,711 triggers with the ViEWS score.
All scores had PPVs less than 25%. As seen in Table 2 and shown graphically in the Figure, all scores performed better on medical patients (blue) than on surgical patients (yellow). The P value was < .0001 for both PPV and sensitivity. The Worthing score had the highest sensitivity (0.78 for medical and 0.68 for surgical) but a very low PPV (0.04 for medical and 0.03 for surgical), while GMEWS was the opposite: low sensitivity (0.10 and 0.07) but the highest PPV (0.22 and 0.18).
The results of the univariable analysis can be seen in Table 3. Most of the criteria performed better (higher sensitivity and PPV) as predictors in the medical hospitalizations than in the surgical hospitalizations.
DISCUSSION
We hypothesized that EWS may perform differently when applied to medical rather than surgical patients. Studies had not analyzed this in a time-dependent manner,24-26 which limited the applicability of the results.8
All analyzed scores performed better in medical patients than in surgical patients (Figure). This could reflect a behavioral difference by the teams on surgical and medical floors in the decision to activate the RRS, or a bias of the clinicians who designed the scores (mostly nonsurgeons). The difference could also mean that physiological deteriorations are intrinsically different in patients who have undergone anesthesia and surgery. For example, in surgical patients, a bleeding episode is more likely to be the cause of their physiological deterioration, or the lingering effects of anesthesia could mask underlying deterioration. Such patients would benefit from scores where variables such as heart rate, blood pressure, or hemoglobin had more influence.
When comparing the different scores, it was much easier for a patient to meet the alerting score with the Worthing score than with GMEWS. In the Worthing score, a respiratory rate greater than 22 breaths per minute, or a systolic blood pressure less than 100 mm Hg, already meet alerting criteria. Similar vital signs result in 0 and 1 points (respectively) in GMEWS, far from its alerting score of 5. This reflects the intrinsic tradeoff of EWS: as the threshold for considering a patient “at risk” drops, not only does the number of true positives (and the sensitivity) increase, but also the number of false positives, thus lowering the PPV.
However, none of the scores analyzed were considered to perform well based on their PPV and sensitivity, particularly in the surgical subpopulation. Focusing on another metric, the area under the receiver operator curve can give misleadingly optimistic results.24,27 However, the extremely low prevalence of acute physiological deterioration can produce low PPVs even when specificity seems acceptable, which is why it is important to evaluate PPV directly.28
To use EWS effectively to activate RRS, they need to be combined with clinical judgment to avoid high levels of false alerts, particularly in surgical patients. It has been reported that RRS is activated only 30% of the time a patient meets RRS calling criteria.29 While there may be cultural characteristics inhibiting the decision to call,30 our study hints at another explanation: if RRS was activated every time a patient met calling criteria based on the scores analyzed, the number of RRS calls would be very high and difficult to manage. So health providers may be doing the right thing when “filtering” RRS calls and not applying the criteria strictly, but in conjunction with clinical judgment.
A limitation of any study like this is how to define “acute physiological deterioration.” We defined an event as recognized episodes of acute physiological deterioration that are signaled by escalations of care (eg, RRS, resuscitation calls, or transfers to an ICU) or unexpected death. By definition, our calculated PPV is affected by clinicians’ recognition of clinical deteriorations. This definition, common in the literature, has the limitation of potentially underestimating EWS’ performance by missing some events that are resolved by the primary care team without an escalation of care. However, we believe our interpretation is not unreasonable since the purpose of EWS is to trigger escalations of care in a timely fashion. Prospective studies could define an event in a way that is less affected by the clinicians’ judgment.
Regarding patient demographics, age was similar between the 2 groups (average, 58.2 years for medical vs 58.9 years for surgical), and there was only a small difference in gender ratios (45.1% male in the medical vs 51.4% in the surgical group). These differences are unlikely to have affected the results significantly, but unknown differences in demographics or other patient characteristics between groups may account for differences in score performance between surgical and medical patients.
Several of the EWS analyzed had overlapping trigger criteria with our own RRS activation criteria (although as single-parameter triggers and not as aggregate). To test how these potential biases could affect our results, we performed a post hoc sensitivity analysis eliminating calls to the RRS as an outcome (so using the alternative outcome of unexpected transfers to the ICU and resuscitation calls). The results are similar to those of our main analysis, with all analyzed scores having lower sensitivity and PPV in surgical hospitalizations when compared to medical hospitalizations.
Our study suggests that, to optimize detection of physiological deterioration events, EWS should try to take into account different patient types, with the most basic distinction being surgical vs medical. This tailoring will make EWS more complex, and less suited for paper-based calculation, but new electronic health records are increasingly able to incorporate decision support, and some EWS have been developed for electronic calculation only. Of particular interest in this regard is the score developed by Escobar et al,31 which groups patients into categories according to the reason for admission, and calculates a different subscore based on that category. While the score by Escobar et al. does not split patients based on medical or surgical status, a more general interpretation of our results suggests that a score may be more accurate if it classifies patients into subgroups with different subscores. This seems to be confirmed by the fact that the score by Escobar et al performs better than MEWS.28 Unfortunately, the paper describing it does not provide enough detail to use it in our database.
A recent systematic review showed increasing evidence that RRS may be effective in reducing CRAs occurring in a non-ICU setting and, more important, overall inhospital mortality.32 While differing implementation strategies (eg, different length of the educational effort, changes in the frequency of vital signs monitoring) can impact the success of such an initiative, it has been speculated that the afferent limb (which often includes an EWS) might be the most critical part of the system.33 Our results show that the most widely used EWS perform significantly worse on surgical patients, and suggest that a way to improve the accuracy of EWS would be to tailor the risk calculation to different patient subgroups (eg, medical and surgical patients). Plausible next steps would be to demonstrate that tailoring risk calculation to medical and surgical patients separately can improve risk predictions and accuracy of EWS.
Disclosure
The authors report no financial conflicts of interest.
1. Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999; 171(1):22-25. PubMed
2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. PubMed
3. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003; 58(3):297-308. PubMed
4. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57. PubMed
5. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463-2478. PubMed
6. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. PubMed
7. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
8. Romero-Brufau S, Huddleston JM, Naessens JM, Johnson MG, Hickman J, Morlan BW, et al. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549-552. PubMed
9. Huddleston JM, Diedrich DA, Kinsey GC, Enzler MJ, Manning DM. Learning from every death. J Patient Saf. 2014;10(1):6-12. PubMed
10. Moriarty JP, Schiebel NE, Johnson MG, Jensen JB, Caples SM, Morlan BW, et al. Evaluating implementation of a rapid response team: considering alternative outcome measures. Int J Qual Health Care. 2014;26(1):49-57. PubMed
11. Kirkland LL, Malinchoc M, O’Byrne M, Benson JT, Kashiwagi DT, Burton MC, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142. PubMed
12. Griffiths JR, Kidney EM. Current use of early warning scores in UK emergency departments. Emerg Med J. 2012;29(1):65-66. PubMed
13. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-526. PubMed
14. Paterson R, MacLeod DC, Thetford D, Beattie A, Graham C, Lam S, et al.. Prediction of in-hospital mortality and length of stay using an early warning scoring system: clinical audit. Clin Med (Lond). 2006;6(3):281-284. PubMed
15. Harrison GA, Jacques T, McLaws ML, Kilborn G. Combinations of early signs of critical illness predict in-hospital death–the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71(3):327-334. PubMed
16. Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007; 98(6):769-774. PubMed
17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-470. PubMed
18. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932-937. PubMed
19. Romero-Brufau S, Huddleston JM. Reply to letter: widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(10):e159. PubMed
20. Efron B, Stein C. The jackknife estimate of variance. Annals of Statistics. 1981;586-596.
21. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. PubMed
22. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. PubMed
23. Goncales PD, Polessi JA, Bass LM, Santos Gde P, Yokota PK, Laselva CR, et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442-448. PubMed
24. Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. PubMed
25. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88(6):571-575. PubMed
26. Stenhouse C, Coates S, Tivey M, Allsop P, Parker T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. British Journal of Anaesthesia. 2000;84(5):663-663.
27. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation. 2008;77(2):170-179. PubMed
28. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015; 19:285. PubMed
29. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. PubMed
30. Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569-575. PubMed
31. Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. PubMed
32. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417-425. PubMed
33. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-146. PubMed
1. Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999; 171(1):22-25. PubMed
2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. PubMed
3. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003; 58(3):297-308. PubMed
4. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57. PubMed
5. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463-2478. PubMed
6. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. PubMed
7. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
8. Romero-Brufau S, Huddleston JM, Naessens JM, Johnson MG, Hickman J, Morlan BW, et al. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549-552. PubMed
9. Huddleston JM, Diedrich DA, Kinsey GC, Enzler MJ, Manning DM. Learning from every death. J Patient Saf. 2014;10(1):6-12. PubMed
10. Moriarty JP, Schiebel NE, Johnson MG, Jensen JB, Caples SM, Morlan BW, et al. Evaluating implementation of a rapid response team: considering alternative outcome measures. Int J Qual Health Care. 2014;26(1):49-57. PubMed
11. Kirkland LL, Malinchoc M, O’Byrne M, Benson JT, Kashiwagi DT, Burton MC, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142. PubMed
12. Griffiths JR, Kidney EM. Current use of early warning scores in UK emergency departments. Emerg Med J. 2012;29(1):65-66. PubMed
13. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-526. PubMed
14. Paterson R, MacLeod DC, Thetford D, Beattie A, Graham C, Lam S, et al.. Prediction of in-hospital mortality and length of stay using an early warning scoring system: clinical audit. Clin Med (Lond). 2006;6(3):281-284. PubMed
15. Harrison GA, Jacques T, McLaws ML, Kilborn G. Combinations of early signs of critical illness predict in-hospital death–the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71(3):327-334. PubMed
16. Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007; 98(6):769-774. PubMed
17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-470. PubMed
18. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932-937. PubMed
19. Romero-Brufau S, Huddleston JM. Reply to letter: widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(10):e159. PubMed
20. Efron B, Stein C. The jackknife estimate of variance. Annals of Statistics. 1981;586-596.
21. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. PubMed
22. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. PubMed
23. Goncales PD, Polessi JA, Bass LM, Santos Gde P, Yokota PK, Laselva CR, et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442-448. PubMed
24. Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. PubMed
25. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88(6):571-575. PubMed
26. Stenhouse C, Coates S, Tivey M, Allsop P, Parker T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. British Journal of Anaesthesia. 2000;84(5):663-663.
27. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation. 2008;77(2):170-179. PubMed
28. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015; 19:285. PubMed
29. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. PubMed
30. Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569-575. PubMed
31. Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. PubMed
32. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417-425. PubMed
33. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-146. PubMed
© 2017 Society of Hospital Medicine
Geriatric Care Approaches in Hospitalist Programs
Between 1996with the first appearance of hospitalists in the medical literatureand the present, the hospitalist workforce has grown to nearly 10,000.1, 2 More remarkable is the estimate that the number of hospitalists will double in the next 5 years.2 The rapid growth of hospital medicine raises significant issues for the care of older patients, who are hospitalized at high rates3 and suffer numerous complications from hospitalization including functional decline,4 delirium,5 and a disproportionate share of adverse events.6 Conversely, the needs of patients older than 65 years of age, whose hospital stays make up nearly 50% of acute‐care bed days, will shape the future of hospital medicine.3
To date, the hospital medicine literature has failed to address the particular challenges of treating older patients, focusing primarily on opportunities for reductions in costs and length of stay for hospitalists' Medicare patients (of about $1000 per admission and 0.5 days, respectively7, 8) when compared with those cared for by other physicians. This focus on economic efficiency reflects the early orientation of the hospitalist movement. More recently, leaders of the hospitalist professional organization, the Society of Hospital Medicine (SHM), have increasingly recognized that caring for the older population will require additional knowledge and clinical skills beyond that taught in internal medicine residencies.9 Beyond educational initiatives, however, hospitalists must reconsider the paradigms of hospital care that make the hospital setting so dangerous for the older patient.
Given the aging population and the predicted growth of hospital medicine, it is essential to develop an understanding of the impact of hospitalists on the care of older patients and to encourage clinical innovation at the intersection of hospital medicine and geriatrics. Consequently, this article 1) identifies and summarizes geriatric care approaches in hospitalist programs, 2) presents a case study of geriatric hospital care by a hospitalist group, and 3) highlights opportunities for innovation and further research.
METHODS
Sample
We conducted a cross‐sectional survey of the hospitalist community via two mailings to SHM Listservs in September 2003 and September 2004. To encourage responses, the e‐mails used terms such as innovating, developing, providing hospitalist services, and caring for the geriatric patient or Medicare population. Respondents to the e‐mail solicitations (n = 14), leaders of SHM and academic hospitalist groups (n = 14), and leaders of the American Geriatrics Society specializing in acute care (n = 3) were queried about additional contacts who might know about programs utilizing geriatric care approaches. Each of these contacts was subsequently solicited and queried.10 Thirteen of the respondents described the current use by their hospitalist groups of one or more geriatric care approaches that represented a departure from usual care. We subsequently refer to these approaches as innovations. The 13 respondents completed in‐depth telephone interviews with one of the authors (H.W.). All respondents were recontacted in the spring of 2005 to update their responses. Two of the 13 programs were eliminated from the analysis after the interviews were completed. The first of these programs was identified in 2003 but had been discontinued by 2004. The second program was eliminated because the innovation was not implemented.
Data Collection
We developed a data collection tool to gather descriptive information from respondents regarding characteristics of the hospitalist group, the clinical program, the primary hospital, and the innovation (focus, target patients, organization, staffing, training, rounding, other). In addition, respondents were queried about motivations for the innovation; successes, opportunities, and future plans; and failures and barriers to implementation.
Analysis
First, we summarized the characteristics of the 11 innovations (Table 1). Second, geriatric care approaches were identified from the innovations on the basis of their objectives and the types of responses we encountered most frequently. The approaches were not mutually exclusive. For instance, a program providing postdischarge care at a skilled nursing facility (SNF) might also use a geriatrician‐hospitalist staffing model.
| Site | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focus | |||||||||||
| Medical care | x | x | x | x | |||||||
| Postdischarge care | x | x | |||||||||
| Perioperative care | x | x | x | ||||||||
| Geriatric assessment | x | x | x | ||||||||
| Quality improvement | x | x | x | x | |||||||
| Staffing | x | ||||||||||
| Generalist‐hospitalist | x | x | x | x | x | x | |||||
| Geriatrician‐hospitalist | x | x | x | x | x | x | |||||
| Advanced‐practice nurse | x | x | x | x | |||||||
| Patient targeting | |||||||||||
| By age | x | x | x | x | x | x | x | x | x | ||
| By diagnosis | x | x | x | x | x | x | |||||
| By location | x | x | x | x | x | x | |||||
| Organization | |||||||||||
| Unit | x | x | x | x | x | x | |||||
| Service | x | x | x | x | x | x | |||||
| Interdisciplinary rounds | x | x | x | x | x | x | |||||
| Geriatrics training | x | x | x | x | x | x | x |
RESULTS
In 2003 the annual survey of the American Hospital Association identified 1415 hospitalist groups in the United States (Joe Miller, SHM senior vice‐president, personal communication). Remarkably, our query identified only 11 hospitalist groups with clinical innovations aimed at the older population. These innovations ranged from single individuals involved in targeted quality‐improvement projects to highly developed programs addressing an array of clinical needs for the hospitalized older patient. These 11 programs are summarized in Table 1 and described below.
Focus
Hospitalists' programs targeted to the older patient were designed to meet various needs arising from an episode of hospital care. These included innovations designed around their core clinical activities in providing acute medical care (four innovations), as well as innovations targeted to postdischarge care at SNFs (two innovations), perioperative care in consultative or comanagement models (four innovations), comprehensive geriatric assessment (three innovations), and clinical quality improvement such as audit tools (four innovations).
Staffing
Four innovations employed physicians without specific geriatrics training (generalist‐hospitalists), four innovations employed 16 fellowship‐trained geriatricians (geriatrician‐hospitalists), and two programs employed both geriatricians and generalist hospitalists. Four innovations employed advanced‐practice nurses, both with and without gerontology training.
Patients
Nine of the 11 innovations targeted patients by age (older than 65, 70, or 75 years). Of the two innovations that did not target patients by age, one focused on improving the quality of care for all patients on a medical ward by focusing on geriatric issues (Site I), and a second was concerned with postdischarge care for all patients discharged to affiliated SNFs (Site K). In addition to targeting by age, six innovations targeted patients on the basis of diagnosis, four of which focused on surgical diagnosis. Finally, patient selection by location occurred in six of the innovations, as described in the next section.
Organization
Six of the innovations were organized to operate within a clinical service (such as a medical or surgical team). In contrast to the service‐based innovations, six clinical innovations for older patients operated in geographic units including acute care for elders (ACE) units (n = 2), SNFs (n = 2), a medical nursing unit (n = 1), and an emergency department (ED; n = 1). Of the two ACE units, one (Site G) existed prior to the establishment of the hospitalist group. In this instance, a geriatrician‐hospitalist appointed jointly by the hospitalist group and the Division of Geriatrics staffed ACE unit patients of select private physicians and unassigned patients. The second ACE unit (Site H), established with the formation of the hospitalist group, was staffed by two hospitalists among eight physicians in a private geriatrics group. Regarding SNFs, one hospitalist group for a large health care organization (Site K) rounded at contract SNFs at which group members held medical directorships; another hospitalist program took over rounding at an SNF owned by its health system (Site A).
Rounding
Six of the innovations incorporated interdisciplinary rounds, including all three innovations with medical care as their focus. Four of the six innovations with interdisciplinary rounds were based in ACE units or SNFs. One of these six innovations (Site C), a perioperative initiative, incorporated twice‐weekly multidisciplinary rounds attended by an attending surgeon, surgical residents, and a hospitalistin addition to the nurses, case managers, and therapists.
Training
Seven of the 11 innovations involved geriatrics training. Four of the training innovations targeted nursing staff, four targeted hospitalist physicians, and one targeted both nurses and physicians. Most institutions developed their own curricula. Three hospitalist groups, however, modified preexisting curricula, struggling to adapt them to the needs of hospital‐based staff. Two innovations (Sites A and K) used a clinical mentoring model in which generalist‐hospitalists learned geriatrics principles while working side by side with geriatrician‐hospitalists.
Case Study
We selected the most comprehensive program for further description. This case illustrates the power of integrating geriatric and hospital medicine paradigms.
Hospital Internal Medicine, Mayo Clinic, Rochester, MN (Site A)
The Mayo Clinic established the Hospital Internal Medicine Group (HIM) in 1998 in response to changing resident workload regulations. The practice initially focused on perioperative medical care for a busy orthopedic trauma surgery (OTS) service. In 2000, noting the average age of the elective orthopedic population was 81, the leadership of HIM made a strategic decision to recruit physicians with geriatrics training. By 2005, 6 of the 22 physicians the group employed were geriatricians.
In mid‐2005 the group's members covered eight services in 1‐ to 2‐week block rotations. Three of the services are uniquely focused on the older patient: the Geriatric Medicine Service (GeM), the OTS, and the SNF. On the GeM, a geriatrician‐hospitalist works alongside a generalist‐hospitalist to for care medical patients triaged to the service based on age (older than 75) and frailty. Although the GeM is based on a medical nursing unit, the unit is neither configured nor staffed like an ACE unit, and up to 20% of the GeM's patients overflow to other units. In addition to providing acute care, the GeM employs standardized documentation to facilitate universal comprehensive geriatric assessment. On the OTS, HIM hospitalists care for postoperative patients in a comanagement model, descriptions of which have been published elsewhere.11, 12 As a reflection of its orientation toward the older surgical patient, every OTS patient is assessed for delirium with the confusion assessment method instrument.13 Finally, the 30‐bed SNF service (on which 75% of admissions are postoperative for subacute rehabilitation) is supervised by a HIM physician and a nurse‐practitioner.
Additional activities of HIM physicians are clinical quality improvement including participation in the creation of inpatient care pathways, revision of the hospital's discharge processes, ongoing review of adverse events, and use of standardized tools for intrahospital transfers. In addition, the HIM group prioritizes geriatrics education for its physicians and hospital medicine fellows. In turn, geriatrics fellows rotate through the GeM, SNF, and OTS services.
DISCUSSION
Although SHM increasingly recognizes the challenges inherent in caring for older patients, few hospitalists are adapting their care for this vulnerable population. We identified only 11 innovations in geriatric care despite there being more than 1000 hospitalist groups. This apparent paucity of innovation in geriatrics might be explained by the relatively recent introduction of hospital medicine. As no hospitalist program is more than 10 years old, most programs are still focused on building core clinical activities or on other competing demands. In addition to time, funding may limit the typical program's ability to innovate without directly increasing revenue. Although the geriatrics literature supports that specialized inpatient care for older patients can result in increased physical functioning and quality of life at no additional cost, it may be that geriatricians have yet to make this case effectively to the hospitalist community.14, 15
The findings of this study were limited by our survey methodology. Specifically, our sample was limited to professional contacts and those using SHM listservs. In addition, some innovative hospitalists may not consider their programs to be geriatric programs and so may not have responded to our queries. Therefore, the reported innovations are not representative of geriatric care among all hospitalist groups, and we are unable to provide a comprehensive picture of geriatric care in hospitalist programs. In addition, we cannot comment on the effectiveness of the care approaches at participating institutions. For example, interdisciplinary care is an important tenet of geriatric medicine. Although six of our programs reported interdisciplinary rounds, it is unclear if these rounds are models of effective collaborative practice. Nonetheless, the information obtained from the structured interviews allowed the identification of several instructive themes discussed below.
Opportunities
The growth of the hospitalist movement provides an opportunity to reconsider clinical paradigms for the hospitalized older population. Hospitalists bring clinical skills in treating acute illness, preventing hospital complications, and providing perioperative care.16, 17 As leaders in institutional quality, safety, and utilization initiatives, hospitalists are often given protected time for such endeavors.18, 19 In so doing, the incentives of hospitalists are aligned with those of hospital administrators. This orientation makes hospitalists open to innovation in clinical care improvement.
The opportunity for hospitalists to bring fresh approaches to acute care geriatrics need not happen in a vacuum. More than 30 years of geriatrics research has provided a framework, literature, and expertise to inform hospitalist groups. The common goal of clinical excellence for the hospitalized older patient should motivate cooperation, collaborative approaches, and a joint clinical research agenda. From our inquiry to hospitalist groups, it appears that this sort of interaction occurs infrequently. The innovations identified and the case study described highlight several ways in which the geriatric medicine and hospital medicine experiences inform one another. These include approaches to staffing, organization, and quality improvement, as well as to clinical areas amenable to innovation.
Approaches
Staffing and Organization
The employment of geriatrics‐trained clinicians by hospitalist programs is one approach to supporting generalist‐hospitalists and inclining group culture toward clinical geriatric concerns. Programs that purposefully hired geriatricians and gerontology nurse‐practitioners used them to staff geriatrics services including ACE units, SNFs and, in the case of HIM, a GeM service that was a modification of a medical service. In addition, two programs relied on geriatrician‐hospitalists to serve as clinical mentors to generalist‐hospitalists.
In particular, the use of geriatrics‐trained staff on specialized services such as ACE units is encouraging, as specialized geriatric units remain an underutilized care model,20 despite compelling evidence of their effectiveness in improving physical functioning and reducing nursing home admissions.14 Although the factors undermining the success of ACE units in the past may also pose challenges for hospitalists, hospitalist groups may be better positioned to maintain the interest and financial commitment of hospital administrators. The HIM's GeM Service is also of interest, given the need to disseminate best practices in geriatrics throughout the hospital. The benefits to older patients of such a service, however, have not been demonstrated. Likewise, comprehensive geriatric assessment and geriatric consultation in the inpatient setting are reported to have had mixed results in the absence of targeting individuals at highest risk for adverse outcomes.21
Patient Safety and Quality Improvement
Hospital medicine has rapidly integrated principles of quality improvement and patient safety, having grown up contemporaneously with the patient safety movement. Several of the hospitalist programs we identified spearheaded quality improvement efforts directed at the particular needs of older patients such as delirium prevention, provision of immunizations, and removal of indwelling Foley catheters.
These efforts can be seen in the context of the many hospitalist programs focusing on standardizing care, understanding iatrogenesis, adopting safe technologies, and generally moving hospital culture forward.22 In choosing to embrace patient safety practices such as medication reconciliation (endorsed by the Institute for Healthcare Improvement),23 hospitalists may confer disproportionate benefits to older patients, who frequently require multiple medications and are at high risk for adverse drug events.6 As the efficacy of many of these interventions is poorly understood, hospitalist and geriatricians (whose work on the hazards of hospitalization anticipated the patient safety movement by many years24) may find a shared clinical research agenda with patient safety as its focus.
Areas of Clinical Opportunity
Perioperative Care
Commentators have noted hospitalists' growing participation in perioperative care,17 much of which concerns the older orthopedic surgery patient.12 Through their embedding in surgical wards, hospitalists may become actual or de facto members of surgical teams with a significant impact on team culture and care delivery. For example, hospitalists at one program implemented a perioperative beta‐blocker protocol for older orthopedic surgery patients, leading to a marked decrease in postoperative cardiac events (Site B).
Although many hospitalist programs participate in similar initiatives, it is likely that additional attention to the needs of older patients will augment the effectiveness of their interventions. For instance, structured geriatrics consultation can reduce the incidence of postoperative delirium among hip fracture patients by 46% (NNT = 5.6).25 Increased attention to postoperative pain control and early mobilization, among others, may affect the functional recovery of the older surgical patient.26, 27
Postdischarge care and care transitions
The hallmark of the hospitalist modelthe handoff of care from a primary care provider to an inpatient provideris commonly considered the major limitation of the hospitalist model because of the risk of lost clinical information.1 Because older patients are particularly susceptible to postdischarge adverse events, their care transitions may require specialized attention.28 Two of the innovations we identified (Sites A and K) have extended care of older patients into the postacute setting by integrating SNF care into their programs as a way to streamline discharge processes, decrease miscommunication, and underscore the limitations of postacute care.
A growing body of evidence supports the role of discharge strategies in improving care transitions. In one study, postdischarge follow‐up with a hospital physician rather than a community physician resulted in a reduction of the combined end point of 30‐day mortality and nonelective readmission.29 In a randomized trial, postdischarge phone calls by a pharmacist reduced the number of ED visits within 30 days of discharge.30 In another trial, older patients receiving a multifactorial intervention aimed at providing the skills for active participation in care transitions resulted in a reduced number of readmissions within 30 days.31 Understanding and implementing these activities may be crucial to both the care of older patients and the success of the hospitalist enterprise.
Barriers
Part of the challenge of treating older patients in hospitals is that the paradigms of geriatrics and hospital medicine differ substantially.32 Notably, geriatric medicine goals of maximizing function and quality of life may conflict with traditional medical goals of diagnosis and cure. This dichotomy is amplified in the hospital setting because hospitals are organized to maximize the physician's ability to stabilize, diagnose, cure, and discharge.33
By design, the hospitalist model introduces additional challenges into the hospital paradigm that affect the older patient, such as the discontinuities addressed above. Additional factors that hospitalists identified as barriers to the effective care of older patients include: 1) poor communication skills, 2) ineffective interdisciplinary collaboration, 3) limited geriatrics knowledge base, and 4) insufficient support for care coordination.34 Despite these recognized challenges, our query to hospitalist groups identified few that had made clinical excellence in geriatrics a focus of their activities.
Even with its prioritization of geriatric medicine, the well‐developed HIM model faces challenges. In particular, the feasibility of the geriatrician‐hospitalist is limited by the many geriatricians who, because of the scarcity of those who are fellowship trained, may be unprepared to care for acutely ill older patients, as their training has not focused on the hospital setting.35, 36 In addition, the surgical comanagement model depends on a unique collaboration with surgical colleagues. Finally, the ability of the HIM group to incorporate geriatrics paradigms into the hospital setting depends on extensive support from the hospital in the form of resources and a shared vision that is unlikely to be found at most institutions.
CONCLUSIONS
The rapid growth of the hospitalist movement will significantly affect clinical care in American hospitals. As most hospital patients are older, the impact on acute care geriatrics cannot be overlooked. In our study, we identified only a small number of hospitalist groups that have made geriatric medicine a priority. These programs prioritize geriatric medicine through the employment of geriatrics‐trained staff, adaptation of geriatric care models such as ACE units, and commitment to clinical quality improvement and patient safety. They also focus on common clinical challenges for older patients, including postoperative and postdischarge care. Although much can be learned from these examples, programs at other institutions will need to be individualized to meet the specific needs of each hospital and community. The common goal of clinical excellence shared by hospitalists and geriatricians should motivate cooperation, collaborative approaches, and a joint clinical research agenda at all levels, as the current paradigm of hospital care remains inadequate to meet the needs of the acutely ill older patient.
- ,.The emerging role of hospitalists in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at URL: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm[accessed January 20, 2005].
- ,.2002 National Hospital Discharge Survey.Advance data from Vital And Health Statistics. No. 342.Hyattsville (MD):National Center for Health Statistics,2002.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156,:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care.Am J Med.1999;106:565–573.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.year="2001"2001.
- .Improving care for older adults: SHM educational initiatives.Hospitalist.2004;8(Suppl):45–47.
- .Qualitative evaluation and research methods.2nd ed.Thousand Oaks (CA):Sage Publications,1990:176.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for the detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,Geriatric evaluation and management units for hospitalized patients. In:Making healthcare safer: a critical analysis of Patient Safety Practices Evidence Report/Technology AssessmentNo. 43.2001. AHRQ Publication No. 01‐E058.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,.The hospitalist movement five years later.JAMA.2002;287:487–494.
- .The hospitalist joins the surgical team.Ann Intern Med.2004;141:67–69.
- .The end of the beginning: patient safety five years after ‘To Err Is Human.’Health Aff.2004;23S2:W534–W545.
- .How hospitalists add value.Hospitalist.2005;9(Suppl 1):6–7.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,, et al.Multidisciplinary geriatric consultation services,Chap. 29.Evidence Report/Technology Assessment No. 43.2001. AHRQ Publication No. 01‐E058.
- .Hospitalists spearhead a wide range of patient safety improvement projects.Hospitalist.2004;8(Suppl.):33–35.
- Institute for Healthcare Improvement.100k Lives campaign. 10‐20‐2005.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,,, et al.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,,, et al.The impact of postoperative pain on outcomes following hip fracture.Pain.2003;103:303–311.
- ,,, et al.Physical therapy and mobility 2 and 6 months after hip fracture.J Am Geriatr Soc.2004;52:1114–1120.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Dis Mon.2002;48:239–248.
- ,,, et al.The care transitions intervention: results from a randomized controlled trial. Society of Hospital Medicine Annual Meeting, Chicago, IL,2005.
- .Case management: high intensity care for frail patients with complex needs.Geriatrics.1998;53:62–68.
- .The care of strangers: the rise of America's hospital system.New York:Basic Books,1987.
- ,,.Hospitalists' role in caring for older Americans: Executive Summary.2002. San Francisco, prepared for the John Hartford Foundation.
- Geriatric medicine training and practice in the United States at the beginning of the 21st century.New York:Association of Directors of Geriatric Academic Programs,2002.
- AGS Education Committee.Guidelines for fellowship training in geriatrics.1998;46:1473–1477.
Between 1996with the first appearance of hospitalists in the medical literatureand the present, the hospitalist workforce has grown to nearly 10,000.1, 2 More remarkable is the estimate that the number of hospitalists will double in the next 5 years.2 The rapid growth of hospital medicine raises significant issues for the care of older patients, who are hospitalized at high rates3 and suffer numerous complications from hospitalization including functional decline,4 delirium,5 and a disproportionate share of adverse events.6 Conversely, the needs of patients older than 65 years of age, whose hospital stays make up nearly 50% of acute‐care bed days, will shape the future of hospital medicine.3
To date, the hospital medicine literature has failed to address the particular challenges of treating older patients, focusing primarily on opportunities for reductions in costs and length of stay for hospitalists' Medicare patients (of about $1000 per admission and 0.5 days, respectively7, 8) when compared with those cared for by other physicians. This focus on economic efficiency reflects the early orientation of the hospitalist movement. More recently, leaders of the hospitalist professional organization, the Society of Hospital Medicine (SHM), have increasingly recognized that caring for the older population will require additional knowledge and clinical skills beyond that taught in internal medicine residencies.9 Beyond educational initiatives, however, hospitalists must reconsider the paradigms of hospital care that make the hospital setting so dangerous for the older patient.
Given the aging population and the predicted growth of hospital medicine, it is essential to develop an understanding of the impact of hospitalists on the care of older patients and to encourage clinical innovation at the intersection of hospital medicine and geriatrics. Consequently, this article 1) identifies and summarizes geriatric care approaches in hospitalist programs, 2) presents a case study of geriatric hospital care by a hospitalist group, and 3) highlights opportunities for innovation and further research.
METHODS
Sample
We conducted a cross‐sectional survey of the hospitalist community via two mailings to SHM Listservs in September 2003 and September 2004. To encourage responses, the e‐mails used terms such as innovating, developing, providing hospitalist services, and caring for the geriatric patient or Medicare population. Respondents to the e‐mail solicitations (n = 14), leaders of SHM and academic hospitalist groups (n = 14), and leaders of the American Geriatrics Society specializing in acute care (n = 3) were queried about additional contacts who might know about programs utilizing geriatric care approaches. Each of these contacts was subsequently solicited and queried.10 Thirteen of the respondents described the current use by their hospitalist groups of one or more geriatric care approaches that represented a departure from usual care. We subsequently refer to these approaches as innovations. The 13 respondents completed in‐depth telephone interviews with one of the authors (H.W.). All respondents were recontacted in the spring of 2005 to update their responses. Two of the 13 programs were eliminated from the analysis after the interviews were completed. The first of these programs was identified in 2003 but had been discontinued by 2004. The second program was eliminated because the innovation was not implemented.
Data Collection
We developed a data collection tool to gather descriptive information from respondents regarding characteristics of the hospitalist group, the clinical program, the primary hospital, and the innovation (focus, target patients, organization, staffing, training, rounding, other). In addition, respondents were queried about motivations for the innovation; successes, opportunities, and future plans; and failures and barriers to implementation.
Analysis
First, we summarized the characteristics of the 11 innovations (Table 1). Second, geriatric care approaches were identified from the innovations on the basis of their objectives and the types of responses we encountered most frequently. The approaches were not mutually exclusive. For instance, a program providing postdischarge care at a skilled nursing facility (SNF) might also use a geriatrician‐hospitalist staffing model.
| Site | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focus | |||||||||||
| Medical care | x | x | x | x | |||||||
| Postdischarge care | x | x | |||||||||
| Perioperative care | x | x | x | ||||||||
| Geriatric assessment | x | x | x | ||||||||
| Quality improvement | x | x | x | x | |||||||
| Staffing | x | ||||||||||
| Generalist‐hospitalist | x | x | x | x | x | x | |||||
| Geriatrician‐hospitalist | x | x | x | x | x | x | |||||
| Advanced‐practice nurse | x | x | x | x | |||||||
| Patient targeting | |||||||||||
| By age | x | x | x | x | x | x | x | x | x | ||
| By diagnosis | x | x | x | x | x | x | |||||
| By location | x | x | x | x | x | x | |||||
| Organization | |||||||||||
| Unit | x | x | x | x | x | x | |||||
| Service | x | x | x | x | x | x | |||||
| Interdisciplinary rounds | x | x | x | x | x | x | |||||
| Geriatrics training | x | x | x | x | x | x | x |
RESULTS
In 2003 the annual survey of the American Hospital Association identified 1415 hospitalist groups in the United States (Joe Miller, SHM senior vice‐president, personal communication). Remarkably, our query identified only 11 hospitalist groups with clinical innovations aimed at the older population. These innovations ranged from single individuals involved in targeted quality‐improvement projects to highly developed programs addressing an array of clinical needs for the hospitalized older patient. These 11 programs are summarized in Table 1 and described below.
Focus
Hospitalists' programs targeted to the older patient were designed to meet various needs arising from an episode of hospital care. These included innovations designed around their core clinical activities in providing acute medical care (four innovations), as well as innovations targeted to postdischarge care at SNFs (two innovations), perioperative care in consultative or comanagement models (four innovations), comprehensive geriatric assessment (three innovations), and clinical quality improvement such as audit tools (four innovations).
Staffing
Four innovations employed physicians without specific geriatrics training (generalist‐hospitalists), four innovations employed 16 fellowship‐trained geriatricians (geriatrician‐hospitalists), and two programs employed both geriatricians and generalist hospitalists. Four innovations employed advanced‐practice nurses, both with and without gerontology training.
Patients
Nine of the 11 innovations targeted patients by age (older than 65, 70, or 75 years). Of the two innovations that did not target patients by age, one focused on improving the quality of care for all patients on a medical ward by focusing on geriatric issues (Site I), and a second was concerned with postdischarge care for all patients discharged to affiliated SNFs (Site K). In addition to targeting by age, six innovations targeted patients on the basis of diagnosis, four of which focused on surgical diagnosis. Finally, patient selection by location occurred in six of the innovations, as described in the next section.
Organization
Six of the innovations were organized to operate within a clinical service (such as a medical or surgical team). In contrast to the service‐based innovations, six clinical innovations for older patients operated in geographic units including acute care for elders (ACE) units (n = 2), SNFs (n = 2), a medical nursing unit (n = 1), and an emergency department (ED; n = 1). Of the two ACE units, one (Site G) existed prior to the establishment of the hospitalist group. In this instance, a geriatrician‐hospitalist appointed jointly by the hospitalist group and the Division of Geriatrics staffed ACE unit patients of select private physicians and unassigned patients. The second ACE unit (Site H), established with the formation of the hospitalist group, was staffed by two hospitalists among eight physicians in a private geriatrics group. Regarding SNFs, one hospitalist group for a large health care organization (Site K) rounded at contract SNFs at which group members held medical directorships; another hospitalist program took over rounding at an SNF owned by its health system (Site A).
Rounding
Six of the innovations incorporated interdisciplinary rounds, including all three innovations with medical care as their focus. Four of the six innovations with interdisciplinary rounds were based in ACE units or SNFs. One of these six innovations (Site C), a perioperative initiative, incorporated twice‐weekly multidisciplinary rounds attended by an attending surgeon, surgical residents, and a hospitalistin addition to the nurses, case managers, and therapists.
Training
Seven of the 11 innovations involved geriatrics training. Four of the training innovations targeted nursing staff, four targeted hospitalist physicians, and one targeted both nurses and physicians. Most institutions developed their own curricula. Three hospitalist groups, however, modified preexisting curricula, struggling to adapt them to the needs of hospital‐based staff. Two innovations (Sites A and K) used a clinical mentoring model in which generalist‐hospitalists learned geriatrics principles while working side by side with geriatrician‐hospitalists.
Case Study
We selected the most comprehensive program for further description. This case illustrates the power of integrating geriatric and hospital medicine paradigms.
Hospital Internal Medicine, Mayo Clinic, Rochester, MN (Site A)
The Mayo Clinic established the Hospital Internal Medicine Group (HIM) in 1998 in response to changing resident workload regulations. The practice initially focused on perioperative medical care for a busy orthopedic trauma surgery (OTS) service. In 2000, noting the average age of the elective orthopedic population was 81, the leadership of HIM made a strategic decision to recruit physicians with geriatrics training. By 2005, 6 of the 22 physicians the group employed were geriatricians.
In mid‐2005 the group's members covered eight services in 1‐ to 2‐week block rotations. Three of the services are uniquely focused on the older patient: the Geriatric Medicine Service (GeM), the OTS, and the SNF. On the GeM, a geriatrician‐hospitalist works alongside a generalist‐hospitalist to for care medical patients triaged to the service based on age (older than 75) and frailty. Although the GeM is based on a medical nursing unit, the unit is neither configured nor staffed like an ACE unit, and up to 20% of the GeM's patients overflow to other units. In addition to providing acute care, the GeM employs standardized documentation to facilitate universal comprehensive geriatric assessment. On the OTS, HIM hospitalists care for postoperative patients in a comanagement model, descriptions of which have been published elsewhere.11, 12 As a reflection of its orientation toward the older surgical patient, every OTS patient is assessed for delirium with the confusion assessment method instrument.13 Finally, the 30‐bed SNF service (on which 75% of admissions are postoperative for subacute rehabilitation) is supervised by a HIM physician and a nurse‐practitioner.
Additional activities of HIM physicians are clinical quality improvement including participation in the creation of inpatient care pathways, revision of the hospital's discharge processes, ongoing review of adverse events, and use of standardized tools for intrahospital transfers. In addition, the HIM group prioritizes geriatrics education for its physicians and hospital medicine fellows. In turn, geriatrics fellows rotate through the GeM, SNF, and OTS services.
DISCUSSION
Although SHM increasingly recognizes the challenges inherent in caring for older patients, few hospitalists are adapting their care for this vulnerable population. We identified only 11 innovations in geriatric care despite there being more than 1000 hospitalist groups. This apparent paucity of innovation in geriatrics might be explained by the relatively recent introduction of hospital medicine. As no hospitalist program is more than 10 years old, most programs are still focused on building core clinical activities or on other competing demands. In addition to time, funding may limit the typical program's ability to innovate without directly increasing revenue. Although the geriatrics literature supports that specialized inpatient care for older patients can result in increased physical functioning and quality of life at no additional cost, it may be that geriatricians have yet to make this case effectively to the hospitalist community.14, 15
The findings of this study were limited by our survey methodology. Specifically, our sample was limited to professional contacts and those using SHM listservs. In addition, some innovative hospitalists may not consider their programs to be geriatric programs and so may not have responded to our queries. Therefore, the reported innovations are not representative of geriatric care among all hospitalist groups, and we are unable to provide a comprehensive picture of geriatric care in hospitalist programs. In addition, we cannot comment on the effectiveness of the care approaches at participating institutions. For example, interdisciplinary care is an important tenet of geriatric medicine. Although six of our programs reported interdisciplinary rounds, it is unclear if these rounds are models of effective collaborative practice. Nonetheless, the information obtained from the structured interviews allowed the identification of several instructive themes discussed below.
Opportunities
The growth of the hospitalist movement provides an opportunity to reconsider clinical paradigms for the hospitalized older population. Hospitalists bring clinical skills in treating acute illness, preventing hospital complications, and providing perioperative care.16, 17 As leaders in institutional quality, safety, and utilization initiatives, hospitalists are often given protected time for such endeavors.18, 19 In so doing, the incentives of hospitalists are aligned with those of hospital administrators. This orientation makes hospitalists open to innovation in clinical care improvement.
The opportunity for hospitalists to bring fresh approaches to acute care geriatrics need not happen in a vacuum. More than 30 years of geriatrics research has provided a framework, literature, and expertise to inform hospitalist groups. The common goal of clinical excellence for the hospitalized older patient should motivate cooperation, collaborative approaches, and a joint clinical research agenda. From our inquiry to hospitalist groups, it appears that this sort of interaction occurs infrequently. The innovations identified and the case study described highlight several ways in which the geriatric medicine and hospital medicine experiences inform one another. These include approaches to staffing, organization, and quality improvement, as well as to clinical areas amenable to innovation.
Approaches
Staffing and Organization
The employment of geriatrics‐trained clinicians by hospitalist programs is one approach to supporting generalist‐hospitalists and inclining group culture toward clinical geriatric concerns. Programs that purposefully hired geriatricians and gerontology nurse‐practitioners used them to staff geriatrics services including ACE units, SNFs and, in the case of HIM, a GeM service that was a modification of a medical service. In addition, two programs relied on geriatrician‐hospitalists to serve as clinical mentors to generalist‐hospitalists.
In particular, the use of geriatrics‐trained staff on specialized services such as ACE units is encouraging, as specialized geriatric units remain an underutilized care model,20 despite compelling evidence of their effectiveness in improving physical functioning and reducing nursing home admissions.14 Although the factors undermining the success of ACE units in the past may also pose challenges for hospitalists, hospitalist groups may be better positioned to maintain the interest and financial commitment of hospital administrators. The HIM's GeM Service is also of interest, given the need to disseminate best practices in geriatrics throughout the hospital. The benefits to older patients of such a service, however, have not been demonstrated. Likewise, comprehensive geriatric assessment and geriatric consultation in the inpatient setting are reported to have had mixed results in the absence of targeting individuals at highest risk for adverse outcomes.21
Patient Safety and Quality Improvement
Hospital medicine has rapidly integrated principles of quality improvement and patient safety, having grown up contemporaneously with the patient safety movement. Several of the hospitalist programs we identified spearheaded quality improvement efforts directed at the particular needs of older patients such as delirium prevention, provision of immunizations, and removal of indwelling Foley catheters.
These efforts can be seen in the context of the many hospitalist programs focusing on standardizing care, understanding iatrogenesis, adopting safe technologies, and generally moving hospital culture forward.22 In choosing to embrace patient safety practices such as medication reconciliation (endorsed by the Institute for Healthcare Improvement),23 hospitalists may confer disproportionate benefits to older patients, who frequently require multiple medications and are at high risk for adverse drug events.6 As the efficacy of many of these interventions is poorly understood, hospitalist and geriatricians (whose work on the hazards of hospitalization anticipated the patient safety movement by many years24) may find a shared clinical research agenda with patient safety as its focus.
Areas of Clinical Opportunity
Perioperative Care
Commentators have noted hospitalists' growing participation in perioperative care,17 much of which concerns the older orthopedic surgery patient.12 Through their embedding in surgical wards, hospitalists may become actual or de facto members of surgical teams with a significant impact on team culture and care delivery. For example, hospitalists at one program implemented a perioperative beta‐blocker protocol for older orthopedic surgery patients, leading to a marked decrease in postoperative cardiac events (Site B).
Although many hospitalist programs participate in similar initiatives, it is likely that additional attention to the needs of older patients will augment the effectiveness of their interventions. For instance, structured geriatrics consultation can reduce the incidence of postoperative delirium among hip fracture patients by 46% (NNT = 5.6).25 Increased attention to postoperative pain control and early mobilization, among others, may affect the functional recovery of the older surgical patient.26, 27
Postdischarge care and care transitions
The hallmark of the hospitalist modelthe handoff of care from a primary care provider to an inpatient provideris commonly considered the major limitation of the hospitalist model because of the risk of lost clinical information.1 Because older patients are particularly susceptible to postdischarge adverse events, their care transitions may require specialized attention.28 Two of the innovations we identified (Sites A and K) have extended care of older patients into the postacute setting by integrating SNF care into their programs as a way to streamline discharge processes, decrease miscommunication, and underscore the limitations of postacute care.
A growing body of evidence supports the role of discharge strategies in improving care transitions. In one study, postdischarge follow‐up with a hospital physician rather than a community physician resulted in a reduction of the combined end point of 30‐day mortality and nonelective readmission.29 In a randomized trial, postdischarge phone calls by a pharmacist reduced the number of ED visits within 30 days of discharge.30 In another trial, older patients receiving a multifactorial intervention aimed at providing the skills for active participation in care transitions resulted in a reduced number of readmissions within 30 days.31 Understanding and implementing these activities may be crucial to both the care of older patients and the success of the hospitalist enterprise.
Barriers
Part of the challenge of treating older patients in hospitals is that the paradigms of geriatrics and hospital medicine differ substantially.32 Notably, geriatric medicine goals of maximizing function and quality of life may conflict with traditional medical goals of diagnosis and cure. This dichotomy is amplified in the hospital setting because hospitals are organized to maximize the physician's ability to stabilize, diagnose, cure, and discharge.33
By design, the hospitalist model introduces additional challenges into the hospital paradigm that affect the older patient, such as the discontinuities addressed above. Additional factors that hospitalists identified as barriers to the effective care of older patients include: 1) poor communication skills, 2) ineffective interdisciplinary collaboration, 3) limited geriatrics knowledge base, and 4) insufficient support for care coordination.34 Despite these recognized challenges, our query to hospitalist groups identified few that had made clinical excellence in geriatrics a focus of their activities.
Even with its prioritization of geriatric medicine, the well‐developed HIM model faces challenges. In particular, the feasibility of the geriatrician‐hospitalist is limited by the many geriatricians who, because of the scarcity of those who are fellowship trained, may be unprepared to care for acutely ill older patients, as their training has not focused on the hospital setting.35, 36 In addition, the surgical comanagement model depends on a unique collaboration with surgical colleagues. Finally, the ability of the HIM group to incorporate geriatrics paradigms into the hospital setting depends on extensive support from the hospital in the form of resources and a shared vision that is unlikely to be found at most institutions.
CONCLUSIONS
The rapid growth of the hospitalist movement will significantly affect clinical care in American hospitals. As most hospital patients are older, the impact on acute care geriatrics cannot be overlooked. In our study, we identified only a small number of hospitalist groups that have made geriatric medicine a priority. These programs prioritize geriatric medicine through the employment of geriatrics‐trained staff, adaptation of geriatric care models such as ACE units, and commitment to clinical quality improvement and patient safety. They also focus on common clinical challenges for older patients, including postoperative and postdischarge care. Although much can be learned from these examples, programs at other institutions will need to be individualized to meet the specific needs of each hospital and community. The common goal of clinical excellence shared by hospitalists and geriatricians should motivate cooperation, collaborative approaches, and a joint clinical research agenda at all levels, as the current paradigm of hospital care remains inadequate to meet the needs of the acutely ill older patient.
Between 1996with the first appearance of hospitalists in the medical literatureand the present, the hospitalist workforce has grown to nearly 10,000.1, 2 More remarkable is the estimate that the number of hospitalists will double in the next 5 years.2 The rapid growth of hospital medicine raises significant issues for the care of older patients, who are hospitalized at high rates3 and suffer numerous complications from hospitalization including functional decline,4 delirium,5 and a disproportionate share of adverse events.6 Conversely, the needs of patients older than 65 years of age, whose hospital stays make up nearly 50% of acute‐care bed days, will shape the future of hospital medicine.3
To date, the hospital medicine literature has failed to address the particular challenges of treating older patients, focusing primarily on opportunities for reductions in costs and length of stay for hospitalists' Medicare patients (of about $1000 per admission and 0.5 days, respectively7, 8) when compared with those cared for by other physicians. This focus on economic efficiency reflects the early orientation of the hospitalist movement. More recently, leaders of the hospitalist professional organization, the Society of Hospital Medicine (SHM), have increasingly recognized that caring for the older population will require additional knowledge and clinical skills beyond that taught in internal medicine residencies.9 Beyond educational initiatives, however, hospitalists must reconsider the paradigms of hospital care that make the hospital setting so dangerous for the older patient.
Given the aging population and the predicted growth of hospital medicine, it is essential to develop an understanding of the impact of hospitalists on the care of older patients and to encourage clinical innovation at the intersection of hospital medicine and geriatrics. Consequently, this article 1) identifies and summarizes geriatric care approaches in hospitalist programs, 2) presents a case study of geriatric hospital care by a hospitalist group, and 3) highlights opportunities for innovation and further research.
METHODS
Sample
We conducted a cross‐sectional survey of the hospitalist community via two mailings to SHM Listservs in September 2003 and September 2004. To encourage responses, the e‐mails used terms such as innovating, developing, providing hospitalist services, and caring for the geriatric patient or Medicare population. Respondents to the e‐mail solicitations (n = 14), leaders of SHM and academic hospitalist groups (n = 14), and leaders of the American Geriatrics Society specializing in acute care (n = 3) were queried about additional contacts who might know about programs utilizing geriatric care approaches. Each of these contacts was subsequently solicited and queried.10 Thirteen of the respondents described the current use by their hospitalist groups of one or more geriatric care approaches that represented a departure from usual care. We subsequently refer to these approaches as innovations. The 13 respondents completed in‐depth telephone interviews with one of the authors (H.W.). All respondents were recontacted in the spring of 2005 to update their responses. Two of the 13 programs were eliminated from the analysis after the interviews were completed. The first of these programs was identified in 2003 but had been discontinued by 2004. The second program was eliminated because the innovation was not implemented.
Data Collection
We developed a data collection tool to gather descriptive information from respondents regarding characteristics of the hospitalist group, the clinical program, the primary hospital, and the innovation (focus, target patients, organization, staffing, training, rounding, other). In addition, respondents were queried about motivations for the innovation; successes, opportunities, and future plans; and failures and barriers to implementation.
Analysis
First, we summarized the characteristics of the 11 innovations (Table 1). Second, geriatric care approaches were identified from the innovations on the basis of their objectives and the types of responses we encountered most frequently. The approaches were not mutually exclusive. For instance, a program providing postdischarge care at a skilled nursing facility (SNF) might also use a geriatrician‐hospitalist staffing model.
| Site | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focus | |||||||||||
| Medical care | x | x | x | x | |||||||
| Postdischarge care | x | x | |||||||||
| Perioperative care | x | x | x | ||||||||
| Geriatric assessment | x | x | x | ||||||||
| Quality improvement | x | x | x | x | |||||||
| Staffing | x | ||||||||||
| Generalist‐hospitalist | x | x | x | x | x | x | |||||
| Geriatrician‐hospitalist | x | x | x | x | x | x | |||||
| Advanced‐practice nurse | x | x | x | x | |||||||
| Patient targeting | |||||||||||
| By age | x | x | x | x | x | x | x | x | x | ||
| By diagnosis | x | x | x | x | x | x | |||||
| By location | x | x | x | x | x | x | |||||
| Organization | |||||||||||
| Unit | x | x | x | x | x | x | |||||
| Service | x | x | x | x | x | x | |||||
| Interdisciplinary rounds | x | x | x | x | x | x | |||||
| Geriatrics training | x | x | x | x | x | x | x |
RESULTS
In 2003 the annual survey of the American Hospital Association identified 1415 hospitalist groups in the United States (Joe Miller, SHM senior vice‐president, personal communication). Remarkably, our query identified only 11 hospitalist groups with clinical innovations aimed at the older population. These innovations ranged from single individuals involved in targeted quality‐improvement projects to highly developed programs addressing an array of clinical needs for the hospitalized older patient. These 11 programs are summarized in Table 1 and described below.
Focus
Hospitalists' programs targeted to the older patient were designed to meet various needs arising from an episode of hospital care. These included innovations designed around their core clinical activities in providing acute medical care (four innovations), as well as innovations targeted to postdischarge care at SNFs (two innovations), perioperative care in consultative or comanagement models (four innovations), comprehensive geriatric assessment (three innovations), and clinical quality improvement such as audit tools (four innovations).
Staffing
Four innovations employed physicians without specific geriatrics training (generalist‐hospitalists), four innovations employed 16 fellowship‐trained geriatricians (geriatrician‐hospitalists), and two programs employed both geriatricians and generalist hospitalists. Four innovations employed advanced‐practice nurses, both with and without gerontology training.
Patients
Nine of the 11 innovations targeted patients by age (older than 65, 70, or 75 years). Of the two innovations that did not target patients by age, one focused on improving the quality of care for all patients on a medical ward by focusing on geriatric issues (Site I), and a second was concerned with postdischarge care for all patients discharged to affiliated SNFs (Site K). In addition to targeting by age, six innovations targeted patients on the basis of diagnosis, four of which focused on surgical diagnosis. Finally, patient selection by location occurred in six of the innovations, as described in the next section.
Organization
Six of the innovations were organized to operate within a clinical service (such as a medical or surgical team). In contrast to the service‐based innovations, six clinical innovations for older patients operated in geographic units including acute care for elders (ACE) units (n = 2), SNFs (n = 2), a medical nursing unit (n = 1), and an emergency department (ED; n = 1). Of the two ACE units, one (Site G) existed prior to the establishment of the hospitalist group. In this instance, a geriatrician‐hospitalist appointed jointly by the hospitalist group and the Division of Geriatrics staffed ACE unit patients of select private physicians and unassigned patients. The second ACE unit (Site H), established with the formation of the hospitalist group, was staffed by two hospitalists among eight physicians in a private geriatrics group. Regarding SNFs, one hospitalist group for a large health care organization (Site K) rounded at contract SNFs at which group members held medical directorships; another hospitalist program took over rounding at an SNF owned by its health system (Site A).
Rounding
Six of the innovations incorporated interdisciplinary rounds, including all three innovations with medical care as their focus. Four of the six innovations with interdisciplinary rounds were based in ACE units or SNFs. One of these six innovations (Site C), a perioperative initiative, incorporated twice‐weekly multidisciplinary rounds attended by an attending surgeon, surgical residents, and a hospitalistin addition to the nurses, case managers, and therapists.
Training
Seven of the 11 innovations involved geriatrics training. Four of the training innovations targeted nursing staff, four targeted hospitalist physicians, and one targeted both nurses and physicians. Most institutions developed their own curricula. Three hospitalist groups, however, modified preexisting curricula, struggling to adapt them to the needs of hospital‐based staff. Two innovations (Sites A and K) used a clinical mentoring model in which generalist‐hospitalists learned geriatrics principles while working side by side with geriatrician‐hospitalists.
Case Study
We selected the most comprehensive program for further description. This case illustrates the power of integrating geriatric and hospital medicine paradigms.
Hospital Internal Medicine, Mayo Clinic, Rochester, MN (Site A)
The Mayo Clinic established the Hospital Internal Medicine Group (HIM) in 1998 in response to changing resident workload regulations. The practice initially focused on perioperative medical care for a busy orthopedic trauma surgery (OTS) service. In 2000, noting the average age of the elective orthopedic population was 81, the leadership of HIM made a strategic decision to recruit physicians with geriatrics training. By 2005, 6 of the 22 physicians the group employed were geriatricians.
In mid‐2005 the group's members covered eight services in 1‐ to 2‐week block rotations. Three of the services are uniquely focused on the older patient: the Geriatric Medicine Service (GeM), the OTS, and the SNF. On the GeM, a geriatrician‐hospitalist works alongside a generalist‐hospitalist to for care medical patients triaged to the service based on age (older than 75) and frailty. Although the GeM is based on a medical nursing unit, the unit is neither configured nor staffed like an ACE unit, and up to 20% of the GeM's patients overflow to other units. In addition to providing acute care, the GeM employs standardized documentation to facilitate universal comprehensive geriatric assessment. On the OTS, HIM hospitalists care for postoperative patients in a comanagement model, descriptions of which have been published elsewhere.11, 12 As a reflection of its orientation toward the older surgical patient, every OTS patient is assessed for delirium with the confusion assessment method instrument.13 Finally, the 30‐bed SNF service (on which 75% of admissions are postoperative for subacute rehabilitation) is supervised by a HIM physician and a nurse‐practitioner.
Additional activities of HIM physicians are clinical quality improvement including participation in the creation of inpatient care pathways, revision of the hospital's discharge processes, ongoing review of adverse events, and use of standardized tools for intrahospital transfers. In addition, the HIM group prioritizes geriatrics education for its physicians and hospital medicine fellows. In turn, geriatrics fellows rotate through the GeM, SNF, and OTS services.
DISCUSSION
Although SHM increasingly recognizes the challenges inherent in caring for older patients, few hospitalists are adapting their care for this vulnerable population. We identified only 11 innovations in geriatric care despite there being more than 1000 hospitalist groups. This apparent paucity of innovation in geriatrics might be explained by the relatively recent introduction of hospital medicine. As no hospitalist program is more than 10 years old, most programs are still focused on building core clinical activities or on other competing demands. In addition to time, funding may limit the typical program's ability to innovate without directly increasing revenue. Although the geriatrics literature supports that specialized inpatient care for older patients can result in increased physical functioning and quality of life at no additional cost, it may be that geriatricians have yet to make this case effectively to the hospitalist community.14, 15
The findings of this study were limited by our survey methodology. Specifically, our sample was limited to professional contacts and those using SHM listservs. In addition, some innovative hospitalists may not consider their programs to be geriatric programs and so may not have responded to our queries. Therefore, the reported innovations are not representative of geriatric care among all hospitalist groups, and we are unable to provide a comprehensive picture of geriatric care in hospitalist programs. In addition, we cannot comment on the effectiveness of the care approaches at participating institutions. For example, interdisciplinary care is an important tenet of geriatric medicine. Although six of our programs reported interdisciplinary rounds, it is unclear if these rounds are models of effective collaborative practice. Nonetheless, the information obtained from the structured interviews allowed the identification of several instructive themes discussed below.
Opportunities
The growth of the hospitalist movement provides an opportunity to reconsider clinical paradigms for the hospitalized older population. Hospitalists bring clinical skills in treating acute illness, preventing hospital complications, and providing perioperative care.16, 17 As leaders in institutional quality, safety, and utilization initiatives, hospitalists are often given protected time for such endeavors.18, 19 In so doing, the incentives of hospitalists are aligned with those of hospital administrators. This orientation makes hospitalists open to innovation in clinical care improvement.
The opportunity for hospitalists to bring fresh approaches to acute care geriatrics need not happen in a vacuum. More than 30 years of geriatrics research has provided a framework, literature, and expertise to inform hospitalist groups. The common goal of clinical excellence for the hospitalized older patient should motivate cooperation, collaborative approaches, and a joint clinical research agenda. From our inquiry to hospitalist groups, it appears that this sort of interaction occurs infrequently. The innovations identified and the case study described highlight several ways in which the geriatric medicine and hospital medicine experiences inform one another. These include approaches to staffing, organization, and quality improvement, as well as to clinical areas amenable to innovation.
Approaches
Staffing and Organization
The employment of geriatrics‐trained clinicians by hospitalist programs is one approach to supporting generalist‐hospitalists and inclining group culture toward clinical geriatric concerns. Programs that purposefully hired geriatricians and gerontology nurse‐practitioners used them to staff geriatrics services including ACE units, SNFs and, in the case of HIM, a GeM service that was a modification of a medical service. In addition, two programs relied on geriatrician‐hospitalists to serve as clinical mentors to generalist‐hospitalists.
In particular, the use of geriatrics‐trained staff on specialized services such as ACE units is encouraging, as specialized geriatric units remain an underutilized care model,20 despite compelling evidence of their effectiveness in improving physical functioning and reducing nursing home admissions.14 Although the factors undermining the success of ACE units in the past may also pose challenges for hospitalists, hospitalist groups may be better positioned to maintain the interest and financial commitment of hospital administrators. The HIM's GeM Service is also of interest, given the need to disseminate best practices in geriatrics throughout the hospital. The benefits to older patients of such a service, however, have not been demonstrated. Likewise, comprehensive geriatric assessment and geriatric consultation in the inpatient setting are reported to have had mixed results in the absence of targeting individuals at highest risk for adverse outcomes.21
Patient Safety and Quality Improvement
Hospital medicine has rapidly integrated principles of quality improvement and patient safety, having grown up contemporaneously with the patient safety movement. Several of the hospitalist programs we identified spearheaded quality improvement efforts directed at the particular needs of older patients such as delirium prevention, provision of immunizations, and removal of indwelling Foley catheters.
These efforts can be seen in the context of the many hospitalist programs focusing on standardizing care, understanding iatrogenesis, adopting safe technologies, and generally moving hospital culture forward.22 In choosing to embrace patient safety practices such as medication reconciliation (endorsed by the Institute for Healthcare Improvement),23 hospitalists may confer disproportionate benefits to older patients, who frequently require multiple medications and are at high risk for adverse drug events.6 As the efficacy of many of these interventions is poorly understood, hospitalist and geriatricians (whose work on the hazards of hospitalization anticipated the patient safety movement by many years24) may find a shared clinical research agenda with patient safety as its focus.
Areas of Clinical Opportunity
Perioperative Care
Commentators have noted hospitalists' growing participation in perioperative care,17 much of which concerns the older orthopedic surgery patient.12 Through their embedding in surgical wards, hospitalists may become actual or de facto members of surgical teams with a significant impact on team culture and care delivery. For example, hospitalists at one program implemented a perioperative beta‐blocker protocol for older orthopedic surgery patients, leading to a marked decrease in postoperative cardiac events (Site B).
Although many hospitalist programs participate in similar initiatives, it is likely that additional attention to the needs of older patients will augment the effectiveness of their interventions. For instance, structured geriatrics consultation can reduce the incidence of postoperative delirium among hip fracture patients by 46% (NNT = 5.6).25 Increased attention to postoperative pain control and early mobilization, among others, may affect the functional recovery of the older surgical patient.26, 27
Postdischarge care and care transitions
The hallmark of the hospitalist modelthe handoff of care from a primary care provider to an inpatient provideris commonly considered the major limitation of the hospitalist model because of the risk of lost clinical information.1 Because older patients are particularly susceptible to postdischarge adverse events, their care transitions may require specialized attention.28 Two of the innovations we identified (Sites A and K) have extended care of older patients into the postacute setting by integrating SNF care into their programs as a way to streamline discharge processes, decrease miscommunication, and underscore the limitations of postacute care.
A growing body of evidence supports the role of discharge strategies in improving care transitions. In one study, postdischarge follow‐up with a hospital physician rather than a community physician resulted in a reduction of the combined end point of 30‐day mortality and nonelective readmission.29 In a randomized trial, postdischarge phone calls by a pharmacist reduced the number of ED visits within 30 days of discharge.30 In another trial, older patients receiving a multifactorial intervention aimed at providing the skills for active participation in care transitions resulted in a reduced number of readmissions within 30 days.31 Understanding and implementing these activities may be crucial to both the care of older patients and the success of the hospitalist enterprise.
Barriers
Part of the challenge of treating older patients in hospitals is that the paradigms of geriatrics and hospital medicine differ substantially.32 Notably, geriatric medicine goals of maximizing function and quality of life may conflict with traditional medical goals of diagnosis and cure. This dichotomy is amplified in the hospital setting because hospitals are organized to maximize the physician's ability to stabilize, diagnose, cure, and discharge.33
By design, the hospitalist model introduces additional challenges into the hospital paradigm that affect the older patient, such as the discontinuities addressed above. Additional factors that hospitalists identified as barriers to the effective care of older patients include: 1) poor communication skills, 2) ineffective interdisciplinary collaboration, 3) limited geriatrics knowledge base, and 4) insufficient support for care coordination.34 Despite these recognized challenges, our query to hospitalist groups identified few that had made clinical excellence in geriatrics a focus of their activities.
Even with its prioritization of geriatric medicine, the well‐developed HIM model faces challenges. In particular, the feasibility of the geriatrician‐hospitalist is limited by the many geriatricians who, because of the scarcity of those who are fellowship trained, may be unprepared to care for acutely ill older patients, as their training has not focused on the hospital setting.35, 36 In addition, the surgical comanagement model depends on a unique collaboration with surgical colleagues. Finally, the ability of the HIM group to incorporate geriatrics paradigms into the hospital setting depends on extensive support from the hospital in the form of resources and a shared vision that is unlikely to be found at most institutions.
CONCLUSIONS
The rapid growth of the hospitalist movement will significantly affect clinical care in American hospitals. As most hospital patients are older, the impact on acute care geriatrics cannot be overlooked. In our study, we identified only a small number of hospitalist groups that have made geriatric medicine a priority. These programs prioritize geriatric medicine through the employment of geriatrics‐trained staff, adaptation of geriatric care models such as ACE units, and commitment to clinical quality improvement and patient safety. They also focus on common clinical challenges for older patients, including postoperative and postdischarge care. Although much can be learned from these examples, programs at other institutions will need to be individualized to meet the specific needs of each hospital and community. The common goal of clinical excellence shared by hospitalists and geriatricians should motivate cooperation, collaborative approaches, and a joint clinical research agenda at all levels, as the current paradigm of hospital care remains inadequate to meet the needs of the acutely ill older patient.
- ,.The emerging role of hospitalists in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at URL: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm[accessed January 20, 2005].
- ,.2002 National Hospital Discharge Survey.Advance data from Vital And Health Statistics. No. 342.Hyattsville (MD):National Center for Health Statistics,2002.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156,:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care.Am J Med.1999;106:565–573.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.year="2001"2001.
- .Improving care for older adults: SHM educational initiatives.Hospitalist.2004;8(Suppl):45–47.
- .Qualitative evaluation and research methods.2nd ed.Thousand Oaks (CA):Sage Publications,1990:176.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for the detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,Geriatric evaluation and management units for hospitalized patients. In:Making healthcare safer: a critical analysis of Patient Safety Practices Evidence Report/Technology AssessmentNo. 43.2001. AHRQ Publication No. 01‐E058.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,.The hospitalist movement five years later.JAMA.2002;287:487–494.
- .The hospitalist joins the surgical team.Ann Intern Med.2004;141:67–69.
- .The end of the beginning: patient safety five years after ‘To Err Is Human.’Health Aff.2004;23S2:W534–W545.
- .How hospitalists add value.Hospitalist.2005;9(Suppl 1):6–7.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,, et al.Multidisciplinary geriatric consultation services,Chap. 29.Evidence Report/Technology Assessment No. 43.2001. AHRQ Publication No. 01‐E058.
- .Hospitalists spearhead a wide range of patient safety improvement projects.Hospitalist.2004;8(Suppl.):33–35.
- Institute for Healthcare Improvement.100k Lives campaign. 10‐20‐2005.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,,, et al.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,,, et al.The impact of postoperative pain on outcomes following hip fracture.Pain.2003;103:303–311.
- ,,, et al.Physical therapy and mobility 2 and 6 months after hip fracture.J Am Geriatr Soc.2004;52:1114–1120.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Dis Mon.2002;48:239–248.
- ,,, et al.The care transitions intervention: results from a randomized controlled trial. Society of Hospital Medicine Annual Meeting, Chicago, IL,2005.
- .Case management: high intensity care for frail patients with complex needs.Geriatrics.1998;53:62–68.
- .The care of strangers: the rise of America's hospital system.New York:Basic Books,1987.
- ,,.Hospitalists' role in caring for older Americans: Executive Summary.2002. San Francisco, prepared for the John Hartford Foundation.
- Geriatric medicine training and practice in the United States at the beginning of the 21st century.New York:Association of Directors of Geriatric Academic Programs,2002.
- AGS Education Committee.Guidelines for fellowship training in geriatrics.1998;46:1473–1477.
- ,.The emerging role of hospitalists in the American health care system.N Engl J Med.1996;335:514–517.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at URL: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm[accessed January 20, 2005].
- ,.2002 National Hospital Discharge Survey.Advance data from Vital And Health Statistics. No. 342.Hyattsville (MD):National Center for Health Statistics,2002.
- ,,, et al.Functional outcomes of acute medical illness and hospitalization in older persons.Arch Intern Med.1996;156,:645–652.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care.Am J Med.1999;106:565–573.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,,.The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.year="2001"2001.
- .Improving care for older adults: SHM educational initiatives.Hospitalist.2004;8(Suppl):45–47.
- .Qualitative evaluation and research methods.2nd ed.Thousand Oaks (CA):Sage Publications,1990:176.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for the detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,Geriatric evaluation and management units for hospitalized patients. In:Making healthcare safer: a critical analysis of Patient Safety Practices Evidence Report/Technology AssessmentNo. 43.2001. AHRQ Publication No. 01‐E058.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,.The hospitalist movement five years later.JAMA.2002;287:487–494.
- .The hospitalist joins the surgical team.Ann Intern Med.2004;141:67–69.
- .The end of the beginning: patient safety five years after ‘To Err Is Human.’Health Aff.2004;23S2:W534–W545.
- .How hospitalists add value.Hospitalist.2005;9(Suppl 1):6–7.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,, et al.Multidisciplinary geriatric consultation services,Chap. 29.Evidence Report/Technology Assessment No. 43.2001. AHRQ Publication No. 01‐E058.
- .Hospitalists spearhead a wide range of patient safety improvement projects.Hospitalist.2004;8(Suppl.):33–35.
- Institute for Healthcare Improvement.100k Lives campaign. 10‐20‐2005.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118:219–223.
- ,,, et al.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,,, et al.The impact of postoperative pain on outcomes following hip fracture.Pain.2003;103:303–311.
- ,,, et al.Physical therapy and mobility 2 and 6 months after hip fracture.J Am Geriatr Soc.2004;52:1114–1120.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Dis Mon.2002;48:239–248.
- ,,, et al.The care transitions intervention: results from a randomized controlled trial. Society of Hospital Medicine Annual Meeting, Chicago, IL,2005.
- .Case management: high intensity care for frail patients with complex needs.Geriatrics.1998;53:62–68.
- .The care of strangers: the rise of America's hospital system.New York:Basic Books,1987.
- ,,.Hospitalists' role in caring for older Americans: Executive Summary.2002. San Francisco, prepared for the John Hartford Foundation.
- Geriatric medicine training and practice in the United States at the beginning of the 21st century.New York:Association of Directors of Geriatric Academic Programs,2002.
- AGS Education Committee.Guidelines for fellowship training in geriatrics.1998;46:1473–1477.