User login
Fifth and Sixth Diseases: More Than a Fever and a Rash
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
FIFTH DISEASE
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
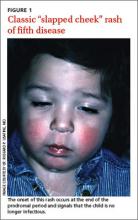
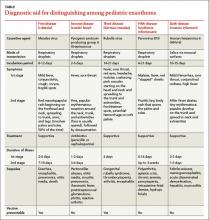
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Next page: Treatment >>
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Continue for information on sixth disease >>
SIXTH DISEASE
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
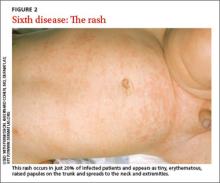
Clinical presentation: Only 20% may exhibit a rash
Continue for complications >>
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; ogrady.jason@mayo.edu
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
FIFTH DISEASE
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Next page: Treatment >>
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Continue for information on sixth disease >>
SIXTH DISEASE
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Continue for complications >>
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; ogrady.jason@mayo.edu
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
FIFTH DISEASE
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Next page: Treatment >>
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Continue for information on sixth disease >>
SIXTH DISEASE
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Continue for complications >>
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; ogrady.jason@mayo.edu
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
Fifth and sixth diseases: More than a fever and a rash
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
Fifth disease
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Sixth disease
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; ogrady.jason@mayo.edu
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
Fifth disease
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Sixth disease
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; ogrady.jason@mayo.edu
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
Fifth disease
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Sixth disease
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; ogrady.jason@mayo.edu
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.