User login
Assessing Outcomes Between Risperidone Microspheres and Paliperidone Palmitate Long-Acting Injectable Antipsychotics Among Veterans
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
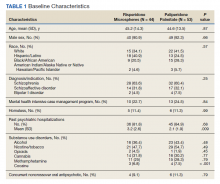
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
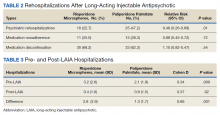
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
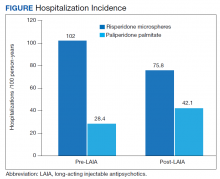
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7