User login
2023 Update on abnormal uterine bleeding
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
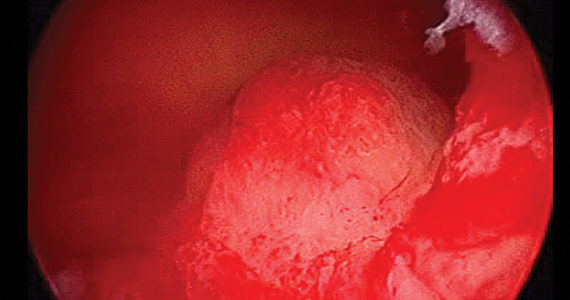
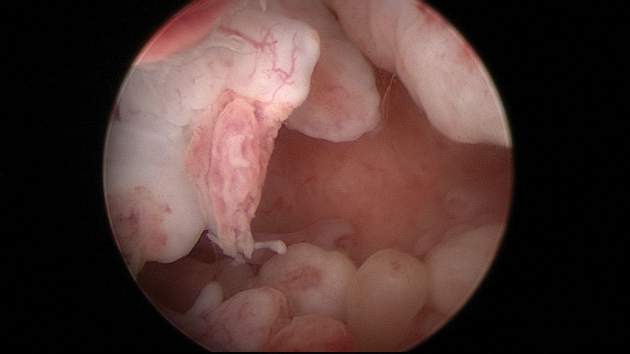
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
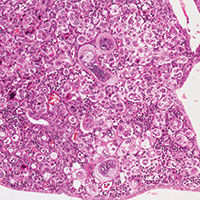
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
2022 Update on abnormal uterine bleeding
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
2021 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
Abnormal uterine bleeding (AUB) continues to be a top-10 reason why women present for gynecologic care, which makes keeping up with clinical therapies important. Over the past year, we have learned a tremendous amount about elagolix with hormonal add-back therapy for the treatment of bleeding associated with uterine fibroids. In this Update, we provide an overview from 3 randomized clinical trials on the recent US Food and Drug Administration (FDA)-approved drug, elagolix with hormonal add-back therapy (approved May 29, 2020). In addition, we review the data on the Cerene cryotherapy device (Channel Medsystems), as one might rightly ask, do we need another endometrial ablation device? We will address that question, as this device has some unique features that gynecologists should be aware of. Last, we review a study on the importance of considering quality of life in patients with uterine fibroids, which provides sobering information on the psychosocial aspects of uterine fibroids that all clinicians who care for such patients should be aware of.
Endometrial ablation with a new cryotherapy device: Is less more?
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
The phrase “less is more,” in the world of architecture and design, is often associated with Ludwig Mies van der Rohe (1886–1969). One could argue that this principle is one key advantage with the addition of yet another non-resectoscopic endometrial ablation device. The Cerene cryotherapy device, FDA approved in 2019, is presented as a simple, disposable device for in-office use that takes advantage of natural cryoanesthesia and results in less tissue destruction than many other ablation methods.
Device reduces bleeding and permits greater ability for future evaluation
Recently, Curlin and colleagues conducted a prospective, multicenter clinical trial to evaluate the safety and efficacy of the Cerene device in reducing menstrual blood loss.1 They followed 230 patients over 12 months and found that 81% (77% with intention-to-treat analysis) met the primary end point of a pictorial blood loss assessment chart (PBLAC) score of 75 or lower. Clinically, this translated to 44% of patients experiencing light bleeding; 27%, eumenorrhea; and 10%, amenorrhea. This is clearly “less” in terms of the rate of amenorrhea in most endometrial ablation studies. However, this also may translate into “more” ability to evaluate the endometrial cavity in the future, as 97% of the patients were able to undergo hysteroscopy at the 12-month mark and, of those, 93% were able to have the entire endometrial cavity assessed.
Further, of 97 patients who had a tubal sterilization, none had symptoms or evidence of postablation tubal sterilization syndrome. Three patients were unable to undergo hysteroscopy due to pain intolerance (2) or cervical stenosis (1). This is important because some gynecologists have expressed concern over intrauterine synechiae, which may result in scarring and associated future difficulty in assessing the endometrium for possible cancer.
Details about the device
The Cerene device is a single use, disposable device that uses cryothermal energy from nitrous oxide that results in a liquid-to-gas phase change within a polyurethane balloon (resulting in a temperature of -86°C) and delivered through a 6-mm sheath. It may be used in uterine cavities that measure between 2.5 and 6.5 cm in length, corresponding to approximately 10 cm in a uterine sound measurement. Treatment time is 2.5 minutes of nitrous oxide flow.
As mentioned, another benefit claimed is that the Cerene device’s cryoanalgesia properties enable the procedure to be more tolerated in the office setting. Of the 230 patients studied in the Curlin trial, no procedures were performed under general anesthesia.1 Medications used included paracervical block (PCB) only (8%), PCB plus nonsteroidal anti-inflammatory drugs (19.8%), PCB plus oral narcotics/anxiolytics (69%), and PCB plus intravenous sedation (2.9%), showing that this device is ideally suited for in-office use.
The rate of serious adverse events was 2.5% (7 total events in 6 patients within 12 months). All serious adverse events were reviewed by a Clinical Events Committee and none were deemed to be device-related events.
Long-term outcomes remain to be seen
For physicians and patients who worry about the ability to access the endometrial cavity in the future, less may be more. It will be interesting to see what the long-term outcomes show with use of the Cerene cryotherapy device, and whether a lower amenorrhea rate will translate into a higher repeat intervention rate or not. Of course, not all are minimalists. As the architect Robert Venturi (1925–2018) was quoted as saying, “Less is a bore.”
The new Cerene cryotherapy endometrial ablation method meets the FDA’s target for reduction of menstrual blood loss, but it has a slightly lower amenorrhea rate than other devices. Its most significant features are the potential for improved analgesia for in-office use and the possibility that there may be less scarring of the endometrial cavity for future assessment if needed.
Continue to: QoL assessment in women with fibroids is useful in evaluating treatment success...
QoL assessment in women with fibroids is useful in evaluating treatment success
Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674- 708.e8.
In many studies that assess AUB, the primary emphasis generally is placed on quantitation of menstrual bleeding by using PBLAC and alkaline hematin scores. In a systematic review, Go and colleagues argue the case for the importance of measuring the psychosocial impact of abnormal bleeding, emphasizing the concerning finding that many women with fibroids report lower vitality and lower social function scores than women with breast cancer.2
Fibroids associated with inconvenience—and anxiety
The authors analyzed and reviewed 18 randomized trials and 39 observational studies after screening 3,625 records from electronic database searches, with the goal to include only studies with validated quality of life (QoL) questionnaires that were administered both before and after treatment. A highlighted aspect of the reviewed studies was that “control” and “concern” subscales were most affected by fibroids, noting the inconvenience and anxiety that are related to the unpredictable onset and intensity of menses and the feeling of loss of control over one’s health and future.
This systematic review is important because although previous research has shown that fibroids significantly affect QoL, the psychosocial burden of fibroid symptoms had not been compared across different QoL instruments for both disease-specific and general validated health subscales.
Disability levels with fibroids are similar to those with other chronic diseases
Go and colleagues further reported that uterine fibroids have considerable psychosocial impact and lead to poor overall QoL physically and emotionally, with diminished sexual function and increased urinary or defecatory issues. Women with fibroids experienced a level of disability that was similar to that seen in other chronic diseases, and their vitality scores were lower than those associated with heart disease, diabetes, and as mentioned, breast cancer.
The authors concluded that “although objective clinical measures are important to establish a comprehensive understanding of health status, patient reported QoL outcomes play a critical role in evaluating success of a therapy.” They suggested that a larger emphasis on patient-centered care may help to mitigate the psychosocial effects of fibroids.
The study by Go and colleagues highlights the significant psychosocial aspects of the heavy menstrual bleeding associated with fibroids, and the authors found that many women with fibroids score in the range of those with other significant diseases, such as breast cancer and diabetes.
We have noted the trend of including QoL in research, and Go and colleagues make an excellent and compelling argument for this trend using quantitative analysis. It is important to consider this not only in our design of future research but also, and perhaps more importantly, in our clinical care of women as we try to better understand what they are experiencing.
Continue to: What have we learned over the past year about elagolix for uterine fibroids?...
What have we learned over the past year about elagolix for uterine fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
Data from the Elaris UF-1 and UF-2 6-month, phase 3 trials3 and the results of the Elaris UF-EXTEND trial with a 6-month extension (totaling 12 months of use)4 were published in 2020, and the 12-month results were discussed in OBG Management (2020;32[7]:35, 39-40). An additional data analysis from the same researchers assessed the effect of elagolix with hormonal add-back therapy in a number of patient subgroups.5 These 3 publications have added to our knowledge of this therapy, and it is worth reviewing them in this context
Design of the elagolix plus hormonal add-back therapy trials
The initial UF-1 and UF-2 trials were 2 identical, double-blind, randomized, placebo-controlled, 6-month, phase 3 trials designed to evaluate the safety and efficacy of elagolix and hormonal add-back therapy.3 UF-1 was conducted at 76 sites in the United States from December 2015 through December 2018, whereas UF-2 was conducted at 77 sites in the United States and Canada from February 2016 through January 2019; the trials were registered separately. Both trials had a 2:1:1 randomization of elagolix (300 mg twice daily) with hormonal add-back therapy (estradiol 1 mg and norethindrone acetate 0.5 mg daily), elagolix alone (300 mg twice daily), or placebo.
In the 6-month studies, the primary end point was both menstrual blood loss of less than 80 mL and at least a 50% reduction of menstrual blood loss as measured by the alkaline hematin method.3 Among several secondary end points was the assessment of QoL using the Uterine Fibroid Symptom QoL questionnaire (UFS-QoL).
Trial results. In UF-1, 68.5% of 206 women, and in UF-2, 76.5% of 189 women, respectively, taking elagolix with add-back therapy met the primary objective. Among women taking elagolix alone, in UF-1, 84.1% of 104 women, and in UF-2, 77% of 95 women, respectively, met criteria. There was improvement in UFS-QoL scores in women receiving elagolix plus add-back therapy with a reduction of symptom severity of -33.2 in UF-1 and -41.4 in UF-2, as compared with the placebo-treated groups (-10.3 and -7.7, respectively).
Adverse effects. Elagolix was associated with a low incidence of serious adverse effects, and the addition of hormonal add-back therapy attenuated the decreases in bone mineral density observed with elagolix alone. In both UF-1 and UF-2 trials, bone mineral density did not differ significantly in the groups of women who received elagolix with hormonal addback therapy versus placebo.
The extension trial results
Of note, in the 12-month study (6-month extension), the authors reported that 87.9% of the women taking elagolix with hormonal add-back therapy met the primary objective.4 Among the women taking elagolix alone, 89.4% met the primary objective.
In a review of the AbbVie-funded extension study, the editorial comments in the Obstetrical and Gynecological Survey expressed concern over the high proportion of data loss, comparing the number of patients joining the extended trial, patients who completed an additional 6 months of treatment, and patients who completed the posttreatment follow-up period of “up to 12 months.”6 Approximately one-third of patients were lost between initial enrollment to the subset who completed follow-up. There was concern that “losses of that magnitude pose a serious threat to validity.”6
Effectiveness in subgroups
Al-Hendy and colleagues analyzed data from the Elaris UF-1 and UF-2 trials to see if the outcomes for elagolix with hormonal addback therapy demonstrated safety and efficacy in subgroups of patients of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume.5
Results. In all subgroups, they found a statistically significant reduction in blood loss in mean menstrual blood loss volume for those treated with elagolix plus hormonal addback therapy compared with those treated with placebo. As well, in terms of QoL, among all subgroups, the mean change in symptom severity score as well as health-related QoL total score from baseline to month 6 was statistically significantly greater than the mean change in the placebo group.
The bottom line
Elagolix with hormonal add-back therapy appears to be a safe and effective method to reduce menstrual blood loss associated with uterine fibroids. It also has a favorable effect on QoL and appears to have benefits in subgroups of women of varying ages, races and ethnicities, baseline menstrual blood loss, body mass indices, fibroid location, and uterine and fibroid volume. ●
Elagolix plus hormonal add-back therapy provides several advantages to fibroid care, including a pill form that, as a gonadotropin-releasing hormone (GnRH) antagonist, provides much quicker action than GnRH agonists. The hormonal add-back feature seems to improve QoL measures and has a favorable reported bleeding reduction rate. It also appears to be reasonably safe. Although the studies reviewed here may have some weaknesses, it helps to have another therapy to offer to women who have blood loss associated with fibroids. Deciding on the drug’s optimal clinical use has not been fully explored, as it may be a short-term solution to a long-term problem and may not be ideal for all patients with fibroids. Elagolix and hormonal add-back therapy may be advantageous for patients who need to stop bleeding quickly and are trying to decide about their reproductive plans, for patients close to menopause who need a therapy to bridge this gap, and for patients trying to obtain relief between pregnancies.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28:899-908.
- Go VAA, Thomas MC, Singh B, et al. A systematic review of the psychosocial impact of fibroids before and after treatment. Am J Obstet Gynecol. 2020;223:674-708.e8.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol. 2021:224-72.e1-72.e50.
- Obstetrical & Gynecological Survey. 2020;75:545-547.
2020 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
2019 Update on abnormal uterine bleeding
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
2018 Update on abnormal uterine bleeding
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.

Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.

Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
2017 Update on abnormal uterine bleeding
Two issues of emerging importance are being addressed in the literature: caring for patients with obesity and the concept of delivering value-based care. Value-based care does not mean providing the cheapest care; “value” places importance on quality as well as cost. In this Update, we present 3 practices that the evidence says will deliver value:
- endometrial biopsy in all obese women. Although performing more endometrial biopsies in younger women with a body mass index (BMI) in the obese range will not be less expensive initially, the procedure’s value likely will be in early diagnosis, which hopefully will translate to eventual health care system savings.
- use of the levonorgestrel-releasing intrauterine device (LNG-IUD) in obese patients experiencing abnormal uterine bleeding (AUB). This practice appears to add value in the context of AUB.
- performance of routine diagnostic hysteroscopy in the office setting. We should reconsider our current habits and traditions of performing routine diagnostic hysteroscopy in the operating room (OR) as we move toward providing value-based care.
Read about obesity as a risk factor for endometrial hyperplasia
Endometrial sampling and obesity: Forget the "age 45" rule
Wise MR, Gill P, Lensen S, Thompson JM, Farquhar CM. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215(5):598.e1-e8.
How do we bring more value to our patients with AUB? We are well aware that heavy menstrual bleeding places a burden on many women; AUB affects 30% of those of reproductive age. The condition often results in lost workdays and diminished quality of life. It also is associated with significant cost expenditures for hygiene products. It is important not only to bring value to women with heavy menstrual bleeding but also to consider our increasingly expensive health care system.
Obesity is a significant problem that likely will increase the number of women presenting with AUB to ObGyns. Recent studies from New Zealand--which has 33% of its population classified as obese--have provided valuable information.1
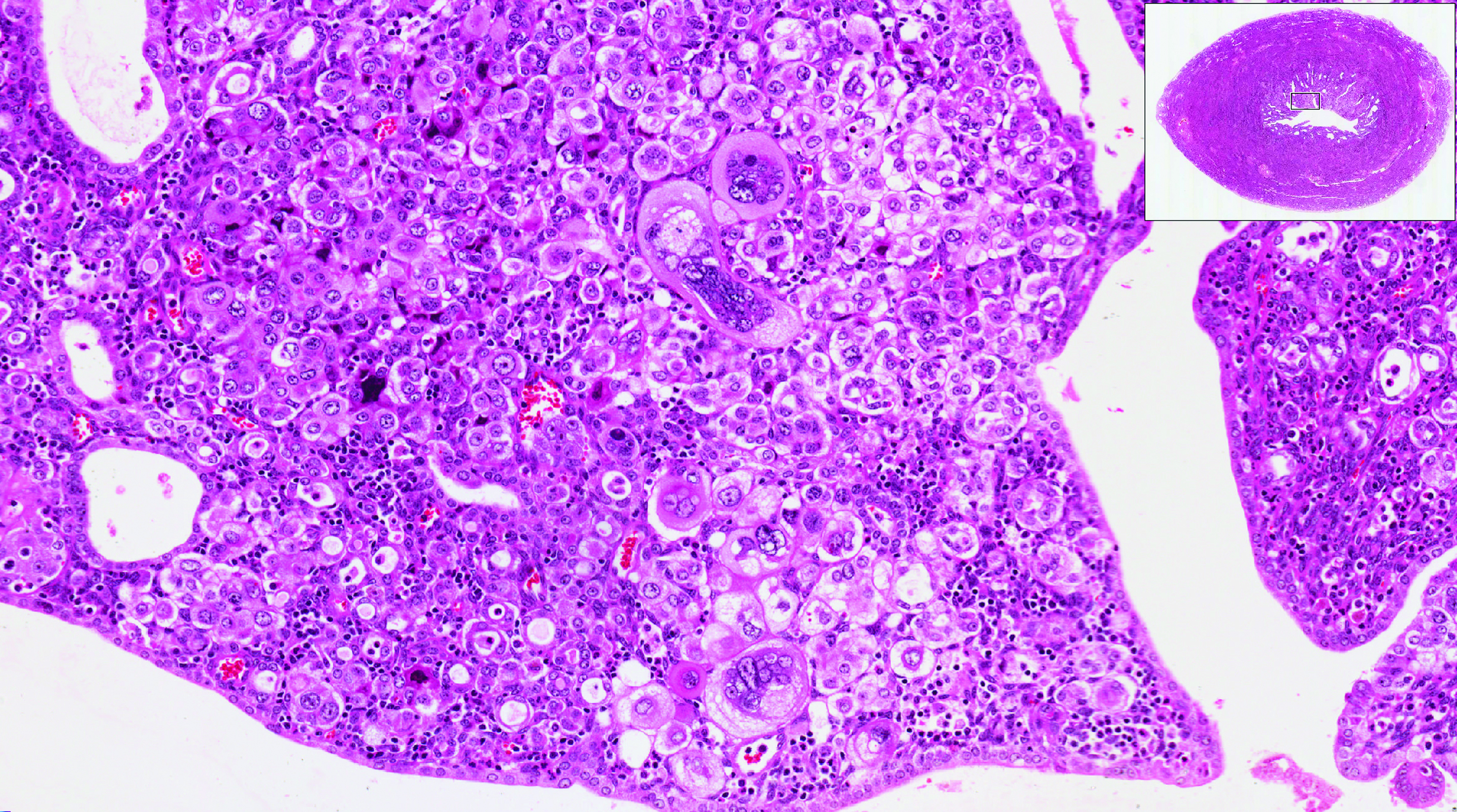
Obesity is a risk factor for endometrial hyperplasia
In a large retrospective cohort study, Wise and colleagues analyzed data from 916 premenopausal women referred for AUB who had an endometrial biopsy from 2008 to 2014. The setting was a single large urban secondary women's health service in New Zealand. This study challenges the concept of age-related biopsy guidelines.
Of the 916 women, half were obese. Almost 5% of the women had complex endometrial hyperplasia with atypia or cancer. This incidence had risen from 3% in the years 1995 to 1997, likely due to the rising incidence of obesity. Women with a BMI ≥30 kg/m2 were 4 times more likely to develop complex hyperplasia or cancer than normal-weight women.
Other factors associated with an increased risk for complex hyperplasia or cancer were nulliparity (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.25-5.05), anemia (OR, 2.38; 95% CI, 1.25-4.56), and a thickened endometrium on ultrasonography (defined as >12 mm; OR, 4.04; 95% CI, 1.69-9.65). Age was not a significant risk factor in this group.
Read about using LNG-IUD to treat AUB in obese women
Small study shows LNG-IUD is effective for treating heavy menstrual bleeding in obese patients
Shaw V, Vandal AC, Coomarasamy C, Ekeroma AJ. The effectiveness of the levonorgestrel intrauterine system in obese women with heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2016;56(6):619-623.
In another recent study from New Zealand, researchers set out to assess the efficacy of the LNG-IUD for the treatment of heavy menstrual bleeding in obese women. This study is important because there are very few studies of the LNG-IUD in the obese population, and none that have studied quality-of-life measures.
Shaw and colleagues conducted the prospective observational study at a tertiary teaching hospital. Twenty obese (BMI >30 kg/m2) women with heavy menstrual bleeding agreed to treatment with an LNG-IUD, and 14 completed the study (2 had a device expulsion, 1 had a device removed for pain, and 1 had a device removed for infection; 2 were lost to follow-up). The women were aged 27 to 52 years (median, 40.5 years), and their BMI ranged from 30 to 68 kg/m2 (median, 40.6 kg/m2). At recruitment, 6 months, and 12 months, participants completed the Menstrual Impact Questionnaire and the Pictorial Bleeding Assessment Chart--2 validated tools.
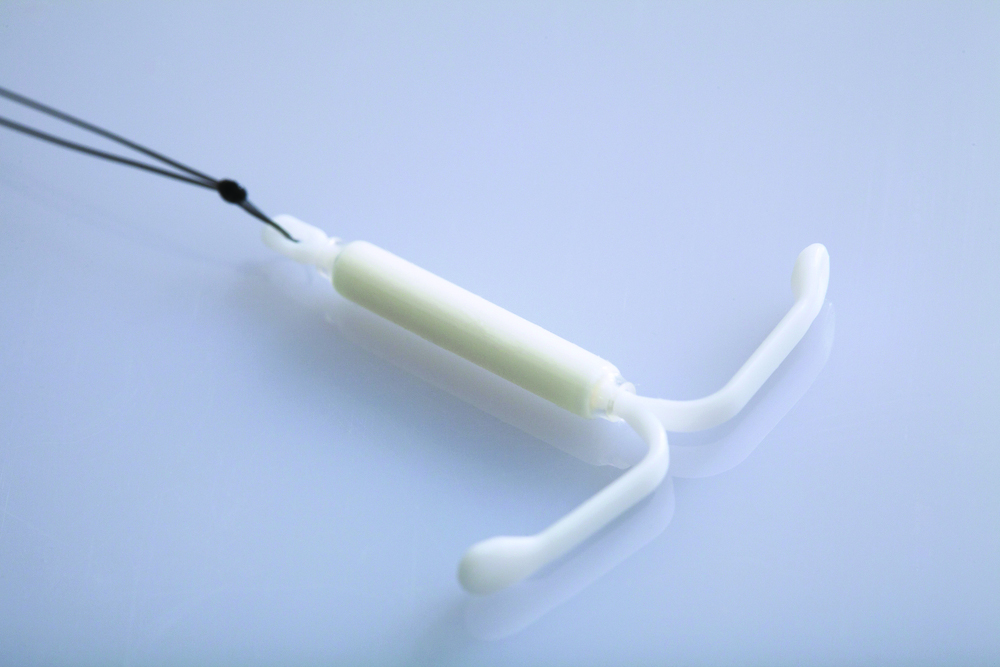
Compared with baseline Pictorial Bleeding Assessment scores, the authors found the LNG-IUD to be effective in 73.2% (95% CI, 55.3%-83.9%) of women at 6 months and in 92.8% (95% CI, 80.0%-97.4%) of women at 12 months. Taking into consideration device failures, including removed and expelled LNG-IUDs (which occurred in 4 women, or 20%, in the intent-to-treat analysis), the actual efficacy rate was 67%. Similarly, there was significant improvement at 6 and 12 months in Menstrual Impact Questionnaire scores for social activities, work performance, tiredness, productivity, hygiene, and depression.
Read about doing more diagnostic hysteroscopy in the office
Is it time to abandon diagnostic hysteroscopy in the OR?
Leung S, Leyland N, Murji A. Decreasing diagnostic hysteroscopy performed in the operating room: a quality improvement initiative. J Obstet Gynaecol Can. 2016;38(4):351-356.
Diagnostic hysteroscopy: Are we stuck in the 1990s? Why are we still performing so many diagnostic hysteroscopies in the OR, thus subjecting our patients to general anesthesia and using our precious OR time? That is the question asked by a group of researchers in Canada.
According to data from the Ontario Ministry of Health and Long Term Care, diagnostic hysteroscopy was performed 10,027 times in the 2013-2014 fiscal year. Ontario researchers designed and implemented a quality improvement initiative at their institution and successfully decreased the number of diagnostic hysteroscopies performed in their hospital by 70% from their baseline 12-month period. The improvements resulted in a savings of 78 hours of case costing, or $126,984. When these data are extrapolated to the Ontario population (in which more than 10,000 diagnostic hysteroscopies were performed), potentially 7,000 women could avoid the risk of general anesthesia and the health care system could save $11 million.
Re-education protocol was key to reducing OR procedures
How did the researchers accomplish their results? The multifaceted intervention had 3 key components:
Staff education and review. Many surgeons were performing diagnostic hysteroscopy in the OR because that is how they were trained, and they were unaware of less invasive options. An awareness campaign was conducted by e-mail, during staff meetings, and at rounds.
Accessible sonohysterography. This diagnostic modality was made more accessible to referring physicians in a timely manner.
Initiation of an operative hysteroscopy education program. To allow more surgeons greater comfort with office hysteroscopy, the authors instituted didactic sessions, dry and wet lab simulations, and mentorship.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- The Organization for Economic Co-operation and Development (OECD). OECD obesity update 2014. http://www.oecd.org/health/Obesity-Update-2014.pdf. Published June 2014. Accessed March 10, 2017.
Two issues of emerging importance are being addressed in the literature: caring for patients with obesity and the concept of delivering value-based care. Value-based care does not mean providing the cheapest care; “value” places importance on quality as well as cost. In this Update, we present 3 practices that the evidence says will deliver value:
- endometrial biopsy in all obese women. Although performing more endometrial biopsies in younger women with a body mass index (BMI) in the obese range will not be less expensive initially, the procedure’s value likely will be in early diagnosis, which hopefully will translate to eventual health care system savings.
- use of the levonorgestrel-releasing intrauterine device (LNG-IUD) in obese patients experiencing abnormal uterine bleeding (AUB). This practice appears to add value in the context of AUB.
- performance of routine diagnostic hysteroscopy in the office setting. We should reconsider our current habits and traditions of performing routine diagnostic hysteroscopy in the operating room (OR) as we move toward providing value-based care.
Read about obesity as a risk factor for endometrial hyperplasia
Endometrial sampling and obesity: Forget the "age 45" rule
Wise MR, Gill P, Lensen S, Thompson JM, Farquhar CM. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215(5):598.e1-e8.
How do we bring more value to our patients with AUB? We are well aware that heavy menstrual bleeding places a burden on many women; AUB affects 30% of those of reproductive age. The condition often results in lost workdays and diminished quality of life. It also is associated with significant cost expenditures for hygiene products. It is important not only to bring value to women with heavy menstrual bleeding but also to consider our increasingly expensive health care system.
Obesity is a significant problem that likely will increase the number of women presenting with AUB to ObGyns. Recent studies from New Zealand--which has 33% of its population classified as obese--have provided valuable information.1

Obesity is a risk factor for endometrial hyperplasia
In a large retrospective cohort study, Wise and colleagues analyzed data from 916 premenopausal women referred for AUB who had an endometrial biopsy from 2008 to 2014. The setting was a single large urban secondary women's health service in New Zealand. This study challenges the concept of age-related biopsy guidelines.
Of the 916 women, half were obese. Almost 5% of the women had complex endometrial hyperplasia with atypia or cancer. This incidence had risen from 3% in the years 1995 to 1997, likely due to the rising incidence of obesity. Women with a BMI ≥30 kg/m2 were 4 times more likely to develop complex hyperplasia or cancer than normal-weight women.
Other factors associated with an increased risk for complex hyperplasia or cancer were nulliparity (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.25-5.05), anemia (OR, 2.38; 95% CI, 1.25-4.56), and a thickened endometrium on ultrasonography (defined as >12 mm; OR, 4.04; 95% CI, 1.69-9.65). Age was not a significant risk factor in this group.
Read about using LNG-IUD to treat AUB in obese women
Small study shows LNG-IUD is effective for treating heavy menstrual bleeding in obese patients
Shaw V, Vandal AC, Coomarasamy C, Ekeroma AJ. The effectiveness of the levonorgestrel intrauterine system in obese women with heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2016;56(6):619-623.
In another recent study from New Zealand, researchers set out to assess the efficacy of the LNG-IUD for the treatment of heavy menstrual bleeding in obese women. This study is important because there are very few studies of the LNG-IUD in the obese population, and none that have studied quality-of-life measures.
Shaw and colleagues conducted the prospective observational study at a tertiary teaching hospital. Twenty obese (BMI >30 kg/m2) women with heavy menstrual bleeding agreed to treatment with an LNG-IUD, and 14 completed the study (2 had a device expulsion, 1 had a device removed for pain, and 1 had a device removed for infection; 2 were lost to follow-up). The women were aged 27 to 52 years (median, 40.5 years), and their BMI ranged from 30 to 68 kg/m2 (median, 40.6 kg/m2). At recruitment, 6 months, and 12 months, participants completed the Menstrual Impact Questionnaire and the Pictorial Bleeding Assessment Chart--2 validated tools.

Compared with baseline Pictorial Bleeding Assessment scores, the authors found the LNG-IUD to be effective in 73.2% (95% CI, 55.3%-83.9%) of women at 6 months and in 92.8% (95% CI, 80.0%-97.4%) of women at 12 months. Taking into consideration device failures, including removed and expelled LNG-IUDs (which occurred in 4 women, or 20%, in the intent-to-treat analysis), the actual efficacy rate was 67%. Similarly, there was significant improvement at 6 and 12 months in Menstrual Impact Questionnaire scores for social activities, work performance, tiredness, productivity, hygiene, and depression.
Read about doing more diagnostic hysteroscopy in the office
Is it time to abandon diagnostic hysteroscopy in the OR?
Leung S, Leyland N, Murji A. Decreasing diagnostic hysteroscopy performed in the operating room: a quality improvement initiative. J Obstet Gynaecol Can. 2016;38(4):351-356.
Diagnostic hysteroscopy: Are we stuck in the 1990s? Why are we still performing so many diagnostic hysteroscopies in the OR, thus subjecting our patients to general anesthesia and using our precious OR time? That is the question asked by a group of researchers in Canada.
According to data from the Ontario Ministry of Health and Long Term Care, diagnostic hysteroscopy was performed 10,027 times in the 2013-2014 fiscal year. Ontario researchers designed and implemented a quality improvement initiative at their institution and successfully decreased the number of diagnostic hysteroscopies performed in their hospital by 70% from their baseline 12-month period. The improvements resulted in a savings of 78 hours of case costing, or $126,984. When these data are extrapolated to the Ontario population (in which more than 10,000 diagnostic hysteroscopies were performed), potentially 7,000 women could avoid the risk of general anesthesia and the health care system could save $11 million.
Re-education protocol was key to reducing OR procedures
How did the researchers accomplish their results? The multifaceted intervention had 3 key components:
Staff education and review. Many surgeons were performing diagnostic hysteroscopy in the OR because that is how they were trained, and they were unaware of less invasive options. An awareness campaign was conducted by e-mail, during staff meetings, and at rounds.
Accessible sonohysterography. This diagnostic modality was made more accessible to referring physicians in a timely manner.
Initiation of an operative hysteroscopy education program. To allow more surgeons greater comfort with office hysteroscopy, the authors instituted didactic sessions, dry and wet lab simulations, and mentorship.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Two issues of emerging importance are being addressed in the literature: caring for patients with obesity and the concept of delivering value-based care. Value-based care does not mean providing the cheapest care; “value” places importance on quality as well as cost. In this Update, we present 3 practices that the evidence says will deliver value:
- endometrial biopsy in all obese women. Although performing more endometrial biopsies in younger women with a body mass index (BMI) in the obese range will not be less expensive initially, the procedure’s value likely will be in early diagnosis, which hopefully will translate to eventual health care system savings.
- use of the levonorgestrel-releasing intrauterine device (LNG-IUD) in obese patients experiencing abnormal uterine bleeding (AUB). This practice appears to add value in the context of AUB.
- performance of routine diagnostic hysteroscopy in the office setting. We should reconsider our current habits and traditions of performing routine diagnostic hysteroscopy in the operating room (OR) as we move toward providing value-based care.
Read about obesity as a risk factor for endometrial hyperplasia
Endometrial sampling and obesity: Forget the "age 45" rule
Wise MR, Gill P, Lensen S, Thompson JM, Farquhar CM. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215(5):598.e1-e8.
How do we bring more value to our patients with AUB? We are well aware that heavy menstrual bleeding places a burden on many women; AUB affects 30% of those of reproductive age. The condition often results in lost workdays and diminished quality of life. It also is associated with significant cost expenditures for hygiene products. It is important not only to bring value to women with heavy menstrual bleeding but also to consider our increasingly expensive health care system.
Obesity is a significant problem that likely will increase the number of women presenting with AUB to ObGyns. Recent studies from New Zealand--which has 33% of its population classified as obese--have provided valuable information.1

Obesity is a risk factor for endometrial hyperplasia
In a large retrospective cohort study, Wise and colleagues analyzed data from 916 premenopausal women referred for AUB who had an endometrial biopsy from 2008 to 2014. The setting was a single large urban secondary women's health service in New Zealand. This study challenges the concept of age-related biopsy guidelines.
Of the 916 women, half were obese. Almost 5% of the women had complex endometrial hyperplasia with atypia or cancer. This incidence had risen from 3% in the years 1995 to 1997, likely due to the rising incidence of obesity. Women with a BMI ≥30 kg/m2 were 4 times more likely to develop complex hyperplasia or cancer than normal-weight women.
Other factors associated with an increased risk for complex hyperplasia or cancer were nulliparity (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.25-5.05), anemia (OR, 2.38; 95% CI, 1.25-4.56), and a thickened endometrium on ultrasonography (defined as >12 mm; OR, 4.04; 95% CI, 1.69-9.65). Age was not a significant risk factor in this group.
Read about using LNG-IUD to treat AUB in obese women
Small study shows LNG-IUD is effective for treating heavy menstrual bleeding in obese patients
Shaw V, Vandal AC, Coomarasamy C, Ekeroma AJ. The effectiveness of the levonorgestrel intrauterine system in obese women with heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2016;56(6):619-623.
In another recent study from New Zealand, researchers set out to assess the efficacy of the LNG-IUD for the treatment of heavy menstrual bleeding in obese women. This study is important because there are very few studies of the LNG-IUD in the obese population, and none that have studied quality-of-life measures.
Shaw and colleagues conducted the prospective observational study at a tertiary teaching hospital. Twenty obese (BMI >30 kg/m2) women with heavy menstrual bleeding agreed to treatment with an LNG-IUD, and 14 completed the study (2 had a device expulsion, 1 had a device removed for pain, and 1 had a device removed for infection; 2 were lost to follow-up). The women were aged 27 to 52 years (median, 40.5 years), and their BMI ranged from 30 to 68 kg/m2 (median, 40.6 kg/m2). At recruitment, 6 months, and 12 months, participants completed the Menstrual Impact Questionnaire and the Pictorial Bleeding Assessment Chart--2 validated tools.

Compared with baseline Pictorial Bleeding Assessment scores, the authors found the LNG-IUD to be effective in 73.2% (95% CI, 55.3%-83.9%) of women at 6 months and in 92.8% (95% CI, 80.0%-97.4%) of women at 12 months. Taking into consideration device failures, including removed and expelled LNG-IUDs (which occurred in 4 women, or 20%, in the intent-to-treat analysis), the actual efficacy rate was 67%. Similarly, there was significant improvement at 6 and 12 months in Menstrual Impact Questionnaire scores for social activities, work performance, tiredness, productivity, hygiene, and depression.
Read about doing more diagnostic hysteroscopy in the office
Is it time to abandon diagnostic hysteroscopy in the OR?
Leung S, Leyland N, Murji A. Decreasing diagnostic hysteroscopy performed in the operating room: a quality improvement initiative. J Obstet Gynaecol Can. 2016;38(4):351-356.
Diagnostic hysteroscopy: Are we stuck in the 1990s? Why are we still performing so many diagnostic hysteroscopies in the OR, thus subjecting our patients to general anesthesia and using our precious OR time? That is the question asked by a group of researchers in Canada.
According to data from the Ontario Ministry of Health and Long Term Care, diagnostic hysteroscopy was performed 10,027 times in the 2013-2014 fiscal year. Ontario researchers designed and implemented a quality improvement initiative at their institution and successfully decreased the number of diagnostic hysteroscopies performed in their hospital by 70% from their baseline 12-month period. The improvements resulted in a savings of 78 hours of case costing, or $126,984. When these data are extrapolated to the Ontario population (in which more than 10,000 diagnostic hysteroscopies were performed), potentially 7,000 women could avoid the risk of general anesthesia and the health care system could save $11 million.
Re-education protocol was key to reducing OR procedures
How did the researchers accomplish their results? The multifaceted intervention had 3 key components:
Staff education and review. Many surgeons were performing diagnostic hysteroscopy in the OR because that is how they were trained, and they were unaware of less invasive options. An awareness campaign was conducted by e-mail, during staff meetings, and at rounds.
Accessible sonohysterography. This diagnostic modality was made more accessible to referring physicians in a timely manner.
Initiation of an operative hysteroscopy education program. To allow more surgeons greater comfort with office hysteroscopy, the authors instituted didactic sessions, dry and wet lab simulations, and mentorship.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- The Organization for Economic Co-operation and Development (OECD). OECD obesity update 2014. http://www.oecd.org/health/Obesity-Update-2014.pdf. Published June 2014. Accessed March 10, 2017.
- The Organization for Economic Co-operation and Development (OECD). OECD obesity update 2014. http://www.oecd.org/health/Obesity-Update-2014.pdf. Published June 2014. Accessed March 10, 2017.
2016 Update on abnormal uterine bleeding
How abnormal uterine bleeding (AUB) is managed has a significant impact on health care. In the United States, almost one-third of all gynecologic visits are related to AUB, with estimated annual direct costs of up to $1.55 billion and indirect costs as high as $36 billion.1 Not surprisingly, office-based procedures for AUB are being emphasized. While in the short term it is more cost efficient to perform surgery in the office rather than in the operating room, questions have arisen regarding the long-term efficacy and durability of in-office procedures. Insurers are undoubtedly raising these questions as well.
Notably, some ObGyns are early adopters of office-based surgery while others tend to adopt in-office procedures more slowly. As the literature for such procedures for AUB matures to provide more data on efficacy and acceptability, we will have a greater evidence base for understanding which procedures are more appropriate for the office. And while practice shifts sometimes occur due to cost-containment initiatives, some shifts are patient driven. Studies that address these driving variables, as well as efficacy considerations, are helpful. As we counsel women about procedures for AUB, the relative advantages and disadvantages of available treatment settings likely will become a greater part of that discussion so that they can make an informed decision.
In this Update, we discuss the results of 3 studies that examined various procedures and settings for AUB management:
- outpatient vs inpatient polypectomy
- hysteroscopic morcellation of polyps and myomas in an office vs ambulatory surgical center
- comparative costs of endometrial ablation and hysterectomy.
Outpatient vs inpatient polypectomy: Similar success rates in the short term
Cooper NA, Clark TJ, Middleton L, et al; OPT Trial Collaborative Group. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ. 2015;350:h1398. doi:10.1136/bmj.h1398.
A collaborative group in the United Kingdom studied the common problem of endometrial polyps. Their objective was to evaluate whether outpatient polypectomy was as effective and well accepted as polypectomy performed in the operating room (OR).
Patients with a hysteroscopically diagnosed polyp were randomly assigned to hysteroscopic polyp removal in either a hysteroscopy clinic or an OR; polyp removal was performed using miniature mechanical or electrosurgical instruments. The primary outcome was successful treatment, determined by the participants’ assessment of their bleeding at 6 months.
Overall, 73% of women (166 of 228) in the clinic group and 80% (168 of 211) in the OR group reported a successful response to surgery at 6 months, with treatment effects being maintained at 12 and 24 months. A “see and treat” approach—that is, treatment carried out at the same time as diagnosis—was possible in 72% of women (174 of 242).
Partial or failed polyp removal occurred in 46 of 242 women (19%) in the clinic group, mostly because of pain issues, and in 18 of 233 women (7%) in the OR group (relative risk, 2.5; 95% confidence interval, 1.5−4.1; P<.001). Four uterine perforations (2% of patients) occurred in the OR group.
Mean pain scores were higher in the clinic group, and treatment was unacceptable for 2% of the women in each group.
The results of this trial show that clinic polypectomy has some limitations, but the outpatient procedure was deemed noninferior to polypectomy performed in the OR for the successful alleviation of uterine bleeding associated with uterine polyps.
What this EVIDENCE means for practice
Office-based polypectomy allowed a “see and treat” model in 72% of cases. Office polypectomy had similar successful therapeutic responses as inpatient polypectomy; however, over a 2-year follow-up period, women treated in the office were twice as likely to undergo at least 1 further polyp removal and were 1.6 times more likely to have further gynecologic surgery.
In-office hysteroscopic morcellation of polyps and myomas improves health-related quality of life
Rubino RJ, Lukes AS. Twelve-month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. J Minim Invasive Gynecol. 2015;22(2):285–290.
Is it feasible to morcellate fibroids, as well as polyps, in the clinic? Rubino and colleagues investigated this question in a randomized, prospective clinical trial. They examined the efficacy of hysteroscopic removal of polyps and myomas on health-related quality of life and symptom severity at 1-year postprocedure. Women aged 18 to 55 years, with hysteroscopic and saline-infusion sonogram–assessed polyps and/or type 0 or I myomas (1.5−3.0 cm), were enrolled from 9 US clinical sites. Some patient populations were excluded, such as women with a long narcotic abuse history, current intrauterine device (IUD), type II submucous myomas, and type I fundal myomas.
A total of 118 pathologies were removed in 74 patients. Forty-two women were treated in the office setting; 32 were treated in the OR setting. Among the 118 pathologies removed, 53 were removed in the office (28 myomas and 25 polyps), and 55 were removed in the OR (14 myomas and 41 polyps).
The percentage of patients who reported being satisfied or highly satisfied was higher in the OR cohort (96.5%) compared with the office cohort (83.3%), although this difference was not statistically significant (P = .06). The percentage of patients who had 100% of their pathology removed was significantly higher in those with polyps compared with patients with myomas (96.0% vs 63.6%, respectively; P<.01).
These findings indicate that there were several cases in which the majority of a myoma was removed but a small residual portion remained. This disparity was especially pronounced in the office setting, where 96% of polyps were completely removed, compared with 52% of fibroids. There was no statistically significant difference in health-related quality of life between patients with complete removal and those with residual pathology, and there was no difference in satisfaction rates between patients who were treated in the office and those treated in the OR.
What this EVIDENCE means for practice
In general, office-based hysteroscopic myomectomy and polypectomy using morcellation for small- to medium-size lesions was associated with low rates of adverse events, high physician acceptance, and significant durable health-related quality-of-life improvements for up to 12 months post‑ procedure. Partial removal of myomas did not seem to be a significant factor in patients’ perceived outcomes.
Endometrial ablation for AUB costs less, has fewer complications at 1 year than hysterectomy
Miller JD, Lenhart GM, Bonafede MM, Lukes AS, Laughlin-Tommaso SK. Cost-effectiveness of global endometrial ablation vs hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. Popul Health Manag. 2015;18(5):373–382.
Endometrial ablation often is performed in the office for AUB management. Miller and colleagues suggested that cost-effectiveness modeling studies of endometrial ablation for AUB treatment from a US perspective are lacking. They therefore designed a study to model the cost-effectiveness of endometrial ablation versus hysterectomy for treatment of AUB from both commercial and Medicaid payer perspectives.
They developed a decision-tree, state-transition (semi-Markov) model to simulate 2 hypothetical patient cohorts of women with AUB: one treated with endometrial ablation and the other with hysterectomy. Twenty-one health states were included in the model of intervention with endometrial ablation or hysterectomy; these comprised postablation reintervention with secondary ablation, tranexamic acid, or a levonorgestrel-containing IUD due to AUB, use of adjunctive pharmacotherapy following ablation, and a small probability of death from hysterectomy or actuarial death from all other causes.
The 1-year direct costs of endometrial ablation were $7,352 and $6,306 in the commercial payer and Medicaid payer perspectives, respectively; these were about half the costs of hysterectomy. The cost differential between the 2 treatments narrowed over time but, even at 5 years, endometrial ablation costs were still one-third less than hysterectomy costs.
In the first year, 35.6% of patients who had a hysterectomy and only 17.1% of patients undergoing ablation had complications. Short-term results were similar under the Medicaid perspective. By 5 years intervention/reintervention, however, complications of endometrial ablation were higher than those for hysterectomy by about 1.6%.
Over a 5-year time frame, direct costs of endometrial ablation were lower than those of hysterectomy from both the commercial payer and Medicaid perspectives. In the commercial payer analysis, the indirect costs of endometrial ablation were also lower than for hysterectomy, with 38.5 workdays lost for endometrial ablation compared with 55.3 days lost for hysterectomy, resulting in indirect costs of $8,976 versus $13,087.
What this EVIDENCE means for practice
Costs and cost-effectiveness of endometrial ablation from a US perspective are understudied. This model estimates a financial advantage for endometrial ablation over hysterectomy from both the commercial payer and Medicaid payer perspectives. Over a variety of time frames, endometrial ablation may save costs while reducing treatment complications and lost workdays. From the patient perspective, this model suggests better quality of life in the short term after endometrial ablation. It will be interesting to see whether longer term impacts show this model to be predictive.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194.
How abnormal uterine bleeding (AUB) is managed has a significant impact on health care. In the United States, almost one-third of all gynecologic visits are related to AUB, with estimated annual direct costs of up to $1.55 billion and indirect costs as high as $36 billion.1 Not surprisingly, office-based procedures for AUB are being emphasized. While in the short term it is more cost efficient to perform surgery in the office rather than in the operating room, questions have arisen regarding the long-term efficacy and durability of in-office procedures. Insurers are undoubtedly raising these questions as well.
Notably, some ObGyns are early adopters of office-based surgery while others tend to adopt in-office procedures more slowly. As the literature for such procedures for AUB matures to provide more data on efficacy and acceptability, we will have a greater evidence base for understanding which procedures are more appropriate for the office. And while practice shifts sometimes occur due to cost-containment initiatives, some shifts are patient driven. Studies that address these driving variables, as well as efficacy considerations, are helpful. As we counsel women about procedures for AUB, the relative advantages and disadvantages of available treatment settings likely will become a greater part of that discussion so that they can make an informed decision.
In this Update, we discuss the results of 3 studies that examined various procedures and settings for AUB management:
- outpatient vs inpatient polypectomy
- hysteroscopic morcellation of polyps and myomas in an office vs ambulatory surgical center
- comparative costs of endometrial ablation and hysterectomy.
Outpatient vs inpatient polypectomy: Similar success rates in the short term
Cooper NA, Clark TJ, Middleton L, et al; OPT Trial Collaborative Group. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ. 2015;350:h1398. doi:10.1136/bmj.h1398.
A collaborative group in the United Kingdom studied the common problem of endometrial polyps. Their objective was to evaluate whether outpatient polypectomy was as effective and well accepted as polypectomy performed in the operating room (OR).
Patients with a hysteroscopically diagnosed polyp were randomly assigned to hysteroscopic polyp removal in either a hysteroscopy clinic or an OR; polyp removal was performed using miniature mechanical or electrosurgical instruments. The primary outcome was successful treatment, determined by the participants’ assessment of their bleeding at 6 months.
Overall, 73% of women (166 of 228) in the clinic group and 80% (168 of 211) in the OR group reported a successful response to surgery at 6 months, with treatment effects being maintained at 12 and 24 months. A “see and treat” approach—that is, treatment carried out at the same time as diagnosis—was possible in 72% of women (174 of 242).
Partial or failed polyp removal occurred in 46 of 242 women (19%) in the clinic group, mostly because of pain issues, and in 18 of 233 women (7%) in the OR group (relative risk, 2.5; 95% confidence interval, 1.5−4.1; P<.001). Four uterine perforations (2% of patients) occurred in the OR group.
Mean pain scores were higher in the clinic group, and treatment was unacceptable for 2% of the women in each group.
The results of this trial show that clinic polypectomy has some limitations, but the outpatient procedure was deemed noninferior to polypectomy performed in the OR for the successful alleviation of uterine bleeding associated with uterine polyps.
What this EVIDENCE means for practice
Office-based polypectomy allowed a “see and treat” model in 72% of cases. Office polypectomy had similar successful therapeutic responses as inpatient polypectomy; however, over a 2-year follow-up period, women treated in the office were twice as likely to undergo at least 1 further polyp removal and were 1.6 times more likely to have further gynecologic surgery.
In-office hysteroscopic morcellation of polyps and myomas improves health-related quality of life
Rubino RJ, Lukes AS. Twelve-month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. J Minim Invasive Gynecol. 2015;22(2):285–290.
Is it feasible to morcellate fibroids, as well as polyps, in the clinic? Rubino and colleagues investigated this question in a randomized, prospective clinical trial. They examined the efficacy of hysteroscopic removal of polyps and myomas on health-related quality of life and symptom severity at 1-year postprocedure. Women aged 18 to 55 years, with hysteroscopic and saline-infusion sonogram–assessed polyps and/or type 0 or I myomas (1.5−3.0 cm), were enrolled from 9 US clinical sites. Some patient populations were excluded, such as women with a long narcotic abuse history, current intrauterine device (IUD), type II submucous myomas, and type I fundal myomas.
A total of 118 pathologies were removed in 74 patients. Forty-two women were treated in the office setting; 32 were treated in the OR setting. Among the 118 pathologies removed, 53 were removed in the office (28 myomas and 25 polyps), and 55 were removed in the OR (14 myomas and 41 polyps).
The percentage of patients who reported being satisfied or highly satisfied was higher in the OR cohort (96.5%) compared with the office cohort (83.3%), although this difference was not statistically significant (P = .06). The percentage of patients who had 100% of their pathology removed was significantly higher in those with polyps compared with patients with myomas (96.0% vs 63.6%, respectively; P<.01).
These findings indicate that there were several cases in which the majority of a myoma was removed but a small residual portion remained. This disparity was especially pronounced in the office setting, where 96% of polyps were completely removed, compared with 52% of fibroids. There was no statistically significant difference in health-related quality of life between patients with complete removal and those with residual pathology, and there was no difference in satisfaction rates between patients who were treated in the office and those treated in the OR.
What this EVIDENCE means for practice
In general, office-based hysteroscopic myomectomy and polypectomy using morcellation for small- to medium-size lesions was associated with low rates of adverse events, high physician acceptance, and significant durable health-related quality-of-life improvements for up to 12 months post‑ procedure. Partial removal of myomas did not seem to be a significant factor in patients’ perceived outcomes.
Endometrial ablation for AUB costs less, has fewer complications at 1 year than hysterectomy
Miller JD, Lenhart GM, Bonafede MM, Lukes AS, Laughlin-Tommaso SK. Cost-effectiveness of global endometrial ablation vs hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. Popul Health Manag. 2015;18(5):373–382.
Endometrial ablation often is performed in the office for AUB management. Miller and colleagues suggested that cost-effectiveness modeling studies of endometrial ablation for AUB treatment from a US perspective are lacking. They therefore designed a study to model the cost-effectiveness of endometrial ablation versus hysterectomy for treatment of AUB from both commercial and Medicaid payer perspectives.
They developed a decision-tree, state-transition (semi-Markov) model to simulate 2 hypothetical patient cohorts of women with AUB: one treated with endometrial ablation and the other with hysterectomy. Twenty-one health states were included in the model of intervention with endometrial ablation or hysterectomy; these comprised postablation reintervention with secondary ablation, tranexamic acid, or a levonorgestrel-containing IUD due to AUB, use of adjunctive pharmacotherapy following ablation, and a small probability of death from hysterectomy or actuarial death from all other causes.
The 1-year direct costs of endometrial ablation were $7,352 and $6,306 in the commercial payer and Medicaid payer perspectives, respectively; these were about half the costs of hysterectomy. The cost differential between the 2 treatments narrowed over time but, even at 5 years, endometrial ablation costs were still one-third less than hysterectomy costs.
In the first year, 35.6% of patients who had a hysterectomy and only 17.1% of patients undergoing ablation had complications. Short-term results were similar under the Medicaid perspective. By 5 years intervention/reintervention, however, complications of endometrial ablation were higher than those for hysterectomy by about 1.6%.
Over a 5-year time frame, direct costs of endometrial ablation were lower than those of hysterectomy from both the commercial payer and Medicaid perspectives. In the commercial payer analysis, the indirect costs of endometrial ablation were also lower than for hysterectomy, with 38.5 workdays lost for endometrial ablation compared with 55.3 days lost for hysterectomy, resulting in indirect costs of $8,976 versus $13,087.
What this EVIDENCE means for practice
Costs and cost-effectiveness of endometrial ablation from a US perspective are understudied. This model estimates a financial advantage for endometrial ablation over hysterectomy from both the commercial payer and Medicaid payer perspectives. Over a variety of time frames, endometrial ablation may save costs while reducing treatment complications and lost workdays. From the patient perspective, this model suggests better quality of life in the short term after endometrial ablation. It will be interesting to see whether longer term impacts show this model to be predictive.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
How abnormal uterine bleeding (AUB) is managed has a significant impact on health care. In the United States, almost one-third of all gynecologic visits are related to AUB, with estimated annual direct costs of up to $1.55 billion and indirect costs as high as $36 billion.1 Not surprisingly, office-based procedures for AUB are being emphasized. While in the short term it is more cost efficient to perform surgery in the office rather than in the operating room, questions have arisen regarding the long-term efficacy and durability of in-office procedures. Insurers are undoubtedly raising these questions as well.
Notably, some ObGyns are early adopters of office-based surgery while others tend to adopt in-office procedures more slowly. As the literature for such procedures for AUB matures to provide more data on efficacy and acceptability, we will have a greater evidence base for understanding which procedures are more appropriate for the office. And while practice shifts sometimes occur due to cost-containment initiatives, some shifts are patient driven. Studies that address these driving variables, as well as efficacy considerations, are helpful. As we counsel women about procedures for AUB, the relative advantages and disadvantages of available treatment settings likely will become a greater part of that discussion so that they can make an informed decision.
In this Update, we discuss the results of 3 studies that examined various procedures and settings for AUB management:
- outpatient vs inpatient polypectomy
- hysteroscopic morcellation of polyps and myomas in an office vs ambulatory surgical center
- comparative costs of endometrial ablation and hysterectomy.
Outpatient vs inpatient polypectomy: Similar success rates in the short term
Cooper NA, Clark TJ, Middleton L, et al; OPT Trial Collaborative Group. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ. 2015;350:h1398. doi:10.1136/bmj.h1398.
A collaborative group in the United Kingdom studied the common problem of endometrial polyps. Their objective was to evaluate whether outpatient polypectomy was as effective and well accepted as polypectomy performed in the operating room (OR).
Patients with a hysteroscopically diagnosed polyp were randomly assigned to hysteroscopic polyp removal in either a hysteroscopy clinic or an OR; polyp removal was performed using miniature mechanical or electrosurgical instruments. The primary outcome was successful treatment, determined by the participants’ assessment of their bleeding at 6 months.
Overall, 73% of women (166 of 228) in the clinic group and 80% (168 of 211) in the OR group reported a successful response to surgery at 6 months, with treatment effects being maintained at 12 and 24 months. A “see and treat” approach—that is, treatment carried out at the same time as diagnosis—was possible in 72% of women (174 of 242).
Partial or failed polyp removal occurred in 46 of 242 women (19%) in the clinic group, mostly because of pain issues, and in 18 of 233 women (7%) in the OR group (relative risk, 2.5; 95% confidence interval, 1.5−4.1; P<.001). Four uterine perforations (2% of patients) occurred in the OR group.
Mean pain scores were higher in the clinic group, and treatment was unacceptable for 2% of the women in each group.
The results of this trial show that clinic polypectomy has some limitations, but the outpatient procedure was deemed noninferior to polypectomy performed in the OR for the successful alleviation of uterine bleeding associated with uterine polyps.
What this EVIDENCE means for practice
Office-based polypectomy allowed a “see and treat” model in 72% of cases. Office polypectomy had similar successful therapeutic responses as inpatient polypectomy; however, over a 2-year follow-up period, women treated in the office were twice as likely to undergo at least 1 further polyp removal and were 1.6 times more likely to have further gynecologic surgery.
In-office hysteroscopic morcellation of polyps and myomas improves health-related quality of life
Rubino RJ, Lukes AS. Twelve-month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. J Minim Invasive Gynecol. 2015;22(2):285–290.
Is it feasible to morcellate fibroids, as well as polyps, in the clinic? Rubino and colleagues investigated this question in a randomized, prospective clinical trial. They examined the efficacy of hysteroscopic removal of polyps and myomas on health-related quality of life and symptom severity at 1-year postprocedure. Women aged 18 to 55 years, with hysteroscopic and saline-infusion sonogram–assessed polyps and/or type 0 or I myomas (1.5−3.0 cm), were enrolled from 9 US clinical sites. Some patient populations were excluded, such as women with a long narcotic abuse history, current intrauterine device (IUD), type II submucous myomas, and type I fundal myomas.
A total of 118 pathologies were removed in 74 patients. Forty-two women were treated in the office setting; 32 were treated in the OR setting. Among the 118 pathologies removed, 53 were removed in the office (28 myomas and 25 polyps), and 55 were removed in the OR (14 myomas and 41 polyps).
The percentage of patients who reported being satisfied or highly satisfied was higher in the OR cohort (96.5%) compared with the office cohort (83.3%), although this difference was not statistically significant (P = .06). The percentage of patients who had 100% of their pathology removed was significantly higher in those with polyps compared with patients with myomas (96.0% vs 63.6%, respectively; P<.01).
These findings indicate that there were several cases in which the majority of a myoma was removed but a small residual portion remained. This disparity was especially pronounced in the office setting, where 96% of polyps were completely removed, compared with 52% of fibroids. There was no statistically significant difference in health-related quality of life between patients with complete removal and those with residual pathology, and there was no difference in satisfaction rates between patients who were treated in the office and those treated in the OR.
What this EVIDENCE means for practice
In general, office-based hysteroscopic myomectomy and polypectomy using morcellation for small- to medium-size lesions was associated with low rates of adverse events, high physician acceptance, and significant durable health-related quality-of-life improvements for up to 12 months post‑ procedure. Partial removal of myomas did not seem to be a significant factor in patients’ perceived outcomes.
Endometrial ablation for AUB costs less, has fewer complications at 1 year than hysterectomy
Miller JD, Lenhart GM, Bonafede MM, Lukes AS, Laughlin-Tommaso SK. Cost-effectiveness of global endometrial ablation vs hysterectomy for treatment of abnormal uterine bleeding: US commercial and Medicaid payer perspectives. Popul Health Manag. 2015;18(5):373–382.
Endometrial ablation often is performed in the office for AUB management. Miller and colleagues suggested that cost-effectiveness modeling studies of endometrial ablation for AUB treatment from a US perspective are lacking. They therefore designed a study to model the cost-effectiveness of endometrial ablation versus hysterectomy for treatment of AUB from both commercial and Medicaid payer perspectives.
They developed a decision-tree, state-transition (semi-Markov) model to simulate 2 hypothetical patient cohorts of women with AUB: one treated with endometrial ablation and the other with hysterectomy. Twenty-one health states were included in the model of intervention with endometrial ablation or hysterectomy; these comprised postablation reintervention with secondary ablation, tranexamic acid, or a levonorgestrel-containing IUD due to AUB, use of adjunctive pharmacotherapy following ablation, and a small probability of death from hysterectomy or actuarial death from all other causes.
The 1-year direct costs of endometrial ablation were $7,352 and $6,306 in the commercial payer and Medicaid payer perspectives, respectively; these were about half the costs of hysterectomy. The cost differential between the 2 treatments narrowed over time but, even at 5 years, endometrial ablation costs were still one-third less than hysterectomy costs.
In the first year, 35.6% of patients who had a hysterectomy and only 17.1% of patients undergoing ablation had complications. Short-term results were similar under the Medicaid perspective. By 5 years intervention/reintervention, however, complications of endometrial ablation were higher than those for hysterectomy by about 1.6%.
Over a 5-year time frame, direct costs of endometrial ablation were lower than those of hysterectomy from both the commercial payer and Medicaid perspectives. In the commercial payer analysis, the indirect costs of endometrial ablation were also lower than for hysterectomy, with 38.5 workdays lost for endometrial ablation compared with 55.3 days lost for hysterectomy, resulting in indirect costs of $8,976 versus $13,087.
What this EVIDENCE means for practice
Costs and cost-effectiveness of endometrial ablation from a US perspective are understudied. This model estimates a financial advantage for endometrial ablation over hysterectomy from both the commercial payer and Medicaid payer perspectives. Over a variety of time frames, endometrial ablation may save costs while reducing treatment complications and lost workdays. From the patient perspective, this model suggests better quality of life in the short term after endometrial ablation. It will be interesting to see whether longer term impacts show this model to be predictive.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194.
Reference
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194.
IN THIS ARTICLE
- Polypectomy in the clinic vs OR
- In-office polyp, fibroid morcellation
- Cost-effectiveness of endometrial ablation
2015 Update on abnormal uterine bleeding
Surgery involves a certain degree of risk. The risk could be in the form of a complication, or it could be surgical failure. When I was a resident, gynecologic oncologist Gary Johnson, MD, used to say: “If you don’t want complications, don’t do surgery.” I was never sure whether he was trying to make me feel better when complications occurred, or was just stating the facts. Maybe both. It could be argued that one should consider offering medical options before exposing a patient to surgical risks.
In this article, I review three recent studies that shed some light on patient selection for surgical intervention—more specifically, on surgical and counseling failures associated with surgical management of abnormal uterine bleeding (AUB):
- an analysis of perioperative hysterectomy data from 52 hospitals in Michigan that showed that alternatives to hysterectomy were underutilized in women with AUB, fibroids, or pelvic pain
- a retrospective cohort study of 300 patients from two large academic medical centers, which explored risk factors for postablation pain
- another retrospective cohort study of 968 women who underwent endometrial ablation. This study was designed to highlight any association between preoperative bleeding patterns and the risk of ablation failure.
Don’t resort to surgery until alternative treatments have been exhausted
Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative [published online ahead of print December 23, 2014]. Am J Obstet Gynecol. pii: S0002-9378(14)02355-2. doi: 10.1016/j.ajog.2014.11.031.
In this analysis, Corona and colleagues evaluated the use of alternative treatments among women who underwent hysterectomy for uterine fibroids, AUB, endometriosis, or pelvic pain at 52 hospitals participating in the Michigan Surgical Quality Collaborative in 2013. They also determined whether the pathology was “supportive” or “unsupportive” of the surgical indication.
A significant percentage of hysterectomies were performed with an indication of AUB or fibroids, or both (49.1%). A combination of pain, AUB, and/or fibroids was the indication in 48.1% of hysterectomies, and endometriosis and/or pain was listed in 9.2%.
In 37.7% of cases (n = 1,281), no offering of alternative treatments was documented.
Although endometrial ablation was offered to 44.1% of women younger than age 40, to 48.3% of women aged 40 to 50 years, and to 31.6% of women older than age 50, the conservative, nonsurgical option of a levonorgestrel intrauterine system (LNG-IUS) was offered in only 12.7%, 12.4%, and 9.3% of these cases, respectively.
Overall, the rate of unsupportive pathology was 18.3% (n = 621). That rate was higher in women younger than age 40, compared with those aged 40 to 50 and older than age 50 (37.8% vs 12.0% and 7.5%, respectively; P<.001).
These data suggest that a significant number of women with AUB associated with ovulatory dysfunction (AUB-O) undergo hysterectomy. The authors point out that the American College of Obstetricians and Gynecologists (ACOG) recommends medical therapy as a first-line therapy for AUB-O rather than surgical therapy.
What this EVIDENCE means for practice
Although AUB is a common indication for hysterectomy, conservative alternative therapies should be offered when appropriate. A particularly cost-effective and effective conservative therapy—the LNG-IUS—is underutilized and should be considered more often.
How to determine who is most likely to benefit from endometrial ablation
Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5):904–910.
Smithling KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211(5):556.e1–e6.
Endometrial ablation has been around a long time—likely since the 1930s. However, it was not until the 1980s that operative hysteroscopy and endometrial ablation became commonplace. As a result of new, more “automated” technology, five nonresectoscopic endometrial ablation techniques were introduced, starting with FDA approval of the thermal balloon device in 1997.
Initially, information on the feasibility of endometrial ablation was presented in the form of case reports. Efficacy and safety were studied through FDA trials, which yielded variable amenorrhea rates but relatively high satisfaction rates in the range of 85% to 95%. In the interim, we have learned more refined details about endometrial ablation as case reports of unintended consequences have cropped up and as this technology has reached a broader physician base. After almost two decades of experience with nonresectoscopic endometrial ablation devices, information on “failure”—ie, the need for additional treatment—is surfacing.
Over the past year, as we have increased adoption of the PALM-COEIN classification system for the causes of AUB in women of reproductive age, we also have gleaned more information about how endometrial ablation works in this context. In general, PALM (polyp, adenomyosis, leiomyoma, and malignancy/hyperplasia) represents lesions that can be seen but may not necessarily be the cause of bleeding. COEIN (coagulopathy, ovulatory dysfunction, endometrial factors, iatrogenic, and not yet classified) represents causes of bleeding that may not be visible.
Although endometrial ablation is ideally suited for women with AUB related to endometrial factors (AUB-E), two studies from 2014 provide insight into endometrial ablation performed when lesions are present within the PALM classification, such as polyps (AUB-P), adenomyosis (AUB-A), and leiomyomas (AUB-L), and in patients with COEIN conditions, such as ovulatory dysfunction (AUB-O) and AUB-E.
Findings of Wishall and colleagues
Three hundred women who underwent endometrial ablation were evaluated in regard to postoperative pain and the need for subsequent hysterectomy. A total of 270 women were available for follow-up in this retrospective cohort (10% lost to follow-up). Wishall and colleagues set out to identify prognostic factors that would put a woman at risk for post-ablation pain. Their secondary outcome was the rate of hysterectomy after ablation.
The study was limited to second-generation endometrial ablation devices, including the thermal balloon, microwave, circulating hot fluid, and bipolar radiofrequency devices.
Wishall and colleagues found that the risk of failure was the highest (a quadrupling) when uterine abnormalities such as leiomyomas, adenomyosis, a thickened endometrial stripe, or polyps were present (adjusted odds ratio [OR], 3.96; 95% confidence interval [CI], 1.25–12.56).
As in other series, 19% of women ultimately required hysterectomy. Twenty-three percent developed new or worsening pain after ablation. Risk factors for postablation pain included a history of dysmenorrhea (OR, 1.74) and tubal sterilization (OR, 2.06).
Findings of Smithling and colleagues
Investigators evaluated the records of 968 women with AUB who had undergone endometrial ablation, categorizing their preoperative bleeding patterns as either regular (presumed AUB-E) or irregular (presumed AUB-O). Of these women, 961 (99.3%) had undergone radiofrequency bipolar endometrial ablation.
Smithling and colleagues hypothesized that women with AUB-O would have a higher failure rate—defined as the need for reablation or subsequent hysterectomy—than women with AUB-E because endometrial ablation does not necessarily address the pathology that underlies AUB-O. However, they found no difference in treatment failure or the need for a subsequent gynecologic procedure between groups during the 3-year period after endometrial ablation. The rate of treatment failure was 16.4% in women with regular bleeding—essentially the same as the rate for women with irregular bleeding (17.6%; P = .7) Risk factors associated with failure included:
- tubal sterilization (16.4% vs 9.0% for women without it)
- pelvic pain or dysmenorrhea (21.8% vs 10.7% for women without it)
- obesity (16.7% vs 9.8%) (P = .003).
Although there was no difference in failure rates between the group with regular bleeding versus the group with irregular bleeding, Smithling and colleagues were careful to avoid interpreting this finding as a recommendation for endometrial ablation in women with AUB-O.
What this EVIDENCE means for practice
When endometrial ablation is performed to treat lesions such as polyps, adenomyosis, and leiomyomata, women are nearly four times more likely to require subsequent hysterectomy.
A history of dysmenorrhea yielded a 74% higher risk of developing postablation pain, and a history of tubal sterilization more than doubled the risk, compared with no history of dysmenorrhea or tubal sterilization.
Women who undergo endometrial ablation for presumed AUB-O and presumed AUB-E have similar failure rates.
Preoperative factors such as dysmenorrhea, prior tubal sterilization, and obesity were identified as risk factors for ablation failure.
The choice between endometrial ablation and hysterectomy for patients with AUB-O depends on an individualized assessment of risks and benefits, including evaluation of medical comorbidities.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Surgery involves a certain degree of risk. The risk could be in the form of a complication, or it could be surgical failure. When I was a resident, gynecologic oncologist Gary Johnson, MD, used to say: “If you don’t want complications, don’t do surgery.” I was never sure whether he was trying to make me feel better when complications occurred, or was just stating the facts. Maybe both. It could be argued that one should consider offering medical options before exposing a patient to surgical risks.
In this article, I review three recent studies that shed some light on patient selection for surgical intervention—more specifically, on surgical and counseling failures associated with surgical management of abnormal uterine bleeding (AUB):
- an analysis of perioperative hysterectomy data from 52 hospitals in Michigan that showed that alternatives to hysterectomy were underutilized in women with AUB, fibroids, or pelvic pain
- a retrospective cohort study of 300 patients from two large academic medical centers, which explored risk factors for postablation pain
- another retrospective cohort study of 968 women who underwent endometrial ablation. This study was designed to highlight any association between preoperative bleeding patterns and the risk of ablation failure.
Don’t resort to surgery until alternative treatments have been exhausted
Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative [published online ahead of print December 23, 2014]. Am J Obstet Gynecol. pii: S0002-9378(14)02355-2. doi: 10.1016/j.ajog.2014.11.031.
In this analysis, Corona and colleagues evaluated the use of alternative treatments among women who underwent hysterectomy for uterine fibroids, AUB, endometriosis, or pelvic pain at 52 hospitals participating in the Michigan Surgical Quality Collaborative in 2013. They also determined whether the pathology was “supportive” or “unsupportive” of the surgical indication.
A significant percentage of hysterectomies were performed with an indication of AUB or fibroids, or both (49.1%). A combination of pain, AUB, and/or fibroids was the indication in 48.1% of hysterectomies, and endometriosis and/or pain was listed in 9.2%.
In 37.7% of cases (n = 1,281), no offering of alternative treatments was documented.
Although endometrial ablation was offered to 44.1% of women younger than age 40, to 48.3% of women aged 40 to 50 years, and to 31.6% of women older than age 50, the conservative, nonsurgical option of a levonorgestrel intrauterine system (LNG-IUS) was offered in only 12.7%, 12.4%, and 9.3% of these cases, respectively.
Overall, the rate of unsupportive pathology was 18.3% (n = 621). That rate was higher in women younger than age 40, compared with those aged 40 to 50 and older than age 50 (37.8% vs 12.0% and 7.5%, respectively; P<.001).
These data suggest that a significant number of women with AUB associated with ovulatory dysfunction (AUB-O) undergo hysterectomy. The authors point out that the American College of Obstetricians and Gynecologists (ACOG) recommends medical therapy as a first-line therapy for AUB-O rather than surgical therapy.
What this EVIDENCE means for practice
Although AUB is a common indication for hysterectomy, conservative alternative therapies should be offered when appropriate. A particularly cost-effective and effective conservative therapy—the LNG-IUS—is underutilized and should be considered more often.
How to determine who is most likely to benefit from endometrial ablation
Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5):904–910.
Smithling KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211(5):556.e1–e6.
Endometrial ablation has been around a long time—likely since the 1930s. However, it was not until the 1980s that operative hysteroscopy and endometrial ablation became commonplace. As a result of new, more “automated” technology, five nonresectoscopic endometrial ablation techniques were introduced, starting with FDA approval of the thermal balloon device in 1997.
Initially, information on the feasibility of endometrial ablation was presented in the form of case reports. Efficacy and safety were studied through FDA trials, which yielded variable amenorrhea rates but relatively high satisfaction rates in the range of 85% to 95%. In the interim, we have learned more refined details about endometrial ablation as case reports of unintended consequences have cropped up and as this technology has reached a broader physician base. After almost two decades of experience with nonresectoscopic endometrial ablation devices, information on “failure”—ie, the need for additional treatment—is surfacing.
Over the past year, as we have increased adoption of the PALM-COEIN classification system for the causes of AUB in women of reproductive age, we also have gleaned more information about how endometrial ablation works in this context. In general, PALM (polyp, adenomyosis, leiomyoma, and malignancy/hyperplasia) represents lesions that can be seen but may not necessarily be the cause of bleeding. COEIN (coagulopathy, ovulatory dysfunction, endometrial factors, iatrogenic, and not yet classified) represents causes of bleeding that may not be visible.
Although endometrial ablation is ideally suited for women with AUB related to endometrial factors (AUB-E), two studies from 2014 provide insight into endometrial ablation performed when lesions are present within the PALM classification, such as polyps (AUB-P), adenomyosis (AUB-A), and leiomyomas (AUB-L), and in patients with COEIN conditions, such as ovulatory dysfunction (AUB-O) and AUB-E.
Findings of Wishall and colleagues
Three hundred women who underwent endometrial ablation were evaluated in regard to postoperative pain and the need for subsequent hysterectomy. A total of 270 women were available for follow-up in this retrospective cohort (10% lost to follow-up). Wishall and colleagues set out to identify prognostic factors that would put a woman at risk for post-ablation pain. Their secondary outcome was the rate of hysterectomy after ablation.
The study was limited to second-generation endometrial ablation devices, including the thermal balloon, microwave, circulating hot fluid, and bipolar radiofrequency devices.
Wishall and colleagues found that the risk of failure was the highest (a quadrupling) when uterine abnormalities such as leiomyomas, adenomyosis, a thickened endometrial stripe, or polyps were present (adjusted odds ratio [OR], 3.96; 95% confidence interval [CI], 1.25–12.56).
As in other series, 19% of women ultimately required hysterectomy. Twenty-three percent developed new or worsening pain after ablation. Risk factors for postablation pain included a history of dysmenorrhea (OR, 1.74) and tubal sterilization (OR, 2.06).
Findings of Smithling and colleagues
Investigators evaluated the records of 968 women with AUB who had undergone endometrial ablation, categorizing their preoperative bleeding patterns as either regular (presumed AUB-E) or irregular (presumed AUB-O). Of these women, 961 (99.3%) had undergone radiofrequency bipolar endometrial ablation.
Smithling and colleagues hypothesized that women with AUB-O would have a higher failure rate—defined as the need for reablation or subsequent hysterectomy—than women with AUB-E because endometrial ablation does not necessarily address the pathology that underlies AUB-O. However, they found no difference in treatment failure or the need for a subsequent gynecologic procedure between groups during the 3-year period after endometrial ablation. The rate of treatment failure was 16.4% in women with regular bleeding—essentially the same as the rate for women with irregular bleeding (17.6%; P = .7) Risk factors associated with failure included:
- tubal sterilization (16.4% vs 9.0% for women without it)
- pelvic pain or dysmenorrhea (21.8% vs 10.7% for women without it)
- obesity (16.7% vs 9.8%) (P = .003).
Although there was no difference in failure rates between the group with regular bleeding versus the group with irregular bleeding, Smithling and colleagues were careful to avoid interpreting this finding as a recommendation for endometrial ablation in women with AUB-O.
What this EVIDENCE means for practice
When endometrial ablation is performed to treat lesions such as polyps, adenomyosis, and leiomyomata, women are nearly four times more likely to require subsequent hysterectomy.
A history of dysmenorrhea yielded a 74% higher risk of developing postablation pain, and a history of tubal sterilization more than doubled the risk, compared with no history of dysmenorrhea or tubal sterilization.
Women who undergo endometrial ablation for presumed AUB-O and presumed AUB-E have similar failure rates.
Preoperative factors such as dysmenorrhea, prior tubal sterilization, and obesity were identified as risk factors for ablation failure.
The choice between endometrial ablation and hysterectomy for patients with AUB-O depends on an individualized assessment of risks and benefits, including evaluation of medical comorbidities.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Surgery involves a certain degree of risk. The risk could be in the form of a complication, or it could be surgical failure. When I was a resident, gynecologic oncologist Gary Johnson, MD, used to say: “If you don’t want complications, don’t do surgery.” I was never sure whether he was trying to make me feel better when complications occurred, or was just stating the facts. Maybe both. It could be argued that one should consider offering medical options before exposing a patient to surgical risks.
In this article, I review three recent studies that shed some light on patient selection for surgical intervention—more specifically, on surgical and counseling failures associated with surgical management of abnormal uterine bleeding (AUB):
- an analysis of perioperative hysterectomy data from 52 hospitals in Michigan that showed that alternatives to hysterectomy were underutilized in women with AUB, fibroids, or pelvic pain
- a retrospective cohort study of 300 patients from two large academic medical centers, which explored risk factors for postablation pain
- another retrospective cohort study of 968 women who underwent endometrial ablation. This study was designed to highlight any association between preoperative bleeding patterns and the risk of ablation failure.
Don’t resort to surgery until alternative treatments have been exhausted
Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative [published online ahead of print December 23, 2014]. Am J Obstet Gynecol. pii: S0002-9378(14)02355-2. doi: 10.1016/j.ajog.2014.11.031.
In this analysis, Corona and colleagues evaluated the use of alternative treatments among women who underwent hysterectomy for uterine fibroids, AUB, endometriosis, or pelvic pain at 52 hospitals participating in the Michigan Surgical Quality Collaborative in 2013. They also determined whether the pathology was “supportive” or “unsupportive” of the surgical indication.
A significant percentage of hysterectomies were performed with an indication of AUB or fibroids, or both (49.1%). A combination of pain, AUB, and/or fibroids was the indication in 48.1% of hysterectomies, and endometriosis and/or pain was listed in 9.2%.
In 37.7% of cases (n = 1,281), no offering of alternative treatments was documented.
Although endometrial ablation was offered to 44.1% of women younger than age 40, to 48.3% of women aged 40 to 50 years, and to 31.6% of women older than age 50, the conservative, nonsurgical option of a levonorgestrel intrauterine system (LNG-IUS) was offered in only 12.7%, 12.4%, and 9.3% of these cases, respectively.
Overall, the rate of unsupportive pathology was 18.3% (n = 621). That rate was higher in women younger than age 40, compared with those aged 40 to 50 and older than age 50 (37.8% vs 12.0% and 7.5%, respectively; P<.001).
These data suggest that a significant number of women with AUB associated with ovulatory dysfunction (AUB-O) undergo hysterectomy. The authors point out that the American College of Obstetricians and Gynecologists (ACOG) recommends medical therapy as a first-line therapy for AUB-O rather than surgical therapy.
What this EVIDENCE means for practice
Although AUB is a common indication for hysterectomy, conservative alternative therapies should be offered when appropriate. A particularly cost-effective and effective conservative therapy—the LNG-IUS—is underutilized and should be considered more often.
How to determine who is most likely to benefit from endometrial ablation
Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5):904–910.
Smithling KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211(5):556.e1–e6.
Endometrial ablation has been around a long time—likely since the 1930s. However, it was not until the 1980s that operative hysteroscopy and endometrial ablation became commonplace. As a result of new, more “automated” technology, five nonresectoscopic endometrial ablation techniques were introduced, starting with FDA approval of the thermal balloon device in 1997.
Initially, information on the feasibility of endometrial ablation was presented in the form of case reports. Efficacy and safety were studied through FDA trials, which yielded variable amenorrhea rates but relatively high satisfaction rates in the range of 85% to 95%. In the interim, we have learned more refined details about endometrial ablation as case reports of unintended consequences have cropped up and as this technology has reached a broader physician base. After almost two decades of experience with nonresectoscopic endometrial ablation devices, information on “failure”—ie, the need for additional treatment—is surfacing.
Over the past year, as we have increased adoption of the PALM-COEIN classification system for the causes of AUB in women of reproductive age, we also have gleaned more information about how endometrial ablation works in this context. In general, PALM (polyp, adenomyosis, leiomyoma, and malignancy/hyperplasia) represents lesions that can be seen but may not necessarily be the cause of bleeding. COEIN (coagulopathy, ovulatory dysfunction, endometrial factors, iatrogenic, and not yet classified) represents causes of bleeding that may not be visible.
Although endometrial ablation is ideally suited for women with AUB related to endometrial factors (AUB-E), two studies from 2014 provide insight into endometrial ablation performed when lesions are present within the PALM classification, such as polyps (AUB-P), adenomyosis (AUB-A), and leiomyomas (AUB-L), and in patients with COEIN conditions, such as ovulatory dysfunction (AUB-O) and AUB-E.
Findings of Wishall and colleagues
Three hundred women who underwent endometrial ablation were evaluated in regard to postoperative pain and the need for subsequent hysterectomy. A total of 270 women were available for follow-up in this retrospective cohort (10% lost to follow-up). Wishall and colleagues set out to identify prognostic factors that would put a woman at risk for post-ablation pain. Their secondary outcome was the rate of hysterectomy after ablation.
The study was limited to second-generation endometrial ablation devices, including the thermal balloon, microwave, circulating hot fluid, and bipolar radiofrequency devices.
Wishall and colleagues found that the risk of failure was the highest (a quadrupling) when uterine abnormalities such as leiomyomas, adenomyosis, a thickened endometrial stripe, or polyps were present (adjusted odds ratio [OR], 3.96; 95% confidence interval [CI], 1.25–12.56).
As in other series, 19% of women ultimately required hysterectomy. Twenty-three percent developed new or worsening pain after ablation. Risk factors for postablation pain included a history of dysmenorrhea (OR, 1.74) and tubal sterilization (OR, 2.06).
Findings of Smithling and colleagues
Investigators evaluated the records of 968 women with AUB who had undergone endometrial ablation, categorizing their preoperative bleeding patterns as either regular (presumed AUB-E) or irregular (presumed AUB-O). Of these women, 961 (99.3%) had undergone radiofrequency bipolar endometrial ablation.
Smithling and colleagues hypothesized that women with AUB-O would have a higher failure rate—defined as the need for reablation or subsequent hysterectomy—than women with AUB-E because endometrial ablation does not necessarily address the pathology that underlies AUB-O. However, they found no difference in treatment failure or the need for a subsequent gynecologic procedure between groups during the 3-year period after endometrial ablation. The rate of treatment failure was 16.4% in women with regular bleeding—essentially the same as the rate for women with irregular bleeding (17.6%; P = .7) Risk factors associated with failure included:
- tubal sterilization (16.4% vs 9.0% for women without it)
- pelvic pain or dysmenorrhea (21.8% vs 10.7% for women without it)
- obesity (16.7% vs 9.8%) (P = .003).
Although there was no difference in failure rates between the group with regular bleeding versus the group with irregular bleeding, Smithling and colleagues were careful to avoid interpreting this finding as a recommendation for endometrial ablation in women with AUB-O.
What this EVIDENCE means for practice
When endometrial ablation is performed to treat lesions such as polyps, adenomyosis, and leiomyomata, women are nearly four times more likely to require subsequent hysterectomy.
A history of dysmenorrhea yielded a 74% higher risk of developing postablation pain, and a history of tubal sterilization more than doubled the risk, compared with no history of dysmenorrhea or tubal sterilization.
Women who undergo endometrial ablation for presumed AUB-O and presumed AUB-E have similar failure rates.
Preoperative factors such as dysmenorrhea, prior tubal sterilization, and obesity were identified as risk factors for ablation failure.
The choice between endometrial ablation and hysterectomy for patients with AUB-O depends on an individualized assessment of risks and benefits, including evaluation of medical comorbidities.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
IN THIS ARTICLE
– When to offer an alternative treatment to patients considering surgery for AUB
– Who is most likely to benefit from endometrial ablation?