User login
Patient Knowledge of Hospital Medication
Inpatient medication errors represent an important patient safety issue. The magnitude of the problem is staggering, with 1 review finding almost 1 in every 5 medication doses in error, with 7% having potential for adverse drug events.1 While mistakes made at the ordering stage are frequently intercepted by pharmacist or nursing review, administration errors are particularly difficult to prevent.2 The patient, as the last link in the medication administration chain, represents the final individual capable of preventing an incorrect medication administration. It is perhaps surprising then that patients generally lack a formal role in detecting and preventing adverse medication administration events.3
There have been some ambitious attempts to improve patient education regarding hospital medications and involve selected patients in the medication administration process. Such initiatives may result in increased patient participation and satisfaction.47 There is also potential that increased patient knowledge of their hospital medications could promote the goal of medication safety, as the actively involved patient may be able to catch medication errors in the hospital.
Knowledge of prescribed medications is a prerequisite to patient involvement in prevention of inpatient medication errors and yet there is little research on patient knowledge of their hospital medications. Furthermore, as the experience of hospitalization may be disorienting and disempowering for patients, it remains to be seen if patient attitudes toward participation in inpatient medication safety are favorable. To that end, we conducted a pilot study in which we assessed current patient awareness of their in‐hospital medications and surveyed attitudes toward increased patient knowledge of hospital medications.
PATIENTS AND METHODS
We conducted a cross‐sectional study of 50 cognitively intact adult internal medicine inpatients at the University of Colorado Hospital, a tertiary‐care academic teaching hospital. This study was part of a larger project designed to examine potential for patient involvement in the medication reconciliation process. A professional research assistant approached eligible patients within 24 hours of admission. To be eligible, patients had to self‐identify as knowing their outpatient medications, speak English, and have been admitted from the community. Nursing home residents and patients with a past medical history of dementia were excluded. Enrollment was tracked during the first half of the study to estimate effect of inclusion/exclusion criteria. Thirty‐eight percent of hospital admissions to medicine services were excluded based on the specified criteria. Thirty‐four percent of eligible patients were approached and 50% of approached patients agreed to participate in the study. Patient knowledge of their outpatient medication regimen was compared to admitting physician medication reconciliation to assess accuracy of patient self‐report of outpatient medication knowledge.
After consenting to participate, study patients completed a structured list of their outpatient medications and a survey of attitudes about being shown their in‐hospital medications, hospital medication errors, and patient involvement in hospital safety. They then completed a list of the medications they believed to be prescribed to them in the hospital.
The primary outcomes were the proportions of as needed (PRN), scheduled, and total hospital medications omitted by the patient, compared to the inpatient medication administration record (MAR) (patient errors of omission). Secondary outcomes included the number of in‐hospital medications listed by the patient that did not appear on the inpatient MAR (patient errors of commission), as well as patient attitudes measured on a 5‐point Likert scale (1 indicated strongly disagree and 5 indicated strongly agree.) Descriptive data included age, race, gender, and number of inpatient medications prescribed. Separate analysis of variance (ANOVA) models provided mean estimates of the primary outcomes and tested differences according to each of the patient characteristics: age in years (65 or 65), self‐reported knowledge of hospital medications, and self‐reported desire to be involved in medication safety. Similar ANOVA models adjusted for number of medications were also examined to determine whether the relationship between the primary outcomes according to patient characteristics were altered by the number of medications. The protocol was approved by the Colorado Multiple Institutional Review Board.
RESULTS
Participants averaged 54 years of age (standard deviation [SD] = 17, range = 21‐89). Forty‐six percent (23/50) were male, and 74% (37/50) were non‐Hispanic white. Using a structured, patient‐completed, outpatient medication list, patients in the study were on an average of 5.3 outpatient prescription medications (range = 0‐17), 2.2 over‐the‐counter medications (range = 0‐8), and 0.2 herbal medications (range = 0‐7). The admitting physician's medication reconciliation list demonstrated similar number of outpatient prescription medications (average = 5.7) to the patient‐generated list. Fifty‐four percent of patient‐completed home medication lists included all of the prescription medications on the physician's medication reconciliation at admission. According to the inpatient MAR, study patients were prescribed an average of 11.3 scheduled and PRN hospital medications (range = 2‐26) at time of study enrollment.
Patient Knowledge of Their Hospital Medication List
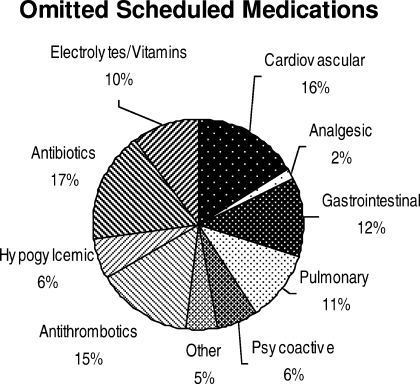
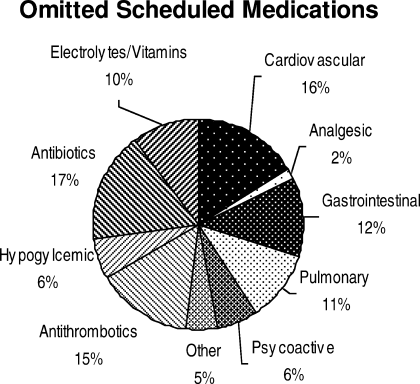
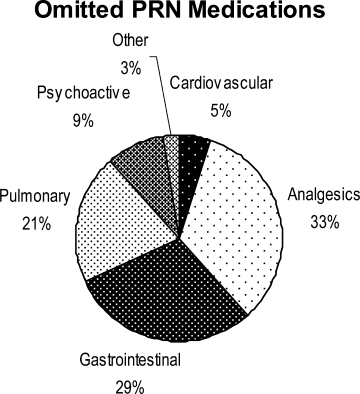
Ninety‐six percent (48/50) of study patients omitted 1 or more of their hospital medications. On average, patients omitted 6.8 medications (range = 0‐22) (Table 1). Among scheduled medications, patients most commonly omitted antibiotics (17%), cardiovascular medications (16%), and antithrombotics (15%) (Figure 1). Among PRN medications, patients most commonly omitted analgesics (33%) and gastrointestinal medications (29%) (Figure 2).
| Total Medications | Scheduled Medications | PRN Medications | |
|---|---|---|---|
| |||
| Percent of patients with at least 1 hospital medication they could not name (95% CI) | 96% (90‐100%) | 94% (87‐100%) | 80% (69‐92%) |
| Average number of hospital medications omitted by patient (range) | 6.8 (0‐22) | 5.2 (0‐15) | 1.6 (0‐7) |
| Percentage of hospital medications omitted by patient (95% CI) | 60% (52‐67%) | 60% (52‐67%) | 68% (57‐78%) |
Patients less than 65 years omitted 60% of their PRN medications whereas patients greater than 65 years omitted 88% (P = 0.01). This difference remained even after adjustment for number of medications. There were no significant differences, based on age, in ability to name scheduled or total medications. Forty‐four percent of patients (22/50) believed they were receiving a medication in the hospital that was not actually prescribed.
Patient Attitudes Toward Increased Knowledge of Hospital Medications
Only 28% (14/50) of patients reported having seen their hospital medication list, although 78% (39/50) favored being given such a list, and 81% (39/48) reported that this would improve their satisfaction with care. Ninety percent (45/50) wanted to review their hospital medication list for accuracy and 94% (47/50) felt patient participation in reviewing hospital medications had potential to reduce errors. No associations were found between self‐reported knowledge of hospital medications or self‐reported desire to be involved in medication safety and the proportion of PRN, scheduled, or total medications omitted.
DISCUSSION
Overall, patients in the study were able to name fewer than one‐half of their hospital medications. Our study suggests that adult medicine inpatients believe learning about their hospital medications would increase their satisfaction and has potential to promote medication safety. At the same time, patients did not know many of their hospital medications and this would limit their ability to fully participate in the medication safety process. Study patients frequently committed both errors of omission (ie, they did not know which medications were prescribed), and errors of commission (ie, they believed they were prescribed medications that were not prescribed). Younger patients were aware of more of their PRN medications than older patients, potentially reflecting greater patient care involvement in younger generations. However, study patients, regardless of age, were able to name fewer than one‐half of their PRN hospital medications. The most common scheduled hospital medications that patients were unable to name come from medication classes which can be associated with significant adverse events, including antibiotics, cardiovascular medications, and antithrombotics.
We posit that without systematically educating patients about their hospital medications, significant deficits in patient knowledge are inevitable. Some might argue that patients should not be asked to know their hospital medications or identify medication errors while sick and vulnerable. Certainly with multiple medication changes, formulary substitutions, and frequent modifications based on changes in clinical status, inpatient medication education could be time consuming and potentially introduce patient confusion or anxiety. Incorrect patient feedback could have potential to introduce new errors. An educational program might use graded participation based on patient interest and ability. Models for this exist in the literature, even extending to patient medication self‐administration.57 In our sample of inpatients, the majority desired a more active role in learning about their hospital medications and believed that their involvement might prevent hospital medication errors from occurring.
Medication literacy, education, and active patient involvement in medication monitoring as a means to improve patient outcomes has received significant attention in the outpatient setting, with lessons applicable to the hospital.8, 9 More broadly, the Joint Commission has established a Hospital National Patient Safety Goal to encourage patients' active involvement in their own care as a patient safety strategy.10 Examples set forth by the Joint Commission include involving patients in infection control measures, marking of procedural sites, and reporting of safety concerns relating to treatment.
While this study identifies patient knowledge deficit as a barrier to utilizing patients as part of the hospital medication safety process, it does not test whether reducing this knowledge deficit would actually reduce medication error. Our study population was limited to cognitively intact adult medicine patients at a single institution, limiting the generalizability of our conclusions. Our enrollment process may have resulted in a study population with less serious illness, greater knowledge of their hospital medications, and greater interest in participating in medication safety potentially overestimating patient knowledge of hospital medications. Finally, our small sample size limits the power to find differences in study comparisons.
Our findings are striking in that we found significant deficits in patient understanding of their hospital medications even among patients who believed they knew, or desired to know, what is being prescribed to them in the hospital. Without a system to incorporate the patient into hospital medication management, these patients will be disenfranchised from participating in inpatient medication safety. These results are a call to reexamine how we educate and involve patients regarding hospital medications. Mechanisms to allow patients to provide feedback to the medical team on their hospital medications might identify errors or improve patient satisfaction with their care. However, the systems and cultural changes needed to provide education on inpatient medications are considerable. Future research is needed to determine if increasing patient knowledge regarding their hospital medications would reduce medication errors in the inpatient setting and how this could be effectively implemented.
Acknowledgements
The authors thank Sue Felton, MA, Professional Research Assistant, for enrolling patients in this trial, and Traci Yamashita, MS, Professional Research Assistant, for statistical analysis.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA.1995;274:29–34.
- ,.Patient Safety: what about the patient?Qual Saf Health Care.2002;11:76–80.
- ,,, et al.Pharmacist involvement in a multidisciplinary inpatient medication education program.Am J Health Syst Pharm.2003;60:1012–1018.
- ,,,.Self‐administration of medication by patients and family members during hospitalization.Patient Educ Couns.1996;27:103–112.
- ,,,.Hospital inpatient self‐administration of medicine programmes: a critical literature review.Pharm World Sci.2006;28:140–151.
- ,,,.Self‐administration of medication in hospital: patients' perspectives.J Adv Nurs.2004;46:194–203.
- ,.Outpatient drug safety: new steps in an old direction.Pharmacoepidemiol Drug Saf.2007;16:160–165.
- ,,.Impact of health literacy on health outcomes in ambulatory care patients: a systematic review.Phamacosociology.2008;42:1272–1281.
- Joint Commission.2009. Standards Improvement Initiative. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed June 2009.
Inpatient medication errors represent an important patient safety issue. The magnitude of the problem is staggering, with 1 review finding almost 1 in every 5 medication doses in error, with 7% having potential for adverse drug events.1 While mistakes made at the ordering stage are frequently intercepted by pharmacist or nursing review, administration errors are particularly difficult to prevent.2 The patient, as the last link in the medication administration chain, represents the final individual capable of preventing an incorrect medication administration. It is perhaps surprising then that patients generally lack a formal role in detecting and preventing adverse medication administration events.3
There have been some ambitious attempts to improve patient education regarding hospital medications and involve selected patients in the medication administration process. Such initiatives may result in increased patient participation and satisfaction.47 There is also potential that increased patient knowledge of their hospital medications could promote the goal of medication safety, as the actively involved patient may be able to catch medication errors in the hospital.
Knowledge of prescribed medications is a prerequisite to patient involvement in prevention of inpatient medication errors and yet there is little research on patient knowledge of their hospital medications. Furthermore, as the experience of hospitalization may be disorienting and disempowering for patients, it remains to be seen if patient attitudes toward participation in inpatient medication safety are favorable. To that end, we conducted a pilot study in which we assessed current patient awareness of their in‐hospital medications and surveyed attitudes toward increased patient knowledge of hospital medications.
PATIENTS AND METHODS
We conducted a cross‐sectional study of 50 cognitively intact adult internal medicine inpatients at the University of Colorado Hospital, a tertiary‐care academic teaching hospital. This study was part of a larger project designed to examine potential for patient involvement in the medication reconciliation process. A professional research assistant approached eligible patients within 24 hours of admission. To be eligible, patients had to self‐identify as knowing their outpatient medications, speak English, and have been admitted from the community. Nursing home residents and patients with a past medical history of dementia were excluded. Enrollment was tracked during the first half of the study to estimate effect of inclusion/exclusion criteria. Thirty‐eight percent of hospital admissions to medicine services were excluded based on the specified criteria. Thirty‐four percent of eligible patients were approached and 50% of approached patients agreed to participate in the study. Patient knowledge of their outpatient medication regimen was compared to admitting physician medication reconciliation to assess accuracy of patient self‐report of outpatient medication knowledge.
After consenting to participate, study patients completed a structured list of their outpatient medications and a survey of attitudes about being shown their in‐hospital medications, hospital medication errors, and patient involvement in hospital safety. They then completed a list of the medications they believed to be prescribed to them in the hospital.
The primary outcomes were the proportions of as needed (PRN), scheduled, and total hospital medications omitted by the patient, compared to the inpatient medication administration record (MAR) (patient errors of omission). Secondary outcomes included the number of in‐hospital medications listed by the patient that did not appear on the inpatient MAR (patient errors of commission), as well as patient attitudes measured on a 5‐point Likert scale (1 indicated strongly disagree and 5 indicated strongly agree.) Descriptive data included age, race, gender, and number of inpatient medications prescribed. Separate analysis of variance (ANOVA) models provided mean estimates of the primary outcomes and tested differences according to each of the patient characteristics: age in years (65 or 65), self‐reported knowledge of hospital medications, and self‐reported desire to be involved in medication safety. Similar ANOVA models adjusted for number of medications were also examined to determine whether the relationship between the primary outcomes according to patient characteristics were altered by the number of medications. The protocol was approved by the Colorado Multiple Institutional Review Board.
RESULTS
Participants averaged 54 years of age (standard deviation [SD] = 17, range = 21‐89). Forty‐six percent (23/50) were male, and 74% (37/50) were non‐Hispanic white. Using a structured, patient‐completed, outpatient medication list, patients in the study were on an average of 5.3 outpatient prescription medications (range = 0‐17), 2.2 over‐the‐counter medications (range = 0‐8), and 0.2 herbal medications (range = 0‐7). The admitting physician's medication reconciliation list demonstrated similar number of outpatient prescription medications (average = 5.7) to the patient‐generated list. Fifty‐four percent of patient‐completed home medication lists included all of the prescription medications on the physician's medication reconciliation at admission. According to the inpatient MAR, study patients were prescribed an average of 11.3 scheduled and PRN hospital medications (range = 2‐26) at time of study enrollment.
Patient Knowledge of Their Hospital Medication List
Ninety‐six percent (48/50) of study patients omitted 1 or more of their hospital medications. On average, patients omitted 6.8 medications (range = 0‐22) (Table 1). Among scheduled medications, patients most commonly omitted antibiotics (17%), cardiovascular medications (16%), and antithrombotics (15%) (Figure 1). Among PRN medications, patients most commonly omitted analgesics (33%) and gastrointestinal medications (29%) (Figure 2).
| Total Medications | Scheduled Medications | PRN Medications | |
|---|---|---|---|
| |||
| Percent of patients with at least 1 hospital medication they could not name (95% CI) | 96% (90‐100%) | 94% (87‐100%) | 80% (69‐92%) |
| Average number of hospital medications omitted by patient (range) | 6.8 (0‐22) | 5.2 (0‐15) | 1.6 (0‐7) |
| Percentage of hospital medications omitted by patient (95% CI) | 60% (52‐67%) | 60% (52‐67%) | 68% (57‐78%) |


Patients less than 65 years omitted 60% of their PRN medications whereas patients greater than 65 years omitted 88% (P = 0.01). This difference remained even after adjustment for number of medications. There were no significant differences, based on age, in ability to name scheduled or total medications. Forty‐four percent of patients (22/50) believed they were receiving a medication in the hospital that was not actually prescribed.
Patient Attitudes Toward Increased Knowledge of Hospital Medications
Only 28% (14/50) of patients reported having seen their hospital medication list, although 78% (39/50) favored being given such a list, and 81% (39/48) reported that this would improve their satisfaction with care. Ninety percent (45/50) wanted to review their hospital medication list for accuracy and 94% (47/50) felt patient participation in reviewing hospital medications had potential to reduce errors. No associations were found between self‐reported knowledge of hospital medications or self‐reported desire to be involved in medication safety and the proportion of PRN, scheduled, or total medications omitted.
DISCUSSION
Overall, patients in the study were able to name fewer than one‐half of their hospital medications. Our study suggests that adult medicine inpatients believe learning about their hospital medications would increase their satisfaction and has potential to promote medication safety. At the same time, patients did not know many of their hospital medications and this would limit their ability to fully participate in the medication safety process. Study patients frequently committed both errors of omission (ie, they did not know which medications were prescribed), and errors of commission (ie, they believed they were prescribed medications that were not prescribed). Younger patients were aware of more of their PRN medications than older patients, potentially reflecting greater patient care involvement in younger generations. However, study patients, regardless of age, were able to name fewer than one‐half of their PRN hospital medications. The most common scheduled hospital medications that patients were unable to name come from medication classes which can be associated with significant adverse events, including antibiotics, cardiovascular medications, and antithrombotics.
We posit that without systematically educating patients about their hospital medications, significant deficits in patient knowledge are inevitable. Some might argue that patients should not be asked to know their hospital medications or identify medication errors while sick and vulnerable. Certainly with multiple medication changes, formulary substitutions, and frequent modifications based on changes in clinical status, inpatient medication education could be time consuming and potentially introduce patient confusion or anxiety. Incorrect patient feedback could have potential to introduce new errors. An educational program might use graded participation based on patient interest and ability. Models for this exist in the literature, even extending to patient medication self‐administration.57 In our sample of inpatients, the majority desired a more active role in learning about their hospital medications and believed that their involvement might prevent hospital medication errors from occurring.
Medication literacy, education, and active patient involvement in medication monitoring as a means to improve patient outcomes has received significant attention in the outpatient setting, with lessons applicable to the hospital.8, 9 More broadly, the Joint Commission has established a Hospital National Patient Safety Goal to encourage patients' active involvement in their own care as a patient safety strategy.10 Examples set forth by the Joint Commission include involving patients in infection control measures, marking of procedural sites, and reporting of safety concerns relating to treatment.
While this study identifies patient knowledge deficit as a barrier to utilizing patients as part of the hospital medication safety process, it does not test whether reducing this knowledge deficit would actually reduce medication error. Our study population was limited to cognitively intact adult medicine patients at a single institution, limiting the generalizability of our conclusions. Our enrollment process may have resulted in a study population with less serious illness, greater knowledge of their hospital medications, and greater interest in participating in medication safety potentially overestimating patient knowledge of hospital medications. Finally, our small sample size limits the power to find differences in study comparisons.
Our findings are striking in that we found significant deficits in patient understanding of their hospital medications even among patients who believed they knew, or desired to know, what is being prescribed to them in the hospital. Without a system to incorporate the patient into hospital medication management, these patients will be disenfranchised from participating in inpatient medication safety. These results are a call to reexamine how we educate and involve patients regarding hospital medications. Mechanisms to allow patients to provide feedback to the medical team on their hospital medications might identify errors or improve patient satisfaction with their care. However, the systems and cultural changes needed to provide education on inpatient medications are considerable. Future research is needed to determine if increasing patient knowledge regarding their hospital medications would reduce medication errors in the inpatient setting and how this could be effectively implemented.
Acknowledgements
The authors thank Sue Felton, MA, Professional Research Assistant, for enrolling patients in this trial, and Traci Yamashita, MS, Professional Research Assistant, for statistical analysis.
Inpatient medication errors represent an important patient safety issue. The magnitude of the problem is staggering, with 1 review finding almost 1 in every 5 medication doses in error, with 7% having potential for adverse drug events.1 While mistakes made at the ordering stage are frequently intercepted by pharmacist or nursing review, administration errors are particularly difficult to prevent.2 The patient, as the last link in the medication administration chain, represents the final individual capable of preventing an incorrect medication administration. It is perhaps surprising then that patients generally lack a formal role in detecting and preventing adverse medication administration events.3
There have been some ambitious attempts to improve patient education regarding hospital medications and involve selected patients in the medication administration process. Such initiatives may result in increased patient participation and satisfaction.47 There is also potential that increased patient knowledge of their hospital medications could promote the goal of medication safety, as the actively involved patient may be able to catch medication errors in the hospital.
Knowledge of prescribed medications is a prerequisite to patient involvement in prevention of inpatient medication errors and yet there is little research on patient knowledge of their hospital medications. Furthermore, as the experience of hospitalization may be disorienting and disempowering for patients, it remains to be seen if patient attitudes toward participation in inpatient medication safety are favorable. To that end, we conducted a pilot study in which we assessed current patient awareness of their in‐hospital medications and surveyed attitudes toward increased patient knowledge of hospital medications.
PATIENTS AND METHODS
We conducted a cross‐sectional study of 50 cognitively intact adult internal medicine inpatients at the University of Colorado Hospital, a tertiary‐care academic teaching hospital. This study was part of a larger project designed to examine potential for patient involvement in the medication reconciliation process. A professional research assistant approached eligible patients within 24 hours of admission. To be eligible, patients had to self‐identify as knowing their outpatient medications, speak English, and have been admitted from the community. Nursing home residents and patients with a past medical history of dementia were excluded. Enrollment was tracked during the first half of the study to estimate effect of inclusion/exclusion criteria. Thirty‐eight percent of hospital admissions to medicine services were excluded based on the specified criteria. Thirty‐four percent of eligible patients were approached and 50% of approached patients agreed to participate in the study. Patient knowledge of their outpatient medication regimen was compared to admitting physician medication reconciliation to assess accuracy of patient self‐report of outpatient medication knowledge.
After consenting to participate, study patients completed a structured list of their outpatient medications and a survey of attitudes about being shown their in‐hospital medications, hospital medication errors, and patient involvement in hospital safety. They then completed a list of the medications they believed to be prescribed to them in the hospital.
The primary outcomes were the proportions of as needed (PRN), scheduled, and total hospital medications omitted by the patient, compared to the inpatient medication administration record (MAR) (patient errors of omission). Secondary outcomes included the number of in‐hospital medications listed by the patient that did not appear on the inpatient MAR (patient errors of commission), as well as patient attitudes measured on a 5‐point Likert scale (1 indicated strongly disagree and 5 indicated strongly agree.) Descriptive data included age, race, gender, and number of inpatient medications prescribed. Separate analysis of variance (ANOVA) models provided mean estimates of the primary outcomes and tested differences according to each of the patient characteristics: age in years (65 or 65), self‐reported knowledge of hospital medications, and self‐reported desire to be involved in medication safety. Similar ANOVA models adjusted for number of medications were also examined to determine whether the relationship between the primary outcomes according to patient characteristics were altered by the number of medications. The protocol was approved by the Colorado Multiple Institutional Review Board.
RESULTS
Participants averaged 54 years of age (standard deviation [SD] = 17, range = 21‐89). Forty‐six percent (23/50) were male, and 74% (37/50) were non‐Hispanic white. Using a structured, patient‐completed, outpatient medication list, patients in the study were on an average of 5.3 outpatient prescription medications (range = 0‐17), 2.2 over‐the‐counter medications (range = 0‐8), and 0.2 herbal medications (range = 0‐7). The admitting physician's medication reconciliation list demonstrated similar number of outpatient prescription medications (average = 5.7) to the patient‐generated list. Fifty‐four percent of patient‐completed home medication lists included all of the prescription medications on the physician's medication reconciliation at admission. According to the inpatient MAR, study patients were prescribed an average of 11.3 scheduled and PRN hospital medications (range = 2‐26) at time of study enrollment.
Patient Knowledge of Their Hospital Medication List
Ninety‐six percent (48/50) of study patients omitted 1 or more of their hospital medications. On average, patients omitted 6.8 medications (range = 0‐22) (Table 1). Among scheduled medications, patients most commonly omitted antibiotics (17%), cardiovascular medications (16%), and antithrombotics (15%) (Figure 1). Among PRN medications, patients most commonly omitted analgesics (33%) and gastrointestinal medications (29%) (Figure 2).
| Total Medications | Scheduled Medications | PRN Medications | |
|---|---|---|---|
| |||
| Percent of patients with at least 1 hospital medication they could not name (95% CI) | 96% (90‐100%) | 94% (87‐100%) | 80% (69‐92%) |
| Average number of hospital medications omitted by patient (range) | 6.8 (0‐22) | 5.2 (0‐15) | 1.6 (0‐7) |
| Percentage of hospital medications omitted by patient (95% CI) | 60% (52‐67%) | 60% (52‐67%) | 68% (57‐78%) |


Patients less than 65 years omitted 60% of their PRN medications whereas patients greater than 65 years omitted 88% (P = 0.01). This difference remained even after adjustment for number of medications. There were no significant differences, based on age, in ability to name scheduled or total medications. Forty‐four percent of patients (22/50) believed they were receiving a medication in the hospital that was not actually prescribed.
Patient Attitudes Toward Increased Knowledge of Hospital Medications
Only 28% (14/50) of patients reported having seen their hospital medication list, although 78% (39/50) favored being given such a list, and 81% (39/48) reported that this would improve their satisfaction with care. Ninety percent (45/50) wanted to review their hospital medication list for accuracy and 94% (47/50) felt patient participation in reviewing hospital medications had potential to reduce errors. No associations were found between self‐reported knowledge of hospital medications or self‐reported desire to be involved in medication safety and the proportion of PRN, scheduled, or total medications omitted.
DISCUSSION
Overall, patients in the study were able to name fewer than one‐half of their hospital medications. Our study suggests that adult medicine inpatients believe learning about their hospital medications would increase their satisfaction and has potential to promote medication safety. At the same time, patients did not know many of their hospital medications and this would limit their ability to fully participate in the medication safety process. Study patients frequently committed both errors of omission (ie, they did not know which medications were prescribed), and errors of commission (ie, they believed they were prescribed medications that were not prescribed). Younger patients were aware of more of their PRN medications than older patients, potentially reflecting greater patient care involvement in younger generations. However, study patients, regardless of age, were able to name fewer than one‐half of their PRN hospital medications. The most common scheduled hospital medications that patients were unable to name come from medication classes which can be associated with significant adverse events, including antibiotics, cardiovascular medications, and antithrombotics.
We posit that without systematically educating patients about their hospital medications, significant deficits in patient knowledge are inevitable. Some might argue that patients should not be asked to know their hospital medications or identify medication errors while sick and vulnerable. Certainly with multiple medication changes, formulary substitutions, and frequent modifications based on changes in clinical status, inpatient medication education could be time consuming and potentially introduce patient confusion or anxiety. Incorrect patient feedback could have potential to introduce new errors. An educational program might use graded participation based on patient interest and ability. Models for this exist in the literature, even extending to patient medication self‐administration.57 In our sample of inpatients, the majority desired a more active role in learning about their hospital medications and believed that their involvement might prevent hospital medication errors from occurring.
Medication literacy, education, and active patient involvement in medication monitoring as a means to improve patient outcomes has received significant attention in the outpatient setting, with lessons applicable to the hospital.8, 9 More broadly, the Joint Commission has established a Hospital National Patient Safety Goal to encourage patients' active involvement in their own care as a patient safety strategy.10 Examples set forth by the Joint Commission include involving patients in infection control measures, marking of procedural sites, and reporting of safety concerns relating to treatment.
While this study identifies patient knowledge deficit as a barrier to utilizing patients as part of the hospital medication safety process, it does not test whether reducing this knowledge deficit would actually reduce medication error. Our study population was limited to cognitively intact adult medicine patients at a single institution, limiting the generalizability of our conclusions. Our enrollment process may have resulted in a study population with less serious illness, greater knowledge of their hospital medications, and greater interest in participating in medication safety potentially overestimating patient knowledge of hospital medications. Finally, our small sample size limits the power to find differences in study comparisons.
Our findings are striking in that we found significant deficits in patient understanding of their hospital medications even among patients who believed they knew, or desired to know, what is being prescribed to them in the hospital. Without a system to incorporate the patient into hospital medication management, these patients will be disenfranchised from participating in inpatient medication safety. These results are a call to reexamine how we educate and involve patients regarding hospital medications. Mechanisms to allow patients to provide feedback to the medical team on their hospital medications might identify errors or improve patient satisfaction with their care. However, the systems and cultural changes needed to provide education on inpatient medications are considerable. Future research is needed to determine if increasing patient knowledge regarding their hospital medications would reduce medication errors in the inpatient setting and how this could be effectively implemented.
Acknowledgements
The authors thank Sue Felton, MA, Professional Research Assistant, for enrolling patients in this trial, and Traci Yamashita, MS, Professional Research Assistant, for statistical analysis.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA.1995;274:29–34.
- ,.Patient Safety: what about the patient?Qual Saf Health Care.2002;11:76–80.
- ,,, et al.Pharmacist involvement in a multidisciplinary inpatient medication education program.Am J Health Syst Pharm.2003;60:1012–1018.
- ,,,.Self‐administration of medication by patients and family members during hospitalization.Patient Educ Couns.1996;27:103–112.
- ,,,.Hospital inpatient self‐administration of medicine programmes: a critical literature review.Pharm World Sci.2006;28:140–151.
- ,,,.Self‐administration of medication in hospital: patients' perspectives.J Adv Nurs.2004;46:194–203.
- ,.Outpatient drug safety: new steps in an old direction.Pharmacoepidemiol Drug Saf.2007;16:160–165.
- ,,.Impact of health literacy on health outcomes in ambulatory care patients: a systematic review.Phamacosociology.2008;42:1272–1281.
- Joint Commission.2009. Standards Improvement Initiative. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed June 2009.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA.1995;274:29–34.
- ,.Patient Safety: what about the patient?Qual Saf Health Care.2002;11:76–80.
- ,,, et al.Pharmacist involvement in a multidisciplinary inpatient medication education program.Am J Health Syst Pharm.2003;60:1012–1018.
- ,,,.Self‐administration of medication by patients and family members during hospitalization.Patient Educ Couns.1996;27:103–112.
- ,,,.Hospital inpatient self‐administration of medicine programmes: a critical literature review.Pharm World Sci.2006;28:140–151.
- ,,,.Self‐administration of medication in hospital: patients' perspectives.J Adv Nurs.2004;46:194–203.
- ,.Outpatient drug safety: new steps in an old direction.Pharmacoepidemiol Drug Saf.2007;16:160–165.
- ,,.Impact of health literacy on health outcomes in ambulatory care patients: a systematic review.Phamacosociology.2008;42:1272–1281.
- Joint Commission.2009. Standards Improvement Initiative. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed June 2009.
How can we Reduce Indwelling Urinary Catheter Use and Complications?
Case
A 68-year-old male with a history of Alzheimer’s dementia and incontinence presents with failure to thrive. A Foley catheter is placed due to the patient’s incontinence and fall risk. Three days after admission while awaiting placement in a skilled nursing facility (SNF), he develops a urinary tract infection (UTI) complicated by delirium delaying his transfer to the SNF. What could have been done to prevent this complication?
Overview
It has been 50 years since Beeson, et al., recognized the potential harms stemming from urethral catheterization and penned an editorial to the American Journal of Medicine titled “The case against the catheter.”1
Since then, there has been considerable exploration of ways to limit urethral catheterization and ultimately decrease catheter-associated urinary tract infections (CAUTIs). Unfortunately, little progress has been made; indwelling urinary catheters remain ubiquitous in hospitals and CAUTIs remain the most common hospital-acquired infection in the United States.2 Given the emphasis on the quality and costs of healthcare, it is an opportune time to revisit catheter management and use as a way to combat the clinical and economic consequences of CAUTIs.
Clinicians may be lulled into thinking the clinical impact of CAUTI is less than that of other nosocomial infections. However, beyond the obvious patient harm from UTIs, associated bacteremia, and even death, the public health implications of CAUTI cannot be denied. Urinary tract infections constitute 40% of all nosocomial infections; accounting for an estimated 1 million cases annually.3 Further, 80% of all UTIs are associated with indwelling catheter use.
On average, nosocomial UTI necessitates one extra hospital day per patient, or approximately one million excess hospital days per year.4 Pooled cost analysis shows that UTIs consume an additional $400-$1,700 per event, or an estimated $425 million per year in the United States.5,6 Clearly, we cannot wait another 50 years to address this problem.
Review of the Data
Catheter duration as a risk factor for CAUTI: The indwelling catheter creates a portal of entry into a usually sterile body cavity and provides a surface on which microorganisms can colonize. At a finite rate of colonization—the incidence of bacteriuria is 3% to 10% per catheter day—the duration of urinary catheterization becomes the strongest predictor of catheter-associated bacteriuria.7 Even in relatively short-term catheter use of two to 10 days, the pooled cumulative incidence of developing bacteriuria is 26%.
Given the magnitude of these numbers, it should be no surprise that after one month of catheterization, bacteriuria develops in almost all patients. Twenty-four percent of patients with bacteriuria develop symptomatic UTIs with close to 5% suffering bacteremia. Consequently, nosocomial UTIs cause 15% of all hospital-acquired bacteremia.
Optimal catheter management: The easiest and most effective means to prevent CAUTI is to limit the use of urinary catheters to clearly identified medical indications (see Table 1, above). However, as simple as this prevention practice may sound, studies have demonstrated that as many as 20% of patients have indwelling catheters initially placed for unjustified or even unknown medical indications.8 Additionally, continued catheter use is inappropriate in one-third to one-half of all catheter days.9 These data confirm misuse and overuse of indwelling urinary catheters in the hospital setting is common.
In 1981, the Centers for Disease Control and Prevention (CDC) recognized the importance of addressing this situation and published a guideline to aid prevention of CAUTIs.10 The CDC urged the limitation of catheter use to a carefully selected patient population. Furthermore, the report strongly stressed the importance of catheter removal as soon as possible and advised against the use of catheters solely for the convenience of healthcare workers.
Evidence-based techniques for insertion and catheter care also were outlined in the guideline (see Table 2, p. 31). However, these recommendations have been poorly implemented, likely due to the competing priorities of providers and the difficulty operationalizing the guidelines. Additionally, evidence from the intervening 25 years has not yet been incorporated into the guideline, although a revision currently is underway.
Until that revision is complete, the Joanna Briggs Institute guideline published in 2001 addresses some of the same management techniques and incorporates newer evidence.11 Of note, practices that have been discredited due to contradictory evidence include aggressive meatal cleaning, bladder irrigation, and the application of antimicrobial agents in the drainage bag.12
Strategies to reduce unnecessary catheter days: One of the remediable reasons for catheter misuse lies in the fact physicians often are unaware of the presence of an indwelling catheter in their hospitalized patients.
Saint, et al., showed physicians were unaware of catheterization in 28% of their patients and that attending physicians were less conscious of a patient’s catheter status than residents, interns, or medical students.13 Further, the “forgotten” catheters were more likely to be unnecessary than those remembered by the healthcare team.
This information has prompted the use of various computer-based and multidisciplinary feedback protocols to readdress and re-evaluate the need for continued catheterization in a patient. For example, a study at the VAMC Puget Sound demonstrated that having a computerized order protocol for urinary catheters significantly increased the rate of documentation as well as decreased the duration of catheterization by an average of three days.14
Similar interventions to encourage early catheter removal have included daily reminders from nursing staff, allowing a nurse to discontinue catheter use independent of a physician’s order, and feedback in which nursing staff is educated about the incidence of UTI.15-17 All these relatively simple interventions showed significant improvement in the catheter removal rate and incidence of CAUTIs as well as documented cost savings.
Alternatives to indwelling catheters: In addition to efforts to decrease catheter days, alternatives to the indwelling catheters also should be explored. One such alternative method is intermittent catheterization.
Several studies in postoperative patients with hip fractures have demonstrated that the development of UTI is lower with intermittent catheterization when compared with indwelling catheterization.18 Nevertheless, since the risk of bacteriuria is 1% to 3% per episode of catheterization, after a few weeks the majority of patients will have bacteriuria. However, as the bulk of this bacteriuria often is asymptomatic, intermittent catheterization may still be an improvement. This is particularly true in postoperative patients undergoing rehabilitation and those patients only requiring catheterization for a limited number of days.
More recent studies have evaluated the use of bedside bladder ultrasound in an attempt to determine when intermittent catheterization is needed and thereby limit its use compared with standard timed catheterization. Frederickson, et al., demonstrated that this intervention resulted in significantly fewer catheterizations in surgical patients, thus delaying or avoiding the need for catheterization in 81% of the cases.19 Given this drastic improvement, it is no surprise bladder ultrasound use reduced the rates of UTI.20
External condom catheters present another alternative to indwelling catheter use but the outcomes data is conflicting. While the risk of bacteriuria is approximately 12% per month, this rate becomes increasingly higher with frequent manipulation of the condom catheter. 21,22
Two parallel cohort studies in a VA nursing home showed the incidence of symptomatic UTI to be 2.5 times greater in men with an indwelling catheter than those with a condom catheter.23 On the other hand, a cross-sectional Danish study reported higher rates of UTI with external condom catheters than urethral catheters in hospitalized patients.24 Complications from condom catheters include phimosis and local skin maceration, necessitating meticulous care with the use of these devices. Although the data surrounding external catheterization is somewhat contradictory, this device warrants consideration in incontinent males without urinary tract obstruction.
There are several other alternatives to urethral catheterization (see Table 3, p. 31), many of which have excellent face validity even in the absence of rigorous evidence.
Antimicrobial catheters: The development of antimicrobial urinary catheters, including silver-alloy and nitrofurazone-coated catheters, has been greeted with much excitement, however, the jury is still out about their best use. A 2006 systematic literature review reported that in comparison to standard catheters, antimicrobial catheters can delay or even prevent the development of bacteriuria with short-term usage.25
However, not all antimicrobial catheters are equally effective; assorted studies lack data about clinically relevant endpoints such as prevention of symptomatic UTI, bloodstream infection or death.26, 27 In addition, there are no good trials comparing nitrofurazone to silver-alloy catheters. Therefore, the level of excitement surrounding antimicrobial catheters—particularly silver-alloy catheters—must be tempered by the additional costs incurred by their use.
To date, the cost-effectiveness of antimicrobial catheters has not been demonstrated. Although additional research in this topic is still needed, some experts currently recommend the consideration of silver-alloy catheters in patients at the highest risk for developing serious consequences from UTIs.
Efforts to reduce CAUTI: In response to significant public interest in hospital-acquired infections including CAUTI, the federal government and many state governments are beginning to demand change. In August 2007, the Centers for Medicare and Medicaid Services instituted a mandate making hospitals financially responsible for selected preventable hospital-acquired harms, including CAUTIs.28 In addition, beginning with Pennsylvania in 2006, several states have mandated public reporting of hospital-acquired infections.29
Given the available information about CAUTI prevalence, risks, and preventive techniques, it is surprising the majority of hospitals in the United States have not taken appropriate measures to limit indwelling catheter use. A recent study by Saint, et al., demonstrated the startling fact that only a minority of hospitals monitor the use of urethral catheters in their patients.30
Among study hospitals, there was no widely used technique to prevent CAUTI including evidence-based practices such as daily catheter reminders. The results of this investigation illustrate the urgent need for a national strategy to reduce CAUTI. Until that time, however, hospital-based physicians must take the lead to champion collaborative efforts, to promote evidence-based catheter use.
Back to the Case
As incontinence and fall risk are not medically appropriate indications for a urethral catheter, a Foley catheter should not have been utilized. Alternatives to indwelling catheterization in this patient would include a bedside commode with nursing assistance, a timed voiding program, intermittent catheterization with or without bladder ultrasound, incontinence pads, or a condom catheter.
Attentiveness to the appropriate medical indications for catheter use, familiarity with catheter alternatives, and recognition of the clinical and economic impact of CAUTI may have prevented this patient’s UTI-induced delirium and facilitated his early transfer to SNF. TH
Dr. Wald is a getriatric hospitalist and assistant professor of medicine at the University of Colorado, Denver. Dr. Furfari is a hospital medicine fellow at the University of Colorado Denver.
References
- Beeson PB. The case against the catheter. Am J Med. 1958;24:1-3.
- Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68-75.
- Sedor J, Mulholland SG. Hospital-acquired UTIs associated with the indwelling catheter. Urol Clin North Am. 1999;26:821-828.
- Foxman B. Epidemiology of UTI: Incidence, morbidity and economic costs. Am J Med. 2002;113(1A):5S-13S.
- Tambyah PA, Knasinski V, Maki D. The direct costs of nosocomial catheter-associated UTI in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27-31.
- Jarvis, WR. Selected aspects of socioeconomic impact of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:552-557.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
- Jain P, Parada JP, David A, Smith L. Overuse of the indwelling urinary catheter in hospitalized medical patients. Arch Internal Med. 1995;155:1425-1429.
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380-386.
- Wong E. Guideline for prevention of catheter-associated urinary tract infections. Center for Disease Control and Prevention 1981. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html . Accessed May 8, 2008.
- Joanna Briggs Institute. Management of short term indwelling urethral catheters to prevent urinary tract infections. 2000;4(1):ISSN 1329-1874.
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Am J Med. 1981;70:655-658.
- Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404-406.
- Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978.
- Topal J, Conklin S, Camp K, Morris TB, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126.
- Goetz AM, Kedzuf S, Wagener M, Muder R. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402-404.
- Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11:651-656.
- Frederickson M, Neitzel JJ, Miller EH, Reuter S, Graner T, Heller J. The implementation of bedside bladder ultrasound technology: Effects of patient and cost postoperative outcomes in tertiary care. Orthop Nurs. 2000;19(3):79-87.
- Slappendel R, Weber EWG. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopedic surgery and its effect on urinary tract infections. Eur J Anesthesiol. 1999;16:503-506.
- Hirsh D, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infections? JAMA. 1979;242:340-341.
- Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11:28-36.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria. Should We? Can We? How? Arch Internal Med. 1999;159:800-808.
- Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infection in Danish hospitals, nursing homes and home care: A national prevalence study. Infect Control Hosp Epidemiol. 1996;17(4):215-221.
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infections in hospitalized patients. Ann Internal Med. 2006;144(2):116-126.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infections; a meta-analysis. Am J Med. 1998;105(3):236-241.
- Bronahan J, Jull A, Tracy C. Cochrane incontinence group. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2004;1:CD004013.
- Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298(23):2782-2784.
- Goldstein J. Hospital infections’ cost tallied. The Philadelphia Inquirer. Nov. 15, 2006.
- Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: A national study. Clin Infect Dis. 2008;46(2):243-250.
Case
A 68-year-old male with a history of Alzheimer’s dementia and incontinence presents with failure to thrive. A Foley catheter is placed due to the patient’s incontinence and fall risk. Three days after admission while awaiting placement in a skilled nursing facility (SNF), he develops a urinary tract infection (UTI) complicated by delirium delaying his transfer to the SNF. What could have been done to prevent this complication?
Overview
It has been 50 years since Beeson, et al., recognized the potential harms stemming from urethral catheterization and penned an editorial to the American Journal of Medicine titled “The case against the catheter.”1
Since then, there has been considerable exploration of ways to limit urethral catheterization and ultimately decrease catheter-associated urinary tract infections (CAUTIs). Unfortunately, little progress has been made; indwelling urinary catheters remain ubiquitous in hospitals and CAUTIs remain the most common hospital-acquired infection in the United States.2 Given the emphasis on the quality and costs of healthcare, it is an opportune time to revisit catheter management and use as a way to combat the clinical and economic consequences of CAUTIs.
Clinicians may be lulled into thinking the clinical impact of CAUTI is less than that of other nosocomial infections. However, beyond the obvious patient harm from UTIs, associated bacteremia, and even death, the public health implications of CAUTI cannot be denied. Urinary tract infections constitute 40% of all nosocomial infections; accounting for an estimated 1 million cases annually.3 Further, 80% of all UTIs are associated with indwelling catheter use.
On average, nosocomial UTI necessitates one extra hospital day per patient, or approximately one million excess hospital days per year.4 Pooled cost analysis shows that UTIs consume an additional $400-$1,700 per event, or an estimated $425 million per year in the United States.5,6 Clearly, we cannot wait another 50 years to address this problem.
Review of the Data
Catheter duration as a risk factor for CAUTI: The indwelling catheter creates a portal of entry into a usually sterile body cavity and provides a surface on which microorganisms can colonize. At a finite rate of colonization—the incidence of bacteriuria is 3% to 10% per catheter day—the duration of urinary catheterization becomes the strongest predictor of catheter-associated bacteriuria.7 Even in relatively short-term catheter use of two to 10 days, the pooled cumulative incidence of developing bacteriuria is 26%.
Given the magnitude of these numbers, it should be no surprise that after one month of catheterization, bacteriuria develops in almost all patients. Twenty-four percent of patients with bacteriuria develop symptomatic UTIs with close to 5% suffering bacteremia. Consequently, nosocomial UTIs cause 15% of all hospital-acquired bacteremia.
Optimal catheter management: The easiest and most effective means to prevent CAUTI is to limit the use of urinary catheters to clearly identified medical indications (see Table 1, above). However, as simple as this prevention practice may sound, studies have demonstrated that as many as 20% of patients have indwelling catheters initially placed for unjustified or even unknown medical indications.8 Additionally, continued catheter use is inappropriate in one-third to one-half of all catheter days.9 These data confirm misuse and overuse of indwelling urinary catheters in the hospital setting is common.
In 1981, the Centers for Disease Control and Prevention (CDC) recognized the importance of addressing this situation and published a guideline to aid prevention of CAUTIs.10 The CDC urged the limitation of catheter use to a carefully selected patient population. Furthermore, the report strongly stressed the importance of catheter removal as soon as possible and advised against the use of catheters solely for the convenience of healthcare workers.
Evidence-based techniques for insertion and catheter care also were outlined in the guideline (see Table 2, p. 31). However, these recommendations have been poorly implemented, likely due to the competing priorities of providers and the difficulty operationalizing the guidelines. Additionally, evidence from the intervening 25 years has not yet been incorporated into the guideline, although a revision currently is underway.
Until that revision is complete, the Joanna Briggs Institute guideline published in 2001 addresses some of the same management techniques and incorporates newer evidence.11 Of note, practices that have been discredited due to contradictory evidence include aggressive meatal cleaning, bladder irrigation, and the application of antimicrobial agents in the drainage bag.12
Strategies to reduce unnecessary catheter days: One of the remediable reasons for catheter misuse lies in the fact physicians often are unaware of the presence of an indwelling catheter in their hospitalized patients.
Saint, et al., showed physicians were unaware of catheterization in 28% of their patients and that attending physicians were less conscious of a patient’s catheter status than residents, interns, or medical students.13 Further, the “forgotten” catheters were more likely to be unnecessary than those remembered by the healthcare team.
This information has prompted the use of various computer-based and multidisciplinary feedback protocols to readdress and re-evaluate the need for continued catheterization in a patient. For example, a study at the VAMC Puget Sound demonstrated that having a computerized order protocol for urinary catheters significantly increased the rate of documentation as well as decreased the duration of catheterization by an average of three days.14
Similar interventions to encourage early catheter removal have included daily reminders from nursing staff, allowing a nurse to discontinue catheter use independent of a physician’s order, and feedback in which nursing staff is educated about the incidence of UTI.15-17 All these relatively simple interventions showed significant improvement in the catheter removal rate and incidence of CAUTIs as well as documented cost savings.
Alternatives to indwelling catheters: In addition to efforts to decrease catheter days, alternatives to the indwelling catheters also should be explored. One such alternative method is intermittent catheterization.
Several studies in postoperative patients with hip fractures have demonstrated that the development of UTI is lower with intermittent catheterization when compared with indwelling catheterization.18 Nevertheless, since the risk of bacteriuria is 1% to 3% per episode of catheterization, after a few weeks the majority of patients will have bacteriuria. However, as the bulk of this bacteriuria often is asymptomatic, intermittent catheterization may still be an improvement. This is particularly true in postoperative patients undergoing rehabilitation and those patients only requiring catheterization for a limited number of days.
More recent studies have evaluated the use of bedside bladder ultrasound in an attempt to determine when intermittent catheterization is needed and thereby limit its use compared with standard timed catheterization. Frederickson, et al., demonstrated that this intervention resulted in significantly fewer catheterizations in surgical patients, thus delaying or avoiding the need for catheterization in 81% of the cases.19 Given this drastic improvement, it is no surprise bladder ultrasound use reduced the rates of UTI.20
External condom catheters present another alternative to indwelling catheter use but the outcomes data is conflicting. While the risk of bacteriuria is approximately 12% per month, this rate becomes increasingly higher with frequent manipulation of the condom catheter. 21,22
Two parallel cohort studies in a VA nursing home showed the incidence of symptomatic UTI to be 2.5 times greater in men with an indwelling catheter than those with a condom catheter.23 On the other hand, a cross-sectional Danish study reported higher rates of UTI with external condom catheters than urethral catheters in hospitalized patients.24 Complications from condom catheters include phimosis and local skin maceration, necessitating meticulous care with the use of these devices. Although the data surrounding external catheterization is somewhat contradictory, this device warrants consideration in incontinent males without urinary tract obstruction.
There are several other alternatives to urethral catheterization (see Table 3, p. 31), many of which have excellent face validity even in the absence of rigorous evidence.
Antimicrobial catheters: The development of antimicrobial urinary catheters, including silver-alloy and nitrofurazone-coated catheters, has been greeted with much excitement, however, the jury is still out about their best use. A 2006 systematic literature review reported that in comparison to standard catheters, antimicrobial catheters can delay or even prevent the development of bacteriuria with short-term usage.25
However, not all antimicrobial catheters are equally effective; assorted studies lack data about clinically relevant endpoints such as prevention of symptomatic UTI, bloodstream infection or death.26, 27 In addition, there are no good trials comparing nitrofurazone to silver-alloy catheters. Therefore, the level of excitement surrounding antimicrobial catheters—particularly silver-alloy catheters—must be tempered by the additional costs incurred by their use.
To date, the cost-effectiveness of antimicrobial catheters has not been demonstrated. Although additional research in this topic is still needed, some experts currently recommend the consideration of silver-alloy catheters in patients at the highest risk for developing serious consequences from UTIs.
Efforts to reduce CAUTI: In response to significant public interest in hospital-acquired infections including CAUTI, the federal government and many state governments are beginning to demand change. In August 2007, the Centers for Medicare and Medicaid Services instituted a mandate making hospitals financially responsible for selected preventable hospital-acquired harms, including CAUTIs.28 In addition, beginning with Pennsylvania in 2006, several states have mandated public reporting of hospital-acquired infections.29
Given the available information about CAUTI prevalence, risks, and preventive techniques, it is surprising the majority of hospitals in the United States have not taken appropriate measures to limit indwelling catheter use. A recent study by Saint, et al., demonstrated the startling fact that only a minority of hospitals monitor the use of urethral catheters in their patients.30
Among study hospitals, there was no widely used technique to prevent CAUTI including evidence-based practices such as daily catheter reminders. The results of this investigation illustrate the urgent need for a national strategy to reduce CAUTI. Until that time, however, hospital-based physicians must take the lead to champion collaborative efforts, to promote evidence-based catheter use.
Back to the Case
As incontinence and fall risk are not medically appropriate indications for a urethral catheter, a Foley catheter should not have been utilized. Alternatives to indwelling catheterization in this patient would include a bedside commode with nursing assistance, a timed voiding program, intermittent catheterization with or without bladder ultrasound, incontinence pads, or a condom catheter.
Attentiveness to the appropriate medical indications for catheter use, familiarity with catheter alternatives, and recognition of the clinical and economic impact of CAUTI may have prevented this patient’s UTI-induced delirium and facilitated his early transfer to SNF. TH
Dr. Wald is a getriatric hospitalist and assistant professor of medicine at the University of Colorado, Denver. Dr. Furfari is a hospital medicine fellow at the University of Colorado Denver.
References
- Beeson PB. The case against the catheter. Am J Med. 1958;24:1-3.
- Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68-75.
- Sedor J, Mulholland SG. Hospital-acquired UTIs associated with the indwelling catheter. Urol Clin North Am. 1999;26:821-828.
- Foxman B. Epidemiology of UTI: Incidence, morbidity and economic costs. Am J Med. 2002;113(1A):5S-13S.
- Tambyah PA, Knasinski V, Maki D. The direct costs of nosocomial catheter-associated UTI in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27-31.
- Jarvis, WR. Selected aspects of socioeconomic impact of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:552-557.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
- Jain P, Parada JP, David A, Smith L. Overuse of the indwelling urinary catheter in hospitalized medical patients. Arch Internal Med. 1995;155:1425-1429.
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380-386.
- Wong E. Guideline for prevention of catheter-associated urinary tract infections. Center for Disease Control and Prevention 1981. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html . Accessed May 8, 2008.
- Joanna Briggs Institute. Management of short term indwelling urethral catheters to prevent urinary tract infections. 2000;4(1):ISSN 1329-1874.
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Am J Med. 1981;70:655-658.
- Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404-406.
- Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978.
- Topal J, Conklin S, Camp K, Morris TB, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126.
- Goetz AM, Kedzuf S, Wagener M, Muder R. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402-404.
- Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11:651-656.
- Frederickson M, Neitzel JJ, Miller EH, Reuter S, Graner T, Heller J. The implementation of bedside bladder ultrasound technology: Effects of patient and cost postoperative outcomes in tertiary care. Orthop Nurs. 2000;19(3):79-87.
- Slappendel R, Weber EWG. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopedic surgery and its effect on urinary tract infections. Eur J Anesthesiol. 1999;16:503-506.
- Hirsh D, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infections? JAMA. 1979;242:340-341.
- Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11:28-36.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria. Should We? Can We? How? Arch Internal Med. 1999;159:800-808.
- Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infection in Danish hospitals, nursing homes and home care: A national prevalence study. Infect Control Hosp Epidemiol. 1996;17(4):215-221.
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infections in hospitalized patients. Ann Internal Med. 2006;144(2):116-126.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infections; a meta-analysis. Am J Med. 1998;105(3):236-241.
- Bronahan J, Jull A, Tracy C. Cochrane incontinence group. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2004;1:CD004013.
- Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298(23):2782-2784.
- Goldstein J. Hospital infections’ cost tallied. The Philadelphia Inquirer. Nov. 15, 2006.
- Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: A national study. Clin Infect Dis. 2008;46(2):243-250.
Case
A 68-year-old male with a history of Alzheimer’s dementia and incontinence presents with failure to thrive. A Foley catheter is placed due to the patient’s incontinence and fall risk. Three days after admission while awaiting placement in a skilled nursing facility (SNF), he develops a urinary tract infection (UTI) complicated by delirium delaying his transfer to the SNF. What could have been done to prevent this complication?
Overview
It has been 50 years since Beeson, et al., recognized the potential harms stemming from urethral catheterization and penned an editorial to the American Journal of Medicine titled “The case against the catheter.”1
Since then, there has been considerable exploration of ways to limit urethral catheterization and ultimately decrease catheter-associated urinary tract infections (CAUTIs). Unfortunately, little progress has been made; indwelling urinary catheters remain ubiquitous in hospitals and CAUTIs remain the most common hospital-acquired infection in the United States.2 Given the emphasis on the quality and costs of healthcare, it is an opportune time to revisit catheter management and use as a way to combat the clinical and economic consequences of CAUTIs.
Clinicians may be lulled into thinking the clinical impact of CAUTI is less than that of other nosocomial infections. However, beyond the obvious patient harm from UTIs, associated bacteremia, and even death, the public health implications of CAUTI cannot be denied. Urinary tract infections constitute 40% of all nosocomial infections; accounting for an estimated 1 million cases annually.3 Further, 80% of all UTIs are associated with indwelling catheter use.
On average, nosocomial UTI necessitates one extra hospital day per patient, or approximately one million excess hospital days per year.4 Pooled cost analysis shows that UTIs consume an additional $400-$1,700 per event, or an estimated $425 million per year in the United States.5,6 Clearly, we cannot wait another 50 years to address this problem.
Review of the Data
Catheter duration as a risk factor for CAUTI: The indwelling catheter creates a portal of entry into a usually sterile body cavity and provides a surface on which microorganisms can colonize. At a finite rate of colonization—the incidence of bacteriuria is 3% to 10% per catheter day—the duration of urinary catheterization becomes the strongest predictor of catheter-associated bacteriuria.7 Even in relatively short-term catheter use of two to 10 days, the pooled cumulative incidence of developing bacteriuria is 26%.
Given the magnitude of these numbers, it should be no surprise that after one month of catheterization, bacteriuria develops in almost all patients. Twenty-four percent of patients with bacteriuria develop symptomatic UTIs with close to 5% suffering bacteremia. Consequently, nosocomial UTIs cause 15% of all hospital-acquired bacteremia.
Optimal catheter management: The easiest and most effective means to prevent CAUTI is to limit the use of urinary catheters to clearly identified medical indications (see Table 1, above). However, as simple as this prevention practice may sound, studies have demonstrated that as many as 20% of patients have indwelling catheters initially placed for unjustified or even unknown medical indications.8 Additionally, continued catheter use is inappropriate in one-third to one-half of all catheter days.9 These data confirm misuse and overuse of indwelling urinary catheters in the hospital setting is common.
In 1981, the Centers for Disease Control and Prevention (CDC) recognized the importance of addressing this situation and published a guideline to aid prevention of CAUTIs.10 The CDC urged the limitation of catheter use to a carefully selected patient population. Furthermore, the report strongly stressed the importance of catheter removal as soon as possible and advised against the use of catheters solely for the convenience of healthcare workers.
Evidence-based techniques for insertion and catheter care also were outlined in the guideline (see Table 2, p. 31). However, these recommendations have been poorly implemented, likely due to the competing priorities of providers and the difficulty operationalizing the guidelines. Additionally, evidence from the intervening 25 years has not yet been incorporated into the guideline, although a revision currently is underway.
Until that revision is complete, the Joanna Briggs Institute guideline published in 2001 addresses some of the same management techniques and incorporates newer evidence.11 Of note, practices that have been discredited due to contradictory evidence include aggressive meatal cleaning, bladder irrigation, and the application of antimicrobial agents in the drainage bag.12
Strategies to reduce unnecessary catheter days: One of the remediable reasons for catheter misuse lies in the fact physicians often are unaware of the presence of an indwelling catheter in their hospitalized patients.
Saint, et al., showed physicians were unaware of catheterization in 28% of their patients and that attending physicians were less conscious of a patient’s catheter status than residents, interns, or medical students.13 Further, the “forgotten” catheters were more likely to be unnecessary than those remembered by the healthcare team.
This information has prompted the use of various computer-based and multidisciplinary feedback protocols to readdress and re-evaluate the need for continued catheterization in a patient. For example, a study at the VAMC Puget Sound demonstrated that having a computerized order protocol for urinary catheters significantly increased the rate of documentation as well as decreased the duration of catheterization by an average of three days.14
Similar interventions to encourage early catheter removal have included daily reminders from nursing staff, allowing a nurse to discontinue catheter use independent of a physician’s order, and feedback in which nursing staff is educated about the incidence of UTI.15-17 All these relatively simple interventions showed significant improvement in the catheter removal rate and incidence of CAUTIs as well as documented cost savings.
Alternatives to indwelling catheters: In addition to efforts to decrease catheter days, alternatives to the indwelling catheters also should be explored. One such alternative method is intermittent catheterization.
Several studies in postoperative patients with hip fractures have demonstrated that the development of UTI is lower with intermittent catheterization when compared with indwelling catheterization.18 Nevertheless, since the risk of bacteriuria is 1% to 3% per episode of catheterization, after a few weeks the majority of patients will have bacteriuria. However, as the bulk of this bacteriuria often is asymptomatic, intermittent catheterization may still be an improvement. This is particularly true in postoperative patients undergoing rehabilitation and those patients only requiring catheterization for a limited number of days.
More recent studies have evaluated the use of bedside bladder ultrasound in an attempt to determine when intermittent catheterization is needed and thereby limit its use compared with standard timed catheterization. Frederickson, et al., demonstrated that this intervention resulted in significantly fewer catheterizations in surgical patients, thus delaying or avoiding the need for catheterization in 81% of the cases.19 Given this drastic improvement, it is no surprise bladder ultrasound use reduced the rates of UTI.20
External condom catheters present another alternative to indwelling catheter use but the outcomes data is conflicting. While the risk of bacteriuria is approximately 12% per month, this rate becomes increasingly higher with frequent manipulation of the condom catheter. 21,22
Two parallel cohort studies in a VA nursing home showed the incidence of symptomatic UTI to be 2.5 times greater in men with an indwelling catheter than those with a condom catheter.23 On the other hand, a cross-sectional Danish study reported higher rates of UTI with external condom catheters than urethral catheters in hospitalized patients.24 Complications from condom catheters include phimosis and local skin maceration, necessitating meticulous care with the use of these devices. Although the data surrounding external catheterization is somewhat contradictory, this device warrants consideration in incontinent males without urinary tract obstruction.
There are several other alternatives to urethral catheterization (see Table 3, p. 31), many of which have excellent face validity even in the absence of rigorous evidence.
Antimicrobial catheters: The development of antimicrobial urinary catheters, including silver-alloy and nitrofurazone-coated catheters, has been greeted with much excitement, however, the jury is still out about their best use. A 2006 systematic literature review reported that in comparison to standard catheters, antimicrobial catheters can delay or even prevent the development of bacteriuria with short-term usage.25
However, not all antimicrobial catheters are equally effective; assorted studies lack data about clinically relevant endpoints such as prevention of symptomatic UTI, bloodstream infection or death.26, 27 In addition, there are no good trials comparing nitrofurazone to silver-alloy catheters. Therefore, the level of excitement surrounding antimicrobial catheters—particularly silver-alloy catheters—must be tempered by the additional costs incurred by their use.
To date, the cost-effectiveness of antimicrobial catheters has not been demonstrated. Although additional research in this topic is still needed, some experts currently recommend the consideration of silver-alloy catheters in patients at the highest risk for developing serious consequences from UTIs.
Efforts to reduce CAUTI: In response to significant public interest in hospital-acquired infections including CAUTI, the federal government and many state governments are beginning to demand change. In August 2007, the Centers for Medicare and Medicaid Services instituted a mandate making hospitals financially responsible for selected preventable hospital-acquired harms, including CAUTIs.28 In addition, beginning with Pennsylvania in 2006, several states have mandated public reporting of hospital-acquired infections.29
Given the available information about CAUTI prevalence, risks, and preventive techniques, it is surprising the majority of hospitals in the United States have not taken appropriate measures to limit indwelling catheter use. A recent study by Saint, et al., demonstrated the startling fact that only a minority of hospitals monitor the use of urethral catheters in their patients.30
Among study hospitals, there was no widely used technique to prevent CAUTI including evidence-based practices such as daily catheter reminders. The results of this investigation illustrate the urgent need for a national strategy to reduce CAUTI. Until that time, however, hospital-based physicians must take the lead to champion collaborative efforts, to promote evidence-based catheter use.
Back to the Case
As incontinence and fall risk are not medically appropriate indications for a urethral catheter, a Foley catheter should not have been utilized. Alternatives to indwelling catheterization in this patient would include a bedside commode with nursing assistance, a timed voiding program, intermittent catheterization with or without bladder ultrasound, incontinence pads, or a condom catheter.
Attentiveness to the appropriate medical indications for catheter use, familiarity with catheter alternatives, and recognition of the clinical and economic impact of CAUTI may have prevented this patient’s UTI-induced delirium and facilitated his early transfer to SNF. TH
Dr. Wald is a getriatric hospitalist and assistant professor of medicine at the University of Colorado, Denver. Dr. Furfari is a hospital medicine fellow at the University of Colorado Denver.
References
- Beeson PB. The case against the catheter. Am J Med. 1958;24:1-3.
- Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68-75.
- Sedor J, Mulholland SG. Hospital-acquired UTIs associated with the indwelling catheter. Urol Clin North Am. 1999;26:821-828.
- Foxman B. Epidemiology of UTI: Incidence, morbidity and economic costs. Am J Med. 2002;113(1A):5S-13S.
- Tambyah PA, Knasinski V, Maki D. The direct costs of nosocomial catheter-associated UTI in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27-31.
- Jarvis, WR. Selected aspects of socioeconomic impact of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:552-557.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
- Jain P, Parada JP, David A, Smith L. Overuse of the indwelling urinary catheter in hospitalized medical patients. Arch Internal Med. 1995;155:1425-1429.
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380-386.
- Wong E. Guideline for prevention of catheter-associated urinary tract infections. Center for Disease Control and Prevention 1981. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html . Accessed May 8, 2008.
- Joanna Briggs Institute. Management of short term indwelling urethral catheters to prevent urinary tract infections. 2000;4(1):ISSN 1329-1874.
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Am J Med. 1981;70:655-658.
- Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404-406.
- Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978.
- Topal J, Conklin S, Camp K, Morris TB, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126.
- Goetz AM, Kedzuf S, Wagener M, Muder R. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402-404.
- Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11:651-656.
- Frederickson M, Neitzel JJ, Miller EH, Reuter S, Graner T, Heller J. The implementation of bedside bladder ultrasound technology: Effects of patient and cost postoperative outcomes in tertiary care. Orthop Nurs. 2000;19(3):79-87.
- Slappendel R, Weber EWG. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopedic surgery and its effect on urinary tract infections. Eur J Anesthesiol. 1999;16:503-506.
- Hirsh D, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infections? JAMA. 1979;242:340-341.
- Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11:28-36.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria. Should We? Can We? How? Arch Internal Med. 1999;159:800-808.
- Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infection in Danish hospitals, nursing homes and home care: A national prevalence study. Infect Control Hosp Epidemiol. 1996;17(4):215-221.
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infections in hospitalized patients. Ann Internal Med. 2006;144(2):116-126.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infections; a meta-analysis. Am J Med. 1998;105(3):236-241.
- Bronahan J, Jull A, Tracy C. Cochrane incontinence group. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2004;1:CD004013.
- Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298(23):2782-2784.
- Goldstein J. Hospital infections’ cost tallied. The Philadelphia Inquirer. Nov. 15, 2006.
- Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: A national study. Clin Infect Dis. 2008;46(2):243-250.