User login
Mentorship in Residency
Mentorship in Residency
The year was 2023, and I was on my way to the American Academy of Dermatology meeting in New Orleans, Louisiana. “Geaux Tigers!” I exclaimed to a stranger as she walked by in her purple and gold shoes and scrubs. We chatted for a minute or two about Louisiana State University (LSU) football, then went our separate ways. Later that day, in the hands-on wound closures workshop, I was surprised to see my new acquaintance step up to the podium to lecture, then make rounds across the room to instruct residents. I didn’t know it at the time, but those purple and gold shoes sparked a conversation with a fellowship program director who would become one of my most valued mentors.
I didn’t set out to find a mentor that day—I simply was excited to connect with a fellow Tigers fan. But mentorship often finds us unexpectedly, and that encounter serves as a reminder that mentorship doesn’t always start in a formal setting. Sometimes it begins with a quick conversation in the right place at the right time. This story is one of many experiences that taught me valuable lessons about mentorship—its importance, how it can grow naturally, and the impact it can have.
Residency is a pivotal time in a physician’s life, filled with rapid learning, complex challenges, and new professional relationships. Amidst the long hours and heavy responsibilities, mentorship stands out as a support system for guiding residents toward professional and personal growth. Herein, I share more about my experiences with mentorship in residency, the lessons I have learned, and how they can serve as guidance for residents.
The Value of Mentorship
Mentorship in residency has been shown to have a major impact on career satisfaction, clinical confidence, and professional development.1 A good mentor offers more than just advice—he or she can provide a model of professionalism and skills that resonates with the mentee’s own aspirations. Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.2
Mentorship can be sought intentionally or arise naturally from shared interests and connections. Some residents reach out to potential mentors directly through emails, set up one-on-one meetings, or shadow them to gain firsthand experience. Others find mentorship simply by putting themselves in situations that foster these connections, such as attending conferences or lectures. Both approaches can lead to impactful relationships that shape a resident’s career and personal growth.
For residents involved in research, an effective faculty research mentor is particularly impactful. Studies show that residents who work with knowledgeable research mentors are more likely to experience success and productivity in their research efforts.3 Research mentors can provide essential guidance—from helping formulate research questions to navigating the complexities of publishing—which makes them invaluable in a resident’s academic development.
If you have interests in specific areas not heavily emphasized within your residency program (eg, transplantation dermatology, hair restoration, cutaneous lymphoma), consider checking within your broader medical community for specialists. Many dermatologists and other specialists welcome the opportunity to mentor residents who express a sincere interest in learning. By reaching out to these professionals, you not only expand your clinical knowledge but also gain access to niche areas of dermatology that can shape and refine your future practice. Often, these experiences lead to invaluable mentorships that may otherwise be unavailable within your immediate training environment.
Networking Through Professional Society Rotational and Mentorship Programs
The Women’s Dermatologic Society (https://www.womensderm.org/), the American Society for Dermatologic Surgery (https://www.asds.net/), and the American Society for Laser Medicine and Surgery (https://www.aslms.org/) all provide excellent formalized mentorship or preceptorship programs. Check their websites for application requirements and timelines. Participating in these programs is a great way to network with experts in dermatology, providing a structured way to interact with physicians who share your interests. Whether you are interested in medical dermatology, surgery, pediatrics, dermatopathology, or cosmetics, there are many mentors who greatly enjoy sharing their knowledge and experience with residents. Oftentimes, these programs include stipends to assist with costs that are awarded as accolades that can enhance your curriculum vitae. Engaging in these recognized preceptorship programs often builds lasting connections and ensures that both mentor and mentee have a vested interest in the relationship’s success.
Making Connections at Conferences and Maximizing Hands-on Learning
Professional conferences offer valuable opportunities to connect with mentors, whether you are proactively seeking mentorship or simply allowing connections to happen naturally. Conferences such as those of the American Academy of Dermatology and American Society for Dermatologic Surgery publish educational booklets and schedules online prior to the event, giving you a chance to explore both topics and speaker names ahead of time. This can be an excellent opportunity to create a day-by-day game plan, identifying sessions and lectures of interest as well as specific authors or experts you might like to meet. Planning in advance makes it easier to engage with leaders in the field, introduce yourself, and make meaningful connections.
Oftentimes, these society meetings offer hands-on courses, which are a great way to meet mentors and learn from direct instruction. Instructors for these courses often are leaders in dermatology who are passionate about teaching. With small group sizes, hands-on courses offer both technical skill-building opportunities and a chance to connect personally with instructors. Take a moment to introduce yourself and engage in a quick conversation, and if you feel it is appropriate, follow up with an email after the conference. This helps keep the connection alive beyond the event and may open doors for future mentorship opportunities.
Away Rotations
For residents looking to build specialized skills and connect with mentors outside their own program—especially those considering fellowship—away rotations can be a great tool. Though it may require using vacation time, an away rotation offers immersive learning in a particular area while providing opportunities to observe new mentors and establish relationships within a desired subspecialty or program. By simply reaching out and expressing interest, residents can connect with physicians who may become lasting mentors and advocates.
Building a Mentor-Mentee Relationship
A meaningful mentor-mentee relationship requires time, effort, and effective communication, with clear expectations around mentorship goals, time commitments, and how both parties envision the relationship evolving.4 Ideally, mentees should feel comfortable sharing their goals with mentors and asking for feedback. In the right context, a simple and effective practice is to send your mentor a brief update on your progress every few months. This could be a quick email sharing your latest projects, ideas, and/or achievements. By regularly checking in, you show your mentor that you are committed to growing from their guidance and respect their time.
The Lasting Impact of Mentorship
The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.5 Residency often is the last time a resident trains under the direct supervision of an attending physician, making it a unique and formative period. After graduation, many new physicians find the transition to independent practice challenging, and the “real world” can be a shock. Having a mentor during this time, or maintaining connections with mentors from residency, can be invaluable. Mentors can offer advice, act as sounding boards, and remind new graduates of the importance of being lifelong learners. These relationships help ease the transition into practice, instilling a commitment to continuous improvement and professional growth. For me, a conversation about LSU football at the AAD meeting in New Orleans exemplifies how mentorship can begin in the most unexpected ways. That casual exchange led to an away rotation, a fellowship interview, connections at national meetings, and the start of what I hope will be a lifelong friendship.
- Ramanan RA, Taylor WC, Davis RB, et al. Mentoring matters. mentoring and career preparation in internal medicine residency training. J Gen Intern Med. 2006;21:340-345.
- Sambunjak D, Straus SE, Marusic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research-a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Allen TD, Eby LT, Poteet ML, et al. Career benefits associated with mentoring for protégeé: a meta-analysis. J Appl Psychol. 2004;89:127-136.
- Kashiwagi DT, Varkey P, Cook DA. Mentoring programs for physicians in academic medicine: a systematic review. Acad Med. 2013;88:1029-1037.
The year was 2023, and I was on my way to the American Academy of Dermatology meeting in New Orleans, Louisiana. “Geaux Tigers!” I exclaimed to a stranger as she walked by in her purple and gold shoes and scrubs. We chatted for a minute or two about Louisiana State University (LSU) football, then went our separate ways. Later that day, in the hands-on wound closures workshop, I was surprised to see my new acquaintance step up to the podium to lecture, then make rounds across the room to instruct residents. I didn’t know it at the time, but those purple and gold shoes sparked a conversation with a fellowship program director who would become one of my most valued mentors.
I didn’t set out to find a mentor that day—I simply was excited to connect with a fellow Tigers fan. But mentorship often finds us unexpectedly, and that encounter serves as a reminder that mentorship doesn’t always start in a formal setting. Sometimes it begins with a quick conversation in the right place at the right time. This story is one of many experiences that taught me valuable lessons about mentorship—its importance, how it can grow naturally, and the impact it can have.
Residency is a pivotal time in a physician’s life, filled with rapid learning, complex challenges, and new professional relationships. Amidst the long hours and heavy responsibilities, mentorship stands out as a support system for guiding residents toward professional and personal growth. Herein, I share more about my experiences with mentorship in residency, the lessons I have learned, and how they can serve as guidance for residents.
The Value of Mentorship
Mentorship in residency has been shown to have a major impact on career satisfaction, clinical confidence, and professional development.1 A good mentor offers more than just advice—he or she can provide a model of professionalism and skills that resonates with the mentee’s own aspirations. Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.2
Mentorship can be sought intentionally or arise naturally from shared interests and connections. Some residents reach out to potential mentors directly through emails, set up one-on-one meetings, or shadow them to gain firsthand experience. Others find mentorship simply by putting themselves in situations that foster these connections, such as attending conferences or lectures. Both approaches can lead to impactful relationships that shape a resident’s career and personal growth.
For residents involved in research, an effective faculty research mentor is particularly impactful. Studies show that residents who work with knowledgeable research mentors are more likely to experience success and productivity in their research efforts.3 Research mentors can provide essential guidance—from helping formulate research questions to navigating the complexities of publishing—which makes them invaluable in a resident’s academic development.
If you have interests in specific areas not heavily emphasized within your residency program (eg, transplantation dermatology, hair restoration, cutaneous lymphoma), consider checking within your broader medical community for specialists. Many dermatologists and other specialists welcome the opportunity to mentor residents who express a sincere interest in learning. By reaching out to these professionals, you not only expand your clinical knowledge but also gain access to niche areas of dermatology that can shape and refine your future practice. Often, these experiences lead to invaluable mentorships that may otherwise be unavailable within your immediate training environment.
Networking Through Professional Society Rotational and Mentorship Programs
The Women’s Dermatologic Society (https://www.womensderm.org/), the American Society for Dermatologic Surgery (https://www.asds.net/), and the American Society for Laser Medicine and Surgery (https://www.aslms.org/) all provide excellent formalized mentorship or preceptorship programs. Check their websites for application requirements and timelines. Participating in these programs is a great way to network with experts in dermatology, providing a structured way to interact with physicians who share your interests. Whether you are interested in medical dermatology, surgery, pediatrics, dermatopathology, or cosmetics, there are many mentors who greatly enjoy sharing their knowledge and experience with residents. Oftentimes, these programs include stipends to assist with costs that are awarded as accolades that can enhance your curriculum vitae. Engaging in these recognized preceptorship programs often builds lasting connections and ensures that both mentor and mentee have a vested interest in the relationship’s success.
Making Connections at Conferences and Maximizing Hands-on Learning
Professional conferences offer valuable opportunities to connect with mentors, whether you are proactively seeking mentorship or simply allowing connections to happen naturally. Conferences such as those of the American Academy of Dermatology and American Society for Dermatologic Surgery publish educational booklets and schedules online prior to the event, giving you a chance to explore both topics and speaker names ahead of time. This can be an excellent opportunity to create a day-by-day game plan, identifying sessions and lectures of interest as well as specific authors or experts you might like to meet. Planning in advance makes it easier to engage with leaders in the field, introduce yourself, and make meaningful connections.
Oftentimes, these society meetings offer hands-on courses, which are a great way to meet mentors and learn from direct instruction. Instructors for these courses often are leaders in dermatology who are passionate about teaching. With small group sizes, hands-on courses offer both technical skill-building opportunities and a chance to connect personally with instructors. Take a moment to introduce yourself and engage in a quick conversation, and if you feel it is appropriate, follow up with an email after the conference. This helps keep the connection alive beyond the event and may open doors for future mentorship opportunities.
Away Rotations
For residents looking to build specialized skills and connect with mentors outside their own program—especially those considering fellowship—away rotations can be a great tool. Though it may require using vacation time, an away rotation offers immersive learning in a particular area while providing opportunities to observe new mentors and establish relationships within a desired subspecialty or program. By simply reaching out and expressing interest, residents can connect with physicians who may become lasting mentors and advocates.
Building a Mentor-Mentee Relationship
A meaningful mentor-mentee relationship requires time, effort, and effective communication, with clear expectations around mentorship goals, time commitments, and how both parties envision the relationship evolving.4 Ideally, mentees should feel comfortable sharing their goals with mentors and asking for feedback. In the right context, a simple and effective practice is to send your mentor a brief update on your progress every few months. This could be a quick email sharing your latest projects, ideas, and/or achievements. By regularly checking in, you show your mentor that you are committed to growing from their guidance and respect their time.
The Lasting Impact of Mentorship
The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.5 Residency often is the last time a resident trains under the direct supervision of an attending physician, making it a unique and formative period. After graduation, many new physicians find the transition to independent practice challenging, and the “real world” can be a shock. Having a mentor during this time, or maintaining connections with mentors from residency, can be invaluable. Mentors can offer advice, act as sounding boards, and remind new graduates of the importance of being lifelong learners. These relationships help ease the transition into practice, instilling a commitment to continuous improvement and professional growth. For me, a conversation about LSU football at the AAD meeting in New Orleans exemplifies how mentorship can begin in the most unexpected ways. That casual exchange led to an away rotation, a fellowship interview, connections at national meetings, and the start of what I hope will be a lifelong friendship.
The year was 2023, and I was on my way to the American Academy of Dermatology meeting in New Orleans, Louisiana. “Geaux Tigers!” I exclaimed to a stranger as she walked by in her purple and gold shoes and scrubs. We chatted for a minute or two about Louisiana State University (LSU) football, then went our separate ways. Later that day, in the hands-on wound closures workshop, I was surprised to see my new acquaintance step up to the podium to lecture, then make rounds across the room to instruct residents. I didn’t know it at the time, but those purple and gold shoes sparked a conversation with a fellowship program director who would become one of my most valued mentors.
I didn’t set out to find a mentor that day—I simply was excited to connect with a fellow Tigers fan. But mentorship often finds us unexpectedly, and that encounter serves as a reminder that mentorship doesn’t always start in a formal setting. Sometimes it begins with a quick conversation in the right place at the right time. This story is one of many experiences that taught me valuable lessons about mentorship—its importance, how it can grow naturally, and the impact it can have.
Residency is a pivotal time in a physician’s life, filled with rapid learning, complex challenges, and new professional relationships. Amidst the long hours and heavy responsibilities, mentorship stands out as a support system for guiding residents toward professional and personal growth. Herein, I share more about my experiences with mentorship in residency, the lessons I have learned, and how they can serve as guidance for residents.
The Value of Mentorship
Mentorship in residency has been shown to have a major impact on career satisfaction, clinical confidence, and professional development.1 A good mentor offers more than just advice—he or she can provide a model of professionalism and skills that resonates with the mentee’s own aspirations. Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.2
Mentorship can be sought intentionally or arise naturally from shared interests and connections. Some residents reach out to potential mentors directly through emails, set up one-on-one meetings, or shadow them to gain firsthand experience. Others find mentorship simply by putting themselves in situations that foster these connections, such as attending conferences or lectures. Both approaches can lead to impactful relationships that shape a resident’s career and personal growth.
For residents involved in research, an effective faculty research mentor is particularly impactful. Studies show that residents who work with knowledgeable research mentors are more likely to experience success and productivity in their research efforts.3 Research mentors can provide essential guidance—from helping formulate research questions to navigating the complexities of publishing—which makes them invaluable in a resident’s academic development.
If you have interests in specific areas not heavily emphasized within your residency program (eg, transplantation dermatology, hair restoration, cutaneous lymphoma), consider checking within your broader medical community for specialists. Many dermatologists and other specialists welcome the opportunity to mentor residents who express a sincere interest in learning. By reaching out to these professionals, you not only expand your clinical knowledge but also gain access to niche areas of dermatology that can shape and refine your future practice. Often, these experiences lead to invaluable mentorships that may otherwise be unavailable within your immediate training environment.
Networking Through Professional Society Rotational and Mentorship Programs
The Women’s Dermatologic Society (https://www.womensderm.org/), the American Society for Dermatologic Surgery (https://www.asds.net/), and the American Society for Laser Medicine and Surgery (https://www.aslms.org/) all provide excellent formalized mentorship or preceptorship programs. Check their websites for application requirements and timelines. Participating in these programs is a great way to network with experts in dermatology, providing a structured way to interact with physicians who share your interests. Whether you are interested in medical dermatology, surgery, pediatrics, dermatopathology, or cosmetics, there are many mentors who greatly enjoy sharing their knowledge and experience with residents. Oftentimes, these programs include stipends to assist with costs that are awarded as accolades that can enhance your curriculum vitae. Engaging in these recognized preceptorship programs often builds lasting connections and ensures that both mentor and mentee have a vested interest in the relationship’s success.
Making Connections at Conferences and Maximizing Hands-on Learning
Professional conferences offer valuable opportunities to connect with mentors, whether you are proactively seeking mentorship or simply allowing connections to happen naturally. Conferences such as those of the American Academy of Dermatology and American Society for Dermatologic Surgery publish educational booklets and schedules online prior to the event, giving you a chance to explore both topics and speaker names ahead of time. This can be an excellent opportunity to create a day-by-day game plan, identifying sessions and lectures of interest as well as specific authors or experts you might like to meet. Planning in advance makes it easier to engage with leaders in the field, introduce yourself, and make meaningful connections.
Oftentimes, these society meetings offer hands-on courses, which are a great way to meet mentors and learn from direct instruction. Instructors for these courses often are leaders in dermatology who are passionate about teaching. With small group sizes, hands-on courses offer both technical skill-building opportunities and a chance to connect personally with instructors. Take a moment to introduce yourself and engage in a quick conversation, and if you feel it is appropriate, follow up with an email after the conference. This helps keep the connection alive beyond the event and may open doors for future mentorship opportunities.
Away Rotations
For residents looking to build specialized skills and connect with mentors outside their own program—especially those considering fellowship—away rotations can be a great tool. Though it may require using vacation time, an away rotation offers immersive learning in a particular area while providing opportunities to observe new mentors and establish relationships within a desired subspecialty or program. By simply reaching out and expressing interest, residents can connect with physicians who may become lasting mentors and advocates.
Building a Mentor-Mentee Relationship
A meaningful mentor-mentee relationship requires time, effort, and effective communication, with clear expectations around mentorship goals, time commitments, and how both parties envision the relationship evolving.4 Ideally, mentees should feel comfortable sharing their goals with mentors and asking for feedback. In the right context, a simple and effective practice is to send your mentor a brief update on your progress every few months. This could be a quick email sharing your latest projects, ideas, and/or achievements. By regularly checking in, you show your mentor that you are committed to growing from their guidance and respect their time.
The Lasting Impact of Mentorship
The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.5 Residency often is the last time a resident trains under the direct supervision of an attending physician, making it a unique and formative period. After graduation, many new physicians find the transition to independent practice challenging, and the “real world” can be a shock. Having a mentor during this time, or maintaining connections with mentors from residency, can be invaluable. Mentors can offer advice, act as sounding boards, and remind new graduates of the importance of being lifelong learners. These relationships help ease the transition into practice, instilling a commitment to continuous improvement and professional growth. For me, a conversation about LSU football at the AAD meeting in New Orleans exemplifies how mentorship can begin in the most unexpected ways. That casual exchange led to an away rotation, a fellowship interview, connections at national meetings, and the start of what I hope will be a lifelong friendship.
- Ramanan RA, Taylor WC, Davis RB, et al. Mentoring matters. mentoring and career preparation in internal medicine residency training. J Gen Intern Med. 2006;21:340-345.
- Sambunjak D, Straus SE, Marusic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research-a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Allen TD, Eby LT, Poteet ML, et al. Career benefits associated with mentoring for protégeé: a meta-analysis. J Appl Psychol. 2004;89:127-136.
- Kashiwagi DT, Varkey P, Cook DA. Mentoring programs for physicians in academic medicine: a systematic review. Acad Med. 2013;88:1029-1037.
- Ramanan RA, Taylor WC, Davis RB, et al. Mentoring matters. mentoring and career preparation in internal medicine residency training. J Gen Intern Med. 2006;21:340-345.
- Sambunjak D, Straus SE, Marusic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research-a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Allen TD, Eby LT, Poteet ML, et al. Career benefits associated with mentoring for protégeé: a meta-analysis. J Appl Psychol. 2004;89:127-136.
- Kashiwagi DT, Varkey P, Cook DA. Mentoring programs for physicians in academic medicine: a systematic review. Acad Med. 2013;88:1029-1037.
Mentorship in Residency
Mentorship in Residency
RESIDENT PEARLS
- Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.
- The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.
A Roadmap to Research Opportunities for Dermatology Residents
Dermatology remains one of the most competitive specialties in the residency match, with successful applicants demonstrating a well-rounded application reflecting not only their academic excellence but also their dedication to research, community service, and hands-on clinical experience.1 A growing emphasis on scholarly activities has made it crucial for applicants to stand out, with an increasing number opting to take gap years to engage in focused research endeavors.2 In highly competitive specialties such as dermatology, successful applicants now report more than 20 research items on average.3,4 This trend also is evident in primary care specialties, which have seen a 2- to 3-fold increase in reported research activities. The average unmatched applicant today lists more research items than the average matched applicant did a decade ago, underscoring the growing emphasis on scholarly activity.3
Ideally, graduate medical education should foster an environment of inquiry and scholarship, where residents develop new knowledge, evaluate research findings, and cultivate lifelong habits of inquiry. The Accreditation Council for Graduate Medical Education requires residents to engage in scholarship, such as case reports, research reviews, and original research.5 Research during residency has been linked to several benefits, including enhanced patient care through improved critical appraisal skills, clinical reasoning, and lifelong learning.6,7 Additionally, students and residents who publish research are more likely to achieve higher rank during residency and pursue careers in academic medicine, potentially helping to address the decline in clinician investigators.8,9 Publishing and presenting research also can enhance a residency program’s reputation, making it more attractive to competitive applicants, and may be beneficial for residents seeking jobs or fellowships.6
Dermatology residency programs vary in their structure and support for resident research. One survey revealed that many programs lack the necessary support, structure, and resources to effectively promote and maintain research training.1 Additionally, residents have less exposure to researchers who could serve as mentors due to the growing demands placed on attending physicians in teaching hospitals.10
The Research Arms Race
The growing emphasis on scholarly activity for residency and fellowship applicants coupled with the use of research productivity to differentiate candidates has led some to declare a “research arms race” in residency selection.3 As one author stated, “We need less research, better research, and research done for the right reasons.”11 Indeed, most articles authored by medical students are short reviews or case reports, with the majority (59% [207/350]) being cited zero times, according to one analysis.12 Given the variable research infrastructure between programs and the decreasing availability of research mentors despite the growing emphasis on scholarly activity, applicants face an unfortunate dilemma. Until the system changes, those who protest this research arms race by not engaging in substantial scholarly activity are less likely to match into competitive specialties. Thus, the race continues.
The Value of Mentorship
Resident research success is impacted by having an effective faculty research mentor.13 Although all medical research at the student or resident levels should be conducted with a faculty mentor to oversee it, finding a mentor can be challenging. If a resident’s program boasts a strong research infrastructure or prolific faculty, building relationships with potential mentors is a logical first step for residents wishing to engage in research; however, if suitable mentors are lacking, efforts should be made by residents to establish these connections elsewhere, such as attending society meetings to network with potential mentors and applying to formal mentorship programs (eg, the American Society for Dermatologic Surgery’s Preceptor Program, the Women’s Dermatologic Society’s Mentorship Award). Unsolicited email inquiries asking, “Hi Dr. X, my name is Y, and I was wondering if you have any research projects I could help with?” often go unanswered. Instead, consider emailing or approaching potential mentors with a more developed proposition, such as the following example:
Hello Dr. X, my name is Y. I have enjoyed reading your publications on A, which inspired me to think about B. I reviewed the literature and noticed a potential to enhance our current understanding on the topic. My team and I conducted a systematic review of the available literature and drafted a manuscript summarizing our findings. Given your expertise in this field, would you be willing to collaborate on this paper? We would be grateful for your critical eye, suggestions for improvement, and overall thoughts.
This approach demonstrates initiative, provides a clear plan, and shows respect for the mentor’s expertise, increasing the likelihood of a positive response and fruitful collaboration. Assuming the resident’s working draft meets the potential mentor’s basic expectations, such a display of initiative is likely to impress them, and they may then offer opportunities to engage in meaningful research projects in the future. Everyone benefits! These efforts to establish connections with mentors can pave the way to further collaboration and meaningful research opportunities for dermatology residents.
The Systematic Review: An Attractive Option For Residents
There are several potential avenues for students or residents interested in pursuing research. Case reports and case series are relatively easy to compile, can be completed quickly, and often require minimal guidance from a faculty mentor; however, case reports rank low in the research hierarchy. Conversely, prospective blinded clinical trials provide some of the highest-quality evidence available but are challenging to conduct without a practicing faculty member to provide a patient cohort, often require extensive funding, and may involve complex statistical analyses beyond the expertise of most students or residents. Additionally, they may take years to complete, often extending beyond residency or fellowship application deadlines.
Most medical applicants likely hold at least some hesitation in churning out vast amounts of low-quality research merely to boost their publication count for the match process. Ideally, those who pursue scholarly activity should be driven by a genuine desire to contribute meaningfully to the medical literature. One particularly valuable avenue for trainees wishing to engage in research is the systematic review, which aims to identify, evaluate, and summarize the findings of all relevant individual studies regarding a research topic and answer a focused question. If performed thoughtfully, a systematic review can meaningfully contribute to the medical literature without requiring access to a prospectively followed cohort of patients or the constant supervision of a faculty mentor. Sure, systematic reviews may not be as robust as prospective cohort clinical trials, but they often provide comprehensive insights and are considered valuable contributions to evidence-based medicine. With the help of co-residents or medical students, a medical reference librarian, and a statistician—along with a working understanding of universally accepted quality measures—a resident physician and their team can produce a systematic review that ultimately may merit publication in a top-tier medical journal.
The remainder of this column will outline a streamlined approach to the systematic review writing process, specifically tailored for medical residents who may not have affiliations to a prolific research department or established relationships with faculty mentors in their field of interest. The aim is to offer a basic framework to help residents navigate the complexities of conducting and writing a high-quality, impactful systematic review. It is important to emphasize that resident research should always be conducted under the guidance of a faculty mentor, and this approach is not intended to encourage independent research and publication by residents. Instead, it provides steps that can be undertaken with a foundational understanding of accepted principles, allowing residents to compile a working draft of a manuscript in collaboration with a trusted faculty mentor.
The Systematic Review: A Simple Approach
Step 1: Choose a Topic—Once a resident has decided to embark on conducting a systematic review, the first step is to choose a topic, which requires consideration of several factors to ensure relevance, feasibility, and impact. Begin by identifying areas of clinical uncertainty or controversy in which a comprehensive synthesis of the literature could provide valuable insights. Often, such a topic can be gleaned from the conclusion section of other primary studies; statements such as “further study is needed to determine the efficacy of X” or “systematic reviews would be beneficial to ascertaining the impact of Y” may be a great place to start.
Next, ensure that sufficient primary studies exist to support a robust review or meta-analysis by conducting a preliminary literature search, which will confirm that the chosen topic is both researchable and relevant. A narrow, focused, well-defined topic likely will prove more feasible to review than a broad, ill-defined one. Once a topic is selected, it is advisable to discuss it with a faculty mentor before starting the literature search to ensure the topic’s feasibility and clinical relevance, helping to guide your research in a meaningful direction.
When deciding between a systematic review and a meta-analysis, the nature of the research question is an influential factor. A systematic review is particularly suitable for addressing broad questions or topics when the aim is to summarize and synthesize all relevant research studies; for example, a systematic review may investigate the various treatment options for atopic dermatitis and their efficacy, which allows for a comprehensive overview of the available treatments—both the interventions and the outcomes. In contrast, a meta-analysis is ideal for collecting and statistically combining quantitative data from multiple primary studies, provided there are enough relevant studies available in the literature.
Step 2: Build a Team—Recruiting a skilled librarian to assist with Medical Subject Headings (MeSH) terms and retrieving relevant papers is crucial for conducting a high-quality systematic review or meta-analysis. Medical librarians specializing in health sciences enhance the efficiency, comprehensiveness, and reliability of your literature search, substantially boosting your work’s credibility. These librarians are well versed in medical databases such as PubMed and Embase. Begin by contacting your institution’s library services, as there often are valuable resources and personnel available to assist you. Personally, I was surprised to find a librarian at my institution specifically dedicated to helping medical residents with such projects! These professionals are eager to help, and if provided with the scope and goal of your project, they can deliver literature search results in a digestible format. Similarly, seeking the expertise of a medical statistician is crucial to the accuracy and legitimacy of your study. In your final paper, it is important to recognize the contributions of the librarian and statistician, either as co-authors or in the acknowledgments section.
In addition, recruiting colleagues or medical students can be an effective strategy to make the project more feasible and offer collaborative benefits for all parties involved. Given the growing emphasis on research for residency and fellowship admissions, there usually is no shortage of motivated volunteers.
Next, identify the software tool you will use for your systematic review. Options range from simple spreadsheets such as Microsoft Excel to reference managers such as EndNote or Mendeley or dedicated systematic review tools. Academic institutions may subscribe to paid services such as Covidence (https://www.covidence.org), or you can utilize free alternatives such as Rayyan (https://www.rayyan.ai). Investing time in learning to navigate dedicated systematic review software can greatly enhance efficiency and reduce frustrations compared to more basic methods. Ultimately, staying organized, thorough, and committed is key.
Step 3: Conduct the Literature Review—At this point, your research topic has been decided, a medical reference librarian has provided the results of a comprehensive literature search, and a software tool has been chosen. The next task is to read hundreds or thousands of papers—easy, right? With your dedicated team assembled, the workload can be divided and conquered. The first step involves screening out duplicate and irrelevant studies based on titles and abstracts. Next, review the remaining papers in more detail. Those that pass this preliminary screen should be read in their entirety, and only the papers relevant to the research topic should be included in the final synthesis. If there are uncertainties about a study’s relevance, consulting a faculty mentor is advisable. To ensure the systematic review is as thorough as possible, pay special attention to the references section of each paper, as cited references can reveal relevant studies that may have been missed in the literature search.
Once all relevant papers are compiled and read, the relevant data points should be extracted and imputed into a data sheet. Collaborating with a medical statistician is crucial at this stage, as they can provide guidance on the most effective ways to structure and input data. After all studies are included, the relevant statistical analyses on the resultant dataset can be run.
Step 4: Write the Paper—In 2020, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was developed to ensure transparent and complete reporting of systematic reviews. A full discussion of PRISMA guidelines is beyond the scope of this paper; Page et al14 provided a summary, checklist, and flow diagram that is available online (https://www.prisma-statement.org). Following the PRISMA checklist and guidelines ensures a high-quality, transparent, and reliable systematic review. These guidelines not only help streamline and simplify the writing process but also enhance its efficiency and effectiveness. Discovering the PRISMA checklist can be transformative, providing a valuable roadmap that guides the author through each step of the reporting process, helping to avoid common pitfalls. This structured approach ultimately leads to a more comprehensive and trustworthy review.
Step 5: Make Finishing Touches—At this stage in the systematic review process, the studies have been compiled and thoroughly analyzed and the statistical analysis has been conducted. The results have been organized within a structured framework following the PRISMA checklist. With these steps completed, the next task is to finalize the manuscript and seek a final review from the senior author or faculty mentor. To streamline this process, it is beneficial to adhere to the formatting guidelines of the specific medical journal you intend to submit to. Check the author guidelines on the journal’s website and review recent systematic reviews published there as a reference. Even if you have not chosen a journal yet, formatting your manuscript according to a prestigious journal’s general style provides a strong foundation that can be easily adapted to fit another journal’s requirements if necessary.
Final Thoughts
Designing and conducting a systematic review is no easy task, but it can be a valuable skill for dermatology residents aiming to contribute meaningfully to the medical literature. The process of compiling a systematic review offers an opportunity for developing critical research skills, from formulating a research question to synthesizing evidence and presenting findings in a clear methodical way. Engaging in systematic review writing not only enhances the resident’s understanding of a particular topic but also demonstrates a commitment to scholarly activity—a key factor in an increasingly competitive residency and fellowship application environment.
The basic steps outlined in this article are just one way in which residents can begin to navigate the complexities of medical research, specifically the systematic review process. By assembling a supportive team, utilizing available resources, and adhering to established guidelines such as PRISMA, one can produce a high-quality, impactful review. Ultimately, the systematic review process is not just about publication—it is about fostering a habit of inquiry, improving patient care, and contributing to the ever-evolving field of medicine. With dedication and collaboration, even the most challenging aspects of research can be tackled, paving the way for future opportunities and professional growth. In this way, perhaps one day the spirit of the “research race” can shift from a frantic sprint to a graceful marathon, where each mile is run with heart and every step is filled with purpose.
- Anand P, Szeto MD, Flaten H, et al. Dermatology residency research policies: a 2021 national survey. Int J Womens Dermatol. 2021;7:787-792.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527.
- MedSchoolCoach. How competitive is a dermatology residency? Updated in 2023. ProspectiveDoctor website. Accessed August 22, 2024. https://www.prospectivedoctor.com/how-competitive-is-a-dermatology-residency/#:~:text=Statistics%20on%20the%20Dermatology%20Match,applied%2C%20169%20did%20not%20match
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education Updated July 1, 2023. Accessed August 22, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2023.pdf
- Bhuiya T, Makaryus AN. The importance of engaging in scientific research during medical training. Int J Angiol. 2023;32:153-157.
- Seaburg LA, Wang AT, West CP, et al. Associations between resident physicians’ publications and clinical performance during residency training. BMC Med Educ. 2016;16:22.
- West CP, Halvorsen AJ, McDonald FS. Scholarship during residency training: a controlled comparison study. Am J Med. 2011;124:983-987.e1.
- Bhattacharya SD, Williams JB, De La Fuente SG, et al. Does protected research time during general surgery training contribute to graduates’ career choice? Am Surg. 2011;77:907-910.
- Kralovec PD, Miller JA, Wellikson L, et al. The status of hospital medicine groups in the United States. J Hosp Med. 2006;1:75-80.
- Altman DG. The scandal of poor medical research. BMJ. 1994;308:283-284.
- Wickramasinghe DP, Perera CS, Senarathna S, et al. Patterns and trends of medical student research. BMC Med Educ. 2013;13:175.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research—a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
Dermatology remains one of the most competitive specialties in the residency match, with successful applicants demonstrating a well-rounded application reflecting not only their academic excellence but also their dedication to research, community service, and hands-on clinical experience.1 A growing emphasis on scholarly activities has made it crucial for applicants to stand out, with an increasing number opting to take gap years to engage in focused research endeavors.2 In highly competitive specialties such as dermatology, successful applicants now report more than 20 research items on average.3,4 This trend also is evident in primary care specialties, which have seen a 2- to 3-fold increase in reported research activities. The average unmatched applicant today lists more research items than the average matched applicant did a decade ago, underscoring the growing emphasis on scholarly activity.3
Ideally, graduate medical education should foster an environment of inquiry and scholarship, where residents develop new knowledge, evaluate research findings, and cultivate lifelong habits of inquiry. The Accreditation Council for Graduate Medical Education requires residents to engage in scholarship, such as case reports, research reviews, and original research.5 Research during residency has been linked to several benefits, including enhanced patient care through improved critical appraisal skills, clinical reasoning, and lifelong learning.6,7 Additionally, students and residents who publish research are more likely to achieve higher rank during residency and pursue careers in academic medicine, potentially helping to address the decline in clinician investigators.8,9 Publishing and presenting research also can enhance a residency program’s reputation, making it more attractive to competitive applicants, and may be beneficial for residents seeking jobs or fellowships.6
Dermatology residency programs vary in their structure and support for resident research. One survey revealed that many programs lack the necessary support, structure, and resources to effectively promote and maintain research training.1 Additionally, residents have less exposure to researchers who could serve as mentors due to the growing demands placed on attending physicians in teaching hospitals.10
The Research Arms Race
The growing emphasis on scholarly activity for residency and fellowship applicants coupled with the use of research productivity to differentiate candidates has led some to declare a “research arms race” in residency selection.3 As one author stated, “We need less research, better research, and research done for the right reasons.”11 Indeed, most articles authored by medical students are short reviews or case reports, with the majority (59% [207/350]) being cited zero times, according to one analysis.12 Given the variable research infrastructure between programs and the decreasing availability of research mentors despite the growing emphasis on scholarly activity, applicants face an unfortunate dilemma. Until the system changes, those who protest this research arms race by not engaging in substantial scholarly activity are less likely to match into competitive specialties. Thus, the race continues.
The Value of Mentorship
Resident research success is impacted by having an effective faculty research mentor.13 Although all medical research at the student or resident levels should be conducted with a faculty mentor to oversee it, finding a mentor can be challenging. If a resident’s program boasts a strong research infrastructure or prolific faculty, building relationships with potential mentors is a logical first step for residents wishing to engage in research; however, if suitable mentors are lacking, efforts should be made by residents to establish these connections elsewhere, such as attending society meetings to network with potential mentors and applying to formal mentorship programs (eg, the American Society for Dermatologic Surgery’s Preceptor Program, the Women’s Dermatologic Society’s Mentorship Award). Unsolicited email inquiries asking, “Hi Dr. X, my name is Y, and I was wondering if you have any research projects I could help with?” often go unanswered. Instead, consider emailing or approaching potential mentors with a more developed proposition, such as the following example:
Hello Dr. X, my name is Y. I have enjoyed reading your publications on A, which inspired me to think about B. I reviewed the literature and noticed a potential to enhance our current understanding on the topic. My team and I conducted a systematic review of the available literature and drafted a manuscript summarizing our findings. Given your expertise in this field, would you be willing to collaborate on this paper? We would be grateful for your critical eye, suggestions for improvement, and overall thoughts.
This approach demonstrates initiative, provides a clear plan, and shows respect for the mentor’s expertise, increasing the likelihood of a positive response and fruitful collaboration. Assuming the resident’s working draft meets the potential mentor’s basic expectations, such a display of initiative is likely to impress them, and they may then offer opportunities to engage in meaningful research projects in the future. Everyone benefits! These efforts to establish connections with mentors can pave the way to further collaboration and meaningful research opportunities for dermatology residents.
The Systematic Review: An Attractive Option For Residents
There are several potential avenues for students or residents interested in pursuing research. Case reports and case series are relatively easy to compile, can be completed quickly, and often require minimal guidance from a faculty mentor; however, case reports rank low in the research hierarchy. Conversely, prospective blinded clinical trials provide some of the highest-quality evidence available but are challenging to conduct without a practicing faculty member to provide a patient cohort, often require extensive funding, and may involve complex statistical analyses beyond the expertise of most students or residents. Additionally, they may take years to complete, often extending beyond residency or fellowship application deadlines.
Most medical applicants likely hold at least some hesitation in churning out vast amounts of low-quality research merely to boost their publication count for the match process. Ideally, those who pursue scholarly activity should be driven by a genuine desire to contribute meaningfully to the medical literature. One particularly valuable avenue for trainees wishing to engage in research is the systematic review, which aims to identify, evaluate, and summarize the findings of all relevant individual studies regarding a research topic and answer a focused question. If performed thoughtfully, a systematic review can meaningfully contribute to the medical literature without requiring access to a prospectively followed cohort of patients or the constant supervision of a faculty mentor. Sure, systematic reviews may not be as robust as prospective cohort clinical trials, but they often provide comprehensive insights and are considered valuable contributions to evidence-based medicine. With the help of co-residents or medical students, a medical reference librarian, and a statistician—along with a working understanding of universally accepted quality measures—a resident physician and their team can produce a systematic review that ultimately may merit publication in a top-tier medical journal.
The remainder of this column will outline a streamlined approach to the systematic review writing process, specifically tailored for medical residents who may not have affiliations to a prolific research department or established relationships with faculty mentors in their field of interest. The aim is to offer a basic framework to help residents navigate the complexities of conducting and writing a high-quality, impactful systematic review. It is important to emphasize that resident research should always be conducted under the guidance of a faculty mentor, and this approach is not intended to encourage independent research and publication by residents. Instead, it provides steps that can be undertaken with a foundational understanding of accepted principles, allowing residents to compile a working draft of a manuscript in collaboration with a trusted faculty mentor.
The Systematic Review: A Simple Approach
Step 1: Choose a Topic—Once a resident has decided to embark on conducting a systematic review, the first step is to choose a topic, which requires consideration of several factors to ensure relevance, feasibility, and impact. Begin by identifying areas of clinical uncertainty or controversy in which a comprehensive synthesis of the literature could provide valuable insights. Often, such a topic can be gleaned from the conclusion section of other primary studies; statements such as “further study is needed to determine the efficacy of X” or “systematic reviews would be beneficial to ascertaining the impact of Y” may be a great place to start.
Next, ensure that sufficient primary studies exist to support a robust review or meta-analysis by conducting a preliminary literature search, which will confirm that the chosen topic is both researchable and relevant. A narrow, focused, well-defined topic likely will prove more feasible to review than a broad, ill-defined one. Once a topic is selected, it is advisable to discuss it with a faculty mentor before starting the literature search to ensure the topic’s feasibility and clinical relevance, helping to guide your research in a meaningful direction.
When deciding between a systematic review and a meta-analysis, the nature of the research question is an influential factor. A systematic review is particularly suitable for addressing broad questions or topics when the aim is to summarize and synthesize all relevant research studies; for example, a systematic review may investigate the various treatment options for atopic dermatitis and their efficacy, which allows for a comprehensive overview of the available treatments—both the interventions and the outcomes. In contrast, a meta-analysis is ideal for collecting and statistically combining quantitative data from multiple primary studies, provided there are enough relevant studies available in the literature.
Step 2: Build a Team—Recruiting a skilled librarian to assist with Medical Subject Headings (MeSH) terms and retrieving relevant papers is crucial for conducting a high-quality systematic review or meta-analysis. Medical librarians specializing in health sciences enhance the efficiency, comprehensiveness, and reliability of your literature search, substantially boosting your work’s credibility. These librarians are well versed in medical databases such as PubMed and Embase. Begin by contacting your institution’s library services, as there often are valuable resources and personnel available to assist you. Personally, I was surprised to find a librarian at my institution specifically dedicated to helping medical residents with such projects! These professionals are eager to help, and if provided with the scope and goal of your project, they can deliver literature search results in a digestible format. Similarly, seeking the expertise of a medical statistician is crucial to the accuracy and legitimacy of your study. In your final paper, it is important to recognize the contributions of the librarian and statistician, either as co-authors or in the acknowledgments section.
In addition, recruiting colleagues or medical students can be an effective strategy to make the project more feasible and offer collaborative benefits for all parties involved. Given the growing emphasis on research for residency and fellowship admissions, there usually is no shortage of motivated volunteers.
Next, identify the software tool you will use for your systematic review. Options range from simple spreadsheets such as Microsoft Excel to reference managers such as EndNote or Mendeley or dedicated systematic review tools. Academic institutions may subscribe to paid services such as Covidence (https://www.covidence.org), or you can utilize free alternatives such as Rayyan (https://www.rayyan.ai). Investing time in learning to navigate dedicated systematic review software can greatly enhance efficiency and reduce frustrations compared to more basic methods. Ultimately, staying organized, thorough, and committed is key.
Step 3: Conduct the Literature Review—At this point, your research topic has been decided, a medical reference librarian has provided the results of a comprehensive literature search, and a software tool has been chosen. The next task is to read hundreds or thousands of papers—easy, right? With your dedicated team assembled, the workload can be divided and conquered. The first step involves screening out duplicate and irrelevant studies based on titles and abstracts. Next, review the remaining papers in more detail. Those that pass this preliminary screen should be read in their entirety, and only the papers relevant to the research topic should be included in the final synthesis. If there are uncertainties about a study’s relevance, consulting a faculty mentor is advisable. To ensure the systematic review is as thorough as possible, pay special attention to the references section of each paper, as cited references can reveal relevant studies that may have been missed in the literature search.
Once all relevant papers are compiled and read, the relevant data points should be extracted and imputed into a data sheet. Collaborating with a medical statistician is crucial at this stage, as they can provide guidance on the most effective ways to structure and input data. After all studies are included, the relevant statistical analyses on the resultant dataset can be run.
Step 4: Write the Paper—In 2020, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was developed to ensure transparent and complete reporting of systematic reviews. A full discussion of PRISMA guidelines is beyond the scope of this paper; Page et al14 provided a summary, checklist, and flow diagram that is available online (https://www.prisma-statement.org). Following the PRISMA checklist and guidelines ensures a high-quality, transparent, and reliable systematic review. These guidelines not only help streamline and simplify the writing process but also enhance its efficiency and effectiveness. Discovering the PRISMA checklist can be transformative, providing a valuable roadmap that guides the author through each step of the reporting process, helping to avoid common pitfalls. This structured approach ultimately leads to a more comprehensive and trustworthy review.
Step 5: Make Finishing Touches—At this stage in the systematic review process, the studies have been compiled and thoroughly analyzed and the statistical analysis has been conducted. The results have been organized within a structured framework following the PRISMA checklist. With these steps completed, the next task is to finalize the manuscript and seek a final review from the senior author or faculty mentor. To streamline this process, it is beneficial to adhere to the formatting guidelines of the specific medical journal you intend to submit to. Check the author guidelines on the journal’s website and review recent systematic reviews published there as a reference. Even if you have not chosen a journal yet, formatting your manuscript according to a prestigious journal’s general style provides a strong foundation that can be easily adapted to fit another journal’s requirements if necessary.
Final Thoughts
Designing and conducting a systematic review is no easy task, but it can be a valuable skill for dermatology residents aiming to contribute meaningfully to the medical literature. The process of compiling a systematic review offers an opportunity for developing critical research skills, from formulating a research question to synthesizing evidence and presenting findings in a clear methodical way. Engaging in systematic review writing not only enhances the resident’s understanding of a particular topic but also demonstrates a commitment to scholarly activity—a key factor in an increasingly competitive residency and fellowship application environment.
The basic steps outlined in this article are just one way in which residents can begin to navigate the complexities of medical research, specifically the systematic review process. By assembling a supportive team, utilizing available resources, and adhering to established guidelines such as PRISMA, one can produce a high-quality, impactful review. Ultimately, the systematic review process is not just about publication—it is about fostering a habit of inquiry, improving patient care, and contributing to the ever-evolving field of medicine. With dedication and collaboration, even the most challenging aspects of research can be tackled, paving the way for future opportunities and professional growth. In this way, perhaps one day the spirit of the “research race” can shift from a frantic sprint to a graceful marathon, where each mile is run with heart and every step is filled with purpose.
Dermatology remains one of the most competitive specialties in the residency match, with successful applicants demonstrating a well-rounded application reflecting not only their academic excellence but also their dedication to research, community service, and hands-on clinical experience.1 A growing emphasis on scholarly activities has made it crucial for applicants to stand out, with an increasing number opting to take gap years to engage in focused research endeavors.2 In highly competitive specialties such as dermatology, successful applicants now report more than 20 research items on average.3,4 This trend also is evident in primary care specialties, which have seen a 2- to 3-fold increase in reported research activities. The average unmatched applicant today lists more research items than the average matched applicant did a decade ago, underscoring the growing emphasis on scholarly activity.3
Ideally, graduate medical education should foster an environment of inquiry and scholarship, where residents develop new knowledge, evaluate research findings, and cultivate lifelong habits of inquiry. The Accreditation Council for Graduate Medical Education requires residents to engage in scholarship, such as case reports, research reviews, and original research.5 Research during residency has been linked to several benefits, including enhanced patient care through improved critical appraisal skills, clinical reasoning, and lifelong learning.6,7 Additionally, students and residents who publish research are more likely to achieve higher rank during residency and pursue careers in academic medicine, potentially helping to address the decline in clinician investigators.8,9 Publishing and presenting research also can enhance a residency program’s reputation, making it more attractive to competitive applicants, and may be beneficial for residents seeking jobs or fellowships.6
Dermatology residency programs vary in their structure and support for resident research. One survey revealed that many programs lack the necessary support, structure, and resources to effectively promote and maintain research training.1 Additionally, residents have less exposure to researchers who could serve as mentors due to the growing demands placed on attending physicians in teaching hospitals.10
The Research Arms Race
The growing emphasis on scholarly activity for residency and fellowship applicants coupled with the use of research productivity to differentiate candidates has led some to declare a “research arms race” in residency selection.3 As one author stated, “We need less research, better research, and research done for the right reasons.”11 Indeed, most articles authored by medical students are short reviews or case reports, with the majority (59% [207/350]) being cited zero times, according to one analysis.12 Given the variable research infrastructure between programs and the decreasing availability of research mentors despite the growing emphasis on scholarly activity, applicants face an unfortunate dilemma. Until the system changes, those who protest this research arms race by not engaging in substantial scholarly activity are less likely to match into competitive specialties. Thus, the race continues.
The Value of Mentorship
Resident research success is impacted by having an effective faculty research mentor.13 Although all medical research at the student or resident levels should be conducted with a faculty mentor to oversee it, finding a mentor can be challenging. If a resident’s program boasts a strong research infrastructure or prolific faculty, building relationships with potential mentors is a logical first step for residents wishing to engage in research; however, if suitable mentors are lacking, efforts should be made by residents to establish these connections elsewhere, such as attending society meetings to network with potential mentors and applying to formal mentorship programs (eg, the American Society for Dermatologic Surgery’s Preceptor Program, the Women’s Dermatologic Society’s Mentorship Award). Unsolicited email inquiries asking, “Hi Dr. X, my name is Y, and I was wondering if you have any research projects I could help with?” often go unanswered. Instead, consider emailing or approaching potential mentors with a more developed proposition, such as the following example:
Hello Dr. X, my name is Y. I have enjoyed reading your publications on A, which inspired me to think about B. I reviewed the literature and noticed a potential to enhance our current understanding on the topic. My team and I conducted a systematic review of the available literature and drafted a manuscript summarizing our findings. Given your expertise in this field, would you be willing to collaborate on this paper? We would be grateful for your critical eye, suggestions for improvement, and overall thoughts.
This approach demonstrates initiative, provides a clear plan, and shows respect for the mentor’s expertise, increasing the likelihood of a positive response and fruitful collaboration. Assuming the resident’s working draft meets the potential mentor’s basic expectations, such a display of initiative is likely to impress them, and they may then offer opportunities to engage in meaningful research projects in the future. Everyone benefits! These efforts to establish connections with mentors can pave the way to further collaboration and meaningful research opportunities for dermatology residents.
The Systematic Review: An Attractive Option For Residents
There are several potential avenues for students or residents interested in pursuing research. Case reports and case series are relatively easy to compile, can be completed quickly, and often require minimal guidance from a faculty mentor; however, case reports rank low in the research hierarchy. Conversely, prospective blinded clinical trials provide some of the highest-quality evidence available but are challenging to conduct without a practicing faculty member to provide a patient cohort, often require extensive funding, and may involve complex statistical analyses beyond the expertise of most students or residents. Additionally, they may take years to complete, often extending beyond residency or fellowship application deadlines.
Most medical applicants likely hold at least some hesitation in churning out vast amounts of low-quality research merely to boost their publication count for the match process. Ideally, those who pursue scholarly activity should be driven by a genuine desire to contribute meaningfully to the medical literature. One particularly valuable avenue for trainees wishing to engage in research is the systematic review, which aims to identify, evaluate, and summarize the findings of all relevant individual studies regarding a research topic and answer a focused question. If performed thoughtfully, a systematic review can meaningfully contribute to the medical literature without requiring access to a prospectively followed cohort of patients or the constant supervision of a faculty mentor. Sure, systematic reviews may not be as robust as prospective cohort clinical trials, but they often provide comprehensive insights and are considered valuable contributions to evidence-based medicine. With the help of co-residents or medical students, a medical reference librarian, and a statistician—along with a working understanding of universally accepted quality measures—a resident physician and their team can produce a systematic review that ultimately may merit publication in a top-tier medical journal.
The remainder of this column will outline a streamlined approach to the systematic review writing process, specifically tailored for medical residents who may not have affiliations to a prolific research department or established relationships with faculty mentors in their field of interest. The aim is to offer a basic framework to help residents navigate the complexities of conducting and writing a high-quality, impactful systematic review. It is important to emphasize that resident research should always be conducted under the guidance of a faculty mentor, and this approach is not intended to encourage independent research and publication by residents. Instead, it provides steps that can be undertaken with a foundational understanding of accepted principles, allowing residents to compile a working draft of a manuscript in collaboration with a trusted faculty mentor.
The Systematic Review: A Simple Approach
Step 1: Choose a Topic—Once a resident has decided to embark on conducting a systematic review, the first step is to choose a topic, which requires consideration of several factors to ensure relevance, feasibility, and impact. Begin by identifying areas of clinical uncertainty or controversy in which a comprehensive synthesis of the literature could provide valuable insights. Often, such a topic can be gleaned from the conclusion section of other primary studies; statements such as “further study is needed to determine the efficacy of X” or “systematic reviews would be beneficial to ascertaining the impact of Y” may be a great place to start.
Next, ensure that sufficient primary studies exist to support a robust review or meta-analysis by conducting a preliminary literature search, which will confirm that the chosen topic is both researchable and relevant. A narrow, focused, well-defined topic likely will prove more feasible to review than a broad, ill-defined one. Once a topic is selected, it is advisable to discuss it with a faculty mentor before starting the literature search to ensure the topic’s feasibility and clinical relevance, helping to guide your research in a meaningful direction.
When deciding between a systematic review and a meta-analysis, the nature of the research question is an influential factor. A systematic review is particularly suitable for addressing broad questions or topics when the aim is to summarize and synthesize all relevant research studies; for example, a systematic review may investigate the various treatment options for atopic dermatitis and their efficacy, which allows for a comprehensive overview of the available treatments—both the interventions and the outcomes. In contrast, a meta-analysis is ideal for collecting and statistically combining quantitative data from multiple primary studies, provided there are enough relevant studies available in the literature.
Step 2: Build a Team—Recruiting a skilled librarian to assist with Medical Subject Headings (MeSH) terms and retrieving relevant papers is crucial for conducting a high-quality systematic review or meta-analysis. Medical librarians specializing in health sciences enhance the efficiency, comprehensiveness, and reliability of your literature search, substantially boosting your work’s credibility. These librarians are well versed in medical databases such as PubMed and Embase. Begin by contacting your institution’s library services, as there often are valuable resources and personnel available to assist you. Personally, I was surprised to find a librarian at my institution specifically dedicated to helping medical residents with such projects! These professionals are eager to help, and if provided with the scope and goal of your project, they can deliver literature search results in a digestible format. Similarly, seeking the expertise of a medical statistician is crucial to the accuracy and legitimacy of your study. In your final paper, it is important to recognize the contributions of the librarian and statistician, either as co-authors or in the acknowledgments section.
In addition, recruiting colleagues or medical students can be an effective strategy to make the project more feasible and offer collaborative benefits for all parties involved. Given the growing emphasis on research for residency and fellowship admissions, there usually is no shortage of motivated volunteers.
Next, identify the software tool you will use for your systematic review. Options range from simple spreadsheets such as Microsoft Excel to reference managers such as EndNote or Mendeley or dedicated systematic review tools. Academic institutions may subscribe to paid services such as Covidence (https://www.covidence.org), or you can utilize free alternatives such as Rayyan (https://www.rayyan.ai). Investing time in learning to navigate dedicated systematic review software can greatly enhance efficiency and reduce frustrations compared to more basic methods. Ultimately, staying organized, thorough, and committed is key.
Step 3: Conduct the Literature Review—At this point, your research topic has been decided, a medical reference librarian has provided the results of a comprehensive literature search, and a software tool has been chosen. The next task is to read hundreds or thousands of papers—easy, right? With your dedicated team assembled, the workload can be divided and conquered. The first step involves screening out duplicate and irrelevant studies based on titles and abstracts. Next, review the remaining papers in more detail. Those that pass this preliminary screen should be read in their entirety, and only the papers relevant to the research topic should be included in the final synthesis. If there are uncertainties about a study’s relevance, consulting a faculty mentor is advisable. To ensure the systematic review is as thorough as possible, pay special attention to the references section of each paper, as cited references can reveal relevant studies that may have been missed in the literature search.
Once all relevant papers are compiled and read, the relevant data points should be extracted and imputed into a data sheet. Collaborating with a medical statistician is crucial at this stage, as they can provide guidance on the most effective ways to structure and input data. After all studies are included, the relevant statistical analyses on the resultant dataset can be run.
Step 4: Write the Paper—In 2020, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was developed to ensure transparent and complete reporting of systematic reviews. A full discussion of PRISMA guidelines is beyond the scope of this paper; Page et al14 provided a summary, checklist, and flow diagram that is available online (https://www.prisma-statement.org). Following the PRISMA checklist and guidelines ensures a high-quality, transparent, and reliable systematic review. These guidelines not only help streamline and simplify the writing process but also enhance its efficiency and effectiveness. Discovering the PRISMA checklist can be transformative, providing a valuable roadmap that guides the author through each step of the reporting process, helping to avoid common pitfalls. This structured approach ultimately leads to a more comprehensive and trustworthy review.
Step 5: Make Finishing Touches—At this stage in the systematic review process, the studies have been compiled and thoroughly analyzed and the statistical analysis has been conducted. The results have been organized within a structured framework following the PRISMA checklist. With these steps completed, the next task is to finalize the manuscript and seek a final review from the senior author or faculty mentor. To streamline this process, it is beneficial to adhere to the formatting guidelines of the specific medical journal you intend to submit to. Check the author guidelines on the journal’s website and review recent systematic reviews published there as a reference. Even if you have not chosen a journal yet, formatting your manuscript according to a prestigious journal’s general style provides a strong foundation that can be easily adapted to fit another journal’s requirements if necessary.
Final Thoughts
Designing and conducting a systematic review is no easy task, but it can be a valuable skill for dermatology residents aiming to contribute meaningfully to the medical literature. The process of compiling a systematic review offers an opportunity for developing critical research skills, from formulating a research question to synthesizing evidence and presenting findings in a clear methodical way. Engaging in systematic review writing not only enhances the resident’s understanding of a particular topic but also demonstrates a commitment to scholarly activity—a key factor in an increasingly competitive residency and fellowship application environment.
The basic steps outlined in this article are just one way in which residents can begin to navigate the complexities of medical research, specifically the systematic review process. By assembling a supportive team, utilizing available resources, and adhering to established guidelines such as PRISMA, one can produce a high-quality, impactful review. Ultimately, the systematic review process is not just about publication—it is about fostering a habit of inquiry, improving patient care, and contributing to the ever-evolving field of medicine. With dedication and collaboration, even the most challenging aspects of research can be tackled, paving the way for future opportunities and professional growth. In this way, perhaps one day the spirit of the “research race” can shift from a frantic sprint to a graceful marathon, where each mile is run with heart and every step is filled with purpose.
- Anand P, Szeto MD, Flaten H, et al. Dermatology residency research policies: a 2021 national survey. Int J Womens Dermatol. 2021;7:787-792.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527.
- MedSchoolCoach. How competitive is a dermatology residency? Updated in 2023. ProspectiveDoctor website. Accessed August 22, 2024. https://www.prospectivedoctor.com/how-competitive-is-a-dermatology-residency/#:~:text=Statistics%20on%20the%20Dermatology%20Match,applied%2C%20169%20did%20not%20match
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education Updated July 1, 2023. Accessed August 22, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2023.pdf
- Bhuiya T, Makaryus AN. The importance of engaging in scientific research during medical training. Int J Angiol. 2023;32:153-157.
- Seaburg LA, Wang AT, West CP, et al. Associations between resident physicians’ publications and clinical performance during residency training. BMC Med Educ. 2016;16:22.
- West CP, Halvorsen AJ, McDonald FS. Scholarship during residency training: a controlled comparison study. Am J Med. 2011;124:983-987.e1.
- Bhattacharya SD, Williams JB, De La Fuente SG, et al. Does protected research time during general surgery training contribute to graduates’ career choice? Am Surg. 2011;77:907-910.
- Kralovec PD, Miller JA, Wellikson L, et al. The status of hospital medicine groups in the United States. J Hosp Med. 2006;1:75-80.
- Altman DG. The scandal of poor medical research. BMJ. 1994;308:283-284.
- Wickramasinghe DP, Perera CS, Senarathna S, et al. Patterns and trends of medical student research. BMC Med Educ. 2013;13:175.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research—a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
- Anand P, Szeto MD, Flaten H, et al. Dermatology residency research policies: a 2021 national survey. Int J Womens Dermatol. 2021;7:787-792.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527.
- MedSchoolCoach. How competitive is a dermatology residency? Updated in 2023. ProspectiveDoctor website. Accessed August 22, 2024. https://www.prospectivedoctor.com/how-competitive-is-a-dermatology-residency/#:~:text=Statistics%20on%20the%20Dermatology%20Match,applied%2C%20169%20did%20not%20match
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education Updated July 1, 2023. Accessed August 22, 2024. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2023.pdf
- Bhuiya T, Makaryus AN. The importance of engaging in scientific research during medical training. Int J Angiol. 2023;32:153-157.
- Seaburg LA, Wang AT, West CP, et al. Associations between resident physicians’ publications and clinical performance during residency training. BMC Med Educ. 2016;16:22.
- West CP, Halvorsen AJ, McDonald FS. Scholarship during residency training: a controlled comparison study. Am J Med. 2011;124:983-987.e1.
- Bhattacharya SD, Williams JB, De La Fuente SG, et al. Does protected research time during general surgery training contribute to graduates’ career choice? Am Surg. 2011;77:907-910.
- Kralovec PD, Miller JA, Wellikson L, et al. The status of hospital medicine groups in the United States. J Hosp Med. 2006;1:75-80.
- Altman DG. The scandal of poor medical research. BMJ. 1994;308:283-284.
- Wickramasinghe DP, Perera CS, Senarathna S, et al. Patterns and trends of medical student research. BMC Med Educ. 2013;13:175.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research—a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372.
Resident Pearls
- Establishing a strong relationship with a research mentor is crucial for success in resident research. If your program lacks the necessary infrastructure, take the initiative to network at society meetings or apply for formal mentorship programs.
- For residents facing limited access to patient cohorts and large datasets or those without access to a robust research infrastructure, conducting a systematic review is a valuable and feasible research option, allowing for meaningful contributions to the medical literature.
Surgical Pearls and Wellness Tips From the American Academy of Dermatology Annual Meeting
Attendees of the 2024 American Academy of Dermatology annual meeting in San Diego, California, were eager to delve into the latest trends and advancements in dermatology and dermatologic surgery. This article provides a few key takeaways for residents from a range of engaging sessions, with an emphasis on procedural dermatology and physician health and well-being.
Practical Applications of Surgical Enhancements
In an informative session dedicated to dermatologic surgeons and their patients, “Simple Tricks and Practical Tips to Optimize the Surgical Experience for You and Your Patients,” attendees learned practical tips for enhancing the surgical experience. The discussion spanned various aspects of surgery, from managing preoperative anxiety with anxiolytics such as midazolam to the strategic use of skin hooks for delicate tissue manipulation. Midazolam is fast acting and its use is tailored to patient factors such as weight, hepatic function, and prior use. An innovative anxiety management algorithm combining “talkesethesia” with other methods such as anodynes and benzodiazepines underscored the importance of a calm patient in successful surgical outcomes. Talkesthesia involves engaging patients in soothing and distracting conversation throughout the procedure. This technique can include discussing nonmedical topics of interest with the patient—such as their hobbies, family, or favorite movies—to divert their attention from the surgical process and reduce anxiety. By creating a friendly and reassuring atmosphere, talkesthesia helps to establish trust between the patient and the medical team, ultimately contributing to a more relaxed and cooperative patient.1
The utility of skin hooks also was discussed, with an emphasis on their role in ensuring gentle tissue handling. The modified buried vertical mattress technique was discussed for its added benefits in wound approximation and strength. Emphasis was placed on the importance of maintaining a clear surgical field by electrocautery to ensure optimal visibility.
Focusing on the treatment of skin cancer, curettage alone was touted as a viable alternative to electrodesiccation and curettage, especially in reducing postoperative hypopigmentation while maintaining high cure rates. This method was shown to be effective in treating basal cell carcinoma and well-differentiated squamous cell carcinoma.2,3
Suturing techniques such as pulley, purse-string, and buried sutures offer efficiencies in time, cost, and improved healing in high-tension areas. These methods can contribute to postsurgical aesthetic and functional outcomes. Additionally, Dr. Desiree Ratner shared her tips for painless local anesthesia techniques, emphasizing the importance of patient comfort through methods such as slow injection and buffering of lidocaine. The next time you give a local anesthetic, try this technique to minimize pain: using a 30-gauge needle, hold the syringe with the bevel up, insert only the bevel into the skin (needle tip goes into the papillary dermis), and numb superficially around the periphery using as little volume as possible. Keep pressure slow and steady without moving the needle, then insert the needle only in previously anesthetized areas, numbing deeply only after the entire periphery has been anesthetized.
The session concluded with the recommendation to provide patients with a goody bag containing postoperative supplies. This thoughtful gesture not only enhances patient satisfaction but also addresses the practical aspect of postsurgery care, offering an inexpensive yet impactful way to ensure patients have the necessary supplies for their recovery.
Take-Home Point—This session distilled essential surgical enhancements into practical applications, emphasizing the importance of anxiety management, delicate tissue handling, innovative suturing techniques, and thoughtful patient care postsurgery. The overarching message highlighted the synergy between technical skill and patient-centric approaches in optimizing surgical outcomes, underscoring the significance of attention to detail in every aspect of patient care, from preoperative preparation to postoperative recovery.
Optimizing Safety and Ergonomics in Surgical Practices
Understanding the dynamics of surgical plume is crucial to safety in the operating room. The carcinogenic risk associated with surgical smoke is not trivial: exposure to the plume generated by monopolar electrocautery in a single day can be equivalent to smoking approximately 30 cigarettes, and a surgeon’s lifetime cancer risk from polycyclic aromatic hydrocarbons exposure is alarmingly high.4 To mitigate these risks, several strategies were recommended, including using lower-energy settings, choosing indirect or bipolar cautery, and ensuring efficient room ventilation with HEPA (high-efficiency particulate absorbing) filters to turn over air frequently. Additionally, employing the use of smoke evacuators and suction devices with proper filters can reduce particulate matter in the operating room.
The importance of the surgeon’s posture during procedures also was emphasized for ergonomic benefits and to minimize fatigue. Maintaining a neutral stance with the core and glutes engaged, standing on the balls of the feet, and aligning the table height to keep the hands at the lower chest level were recommended; this not only helps in reducing strain but also in maintaining precision during surgical tasks.
The surgeons on the panel also highlighted the novel use of hydrocolloid dressings with tattoo lasers, electrodesiccation and curettage for treating rhinophyma, and purse-string closure for chest defects as evolving practices to enhance outcomes and safety.
The session offered valuable insights into suturing techniques, advocating for the use of deep sutures—ideally Monocryl (Ethicon US, LLC)—for superficial closures and fast-absorbing gut sutures for patients who are not expected to return for suture removal. Keith LeBlanc Jr, MD, shared one of his favorite tricks for suturing fragile, sun-damaged skin on the forearm in elderly patients: apply adhesive skin closures aligned parallel to the suture line, then suture through them for extra support. This can help ensure a more secure closure.
In situations when no deep sutures are required, such as on the hair-bearing scalp, large bites through the galea using monofilament nonabsorbable sutures for up to 14 days or staples can offer favorable closures and enhanced hemostasis. Tranexamic acid has emerged as a versatile hemostatic agent—available in multiple forms ranging from direct injection to topical applications—and is cost-effective, enhancing its accessibility in various surgical settings.
A high proportion of patients are taken aback by the length of the scar following removal of what they perceive as a small skin cancer. Leslie Storey, MD, cleverly recommended using the back of a glove to mark surgical planning, giving the patient a visual guide for anticipating the size of the excision. This is a simple yet effective approach to enhance patient understanding and informed consent.
Lastly, the notion that “patients remember you if you don’t cause them pain” resonated deeply, underlining the importance of gentle techniques such as pinching the suture rather than pushing the wound edges together and asking assistants to maintain tension without obstructing the field. In the words of Seth Matarasso, MD: “If you pain ‘em, you won’t retain ‘em!”
Take-Home Point—The take-home message from the session was a comprehensive approach to surgical excellence that aligns technical proficiency with a strong emphasis on safety, patient comfort, and operative efficiency. Surgeons were advised to adopt practices that reduce the risks associated with surgical plume, maintain ergonomic discipline, and apply innovative suturing techniques to enhance patient outcomes. Compassionate patient care, innovative use of materials and methods, and a commitment to continual learning and adaptation of new evidence-based practices are paramount for the modern surgeon.
Approaches for Facial Reconstruction
The intricacies of multisubunit facial reconstruction were explored in a session that blended the pursuit of aesthetic harmony with functional restoration, “Simplifying the Complex: Reconstructing Multisubunit Defects.” The session began with an introduction to flap design principles, emphasizing the importance of thorough defect analysis and the strategic design of flaps. A key objective within this framework is the integration of the flap within existing cosmetic subunits to avoid unwanted effects such as unintended eyebrow elevation.
The concept of tissue reservoirs was discussed,focusing on regions such as the glabella as potential sources for skin recruitment. This then transitioned into a nuanced discussion on incision planning, underscoring the significance of aligning incision lines with relaxed skin tension lines to enhance healing and minimize scarring.
The topic of delayed reconstruction also was introduced as a deliberate tactic for high-risk tumor management. This approach allows for an in-depth pathologic examination and provides patients with more time for psychological adjustment, which may be particularly important for those with complex medical histories or those who require staged surgical interventions.
In a thorough examination of flap design techniques, the session highlighted the bilobed transposition flap as a versatile choice for nasal reconstruction, particularly apt for the distal third of the nose due to its design that harnesses skin from nonadjacent areas. Accompanying this was an exploration of Zitelli modifications, which enhance the bilobed flap by reducing issues such as pincushioning through a moderated rotation angle and the strategic incorporation of a Burow triangle.
Finally, the interpolated paranasal flap was discussed. This technique is designed to reduce the risk for cheek asymmetry and is suitable for patients with generous donor sites; however, this method requires diligent evaluation to avoid complications such as external nasal valve collapse.
Take-Home Point—This session highlighted approaches in facial reconstruction, emphasizing the necessity of strategic flap design and meticulous incision planning to maintain aesthetic harmony and functional integrity.
Strategies for Improving Physician Well-Being
Evidence-based recommendations to support physicians’ well-being are crucial as the health care system becomes increasingly demanding. Instead of focusing on aspects of the health care system that frequently are outside of physicians’ control, the session “A Realistic and Evidence-Based Roadmap for Thriving in Life and Career” discussed many practical, self-empowering tools and strategies to lead a happier and healthier life—both personally and professionally.
The speakers cautioned against the concept of an “unlimited ceiling” for achieving a certain goal, where an unlimited amount of time and energy is allowed to be dedicated to a given task over a period of time. They highlighted the potential consequences of this approach, such as stress, dissatisfaction, and ultimately burnout. The speakers explored the concept of well-being as a continuous journey rather than a destination, emphasizing that it is not the opposite of burnout. To promote well-being, the speakers advocated for utilizing concepts rooted in positive psychology to empower the individual rather than longing for a different environment. They hypothesized that changing one’s life can be accomplished by changing one’s mind, independent of the environment.
The roadmap for physician well-being, as presented by clinical psychologist Amy MacDonald, PsyD, commenced with urging the audience to introspect on situations and experiences, categorizing them into “feel good” and “feel bad” buckets. For every feel-good event, Dr. MacDonald proposed 5 mental exercises for optimized well-being: (1) control/increase: evaluate whether one can control or increase the frequency of the event; (2) consider: reflect on why this event feels good and explore other aspects to gain any additional joy from the event; (3) share: recognize that some feel goods are more joyous when shared; (4) value: connect the feel-good experiences with personal core values, as research shows value affirmations can buffer neuroendocrine and psychological stress responses; and (5) savor: deliberately relish each small or notable feel-good moment.
Similarly, after labeling an event as a feel-bad experience, Dr. MacDonald encouraged the audience to go through mental exercises to strengthen their well-being journey; however, before proceeding, she highlighted the importance of arming ourselves with self-compassion. The 5 mental exercises to address feel bads include (1) solve: assess whether we have control over the situation and attempt to make changes if possible; (2) reframe: explore new perspectives and assess assumptions without minimizing the situation; (3) connect: embrace the positive impact of safe human connections on our stress response; (4) reflect: search curiously using a compassionate lens for any existing patterns of reactions; and (5) accept and pivot: allow thoughts and feelings to exist and pivot to values-based engagement without waiting for the environment to change. Consistently seeking and appreciating feel goods while addressing rather than suppressing the feel bads can lead to joyful satisfaction and overall well-being.
Additional pearls for optimizing physician well-being included accurately labeling emotions rather than lumping them into an overarching theme (eg, stressed), avoiding comparisons with others, choosing courage over comfort, celebrating vulnerability, and embracing the ability to say no to prioritize engagements aligned with one’s purpose and values. Additional resources were shared for further reading, including Emotional Agility by Susan David, Daring Greatly and Rising Strong by Brené Brown, and Self-Compassion by Kristin Neff.
Take-Home Point—This lecture highlighted key strategies for physicians to improve their well-being, emphasizing self-empowerment and practical tools over external circumstances. It distinguished between productive and destructive influences on satisfaction, and emphasized decision-making aligned with personal values. The concept of well-being as a journey, not a destination, was central, encouraging positive psychology and self-reflection to enhance fulfillment. By focusing on amplifying feel-good experiences and addressing feel-bad experiences with resilience, the lecture advocated for internal over external change, offering a pathway to a balanced and satisfying professional and personal life for physicians.
Final Thoughts
The recent American Academy of Dermatology meeting offered valuable insights and practical pearls to enhance surgical practices and promote physician well-being, in addition to a wide range of topics beyond what is mentioned in this article. From optimizing surgical techniques to prioritizing patient care and safety, the sessions underscored the importance of continuous learning and adaptation in the ever-evolving field of dermatology. As we reflect on the lessons learned and the camaraderie shared during this gathering, let us carry forward these teachings to improve patient outcomes, foster innovation, and cultivate resilience in our pursuit of excellence. Together, we can continue to push the boundaries of dermatologic care while nurturing our own well-being and that of our colleagues, ensuring a brighter future for both patients and practitioners alike.
Acknowledgments—Sultan H. Qiblawi, MD, MBA; Eva Shelton, MD; and Christy T. Behnam, MD (all from Madison, Wisconsin), shared their insights and key takeaways from American Academy of Dermatology lecturers, which enriched the content of this article.
- Hills LS. Putting patients at ease with conversation. J Med Pract Manage. 2006;22:168-170.
- Barlow JO, Zalla MJ, Kyle A, et al. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54:1039-1045.
- Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol. 2017;77:582-584.
- Shah NR. Commentary on: “surgical smoke—a health hazard in the operating theatre: a study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units.”Ann Med Surg (Lond). 2012;1:23-24.
Attendees of the 2024 American Academy of Dermatology annual meeting in San Diego, California, were eager to delve into the latest trends and advancements in dermatology and dermatologic surgery. This article provides a few key takeaways for residents from a range of engaging sessions, with an emphasis on procedural dermatology and physician health and well-being.
Practical Applications of Surgical Enhancements
In an informative session dedicated to dermatologic surgeons and their patients, “Simple Tricks and Practical Tips to Optimize the Surgical Experience for You and Your Patients,” attendees learned practical tips for enhancing the surgical experience. The discussion spanned various aspects of surgery, from managing preoperative anxiety with anxiolytics such as midazolam to the strategic use of skin hooks for delicate tissue manipulation. Midazolam is fast acting and its use is tailored to patient factors such as weight, hepatic function, and prior use. An innovative anxiety management algorithm combining “talkesethesia” with other methods such as anodynes and benzodiazepines underscored the importance of a calm patient in successful surgical outcomes. Talkesthesia involves engaging patients in soothing and distracting conversation throughout the procedure. This technique can include discussing nonmedical topics of interest with the patient—such as their hobbies, family, or favorite movies—to divert their attention from the surgical process and reduce anxiety. By creating a friendly and reassuring atmosphere, talkesthesia helps to establish trust between the patient and the medical team, ultimately contributing to a more relaxed and cooperative patient.1
The utility of skin hooks also was discussed, with an emphasis on their role in ensuring gentle tissue handling. The modified buried vertical mattress technique was discussed for its added benefits in wound approximation and strength. Emphasis was placed on the importance of maintaining a clear surgical field by electrocautery to ensure optimal visibility.
Focusing on the treatment of skin cancer, curettage alone was touted as a viable alternative to electrodesiccation and curettage, especially in reducing postoperative hypopigmentation while maintaining high cure rates. This method was shown to be effective in treating basal cell carcinoma and well-differentiated squamous cell carcinoma.2,3
Suturing techniques such as pulley, purse-string, and buried sutures offer efficiencies in time, cost, and improved healing in high-tension areas. These methods can contribute to postsurgical aesthetic and functional outcomes. Additionally, Dr. Desiree Ratner shared her tips for painless local anesthesia techniques, emphasizing the importance of patient comfort through methods such as slow injection and buffering of lidocaine. The next time you give a local anesthetic, try this technique to minimize pain: using a 30-gauge needle, hold the syringe with the bevel up, insert only the bevel into the skin (needle tip goes into the papillary dermis), and numb superficially around the periphery using as little volume as possible. Keep pressure slow and steady without moving the needle, then insert the needle only in previously anesthetized areas, numbing deeply only after the entire periphery has been anesthetized.
The session concluded with the recommendation to provide patients with a goody bag containing postoperative supplies. This thoughtful gesture not only enhances patient satisfaction but also addresses the practical aspect of postsurgery care, offering an inexpensive yet impactful way to ensure patients have the necessary supplies for their recovery.
Take-Home Point—This session distilled essential surgical enhancements into practical applications, emphasizing the importance of anxiety management, delicate tissue handling, innovative suturing techniques, and thoughtful patient care postsurgery. The overarching message highlighted the synergy between technical skill and patient-centric approaches in optimizing surgical outcomes, underscoring the significance of attention to detail in every aspect of patient care, from preoperative preparation to postoperative recovery.
Optimizing Safety and Ergonomics in Surgical Practices
Understanding the dynamics of surgical plume is crucial to safety in the operating room. The carcinogenic risk associated with surgical smoke is not trivial: exposure to the plume generated by monopolar electrocautery in a single day can be equivalent to smoking approximately 30 cigarettes, and a surgeon’s lifetime cancer risk from polycyclic aromatic hydrocarbons exposure is alarmingly high.4 To mitigate these risks, several strategies were recommended, including using lower-energy settings, choosing indirect or bipolar cautery, and ensuring efficient room ventilation with HEPA (high-efficiency particulate absorbing) filters to turn over air frequently. Additionally, employing the use of smoke evacuators and suction devices with proper filters can reduce particulate matter in the operating room.
The importance of the surgeon’s posture during procedures also was emphasized for ergonomic benefits and to minimize fatigue. Maintaining a neutral stance with the core and glutes engaged, standing on the balls of the feet, and aligning the table height to keep the hands at the lower chest level were recommended; this not only helps in reducing strain but also in maintaining precision during surgical tasks.
The surgeons on the panel also highlighted the novel use of hydrocolloid dressings with tattoo lasers, electrodesiccation and curettage for treating rhinophyma, and purse-string closure for chest defects as evolving practices to enhance outcomes and safety.
The session offered valuable insights into suturing techniques, advocating for the use of deep sutures—ideally Monocryl (Ethicon US, LLC)—for superficial closures and fast-absorbing gut sutures for patients who are not expected to return for suture removal. Keith LeBlanc Jr, MD, shared one of his favorite tricks for suturing fragile, sun-damaged skin on the forearm in elderly patients: apply adhesive skin closures aligned parallel to the suture line, then suture through them for extra support. This can help ensure a more secure closure.
In situations when no deep sutures are required, such as on the hair-bearing scalp, large bites through the galea using monofilament nonabsorbable sutures for up to 14 days or staples can offer favorable closures and enhanced hemostasis. Tranexamic acid has emerged as a versatile hemostatic agent—available in multiple forms ranging from direct injection to topical applications—and is cost-effective, enhancing its accessibility in various surgical settings.
A high proportion of patients are taken aback by the length of the scar following removal of what they perceive as a small skin cancer. Leslie Storey, MD, cleverly recommended using the back of a glove to mark surgical planning, giving the patient a visual guide for anticipating the size of the excision. This is a simple yet effective approach to enhance patient understanding and informed consent.
Lastly, the notion that “patients remember you if you don’t cause them pain” resonated deeply, underlining the importance of gentle techniques such as pinching the suture rather than pushing the wound edges together and asking assistants to maintain tension without obstructing the field. In the words of Seth Matarasso, MD: “If you pain ‘em, you won’t retain ‘em!”
Take-Home Point—The take-home message from the session was a comprehensive approach to surgical excellence that aligns technical proficiency with a strong emphasis on safety, patient comfort, and operative efficiency. Surgeons were advised to adopt practices that reduce the risks associated with surgical plume, maintain ergonomic discipline, and apply innovative suturing techniques to enhance patient outcomes. Compassionate patient care, innovative use of materials and methods, and a commitment to continual learning and adaptation of new evidence-based practices are paramount for the modern surgeon.
Approaches for Facial Reconstruction
The intricacies of multisubunit facial reconstruction were explored in a session that blended the pursuit of aesthetic harmony with functional restoration, “Simplifying the Complex: Reconstructing Multisubunit Defects.” The session began with an introduction to flap design principles, emphasizing the importance of thorough defect analysis and the strategic design of flaps. A key objective within this framework is the integration of the flap within existing cosmetic subunits to avoid unwanted effects such as unintended eyebrow elevation.
The concept of tissue reservoirs was discussed,focusing on regions such as the glabella as potential sources for skin recruitment. This then transitioned into a nuanced discussion on incision planning, underscoring the significance of aligning incision lines with relaxed skin tension lines to enhance healing and minimize scarring.
The topic of delayed reconstruction also was introduced as a deliberate tactic for high-risk tumor management. This approach allows for an in-depth pathologic examination and provides patients with more time for psychological adjustment, which may be particularly important for those with complex medical histories or those who require staged surgical interventions.
In a thorough examination of flap design techniques, the session highlighted the bilobed transposition flap as a versatile choice for nasal reconstruction, particularly apt for the distal third of the nose due to its design that harnesses skin from nonadjacent areas. Accompanying this was an exploration of Zitelli modifications, which enhance the bilobed flap by reducing issues such as pincushioning through a moderated rotation angle and the strategic incorporation of a Burow triangle.
Finally, the interpolated paranasal flap was discussed. This technique is designed to reduce the risk for cheek asymmetry and is suitable for patients with generous donor sites; however, this method requires diligent evaluation to avoid complications such as external nasal valve collapse.
Take-Home Point—This session highlighted approaches in facial reconstruction, emphasizing the necessity of strategic flap design and meticulous incision planning to maintain aesthetic harmony and functional integrity.
Strategies for Improving Physician Well-Being
Evidence-based recommendations to support physicians’ well-being are crucial as the health care system becomes increasingly demanding. Instead of focusing on aspects of the health care system that frequently are outside of physicians’ control, the session “A Realistic and Evidence-Based Roadmap for Thriving in Life and Career” discussed many practical, self-empowering tools and strategies to lead a happier and healthier life—both personally and professionally.
The speakers cautioned against the concept of an “unlimited ceiling” for achieving a certain goal, where an unlimited amount of time and energy is allowed to be dedicated to a given task over a period of time. They highlighted the potential consequences of this approach, such as stress, dissatisfaction, and ultimately burnout. The speakers explored the concept of well-being as a continuous journey rather than a destination, emphasizing that it is not the opposite of burnout. To promote well-being, the speakers advocated for utilizing concepts rooted in positive psychology to empower the individual rather than longing for a different environment. They hypothesized that changing one’s life can be accomplished by changing one’s mind, independent of the environment.
The roadmap for physician well-being, as presented by clinical psychologist Amy MacDonald, PsyD, commenced with urging the audience to introspect on situations and experiences, categorizing them into “feel good” and “feel bad” buckets. For every feel-good event, Dr. MacDonald proposed 5 mental exercises for optimized well-being: (1) control/increase: evaluate whether one can control or increase the frequency of the event; (2) consider: reflect on why this event feels good and explore other aspects to gain any additional joy from the event; (3) share: recognize that some feel goods are more joyous when shared; (4) value: connect the feel-good experiences with personal core values, as research shows value affirmations can buffer neuroendocrine and psychological stress responses; and (5) savor: deliberately relish each small or notable feel-good moment.
Similarly, after labeling an event as a feel-bad experience, Dr. MacDonald encouraged the audience to go through mental exercises to strengthen their well-being journey; however, before proceeding, she highlighted the importance of arming ourselves with self-compassion. The 5 mental exercises to address feel bads include (1) solve: assess whether we have control over the situation and attempt to make changes if possible; (2) reframe: explore new perspectives and assess assumptions without minimizing the situation; (3) connect: embrace the positive impact of safe human connections on our stress response; (4) reflect: search curiously using a compassionate lens for any existing patterns of reactions; and (5) accept and pivot: allow thoughts and feelings to exist and pivot to values-based engagement without waiting for the environment to change. Consistently seeking and appreciating feel goods while addressing rather than suppressing the feel bads can lead to joyful satisfaction and overall well-being.
Additional pearls for optimizing physician well-being included accurately labeling emotions rather than lumping them into an overarching theme (eg, stressed), avoiding comparisons with others, choosing courage over comfort, celebrating vulnerability, and embracing the ability to say no to prioritize engagements aligned with one’s purpose and values. Additional resources were shared for further reading, including Emotional Agility by Susan David, Daring Greatly and Rising Strong by Brené Brown, and Self-Compassion by Kristin Neff.
Take-Home Point—This lecture highlighted key strategies for physicians to improve their well-being, emphasizing self-empowerment and practical tools over external circumstances. It distinguished between productive and destructive influences on satisfaction, and emphasized decision-making aligned with personal values. The concept of well-being as a journey, not a destination, was central, encouraging positive psychology and self-reflection to enhance fulfillment. By focusing on amplifying feel-good experiences and addressing feel-bad experiences with resilience, the lecture advocated for internal over external change, offering a pathway to a balanced and satisfying professional and personal life for physicians.
Final Thoughts
The recent American Academy of Dermatology meeting offered valuable insights and practical pearls to enhance surgical practices and promote physician well-being, in addition to a wide range of topics beyond what is mentioned in this article. From optimizing surgical techniques to prioritizing patient care and safety, the sessions underscored the importance of continuous learning and adaptation in the ever-evolving field of dermatology. As we reflect on the lessons learned and the camaraderie shared during this gathering, let us carry forward these teachings to improve patient outcomes, foster innovation, and cultivate resilience in our pursuit of excellence. Together, we can continue to push the boundaries of dermatologic care while nurturing our own well-being and that of our colleagues, ensuring a brighter future for both patients and practitioners alike.
Acknowledgments—Sultan H. Qiblawi, MD, MBA; Eva Shelton, MD; and Christy T. Behnam, MD (all from Madison, Wisconsin), shared their insights and key takeaways from American Academy of Dermatology lecturers, which enriched the content of this article.
Attendees of the 2024 American Academy of Dermatology annual meeting in San Diego, California, were eager to delve into the latest trends and advancements in dermatology and dermatologic surgery. This article provides a few key takeaways for residents from a range of engaging sessions, with an emphasis on procedural dermatology and physician health and well-being.
Practical Applications of Surgical Enhancements
In an informative session dedicated to dermatologic surgeons and their patients, “Simple Tricks and Practical Tips to Optimize the Surgical Experience for You and Your Patients,” attendees learned practical tips for enhancing the surgical experience. The discussion spanned various aspects of surgery, from managing preoperative anxiety with anxiolytics such as midazolam to the strategic use of skin hooks for delicate tissue manipulation. Midazolam is fast acting and its use is tailored to patient factors such as weight, hepatic function, and prior use. An innovative anxiety management algorithm combining “talkesethesia” with other methods such as anodynes and benzodiazepines underscored the importance of a calm patient in successful surgical outcomes. Talkesthesia involves engaging patients in soothing and distracting conversation throughout the procedure. This technique can include discussing nonmedical topics of interest with the patient—such as their hobbies, family, or favorite movies—to divert their attention from the surgical process and reduce anxiety. By creating a friendly and reassuring atmosphere, talkesthesia helps to establish trust between the patient and the medical team, ultimately contributing to a more relaxed and cooperative patient.1
The utility of skin hooks also was discussed, with an emphasis on their role in ensuring gentle tissue handling. The modified buried vertical mattress technique was discussed for its added benefits in wound approximation and strength. Emphasis was placed on the importance of maintaining a clear surgical field by electrocautery to ensure optimal visibility.
Focusing on the treatment of skin cancer, curettage alone was touted as a viable alternative to electrodesiccation and curettage, especially in reducing postoperative hypopigmentation while maintaining high cure rates. This method was shown to be effective in treating basal cell carcinoma and well-differentiated squamous cell carcinoma.2,3
Suturing techniques such as pulley, purse-string, and buried sutures offer efficiencies in time, cost, and improved healing in high-tension areas. These methods can contribute to postsurgical aesthetic and functional outcomes. Additionally, Dr. Desiree Ratner shared her tips for painless local anesthesia techniques, emphasizing the importance of patient comfort through methods such as slow injection and buffering of lidocaine. The next time you give a local anesthetic, try this technique to minimize pain: using a 30-gauge needle, hold the syringe with the bevel up, insert only the bevel into the skin (needle tip goes into the papillary dermis), and numb superficially around the periphery using as little volume as possible. Keep pressure slow and steady without moving the needle, then insert the needle only in previously anesthetized areas, numbing deeply only after the entire periphery has been anesthetized.
The session concluded with the recommendation to provide patients with a goody bag containing postoperative supplies. This thoughtful gesture not only enhances patient satisfaction but also addresses the practical aspect of postsurgery care, offering an inexpensive yet impactful way to ensure patients have the necessary supplies for their recovery.
Take-Home Point—This session distilled essential surgical enhancements into practical applications, emphasizing the importance of anxiety management, delicate tissue handling, innovative suturing techniques, and thoughtful patient care postsurgery. The overarching message highlighted the synergy between technical skill and patient-centric approaches in optimizing surgical outcomes, underscoring the significance of attention to detail in every aspect of patient care, from preoperative preparation to postoperative recovery.
Optimizing Safety and Ergonomics in Surgical Practices
Understanding the dynamics of surgical plume is crucial to safety in the operating room. The carcinogenic risk associated with surgical smoke is not trivial: exposure to the plume generated by monopolar electrocautery in a single day can be equivalent to smoking approximately 30 cigarettes, and a surgeon’s lifetime cancer risk from polycyclic aromatic hydrocarbons exposure is alarmingly high.4 To mitigate these risks, several strategies were recommended, including using lower-energy settings, choosing indirect or bipolar cautery, and ensuring efficient room ventilation with HEPA (high-efficiency particulate absorbing) filters to turn over air frequently. Additionally, employing the use of smoke evacuators and suction devices with proper filters can reduce particulate matter in the operating room.
The importance of the surgeon’s posture during procedures also was emphasized for ergonomic benefits and to minimize fatigue. Maintaining a neutral stance with the core and glutes engaged, standing on the balls of the feet, and aligning the table height to keep the hands at the lower chest level were recommended; this not only helps in reducing strain but also in maintaining precision during surgical tasks.
The surgeons on the panel also highlighted the novel use of hydrocolloid dressings with tattoo lasers, electrodesiccation and curettage for treating rhinophyma, and purse-string closure for chest defects as evolving practices to enhance outcomes and safety.
The session offered valuable insights into suturing techniques, advocating for the use of deep sutures—ideally Monocryl (Ethicon US, LLC)—for superficial closures and fast-absorbing gut sutures for patients who are not expected to return for suture removal. Keith LeBlanc Jr, MD, shared one of his favorite tricks for suturing fragile, sun-damaged skin on the forearm in elderly patients: apply adhesive skin closures aligned parallel to the suture line, then suture through them for extra support. This can help ensure a more secure closure.
In situations when no deep sutures are required, such as on the hair-bearing scalp, large bites through the galea using monofilament nonabsorbable sutures for up to 14 days or staples can offer favorable closures and enhanced hemostasis. Tranexamic acid has emerged as a versatile hemostatic agent—available in multiple forms ranging from direct injection to topical applications—and is cost-effective, enhancing its accessibility in various surgical settings.
A high proportion of patients are taken aback by the length of the scar following removal of what they perceive as a small skin cancer. Leslie Storey, MD, cleverly recommended using the back of a glove to mark surgical planning, giving the patient a visual guide for anticipating the size of the excision. This is a simple yet effective approach to enhance patient understanding and informed consent.
Lastly, the notion that “patients remember you if you don’t cause them pain” resonated deeply, underlining the importance of gentle techniques such as pinching the suture rather than pushing the wound edges together and asking assistants to maintain tension without obstructing the field. In the words of Seth Matarasso, MD: “If you pain ‘em, you won’t retain ‘em!”
Take-Home Point—The take-home message from the session was a comprehensive approach to surgical excellence that aligns technical proficiency with a strong emphasis on safety, patient comfort, and operative efficiency. Surgeons were advised to adopt practices that reduce the risks associated with surgical plume, maintain ergonomic discipline, and apply innovative suturing techniques to enhance patient outcomes. Compassionate patient care, innovative use of materials and methods, and a commitment to continual learning and adaptation of new evidence-based practices are paramount for the modern surgeon.
Approaches for Facial Reconstruction
The intricacies of multisubunit facial reconstruction were explored in a session that blended the pursuit of aesthetic harmony with functional restoration, “Simplifying the Complex: Reconstructing Multisubunit Defects.” The session began with an introduction to flap design principles, emphasizing the importance of thorough defect analysis and the strategic design of flaps. A key objective within this framework is the integration of the flap within existing cosmetic subunits to avoid unwanted effects such as unintended eyebrow elevation.
The concept of tissue reservoirs was discussed,focusing on regions such as the glabella as potential sources for skin recruitment. This then transitioned into a nuanced discussion on incision planning, underscoring the significance of aligning incision lines with relaxed skin tension lines to enhance healing and minimize scarring.
The topic of delayed reconstruction also was introduced as a deliberate tactic for high-risk tumor management. This approach allows for an in-depth pathologic examination and provides patients with more time for psychological adjustment, which may be particularly important for those with complex medical histories or those who require staged surgical interventions.
In a thorough examination of flap design techniques, the session highlighted the bilobed transposition flap as a versatile choice for nasal reconstruction, particularly apt for the distal third of the nose due to its design that harnesses skin from nonadjacent areas. Accompanying this was an exploration of Zitelli modifications, which enhance the bilobed flap by reducing issues such as pincushioning through a moderated rotation angle and the strategic incorporation of a Burow triangle.
Finally, the interpolated paranasal flap was discussed. This technique is designed to reduce the risk for cheek asymmetry and is suitable for patients with generous donor sites; however, this method requires diligent evaluation to avoid complications such as external nasal valve collapse.
Take-Home Point—This session highlighted approaches in facial reconstruction, emphasizing the necessity of strategic flap design and meticulous incision planning to maintain aesthetic harmony and functional integrity.
Strategies for Improving Physician Well-Being
Evidence-based recommendations to support physicians’ well-being are crucial as the health care system becomes increasingly demanding. Instead of focusing on aspects of the health care system that frequently are outside of physicians’ control, the session “A Realistic and Evidence-Based Roadmap for Thriving in Life and Career” discussed many practical, self-empowering tools and strategies to lead a happier and healthier life—both personally and professionally.
The speakers cautioned against the concept of an “unlimited ceiling” for achieving a certain goal, where an unlimited amount of time and energy is allowed to be dedicated to a given task over a period of time. They highlighted the potential consequences of this approach, such as stress, dissatisfaction, and ultimately burnout. The speakers explored the concept of well-being as a continuous journey rather than a destination, emphasizing that it is not the opposite of burnout. To promote well-being, the speakers advocated for utilizing concepts rooted in positive psychology to empower the individual rather than longing for a different environment. They hypothesized that changing one’s life can be accomplished by changing one’s mind, independent of the environment.
The roadmap for physician well-being, as presented by clinical psychologist Amy MacDonald, PsyD, commenced with urging the audience to introspect on situations and experiences, categorizing them into “feel good” and “feel bad” buckets. For every feel-good event, Dr. MacDonald proposed 5 mental exercises for optimized well-being: (1) control/increase: evaluate whether one can control or increase the frequency of the event; (2) consider: reflect on why this event feels good and explore other aspects to gain any additional joy from the event; (3) share: recognize that some feel goods are more joyous when shared; (4) value: connect the feel-good experiences with personal core values, as research shows value affirmations can buffer neuroendocrine and psychological stress responses; and (5) savor: deliberately relish each small or notable feel-good moment.
Similarly, after labeling an event as a feel-bad experience, Dr. MacDonald encouraged the audience to go through mental exercises to strengthen their well-being journey; however, before proceeding, she highlighted the importance of arming ourselves with self-compassion. The 5 mental exercises to address feel bads include (1) solve: assess whether we have control over the situation and attempt to make changes if possible; (2) reframe: explore new perspectives and assess assumptions without minimizing the situation; (3) connect: embrace the positive impact of safe human connections on our stress response; (4) reflect: search curiously using a compassionate lens for any existing patterns of reactions; and (5) accept and pivot: allow thoughts and feelings to exist and pivot to values-based engagement without waiting for the environment to change. Consistently seeking and appreciating feel goods while addressing rather than suppressing the feel bads can lead to joyful satisfaction and overall well-being.
Additional pearls for optimizing physician well-being included accurately labeling emotions rather than lumping them into an overarching theme (eg, stressed), avoiding comparisons with others, choosing courage over comfort, celebrating vulnerability, and embracing the ability to say no to prioritize engagements aligned with one’s purpose and values. Additional resources were shared for further reading, including Emotional Agility by Susan David, Daring Greatly and Rising Strong by Brené Brown, and Self-Compassion by Kristin Neff.
Take-Home Point—This lecture highlighted key strategies for physicians to improve their well-being, emphasizing self-empowerment and practical tools over external circumstances. It distinguished between productive and destructive influences on satisfaction, and emphasized decision-making aligned with personal values. The concept of well-being as a journey, not a destination, was central, encouraging positive psychology and self-reflection to enhance fulfillment. By focusing on amplifying feel-good experiences and addressing feel-bad experiences with resilience, the lecture advocated for internal over external change, offering a pathway to a balanced and satisfying professional and personal life for physicians.
Final Thoughts
The recent American Academy of Dermatology meeting offered valuable insights and practical pearls to enhance surgical practices and promote physician well-being, in addition to a wide range of topics beyond what is mentioned in this article. From optimizing surgical techniques to prioritizing patient care and safety, the sessions underscored the importance of continuous learning and adaptation in the ever-evolving field of dermatology. As we reflect on the lessons learned and the camaraderie shared during this gathering, let us carry forward these teachings to improve patient outcomes, foster innovation, and cultivate resilience in our pursuit of excellence. Together, we can continue to push the boundaries of dermatologic care while nurturing our own well-being and that of our colleagues, ensuring a brighter future for both patients and practitioners alike.
Acknowledgments—Sultan H. Qiblawi, MD, MBA; Eva Shelton, MD; and Christy T. Behnam, MD (all from Madison, Wisconsin), shared their insights and key takeaways from American Academy of Dermatology lecturers, which enriched the content of this article.
- Hills LS. Putting patients at ease with conversation. J Med Pract Manage. 2006;22:168-170.
- Barlow JO, Zalla MJ, Kyle A, et al. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54:1039-1045.
- Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol. 2017;77:582-584.
- Shah NR. Commentary on: “surgical smoke—a health hazard in the operating theatre: a study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units.”Ann Med Surg (Lond). 2012;1:23-24.
- Hills LS. Putting patients at ease with conversation. J Med Pract Manage. 2006;22:168-170.
- Barlow JO, Zalla MJ, Kyle A, et al. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol. 2006;54:1039-1045.
- Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol. 2017;77:582-584.
- Shah NR. Commentary on: “surgical smoke—a health hazard in the operating theatre: a study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units.”Ann Med Surg (Lond). 2012;1:23-24.
RESIDENT PEARLS
- By protecting yourself and ensuring your own longevity as a practicing physician, you will be better able to care for your patients over the long term. Focus on self-empowerment and positive psychology for a balanced life.
- Protect yourself from surgical plume by using smoke evacuators and ensuring proper room ventilation with HEPA (high-efficiency particulate absorbing) filters whenever possible. Stick to low-energy settings for electrocautery.
- During surgical procedures, maintain a neutral posture, keep your core and glutes engaged, and adjust the table height to reduce strain and improve precision.
Recurrence Rates of Mohs Micrographic Surgery vs Radiation Therapy for Basal Cell Carcinoma of the Ear
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.
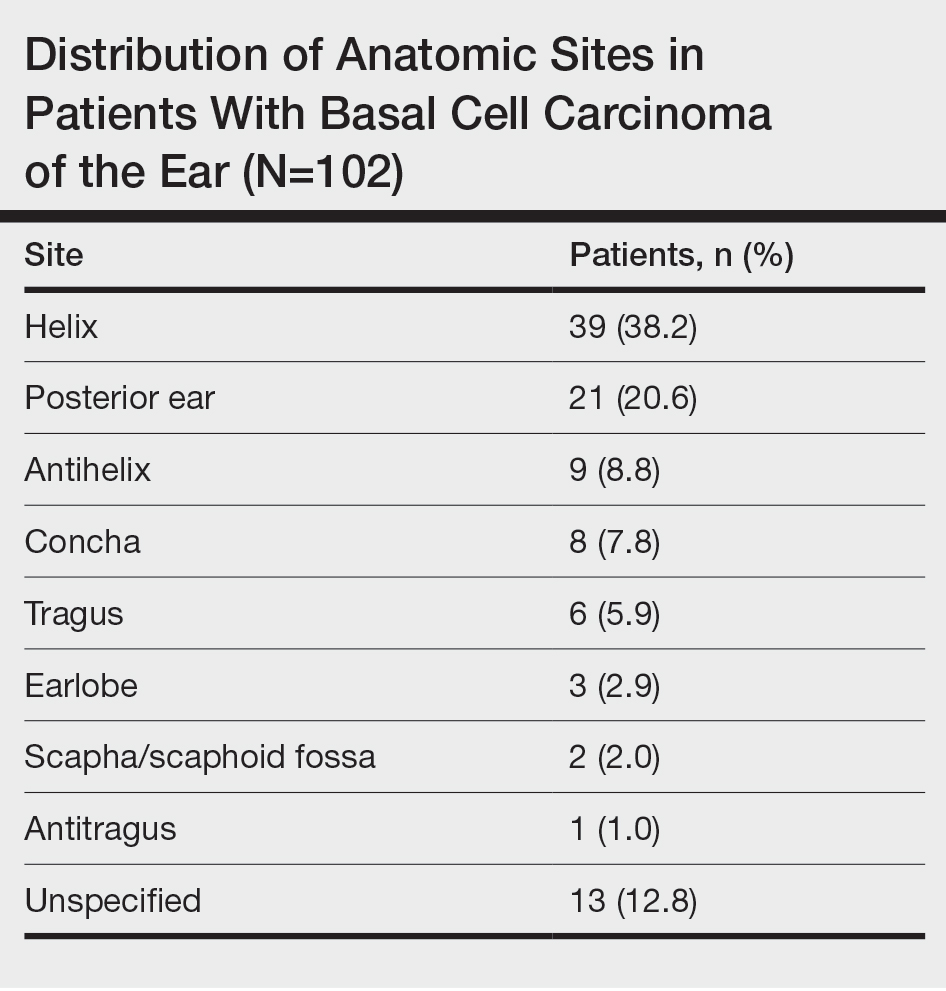
Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
PRACTICE POINTS
- Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations, highlighting the importance of careful management and follow-up.
- Although Mohs micrographic surgery remains the gold standard for treating BCC of the ear, radiation therapy can be considered as a suitable alternative for nonsurgical candidates.
Management of Acute and Chronic Pain Associated With Hidradenitis Suppurativa: A Comprehensive Review of Pharmacologic and Therapeutic Considerations in Clinical Practice
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
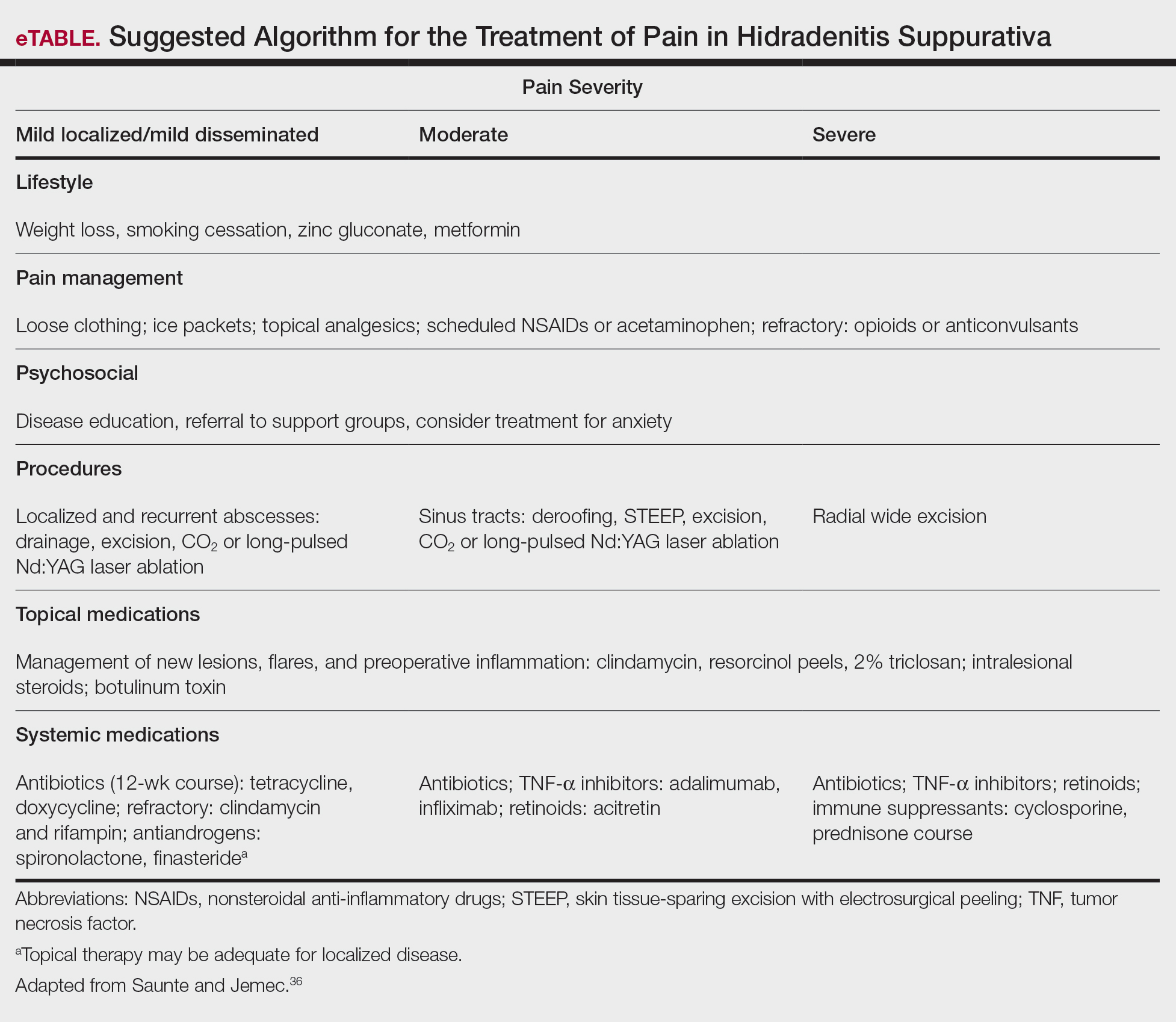
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36

Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36

Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Practice Points
- First-line therapies may not provide adequate pain control in many patients with hidradenitis suppurativa.
- Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.
- Long-term management involves lifestyle modifications and pharmacologic agents.
- The most effective pain remedies developed thus far are limited to surgery and tumor necrosis factor α inhibitors.