User login
How Should the Treatment Costs of Distal Radius Fractures Be Measured?
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
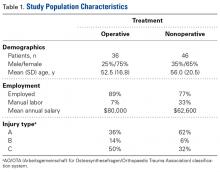
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
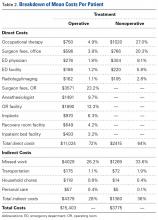
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
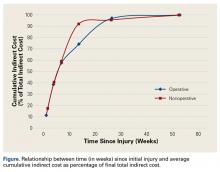
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
The Effect of Ligament Injuries on Outcomes of Operatively Treated Distal Radius Fractures
Take-Home Points
- Patients sustaining DRFs commonly have associated ligament injuries and chondral damage as well.
- Many of these associated injuries do not seem to affect outcomes up to 1 year after surgery.
- Plain radiographs have a 74% sensitivity and 73% specificity for detecting intra-articular fractures.
- ”Minor” injuries identified incidentally by arthroscopy during fixation of DRFs may not require dedicated treatment.
- The optimal treatment for high-grade ligament or chondral injuries in patients with DRFs remains incompletely understood.
Distal radius fracture (DRF) is one of the most common upper extremity injuries, with up to 20% to 50% requiring surgical fixation.1 With increasing use of wrist arthroscopy to assist in managing these fractures,2-6 it has become easier to accurately assess concomitant wrist ligament injuries. Reported injury rates are 18% to 86% for the scapholunate interosseous ligament (SLIL),7,8 5% to 29% for the lunotriquetral ligament (LTL),8,9 and 17% to 60% for the triangular fibrocartilage complex (TFCC).10,11 Reported chondral injury rates range from 18% to 60%.7,9,12 Despite the common occurrence of these injuries, it is unclear how they affect outcomes and how aggressively they should be treated when detected during fracture surgery.
As the use of arthroscopy in DRF management becomes more common, surgeons often must decide how to treat ligamentous/chondral injuries incidentally discovered during surgery. To date, only 1 study prospectively evaluated how these injuries affect DRF outcomes,8 though it did not use a validated, patient-based outcome measure.
We conducted a study to address a common clinical scenario: When arthroscopy is used to assist with intra-articular reduction during DRF fixation, how should the surgeon respond to incidentally identified ligament and chondral injuries? Specifically, we wanted to address 3 questions: What is the overall incidence of SLIL, TFCC, and chondral surface injuries in patients undergoing operative fracture fixation? On initial injury films, do any radiographic parameters predict specific soft-tissue injuries or ultimate functional outcomes? Do wrist ligament and chondral injuries affect patient-rated outcomes (disability, pain) and objective measures (range of motion [ROM], grip strength, pinch strength) up to 1 year after fracture surgery?
Materials and Methods
Patient Selection/Population
This observational, prognostic study was approved by our Institutional Review Board. Inclusion criteria were age over 18 years, isolated acute operatively treated DRF (surgery within 14 days of injury), and informed consent. All patients were treated by the same surgeon. Exclusion criteria were open DRF, dorsal shear pattern, fractures requiring dorsal arthrotomy for reduction because of significant intra-articular damage, prior ipsilateral DRF, and prior SLIL or TFCC injury.
Surgery was indicated according to general radiographic parameters as measured on postreduction films: radial height, <8 mm; radial inclination, <15°; positive ulnar variance, >3 mm, or 3 mm more than contralateral side; dorsal tilt, >10°; and volar tilt, >15°. With these parameters within acceptable limits, surgery was also indicated when fractures were deemed unstable and likely to displace because of dorsal tilt >20°, dorsal comminution, intra-articular step-off of ≥2 mm on the posterior-anterior (PA) film, associated ulnar fracture, and age >60 years.13Over a 2-year period, 42 patients (12 male, 30 female) met the inclusion criteria and were enrolled in the study. The dominant arm was affected in 17 patients (40%). Mean (SD) age at time of injury was 56.6 (16.4) years (median, 54 years; range, 20-85 years).
Operative Technique
During surgery, damage to the SLIL, the TFCC, and chondral surfaces (scaphoid, lunate, scaphoid fossa, lunate fossa) and to the intra-articular extension of the DRF was assessed and recorded. Wrist arthroscopy was performed with the 3, 4 portal as the primary portal. When significant damage to the TFCC warranted débridement, the 6R (radial) portal was used as an accessory portal. As a midcarpal portal was not used for SLIL assessment, we used a novel classification system: 0 = no injury, normal-appearing ligament without hemorrhage and smooth transition from scaphoid to lunate surface except for slight concave indentation at the ligament; 1 = attenuation, no visible tear with convex shape of ligament with or without hemorrhage; 2 = partial tear with or without step-off at junction between scaphoid and lunate, but 2.7-mm arthroscope cannot “drive through” to midcarpal joint; and 3 = complete tear with positive “drive-through” sign. TFCC injuries were classified according to the system described by Palmer14: Avulsions were central (1A), ulnar (1B), distal (1C), or radial (1D). The trampoline test was performed through a 6R portal by using a probe to evaluate ligament tension/laxity. In some cases, a 6R portal was deemed unnecessary, and a modified trampoline test was performed—tension/laxity/displacement was evaluated by manually palpating at the fovea and observing TFCC motion with the arthroscope. When appropriate, the TFCC was débrided with a shaver through the 6R portal. In cases of significant instability at the SLIL interval, two 0.062-inch K-wires were placed percutaneously through the scaphoid and lunate, and one was placed from the scaphoid to the capitate.
All DRFs underwent internal fixation with a locked volar plate. When necessary, K-wires and/or a locked radial column plate was used for additional fixation. External fixation was not used. The postoperative protocol began with a dorsal wrist splint placed on the patient in the operating room and worn for 10 to 14 days. At the first postoperative visit, the patient received a removable splint that was to be worn at all times except during showers, therapy, and home exercises. Occupational therapy, initiated the week of the first postoperative visit, consisted of active and passive ROM exercises. At 6 weeks, the splint was removed and strengthening initiated.
Outcome Measures
Our primary outcome measure was the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire at 1 year.15 Secondary outcome measures were visual analog scale (VAS) pain rating, ROM, and radiographic measurements. Patients returned for evaluation 2, 6, 12, 24, and 52 weeks after surgery. At each follow-up visit, the DASH questionnaire and the pain VAS were administered, and ROM and strength were measured. Patient-reported pain was recorded on a standard VAS and measured on a scale from 0 (no pain) to 10 (worst possible pain). Wrist flexion and extension and radioulnar deviation were assessed with a goniometer. Forearm supination and pronation were assessed with the elbow flexed 90° at the patient’s side. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan), and lateral pinch strength was measured with a hydraulic pinch gauge (Sammons Preston Rolyan). The average of 3 trials for both hands was recorded for all strength measurements.
Radiographs were obtained on presentation. When appropriate, the fracture was manually reduced with a hematoma block, and postreduction radiographs were obtained. Then, radiographs were obtained at each postoperative visit until union. Radial height, radial inclination, tilt, and ulnar variance were measured on preoperative and postoperative radiographs according to standard methods.16 Radiographs were used to classify the fracture patterns according to the AO/ASIF (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) classification. Union was determined by radiographic healing, absence of tenderness to palpation, absence of pain with motion, and continued functional improvement.
Data Analysis
To evaluate for relationships between patient injury parameters and outcome measures, we used a 1-way analysis of variance seeking statistically significant differences between groups. Patients were divided into 4 groups: no ligament injuries; isolated SLIL injuries; isolated TFCC injuries; and both SLIL and TFCC injuries. These injury classification categories were then evaluated independently against our chosen outcome measures, which included DASH and VAS pain scores, ROM, and grip/pinch strength.
To determine the optimal sample size, we performed a power analysis to estimate the number of patients required to detect a clinically significant difference in DASH scores at 1 year among the 4 groups. According to the literature, standard deviations of DASH scores in healthy volunteers range from 10 to 15,17 consistent with values found in other recent trials of patients with DRFs.18 The recent literature on DASH construct validity has established a DASH score difference of 19 as representing a disability change being “much better or much worse.”19 As such, power analysis for a 1-way analysis of variance among 4 categories, detecting a DASH score difference of 19 with a standard deviation ranging from 10 to 15, would require 28 to 60 patients to detect a difference with an α of 0.05 and a power of 0.8.
In addition, radiographic parameters at time of injury were compared with injury characteristics to assess for significant relationships. Multivariate linear regression analysis was performed to evaluate radial height, radial inclination, and volar tilt as possible predictors of SLIL injury, TFCC injury, and chondral surface damage. A statistically significant result was defined as a correlation with P < .05.
Results
Of the 42 patients included in the study, 11 (26%) had no ligament injuries, 10 (24%) had isolated SLIL injuries, 12 (29%) had isolated TFCC injuries, and 9 (21%) had injuries to both the SLIL and the TFCC. In addition, in 12 patients (29%), the articular cartilage had visible damage (Table 1).
In all patients, bony union occurred. After union, 1 patient underwent hardware removal for hardware-related pain. The same patient had a dorsal ulnar cutaneous nerve neurolysis at the ulnar styloid fixation site. Another patient developed a partial extensor pollicis longus tear from a prominent dorsal screw tip.
All patients returned for their 2- and 6-week follow-ups. At 1 year, 30 patients (71%) returned for follow-up, 11 could not be contacted, and 1 was removed because of an olecranon fracture from a subsequent fall.
Regarding the primary outcome measure, mean DASH score at 1-year follow-up was 30.8 for the group without injuries, 10.8 for the group with SLIL injuries, 14.7 for the group with TFCC injuries, and 21.9 for the group with SLIL and TFCC injuries (Table 2).
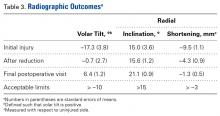
Radiographic parameters were restored to acceptable limits in all patients (Table 3).
Discussion
Use of wrist arthroscopy in DRF management has allowed assessment of the incidence of intra-articular injuries, including ligament and chondral surface injuries. Although the literature on the incidence of these injuries has been expanding, their clinical significance remains unclear.
Authors have postulated that some patients do not do well after DRF repair because of undetected ligament injuries. With the current trend of internal fixation, locked plating, and early motion—contrasting with older trends of prolonged immobilization in a cast or external fixation—concerns have been raised that early mobilization results in inadequate treatment of ligament injuries. However, data from the present study suggest no significant morbidity from early mobilization despite the presence of ligament injuries in more than half of all operatively treated DRFs. It is possible morbidity was not appreciated, as most patients with DRFs end up with some stiffness, which masks the effects of ligament injuries during healing.
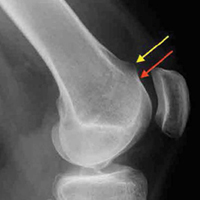
We found no correlation between injury radiographic parameters, observed soft-tissue injuries, or final subjective outcomes. Interestingly, in this study, there was some discordance between the appearance of intra-articular fractures on radiographs and the direct arthroscopic observation of intra-articular fracture extension. With the present data and with arthroscopic visualization as the gold standard, radiographs had 74% sensitivity and 73% specificity for detecting intra-articular fractures (the corresponding positive predictive value was 83%, and the negative predictive value was 61%). As we typically rely on radiographs as the primary tool in assessing the articular component of a fracture, these results should be taken into account when basing management decisions exclusively on static injury films.
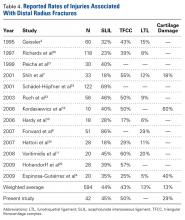
Observational studies of arthroscopy in DRFs have revealed a wide range of injury rates: For SLILs, the average injury rate was 44%; for LTLs, 13%; for TFCCs, 43%; and for chondral surfaces, 32% (Table 4).
This study had several limitations, including loss to follow-up at the primary endpoint (we were unable to contact 29% of patients). In addition, because of resource limitations, we were able to enroll only a limited number of patients, and as a result were able to power the study to detect only major effects on DASH scores. Therefore, although our 32 patients with long-term follow-up are within the range dictated by the power analysis, this study was not powered to capture more subtle differences in disability. Furthermore, because we used 1 year as the longest follow-up point, the long-term sequelae (eg, arthritis) of these injuries may not have been captured. Last, despite the high incidence of soft-tissue injuries overall, the number of patients with severe ligament injuries was relatively low, which makes it difficult to make definitive statements about their contribution to outcomes. A likely explanation is that patients with high-energy injuries and significant intra-articular displacement requiring open arthrotomies were excluded.
At 1-year follow-up, with use of DASH as the gold standard for disability, we found no major difference in subjective or objective outcome measures between patients with and without ligament injuries. Radiographs did not predict soft-tissue injury or ultimate outcome. Rates of ligament injuries in our operatively treated DRFs were similar to those in the literature. Overall, these findings suggest that “minor” injuries incidentally discovered with arthroscopy during DRF surgery may not have a significant effect on outcomes, with the caveat that the significance of very severe injuries (eg, Geissler grade 4 injuries with frank scapholunate diastasis) remains incompletely understood. The decision by the treating surgeon to perform arthroscopy and/or to repair soft-tissue injuries should be made on a case-by-case basis.
Am J Orthop. 2017;46(1):E41-E46. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand. 1990;61(5):457-459.
2. Geissler WB. Arthroscopically assisted reduction of intra-articular fractures of the distal radius. Hand Clin. 1995;11(1):19-29.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Wolfe SW, Easterling KJ, Yoo HH. Arthroscopic-assisted reduction of distal radius fractures. Arthroscopy. 1995;11(6):706-714.
5. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
6. Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093-1110.
7. Shih JT, Lee HM, Hou YT, Tan CM. Arthroscopically-assisted reduction of intra-articular fractures and soft tissue management of distal radius. Hand Surg. 2001;6(2):127-135.
8. Forward DP, Lindau TR, Melsom DS. Intercarpal ligament injuries associated with fractures of the distal part of the radius. J Bone Joint Surg Am. 2007;89(11):2334-2340.
9. Espinosa-Gutiérrez A, Rivas-Montero JA, Elias-Escobedo A, Alisedo-Ochoa PG. Wrist arthroscopy for fractures of the distal end of the radius [in Spanish]. Acta Ortop Mex. 2009;23(6):358-365.
10. Hardy P, Gomes N, Chebil M, Bauer T. Wrist arthroscopy and intra-articular fractures of the distal radius in young adults. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1225-1230.
11. Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778-785.
12. Kordasiewicz B, Pomianowski S, Rylski W, Antolak L, Marczak D. Intraarticular distal radius fractures—arthroscopic assessment of injuries [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2006;71(2):113-116.
13. Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20(4):208-210.
14. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989;14(4):594-606.
15. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med. 1996;30(3):372]. Am J Ind Med. 1996;29(6):602-608.
16. Fernandez DL, Jupiter JB. Fractures of the Distal Radius: A Practical Approach to Management. New York, NY: Springer; 1996.
17. Jester A, Harth A, Wind G, Germann G, Sauerbier M. Does the Disability of Shoulder, Arm and Hand questionnaire (DASH) replace grip strength and range of motion in outcome-evaluation? [in German]. Handchir Mikrochir Plast Chir. 2005;37(2):126-130.
18. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
19. Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
20. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
21. Peicha G, Seibert F, Fellinger M, Grechenig W. Midterm results of arthroscopic treatment of scapholunate ligament lesions associated with intra-articular distal radius fractures. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):327-333.
22. Schädel-Höpfner M, Böhringer G, Junge A, Celik I, Gotzen L. [Arthroscopic diagnosis of concomitant scapholunate ligament injuries in fractures of the distal radius]. Handchir Mikrochir Plast Chir. 2001;33(4):229-233.
23. Ruch DS, Yang CC, Smith BP. Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19(5):511-516.
24. Hattori Y, Doi K, Estrella EP, Chen G. Arthroscopically assisted reduction with volar plating or external fixation for displaced intra-articular fractures of the distal radius in the elderly patients. Hand Surg. 2007;12(1):1-12.
25. Hohendorff B, Eck M, Mühldorfer M, Fodor S, Schmitt R, Prommersberger KJ. [Palmar wrist arthroscopy for evaluation of concomitant carpal lesions in operative treatment of distal intraarticular radius fractures]. Handchir Mikrochir Plast Chir. 2009;41(5):295-299.
Take-Home Points
- Patients sustaining DRFs commonly have associated ligament injuries and chondral damage as well.
- Many of these associated injuries do not seem to affect outcomes up to 1 year after surgery.
- Plain radiographs have a 74% sensitivity and 73% specificity for detecting intra-articular fractures.
- ”Minor” injuries identified incidentally by arthroscopy during fixation of DRFs may not require dedicated treatment.
- The optimal treatment for high-grade ligament or chondral injuries in patients with DRFs remains incompletely understood.
Distal radius fracture (DRF) is one of the most common upper extremity injuries, with up to 20% to 50% requiring surgical fixation.1 With increasing use of wrist arthroscopy to assist in managing these fractures,2-6 it has become easier to accurately assess concomitant wrist ligament injuries. Reported injury rates are 18% to 86% for the scapholunate interosseous ligament (SLIL),7,8 5% to 29% for the lunotriquetral ligament (LTL),8,9 and 17% to 60% for the triangular fibrocartilage complex (TFCC).10,11 Reported chondral injury rates range from 18% to 60%.7,9,12 Despite the common occurrence of these injuries, it is unclear how they affect outcomes and how aggressively they should be treated when detected during fracture surgery.
As the use of arthroscopy in DRF management becomes more common, surgeons often must decide how to treat ligamentous/chondral injuries incidentally discovered during surgery. To date, only 1 study prospectively evaluated how these injuries affect DRF outcomes,8 though it did not use a validated, patient-based outcome measure.
We conducted a study to address a common clinical scenario: When arthroscopy is used to assist with intra-articular reduction during DRF fixation, how should the surgeon respond to incidentally identified ligament and chondral injuries? Specifically, we wanted to address 3 questions: What is the overall incidence of SLIL, TFCC, and chondral surface injuries in patients undergoing operative fracture fixation? On initial injury films, do any radiographic parameters predict specific soft-tissue injuries or ultimate functional outcomes? Do wrist ligament and chondral injuries affect patient-rated outcomes (disability, pain) and objective measures (range of motion [ROM], grip strength, pinch strength) up to 1 year after fracture surgery?
Materials and Methods
Patient Selection/Population
This observational, prognostic study was approved by our Institutional Review Board. Inclusion criteria were age over 18 years, isolated acute operatively treated DRF (surgery within 14 days of injury), and informed consent. All patients were treated by the same surgeon. Exclusion criteria were open DRF, dorsal shear pattern, fractures requiring dorsal arthrotomy for reduction because of significant intra-articular damage, prior ipsilateral DRF, and prior SLIL or TFCC injury.
Surgery was indicated according to general radiographic parameters as measured on postreduction films: radial height, <8 mm; radial inclination, <15°; positive ulnar variance, >3 mm, or 3 mm more than contralateral side; dorsal tilt, >10°; and volar tilt, >15°. With these parameters within acceptable limits, surgery was also indicated when fractures were deemed unstable and likely to displace because of dorsal tilt >20°, dorsal comminution, intra-articular step-off of ≥2 mm on the posterior-anterior (PA) film, associated ulnar fracture, and age >60 years.13Over a 2-year period, 42 patients (12 male, 30 female) met the inclusion criteria and were enrolled in the study. The dominant arm was affected in 17 patients (40%). Mean (SD) age at time of injury was 56.6 (16.4) years (median, 54 years; range, 20-85 years).
Operative Technique
During surgery, damage to the SLIL, the TFCC, and chondral surfaces (scaphoid, lunate, scaphoid fossa, lunate fossa) and to the intra-articular extension of the DRF was assessed and recorded. Wrist arthroscopy was performed with the 3, 4 portal as the primary portal. When significant damage to the TFCC warranted débridement, the 6R (radial) portal was used as an accessory portal. As a midcarpal portal was not used for SLIL assessment, we used a novel classification system: 0 = no injury, normal-appearing ligament without hemorrhage and smooth transition from scaphoid to lunate surface except for slight concave indentation at the ligament; 1 = attenuation, no visible tear with convex shape of ligament with or without hemorrhage; 2 = partial tear with or without step-off at junction between scaphoid and lunate, but 2.7-mm arthroscope cannot “drive through” to midcarpal joint; and 3 = complete tear with positive “drive-through” sign. TFCC injuries were classified according to the system described by Palmer14: Avulsions were central (1A), ulnar (1B), distal (1C), or radial (1D). The trampoline test was performed through a 6R portal by using a probe to evaluate ligament tension/laxity. In some cases, a 6R portal was deemed unnecessary, and a modified trampoline test was performed—tension/laxity/displacement was evaluated by manually palpating at the fovea and observing TFCC motion with the arthroscope. When appropriate, the TFCC was débrided with a shaver through the 6R portal. In cases of significant instability at the SLIL interval, two 0.062-inch K-wires were placed percutaneously through the scaphoid and lunate, and one was placed from the scaphoid to the capitate.
All DRFs underwent internal fixation with a locked volar plate. When necessary, K-wires and/or a locked radial column plate was used for additional fixation. External fixation was not used. The postoperative protocol began with a dorsal wrist splint placed on the patient in the operating room and worn for 10 to 14 days. At the first postoperative visit, the patient received a removable splint that was to be worn at all times except during showers, therapy, and home exercises. Occupational therapy, initiated the week of the first postoperative visit, consisted of active and passive ROM exercises. At 6 weeks, the splint was removed and strengthening initiated.
Outcome Measures
Our primary outcome measure was the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire at 1 year.15 Secondary outcome measures were visual analog scale (VAS) pain rating, ROM, and radiographic measurements. Patients returned for evaluation 2, 6, 12, 24, and 52 weeks after surgery. At each follow-up visit, the DASH questionnaire and the pain VAS were administered, and ROM and strength were measured. Patient-reported pain was recorded on a standard VAS and measured on a scale from 0 (no pain) to 10 (worst possible pain). Wrist flexion and extension and radioulnar deviation were assessed with a goniometer. Forearm supination and pronation were assessed with the elbow flexed 90° at the patient’s side. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan), and lateral pinch strength was measured with a hydraulic pinch gauge (Sammons Preston Rolyan). The average of 3 trials for both hands was recorded for all strength measurements.
Radiographs were obtained on presentation. When appropriate, the fracture was manually reduced with a hematoma block, and postreduction radiographs were obtained. Then, radiographs were obtained at each postoperative visit until union. Radial height, radial inclination, tilt, and ulnar variance were measured on preoperative and postoperative radiographs according to standard methods.16 Radiographs were used to classify the fracture patterns according to the AO/ASIF (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) classification. Union was determined by radiographic healing, absence of tenderness to palpation, absence of pain with motion, and continued functional improvement.
Data Analysis
To evaluate for relationships between patient injury parameters and outcome measures, we used a 1-way analysis of variance seeking statistically significant differences between groups. Patients were divided into 4 groups: no ligament injuries; isolated SLIL injuries; isolated TFCC injuries; and both SLIL and TFCC injuries. These injury classification categories were then evaluated independently against our chosen outcome measures, which included DASH and VAS pain scores, ROM, and grip/pinch strength.
To determine the optimal sample size, we performed a power analysis to estimate the number of patients required to detect a clinically significant difference in DASH scores at 1 year among the 4 groups. According to the literature, standard deviations of DASH scores in healthy volunteers range from 10 to 15,17 consistent with values found in other recent trials of patients with DRFs.18 The recent literature on DASH construct validity has established a DASH score difference of 19 as representing a disability change being “much better or much worse.”19 As such, power analysis for a 1-way analysis of variance among 4 categories, detecting a DASH score difference of 19 with a standard deviation ranging from 10 to 15, would require 28 to 60 patients to detect a difference with an α of 0.05 and a power of 0.8.
In addition, radiographic parameters at time of injury were compared with injury characteristics to assess for significant relationships. Multivariate linear regression analysis was performed to evaluate radial height, radial inclination, and volar tilt as possible predictors of SLIL injury, TFCC injury, and chondral surface damage. A statistically significant result was defined as a correlation with P < .05.
Results
Of the 42 patients included in the study, 11 (26%) had no ligament injuries, 10 (24%) had isolated SLIL injuries, 12 (29%) had isolated TFCC injuries, and 9 (21%) had injuries to both the SLIL and the TFCC. In addition, in 12 patients (29%), the articular cartilage had visible damage (Table 1).
In all patients, bony union occurred. After union, 1 patient underwent hardware removal for hardware-related pain. The same patient had a dorsal ulnar cutaneous nerve neurolysis at the ulnar styloid fixation site. Another patient developed a partial extensor pollicis longus tear from a prominent dorsal screw tip.
All patients returned for their 2- and 6-week follow-ups. At 1 year, 30 patients (71%) returned for follow-up, 11 could not be contacted, and 1 was removed because of an olecranon fracture from a subsequent fall.
Regarding the primary outcome measure, mean DASH score at 1-year follow-up was 30.8 for the group without injuries, 10.8 for the group with SLIL injuries, 14.7 for the group with TFCC injuries, and 21.9 for the group with SLIL and TFCC injuries (Table 2).
Radiographic parameters were restored to acceptable limits in all patients (Table 3).
Discussion
Use of wrist arthroscopy in DRF management has allowed assessment of the incidence of intra-articular injuries, including ligament and chondral surface injuries. Although the literature on the incidence of these injuries has been expanding, their clinical significance remains unclear.
Authors have postulated that some patients do not do well after DRF repair because of undetected ligament injuries. With the current trend of internal fixation, locked plating, and early motion—contrasting with older trends of prolonged immobilization in a cast or external fixation—concerns have been raised that early mobilization results in inadequate treatment of ligament injuries. However, data from the present study suggest no significant morbidity from early mobilization despite the presence of ligament injuries in more than half of all operatively treated DRFs. It is possible morbidity was not appreciated, as most patients with DRFs end up with some stiffness, which masks the effects of ligament injuries during healing.
We found no correlation between injury radiographic parameters, observed soft-tissue injuries, or final subjective outcomes. Interestingly, in this study, there was some discordance between the appearance of intra-articular fractures on radiographs and the direct arthroscopic observation of intra-articular fracture extension. With the present data and with arthroscopic visualization as the gold standard, radiographs had 74% sensitivity and 73% specificity for detecting intra-articular fractures (the corresponding positive predictive value was 83%, and the negative predictive value was 61%). As we typically rely on radiographs as the primary tool in assessing the articular component of a fracture, these results should be taken into account when basing management decisions exclusively on static injury films.
Observational studies of arthroscopy in DRFs have revealed a wide range of injury rates: For SLILs, the average injury rate was 44%; for LTLs, 13%; for TFCCs, 43%; and for chondral surfaces, 32% (Table 4).
This study had several limitations, including loss to follow-up at the primary endpoint (we were unable to contact 29% of patients). In addition, because of resource limitations, we were able to enroll only a limited number of patients, and as a result were able to power the study to detect only major effects on DASH scores. Therefore, although our 32 patients with long-term follow-up are within the range dictated by the power analysis, this study was not powered to capture more subtle differences in disability. Furthermore, because we used 1 year as the longest follow-up point, the long-term sequelae (eg, arthritis) of these injuries may not have been captured. Last, despite the high incidence of soft-tissue injuries overall, the number of patients with severe ligament injuries was relatively low, which makes it difficult to make definitive statements about their contribution to outcomes. A likely explanation is that patients with high-energy injuries and significant intra-articular displacement requiring open arthrotomies were excluded.
At 1-year follow-up, with use of DASH as the gold standard for disability, we found no major difference in subjective or objective outcome measures between patients with and without ligament injuries. Radiographs did not predict soft-tissue injury or ultimate outcome. Rates of ligament injuries in our operatively treated DRFs were similar to those in the literature. Overall, these findings suggest that “minor” injuries incidentally discovered with arthroscopy during DRF surgery may not have a significant effect on outcomes, with the caveat that the significance of very severe injuries (eg, Geissler grade 4 injuries with frank scapholunate diastasis) remains incompletely understood. The decision by the treating surgeon to perform arthroscopy and/or to repair soft-tissue injuries should be made on a case-by-case basis.
Am J Orthop. 2017;46(1):E41-E46. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patients sustaining DRFs commonly have associated ligament injuries and chondral damage as well.
- Many of these associated injuries do not seem to affect outcomes up to 1 year after surgery.
- Plain radiographs have a 74% sensitivity and 73% specificity for detecting intra-articular fractures.
- ”Minor” injuries identified incidentally by arthroscopy during fixation of DRFs may not require dedicated treatment.
- The optimal treatment for high-grade ligament or chondral injuries in patients with DRFs remains incompletely understood.
Distal radius fracture (DRF) is one of the most common upper extremity injuries, with up to 20% to 50% requiring surgical fixation.1 With increasing use of wrist arthroscopy to assist in managing these fractures,2-6 it has become easier to accurately assess concomitant wrist ligament injuries. Reported injury rates are 18% to 86% for the scapholunate interosseous ligament (SLIL),7,8 5% to 29% for the lunotriquetral ligament (LTL),8,9 and 17% to 60% for the triangular fibrocartilage complex (TFCC).10,11 Reported chondral injury rates range from 18% to 60%.7,9,12 Despite the common occurrence of these injuries, it is unclear how they affect outcomes and how aggressively they should be treated when detected during fracture surgery.
As the use of arthroscopy in DRF management becomes more common, surgeons often must decide how to treat ligamentous/chondral injuries incidentally discovered during surgery. To date, only 1 study prospectively evaluated how these injuries affect DRF outcomes,8 though it did not use a validated, patient-based outcome measure.
We conducted a study to address a common clinical scenario: When arthroscopy is used to assist with intra-articular reduction during DRF fixation, how should the surgeon respond to incidentally identified ligament and chondral injuries? Specifically, we wanted to address 3 questions: What is the overall incidence of SLIL, TFCC, and chondral surface injuries in patients undergoing operative fracture fixation? On initial injury films, do any radiographic parameters predict specific soft-tissue injuries or ultimate functional outcomes? Do wrist ligament and chondral injuries affect patient-rated outcomes (disability, pain) and objective measures (range of motion [ROM], grip strength, pinch strength) up to 1 year after fracture surgery?
Materials and Methods
Patient Selection/Population
This observational, prognostic study was approved by our Institutional Review Board. Inclusion criteria were age over 18 years, isolated acute operatively treated DRF (surgery within 14 days of injury), and informed consent. All patients were treated by the same surgeon. Exclusion criteria were open DRF, dorsal shear pattern, fractures requiring dorsal arthrotomy for reduction because of significant intra-articular damage, prior ipsilateral DRF, and prior SLIL or TFCC injury.
Surgery was indicated according to general radiographic parameters as measured on postreduction films: radial height, <8 mm; radial inclination, <15°; positive ulnar variance, >3 mm, or 3 mm more than contralateral side; dorsal tilt, >10°; and volar tilt, >15°. With these parameters within acceptable limits, surgery was also indicated when fractures were deemed unstable and likely to displace because of dorsal tilt >20°, dorsal comminution, intra-articular step-off of ≥2 mm on the posterior-anterior (PA) film, associated ulnar fracture, and age >60 years.13Over a 2-year period, 42 patients (12 male, 30 female) met the inclusion criteria and were enrolled in the study. The dominant arm was affected in 17 patients (40%). Mean (SD) age at time of injury was 56.6 (16.4) years (median, 54 years; range, 20-85 years).
Operative Technique
During surgery, damage to the SLIL, the TFCC, and chondral surfaces (scaphoid, lunate, scaphoid fossa, lunate fossa) and to the intra-articular extension of the DRF was assessed and recorded. Wrist arthroscopy was performed with the 3, 4 portal as the primary portal. When significant damage to the TFCC warranted débridement, the 6R (radial) portal was used as an accessory portal. As a midcarpal portal was not used for SLIL assessment, we used a novel classification system: 0 = no injury, normal-appearing ligament without hemorrhage and smooth transition from scaphoid to lunate surface except for slight concave indentation at the ligament; 1 = attenuation, no visible tear with convex shape of ligament with or without hemorrhage; 2 = partial tear with or without step-off at junction between scaphoid and lunate, but 2.7-mm arthroscope cannot “drive through” to midcarpal joint; and 3 = complete tear with positive “drive-through” sign. TFCC injuries were classified according to the system described by Palmer14: Avulsions were central (1A), ulnar (1B), distal (1C), or radial (1D). The trampoline test was performed through a 6R portal by using a probe to evaluate ligament tension/laxity. In some cases, a 6R portal was deemed unnecessary, and a modified trampoline test was performed—tension/laxity/displacement was evaluated by manually palpating at the fovea and observing TFCC motion with the arthroscope. When appropriate, the TFCC was débrided with a shaver through the 6R portal. In cases of significant instability at the SLIL interval, two 0.062-inch K-wires were placed percutaneously through the scaphoid and lunate, and one was placed from the scaphoid to the capitate.
All DRFs underwent internal fixation with a locked volar plate. When necessary, K-wires and/or a locked radial column plate was used for additional fixation. External fixation was not used. The postoperative protocol began with a dorsal wrist splint placed on the patient in the operating room and worn for 10 to 14 days. At the first postoperative visit, the patient received a removable splint that was to be worn at all times except during showers, therapy, and home exercises. Occupational therapy, initiated the week of the first postoperative visit, consisted of active and passive ROM exercises. At 6 weeks, the splint was removed and strengthening initiated.
Outcome Measures
Our primary outcome measure was the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire at 1 year.15 Secondary outcome measures were visual analog scale (VAS) pain rating, ROM, and radiographic measurements. Patients returned for evaluation 2, 6, 12, 24, and 52 weeks after surgery. At each follow-up visit, the DASH questionnaire and the pain VAS were administered, and ROM and strength were measured. Patient-reported pain was recorded on a standard VAS and measured on a scale from 0 (no pain) to 10 (worst possible pain). Wrist flexion and extension and radioulnar deviation were assessed with a goniometer. Forearm supination and pronation were assessed with the elbow flexed 90° at the patient’s side. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan), and lateral pinch strength was measured with a hydraulic pinch gauge (Sammons Preston Rolyan). The average of 3 trials for both hands was recorded for all strength measurements.
Radiographs were obtained on presentation. When appropriate, the fracture was manually reduced with a hematoma block, and postreduction radiographs were obtained. Then, radiographs were obtained at each postoperative visit until union. Radial height, radial inclination, tilt, and ulnar variance were measured on preoperative and postoperative radiographs according to standard methods.16 Radiographs were used to classify the fracture patterns according to the AO/ASIF (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) classification. Union was determined by radiographic healing, absence of tenderness to palpation, absence of pain with motion, and continued functional improvement.
Data Analysis
To evaluate for relationships between patient injury parameters and outcome measures, we used a 1-way analysis of variance seeking statistically significant differences between groups. Patients were divided into 4 groups: no ligament injuries; isolated SLIL injuries; isolated TFCC injuries; and both SLIL and TFCC injuries. These injury classification categories were then evaluated independently against our chosen outcome measures, which included DASH and VAS pain scores, ROM, and grip/pinch strength.
To determine the optimal sample size, we performed a power analysis to estimate the number of patients required to detect a clinically significant difference in DASH scores at 1 year among the 4 groups. According to the literature, standard deviations of DASH scores in healthy volunteers range from 10 to 15,17 consistent with values found in other recent trials of patients with DRFs.18 The recent literature on DASH construct validity has established a DASH score difference of 19 as representing a disability change being “much better or much worse.”19 As such, power analysis for a 1-way analysis of variance among 4 categories, detecting a DASH score difference of 19 with a standard deviation ranging from 10 to 15, would require 28 to 60 patients to detect a difference with an α of 0.05 and a power of 0.8.
In addition, radiographic parameters at time of injury were compared with injury characteristics to assess for significant relationships. Multivariate linear regression analysis was performed to evaluate radial height, radial inclination, and volar tilt as possible predictors of SLIL injury, TFCC injury, and chondral surface damage. A statistically significant result was defined as a correlation with P < .05.
Results
Of the 42 patients included in the study, 11 (26%) had no ligament injuries, 10 (24%) had isolated SLIL injuries, 12 (29%) had isolated TFCC injuries, and 9 (21%) had injuries to both the SLIL and the TFCC. In addition, in 12 patients (29%), the articular cartilage had visible damage (Table 1).
In all patients, bony union occurred. After union, 1 patient underwent hardware removal for hardware-related pain. The same patient had a dorsal ulnar cutaneous nerve neurolysis at the ulnar styloid fixation site. Another patient developed a partial extensor pollicis longus tear from a prominent dorsal screw tip.
All patients returned for their 2- and 6-week follow-ups. At 1 year, 30 patients (71%) returned for follow-up, 11 could not be contacted, and 1 was removed because of an olecranon fracture from a subsequent fall.
Regarding the primary outcome measure, mean DASH score at 1-year follow-up was 30.8 for the group without injuries, 10.8 for the group with SLIL injuries, 14.7 for the group with TFCC injuries, and 21.9 for the group with SLIL and TFCC injuries (Table 2).
Radiographic parameters were restored to acceptable limits in all patients (Table 3).
Discussion
Use of wrist arthroscopy in DRF management has allowed assessment of the incidence of intra-articular injuries, including ligament and chondral surface injuries. Although the literature on the incidence of these injuries has been expanding, their clinical significance remains unclear.
Authors have postulated that some patients do not do well after DRF repair because of undetected ligament injuries. With the current trend of internal fixation, locked plating, and early motion—contrasting with older trends of prolonged immobilization in a cast or external fixation—concerns have been raised that early mobilization results in inadequate treatment of ligament injuries. However, data from the present study suggest no significant morbidity from early mobilization despite the presence of ligament injuries in more than half of all operatively treated DRFs. It is possible morbidity was not appreciated, as most patients with DRFs end up with some stiffness, which masks the effects of ligament injuries during healing.
We found no correlation between injury radiographic parameters, observed soft-tissue injuries, or final subjective outcomes. Interestingly, in this study, there was some discordance between the appearance of intra-articular fractures on radiographs and the direct arthroscopic observation of intra-articular fracture extension. With the present data and with arthroscopic visualization as the gold standard, radiographs had 74% sensitivity and 73% specificity for detecting intra-articular fractures (the corresponding positive predictive value was 83%, and the negative predictive value was 61%). As we typically rely on radiographs as the primary tool in assessing the articular component of a fracture, these results should be taken into account when basing management decisions exclusively on static injury films.
Observational studies of arthroscopy in DRFs have revealed a wide range of injury rates: For SLILs, the average injury rate was 44%; for LTLs, 13%; for TFCCs, 43%; and for chondral surfaces, 32% (Table 4).
This study had several limitations, including loss to follow-up at the primary endpoint (we were unable to contact 29% of patients). In addition, because of resource limitations, we were able to enroll only a limited number of patients, and as a result were able to power the study to detect only major effects on DASH scores. Therefore, although our 32 patients with long-term follow-up are within the range dictated by the power analysis, this study was not powered to capture more subtle differences in disability. Furthermore, because we used 1 year as the longest follow-up point, the long-term sequelae (eg, arthritis) of these injuries may not have been captured. Last, despite the high incidence of soft-tissue injuries overall, the number of patients with severe ligament injuries was relatively low, which makes it difficult to make definitive statements about their contribution to outcomes. A likely explanation is that patients with high-energy injuries and significant intra-articular displacement requiring open arthrotomies were excluded.
At 1-year follow-up, with use of DASH as the gold standard for disability, we found no major difference in subjective or objective outcome measures between patients with and without ligament injuries. Radiographs did not predict soft-tissue injury or ultimate outcome. Rates of ligament injuries in our operatively treated DRFs were similar to those in the literature. Overall, these findings suggest that “minor” injuries incidentally discovered with arthroscopy during DRF surgery may not have a significant effect on outcomes, with the caveat that the significance of very severe injuries (eg, Geissler grade 4 injuries with frank scapholunate diastasis) remains incompletely understood. The decision by the treating surgeon to perform arthroscopy and/or to repair soft-tissue injuries should be made on a case-by-case basis.
Am J Orthop. 2017;46(1):E41-E46. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand. 1990;61(5):457-459.
2. Geissler WB. Arthroscopically assisted reduction of intra-articular fractures of the distal radius. Hand Clin. 1995;11(1):19-29.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Wolfe SW, Easterling KJ, Yoo HH. Arthroscopic-assisted reduction of distal radius fractures. Arthroscopy. 1995;11(6):706-714.
5. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
6. Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093-1110.
7. Shih JT, Lee HM, Hou YT, Tan CM. Arthroscopically-assisted reduction of intra-articular fractures and soft tissue management of distal radius. Hand Surg. 2001;6(2):127-135.
8. Forward DP, Lindau TR, Melsom DS. Intercarpal ligament injuries associated with fractures of the distal part of the radius. J Bone Joint Surg Am. 2007;89(11):2334-2340.
9. Espinosa-Gutiérrez A, Rivas-Montero JA, Elias-Escobedo A, Alisedo-Ochoa PG. Wrist arthroscopy for fractures of the distal end of the radius [in Spanish]. Acta Ortop Mex. 2009;23(6):358-365.
10. Hardy P, Gomes N, Chebil M, Bauer T. Wrist arthroscopy and intra-articular fractures of the distal radius in young adults. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1225-1230.
11. Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778-785.
12. Kordasiewicz B, Pomianowski S, Rylski W, Antolak L, Marczak D. Intraarticular distal radius fractures—arthroscopic assessment of injuries [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2006;71(2):113-116.
13. Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20(4):208-210.
14. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989;14(4):594-606.
15. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med. 1996;30(3):372]. Am J Ind Med. 1996;29(6):602-608.
16. Fernandez DL, Jupiter JB. Fractures of the Distal Radius: A Practical Approach to Management. New York, NY: Springer; 1996.
17. Jester A, Harth A, Wind G, Germann G, Sauerbier M. Does the Disability of Shoulder, Arm and Hand questionnaire (DASH) replace grip strength and range of motion in outcome-evaluation? [in German]. Handchir Mikrochir Plast Chir. 2005;37(2):126-130.
18. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
19. Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
20. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
21. Peicha G, Seibert F, Fellinger M, Grechenig W. Midterm results of arthroscopic treatment of scapholunate ligament lesions associated with intra-articular distal radius fractures. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):327-333.
22. Schädel-Höpfner M, Böhringer G, Junge A, Celik I, Gotzen L. [Arthroscopic diagnosis of concomitant scapholunate ligament injuries in fractures of the distal radius]. Handchir Mikrochir Plast Chir. 2001;33(4):229-233.
23. Ruch DS, Yang CC, Smith BP. Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19(5):511-516.
24. Hattori Y, Doi K, Estrella EP, Chen G. Arthroscopically assisted reduction with volar plating or external fixation for displaced intra-articular fractures of the distal radius in the elderly patients. Hand Surg. 2007;12(1):1-12.
25. Hohendorff B, Eck M, Mühldorfer M, Fodor S, Schmitt R, Prommersberger KJ. [Palmar wrist arthroscopy for evaluation of concomitant carpal lesions in operative treatment of distal intraarticular radius fractures]. Handchir Mikrochir Plast Chir. 2009;41(5):295-299.
1. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand. 1990;61(5):457-459.
2. Geissler WB. Arthroscopically assisted reduction of intra-articular fractures of the distal radius. Hand Clin. 1995;11(1):19-29.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Wolfe SW, Easterling KJ, Yoo HH. Arthroscopic-assisted reduction of distal radius fractures. Arthroscopy. 1995;11(6):706-714.
5. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
6. Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093-1110.
7. Shih JT, Lee HM, Hou YT, Tan CM. Arthroscopically-assisted reduction of intra-articular fractures and soft tissue management of distal radius. Hand Surg. 2001;6(2):127-135.
8. Forward DP, Lindau TR, Melsom DS. Intercarpal ligament injuries associated with fractures of the distal part of the radius. J Bone Joint Surg Am. 2007;89(11):2334-2340.
9. Espinosa-Gutiérrez A, Rivas-Montero JA, Elias-Escobedo A, Alisedo-Ochoa PG. Wrist arthroscopy for fractures of the distal end of the radius [in Spanish]. Acta Ortop Mex. 2009;23(6):358-365.
10. Hardy P, Gomes N, Chebil M, Bauer T. Wrist arthroscopy and intra-articular fractures of the distal radius in young adults. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1225-1230.
11. Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778-785.
12. Kordasiewicz B, Pomianowski S, Rylski W, Antolak L, Marczak D. Intraarticular distal radius fractures—arthroscopic assessment of injuries [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2006;71(2):113-116.
13. Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20(4):208-210.
14. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989;14(4):594-606.
15. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med. 1996;30(3):372]. Am J Ind Med. 1996;29(6):602-608.
16. Fernandez DL, Jupiter JB. Fractures of the Distal Radius: A Practical Approach to Management. New York, NY: Springer; 1996.
17. Jester A, Harth A, Wind G, Germann G, Sauerbier M. Does the Disability of Shoulder, Arm and Hand questionnaire (DASH) replace grip strength and range of motion in outcome-evaluation? [in German]. Handchir Mikrochir Plast Chir. 2005;37(2):126-130.
18. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
19. Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
20. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
21. Peicha G, Seibert F, Fellinger M, Grechenig W. Midterm results of arthroscopic treatment of scapholunate ligament lesions associated with intra-articular distal radius fractures. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):327-333.
22. Schädel-Höpfner M, Böhringer G, Junge A, Celik I, Gotzen L. [Arthroscopic diagnosis of concomitant scapholunate ligament injuries in fractures of the distal radius]. Handchir Mikrochir Plast Chir. 2001;33(4):229-233.
23. Ruch DS, Yang CC, Smith BP. Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19(5):511-516.
24. Hattori Y, Doi K, Estrella EP, Chen G. Arthroscopically assisted reduction with volar plating or external fixation for displaced intra-articular fractures of the distal radius in the elderly patients. Hand Surg. 2007;12(1):1-12.
25. Hohendorff B, Eck M, Mühldorfer M, Fodor S, Schmitt R, Prommersberger KJ. [Palmar wrist arthroscopy for evaluation of concomitant carpal lesions in operative treatment of distal intraarticular radius fractures]. Handchir Mikrochir Plast Chir. 2009;41(5):295-299.