User login
Focusing on Inattention: The Diagnostic Accuracy of Brief Measures of Inattention for Detecting Delirium
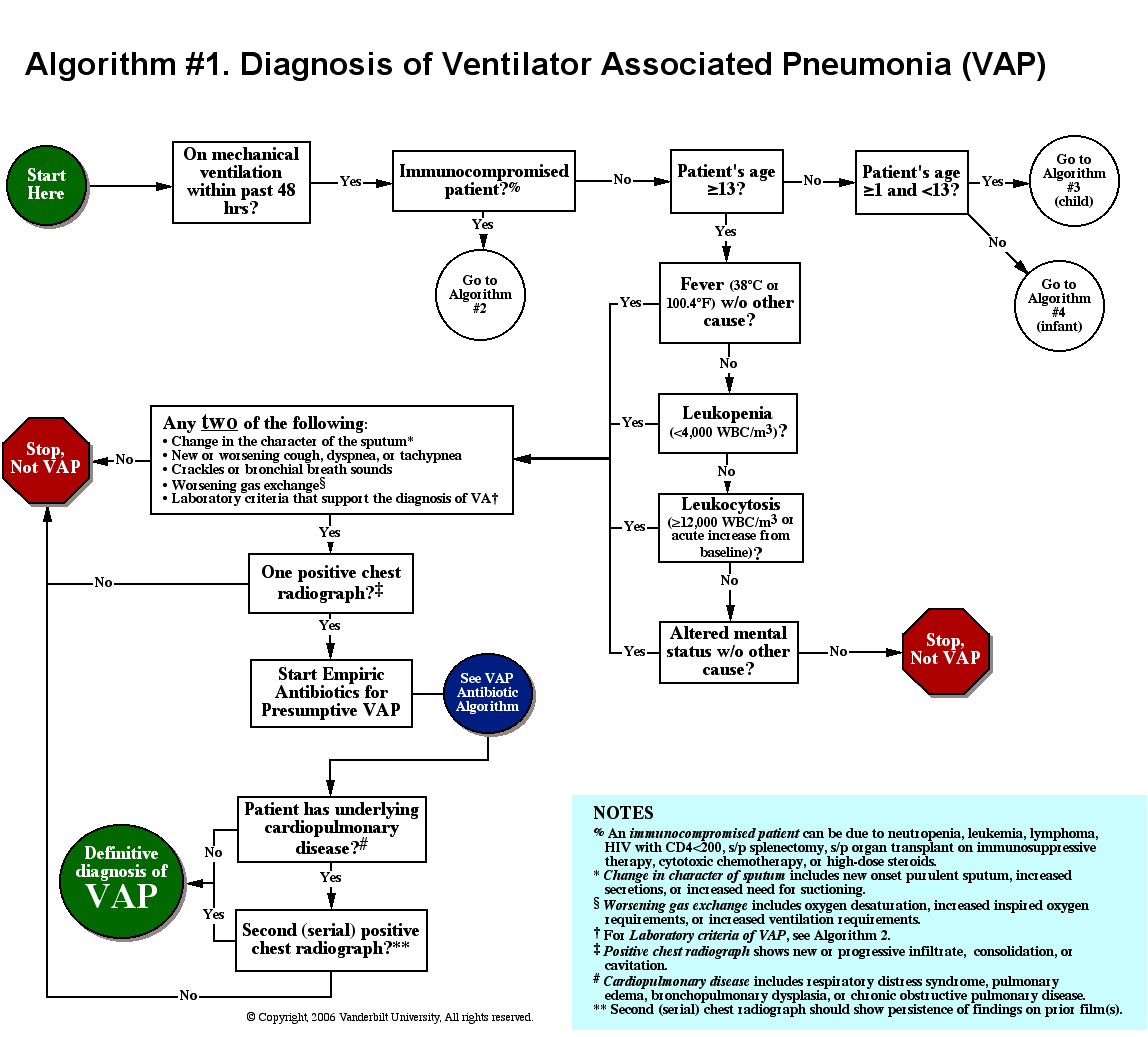
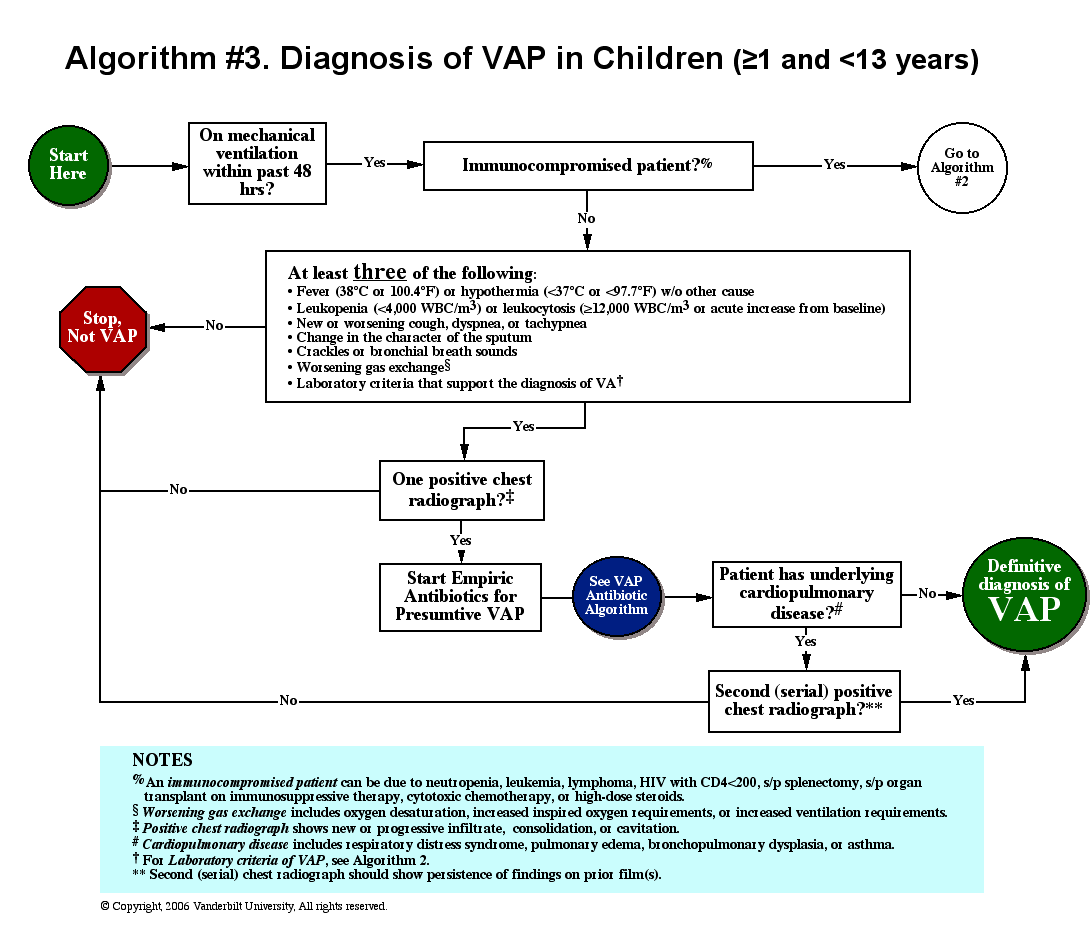
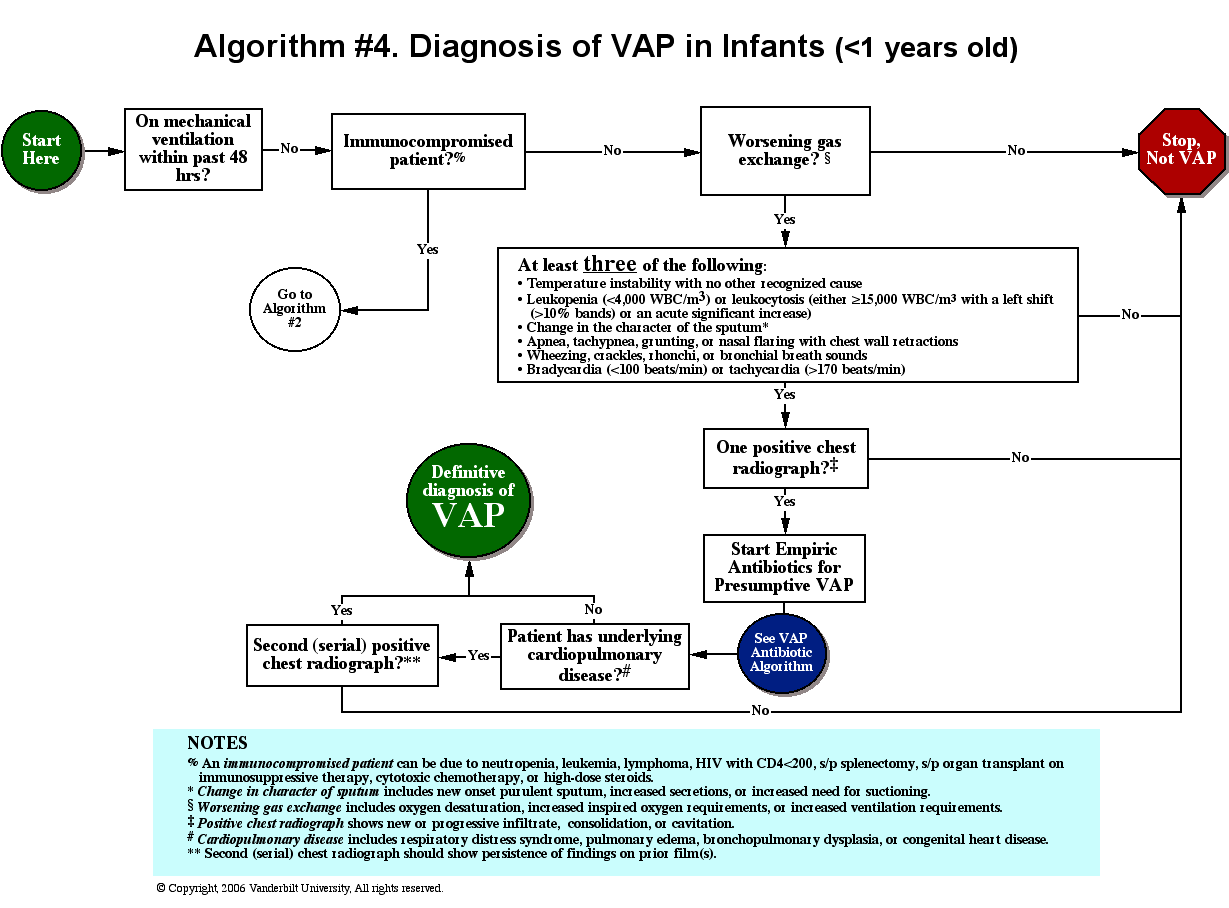
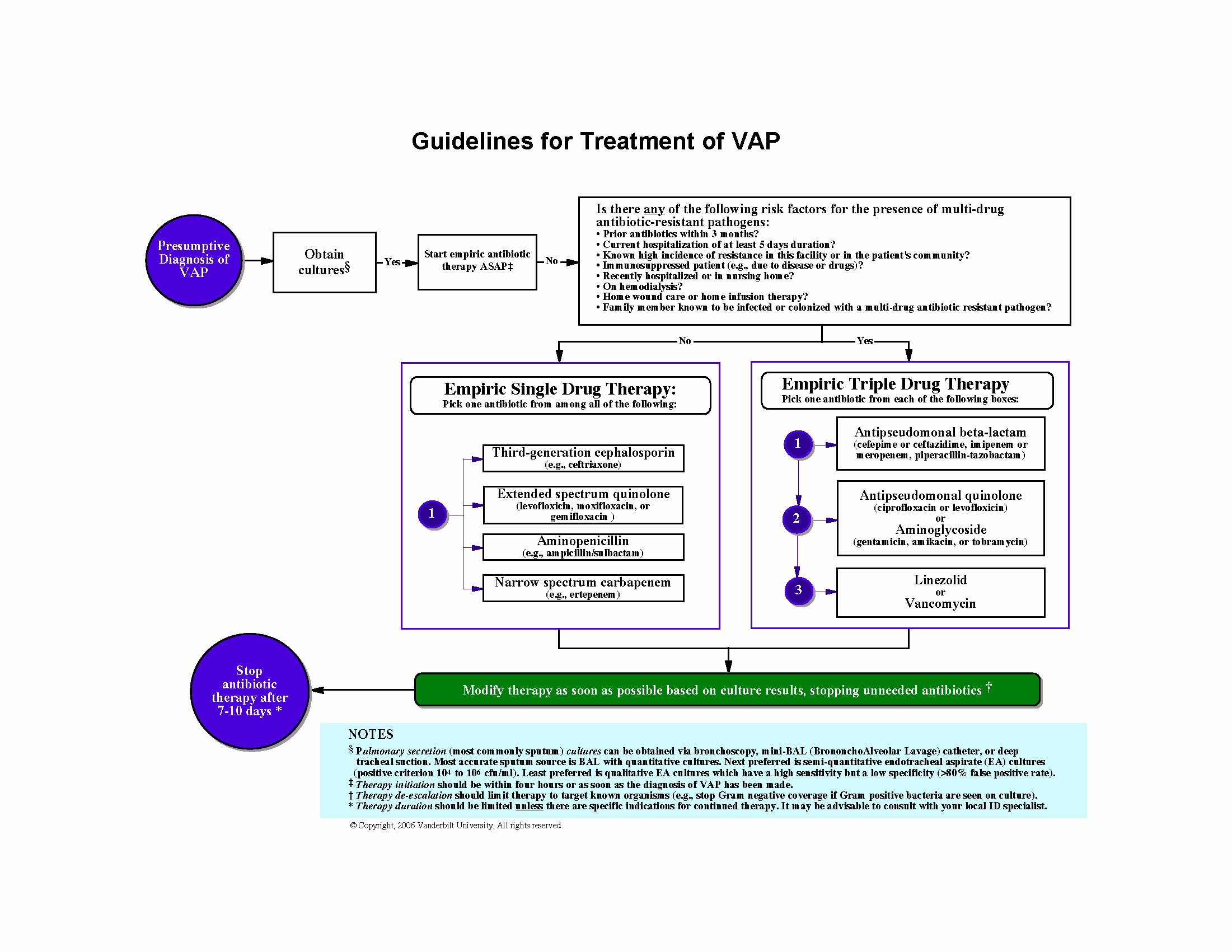
Delirium is an acute neurocognitive disorder1 that affects up to 25% of older emergency department (ED) and hospitalized patients.2-4 The relationship between delirium and adverse outcomes is well documented.5-7 Delirium is a strong predictor of increased length of mechanical ventilation, longer intensive care unit and hospital stays, increased risk of falls, long-term cognitive impairment, and mortality.8-13 Delirium is frequently missed by healthcare professionals2,14-16 and goes undetected in up to 3 out of 4 patients by bedside nurses and medical practitioners in many hospital settings.14,17-22 A significant barrier to recognizing delirium is the absence of brief delirium assessments.
In an effort to improve delirium recognition in the acute care setting, there has been a concerted effort to develop and validate brief delirium assessments. To address this unmet need, 4 ‘A’s Test (4AT), the Brief Confusion Assessment Method (bCAM), and the 3-minute diagnostic assessment for CAM-defined delirium (3D-CAM) are 1- to 3-minute delirium assessments that were validated in acutely ill older patients.23 However, 1 to 3 minutes may still be too long in busy clinical environments, and briefer (<30 seconds) delirium assessments may be needed.
One potential more-rapid method to screen for delirium is to specifically test for the presence of inattention, which is a cardinal feature of delirium.24,25 Inattention can be ascertained by having the patient recite the months backwards, recite the days of the week backwards, or spell a word backwards.26 Recent studies have evaluated the diagnostic accuracy of reciting the months of the year backwards for delirium. O’Regan et al.27 evaluated the diagnostic accuracy of the month of the year backwards from December to July (MOTYB-6) and observed that this task was 84% sensitive and 90% specific for delirium in older patients. However, they performed the reference standard delirium assessments in patients who had a positive MOTYB-6, which can overestimate sensitivity and underestimate specificity (verification bias).28 Fick et al.29 examined the diagnostic accuracy of 20 individual elements of the 3D-CAM and observed that reciting the months of the year backwards from December to January (MOTYB-12) was 83% sensitive and 69% specific for delirium. However, this was an exploratory study that was designed to identify an element of the 3D-CAM that had the best diagnostic accuracy.
To address these limitations, we sought to evaluate the diagnostic performance of the MOTYB-6 and MOTYB-12 for delirium as diagnosed by a reference standard. We also explored other brief tests of inattention such as spelling a word (“LUNCH”) backwards, reciting the days of the week backwards, 10-letter vigilance “A” task, and 5 picture recognition task.
METHODS
Study Design and Setting
This was a preplanned secondary analysis of a prospective observational study that validated 3 delirium assessments.30,31 This study was conducted at a tertiary care, academic ED. The local institutional review board (IRB) reviewed and approved this study. Informed consent from the patient or an authorized surrogate was obtained whenever possible. Because this was an observational study and posed minimal risk to the patient, the IRB granted a waiver of consent for patients who were both unable to provide consent and were without an authorized surrogate available in the ED or by phone.
Selection of Participants
We enrolled a convenience sample of patients between June 2010 and February 2012 Monday through Friday from 8
Research assistants approached patients who met inclusion criteria and determined if any exclusion criteria were present. If none of the exclusion criteria were present, then the research assistant reviewed the informed consent document with the patient or authorized surrogate if the patient was not capable of providing consent. If a patient was not capable of providing consent and no authorized surrogate was available, then the patient was enrolled (under the waiver of consent) as long as the patient assented to be a part of the study. Once the patient was enrolled, the research assistant contacted the physician rater and reference standard psychiatrists to approach the patient.
Measures of Inattention
An emergency physician (JHH) who had no formal training in the mental status assessment of elders administered a cognitive battery to the patient, including tests of inattention. The following inattention tasks were administered:
- Spell the word “LUNCH” backwards.30 Patients were initially allowed to spell the word “LUNCH” forwards. Patients who were unable to perform the task were assigned 5 errors.
- Recite the months of the year backwards from December to July.23,26,27,30,32 Patients who were unable to perform the task were assigned 6 errors.
- Recite the days of the week backwards.23,26,33 Patients who were unable to perform the task were assigned 7 errors.
- Ten-letter vigilance “A” task.34 The patient was given a series of 10 letters (“S-A-V-E-A-H-A-A-R-T”) every 3 seconds and was asked to squeeze the rater’s hand every time the patient heard the letter “A.” Patients who were unable to perform the task were assigned 10 errors.
- Five picture recognition task.34 Patients were shown 5 objects on picture cards. Afterwards, patients were shown 10 pictures with the previously shown objects intermingled. The patient had to identify which objects were seen previously in the first 5 pictures. Patients who were unable to perform the task were assigned 10 errors.
- Recite the months of the year backwards from December to January.29 Patients who were unable to perform the task were assigned 12 errors.
Reference Standard for Delirium
A comprehensive consultation-liaison psychiatrist assessment was the reference standard for delirium; the diagnosis of delirium was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria.35 Three psychiatrists who each had an average of 11 years of clinical experience and regularly diagnosed delirium as part of their daily clinical practice were available to perform these assessments. To arrive at the diagnosis of delirium, they interviewed those who best understood the patient’s mental status (eg, the patient’s family members or caregivers, physician, and nurses). They also reviewed the patient’s medical record and radiology and laboratory test results. They performed bedside cognitive testing that included, but was not limited to, the Mini-Mental State Examination, Clock Drawing Test, Luria hand sequencing task, and tests for verbal fluency. A focused neurological examination was also performed (ie, screening for paraphasic errors, tremors, tone, asterixis, frontal release signs, etc.), and they also evaluated the patient for affective lability, hallucinations, and level of alertness. If the presence of delirium was still questionable, then confrontational naming, proverb interpretation or similarities, and assessments for apraxias were performed at the discretion of the psychiatrist. The psychiatrists were blinded to the physician’s assessments, and the assessments were conducted within 3 hours of each other.
Additional Variables Collected
Using medical record review, comorbidity burden, severity of illness, and premorbid cognition were ascertained. The Charlson Comorbidity Index, a weighted index that takes into account the number and seriousness of 19 preexisting comorbid conditions, was used to quantify comorbidity burden; higher scores indicate higher comorbid burden.36,37 The Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II was used to quantify severity of illness.38 This score is based upon the initial values of 12 routine physiologic measurements such as vital sign and laboratory abnormalities; higher scores represent higher severities of illness.38 The medical record was reviewed to ascertain the presence of premorbid cognitive impairment; any documentation of dementia in the patient’s clinical problem list or physician history and physical examination from the outpatient or inpatient settings was considered positive. The medical record review was performed by a research assistant and was double-checked for accuracy by one of the investigators (JHH).
Data Analyses
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. Receiver operating characteristic curves were constructed for each inattention task. Area under the receiver operating characteristic curves (AUC) was reported to provide a global measure of diagnostic accuracy. Sensitivities, specificities, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with their 95% CIs were calculated using the psychiatrist’s assessment as the reference standard.39 Cut-points with PLRs greater than 10 (strongly increased the likelihood of delirium) or NLRs less than 0.1 (strongly decreased the likelihood of delirium) were preferentially reported whenever possible.
All statistical analyses were performed with open source R statistical software version 3.0.1 (http://www.r-project.org/), SAS 9.4 (SAS Institute, Cary, NC), and Microsoft Excel 2010 (Microsoft Inc., Redmond, WA).
RESULTS
DISCUSSION
Delirium is frequently missed by healthcare providers because it is not routinely screened for in the acute care setting. To help address this deficiency of care, we evaluated several brief measures of inattention that take less than 30 seconds to complete. We observed that any errors made on the MOTYB-6 and MOTYB-12 tasks had very good sensitivities (80% and 84%) but were limited by their modest specificities (approximately 50%) for delirium. As a result, these assessments have limited clinical utility as standalone delirium screens. We also explored other commonly used brief measures of inattention and at a variety of error cutoffs. Reciting the days of the week backwards appeared to best balance sensitivity and specificity. None of the inattention measures could convincingly rule out delirium (NLR < 0.10), but the vigilance “A” and picture recognition tasks may have clinical utility in ruling in delirium (PLR > 10). Overall, all the inattention tasks, including MOTYB-6 and MOTYB-12, had very good diagnostic performances based upon their AUC. However, achieving a high sensitivity often had to be sacrificed for specificity or, alternatively, achieving a high specificity had to be sacrificed for sensitivity.
Inattention has been shown to be the cardinal feature for delirium,40 and its assessment using cognitive testing has been recommended to help identify the presence of delirium according to an expert consensus panel.26 The diagnostic performance of the MOTYB-12 observed in our study is similar to a study by Fick et al., who reported that MOTYB-12 had very good sensitivity (83%) but had modest specificity (69%) with a cutoff of 1 or more errors. Hendry et al. observed that the MOTYB-12 was 91% sensitive and 50% specific using a cutoff of 4 or more errors. With regard to the MOTYB-6, our reported specificity was different from what was observed by O’Regan et al.27 Using 1 or more errors as a cutoff, they observed a much higher specificity for delirium than we did (90% vs 57%). Discordant observations regarding the diagnostic accuracy for other inattention tasks also exist. We observed that making any error on the days of the week backwards task was 84% sensitive and 82% specific for delirium, whereas Fick et al. observed a sensitivity and specificity of 50% and 94%, respectively. For the vigilance “A” task, we observed that making 2 or more errors over a series of 10 letters was 64.0% sensitive and 91.4% specific for delirium, whereas Pompei et al.41 observed that making 2 or more errors over a series of 60 letters was 51% sensitive and 77% specific for delirium.
The abovementioned discordant findings may be driven by spectrum bias, wherein the sensitivities and specificities for each inattention task may differ in different subgroups. As a result, differences in the age distribution, proportion of college graduates, history of dementia, and susceptibility to delirium can influence overall sensitivity and specificity. Objective measures of delirium, including the inattention screens studied, are particularly prone to spectrum bias.31,34 However, the strength of this approach is that the assessment of inattention becomes less reliant upon clinical judgment and allows it to be used by raters from a wide range of clinical backgrounds. On the other hand, a subjective interpretation of these inattention tasks may allow the rater to capture the subtleties of inattention (ie, decreased speed of performance in a highly intelligent and well-educated patient without dementia). The disadvantage of this approach, however, is that it is more dependent on clinical judgment and may have decreased diagnostic accuracy in those with less clinical experience or with limited training.14,42,43 These factors must be carefully considered when determining which delirium assessment to use.
Additional research is required to determine the clinical utility of these brief inattention assessments. These findings need to be further validated in larger studies, and the optimal cutoff of each task for different subgroup of patients (eg, demented vs nondemented) needs to be further clarified. It is not completely clear whether these inattention tests can serve as standalone assessments. Depending on the cutoff used, some of these assessments may have unacceptable false negative or false positive rates that may lead to increased adverse patient outcomes or increased resource utilization, respectively. Additional components or assessments may be needed to improve the diagnostic accuracy of these assessments. In addition to understanding these inattention assessments’ diagnostic accuracies, their ability to predict adverse outcomes also needs to be investigated. While a previous study observed that making any error on the MOTYB-12 task was associated with increased physical restraint use and prolonged hospital length of stay,44 these assessments’ ability to prognosticate long-term outcomes such as mortality or long-term cognition or function need to be studied. Lastly, studies should also evaluate how easily implementable these assessments are and whether improved delirium recognition leads to improved patient outcomes.
This study has several notable limitations. Though planned a priori, this was a secondary analysis of a larger investigation designed to validate 3 delirium assessments. Our sample size was also relatively small, causing our 95% CIs to overlap in most cases and limiting the statistical power to truly determine whether one measure is better than the other. We also asked the patient to recite the months backwards from December to July as well as recite the months backwards from December to January. It is possible that the patient may have performed better at going from December to January because of learning effect. Our reference standard for delirium was based upon DSM-IV-TR criteria. The new DSM-V criteria may be more restrictive and may slightly change the sensitivities and specificities of the inattention tasks. We enrolled a convenience sample and enrolled patients who were more likely to be male, have cardiovascular chief complaints, and be admitted to the hospital; as a result, selection bias may have been introduced. Lastly, this study was conducted in a single center and enrolled patients who were 65 years and older. Our findings may not be generalizable to other settings and in those who are less than 65 years of age.
CONCLUSIONS
The MOTYB-6 and MOTYB-12 tasks had very good sensitivities but modest specificities (approximately 50%) using any error made as a cutoff; increasing cutoff to 2 errors and 3 errors, respectively, improved their specificities (approximately 70%) with minimal impact to their sensitivities. Reciting the days of the week backwards, spelling the word “LUNCH” backwards, and the 10-letter vigilance “A” task appeared to perform the best in ruling out delirium but only moderately decreased the likelihood of delirium. The 10-letter Vigilance “A” and picture recognition task
Disclosure
This study was funded by the Emergency Medicine Foundation Career Development Award, National Institutes of Health K23AG032355, and National Center for Research Resources, Grant UL1 RR024975-01. The authors report no financial conflicts of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
33. Hamrick I, Hafiz R, Cummings DM. Use of days of the week in a modified mini-mental state exam (M-MMSE) for detecting geriatric cognitive impairment. J Am Board Fam Med. 2013;26(4):429-435.
Delirium is an acute neurocognitive disorder1 that affects up to 25% of older emergency department (ED) and hospitalized patients.2-4 The relationship between delirium and adverse outcomes is well documented.5-7 Delirium is a strong predictor of increased length of mechanical ventilation, longer intensive care unit and hospital stays, increased risk of falls, long-term cognitive impairment, and mortality.8-13 Delirium is frequently missed by healthcare professionals2,14-16 and goes undetected in up to 3 out of 4 patients by bedside nurses and medical practitioners in many hospital settings.14,17-22 A significant barrier to recognizing delirium is the absence of brief delirium assessments.
In an effort to improve delirium recognition in the acute care setting, there has been a concerted effort to develop and validate brief delirium assessments. To address this unmet need, 4 ‘A’s Test (4AT), the Brief Confusion Assessment Method (bCAM), and the 3-minute diagnostic assessment for CAM-defined delirium (3D-CAM) are 1- to 3-minute delirium assessments that were validated in acutely ill older patients.23 However, 1 to 3 minutes may still be too long in busy clinical environments, and briefer (<30 seconds) delirium assessments may be needed.
One potential more-rapid method to screen for delirium is to specifically test for the presence of inattention, which is a cardinal feature of delirium.24,25 Inattention can be ascertained by having the patient recite the months backwards, recite the days of the week backwards, or spell a word backwards.26 Recent studies have evaluated the diagnostic accuracy of reciting the months of the year backwards for delirium. O’Regan et al.27 evaluated the diagnostic accuracy of the month of the year backwards from December to July (MOTYB-6) and observed that this task was 84% sensitive and 90% specific for delirium in older patients. However, they performed the reference standard delirium assessments in patients who had a positive MOTYB-6, which can overestimate sensitivity and underestimate specificity (verification bias).28 Fick et al.29 examined the diagnostic accuracy of 20 individual elements of the 3D-CAM and observed that reciting the months of the year backwards from December to January (MOTYB-12) was 83% sensitive and 69% specific for delirium. However, this was an exploratory study that was designed to identify an element of the 3D-CAM that had the best diagnostic accuracy.
To address these limitations, we sought to evaluate the diagnostic performance of the MOTYB-6 and MOTYB-12 for delirium as diagnosed by a reference standard. We also explored other brief tests of inattention such as spelling a word (“LUNCH”) backwards, reciting the days of the week backwards, 10-letter vigilance “A” task, and 5 picture recognition task.
METHODS
Study Design and Setting
This was a preplanned secondary analysis of a prospective observational study that validated 3 delirium assessments.30,31 This study was conducted at a tertiary care, academic ED. The local institutional review board (IRB) reviewed and approved this study. Informed consent from the patient or an authorized surrogate was obtained whenever possible. Because this was an observational study and posed minimal risk to the patient, the IRB granted a waiver of consent for patients who were both unable to provide consent and were without an authorized surrogate available in the ED or by phone.
Selection of Participants
We enrolled a convenience sample of patients between June 2010 and February 2012 Monday through Friday from 8
Research assistants approached patients who met inclusion criteria and determined if any exclusion criteria were present. If none of the exclusion criteria were present, then the research assistant reviewed the informed consent document with the patient or authorized surrogate if the patient was not capable of providing consent. If a patient was not capable of providing consent and no authorized surrogate was available, then the patient was enrolled (under the waiver of consent) as long as the patient assented to be a part of the study. Once the patient was enrolled, the research assistant contacted the physician rater and reference standard psychiatrists to approach the patient.
Measures of Inattention
An emergency physician (JHH) who had no formal training in the mental status assessment of elders administered a cognitive battery to the patient, including tests of inattention. The following inattention tasks were administered:
- Spell the word “LUNCH” backwards.30 Patients were initially allowed to spell the word “LUNCH” forwards. Patients who were unable to perform the task were assigned 5 errors.
- Recite the months of the year backwards from December to July.23,26,27,30,32 Patients who were unable to perform the task were assigned 6 errors.
- Recite the days of the week backwards.23,26,33 Patients who were unable to perform the task were assigned 7 errors.
- Ten-letter vigilance “A” task.34 The patient was given a series of 10 letters (“S-A-V-E-A-H-A-A-R-T”) every 3 seconds and was asked to squeeze the rater’s hand every time the patient heard the letter “A.” Patients who were unable to perform the task were assigned 10 errors.
- Five picture recognition task.34 Patients were shown 5 objects on picture cards. Afterwards, patients were shown 10 pictures with the previously shown objects intermingled. The patient had to identify which objects were seen previously in the first 5 pictures. Patients who were unable to perform the task were assigned 10 errors.
- Recite the months of the year backwards from December to January.29 Patients who were unable to perform the task were assigned 12 errors.
Reference Standard for Delirium
A comprehensive consultation-liaison psychiatrist assessment was the reference standard for delirium; the diagnosis of delirium was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria.35 Three psychiatrists who each had an average of 11 years of clinical experience and regularly diagnosed delirium as part of their daily clinical practice were available to perform these assessments. To arrive at the diagnosis of delirium, they interviewed those who best understood the patient’s mental status (eg, the patient’s family members or caregivers, physician, and nurses). They also reviewed the patient’s medical record and radiology and laboratory test results. They performed bedside cognitive testing that included, but was not limited to, the Mini-Mental State Examination, Clock Drawing Test, Luria hand sequencing task, and tests for verbal fluency. A focused neurological examination was also performed (ie, screening for paraphasic errors, tremors, tone, asterixis, frontal release signs, etc.), and they also evaluated the patient for affective lability, hallucinations, and level of alertness. If the presence of delirium was still questionable, then confrontational naming, proverb interpretation or similarities, and assessments for apraxias were performed at the discretion of the psychiatrist. The psychiatrists were blinded to the physician’s assessments, and the assessments were conducted within 3 hours of each other.
Additional Variables Collected
Using medical record review, comorbidity burden, severity of illness, and premorbid cognition were ascertained. The Charlson Comorbidity Index, a weighted index that takes into account the number and seriousness of 19 preexisting comorbid conditions, was used to quantify comorbidity burden; higher scores indicate higher comorbid burden.36,37 The Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II was used to quantify severity of illness.38 This score is based upon the initial values of 12 routine physiologic measurements such as vital sign and laboratory abnormalities; higher scores represent higher severities of illness.38 The medical record was reviewed to ascertain the presence of premorbid cognitive impairment; any documentation of dementia in the patient’s clinical problem list or physician history and physical examination from the outpatient or inpatient settings was considered positive. The medical record review was performed by a research assistant and was double-checked for accuracy by one of the investigators (JHH).
Data Analyses
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. Receiver operating characteristic curves were constructed for each inattention task. Area under the receiver operating characteristic curves (AUC) was reported to provide a global measure of diagnostic accuracy. Sensitivities, specificities, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with their 95% CIs were calculated using the psychiatrist’s assessment as the reference standard.39 Cut-points with PLRs greater than 10 (strongly increased the likelihood of delirium) or NLRs less than 0.1 (strongly decreased the likelihood of delirium) were preferentially reported whenever possible.
All statistical analyses were performed with open source R statistical software version 3.0.1 (http://www.r-project.org/), SAS 9.4 (SAS Institute, Cary, NC), and Microsoft Excel 2010 (Microsoft Inc., Redmond, WA).
RESULTS
DISCUSSION
Delirium is frequently missed by healthcare providers because it is not routinely screened for in the acute care setting. To help address this deficiency of care, we evaluated several brief measures of inattention that take less than 30 seconds to complete. We observed that any errors made on the MOTYB-6 and MOTYB-12 tasks had very good sensitivities (80% and 84%) but were limited by their modest specificities (approximately 50%) for delirium. As a result, these assessments have limited clinical utility as standalone delirium screens. We also explored other commonly used brief measures of inattention and at a variety of error cutoffs. Reciting the days of the week backwards appeared to best balance sensitivity and specificity. None of the inattention measures could convincingly rule out delirium (NLR < 0.10), but the vigilance “A” and picture recognition tasks may have clinical utility in ruling in delirium (PLR > 10). Overall, all the inattention tasks, including MOTYB-6 and MOTYB-12, had very good diagnostic performances based upon their AUC. However, achieving a high sensitivity often had to be sacrificed for specificity or, alternatively, achieving a high specificity had to be sacrificed for sensitivity.
Inattention has been shown to be the cardinal feature for delirium,40 and its assessment using cognitive testing has been recommended to help identify the presence of delirium according to an expert consensus panel.26 The diagnostic performance of the MOTYB-12 observed in our study is similar to a study by Fick et al., who reported that MOTYB-12 had very good sensitivity (83%) but had modest specificity (69%) with a cutoff of 1 or more errors. Hendry et al. observed that the MOTYB-12 was 91% sensitive and 50% specific using a cutoff of 4 or more errors. With regard to the MOTYB-6, our reported specificity was different from what was observed by O’Regan et al.27 Using 1 or more errors as a cutoff, they observed a much higher specificity for delirium than we did (90% vs 57%). Discordant observations regarding the diagnostic accuracy for other inattention tasks also exist. We observed that making any error on the days of the week backwards task was 84% sensitive and 82% specific for delirium, whereas Fick et al. observed a sensitivity and specificity of 50% and 94%, respectively. For the vigilance “A” task, we observed that making 2 or more errors over a series of 10 letters was 64.0% sensitive and 91.4% specific for delirium, whereas Pompei et al.41 observed that making 2 or more errors over a series of 60 letters was 51% sensitive and 77% specific for delirium.
The abovementioned discordant findings may be driven by spectrum bias, wherein the sensitivities and specificities for each inattention task may differ in different subgroups. As a result, differences in the age distribution, proportion of college graduates, history of dementia, and susceptibility to delirium can influence overall sensitivity and specificity. Objective measures of delirium, including the inattention screens studied, are particularly prone to spectrum bias.31,34 However, the strength of this approach is that the assessment of inattention becomes less reliant upon clinical judgment and allows it to be used by raters from a wide range of clinical backgrounds. On the other hand, a subjective interpretation of these inattention tasks may allow the rater to capture the subtleties of inattention (ie, decreased speed of performance in a highly intelligent and well-educated patient without dementia). The disadvantage of this approach, however, is that it is more dependent on clinical judgment and may have decreased diagnostic accuracy in those with less clinical experience or with limited training.14,42,43 These factors must be carefully considered when determining which delirium assessment to use.
Additional research is required to determine the clinical utility of these brief inattention assessments. These findings need to be further validated in larger studies, and the optimal cutoff of each task for different subgroup of patients (eg, demented vs nondemented) needs to be further clarified. It is not completely clear whether these inattention tests can serve as standalone assessments. Depending on the cutoff used, some of these assessments may have unacceptable false negative or false positive rates that may lead to increased adverse patient outcomes or increased resource utilization, respectively. Additional components or assessments may be needed to improve the diagnostic accuracy of these assessments. In addition to understanding these inattention assessments’ diagnostic accuracies, their ability to predict adverse outcomes also needs to be investigated. While a previous study observed that making any error on the MOTYB-12 task was associated with increased physical restraint use and prolonged hospital length of stay,44 these assessments’ ability to prognosticate long-term outcomes such as mortality or long-term cognition or function need to be studied. Lastly, studies should also evaluate how easily implementable these assessments are and whether improved delirium recognition leads to improved patient outcomes.
This study has several notable limitations. Though planned a priori, this was a secondary analysis of a larger investigation designed to validate 3 delirium assessments. Our sample size was also relatively small, causing our 95% CIs to overlap in most cases and limiting the statistical power to truly determine whether one measure is better than the other. We also asked the patient to recite the months backwards from December to July as well as recite the months backwards from December to January. It is possible that the patient may have performed better at going from December to January because of learning effect. Our reference standard for delirium was based upon DSM-IV-TR criteria. The new DSM-V criteria may be more restrictive and may slightly change the sensitivities and specificities of the inattention tasks. We enrolled a convenience sample and enrolled patients who were more likely to be male, have cardiovascular chief complaints, and be admitted to the hospital; as a result, selection bias may have been introduced. Lastly, this study was conducted in a single center and enrolled patients who were 65 years and older. Our findings may not be generalizable to other settings and in those who are less than 65 years of age.
CONCLUSIONS
The MOTYB-6 and MOTYB-12 tasks had very good sensitivities but modest specificities (approximately 50%) using any error made as a cutoff; increasing cutoff to 2 errors and 3 errors, respectively, improved their specificities (approximately 70%) with minimal impact to their sensitivities. Reciting the days of the week backwards, spelling the word “LUNCH” backwards, and the 10-letter vigilance “A” task appeared to perform the best in ruling out delirium but only moderately decreased the likelihood of delirium. The 10-letter Vigilance “A” and picture recognition task
Disclosure
This study was funded by the Emergency Medicine Foundation Career Development Award, National Institutes of Health K23AG032355, and National Center for Research Resources, Grant UL1 RR024975-01. The authors report no financial conflicts of interest.
Delirium is an acute neurocognitive disorder1 that affects up to 25% of older emergency department (ED) and hospitalized patients.2-4 The relationship between delirium and adverse outcomes is well documented.5-7 Delirium is a strong predictor of increased length of mechanical ventilation, longer intensive care unit and hospital stays, increased risk of falls, long-term cognitive impairment, and mortality.8-13 Delirium is frequently missed by healthcare professionals2,14-16 and goes undetected in up to 3 out of 4 patients by bedside nurses and medical practitioners in many hospital settings.14,17-22 A significant barrier to recognizing delirium is the absence of brief delirium assessments.
In an effort to improve delirium recognition in the acute care setting, there has been a concerted effort to develop and validate brief delirium assessments. To address this unmet need, 4 ‘A’s Test (4AT), the Brief Confusion Assessment Method (bCAM), and the 3-minute diagnostic assessment for CAM-defined delirium (3D-CAM) are 1- to 3-minute delirium assessments that were validated in acutely ill older patients.23 However, 1 to 3 minutes may still be too long in busy clinical environments, and briefer (<30 seconds) delirium assessments may be needed.
One potential more-rapid method to screen for delirium is to specifically test for the presence of inattention, which is a cardinal feature of delirium.24,25 Inattention can be ascertained by having the patient recite the months backwards, recite the days of the week backwards, or spell a word backwards.26 Recent studies have evaluated the diagnostic accuracy of reciting the months of the year backwards for delirium. O’Regan et al.27 evaluated the diagnostic accuracy of the month of the year backwards from December to July (MOTYB-6) and observed that this task was 84% sensitive and 90% specific for delirium in older patients. However, they performed the reference standard delirium assessments in patients who had a positive MOTYB-6, which can overestimate sensitivity and underestimate specificity (verification bias).28 Fick et al.29 examined the diagnostic accuracy of 20 individual elements of the 3D-CAM and observed that reciting the months of the year backwards from December to January (MOTYB-12) was 83% sensitive and 69% specific for delirium. However, this was an exploratory study that was designed to identify an element of the 3D-CAM that had the best diagnostic accuracy.
To address these limitations, we sought to evaluate the diagnostic performance of the MOTYB-6 and MOTYB-12 for delirium as diagnosed by a reference standard. We also explored other brief tests of inattention such as spelling a word (“LUNCH”) backwards, reciting the days of the week backwards, 10-letter vigilance “A” task, and 5 picture recognition task.
METHODS
Study Design and Setting
This was a preplanned secondary analysis of a prospective observational study that validated 3 delirium assessments.30,31 This study was conducted at a tertiary care, academic ED. The local institutional review board (IRB) reviewed and approved this study. Informed consent from the patient or an authorized surrogate was obtained whenever possible. Because this was an observational study and posed minimal risk to the patient, the IRB granted a waiver of consent for patients who were both unable to provide consent and were without an authorized surrogate available in the ED or by phone.
Selection of Participants
We enrolled a convenience sample of patients between June 2010 and February 2012 Monday through Friday from 8
Research assistants approached patients who met inclusion criteria and determined if any exclusion criteria were present. If none of the exclusion criteria were present, then the research assistant reviewed the informed consent document with the patient or authorized surrogate if the patient was not capable of providing consent. If a patient was not capable of providing consent and no authorized surrogate was available, then the patient was enrolled (under the waiver of consent) as long as the patient assented to be a part of the study. Once the patient was enrolled, the research assistant contacted the physician rater and reference standard psychiatrists to approach the patient.
Measures of Inattention
An emergency physician (JHH) who had no formal training in the mental status assessment of elders administered a cognitive battery to the patient, including tests of inattention. The following inattention tasks were administered:
- Spell the word “LUNCH” backwards.30 Patients were initially allowed to spell the word “LUNCH” forwards. Patients who were unable to perform the task were assigned 5 errors.
- Recite the months of the year backwards from December to July.23,26,27,30,32 Patients who were unable to perform the task were assigned 6 errors.
- Recite the days of the week backwards.23,26,33 Patients who were unable to perform the task were assigned 7 errors.
- Ten-letter vigilance “A” task.34 The patient was given a series of 10 letters (“S-A-V-E-A-H-A-A-R-T”) every 3 seconds and was asked to squeeze the rater’s hand every time the patient heard the letter “A.” Patients who were unable to perform the task were assigned 10 errors.
- Five picture recognition task.34 Patients were shown 5 objects on picture cards. Afterwards, patients were shown 10 pictures with the previously shown objects intermingled. The patient had to identify which objects were seen previously in the first 5 pictures. Patients who were unable to perform the task were assigned 10 errors.
- Recite the months of the year backwards from December to January.29 Patients who were unable to perform the task were assigned 12 errors.
Reference Standard for Delirium
A comprehensive consultation-liaison psychiatrist assessment was the reference standard for delirium; the diagnosis of delirium was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria.35 Three psychiatrists who each had an average of 11 years of clinical experience and regularly diagnosed delirium as part of their daily clinical practice were available to perform these assessments. To arrive at the diagnosis of delirium, they interviewed those who best understood the patient’s mental status (eg, the patient’s family members or caregivers, physician, and nurses). They also reviewed the patient’s medical record and radiology and laboratory test results. They performed bedside cognitive testing that included, but was not limited to, the Mini-Mental State Examination, Clock Drawing Test, Luria hand sequencing task, and tests for verbal fluency. A focused neurological examination was also performed (ie, screening for paraphasic errors, tremors, tone, asterixis, frontal release signs, etc.), and they also evaluated the patient for affective lability, hallucinations, and level of alertness. If the presence of delirium was still questionable, then confrontational naming, proverb interpretation or similarities, and assessments for apraxias were performed at the discretion of the psychiatrist. The psychiatrists were blinded to the physician’s assessments, and the assessments were conducted within 3 hours of each other.
Additional Variables Collected
Using medical record review, comorbidity burden, severity of illness, and premorbid cognition were ascertained. The Charlson Comorbidity Index, a weighted index that takes into account the number and seriousness of 19 preexisting comorbid conditions, was used to quantify comorbidity burden; higher scores indicate higher comorbid burden.36,37 The Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II was used to quantify severity of illness.38 This score is based upon the initial values of 12 routine physiologic measurements such as vital sign and laboratory abnormalities; higher scores represent higher severities of illness.38 The medical record was reviewed to ascertain the presence of premorbid cognitive impairment; any documentation of dementia in the patient’s clinical problem list or physician history and physical examination from the outpatient or inpatient settings was considered positive. The medical record review was performed by a research assistant and was double-checked for accuracy by one of the investigators (JHH).
Data Analyses
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. Receiver operating characteristic curves were constructed for each inattention task. Area under the receiver operating characteristic curves (AUC) was reported to provide a global measure of diagnostic accuracy. Sensitivities, specificities, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with their 95% CIs were calculated using the psychiatrist’s assessment as the reference standard.39 Cut-points with PLRs greater than 10 (strongly increased the likelihood of delirium) or NLRs less than 0.1 (strongly decreased the likelihood of delirium) were preferentially reported whenever possible.
All statistical analyses were performed with open source R statistical software version 3.0.1 (http://www.r-project.org/), SAS 9.4 (SAS Institute, Cary, NC), and Microsoft Excel 2010 (Microsoft Inc., Redmond, WA).
RESULTS
DISCUSSION
Delirium is frequently missed by healthcare providers because it is not routinely screened for in the acute care setting. To help address this deficiency of care, we evaluated several brief measures of inattention that take less than 30 seconds to complete. We observed that any errors made on the MOTYB-6 and MOTYB-12 tasks had very good sensitivities (80% and 84%) but were limited by their modest specificities (approximately 50%) for delirium. As a result, these assessments have limited clinical utility as standalone delirium screens. We also explored other commonly used brief measures of inattention and at a variety of error cutoffs. Reciting the days of the week backwards appeared to best balance sensitivity and specificity. None of the inattention measures could convincingly rule out delirium (NLR < 0.10), but the vigilance “A” and picture recognition tasks may have clinical utility in ruling in delirium (PLR > 10). Overall, all the inattention tasks, including MOTYB-6 and MOTYB-12, had very good diagnostic performances based upon their AUC. However, achieving a high sensitivity often had to be sacrificed for specificity or, alternatively, achieving a high specificity had to be sacrificed for sensitivity.
Inattention has been shown to be the cardinal feature for delirium,40 and its assessment using cognitive testing has been recommended to help identify the presence of delirium according to an expert consensus panel.26 The diagnostic performance of the MOTYB-12 observed in our study is similar to a study by Fick et al., who reported that MOTYB-12 had very good sensitivity (83%) but had modest specificity (69%) with a cutoff of 1 or more errors. Hendry et al. observed that the MOTYB-12 was 91% sensitive and 50% specific using a cutoff of 4 or more errors. With regard to the MOTYB-6, our reported specificity was different from what was observed by O’Regan et al.27 Using 1 or more errors as a cutoff, they observed a much higher specificity for delirium than we did (90% vs 57%). Discordant observations regarding the diagnostic accuracy for other inattention tasks also exist. We observed that making any error on the days of the week backwards task was 84% sensitive and 82% specific for delirium, whereas Fick et al. observed a sensitivity and specificity of 50% and 94%, respectively. For the vigilance “A” task, we observed that making 2 or more errors over a series of 10 letters was 64.0% sensitive and 91.4% specific for delirium, whereas Pompei et al.41 observed that making 2 or more errors over a series of 60 letters was 51% sensitive and 77% specific for delirium.
The abovementioned discordant findings may be driven by spectrum bias, wherein the sensitivities and specificities for each inattention task may differ in different subgroups. As a result, differences in the age distribution, proportion of college graduates, history of dementia, and susceptibility to delirium can influence overall sensitivity and specificity. Objective measures of delirium, including the inattention screens studied, are particularly prone to spectrum bias.31,34 However, the strength of this approach is that the assessment of inattention becomes less reliant upon clinical judgment and allows it to be used by raters from a wide range of clinical backgrounds. On the other hand, a subjective interpretation of these inattention tasks may allow the rater to capture the subtleties of inattention (ie, decreased speed of performance in a highly intelligent and well-educated patient without dementia). The disadvantage of this approach, however, is that it is more dependent on clinical judgment and may have decreased diagnostic accuracy in those with less clinical experience or with limited training.14,42,43 These factors must be carefully considered when determining which delirium assessment to use.
Additional research is required to determine the clinical utility of these brief inattention assessments. These findings need to be further validated in larger studies, and the optimal cutoff of each task for different subgroup of patients (eg, demented vs nondemented) needs to be further clarified. It is not completely clear whether these inattention tests can serve as standalone assessments. Depending on the cutoff used, some of these assessments may have unacceptable false negative or false positive rates that may lead to increased adverse patient outcomes or increased resource utilization, respectively. Additional components or assessments may be needed to improve the diagnostic accuracy of these assessments. In addition to understanding these inattention assessments’ diagnostic accuracies, their ability to predict adverse outcomes also needs to be investigated. While a previous study observed that making any error on the MOTYB-12 task was associated with increased physical restraint use and prolonged hospital length of stay,44 these assessments’ ability to prognosticate long-term outcomes such as mortality or long-term cognition or function need to be studied. Lastly, studies should also evaluate how easily implementable these assessments are and whether improved delirium recognition leads to improved patient outcomes.
This study has several notable limitations. Though planned a priori, this was a secondary analysis of a larger investigation designed to validate 3 delirium assessments. Our sample size was also relatively small, causing our 95% CIs to overlap in most cases and limiting the statistical power to truly determine whether one measure is better than the other. We also asked the patient to recite the months backwards from December to July as well as recite the months backwards from December to January. It is possible that the patient may have performed better at going from December to January because of learning effect. Our reference standard for delirium was based upon DSM-IV-TR criteria. The new DSM-V criteria may be more restrictive and may slightly change the sensitivities and specificities of the inattention tasks. We enrolled a convenience sample and enrolled patients who were more likely to be male, have cardiovascular chief complaints, and be admitted to the hospital; as a result, selection bias may have been introduced. Lastly, this study was conducted in a single center and enrolled patients who were 65 years and older. Our findings may not be generalizable to other settings and in those who are less than 65 years of age.
CONCLUSIONS
The MOTYB-6 and MOTYB-12 tasks had very good sensitivities but modest specificities (approximately 50%) using any error made as a cutoff; increasing cutoff to 2 errors and 3 errors, respectively, improved their specificities (approximately 70%) with minimal impact to their sensitivities. Reciting the days of the week backwards, spelling the word “LUNCH” backwards, and the 10-letter vigilance “A” task appeared to perform the best in ruling out delirium but only moderately decreased the likelihood of delirium. The 10-letter Vigilance “A” and picture recognition task
Disclosure
This study was funded by the Emergency Medicine Foundation Career Development Award, National Institutes of Health K23AG032355, and National Center for Research Resources, Grant UL1 RR024975-01. The authors report no financial conflicts of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
33. Hamrick I, Hafiz R, Cummings DM. Use of days of the week in a modified mini-mental state exam (M-MMSE) for detecting geriatric cognitive impairment. J Am Board Fam Med. 2013;26(4):429-435.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
33. Hamrick I, Hafiz R, Cummings DM. Use of days of the week in a modified mini-mental state exam (M-MMSE) for detecting geriatric cognitive impairment. J Am Board Fam Med. 2013;26(4):429-435.
© 2018 Society of Hospital Medicine
Impaired Arousal and Mortality
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
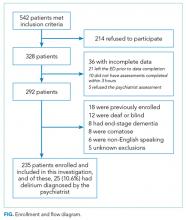
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
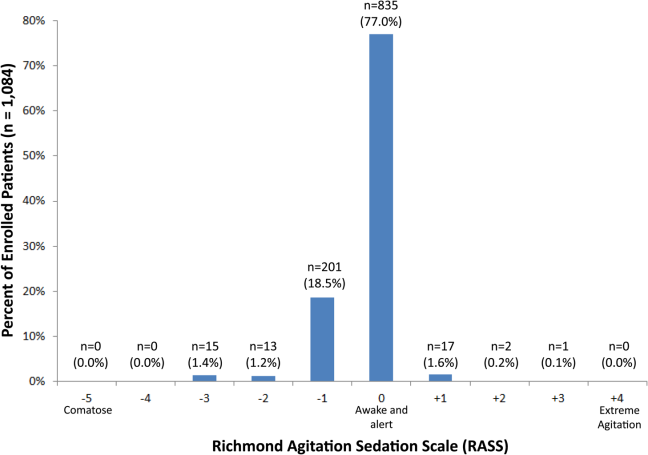
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
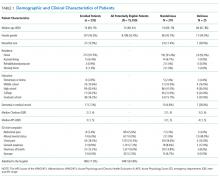
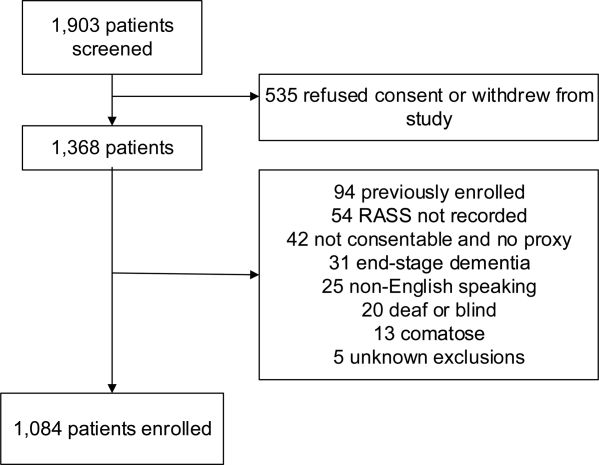
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).
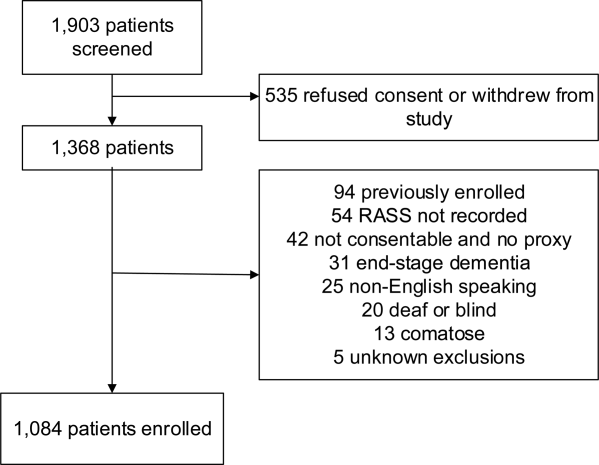
| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.
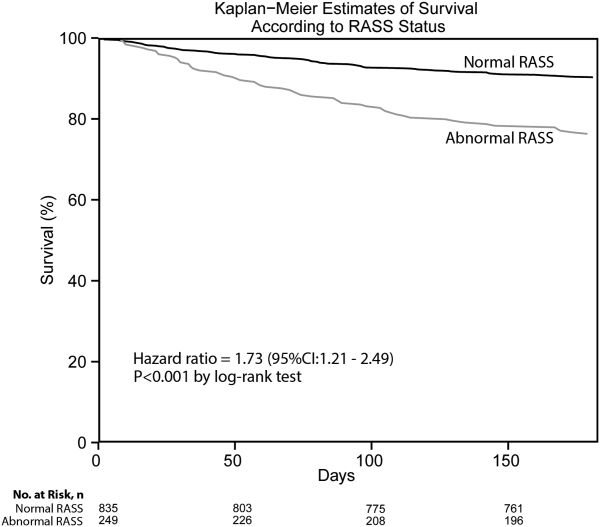
Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).

| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.


Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).

| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.


Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
© 2014 Society of Hospital Medicine
Algorithms for Diagnosing and Treating VAP
Ventilator‐associated pneumonia (VAP) is a serious and common complication for patients in the intensive care unit (ICU).1 VAP is defined as a pulmonary infection occurring after hospital admission in a mechanically‐ventilated patient with a tracheostomy or endotracheal tube.2, 3 With an attributable mortality that may exceed 20% and an estimated cost of $5000‐$20,000 per episode,49 the management of VAP is an important issue for both patient safety and cost of care.
The diagnosis of VAP is a controversial topic in critical care, primarily because of the difficulty distinguishing between airway colonization, upper respiratory tract infection (eg, tracheobronchitis), and early‐onset pneumonia. Some clinicians insist that an invasive sampling technique (eg, bronchoalveolar lavage) with quantitative cultures is essential for determining the presence of VAP.10 However, other clinicians suggest that a noninvasive approach using qualitative cultures (eg, tracheal suctioning) is an acceptable alternative.11 Regardless, nearly all experts agree that a specimen for microbiologic culture should be obtained prior to initiating antibiotics. Subsequent therapy should then be adjusted according to culture results.
Studies from both Europe and North America have demonstrated considerable variation in the diagnostic approaches used for patients with suspected VAP.12, 13 This variation is likely a result of several factors including controversy about the best diagnostic approach, variation in clinician knowledge and experience, and variation in ICU management protocols. Such practice variability is common for many ICU behaviors.1416 Quality‐of‐care proponents view this variation as an important opportunity for improvement.17
During a recent national collaborative aimed at reducing health careassociated infections in the ICU, we discovered many participants were uncertain about how to diagnose and manage VAP, and considerable practice variability existed among participating hospitals. This uncertainty provided an important opportunity for developing consensus on VAP management. On the basis of diagnostic criteria outlined by the Centers for Disease Control and Prevention (CDC), we developed algorithms as tools for diagnosing VAP in 4 ICU populations: infant, pediatric, immunocompromised, and adult ICU patients. We also developed an algorithm for initial VAP treatment. An interdisciplinary team of experts reviewed the current literature and developed these evidence‐based consensus guidelines. Our intent is that the algorithms provide guidance to clinicians looking for a standardized approach to the diagnosis and management of this complicated clinical situation.
METHODS
Our primary goal was to develop practical algorithms that assist ICU clinicians in the diagnosis and management of VAP during daily practice. To improve the quality and credibility of these algorithms, the development process used a stepwise approach that included assembling an interdisciplinary team of experts, appraising the published evidence, and formulating the algorithms through a consensus process.18
AHRQ National Collaborative
We developed these diagnostic algorithms as part of a national collaborative effort aimed at reducing VAP and central venous catheterrelated bloodstream infections in the ICU. This effort was possible through a 2‐year Partnerships in Implementing Patient Safety grant funded by the Agency for Healthcare Research and Quality (AHRQ).19 The voluntary collaborative was conducted in 61 medical/surgical and children's hospitals across the Hospital Corporation of America (HCA), a company that owns and/or operates 173 hospitals and 107 freestanding surgery centers in 20 states, England, and Switzerland. HCA is one of the largest providers of health care in the United States. All participating hospitals had at least 1 ICU, and a total of 110 ICUs were included in the project. Most hospitals were in the southern or southeastern regions of the United States.
Interdisciplinary Team
We assembled an interdisciplinary team to develop the diagnostic algorithms. Individuals on the team represented the specialties of infectious diseases, infection control, anesthesia, critical care medicine, hospital medicine, critical care nursing, pharmacy, and biostatistics. The development phase occurred over 34 months and used an iterative process that consisted of both group conference calls and in‐person meetings.
Our goal was not to conduct a systematic review but rather to develop practical algorithms for collaborative participants in a timely manner. Our literature search strategy included MEDLINE and the Cochrane Library. We focused on articles that addressed key diagnostic issues, proposed an algorithm, or summarized a topic relevant to practicing clinicians. Extra attention was given to articles that were randomized trials, meta‐analyses, or systematic reviews. No explicit grading of articles was performed. We examined studies with outcomes of interest to clinicians, including mortality, number of ventilator days, length of stay, antibiotic utilization, and antibiotic resistance.
We screened potentially relevant articles and the references of these articles. The search results were reviewed by all members of the team, and an iterative consensus process was used to derive the current algorithms. Preliminary versions of the algorithms were shown to other AHRQ investigators and outside experts in the field, and additional modifications were made based on their feedback. The final algorithms were approved by all study investigators.
RESULTS
Literature Overview
Overall, there is an enormous body of published literature on diagnosing and managing VAP. The Medline database has listed more than 500 articles on VAP diagnosis in the past decade. Nonetheless, the best diagnostic approach remains unclear. The gold standard for diagnosing VAP is lung biopsy with histopathologic examination and tissue culture. However, this procedure is fraught with potential dangers and impractical for most critically ill patients.20 Therefore, practitioners traditionally combine their clinical suspicion (based on fever, leukocytosis, character of sputum, and radiographic changes), epidemiologic data (eg, patient demographics, medical history, and ICU infection surveillance data), and microbiologic data.
Several issues relevant to practicing clinicians deserve further mention.
Definition of VAP
Although early articles used variable criteria for diagnosing VAP, recent studies have traditionally defined VAP as an infection occurring more than 48 hours after hospital admission in a mechanically ventilated patient with a tracheostomy or endotracheal tube.2 In early 2007, the CDC revised their definition for diagnosing VAP.3 These latest criteria state there is no minimum period that the ventilator must be in place in order to diagnose VAP. This important change must be kept in mind when examining future studies.
The term VAP is more specific than the term health careassociated pneumonia. The latter encompasses patients residing in a nursing home or long‐term care facility; hospitalized in an acute care hospital for more than 48 hours in the past 90 days; receiving antibiotics, chemotherapy, or wound care within the past 30 days; or attending a hospital or hemodialysis clinic.
The CDC published detailed criteria for diagnosing VAP in its member hospitals (Tables 1 and 2).3 Because diagnosing VAP in infants, children, elderly, and immunocompromised patients is often confusing because of other conditions with similar signs and symptoms, the CDC published alternate criteria for these populations. A key objective during development of our algorithms was to consolidate and simplify these diagnostic criteria for ICU clinicians.
| Radiology | Signs/symptoms/laboratory |
|---|---|
| |
| Two or more serial chest radiographs with at least 1 of the following*: | CRITERIA FOR ANY PATIENT |
| New or progressive and persistent infiltrate | At least 1 of the following: |
| Consolidation | Fever (>38C or >100.4F) with no other recognized cause |
| Cavitation | Leukopenia (4000 WBC/mm3) or leukocytosis (12,000 WBC/mm3) |
| Pneumatoceles, in infants 1 year old | For adults 70 years old, altered mental status with no other recognized causeand |
| Note: In patients without underlying pulmonary or cardiac disease (eg, respiratory distress syndrome, bronchopulmonary dysplasia, pulmonary edema, or chronic obstructive pulmonary disease), 1 definitive chest radiograph is acceptable.* | |
| At least 2 of the following: | |
| New onset of purulent sputum, or change in character of sputum, or increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough or dyspnea or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation [eg, PaO2/FiO2 240],** increased oxygen requirement, or increased ventilation demand) | |
|
Any laboratory criterion from Table 2 |
|
| ALTERNATE CRITERIA FOR INFANTS 1 YEAR OLD | |
| Worsening gas exchange (eg, O2 desaturation, increased ventilation demand or O2 requirement) | |
| and | |
| At least 3 of the following: | |
| Temperature instability with no other recognized cause | |
| Leukopenia (4000 WBC/mm3) or leukocytosis (15,000 WBC/mm3) and left shift (10% bands) | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| Apnea, tachypnea,‖ nasal flaring with retraction of chest wall, or grunting | |
| Wheezing, rales, or rhonchi | |
| Cough | |
| Bradycadia (100 beats/min) or tachycardia (>170 beats/min) | |
| ALTERNATE CRITERIA FOR CHILD >1 OR 12 YEARS OLD | |
| At least 3 of the following: | |
| Fever (>38.4C or >101.1F) or hypothermia (36.5C or 97.7F) with no other recognized cause | |
| Leukopenia (4000 WBC/mm3) or leukocytosis (15,000 WBC/mm3) | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough or dyspnea, apnea, or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation 94%, increased ventilation demand or O2 requirement) | |
|
Any laboratory criterion from Table 2 |
|
| ALTERNATE CRITERIA FOR IMMUNOCOMPROMISED PATIENTS*** | |
| At least 1 of the following: | |
| Fever (>38.4C or >101.1F) with no other recognized cause | |
| For adults > 70 years old, altered mental status with no other recognized cause | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough, dyspnea, or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation [eg, PaO2/FiO2 240],** increased oxygen requirement, or increased ventilation demand) | |
| Hemoptysis | |
| Pleuritic chest pain | |
| Matching positive blood and sputum cultures with Candida spp. | |
| Evidence of fungi or Pneumocytis from minimally contaminated LRT specimen (eg, BAL or protected specimen brushing) from 1 of the following: | |
| Direct microscopic exam | |
| Positive culture of fungi | |
|
Any laboratory criterion from Table 2 |
|
|
| Positive growth in blood culture* not related to another source of infection |
| Positive growth in culture of pleural fluid |
| Positive quantitative culture from minimally contaminated LRT specimen (eg, BAL) |
| 5% BAL‐obtained cells contain intracellular bacteria on direct microscopic exam (eg, gram stain) |
| Histopathologic exam shows at least 1 of the following: |
| Abscess formation or foci of consolidation with intense PMN accumulation in bronchioles and alveoli |
| Positive quantitative culture of lung parenchyma |
| Evidence of lung parenchyma invasion by fungal hyphae or pseudohyphae |
| Positive culture of virus or Chlamydia from respiratory secretions |
| Positive detection of viral antigen or antibody from respiratory secretions (eg, EIA, FAMA, shell vial assay, PCR) |
| Fourfold rise in paired sera (IgG) for pathogen (eg, influenza viruses, Chlamydia) |
| Positive PCR for Chlamydia or Mycoplasma |
| Positive micro‐IF test for Chlamydia |
| Positive culture or visualization by micro‐IF of Legionella spp. from respiratory secretions or tissue |
| Detection of Legionella pneumophila serogroup 1 antigens in urine by RIA or EIA |
| Fourfold rise in L. pneumophila serogroup 1 antibody titer to 1:128 in paired acute and convalescent sera by indirect IFA |
Etiology
The most commonly isolated VAP pathogens in all patients are bacteria.21 Most of these organisms normally colonize the respiratory and gastrointestinal tracts, but some are unique to health care settings. Tracheal intubation disrupts the body's natural anatomic and physiologic defenses and facilitates easier entry of these pathogens. Typical organisms include Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter species, Klebsiella pneumoniae, Acinetobacter species, Escherichia coli, and Haemophilus influenzae.22, 23 Unfortunately, the prevalence of antimicrobial resistance among VAP pathogens is increasing.24 Risk factors for antibiotic resistance are common to ICU patients and include recent antibiotics, hemodialysis, nursing home residence, immunosuppression, and chronic wound care.5 Polymicrobial infections are frequently seen in VAP, with up to 50% of all VAP episodes caused by more than 1 organism.25
Viral VAP is rare in immunocompetent hosts, and seasonal outbreaks of influenza and other similar viruses are usually limited to nonventilated patients.26 However, influenza is underrecognized as a potential nosocomial pathogen, and numerous nosocomial outbreaks because of influenza have been reported.2731 Although herpes simplex virus is often detected in the respiratory tract of critically ill patients, its clinical importance remains unclear.32
Fungal VAP is also rare in immunocompetent hosts. On the other hand, pulmonary fungal infections are common in immunocompromised patients, especially following chemotherapy and transplantation. Candida species are often isolated from the airways of normal hosts, but most cases traditionally have been considered clinically unimportant because these organisms are normal oropharyngeal flora and rarely invade lung tissue.33, 34 It is unclear whether recent studies suggesting Candida colonization is associated with a higher risk for Pseudomonas VAP will change this conventional wisdom.3537
Immunocompromised patients with suspected VAP are unique because they are at risk not only for typical bacteria (which are the most common causes of VAP) but also for rarer opportunistic infections and noninfectious processes that mimic pneumonia.3840 While assessing these patients, clinicians must consider the status of the underlying disease, duration and type of immunosuppression, prophylactic regimens, and risk factors for noninfectious causes of pulmonary infiltrates.41 Common opportunistic infections include viruses, mycobacteria, fungi, and Pneumocystis. Noninfectious processes include pulmonary edema, drug toxicity, radiation pneumonitis, engraftment syndrome, bronchiolitis obliterans organizing pneumonia, alveolar proteinosis, transfusion‐related lung injury, alveolar hemorrhage, and progression of underlying disease. In general, diagnosing VAP in the immunocompromised patient requires a prompt, comprehensive, and multidisciplinary approach.38
In preterm and term infants, the most common VAP pathogens are gram‐negative organisms such as E. coli and P. aeruginosa. Other less common pathogens are Enterobacter, Klebsiella, Acinetobacter, Proteus, Citrobacter, and Stenotrophomonas maltophilia.42, 43 Infants with a preceding bloodstream infection or prolonged intubation are more likely to develop VAP.43, 44 Unfortunately, gram‐negative bacteria often colonize the airways of mechanically ventilated infants, and tracheal aspirate culture data are difficult to interpret in this population.42
Children are more likely to develop VAP if they are intubated for more than 48 hours. The most common pathogens isolated from tracheal aspirates in mechanically ventilated children are enteric gram‐negative bacteria, P. aeruginosa, and S. aureus.45, 46 Few studies have precisely delineated the pathogenesis of VAP in the pediatric ICU population.
Overall, the causes of VAP vary by hospital, patient population, and ICU type. Therefore, it is essential that ICU clinicians remain knowledgeable about their local surveillance data.21 Awareness of VAP microbiology is essential for optimizing initial antibiotic therapy and improving outcomes.
Early Versus Late VAP
Distinguishing between early and late VAP is important for initial antibiotic selection because the etiologic pathogens vary between these 2 periods.4749 Early VAP (days 14 of hospitalization) usually involves antibiotic‐sensitive community‐acquired bacteria and carries a better prognosis. In contrast, late VAP (5 days after hospital admission) is more likely to be caused by antibiotic‐resistant nosocomial bacteria that lead to increased morbidity and mortality. All patients who have been hospitalized or have received antibiotics during the prior 90 days should be treated as having late VAP because they are at much higher risk for colonization and infection with antibiotic‐resistant bacteria.47 Of note, 2 recent studies suggest that pathogens in the early and late periods are becoming similar at some institutions.50, 51 Overall, the distinction between early and late VAP is important because it affects the likelihood that a patient has antibiotic‐resistant bacteria. If antibiotic‐resistant pathogens are suspected, initial therapy should include empiric triple antibiotics until culture data are available.
Culturing Approaches
Because clinical criteria alone are rarely able to accurately diagnose VAP,52, 53 clinicians should also obtain a respiratory specimen for microbiologic culture. Despite the convenience of blood cultures, their sensitivity for diagnosing VAP is poor, and they rarely make the diagnosis alone.54 Two methods are available for culturing the lungsan invasive approach (eg, bronchoscopy with bronchoalveolar lavage) and a noninvasive approach (eg, tracheal aspirate).
Some investigators believe that adult patients with suspected VAP should always undergo an invasive sampling of lower‐respiratory‐tract secretions.55 Proponents of the invasive approach cite the frequency with which potential pathogens colonize the trachea of ICU patients and create spurious results on tracheal aspirates.22 In addition, several studies have shown that clinicians are more likely to narrow the spectrum of antibiotics after obtaining an invasive diagnostic sample.56 In other words, the invasive approach has been associated with better antimicrobial stewardship.
Other investigators believe that a noninvasive approach is equally safe and effective for diagnosing VAP.57 This clinical approach involves culturing a tracheal aspirate and using a pneumonia prediction score such as the clinical pulmonary infection score (CPIS; Table 3). The CPIS assigns 012 points based on 6 clinical criteria: fever, leukocyte count, oxygenation, quantity and purulence of secretions, type of radiographic abnormality, and results of sputum gram stain and culture.58 As developed, a CPIS > 6 has a sensitivity of 93% and a specificity of 100% for diagnosing VAP.58 However, the CPIS requires that nurses record sputum volume and that the laboratory stains the specimen. When the CPIS has been modified based on the unavailability of such resources, the results have been less impressive.5961 Despite studies showing that a noninvasive clinical approach can achieve adequate initial antibiotic coverage and reduce overuse of broad‐spectrum agents,62, 63 clinicians who use the CPIS must understand its inherent limitations.
| Criterion | Range | Score |
|---|---|---|
| ||
| Temperature (C) | 36.138.4 | 0 |
| 38.538.9 | 1 | |
| 39 or 36 | 2 | |
| Blood leukocytes (/mm3) | 4000 and 11,000 | 0 |
| 4000 or >11,000 | 1 | |
| + band forms 500 | 2 | |
| Oxygenation: PaO2/FiO2 (mmHg) | >240 or ARDS | 0 |
| 240 and no evidence of ARDS | 2 | |
| Chest radiograph | No infiltrate | 0 |
| Diffuse (or patchy) infiltrate | 1 | |
| Localized infiltrate | 2 | |
| Tracheal secretions | Absence of tracheal secretions | 0 |
| Nonpurulent tracheal secretions | 1 | |
| Purulent tracheal secretions | 2 | |
| Culture of tracheal aspirate | Pathogenic bacteria culture: no growth or light growth | 0 |
| Pathogenic bacteria culture: moderate/heavy growth | 1 | |
| Same pathogenic bacteria seen on gram stain (add 1 point) | 2 | |
A meta‐analysis56 comparing the utility of an invasive versus a noninvasive culturing approach identified 4 randomized trials examining this issue.6669 Overall, an invasive approach did not alter mortality, but patients undergoing bronchoscopy were much more likely to have their antibiotic regimens modified by clinicians. This suggests that the invasive approach may allow more directed use of antibiotics. Recently, the Canadian Critical Care Trials Group conducted a multicenter randomized trial looking at this issue.11 There was no difference between the 2 approaches in mortality, number of ventilator days, and antibiotic usage. However, all patients in this study were immediately treated with empiric broad‐spectrum antibiotics until culture results were available, and the investigators did not have a protocol for stopping antibiotics after culture data were available.
In summary, both invasive and noninvasive culturing approaches are considered acceptable options for diagnosing VAP. Readers interested in learning more about this topic should read the worthwhile Expert Discussion70 by Chastre and colleagues55 at the end of this article. In general, we recommend that ICU clinicians use a combination of clinical suspicion (based on the CPIS or other objective data) and cultures ideally obtained prior to antibiotics. Regardless of the chosen culturing approach, clinicians must recognize that 1 of the most important determinants of patient outcome is prompt administration of adequate initial antibiotics.7175
Initial Antibiotic Administration
Delaying initial antibiotics in VAP increases the risk of death.7175 If a patient receives ineffective initial therapy, a later switch to appropriate therapy does not eliminate the increased mortality risk. Therefore, a comprehensive approach to VAP diagnosis requires consideration of initial empiric antibiotic administration.
Whenever possible, clinicians should obtain a lower respiratory tract sample for microscopy and culture before administering antibiotics because performing cultures after antibiotics have been recently started will lead to a higher rate of false‐negative results.76 Unless the patient has no signs of sepsis and microscopy is completely negative, clinicians should then immediately start empiric broad‐spectrum antibiotics.57 Once the culture sensitivities are known, therapy can be deescalated to a narrower spectrum.77 Recent studies suggest that shorter durations of therapy (8 days) are as effective as longer courses and are associated with lower colonization rates by antibiotic‐resistant bacteria.62, 78
Initial broad‐spectrum antibiotics should be chosen based on local bacteriology and resistance patterns. Clinicians must remain aware of the most common bacterial pathogens in their local community, hospital, and ICU. This is essential for both ensuring adequate initial antibiotic coverage and reducing overall antibiotic days.65 Unrestrained use of broad‐spectrum antibiotics increases the risk of resistant pathogens. Clinicians must continually deescalate therapy and use narrow‐spectrum drugs as pathogens are identified.79
Prevention of VAP
In 2005, the American Thoracic Society published guidelines for the management of adults with VAP.5 These guidelines included a discussion of modifiable risk factors for preventing VAP and used an evidence‐based grading system to rank the various recommendations. The highest evidence (level 1) comes from randomized clinical trials, moderate evidence (level 2) comes from nonrandomized studies, and the lowest evidence (level 3) comes from case studies or expert opinion. Others have also published their own guidelines and recommendations for preventing VAP.8082 Table 4 shows the key VAP preventive strategies.
| Strategy | Level of evidence | References |
|---|---|---|
| ||
| General infection control measures (hand hygiene, staff education, isolate MDR pathogens, etc.) | 1 | 2,83,84 |
| ICU infection surveillance | 2 | 2,8385 |
| Avoid reintubation if possible, but promptly reintubate if a patients inexorably fails extubation | 1 | 2,83,86,87 |
| Use NPPV when appropriate (in selected patients) | 1 | 88 |
| Use oral route for endotracheal and gastric tubes (vs. nasal route) | 2 | 89 |
| Continuous suctioning of subglottic secretions (to avoid pooling on cuff and leakage into LRT) | 1 | 9092 |
| Maintain endotracheal cuff pressure > 20 cm H2O (to prevent secretion leakage into LRT) | 2 | 93 |
| Avoid unnecessary ventilator circuit changes | 1 | 94 |
| Routinely empty condensate in ventilator circuit | 2 | 95 |
| Maintain adequate nursing and therapist staffing | 2 | 9698 |
| Implement ventilator weaning and sedation protocols | 2 | 99101 |
| Semierect patient positioning (vs. supine) | 1 | 102 |
| Avoid aspiration when using enteral nutrition | 1 | 103,104 |
| Topical oral antisepsis (eg, chlorhexidine) | 1 | 105108 |
| Control blood sugar with insulin | 1 | 109 |
| Use heat‐moisture exchanger (vs. conventional humidifier) to reduce tubing condensate | 1 | 95 |
| Avoid unnecessary red blood cell transfusions | 1 | 110 |
| Use of sucralfate for GI prophylaxis | 1 | 111,112 |
| Influenza vaccination for health care workers | 2 | 2 |
Some strategies are not recommended for VAP prevention in general ICU patients. Selective decontamination of the digestive tract (ie, prophylactic oral antibiotics) has been shown to reduce respiratory infections in ICU patients,113 but its overall role remains controversial because of concerns it may increase the incidence of multi‐drug‐resistant pathogens.114 Similarly, prophylactic intravenous antibiotics administered at the time of intubation can reduce VAP in certain patient populations,115 but this strategy is also associated with an increased risk of antibiotic‐resistant nosocomial infections.116 Using kinetic beds and scheduled chest physiotherapy to reduce VAP is based on the premise that critically ill patients often develop atelectasis and cannot effectively clear their secretions. Unfortunately, neither of these modalities has been shown to consistently reduce VAP in medical ICU patients.117119
Algorithms for Diagnosis and Treatment of VAP
We present algorithms for diagnosing VAP in 4 ICU populations: infant (1 year old), pediatric (1‐12 years old), immunocompromised, and adult ICU patients (Figs. 14). Because clinicians face considerable uncertainty when diagnosing VAP, we sought to develop practical algorithms for use in daily ICU practice. Although we provided the algorithms to collaborative participants as a tool for improving care, we never mandated use, and we did not monitor levels of adherence.
Five teaching cases are presented in the Appendix. We demonstrate how to utilize the diagnostic algorithms in these clinical scenarios and offer tips for clinicians wishing to employ these tools in their daily practice. These cases are useful for educating residents, nurses, and hospitalists.
Overall, our intent is that the combined use of these VAP algorithms facilitate a streamlined diagnostic approach and minimize delays in initial antibiotic administration. A primary focus of any VAP guideline should be early and appropriate antibiotics in adequate doses, with deescalation of therapy as culture data permit.5 In general, the greatest risk to a patient with VAP is delaying initial adequate antibiotic coverage, and for this reason, antibiotics must always be administered promptly. However, if culture data are negative, the clinician should consider withdrawing unnecessary antibiotics. For example, the absence of gram‐positive organisms on BAL after 72 hours would strongly suggest that MRSA is not playing a role and that vancomycin can be safely stopped. We agree with Neiderman that the decision point is not whether to start antibiotics, but whether to continue them at day 23.57
DISCUSSION
In this article, we introduce algorithms for diagnosing and managing VAP in infant, pediatric, immunocompromised, and adult ICU patients. We developed 4 algorithms because the hospitals in our system care for a wide range of patients. Our definitions for VAP were based on criteria outlined by the CDC because these rigorously developed criteria have been widely disseminated as components of the Institute for Healthcare Improvement's ventilator bundle.120 Clinicians should be able to easily incorporate these practical algorithms into their current practice.
The algorithms were developed during a collaborative across a large national health care system. We undertook this task because many clinicians were uncertain how to integrate the enormous volume of VAP literature into their daily practice, and we suspected there was large variation in practice in our ICUs. Recent studies from other health care systems provided empiric evidence to support this notion.12, 13
We offer these algorithms as practical tools to assist ICU clinicians and not as proscriptive mandates. We realize that the algorithms may need modification based on a hospital's unique bacteriology and patient populations. We also anticipate that the algorithms will adapt to future changes in VAP epidemiology, preventive strategies, emerging pathogens, and new antibiotics.
Numerous resources are available to learn more about VAP management. An excellent guideline from the Infectious Diseases Society of America and the American Thoracic Society discusses VAP issues in detail,5 although this guideline only focuses on immunocompetent adult patients. The journal Respiratory Care organized an international conference with numerous VAP experts in 2005 and subsequently devoted an entire issue to this topic.81 The Canadian Critical Care Trials Group and the Canadian Critical Care Society conducted systematic reviews and developed separate guidelines for the prevention, diagnosis, and treatment of VAP.80, 121
In summary, we present diagnostic and treatment algorithms for VAP. Our intent is that these algorithms may provide evidence‐based practical guidance to clinicians seeking a standardized approach to diagnosing and managing this challenging problem.
- ,,,.Nosocomial infections in combined medical‐surgical intensive care units in the United States.Infect Control Hosp Epidemiol.2000;21:510–515.
- Centers for Disease Control and Prevention.Guidelines for preventing health‐care—associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee.MMWR Recomm Rep.2004;53:1–36.
- Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) manual: patient safety component protocol (updated May 24,2007). Available at: http://www.cdc.gov/ncidod/dhqp/pdf/nhsn/NHSN_Manual_Patient_Safety_Protocol052407.pdf. Accessed October 1, 2007.
- ,,, et al.Outcome and attributable cost of ventilator‐associated pneumonia among intensive care unit patients in a suburban medical center.Crit Care Med.2003;31:1312–1317.
- ATS.Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,,.Nosocomial pneumonia in Medicare patients. Hospital costs and reimbursement patterns under the prospective payment system.Arch Intern Med.1991;151:1109–1114.
- ,,,,,.Oral decontamination is cost‐saving in the prevention of ventilator‐associated pneumonia in intensive care units.Crit Care Med.2004;32:126–130.
- ,,,.Clinical and economic consequences of ventilator‐associated pneumonia: a systematic review.Crit Care Med.2005;33:2184–2193.
- .Cost‐effectiveness issues in ventilator‐associated pneumonia.Respir Care.2005;50:956–963; discussion 963–964.
- .Diagnosis and treatment of ventilator‐associated pneumonia: fiberoptic bronchoscopy with bronchoalveolar lavage is essential.Semin Respir Crit Care Med.2006;27:34–44.
- ,,,.A randomized trial of diagnostic techniques for ventilator‐associated pneumonia.N Engl J Med.2006;355:2619–2630.
- ,,, et al.Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia.Chest.2006;129:1210–1218.
- ,,,.Prevention and diagnosis of ventilator‐associated pneumonia: a survey on current practices in Southern Spanish ICUs.Chest.2005;128:1667–1673.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,,,,,.Why do physicians not follow evidence‐based guidelines for preventing ventilator‐associated pneumonia?: a survey based on the opinions of an international panel of intensivists.Chest.2002;122:656–661.
- ,,, et al.Invitation to a dialogue between researchers and clinicians about evidence‐based behavioral medicine.Ann Behav Med.2005;30:125–137.
- ,,.Connections between quality measurement and improvement.Med Care.2003;41:I30–I38.
- ,,,,.Practice guidelines developed by specialty societies: the need for a critical appraisal.Lancet.2000;355:103–106.
- Agency for Healthcare Research and Quality (AHRQ). Partnerships in Implementing Patient Safety. Online at http://www.ahrq.gov/qual/pips.htm. Accessed March 1,2007.
- ,.The utility of open lung biopsy in patients requiring mechanical ventilation.Chest.1999;115:811–817.
- .The microbiology of ventilator‐associated pneumonia.Respir Care.2005;50:742–763; discussion 763–765.
- ,.Ventilator‐associated pneumonia.Am J Respir Crit Care Med.2002;165:867–903.
- ,.Overview of nosocomial infections caused by gram‐negative bacilli.Clin Infect Dis.2005;41:848–854.
- .Increasing prevalence of antimicrobial resistance in intensive care units.Crit Care Med.2001;29:N64–N68.
- ,,, et al.Incidence and outcome of polymicrobial ventilator‐associated pneumonia.Chest.2002;121:1618–1623.
- ,,.Nosocomial transmission of influenza.Occup Med (Lond).2002;52:249–253.
- ,.Nosocomial influenza at a Canadian pediatric hospital from 1995 to 1999: opportunities for prevention.Infect Control Hosp Epidemiol.2002;23:627–629.
- ,Nosocomial influenza infection as a cause of intercurrent fevers in infants.Pediatrics.1975;55:673–677.
- ,.Nosocomial influenza infection.Lancet.2000;355:1187.
- ,,.Nosocomial influenza B virus infection in the elderly.Ann Intern Med.1982;96:153–158.
- ,,,,,.Influenza vaccination of healthcare workers and vaccine allocation for healthcare workers during vaccine shortages.Infect Control Hosp Epidemiol.2005;26:882–890.
- ,,, et al.Herpes simplex virus in the respiratory tract of critical care patients: a prospective study.Lancet.2003;362:1536–1541.
- ,,, et al.Significance of the isolation of Candida species from respiratory samples in critically ill, non‐neutropenic patients. An immediate postmortem histologic study.Am J Respir Crit Care Med.1997;156:583–590.
- ,,,,,.The role of Candida sp isolated from bronchoscopic samples in nonneutropenic patients.Chest.1998;114:146– 149.
- ,.Pseudomonas‐Candida interactions: an ecological role for virulence factors.Science.2002;296:2229–2232.
- ,,, et al.Candida colonization of the respiratory tract and subsequent pseudomonas ventilator‐associated pneumonia.Chest.2006;129:110–117.
- ,,, et al.Impact of antifungal treatment on Candida‐Pseudomonas interaction: a preliminary retrospective case‐control study.Intensive Care Med.2007;33:137–142.
- ,,.Pulmonary infiltrates in the non‐HIV‐infected immunocompromised patient: etiologies, diagnostic strategies, and outcomes.Chest.2004;125:260–271.
- ,,.Pulmonary complications of solid organ and hematopoietic stem cell transplantation.Am J Respir Crit Care Med.2004;170:22–48.
- ,,, et al.Incidence, outcome, and risk factors of late‐onset noninfectious pulmonary complications after unrelated donor stem cell transplantation.Bone Marrow Transplant.2004;33:751–758.
- ,.Infection in organ‐transplant recipients.N Engl J Med.1998;338:1741–1751.
- ,,,,.Surveillance of ventilator‐associated pneumonia in very‐low‐birth‐weight infants.Am J Infect Control.2002;30:32–39.
- ,,,,.Ventilator‐associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes.Pediatrics.2003;112:1283–1289.
- ,,.Risk factors for nosocomial infection in a high‐risk nursery.Infect Control Hosp Epidemiol.2000;21:250–251.
- .Pediatric ventilator‐associated pneumonia.Pediatr Infect Dis J.2003;22:445–446.
- ,,.Ventilator‐associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes.Pediatrics.2002;109:758–764.
- ,,, et al.Ventilator‐associated pneumonia caused by potentially drug‐resistant bacteria.Am J Respir Crit Care Med.1998;157:531–539.
- ,,.Pneumonia in intubated trauma patients. Microbiology and outcomes.Am J Respir Crit Care Med.1996;153:343–349.
- ,,,,.Pneumonia due to Haemophilus influenzae among mechanically ventilated patients. Incidence, outcome, and risk factors.Chest.1992;102:1562–1565.
- ,,, et al.Both early‐onset and late‐onset ventilator‐associated pneumonia are caused mainly by potentially multiresistant bacteria.Intensive Care Med2005;31:1488–1494.
- ,,,.A comparative analysis of patients with early‐onset vs late‐onset nosocomial pneumonia in the ICU setting.Chest.2000;117:1434–1442.
- .Diagnosis of ventilator‐associated pneumonia.Curr Opin Crit Care.2003;9:397–402.
- ,.Use and limitations of clinical and radiologic diagnosis of pneumonia.Semin Respir Infect.2003;18:72–79.
- ,,, et al.Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator‐associated pneumonia.Chest.1999;116:1075–1084.
- ,,.The invasive (quantitative) diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:797–807.
- ,,,.Invasive approaches to the diagnosis of ventilator‐associated pneumonia: a meta‐analysis.Crit Care Med.2005;33:46– 53.
- .The clinical diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:788–796; discussion 807–812.
- ,,,,,.Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid.Am Rev Respir Dis.1991;143:1121–1129.
- ,,,,,.Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited.Am J Respir Crit Care Med.2003;168:173–179.
- ,,,,,.The diagnosis of ventilator‐associated pneumonia using non‐bronchoscopic, non‐directed lung lavages.Intensive Care Med.2000;26:20–30.
- ,,, et al.Clinical pulmonary infection score for ventilator‐associated pneumonia: accuracy and inter‐observer variability.Intensive Care Med.2004;30:217–224.
- ,,,.A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator‐associated pneumonia.Chest.2004;125:1791–1799.
- ,,, et al.Early antibiotic treatment for BAL‐confirmed ventilator‐associated pneumonia: a role for routine endotracheal aspirate cultures.Chest.2005;127:589–597.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,,,,.Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia.Crit Care Med.2001;29:1109–1115.
- ,,, et al.Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator‐associated pneumonia: a pilot study.Am J Respir Crit Care Med.1998;157:371–376.
- ,,, et al.Noninvasive versus invasive microbial investigation in ventilator‐associated pneumonia: evaluation of outcome.Am J Respir Crit Care Med.2000;162:119–125.
- ,,,,.Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia.Crit Care Med.2000;28:2737–2741.
- ,,, et al.Invasive and noninvasive strategies for management of suspected ventilator‐associated pneumonia. A randomized trial.Ann Intern Med.2000;132:621–630.
- ,,.Expert discussion: The invasive (quantitative) diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:807–812.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,,,.Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator‐associated pneumonia.Intensive Care Med.2001;27:355–362.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Appropriateness and delay to initiate therapy in ventilator‐associated pneumonia.Eur Respir J.2006;27:158–164.
- .The importance of appropriate initial antibiotic therapy for hospital‐acquired infections.Am J Med.2003;115:582–584.
- ,,, et al.Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments.Crit Care Med.1998;26:236–244.
- ,,, et al.De‐escalation therapy in ventilator‐associated pneumonia.Crit Care Med.2004;32:2183–2190.
- ,,, et al.Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial.JAMA.2003;290:2588–2598.
- .De‐escalation therapy in ventilator‐associated pneumonia.Curr Opin Crit Care.2006;12:452–457.
- ,,, et al.Evidence‐based clinical practice guideline for the prevention of ventilator‐associated pneumonia.Ann Intern Med.2004;141:305–313.
- .Conference summary: ventilator‐associated pneumonia.Respir Care.2005;50:975–983.
- ,,.Prevention of ventilator‐associated pneumonia: an evidence‐based systematic review.Ann Intern Med.2003;138:494–501.
- .The prevention of ventilator‐associated pneumonia.N Engl J Med.1999;340:627–634.
- .Epidemiology and control of nosocomial infections in adult intensive care units.Am J Med.1991;91:179S–184S.
- ,,, et al.Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene. Infection Control Programme.Lancet.2000;356:1307–1312.
- ,,, et al.Re‐intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation.Am J Respir Crit Care Med.1995;152:137–41.
- ,,,,,.Nosocomial pneumonia. A multivariate analysis of risk and prognosis.Chest.1988;93:318–324.
- ,,, et al.Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients.JAMA.2000;284:2361–2367.
- ,,, et al.Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill.Am J Respir Crit Care Med.1994;150:776–783.
- ,,.Prevention measures for ventilator‐associated pneumonia: a new focus on the endotracheal tube.Curr Opin Infect Dis.2007;20:190–197.
- ,,,.A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation.Chest.2002;121:858–862.
- ,,, et al.Continuous aspiration of subglottic secretions in preventing ventilator‐associated pneumonia.Ann Intern Med.1995;122:179–186.
- ,,,,,.Pneumonia in intubated patients: role of respiratory airway care.Am J Respir Crit Care Med.1996;154:111–115.
- ,,, et al.Mechanical ventilation with or without 7‐day circuit changes. A randomized controlled trial.Ann Intern Med.1995;123:168–174.
- ,,.Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia?Am Rev Respir Dis.1984;129:625–628.
- ,,,,.Outbreak of Enterobacter cloacae related to understaffing, overcrowding, and poor hygiene practices.Infect Control Hosp. Epidemiol.1999;20:598–603.
- ,,,,.Nursing resources: a major determinant of nosocomial infection?Curr Opin Infect Dis.2004;17:329–333.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346:1715–1722.
- ,,, et al.Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously.N Engl J Med.1996;335:1864–1869.
- ,,, et al.A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation.Crit Care Med.1997;25:567–574.
- ,,,.Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation.N Engl J Med.2000;342:1471–1477.
- ,,,,,.Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial.Lancet.1999;354:1851–1858.
- ,,, et al.Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial.JPEN J Parenter Enteral Nutr.2002;26:174–181.
- ,,,.Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: role of small bowel feeding.JPEN J Parenter Enteral Nutr.2002;26:S51–S55; discussion S56–S57.
- ,,,.Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta‐analysis.BMJ.2007;334:889.
- ,.Topical chlorhexidine for prevention of ventilator‐associated pneumonia: a meta‐analysis.Crit Care Med.2007;35:595–602.
- ,,, et al.Oral decontamination with chlorhexidine reduces the incidence of ventilator‐associated pneumonia.Am J Respir Crit Care Med.2006;173:1348–1355.
- ,.Efficacy of oral chlorhexidine in preventing lower respiratory tract infections. Meta‐analysis of randomized controlled trials.J Hosp Infect.2007;66:207– 216.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Red blood cell transfusion and ventilator‐associated pneumonia: A potential link?Crit Care Med.2004;32:666–674.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- ,,, et al.Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization.N Engl J Med.1987;317:1376–1382.
- ,,,,.Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care.Cochrane Database Syst Rev.2004:CD000022.
- ,.Selective decontamination of the digestive tract: cumulating evidence, at last?Semin Respir Crit Care Med.2006;27:18–22.
- ,,,,,.Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma.Am J Respir Crit Care Med.1997;155:1729–1734.
- ,,,,,.Prophylactic antibiotics adversely affect nosocomial pneumonia in trauma patients.J Trauma.2003;55:249–254.
- ,.Kinetic therapy in critically ill trauma patients.Clin Intensive Care.1992;3:248–252.
- ,,,,.Continuous oscillation: outcome in critically ill patients.JCrit Care.1995;10:97–103.
- ,,,.Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: a systematic review and meta‐analysis.Crit Care.2006;10:R70.
- ,,,.The 100,000 lives campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295:324–327.
- Zap the VAP. Available at: http://www.zapthevap.com. Accessed March 1,2007.
- .Aspiration pneumonitis and aspiration pneumonia.N Engl J Med.2001;344:665–671.
Ventilator‐associated pneumonia (VAP) is a serious and common complication for patients in the intensive care unit (ICU).1 VAP is defined as a pulmonary infection occurring after hospital admission in a mechanically‐ventilated patient with a tracheostomy or endotracheal tube.2, 3 With an attributable mortality that may exceed 20% and an estimated cost of $5000‐$20,000 per episode,49 the management of VAP is an important issue for both patient safety and cost of care.
The diagnosis of VAP is a controversial topic in critical care, primarily because of the difficulty distinguishing between airway colonization, upper respiratory tract infection (eg, tracheobronchitis), and early‐onset pneumonia. Some clinicians insist that an invasive sampling technique (eg, bronchoalveolar lavage) with quantitative cultures is essential for determining the presence of VAP.10 However, other clinicians suggest that a noninvasive approach using qualitative cultures (eg, tracheal suctioning) is an acceptable alternative.11 Regardless, nearly all experts agree that a specimen for microbiologic culture should be obtained prior to initiating antibiotics. Subsequent therapy should then be adjusted according to culture results.
Studies from both Europe and North America have demonstrated considerable variation in the diagnostic approaches used for patients with suspected VAP.12, 13 This variation is likely a result of several factors including controversy about the best diagnostic approach, variation in clinician knowledge and experience, and variation in ICU management protocols. Such practice variability is common for many ICU behaviors.1416 Quality‐of‐care proponents view this variation as an important opportunity for improvement.17
During a recent national collaborative aimed at reducing health careassociated infections in the ICU, we discovered many participants were uncertain about how to diagnose and manage VAP, and considerable practice variability existed among participating hospitals. This uncertainty provided an important opportunity for developing consensus on VAP management. On the basis of diagnostic criteria outlined by the Centers for Disease Control and Prevention (CDC), we developed algorithms as tools for diagnosing VAP in 4 ICU populations: infant, pediatric, immunocompromised, and adult ICU patients. We also developed an algorithm for initial VAP treatment. An interdisciplinary team of experts reviewed the current literature and developed these evidence‐based consensus guidelines. Our intent is that the algorithms provide guidance to clinicians looking for a standardized approach to the diagnosis and management of this complicated clinical situation.
METHODS
Our primary goal was to develop practical algorithms that assist ICU clinicians in the diagnosis and management of VAP during daily practice. To improve the quality and credibility of these algorithms, the development process used a stepwise approach that included assembling an interdisciplinary team of experts, appraising the published evidence, and formulating the algorithms through a consensus process.18
AHRQ National Collaborative
We developed these diagnostic algorithms as part of a national collaborative effort aimed at reducing VAP and central venous catheterrelated bloodstream infections in the ICU. This effort was possible through a 2‐year Partnerships in Implementing Patient Safety grant funded by the Agency for Healthcare Research and Quality (AHRQ).19 The voluntary collaborative was conducted in 61 medical/surgical and children's hospitals across the Hospital Corporation of America (HCA), a company that owns and/or operates 173 hospitals and 107 freestanding surgery centers in 20 states, England, and Switzerland. HCA is one of the largest providers of health care in the United States. All participating hospitals had at least 1 ICU, and a total of 110 ICUs were included in the project. Most hospitals were in the southern or southeastern regions of the United States.
Interdisciplinary Team
We assembled an interdisciplinary team to develop the diagnostic algorithms. Individuals on the team represented the specialties of infectious diseases, infection control, anesthesia, critical care medicine, hospital medicine, critical care nursing, pharmacy, and biostatistics. The development phase occurred over 34 months and used an iterative process that consisted of both group conference calls and in‐person meetings.
Our goal was not to conduct a systematic review but rather to develop practical algorithms for collaborative participants in a timely manner. Our literature search strategy included MEDLINE and the Cochrane Library. We focused on articles that addressed key diagnostic issues, proposed an algorithm, or summarized a topic relevant to practicing clinicians. Extra attention was given to articles that were randomized trials, meta‐analyses, or systematic reviews. No explicit grading of articles was performed. We examined studies with outcomes of interest to clinicians, including mortality, number of ventilator days, length of stay, antibiotic utilization, and antibiotic resistance.
We screened potentially relevant articles and the references of these articles. The search results were reviewed by all members of the team, and an iterative consensus process was used to derive the current algorithms. Preliminary versions of the algorithms were shown to other AHRQ investigators and outside experts in the field, and additional modifications were made based on their feedback. The final algorithms were approved by all study investigators.
RESULTS
Literature Overview
Overall, there is an enormous body of published literature on diagnosing and managing VAP. The Medline database has listed more than 500 articles on VAP diagnosis in the past decade. Nonetheless, the best diagnostic approach remains unclear. The gold standard for diagnosing VAP is lung biopsy with histopathologic examination and tissue culture. However, this procedure is fraught with potential dangers and impractical for most critically ill patients.20 Therefore, practitioners traditionally combine their clinical suspicion (based on fever, leukocytosis, character of sputum, and radiographic changes), epidemiologic data (eg, patient demographics, medical history, and ICU infection surveillance data), and microbiologic data.
Several issues relevant to practicing clinicians deserve further mention.
Definition of VAP
Although early articles used variable criteria for diagnosing VAP, recent studies have traditionally defined VAP as an infection occurring more than 48 hours after hospital admission in a mechanically ventilated patient with a tracheostomy or endotracheal tube.2 In early 2007, the CDC revised their definition for diagnosing VAP.3 These latest criteria state there is no minimum period that the ventilator must be in place in order to diagnose VAP. This important change must be kept in mind when examining future studies.
The term VAP is more specific than the term health careassociated pneumonia. The latter encompasses patients residing in a nursing home or long‐term care facility; hospitalized in an acute care hospital for more than 48 hours in the past 90 days; receiving antibiotics, chemotherapy, or wound care within the past 30 days; or attending a hospital or hemodialysis clinic.
The CDC published detailed criteria for diagnosing VAP in its member hospitals (Tables 1 and 2).3 Because diagnosing VAP in infants, children, elderly, and immunocompromised patients is often confusing because of other conditions with similar signs and symptoms, the CDC published alternate criteria for these populations. A key objective during development of our algorithms was to consolidate and simplify these diagnostic criteria for ICU clinicians.
| Radiology | Signs/symptoms/laboratory |
|---|---|
| |
| Two or more serial chest radiographs with at least 1 of the following*: | CRITERIA FOR ANY PATIENT |
| New or progressive and persistent infiltrate | At least 1 of the following: |
| Consolidation | Fever (>38C or >100.4F) with no other recognized cause |
| Cavitation | Leukopenia (4000 WBC/mm3) or leukocytosis (12,000 WBC/mm3) |
| Pneumatoceles, in infants 1 year old | For adults 70 years old, altered mental status with no other recognized causeand |
| Note: In patients without underlying pulmonary or cardiac disease (eg, respiratory distress syndrome, bronchopulmonary dysplasia, pulmonary edema, or chronic obstructive pulmonary disease), 1 definitive chest radiograph is acceptable.* | |
| At least 2 of the following: | |
| New onset of purulent sputum, or change in character of sputum, or increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough or dyspnea or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation [eg, PaO2/FiO2 240],** increased oxygen requirement, or increased ventilation demand) | |
|
Any laboratory criterion from Table 2 |
|
| ALTERNATE CRITERIA FOR INFANTS 1 YEAR OLD | |
| Worsening gas exchange (eg, O2 desaturation, increased ventilation demand or O2 requirement) | |
| and | |
| At least 3 of the following: | |
| Temperature instability with no other recognized cause | |
| Leukopenia (4000 WBC/mm3) or leukocytosis (15,000 WBC/mm3) and left shift (10% bands) | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| Apnea, tachypnea,‖ nasal flaring with retraction of chest wall, or grunting | |
| Wheezing, rales, or rhonchi | |
| Cough | |
| Bradycadia (100 beats/min) or tachycardia (>170 beats/min) | |
| ALTERNATE CRITERIA FOR CHILD >1 OR 12 YEARS OLD | |
| At least 3 of the following: | |
| Fever (>38.4C or >101.1F) or hypothermia (36.5C or 97.7F) with no other recognized cause | |
| Leukopenia (4000 WBC/mm3) or leukocytosis (15,000 WBC/mm3) | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough or dyspnea, apnea, or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation 94%, increased ventilation demand or O2 requirement) | |
|
Any laboratory criterion from Table 2 |
|
| ALTERNATE CRITERIA FOR IMMUNOCOMPROMISED PATIENTS*** | |
| At least 1 of the following: | |
| Fever (>38.4C or >101.1F) with no other recognized cause | |
| For adults > 70 years old, altered mental status with no other recognized cause | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough, dyspnea, or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation [eg, PaO2/FiO2 240],** increased oxygen requirement, or increased ventilation demand) | |
| Hemoptysis | |
| Pleuritic chest pain | |
| Matching positive blood and sputum cultures with Candida spp. | |
| Evidence of fungi or Pneumocytis from minimally contaminated LRT specimen (eg, BAL or protected specimen brushing) from 1 of the following: | |
| Direct microscopic exam | |
| Positive culture of fungi | |
|
Any laboratory criterion from Table 2 |
|
|
| Positive growth in blood culture* not related to another source of infection |
| Positive growth in culture of pleural fluid |
| Positive quantitative culture from minimally contaminated LRT specimen (eg, BAL) |
| 5% BAL‐obtained cells contain intracellular bacteria on direct microscopic exam (eg, gram stain) |
| Histopathologic exam shows at least 1 of the following: |
| Abscess formation or foci of consolidation with intense PMN accumulation in bronchioles and alveoli |
| Positive quantitative culture of lung parenchyma |
| Evidence of lung parenchyma invasion by fungal hyphae or pseudohyphae |
| Positive culture of virus or Chlamydia from respiratory secretions |
| Positive detection of viral antigen or antibody from respiratory secretions (eg, EIA, FAMA, shell vial assay, PCR) |
| Fourfold rise in paired sera (IgG) for pathogen (eg, influenza viruses, Chlamydia) |
| Positive PCR for Chlamydia or Mycoplasma |
| Positive micro‐IF test for Chlamydia |
| Positive culture or visualization by micro‐IF of Legionella spp. from respiratory secretions or tissue |
| Detection of Legionella pneumophila serogroup 1 antigens in urine by RIA or EIA |
| Fourfold rise in L. pneumophila serogroup 1 antibody titer to 1:128 in paired acute and convalescent sera by indirect IFA |
Etiology
The most commonly isolated VAP pathogens in all patients are bacteria.21 Most of these organisms normally colonize the respiratory and gastrointestinal tracts, but some are unique to health care settings. Tracheal intubation disrupts the body's natural anatomic and physiologic defenses and facilitates easier entry of these pathogens. Typical organisms include Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter species, Klebsiella pneumoniae, Acinetobacter species, Escherichia coli, and Haemophilus influenzae.22, 23 Unfortunately, the prevalence of antimicrobial resistance among VAP pathogens is increasing.24 Risk factors for antibiotic resistance are common to ICU patients and include recent antibiotics, hemodialysis, nursing home residence, immunosuppression, and chronic wound care.5 Polymicrobial infections are frequently seen in VAP, with up to 50% of all VAP episodes caused by more than 1 organism.25
Viral VAP is rare in immunocompetent hosts, and seasonal outbreaks of influenza and other similar viruses are usually limited to nonventilated patients.26 However, influenza is underrecognized as a potential nosocomial pathogen, and numerous nosocomial outbreaks because of influenza have been reported.2731 Although herpes simplex virus is often detected in the respiratory tract of critically ill patients, its clinical importance remains unclear.32
Fungal VAP is also rare in immunocompetent hosts. On the other hand, pulmonary fungal infections are common in immunocompromised patients, especially following chemotherapy and transplantation. Candida species are often isolated from the airways of normal hosts, but most cases traditionally have been considered clinically unimportant because these organisms are normal oropharyngeal flora and rarely invade lung tissue.33, 34 It is unclear whether recent studies suggesting Candida colonization is associated with a higher risk for Pseudomonas VAP will change this conventional wisdom.3537
Immunocompromised patients with suspected VAP are unique because they are at risk not only for typical bacteria (which are the most common causes of VAP) but also for rarer opportunistic infections and noninfectious processes that mimic pneumonia.3840 While assessing these patients, clinicians must consider the status of the underlying disease, duration and type of immunosuppression, prophylactic regimens, and risk factors for noninfectious causes of pulmonary infiltrates.41 Common opportunistic infections include viruses, mycobacteria, fungi, and Pneumocystis. Noninfectious processes include pulmonary edema, drug toxicity, radiation pneumonitis, engraftment syndrome, bronchiolitis obliterans organizing pneumonia, alveolar proteinosis, transfusion‐related lung injury, alveolar hemorrhage, and progression of underlying disease. In general, diagnosing VAP in the immunocompromised patient requires a prompt, comprehensive, and multidisciplinary approach.38
In preterm and term infants, the most common VAP pathogens are gram‐negative organisms such as E. coli and P. aeruginosa. Other less common pathogens are Enterobacter, Klebsiella, Acinetobacter, Proteus, Citrobacter, and Stenotrophomonas maltophilia.42, 43 Infants with a preceding bloodstream infection or prolonged intubation are more likely to develop VAP.43, 44 Unfortunately, gram‐negative bacteria often colonize the airways of mechanically ventilated infants, and tracheal aspirate culture data are difficult to interpret in this population.42
Children are more likely to develop VAP if they are intubated for more than 48 hours. The most common pathogens isolated from tracheal aspirates in mechanically ventilated children are enteric gram‐negative bacteria, P. aeruginosa, and S. aureus.45, 46 Few studies have precisely delineated the pathogenesis of VAP in the pediatric ICU population.
Overall, the causes of VAP vary by hospital, patient population, and ICU type. Therefore, it is essential that ICU clinicians remain knowledgeable about their local surveillance data.21 Awareness of VAP microbiology is essential for optimizing initial antibiotic therapy and improving outcomes.
Early Versus Late VAP
Distinguishing between early and late VAP is important for initial antibiotic selection because the etiologic pathogens vary between these 2 periods.4749 Early VAP (days 14 of hospitalization) usually involves antibiotic‐sensitive community‐acquired bacteria and carries a better prognosis. In contrast, late VAP (5 days after hospital admission) is more likely to be caused by antibiotic‐resistant nosocomial bacteria that lead to increased morbidity and mortality. All patients who have been hospitalized or have received antibiotics during the prior 90 days should be treated as having late VAP because they are at much higher risk for colonization and infection with antibiotic‐resistant bacteria.47 Of note, 2 recent studies suggest that pathogens in the early and late periods are becoming similar at some institutions.50, 51 Overall, the distinction between early and late VAP is important because it affects the likelihood that a patient has antibiotic‐resistant bacteria. If antibiotic‐resistant pathogens are suspected, initial therapy should include empiric triple antibiotics until culture data are available.
Culturing Approaches
Because clinical criteria alone are rarely able to accurately diagnose VAP,52, 53 clinicians should also obtain a respiratory specimen for microbiologic culture. Despite the convenience of blood cultures, their sensitivity for diagnosing VAP is poor, and they rarely make the diagnosis alone.54 Two methods are available for culturing the lungsan invasive approach (eg, bronchoscopy with bronchoalveolar lavage) and a noninvasive approach (eg, tracheal aspirate).
Some investigators believe that adult patients with suspected VAP should always undergo an invasive sampling of lower‐respiratory‐tract secretions.55 Proponents of the invasive approach cite the frequency with which potential pathogens colonize the trachea of ICU patients and create spurious results on tracheal aspirates.22 In addition, several studies have shown that clinicians are more likely to narrow the spectrum of antibiotics after obtaining an invasive diagnostic sample.56 In other words, the invasive approach has been associated with better antimicrobial stewardship.
Other investigators believe that a noninvasive approach is equally safe and effective for diagnosing VAP.57 This clinical approach involves culturing a tracheal aspirate and using a pneumonia prediction score such as the clinical pulmonary infection score (CPIS; Table 3). The CPIS assigns 012 points based on 6 clinical criteria: fever, leukocyte count, oxygenation, quantity and purulence of secretions, type of radiographic abnormality, and results of sputum gram stain and culture.58 As developed, a CPIS > 6 has a sensitivity of 93% and a specificity of 100% for diagnosing VAP.58 However, the CPIS requires that nurses record sputum volume and that the laboratory stains the specimen. When the CPIS has been modified based on the unavailability of such resources, the results have been less impressive.5961 Despite studies showing that a noninvasive clinical approach can achieve adequate initial antibiotic coverage and reduce overuse of broad‐spectrum agents,62, 63 clinicians who use the CPIS must understand its inherent limitations.
| Criterion | Range | Score |
|---|---|---|
| ||
| Temperature (C) | 36.138.4 | 0 |
| 38.538.9 | 1 | |
| 39 or 36 | 2 | |
| Blood leukocytes (/mm3) | 4000 and 11,000 | 0 |
| 4000 or >11,000 | 1 | |
| + band forms 500 | 2 | |
| Oxygenation: PaO2/FiO2 (mmHg) | >240 or ARDS | 0 |
| 240 and no evidence of ARDS | 2 | |
| Chest radiograph | No infiltrate | 0 |
| Diffuse (or patchy) infiltrate | 1 | |
| Localized infiltrate | 2 | |
| Tracheal secretions | Absence of tracheal secretions | 0 |
| Nonpurulent tracheal secretions | 1 | |
| Purulent tracheal secretions | 2 | |
| Culture of tracheal aspirate | Pathogenic bacteria culture: no growth or light growth | 0 |
| Pathogenic bacteria culture: moderate/heavy growth | 1 | |
| Same pathogenic bacteria seen on gram stain (add 1 point) | 2 | |
A meta‐analysis56 comparing the utility of an invasive versus a noninvasive culturing approach identified 4 randomized trials examining this issue.6669 Overall, an invasive approach did not alter mortality, but patients undergoing bronchoscopy were much more likely to have their antibiotic regimens modified by clinicians. This suggests that the invasive approach may allow more directed use of antibiotics. Recently, the Canadian Critical Care Trials Group conducted a multicenter randomized trial looking at this issue.11 There was no difference between the 2 approaches in mortality, number of ventilator days, and antibiotic usage. However, all patients in this study were immediately treated with empiric broad‐spectrum antibiotics until culture results were available, and the investigators did not have a protocol for stopping antibiotics after culture data were available.
In summary, both invasive and noninvasive culturing approaches are considered acceptable options for diagnosing VAP. Readers interested in learning more about this topic should read the worthwhile Expert Discussion70 by Chastre and colleagues55 at the end of this article. In general, we recommend that ICU clinicians use a combination of clinical suspicion (based on the CPIS or other objective data) and cultures ideally obtained prior to antibiotics. Regardless of the chosen culturing approach, clinicians must recognize that 1 of the most important determinants of patient outcome is prompt administration of adequate initial antibiotics.7175
Initial Antibiotic Administration
Delaying initial antibiotics in VAP increases the risk of death.7175 If a patient receives ineffective initial therapy, a later switch to appropriate therapy does not eliminate the increased mortality risk. Therefore, a comprehensive approach to VAP diagnosis requires consideration of initial empiric antibiotic administration.
Whenever possible, clinicians should obtain a lower respiratory tract sample for microscopy and culture before administering antibiotics because performing cultures after antibiotics have been recently started will lead to a higher rate of false‐negative results.76 Unless the patient has no signs of sepsis and microscopy is completely negative, clinicians should then immediately start empiric broad‐spectrum antibiotics.57 Once the culture sensitivities are known, therapy can be deescalated to a narrower spectrum.77 Recent studies suggest that shorter durations of therapy (8 days) are as effective as longer courses and are associated with lower colonization rates by antibiotic‐resistant bacteria.62, 78
Initial broad‐spectrum antibiotics should be chosen based on local bacteriology and resistance patterns. Clinicians must remain aware of the most common bacterial pathogens in their local community, hospital, and ICU. This is essential for both ensuring adequate initial antibiotic coverage and reducing overall antibiotic days.65 Unrestrained use of broad‐spectrum antibiotics increases the risk of resistant pathogens. Clinicians must continually deescalate therapy and use narrow‐spectrum drugs as pathogens are identified.79
Prevention of VAP
In 2005, the American Thoracic Society published guidelines for the management of adults with VAP.5 These guidelines included a discussion of modifiable risk factors for preventing VAP and used an evidence‐based grading system to rank the various recommendations. The highest evidence (level 1) comes from randomized clinical trials, moderate evidence (level 2) comes from nonrandomized studies, and the lowest evidence (level 3) comes from case studies or expert opinion. Others have also published their own guidelines and recommendations for preventing VAP.8082 Table 4 shows the key VAP preventive strategies.
| Strategy | Level of evidence | References |
|---|---|---|
| ||
| General infection control measures (hand hygiene, staff education, isolate MDR pathogens, etc.) | 1 | 2,83,84 |
| ICU infection surveillance | 2 | 2,8385 |
| Avoid reintubation if possible, but promptly reintubate if a patients inexorably fails extubation | 1 | 2,83,86,87 |
| Use NPPV when appropriate (in selected patients) | 1 | 88 |
| Use oral route for endotracheal and gastric tubes (vs. nasal route) | 2 | 89 |
| Continuous suctioning of subglottic secretions (to avoid pooling on cuff and leakage into LRT) | 1 | 9092 |
| Maintain endotracheal cuff pressure > 20 cm H2O (to prevent secretion leakage into LRT) | 2 | 93 |
| Avoid unnecessary ventilator circuit changes | 1 | 94 |
| Routinely empty condensate in ventilator circuit | 2 | 95 |
| Maintain adequate nursing and therapist staffing | 2 | 9698 |
| Implement ventilator weaning and sedation protocols | 2 | 99101 |
| Semierect patient positioning (vs. supine) | 1 | 102 |
| Avoid aspiration when using enteral nutrition | 1 | 103,104 |
| Topical oral antisepsis (eg, chlorhexidine) | 1 | 105108 |
| Control blood sugar with insulin | 1 | 109 |
| Use heat‐moisture exchanger (vs. conventional humidifier) to reduce tubing condensate | 1 | 95 |
| Avoid unnecessary red blood cell transfusions | 1 | 110 |
| Use of sucralfate for GI prophylaxis | 1 | 111,112 |
| Influenza vaccination for health care workers | 2 | 2 |
Some strategies are not recommended for VAP prevention in general ICU patients. Selective decontamination of the digestive tract (ie, prophylactic oral antibiotics) has been shown to reduce respiratory infections in ICU patients,113 but its overall role remains controversial because of concerns it may increase the incidence of multi‐drug‐resistant pathogens.114 Similarly, prophylactic intravenous antibiotics administered at the time of intubation can reduce VAP in certain patient populations,115 but this strategy is also associated with an increased risk of antibiotic‐resistant nosocomial infections.116 Using kinetic beds and scheduled chest physiotherapy to reduce VAP is based on the premise that critically ill patients often develop atelectasis and cannot effectively clear their secretions. Unfortunately, neither of these modalities has been shown to consistently reduce VAP in medical ICU patients.117119
Algorithms for Diagnosis and Treatment of VAP
We present algorithms for diagnosing VAP in 4 ICU populations: infant (1 year old), pediatric (1‐12 years old), immunocompromised, and adult ICU patients (Figs. 14). Because clinicians face considerable uncertainty when diagnosing VAP, we sought to develop practical algorithms for use in daily ICU practice. Although we provided the algorithms to collaborative participants as a tool for improving care, we never mandated use, and we did not monitor levels of adherence.
Five teaching cases are presented in the Appendix. We demonstrate how to utilize the diagnostic algorithms in these clinical scenarios and offer tips for clinicians wishing to employ these tools in their daily practice. These cases are useful for educating residents, nurses, and hospitalists.
Overall, our intent is that the combined use of these VAP algorithms facilitate a streamlined diagnostic approach and minimize delays in initial antibiotic administration. A primary focus of any VAP guideline should be early and appropriate antibiotics in adequate doses, with deescalation of therapy as culture data permit.5 In general, the greatest risk to a patient with VAP is delaying initial adequate antibiotic coverage, and for this reason, antibiotics must always be administered promptly. However, if culture data are negative, the clinician should consider withdrawing unnecessary antibiotics. For example, the absence of gram‐positive organisms on BAL after 72 hours would strongly suggest that MRSA is not playing a role and that vancomycin can be safely stopped. We agree with Neiderman that the decision point is not whether to start antibiotics, but whether to continue them at day 23.57
DISCUSSION
In this article, we introduce algorithms for diagnosing and managing VAP in infant, pediatric, immunocompromised, and adult ICU patients. We developed 4 algorithms because the hospitals in our system care for a wide range of patients. Our definitions for VAP were based on criteria outlined by the CDC because these rigorously developed criteria have been widely disseminated as components of the Institute for Healthcare Improvement's ventilator bundle.120 Clinicians should be able to easily incorporate these practical algorithms into their current practice.
The algorithms were developed during a collaborative across a large national health care system. We undertook this task because many clinicians were uncertain how to integrate the enormous volume of VAP literature into their daily practice, and we suspected there was large variation in practice in our ICUs. Recent studies from other health care systems provided empiric evidence to support this notion.12, 13
We offer these algorithms as practical tools to assist ICU clinicians and not as proscriptive mandates. We realize that the algorithms may need modification based on a hospital's unique bacteriology and patient populations. We also anticipate that the algorithms will adapt to future changes in VAP epidemiology, preventive strategies, emerging pathogens, and new antibiotics.
Numerous resources are available to learn more about VAP management. An excellent guideline from the Infectious Diseases Society of America and the American Thoracic Society discusses VAP issues in detail,5 although this guideline only focuses on immunocompetent adult patients. The journal Respiratory Care organized an international conference with numerous VAP experts in 2005 and subsequently devoted an entire issue to this topic.81 The Canadian Critical Care Trials Group and the Canadian Critical Care Society conducted systematic reviews and developed separate guidelines for the prevention, diagnosis, and treatment of VAP.80, 121
In summary, we present diagnostic and treatment algorithms for VAP. Our intent is that these algorithms may provide evidence‐based practical guidance to clinicians seeking a standardized approach to diagnosing and managing this challenging problem.
Ventilator‐associated pneumonia (VAP) is a serious and common complication for patients in the intensive care unit (ICU).1 VAP is defined as a pulmonary infection occurring after hospital admission in a mechanically‐ventilated patient with a tracheostomy or endotracheal tube.2, 3 With an attributable mortality that may exceed 20% and an estimated cost of $5000‐$20,000 per episode,49 the management of VAP is an important issue for both patient safety and cost of care.
The diagnosis of VAP is a controversial topic in critical care, primarily because of the difficulty distinguishing between airway colonization, upper respiratory tract infection (eg, tracheobronchitis), and early‐onset pneumonia. Some clinicians insist that an invasive sampling technique (eg, bronchoalveolar lavage) with quantitative cultures is essential for determining the presence of VAP.10 However, other clinicians suggest that a noninvasive approach using qualitative cultures (eg, tracheal suctioning) is an acceptable alternative.11 Regardless, nearly all experts agree that a specimen for microbiologic culture should be obtained prior to initiating antibiotics. Subsequent therapy should then be adjusted according to culture results.
Studies from both Europe and North America have demonstrated considerable variation in the diagnostic approaches used for patients with suspected VAP.12, 13 This variation is likely a result of several factors including controversy about the best diagnostic approach, variation in clinician knowledge and experience, and variation in ICU management protocols. Such practice variability is common for many ICU behaviors.1416 Quality‐of‐care proponents view this variation as an important opportunity for improvement.17
During a recent national collaborative aimed at reducing health careassociated infections in the ICU, we discovered many participants were uncertain about how to diagnose and manage VAP, and considerable practice variability existed among participating hospitals. This uncertainty provided an important opportunity for developing consensus on VAP management. On the basis of diagnostic criteria outlined by the Centers for Disease Control and Prevention (CDC), we developed algorithms as tools for diagnosing VAP in 4 ICU populations: infant, pediatric, immunocompromised, and adult ICU patients. We also developed an algorithm for initial VAP treatment. An interdisciplinary team of experts reviewed the current literature and developed these evidence‐based consensus guidelines. Our intent is that the algorithms provide guidance to clinicians looking for a standardized approach to the diagnosis and management of this complicated clinical situation.
METHODS
Our primary goal was to develop practical algorithms that assist ICU clinicians in the diagnosis and management of VAP during daily practice. To improve the quality and credibility of these algorithms, the development process used a stepwise approach that included assembling an interdisciplinary team of experts, appraising the published evidence, and formulating the algorithms through a consensus process.18
AHRQ National Collaborative
We developed these diagnostic algorithms as part of a national collaborative effort aimed at reducing VAP and central venous catheterrelated bloodstream infections in the ICU. This effort was possible through a 2‐year Partnerships in Implementing Patient Safety grant funded by the Agency for Healthcare Research and Quality (AHRQ).19 The voluntary collaborative was conducted in 61 medical/surgical and children's hospitals across the Hospital Corporation of America (HCA), a company that owns and/or operates 173 hospitals and 107 freestanding surgery centers in 20 states, England, and Switzerland. HCA is one of the largest providers of health care in the United States. All participating hospitals had at least 1 ICU, and a total of 110 ICUs were included in the project. Most hospitals were in the southern or southeastern regions of the United States.
Interdisciplinary Team
We assembled an interdisciplinary team to develop the diagnostic algorithms. Individuals on the team represented the specialties of infectious diseases, infection control, anesthesia, critical care medicine, hospital medicine, critical care nursing, pharmacy, and biostatistics. The development phase occurred over 34 months and used an iterative process that consisted of both group conference calls and in‐person meetings.
Our goal was not to conduct a systematic review but rather to develop practical algorithms for collaborative participants in a timely manner. Our literature search strategy included MEDLINE and the Cochrane Library. We focused on articles that addressed key diagnostic issues, proposed an algorithm, or summarized a topic relevant to practicing clinicians. Extra attention was given to articles that were randomized trials, meta‐analyses, or systematic reviews. No explicit grading of articles was performed. We examined studies with outcomes of interest to clinicians, including mortality, number of ventilator days, length of stay, antibiotic utilization, and antibiotic resistance.
We screened potentially relevant articles and the references of these articles. The search results were reviewed by all members of the team, and an iterative consensus process was used to derive the current algorithms. Preliminary versions of the algorithms were shown to other AHRQ investigators and outside experts in the field, and additional modifications were made based on their feedback. The final algorithms were approved by all study investigators.
RESULTS
Literature Overview
Overall, there is an enormous body of published literature on diagnosing and managing VAP. The Medline database has listed more than 500 articles on VAP diagnosis in the past decade. Nonetheless, the best diagnostic approach remains unclear. The gold standard for diagnosing VAP is lung biopsy with histopathologic examination and tissue culture. However, this procedure is fraught with potential dangers and impractical for most critically ill patients.20 Therefore, practitioners traditionally combine their clinical suspicion (based on fever, leukocytosis, character of sputum, and radiographic changes), epidemiologic data (eg, patient demographics, medical history, and ICU infection surveillance data), and microbiologic data.
Several issues relevant to practicing clinicians deserve further mention.
Definition of VAP
Although early articles used variable criteria for diagnosing VAP, recent studies have traditionally defined VAP as an infection occurring more than 48 hours after hospital admission in a mechanically ventilated patient with a tracheostomy or endotracheal tube.2 In early 2007, the CDC revised their definition for diagnosing VAP.3 These latest criteria state there is no minimum period that the ventilator must be in place in order to diagnose VAP. This important change must be kept in mind when examining future studies.
The term VAP is more specific than the term health careassociated pneumonia. The latter encompasses patients residing in a nursing home or long‐term care facility; hospitalized in an acute care hospital for more than 48 hours in the past 90 days; receiving antibiotics, chemotherapy, or wound care within the past 30 days; or attending a hospital or hemodialysis clinic.
The CDC published detailed criteria for diagnosing VAP in its member hospitals (Tables 1 and 2).3 Because diagnosing VAP in infants, children, elderly, and immunocompromised patients is often confusing because of other conditions with similar signs and symptoms, the CDC published alternate criteria for these populations. A key objective during development of our algorithms was to consolidate and simplify these diagnostic criteria for ICU clinicians.
| Radiology | Signs/symptoms/laboratory |
|---|---|
| |
| Two or more serial chest radiographs with at least 1 of the following*: | CRITERIA FOR ANY PATIENT |
| New or progressive and persistent infiltrate | At least 1 of the following: |
| Consolidation | Fever (>38C or >100.4F) with no other recognized cause |
| Cavitation | Leukopenia (4000 WBC/mm3) or leukocytosis (12,000 WBC/mm3) |
| Pneumatoceles, in infants 1 year old | For adults 70 years old, altered mental status with no other recognized causeand |
| Note: In patients without underlying pulmonary or cardiac disease (eg, respiratory distress syndrome, bronchopulmonary dysplasia, pulmonary edema, or chronic obstructive pulmonary disease), 1 definitive chest radiograph is acceptable.* | |
| At least 2 of the following: | |
| New onset of purulent sputum, or change in character of sputum, or increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough or dyspnea or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation [eg, PaO2/FiO2 240],** increased oxygen requirement, or increased ventilation demand) | |
|
Any laboratory criterion from Table 2 |
|
| ALTERNATE CRITERIA FOR INFANTS 1 YEAR OLD | |
| Worsening gas exchange (eg, O2 desaturation, increased ventilation demand or O2 requirement) | |
| and | |
| At least 3 of the following: | |
| Temperature instability with no other recognized cause | |
| Leukopenia (4000 WBC/mm3) or leukocytosis (15,000 WBC/mm3) and left shift (10% bands) | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| Apnea, tachypnea,‖ nasal flaring with retraction of chest wall, or grunting | |
| Wheezing, rales, or rhonchi | |
| Cough | |
| Bradycadia (100 beats/min) or tachycardia (>170 beats/min) | |
| ALTERNATE CRITERIA FOR CHILD >1 OR 12 YEARS OLD | |
| At least 3 of the following: | |
| Fever (>38.4C or >101.1F) or hypothermia (36.5C or 97.7F) with no other recognized cause | |
| Leukopenia (4000 WBC/mm3) or leukocytosis (15,000 WBC/mm3) | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough or dyspnea, apnea, or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation 94%, increased ventilation demand or O2 requirement) | |
|
Any laboratory criterion from Table 2 |
|
| ALTERNATE CRITERIA FOR IMMUNOCOMPROMISED PATIENTS*** | |
| At least 1 of the following: | |
| Fever (>38.4C or >101.1F) with no other recognized cause | |
| For adults > 70 years old, altered mental status with no other recognized cause | |
| New‐onset purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements | |
| New‐onset or worsening cough, dyspnea, or tachypnea‖ | |
| Rales or bronchial breath sounds | |
| Worsening gas exchange (eg, O2 desaturation [eg, PaO2/FiO2 240],** increased oxygen requirement, or increased ventilation demand) | |
| Hemoptysis | |
| Pleuritic chest pain | |
| Matching positive blood and sputum cultures with Candida spp. | |
| Evidence of fungi or Pneumocytis from minimally contaminated LRT specimen (eg, BAL or protected specimen brushing) from 1 of the following: | |
| Direct microscopic exam | |
| Positive culture of fungi | |
|
Any laboratory criterion from Table 2 |
|
|
| Positive growth in blood culture* not related to another source of infection |
| Positive growth in culture of pleural fluid |
| Positive quantitative culture from minimally contaminated LRT specimen (eg, BAL) |
| 5% BAL‐obtained cells contain intracellular bacteria on direct microscopic exam (eg, gram stain) |
| Histopathologic exam shows at least 1 of the following: |
| Abscess formation or foci of consolidation with intense PMN accumulation in bronchioles and alveoli |
| Positive quantitative culture of lung parenchyma |
| Evidence of lung parenchyma invasion by fungal hyphae or pseudohyphae |
| Positive culture of virus or Chlamydia from respiratory secretions |
| Positive detection of viral antigen or antibody from respiratory secretions (eg, EIA, FAMA, shell vial assay, PCR) |
| Fourfold rise in paired sera (IgG) for pathogen (eg, influenza viruses, Chlamydia) |
| Positive PCR for Chlamydia or Mycoplasma |
| Positive micro‐IF test for Chlamydia |
| Positive culture or visualization by micro‐IF of Legionella spp. from respiratory secretions or tissue |
| Detection of Legionella pneumophila serogroup 1 antigens in urine by RIA or EIA |
| Fourfold rise in L. pneumophila serogroup 1 antibody titer to 1:128 in paired acute and convalescent sera by indirect IFA |
Etiology
The most commonly isolated VAP pathogens in all patients are bacteria.21 Most of these organisms normally colonize the respiratory and gastrointestinal tracts, but some are unique to health care settings. Tracheal intubation disrupts the body's natural anatomic and physiologic defenses and facilitates easier entry of these pathogens. Typical organisms include Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter species, Klebsiella pneumoniae, Acinetobacter species, Escherichia coli, and Haemophilus influenzae.22, 23 Unfortunately, the prevalence of antimicrobial resistance among VAP pathogens is increasing.24 Risk factors for antibiotic resistance are common to ICU patients and include recent antibiotics, hemodialysis, nursing home residence, immunosuppression, and chronic wound care.5 Polymicrobial infections are frequently seen in VAP, with up to 50% of all VAP episodes caused by more than 1 organism.25
Viral VAP is rare in immunocompetent hosts, and seasonal outbreaks of influenza and other similar viruses are usually limited to nonventilated patients.26 However, influenza is underrecognized as a potential nosocomial pathogen, and numerous nosocomial outbreaks because of influenza have been reported.2731 Although herpes simplex virus is often detected in the respiratory tract of critically ill patients, its clinical importance remains unclear.32
Fungal VAP is also rare in immunocompetent hosts. On the other hand, pulmonary fungal infections are common in immunocompromised patients, especially following chemotherapy and transplantation. Candida species are often isolated from the airways of normal hosts, but most cases traditionally have been considered clinically unimportant because these organisms are normal oropharyngeal flora and rarely invade lung tissue.33, 34 It is unclear whether recent studies suggesting Candida colonization is associated with a higher risk for Pseudomonas VAP will change this conventional wisdom.3537
Immunocompromised patients with suspected VAP are unique because they are at risk not only for typical bacteria (which are the most common causes of VAP) but also for rarer opportunistic infections and noninfectious processes that mimic pneumonia.3840 While assessing these patients, clinicians must consider the status of the underlying disease, duration and type of immunosuppression, prophylactic regimens, and risk factors for noninfectious causes of pulmonary infiltrates.41 Common opportunistic infections include viruses, mycobacteria, fungi, and Pneumocystis. Noninfectious processes include pulmonary edema, drug toxicity, radiation pneumonitis, engraftment syndrome, bronchiolitis obliterans organizing pneumonia, alveolar proteinosis, transfusion‐related lung injury, alveolar hemorrhage, and progression of underlying disease. In general, diagnosing VAP in the immunocompromised patient requires a prompt, comprehensive, and multidisciplinary approach.38
In preterm and term infants, the most common VAP pathogens are gram‐negative organisms such as E. coli and P. aeruginosa. Other less common pathogens are Enterobacter, Klebsiella, Acinetobacter, Proteus, Citrobacter, and Stenotrophomonas maltophilia.42, 43 Infants with a preceding bloodstream infection or prolonged intubation are more likely to develop VAP.43, 44 Unfortunately, gram‐negative bacteria often colonize the airways of mechanically ventilated infants, and tracheal aspirate culture data are difficult to interpret in this population.42
Children are more likely to develop VAP if they are intubated for more than 48 hours. The most common pathogens isolated from tracheal aspirates in mechanically ventilated children are enteric gram‐negative bacteria, P. aeruginosa, and S. aureus.45, 46 Few studies have precisely delineated the pathogenesis of VAP in the pediatric ICU population.
Overall, the causes of VAP vary by hospital, patient population, and ICU type. Therefore, it is essential that ICU clinicians remain knowledgeable about their local surveillance data.21 Awareness of VAP microbiology is essential for optimizing initial antibiotic therapy and improving outcomes.
Early Versus Late VAP
Distinguishing between early and late VAP is important for initial antibiotic selection because the etiologic pathogens vary between these 2 periods.4749 Early VAP (days 14 of hospitalization) usually involves antibiotic‐sensitive community‐acquired bacteria and carries a better prognosis. In contrast, late VAP (5 days after hospital admission) is more likely to be caused by antibiotic‐resistant nosocomial bacteria that lead to increased morbidity and mortality. All patients who have been hospitalized or have received antibiotics during the prior 90 days should be treated as having late VAP because they are at much higher risk for colonization and infection with antibiotic‐resistant bacteria.47 Of note, 2 recent studies suggest that pathogens in the early and late periods are becoming similar at some institutions.50, 51 Overall, the distinction between early and late VAP is important because it affects the likelihood that a patient has antibiotic‐resistant bacteria. If antibiotic‐resistant pathogens are suspected, initial therapy should include empiric triple antibiotics until culture data are available.
Culturing Approaches
Because clinical criteria alone are rarely able to accurately diagnose VAP,52, 53 clinicians should also obtain a respiratory specimen for microbiologic culture. Despite the convenience of blood cultures, their sensitivity for diagnosing VAP is poor, and they rarely make the diagnosis alone.54 Two methods are available for culturing the lungsan invasive approach (eg, bronchoscopy with bronchoalveolar lavage) and a noninvasive approach (eg, tracheal aspirate).
Some investigators believe that adult patients with suspected VAP should always undergo an invasive sampling of lower‐respiratory‐tract secretions.55 Proponents of the invasive approach cite the frequency with which potential pathogens colonize the trachea of ICU patients and create spurious results on tracheal aspirates.22 In addition, several studies have shown that clinicians are more likely to narrow the spectrum of antibiotics after obtaining an invasive diagnostic sample.56 In other words, the invasive approach has been associated with better antimicrobial stewardship.
Other investigators believe that a noninvasive approach is equally safe and effective for diagnosing VAP.57 This clinical approach involves culturing a tracheal aspirate and using a pneumonia prediction score such as the clinical pulmonary infection score (CPIS; Table 3). The CPIS assigns 012 points based on 6 clinical criteria: fever, leukocyte count, oxygenation, quantity and purulence of secretions, type of radiographic abnormality, and results of sputum gram stain and culture.58 As developed, a CPIS > 6 has a sensitivity of 93% and a specificity of 100% for diagnosing VAP.58 However, the CPIS requires that nurses record sputum volume and that the laboratory stains the specimen. When the CPIS has been modified based on the unavailability of such resources, the results have been less impressive.5961 Despite studies showing that a noninvasive clinical approach can achieve adequate initial antibiotic coverage and reduce overuse of broad‐spectrum agents,62, 63 clinicians who use the CPIS must understand its inherent limitations.
| Criterion | Range | Score |
|---|---|---|
| ||
| Temperature (C) | 36.138.4 | 0 |
| 38.538.9 | 1 | |
| 39 or 36 | 2 | |
| Blood leukocytes (/mm3) | 4000 and 11,000 | 0 |
| 4000 or >11,000 | 1 | |
| + band forms 500 | 2 | |
| Oxygenation: PaO2/FiO2 (mmHg) | >240 or ARDS | 0 |
| 240 and no evidence of ARDS | 2 | |
| Chest radiograph | No infiltrate | 0 |
| Diffuse (or patchy) infiltrate | 1 | |
| Localized infiltrate | 2 | |
| Tracheal secretions | Absence of tracheal secretions | 0 |
| Nonpurulent tracheal secretions | 1 | |
| Purulent tracheal secretions | 2 | |
| Culture of tracheal aspirate | Pathogenic bacteria culture: no growth or light growth | 0 |
| Pathogenic bacteria culture: moderate/heavy growth | 1 | |
| Same pathogenic bacteria seen on gram stain (add 1 point) | 2 | |
A meta‐analysis56 comparing the utility of an invasive versus a noninvasive culturing approach identified 4 randomized trials examining this issue.6669 Overall, an invasive approach did not alter mortality, but patients undergoing bronchoscopy were much more likely to have their antibiotic regimens modified by clinicians. This suggests that the invasive approach may allow more directed use of antibiotics. Recently, the Canadian Critical Care Trials Group conducted a multicenter randomized trial looking at this issue.11 There was no difference between the 2 approaches in mortality, number of ventilator days, and antibiotic usage. However, all patients in this study were immediately treated with empiric broad‐spectrum antibiotics until culture results were available, and the investigators did not have a protocol for stopping antibiotics after culture data were available.
In summary, both invasive and noninvasive culturing approaches are considered acceptable options for diagnosing VAP. Readers interested in learning more about this topic should read the worthwhile Expert Discussion70 by Chastre and colleagues55 at the end of this article. In general, we recommend that ICU clinicians use a combination of clinical suspicion (based on the CPIS or other objective data) and cultures ideally obtained prior to antibiotics. Regardless of the chosen culturing approach, clinicians must recognize that 1 of the most important determinants of patient outcome is prompt administration of adequate initial antibiotics.7175
Initial Antibiotic Administration
Delaying initial antibiotics in VAP increases the risk of death.7175 If a patient receives ineffective initial therapy, a later switch to appropriate therapy does not eliminate the increased mortality risk. Therefore, a comprehensive approach to VAP diagnosis requires consideration of initial empiric antibiotic administration.
Whenever possible, clinicians should obtain a lower respiratory tract sample for microscopy and culture before administering antibiotics because performing cultures after antibiotics have been recently started will lead to a higher rate of false‐negative results.76 Unless the patient has no signs of sepsis and microscopy is completely negative, clinicians should then immediately start empiric broad‐spectrum antibiotics.57 Once the culture sensitivities are known, therapy can be deescalated to a narrower spectrum.77 Recent studies suggest that shorter durations of therapy (8 days) are as effective as longer courses and are associated with lower colonization rates by antibiotic‐resistant bacteria.62, 78
Initial broad‐spectrum antibiotics should be chosen based on local bacteriology and resistance patterns. Clinicians must remain aware of the most common bacterial pathogens in their local community, hospital, and ICU. This is essential for both ensuring adequate initial antibiotic coverage and reducing overall antibiotic days.65 Unrestrained use of broad‐spectrum antibiotics increases the risk of resistant pathogens. Clinicians must continually deescalate therapy and use narrow‐spectrum drugs as pathogens are identified.79
Prevention of VAP
In 2005, the American Thoracic Society published guidelines for the management of adults with VAP.5 These guidelines included a discussion of modifiable risk factors for preventing VAP and used an evidence‐based grading system to rank the various recommendations. The highest evidence (level 1) comes from randomized clinical trials, moderate evidence (level 2) comes from nonrandomized studies, and the lowest evidence (level 3) comes from case studies or expert opinion. Others have also published their own guidelines and recommendations for preventing VAP.8082 Table 4 shows the key VAP preventive strategies.
| Strategy | Level of evidence | References |
|---|---|---|
| ||
| General infection control measures (hand hygiene, staff education, isolate MDR pathogens, etc.) | 1 | 2,83,84 |
| ICU infection surveillance | 2 | 2,8385 |
| Avoid reintubation if possible, but promptly reintubate if a patients inexorably fails extubation | 1 | 2,83,86,87 |
| Use NPPV when appropriate (in selected patients) | 1 | 88 |
| Use oral route for endotracheal and gastric tubes (vs. nasal route) | 2 | 89 |
| Continuous suctioning of subglottic secretions (to avoid pooling on cuff and leakage into LRT) | 1 | 9092 |
| Maintain endotracheal cuff pressure > 20 cm H2O (to prevent secretion leakage into LRT) | 2 | 93 |
| Avoid unnecessary ventilator circuit changes | 1 | 94 |
| Routinely empty condensate in ventilator circuit | 2 | 95 |
| Maintain adequate nursing and therapist staffing | 2 | 9698 |
| Implement ventilator weaning and sedation protocols | 2 | 99101 |
| Semierect patient positioning (vs. supine) | 1 | 102 |
| Avoid aspiration when using enteral nutrition | 1 | 103,104 |
| Topical oral antisepsis (eg, chlorhexidine) | 1 | 105108 |
| Control blood sugar with insulin | 1 | 109 |
| Use heat‐moisture exchanger (vs. conventional humidifier) to reduce tubing condensate | 1 | 95 |
| Avoid unnecessary red blood cell transfusions | 1 | 110 |
| Use of sucralfate for GI prophylaxis | 1 | 111,112 |
| Influenza vaccination for health care workers | 2 | 2 |
Some strategies are not recommended for VAP prevention in general ICU patients. Selective decontamination of the digestive tract (ie, prophylactic oral antibiotics) has been shown to reduce respiratory infections in ICU patients,113 but its overall role remains controversial because of concerns it may increase the incidence of multi‐drug‐resistant pathogens.114 Similarly, prophylactic intravenous antibiotics administered at the time of intubation can reduce VAP in certain patient populations,115 but this strategy is also associated with an increased risk of antibiotic‐resistant nosocomial infections.116 Using kinetic beds and scheduled chest physiotherapy to reduce VAP is based on the premise that critically ill patients often develop atelectasis and cannot effectively clear their secretions. Unfortunately, neither of these modalities has been shown to consistently reduce VAP in medical ICU patients.117119
Algorithms for Diagnosis and Treatment of VAP
We present algorithms for diagnosing VAP in 4 ICU populations: infant (1 year old), pediatric (1‐12 years old), immunocompromised, and adult ICU patients (Figs. 14). Because clinicians face considerable uncertainty when diagnosing VAP, we sought to develop practical algorithms for use in daily ICU practice. Although we provided the algorithms to collaborative participants as a tool for improving care, we never mandated use, and we did not monitor levels of adherence.
Five teaching cases are presented in the Appendix. We demonstrate how to utilize the diagnostic algorithms in these clinical scenarios and offer tips for clinicians wishing to employ these tools in their daily practice. These cases are useful for educating residents, nurses, and hospitalists.
Overall, our intent is that the combined use of these VAP algorithms facilitate a streamlined diagnostic approach and minimize delays in initial antibiotic administration. A primary focus of any VAP guideline should be early and appropriate antibiotics in adequate doses, with deescalation of therapy as culture data permit.5 In general, the greatest risk to a patient with VAP is delaying initial adequate antibiotic coverage, and for this reason, antibiotics must always be administered promptly. However, if culture data are negative, the clinician should consider withdrawing unnecessary antibiotics. For example, the absence of gram‐positive organisms on BAL after 72 hours would strongly suggest that MRSA is not playing a role and that vancomycin can be safely stopped. We agree with Neiderman that the decision point is not whether to start antibiotics, but whether to continue them at day 23.57
DISCUSSION
In this article, we introduce algorithms for diagnosing and managing VAP in infant, pediatric, immunocompromised, and adult ICU patients. We developed 4 algorithms because the hospitals in our system care for a wide range of patients. Our definitions for VAP were based on criteria outlined by the CDC because these rigorously developed criteria have been widely disseminated as components of the Institute for Healthcare Improvement's ventilator bundle.120 Clinicians should be able to easily incorporate these practical algorithms into their current practice.
The algorithms were developed during a collaborative across a large national health care system. We undertook this task because many clinicians were uncertain how to integrate the enormous volume of VAP literature into their daily practice, and we suspected there was large variation in practice in our ICUs. Recent studies from other health care systems provided empiric evidence to support this notion.12, 13
We offer these algorithms as practical tools to assist ICU clinicians and not as proscriptive mandates. We realize that the algorithms may need modification based on a hospital's unique bacteriology and patient populations. We also anticipate that the algorithms will adapt to future changes in VAP epidemiology, preventive strategies, emerging pathogens, and new antibiotics.
Numerous resources are available to learn more about VAP management. An excellent guideline from the Infectious Diseases Society of America and the American Thoracic Society discusses VAP issues in detail,5 although this guideline only focuses on immunocompetent adult patients. The journal Respiratory Care organized an international conference with numerous VAP experts in 2005 and subsequently devoted an entire issue to this topic.81 The Canadian Critical Care Trials Group and the Canadian Critical Care Society conducted systematic reviews and developed separate guidelines for the prevention, diagnosis, and treatment of VAP.80, 121
In summary, we present diagnostic and treatment algorithms for VAP. Our intent is that these algorithms may provide evidence‐based practical guidance to clinicians seeking a standardized approach to diagnosing and managing this challenging problem.
- ,,,.Nosocomial infections in combined medical‐surgical intensive care units in the United States.Infect Control Hosp Epidemiol.2000;21:510–515.
- Centers for Disease Control and Prevention.Guidelines for preventing health‐care—associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee.MMWR Recomm Rep.2004;53:1–36.
- Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) manual: patient safety component protocol (updated May 24,2007). Available at: http://www.cdc.gov/ncidod/dhqp/pdf/nhsn/NHSN_Manual_Patient_Safety_Protocol052407.pdf. Accessed October 1, 2007.
- ,,, et al.Outcome and attributable cost of ventilator‐associated pneumonia among intensive care unit patients in a suburban medical center.Crit Care Med.2003;31:1312–1317.
- ATS.Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,,.Nosocomial pneumonia in Medicare patients. Hospital costs and reimbursement patterns under the prospective payment system.Arch Intern Med.1991;151:1109–1114.
- ,,,,,.Oral decontamination is cost‐saving in the prevention of ventilator‐associated pneumonia in intensive care units.Crit Care Med.2004;32:126–130.
- ,,,.Clinical and economic consequences of ventilator‐associated pneumonia: a systematic review.Crit Care Med.2005;33:2184–2193.
- .Cost‐effectiveness issues in ventilator‐associated pneumonia.Respir Care.2005;50:956–963; discussion 963–964.
- .Diagnosis and treatment of ventilator‐associated pneumonia: fiberoptic bronchoscopy with bronchoalveolar lavage is essential.Semin Respir Crit Care Med.2006;27:34–44.
- ,,,.A randomized trial of diagnostic techniques for ventilator‐associated pneumonia.N Engl J Med.2006;355:2619–2630.
- ,,, et al.Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia.Chest.2006;129:1210–1218.
- ,,,.Prevention and diagnosis of ventilator‐associated pneumonia: a survey on current practices in Southern Spanish ICUs.Chest.2005;128:1667–1673.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,,,,,.Why do physicians not follow evidence‐based guidelines for preventing ventilator‐associated pneumonia?: a survey based on the opinions of an international panel of intensivists.Chest.2002;122:656–661.
- ,,, et al.Invitation to a dialogue between researchers and clinicians about evidence‐based behavioral medicine.Ann Behav Med.2005;30:125–137.
- ,,.Connections between quality measurement and improvement.Med Care.2003;41:I30–I38.
- ,,,,.Practice guidelines developed by specialty societies: the need for a critical appraisal.Lancet.2000;355:103–106.
- Agency for Healthcare Research and Quality (AHRQ). Partnerships in Implementing Patient Safety. Online at http://www.ahrq.gov/qual/pips.htm. Accessed March 1,2007.
- ,.The utility of open lung biopsy in patients requiring mechanical ventilation.Chest.1999;115:811–817.
- .The microbiology of ventilator‐associated pneumonia.Respir Care.2005;50:742–763; discussion 763–765.
- ,.Ventilator‐associated pneumonia.Am J Respir Crit Care Med.2002;165:867–903.
- ,.Overview of nosocomial infections caused by gram‐negative bacilli.Clin Infect Dis.2005;41:848–854.
- .Increasing prevalence of antimicrobial resistance in intensive care units.Crit Care Med.2001;29:N64–N68.
- ,,, et al.Incidence and outcome of polymicrobial ventilator‐associated pneumonia.Chest.2002;121:1618–1623.
- ,,.Nosocomial transmission of influenza.Occup Med (Lond).2002;52:249–253.
- ,.Nosocomial influenza at a Canadian pediatric hospital from 1995 to 1999: opportunities for prevention.Infect Control Hosp Epidemiol.2002;23:627–629.
- ,Nosocomial influenza infection as a cause of intercurrent fevers in infants.Pediatrics.1975;55:673–677.
- ,.Nosocomial influenza infection.Lancet.2000;355:1187.
- ,,.Nosocomial influenza B virus infection in the elderly.Ann Intern Med.1982;96:153–158.
- ,,,,,.Influenza vaccination of healthcare workers and vaccine allocation for healthcare workers during vaccine shortages.Infect Control Hosp Epidemiol.2005;26:882–890.
- ,,, et al.Herpes simplex virus in the respiratory tract of critical care patients: a prospective study.Lancet.2003;362:1536–1541.
- ,,, et al.Significance of the isolation of Candida species from respiratory samples in critically ill, non‐neutropenic patients. An immediate postmortem histologic study.Am J Respir Crit Care Med.1997;156:583–590.
- ,,,,,.The role of Candida sp isolated from bronchoscopic samples in nonneutropenic patients.Chest.1998;114:146– 149.
- ,.Pseudomonas‐Candida interactions: an ecological role for virulence factors.Science.2002;296:2229–2232.
- ,,, et al.Candida colonization of the respiratory tract and subsequent pseudomonas ventilator‐associated pneumonia.Chest.2006;129:110–117.
- ,,, et al.Impact of antifungal treatment on Candida‐Pseudomonas interaction: a preliminary retrospective case‐control study.Intensive Care Med.2007;33:137–142.
- ,,.Pulmonary infiltrates in the non‐HIV‐infected immunocompromised patient: etiologies, diagnostic strategies, and outcomes.Chest.2004;125:260–271.
- ,,.Pulmonary complications of solid organ and hematopoietic stem cell transplantation.Am J Respir Crit Care Med.2004;170:22–48.
- ,,, et al.Incidence, outcome, and risk factors of late‐onset noninfectious pulmonary complications after unrelated donor stem cell transplantation.Bone Marrow Transplant.2004;33:751–758.
- ,.Infection in organ‐transplant recipients.N Engl J Med.1998;338:1741–1751.
- ,,,,.Surveillance of ventilator‐associated pneumonia in very‐low‐birth‐weight infants.Am J Infect Control.2002;30:32–39.
- ,,,,.Ventilator‐associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes.Pediatrics.2003;112:1283–1289.
- ,,.Risk factors for nosocomial infection in a high‐risk nursery.Infect Control Hosp Epidemiol.2000;21:250–251.
- .Pediatric ventilator‐associated pneumonia.Pediatr Infect Dis J.2003;22:445–446.
- ,,.Ventilator‐associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes.Pediatrics.2002;109:758–764.
- ,,, et al.Ventilator‐associated pneumonia caused by potentially drug‐resistant bacteria.Am J Respir Crit Care Med.1998;157:531–539.
- ,,.Pneumonia in intubated trauma patients. Microbiology and outcomes.Am J Respir Crit Care Med.1996;153:343–349.
- ,,,,.Pneumonia due to Haemophilus influenzae among mechanically ventilated patients. Incidence, outcome, and risk factors.Chest.1992;102:1562–1565.
- ,,, et al.Both early‐onset and late‐onset ventilator‐associated pneumonia are caused mainly by potentially multiresistant bacteria.Intensive Care Med2005;31:1488–1494.
- ,,,.A comparative analysis of patients with early‐onset vs late‐onset nosocomial pneumonia in the ICU setting.Chest.2000;117:1434–1442.
- .Diagnosis of ventilator‐associated pneumonia.Curr Opin Crit Care.2003;9:397–402.
- ,.Use and limitations of clinical and radiologic diagnosis of pneumonia.Semin Respir Infect.2003;18:72–79.
- ,,, et al.Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator‐associated pneumonia.Chest.1999;116:1075–1084.
- ,,.The invasive (quantitative) diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:797–807.
- ,,,.Invasive approaches to the diagnosis of ventilator‐associated pneumonia: a meta‐analysis.Crit Care Med.2005;33:46– 53.
- .The clinical diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:788–796; discussion 807–812.
- ,,,,,.Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid.Am Rev Respir Dis.1991;143:1121–1129.
- ,,,,,.Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited.Am J Respir Crit Care Med.2003;168:173–179.
- ,,,,,.The diagnosis of ventilator‐associated pneumonia using non‐bronchoscopic, non‐directed lung lavages.Intensive Care Med.2000;26:20–30.
- ,,, et al.Clinical pulmonary infection score for ventilator‐associated pneumonia: accuracy and inter‐observer variability.Intensive Care Med.2004;30:217–224.
- ,,,.A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator‐associated pneumonia.Chest.2004;125:1791–1799.
- ,,, et al.Early antibiotic treatment for BAL‐confirmed ventilator‐associated pneumonia: a role for routine endotracheal aspirate cultures.Chest.2005;127:589–597.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,,,,.Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia.Crit Care Med.2001;29:1109–1115.
- ,,, et al.Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator‐associated pneumonia: a pilot study.Am J Respir Crit Care Med.1998;157:371–376.
- ,,, et al.Noninvasive versus invasive microbial investigation in ventilator‐associated pneumonia: evaluation of outcome.Am J Respir Crit Care Med.2000;162:119–125.
- ,,,,.Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia.Crit Care Med.2000;28:2737–2741.
- ,,, et al.Invasive and noninvasive strategies for management of suspected ventilator‐associated pneumonia. A randomized trial.Ann Intern Med.2000;132:621–630.
- ,,.Expert discussion: The invasive (quantitative) diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:807–812.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,,,.Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator‐associated pneumonia.Intensive Care Med.2001;27:355–362.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Appropriateness and delay to initiate therapy in ventilator‐associated pneumonia.Eur Respir J.2006;27:158–164.
- .The importance of appropriate initial antibiotic therapy for hospital‐acquired infections.Am J Med.2003;115:582–584.
- ,,, et al.Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments.Crit Care Med.1998;26:236–244.
- ,,, et al.De‐escalation therapy in ventilator‐associated pneumonia.Crit Care Med.2004;32:2183–2190.
- ,,, et al.Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial.JAMA.2003;290:2588–2598.
- .De‐escalation therapy in ventilator‐associated pneumonia.Curr Opin Crit Care.2006;12:452–457.
- ,,, et al.Evidence‐based clinical practice guideline for the prevention of ventilator‐associated pneumonia.Ann Intern Med.2004;141:305–313.
- .Conference summary: ventilator‐associated pneumonia.Respir Care.2005;50:975–983.
- ,,.Prevention of ventilator‐associated pneumonia: an evidence‐based systematic review.Ann Intern Med.2003;138:494–501.
- .The prevention of ventilator‐associated pneumonia.N Engl J Med.1999;340:627–634.
- .Epidemiology and control of nosocomial infections in adult intensive care units.Am J Med.1991;91:179S–184S.
- ,,, et al.Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene. Infection Control Programme.Lancet.2000;356:1307–1312.
- ,,, et al.Re‐intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation.Am J Respir Crit Care Med.1995;152:137–41.
- ,,,,,.Nosocomial pneumonia. A multivariate analysis of risk and prognosis.Chest.1988;93:318–324.
- ,,, et al.Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients.JAMA.2000;284:2361–2367.
- ,,, et al.Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill.Am J Respir Crit Care Med.1994;150:776–783.
- ,,.Prevention measures for ventilator‐associated pneumonia: a new focus on the endotracheal tube.Curr Opin Infect Dis.2007;20:190–197.
- ,,,.A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation.Chest.2002;121:858–862.
- ,,, et al.Continuous aspiration of subglottic secretions in preventing ventilator‐associated pneumonia.Ann Intern Med.1995;122:179–186.
- ,,,,,.Pneumonia in intubated patients: role of respiratory airway care.Am J Respir Crit Care Med.1996;154:111–115.
- ,,, et al.Mechanical ventilation with or without 7‐day circuit changes. A randomized controlled trial.Ann Intern Med.1995;123:168–174.
- ,,.Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia?Am Rev Respir Dis.1984;129:625–628.
- ,,,,.Outbreak of Enterobacter cloacae related to understaffing, overcrowding, and poor hygiene practices.Infect Control Hosp. Epidemiol.1999;20:598–603.
- ,,,,.Nursing resources: a major determinant of nosocomial infection?Curr Opin Infect Dis.2004;17:329–333.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346:1715–1722.
- ,,, et al.Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously.N Engl J Med.1996;335:1864–1869.
- ,,, et al.A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation.Crit Care Med.1997;25:567–574.
- ,,,.Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation.N Engl J Med.2000;342:1471–1477.
- ,,,,,.Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial.Lancet.1999;354:1851–1858.
- ,,, et al.Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial.JPEN J Parenter Enteral Nutr.2002;26:174–181.
- ,,,.Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: role of small bowel feeding.JPEN J Parenter Enteral Nutr.2002;26:S51–S55; discussion S56–S57.
- ,,,.Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta‐analysis.BMJ.2007;334:889.
- ,.Topical chlorhexidine for prevention of ventilator‐associated pneumonia: a meta‐analysis.Crit Care Med.2007;35:595–602.
- ,,, et al.Oral decontamination with chlorhexidine reduces the incidence of ventilator‐associated pneumonia.Am J Respir Crit Care Med.2006;173:1348–1355.
- ,.Efficacy of oral chlorhexidine in preventing lower respiratory tract infections. Meta‐analysis of randomized controlled trials.J Hosp Infect.2007;66:207– 216.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Red blood cell transfusion and ventilator‐associated pneumonia: A potential link?Crit Care Med.2004;32:666–674.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- ,,, et al.Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization.N Engl J Med.1987;317:1376–1382.
- ,,,,.Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care.Cochrane Database Syst Rev.2004:CD000022.
- ,.Selective decontamination of the digestive tract: cumulating evidence, at last?Semin Respir Crit Care Med.2006;27:18–22.
- ,,,,,.Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma.Am J Respir Crit Care Med.1997;155:1729–1734.
- ,,,,,.Prophylactic antibiotics adversely affect nosocomial pneumonia in trauma patients.J Trauma.2003;55:249–254.
- ,.Kinetic therapy in critically ill trauma patients.Clin Intensive Care.1992;3:248–252.
- ,,,,.Continuous oscillation: outcome in critically ill patients.JCrit Care.1995;10:97–103.
- ,,,.Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: a systematic review and meta‐analysis.Crit Care.2006;10:R70.
- ,,,.The 100,000 lives campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295:324–327.
- Zap the VAP. Available at: http://www.zapthevap.com. Accessed March 1,2007.
- .Aspiration pneumonitis and aspiration pneumonia.N Engl J Med.2001;344:665–671.
- ,,,.Nosocomial infections in combined medical‐surgical intensive care units in the United States.Infect Control Hosp Epidemiol.2000;21:510–515.
- Centers for Disease Control and Prevention.Guidelines for preventing health‐care—associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee.MMWR Recomm Rep.2004;53:1–36.
- Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) manual: patient safety component protocol (updated May 24,2007). Available at: http://www.cdc.gov/ncidod/dhqp/pdf/nhsn/NHSN_Manual_Patient_Safety_Protocol052407.pdf. Accessed October 1, 2007.
- ,,, et al.Outcome and attributable cost of ventilator‐associated pneumonia among intensive care unit patients in a suburban medical center.Crit Care Med.2003;31:1312–1317.
- ATS.Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,,.Nosocomial pneumonia in Medicare patients. Hospital costs and reimbursement patterns under the prospective payment system.Arch Intern Med.1991;151:1109–1114.
- ,,,,,.Oral decontamination is cost‐saving in the prevention of ventilator‐associated pneumonia in intensive care units.Crit Care Med.2004;32:126–130.
- ,,,.Clinical and economic consequences of ventilator‐associated pneumonia: a systematic review.Crit Care Med.2005;33:2184–2193.
- .Cost‐effectiveness issues in ventilator‐associated pneumonia.Respir Care.2005;50:956–963; discussion 963–964.
- .Diagnosis and treatment of ventilator‐associated pneumonia: fiberoptic bronchoscopy with bronchoalveolar lavage is essential.Semin Respir Crit Care Med.2006;27:34–44.
- ,,,.A randomized trial of diagnostic techniques for ventilator‐associated pneumonia.N Engl J Med.2006;355:2619–2630.
- ,,, et al.Clinical characteristics and treatment patterns among patients with ventilator‐associated pneumonia.Chest.2006;129:1210–1218.
- ,,,.Prevention and diagnosis of ventilator‐associated pneumonia: a survey on current practices in Southern Spanish ICUs.Chest.2005;128:1667–1673.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,,,,,.Why do physicians not follow evidence‐based guidelines for preventing ventilator‐associated pneumonia?: a survey based on the opinions of an international panel of intensivists.Chest.2002;122:656–661.
- ,,, et al.Invitation to a dialogue between researchers and clinicians about evidence‐based behavioral medicine.Ann Behav Med.2005;30:125–137.
- ,,.Connections between quality measurement and improvement.Med Care.2003;41:I30–I38.
- ,,,,.Practice guidelines developed by specialty societies: the need for a critical appraisal.Lancet.2000;355:103–106.
- Agency for Healthcare Research and Quality (AHRQ). Partnerships in Implementing Patient Safety. Online at http://www.ahrq.gov/qual/pips.htm. Accessed March 1,2007.
- ,.The utility of open lung biopsy in patients requiring mechanical ventilation.Chest.1999;115:811–817.
- .The microbiology of ventilator‐associated pneumonia.Respir Care.2005;50:742–763; discussion 763–765.
- ,.Ventilator‐associated pneumonia.Am J Respir Crit Care Med.2002;165:867–903.
- ,.Overview of nosocomial infections caused by gram‐negative bacilli.Clin Infect Dis.2005;41:848–854.
- .Increasing prevalence of antimicrobial resistance in intensive care units.Crit Care Med.2001;29:N64–N68.
- ,,, et al.Incidence and outcome of polymicrobial ventilator‐associated pneumonia.Chest.2002;121:1618–1623.
- ,,.Nosocomial transmission of influenza.Occup Med (Lond).2002;52:249–253.
- ,.Nosocomial influenza at a Canadian pediatric hospital from 1995 to 1999: opportunities for prevention.Infect Control Hosp Epidemiol.2002;23:627–629.
- ,Nosocomial influenza infection as a cause of intercurrent fevers in infants.Pediatrics.1975;55:673–677.
- ,.Nosocomial influenza infection.Lancet.2000;355:1187.
- ,,.Nosocomial influenza B virus infection in the elderly.Ann Intern Med.1982;96:153–158.
- ,,,,,.Influenza vaccination of healthcare workers and vaccine allocation for healthcare workers during vaccine shortages.Infect Control Hosp Epidemiol.2005;26:882–890.
- ,,, et al.Herpes simplex virus in the respiratory tract of critical care patients: a prospective study.Lancet.2003;362:1536–1541.
- ,,, et al.Significance of the isolation of Candida species from respiratory samples in critically ill, non‐neutropenic patients. An immediate postmortem histologic study.Am J Respir Crit Care Med.1997;156:583–590.
- ,,,,,.The role of Candida sp isolated from bronchoscopic samples in nonneutropenic patients.Chest.1998;114:146– 149.
- ,.Pseudomonas‐Candida interactions: an ecological role for virulence factors.Science.2002;296:2229–2232.
- ,,, et al.Candida colonization of the respiratory tract and subsequent pseudomonas ventilator‐associated pneumonia.Chest.2006;129:110–117.
- ,,, et al.Impact of antifungal treatment on Candida‐Pseudomonas interaction: a preliminary retrospective case‐control study.Intensive Care Med.2007;33:137–142.
- ,,.Pulmonary infiltrates in the non‐HIV‐infected immunocompromised patient: etiologies, diagnostic strategies, and outcomes.Chest.2004;125:260–271.
- ,,.Pulmonary complications of solid organ and hematopoietic stem cell transplantation.Am J Respir Crit Care Med.2004;170:22–48.
- ,,, et al.Incidence, outcome, and risk factors of late‐onset noninfectious pulmonary complications after unrelated donor stem cell transplantation.Bone Marrow Transplant.2004;33:751–758.
- ,.Infection in organ‐transplant recipients.N Engl J Med.1998;338:1741–1751.
- ,,,,.Surveillance of ventilator‐associated pneumonia in very‐low‐birth‐weight infants.Am J Infect Control.2002;30:32–39.
- ,,,,.Ventilator‐associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes.Pediatrics.2003;112:1283–1289.
- ,,.Risk factors for nosocomial infection in a high‐risk nursery.Infect Control Hosp Epidemiol.2000;21:250–251.
- .Pediatric ventilator‐associated pneumonia.Pediatr Infect Dis J.2003;22:445–446.
- ,,.Ventilator‐associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes.Pediatrics.2002;109:758–764.
- ,,, et al.Ventilator‐associated pneumonia caused by potentially drug‐resistant bacteria.Am J Respir Crit Care Med.1998;157:531–539.
- ,,.Pneumonia in intubated trauma patients. Microbiology and outcomes.Am J Respir Crit Care Med.1996;153:343–349.
- ,,,,.Pneumonia due to Haemophilus influenzae among mechanically ventilated patients. Incidence, outcome, and risk factors.Chest.1992;102:1562–1565.
- ,,, et al.Both early‐onset and late‐onset ventilator‐associated pneumonia are caused mainly by potentially multiresistant bacteria.Intensive Care Med2005;31:1488–1494.
- ,,,.A comparative analysis of patients with early‐onset vs late‐onset nosocomial pneumonia in the ICU setting.Chest.2000;117:1434–1442.
- .Diagnosis of ventilator‐associated pneumonia.Curr Opin Crit Care.2003;9:397–402.
- ,.Use and limitations of clinical and radiologic diagnosis of pneumonia.Semin Respir Infect.2003;18:72–79.
- ,,, et al.Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator‐associated pneumonia.Chest.1999;116:1075–1084.
- ,,.The invasive (quantitative) diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:797–807.
- ,,,.Invasive approaches to the diagnosis of ventilator‐associated pneumonia: a meta‐analysis.Crit Care Med.2005;33:46– 53.
- .The clinical diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:788–796; discussion 807–812.
- ,,,,,.Diagnosis of ventilator‐associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid.Am Rev Respir Dis.1991;143:1121–1129.
- ,,,,,.Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited.Am J Respir Crit Care Med.2003;168:173–179.
- ,,,,,.The diagnosis of ventilator‐associated pneumonia using non‐bronchoscopic, non‐directed lung lavages.Intensive Care Med.2000;26:20–30.
- ,,, et al.Clinical pulmonary infection score for ventilator‐associated pneumonia: accuracy and inter‐observer variability.Intensive Care Med.2004;30:217–224.
- ,,,.A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator‐associated pneumonia.Chest.2004;125:1791–1799.
- ,,, et al.Early antibiotic treatment for BAL‐confirmed ventilator‐associated pneumonia: a role for routine endotracheal aspirate cultures.Chest.2005;127:589–597.
- ,,,,.Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription.Am J Respir Crit Care Med.2000;162:505–511.
- ,,,,,.Experience with a clinical guideline for the treatment of ventilator‐associated pneumonia.Crit Care Med.2001;29:1109–1115.
- ,,, et al.Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator‐associated pneumonia: a pilot study.Am J Respir Crit Care Med.1998;157:371–376.
- ,,, et al.Noninvasive versus invasive microbial investigation in ventilator‐associated pneumonia: evaluation of outcome.Am J Respir Crit Care Med.2000;162:119–125.
- ,,,,.Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia.Crit Care Med.2000;28:2737–2741.
- ,,, et al.Invasive and noninvasive strategies for management of suspected ventilator‐associated pneumonia. A randomized trial.Ann Intern Med.2000;132:621–630.
- ,,.Expert discussion: The invasive (quantitative) diagnosis of ventilator‐associated pneumonia.Respir Care.2005;50:807–812.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,,,.Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator‐associated pneumonia.Intensive Care Med.2001;27:355–362.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Appropriateness and delay to initiate therapy in ventilator‐associated pneumonia.Eur Respir J.2006;27:158–164.
- .The importance of appropriate initial antibiotic therapy for hospital‐acquired infections.Am J Med.2003;115:582–584.
- ,,, et al.Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments.Crit Care Med.1998;26:236–244.
- ,,, et al.De‐escalation therapy in ventilator‐associated pneumonia.Crit Care Med.2004;32:2183–2190.
- ,,, et al.Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial.JAMA.2003;290:2588–2598.
- .De‐escalation therapy in ventilator‐associated pneumonia.Curr Opin Crit Care.2006;12:452–457.
- ,,, et al.Evidence‐based clinical practice guideline for the prevention of ventilator‐associated pneumonia.Ann Intern Med.2004;141:305–313.
- .Conference summary: ventilator‐associated pneumonia.Respir Care.2005;50:975–983.
- ,,.Prevention of ventilator‐associated pneumonia: an evidence‐based systematic review.Ann Intern Med.2003;138:494–501.
- .The prevention of ventilator‐associated pneumonia.N Engl J Med.1999;340:627–634.
- .Epidemiology and control of nosocomial infections in adult intensive care units.Am J Med.1991;91:179S–184S.
- ,,, et al.Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene. Infection Control Programme.Lancet.2000;356:1307–1312.
- ,,, et al.Re‐intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation.Am J Respir Crit Care Med.1995;152:137–41.
- ,,,,,.Nosocomial pneumonia. A multivariate analysis of risk and prognosis.Chest.1988;93:318–324.
- ,,, et al.Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients.JAMA.2000;284:2361–2367.
- ,,, et al.Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill.Am J Respir Crit Care Med.1994;150:776–783.
- ,,.Prevention measures for ventilator‐associated pneumonia: a new focus on the endotracheal tube.Curr Opin Infect Dis.2007;20:190–197.
- ,,,.A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation.Chest.2002;121:858–862.
- ,,, et al.Continuous aspiration of subglottic secretions in preventing ventilator‐associated pneumonia.Ann Intern Med.1995;122:179–186.
- ,,,,,.Pneumonia in intubated patients: role of respiratory airway care.Am J Respir Crit Care Med.1996;154:111–115.
- ,,, et al.Mechanical ventilation with or without 7‐day circuit changes. A randomized controlled trial.Ann Intern Med.1995;123:168–174.
- ,,.Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia?Am Rev Respir Dis.1984;129:625–628.
- ,,,,.Outbreak of Enterobacter cloacae related to understaffing, overcrowding, and poor hygiene practices.Infect Control Hosp. Epidemiol.1999;20:598–603.
- ,,,,.Nursing resources: a major determinant of nosocomial infection?Curr Opin Infect Dis.2004;17:329–333.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346:1715–1722.
- ,,, et al.Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously.N Engl J Med.1996;335:1864–1869.
- ,,, et al.A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation.Crit Care Med.1997;25:567–574.
- ,,,.Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation.N Engl J Med.2000;342:1471–1477.
- ,,,,,.Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial.Lancet.1999;354:1851–1858.
- ,,, et al.Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial.JPEN J Parenter Enteral Nutr.2002;26:174–181.
- ,,,.Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: role of small bowel feeding.JPEN J Parenter Enteral Nutr.2002;26:S51–S55; discussion S56–S57.
- ,,,.Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta‐analysis.BMJ.2007;334:889.
- ,.Topical chlorhexidine for prevention of ventilator‐associated pneumonia: a meta‐analysis.Crit Care Med.2007;35:595–602.
- ,,, et al.Oral decontamination with chlorhexidine reduces the incidence of ventilator‐associated pneumonia.Am J Respir Crit Care Med.2006;173:1348–1355.
- ,.Efficacy of oral chlorhexidine in preventing lower respiratory tract infections. Meta‐analysis of randomized controlled trials.J Hosp Infect.2007;66:207– 216.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Red blood cell transfusion and ventilator‐associated pneumonia: A potential link?Crit Care Med.2004;32:666–674.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- ,,, et al.Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization.N Engl J Med.1987;317:1376–1382.
- ,,,,.Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care.Cochrane Database Syst Rev.2004:CD000022.
- ,.Selective decontamination of the digestive tract: cumulating evidence, at last?Semin Respir Crit Care Med.2006;27:18–22.
- ,,,,,.Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma.Am J Respir Crit Care Med.1997;155:1729–1734.
- ,,,,,.Prophylactic antibiotics adversely affect nosocomial pneumonia in trauma patients.J Trauma.2003;55:249–254.
- ,.Kinetic therapy in critically ill trauma patients.Clin Intensive Care.1992;3:248–252.
- ,,,,.Continuous oscillation: outcome in critically ill patients.JCrit Care.1995;10:97–103.
- ,,,.Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: a systematic review and meta‐analysis.Crit Care.2006;10:R70.
- ,,,.The 100,000 lives campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295:324–327.
- Zap the VAP. Available at: http://www.zapthevap.com. Accessed March 1,2007.
- .Aspiration pneumonitis and aspiration pneumonia.N Engl J Med.2001;344:665–671.