User login
Steps to minimize morbidity from unanticipated placenta accreta spectrum
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
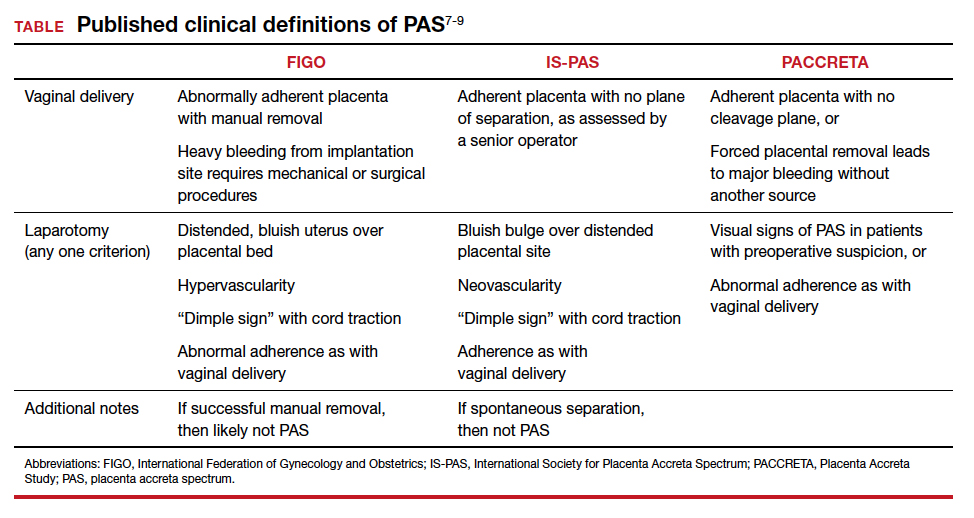
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.
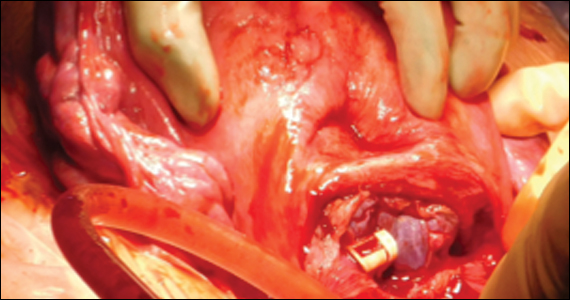
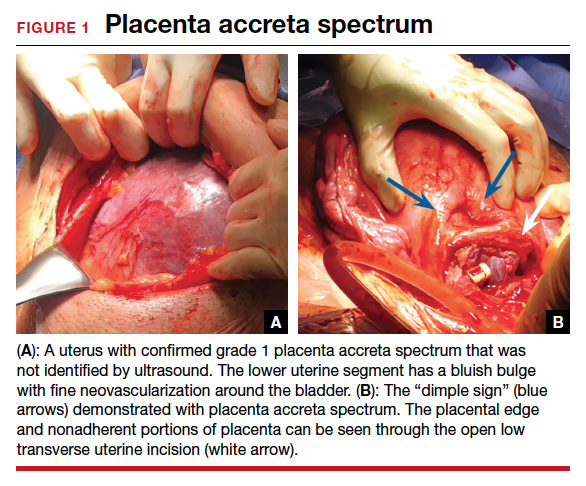
At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8
Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
Reduce the use of perioperative opioids with a multimodal pain management strategy
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
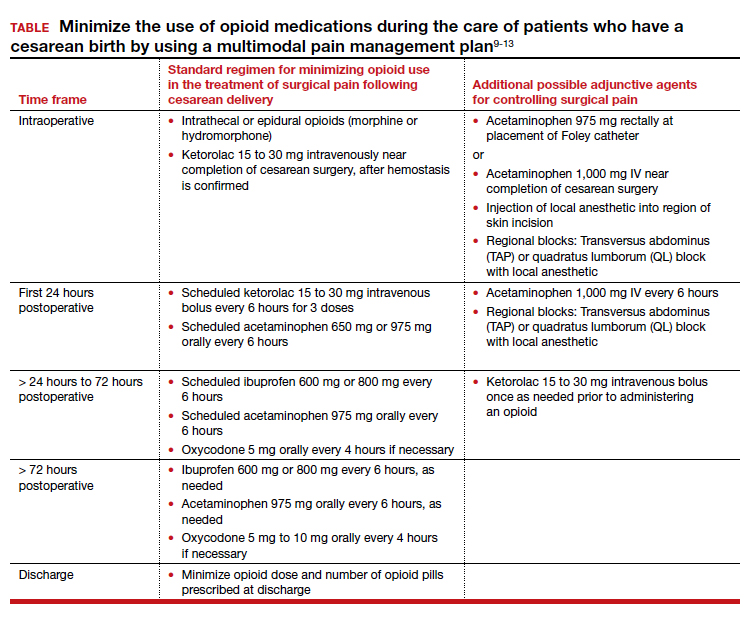
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.
Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.

Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.

Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.