User login
Bariatric surgery cuts risk for obesity-related cancers in half: Study
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).
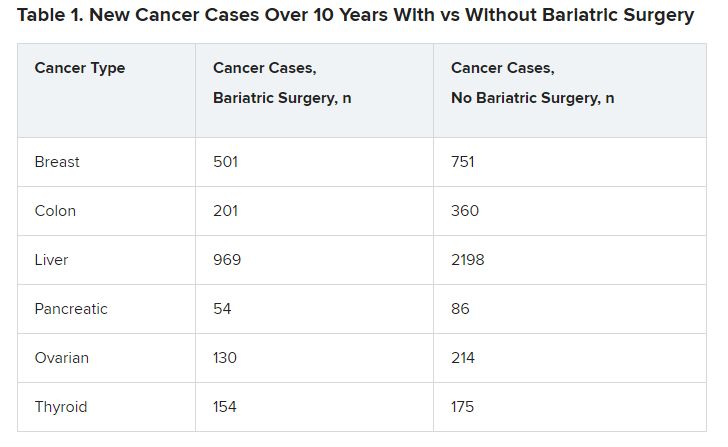
The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
Likely cause of mysterious hepatitis outbreak in children identified
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
Nasal COVID treatment shows early promise against multiple variants
if used within 4 hours after infection inside the nose, new research reveals.
Known as TriSb92 (brand name Covidin, from drugmaker Pandemblock Oy in Finland), the viral inhibitor also appears effective against all coronavirus variants of concern, neutralizing even the Omicron variants BA.5, XBB, and BQ.1.1 in laboratory and mice studies.
Unlike a COVID vaccine that boosts a person’s immune system as protection, the antiviral nasal spray works more directly by blocking the virus, acting as a “biological mask in the nasal cavity,” according to the biotechnology company set up to develop the treatment.
The product targets a stable site on the spike protein of the virus that is not known to mutate. This same site is shared among many variants of the COVID virus, so it could be effective against future variants as well, researchers note.
“In animal models, by directly inactivating the virus, TriSb92 offers immediate and robust protection” against coronavirus infection and severe COVID, said Anna R. Mäkelä, PhD, lead author of the study and a senior scientist in the department of virology at the University of Helsinki.
The study was published online in Nature Communications.
A potential first line of defense
Even in cases where the antiviral does not prevent coronavirus infection, the treatment could slow infection. This could happen by limiting how much virus could replicate early in the skin inside the nose and nasopharynx (the upper part of the throat), said Dr. Mäkelä, who is also CEO of Pandemblock Oy, the company set up to develop the product.
“TriSb92 could effectively tip the balance in favor of the [the person] and thereby help to reduce the risk of severe COVID-19 disease,” she said.
The antiviral also could offer an alternative to people who cannot or do not respond to a vaccine.
“Many elderly people as well as individuals who are immunodeficient for various reasons do not respond to vaccines and are in the need of other protective measures,” said Kalle Saksela, MD, PhD, senior author of the study and a virologist at the University of Helsinki.
Multiple doses needed?
TriSb92 is “one of multiple nasal spray approaches but unlikely to be as durable as effective nasal vaccines,” said Eric Topol, MD, a professor of molecular medicine and executive vice president of Scripps Research in La Jolla, Calif. Dr. Topol is also editor-in-chief of Medscape, WebMD’s sister site for medical professionals.
“The sprays generally require multiple doses per day, whereas a single dose of a nasal vaccine may protect for months,” he said.
“Both have the allure of being variant-proof,” Dr. Topol added.
Thinking small
Many laboratories are shifting from treatments using monoclonal antibodies to treatments using smaller antibody fragments called “nanobodies” because they are more cost-effective and are able to last longer in storage, Dr. Mäkelä and colleagues noted.
Several of these nanobodies have shown promise against viruses in cell culture or animal models, including as an intranasal preventive treatment for SARS-CoV-2.
One of these smaller antibodies is being developed from llamas for example; another comes from experiments with yeast to develop synthetic nanobodies; and in a third case, researchers isolated nanobodies from llamas and from mice and showed they could neutralize the SARS-CoV-2 virus.
These nanobodies and TriSb92 target a specific part of the coronavirus spike protein called the receptor-binding domain (RBD). The RBD is where the coronavirus attaches to cells in the body. These agents essentially trick the virus by changing the structure of the outside of cells, so they look like a virus has already fused to them. This way, the virus moves on.
Key findings
The researchers compared mice treated with TriSb92 before and after exposure to SARS-CoV-2. When given in advance, none of the treated mice had SARS-CoV-2 RNA in their lungs, while untreated mice in the comparison group had “abundant” levels.
Other evidence of viral infection showed similar differences between treated and untreated mice in the protective lining of cells called the epithelium inside the nose, nasal mucosa, and airways.
Similarly, when given 2 or 4 hours after SARS-CoV-2 had already infected the epithelium, TriSb92 was linked to a complete lack of the virus’s RNA in the lungs.
It was more effective against the virus, though, when given before infection rather than after, “perhaps due to the initial establishment of the infection,” the researchers note.
The company led by Dr. Mäkelä is now working to secure funding for clinical trials of TriSb92 in humans.
A version of this article first appeared on WebMD.com.
if used within 4 hours after infection inside the nose, new research reveals.
Known as TriSb92 (brand name Covidin, from drugmaker Pandemblock Oy in Finland), the viral inhibitor also appears effective against all coronavirus variants of concern, neutralizing even the Omicron variants BA.5, XBB, and BQ.1.1 in laboratory and mice studies.
Unlike a COVID vaccine that boosts a person’s immune system as protection, the antiviral nasal spray works more directly by blocking the virus, acting as a “biological mask in the nasal cavity,” according to the biotechnology company set up to develop the treatment.
The product targets a stable site on the spike protein of the virus that is not known to mutate. This same site is shared among many variants of the COVID virus, so it could be effective against future variants as well, researchers note.
“In animal models, by directly inactivating the virus, TriSb92 offers immediate and robust protection” against coronavirus infection and severe COVID, said Anna R. Mäkelä, PhD, lead author of the study and a senior scientist in the department of virology at the University of Helsinki.
The study was published online in Nature Communications.
A potential first line of defense
Even in cases where the antiviral does not prevent coronavirus infection, the treatment could slow infection. This could happen by limiting how much virus could replicate early in the skin inside the nose and nasopharynx (the upper part of the throat), said Dr. Mäkelä, who is also CEO of Pandemblock Oy, the company set up to develop the product.
“TriSb92 could effectively tip the balance in favor of the [the person] and thereby help to reduce the risk of severe COVID-19 disease,” she said.
The antiviral also could offer an alternative to people who cannot or do not respond to a vaccine.
“Many elderly people as well as individuals who are immunodeficient for various reasons do not respond to vaccines and are in the need of other protective measures,” said Kalle Saksela, MD, PhD, senior author of the study and a virologist at the University of Helsinki.
Multiple doses needed?
TriSb92 is “one of multiple nasal spray approaches but unlikely to be as durable as effective nasal vaccines,” said Eric Topol, MD, a professor of molecular medicine and executive vice president of Scripps Research in La Jolla, Calif. Dr. Topol is also editor-in-chief of Medscape, WebMD’s sister site for medical professionals.
“The sprays generally require multiple doses per day, whereas a single dose of a nasal vaccine may protect for months,” he said.
“Both have the allure of being variant-proof,” Dr. Topol added.
Thinking small
Many laboratories are shifting from treatments using monoclonal antibodies to treatments using smaller antibody fragments called “nanobodies” because they are more cost-effective and are able to last longer in storage, Dr. Mäkelä and colleagues noted.
Several of these nanobodies have shown promise against viruses in cell culture or animal models, including as an intranasal preventive treatment for SARS-CoV-2.
One of these smaller antibodies is being developed from llamas for example; another comes from experiments with yeast to develop synthetic nanobodies; and in a third case, researchers isolated nanobodies from llamas and from mice and showed they could neutralize the SARS-CoV-2 virus.
These nanobodies and TriSb92 target a specific part of the coronavirus spike protein called the receptor-binding domain (RBD). The RBD is where the coronavirus attaches to cells in the body. These agents essentially trick the virus by changing the structure of the outside of cells, so they look like a virus has already fused to them. This way, the virus moves on.
Key findings
The researchers compared mice treated with TriSb92 before and after exposure to SARS-CoV-2. When given in advance, none of the treated mice had SARS-CoV-2 RNA in their lungs, while untreated mice in the comparison group had “abundant” levels.
Other evidence of viral infection showed similar differences between treated and untreated mice in the protective lining of cells called the epithelium inside the nose, nasal mucosa, and airways.
Similarly, when given 2 or 4 hours after SARS-CoV-2 had already infected the epithelium, TriSb92 was linked to a complete lack of the virus’s RNA in the lungs.
It was more effective against the virus, though, when given before infection rather than after, “perhaps due to the initial establishment of the infection,” the researchers note.
The company led by Dr. Mäkelä is now working to secure funding for clinical trials of TriSb92 in humans.
A version of this article first appeared on WebMD.com.
if used within 4 hours after infection inside the nose, new research reveals.
Known as TriSb92 (brand name Covidin, from drugmaker Pandemblock Oy in Finland), the viral inhibitor also appears effective against all coronavirus variants of concern, neutralizing even the Omicron variants BA.5, XBB, and BQ.1.1 in laboratory and mice studies.
Unlike a COVID vaccine that boosts a person’s immune system as protection, the antiviral nasal spray works more directly by blocking the virus, acting as a “biological mask in the nasal cavity,” according to the biotechnology company set up to develop the treatment.
The product targets a stable site on the spike protein of the virus that is not known to mutate. This same site is shared among many variants of the COVID virus, so it could be effective against future variants as well, researchers note.
“In animal models, by directly inactivating the virus, TriSb92 offers immediate and robust protection” against coronavirus infection and severe COVID, said Anna R. Mäkelä, PhD, lead author of the study and a senior scientist in the department of virology at the University of Helsinki.
The study was published online in Nature Communications.
A potential first line of defense
Even in cases where the antiviral does not prevent coronavirus infection, the treatment could slow infection. This could happen by limiting how much virus could replicate early in the skin inside the nose and nasopharynx (the upper part of the throat), said Dr. Mäkelä, who is also CEO of Pandemblock Oy, the company set up to develop the product.
“TriSb92 could effectively tip the balance in favor of the [the person] and thereby help to reduce the risk of severe COVID-19 disease,” she said.
The antiviral also could offer an alternative to people who cannot or do not respond to a vaccine.
“Many elderly people as well as individuals who are immunodeficient for various reasons do not respond to vaccines and are in the need of other protective measures,” said Kalle Saksela, MD, PhD, senior author of the study and a virologist at the University of Helsinki.
Multiple doses needed?
TriSb92 is “one of multiple nasal spray approaches but unlikely to be as durable as effective nasal vaccines,” said Eric Topol, MD, a professor of molecular medicine and executive vice president of Scripps Research in La Jolla, Calif. Dr. Topol is also editor-in-chief of Medscape, WebMD’s sister site for medical professionals.
“The sprays generally require multiple doses per day, whereas a single dose of a nasal vaccine may protect for months,” he said.
“Both have the allure of being variant-proof,” Dr. Topol added.
Thinking small
Many laboratories are shifting from treatments using monoclonal antibodies to treatments using smaller antibody fragments called “nanobodies” because they are more cost-effective and are able to last longer in storage, Dr. Mäkelä and colleagues noted.
Several of these nanobodies have shown promise against viruses in cell culture or animal models, including as an intranasal preventive treatment for SARS-CoV-2.
One of these smaller antibodies is being developed from llamas for example; another comes from experiments with yeast to develop synthetic nanobodies; and in a third case, researchers isolated nanobodies from llamas and from mice and showed they could neutralize the SARS-CoV-2 virus.
These nanobodies and TriSb92 target a specific part of the coronavirus spike protein called the receptor-binding domain (RBD). The RBD is where the coronavirus attaches to cells in the body. These agents essentially trick the virus by changing the structure of the outside of cells, so they look like a virus has already fused to them. This way, the virus moves on.
Key findings
The researchers compared mice treated with TriSb92 before and after exposure to SARS-CoV-2. When given in advance, none of the treated mice had SARS-CoV-2 RNA in their lungs, while untreated mice in the comparison group had “abundant” levels.
Other evidence of viral infection showed similar differences between treated and untreated mice in the protective lining of cells called the epithelium inside the nose, nasal mucosa, and airways.
Similarly, when given 2 or 4 hours after SARS-CoV-2 had already infected the epithelium, TriSb92 was linked to a complete lack of the virus’s RNA in the lungs.
It was more effective against the virus, though, when given before infection rather than after, “perhaps due to the initial establishment of the infection,” the researchers note.
The company led by Dr. Mäkelä is now working to secure funding for clinical trials of TriSb92 in humans.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
Upadacitinib shows positive endoscopic outcomes in Crohn’s disease at 1 year
The findings of this subanalysis come from two phase 3 induction trials (U-EXCEL and U-EXCEED) and one maintenance study (U-ENDURE) of upadacitinib in this patient population.
“Upadacitinib shows large differences relative to placebo in endoscopic response and remission ... in a difficult-to-treat population of patients, the majority of whom had failed an advanced therapy,” lead investigator Brian Feagan, MD, senior scientific director of the GI contract research firm Alimentiv in London, Ontario, said in an interview.
“The absolute magnitude of the finding was unanticipated – a greater treatment effect than might be anticipated for these outcomes compared with other advanced treatments for Crohn’s disease in these higher-risk patients,” he said.
Dr. Feagan presented the research at the annual congress of the European Crohn’s and Colitis Organisation.
Research methodology
At baseline, participants had an average daily stool frequency of 4 or more and/or an abdominal pain score of 2 or greater. They also had a Simple Endoscopic Score for Crohn’s disease of 6 or more, excluding a narrowing component, or a score of 4 or more for isolated ileal Crohn’s disease.
In the treatment induction phase, patients were randomly assigned 2:1, with 674 people receiving 45 mg upadacitinib and 347 taking a placebo once daily for 12 weeks.
Participants who experienced at least a 30% decrease in stool frequency and/or daily abdominal pain scores were enrolled in the maintenance phase of the study. For this phase, patients were randomly assigned again, with 168 receiving 30 mg upadacitinib, 169 receiving 15 mg upadacitinib, and 165 taking a placebo once daily for 52 weeks.
In each induction and maintenance cohort, more than 70% of patients had failed one prior biologic therapy, with failure defined as inadequate response or intolerance. Among those who failed a previous biologic in induction, 96% had also failed prior treatment with an anti–tumor necrosis factor (anti-TNF) inhibitor.
Participants’ mean age was 38-40 years, and 52%-55% were men. Patients who had not failed previous therapy had Crohn’s disease for a median of 6-7 years. In contrast, the prior-failure group had Crohn’s disease for a median of 9-10 years.
Key outcomes
At 12 weeks, endoscopic response among patients who had not failed a prior biologic was 52% in the treatment group versus 16% of the placebo group. In the prior-failure group, endoscopic response was observed in 36% and 5%, respectively.
Endoscopic remission at 12 weeks among patients who had not failed a prior biologic was 36% in the treatment group versus 10% in the placebo group. In the prior-failure group, endoscopic remission was 20% in the treatment group versus 3% in those who took placebo.
Participants in the treatment groups of the 52-week maintenance phase of the study experienced higher endoscopic response and endoscopic remission rates compared with those who received placebo.
Endoscopic response in the group without prior biologic failure was 44% in the 30-mg upadacitinib group, 40% in the 15-mg group, and 18% in the placebo group. Among those with prior biologic failure, endoscopic response was seen in 39% of the 30-mg upadacitinib group, 23% of the 15-mg group, and 4% of the placebo group.
There is a “very striking difference in endoscopic response rates between the high dose and placebo,” Dr. Feagan said. “That difference here is in the response rate. You see dose separation.”
Endoscopic remission among those without prior biologic failure was observed in 34% of the 30-mg upadacitinib group, 27% of the 15-mg group, and 16% of the placebo group. Among those with prior biologic failure, endoscopic remission was seen in 27% of the 30-mg upadacitinib group, 16% of the 15-mg group, and 2% of the placebo group.
The results show “a clear advantage for the 30-mg dose versus the 15-mg in the maintenance component, especially in patients who had failed an advanced therapy,” Dr. Feagan said.
Safety signals
Upadacitinib was well tolerated in the induction and maintenance phases, and no new safety risks were observed compared with the known safety profile of the drug, the researchers noted.
For example, during the induction studies, the rate of any adverse event among patients without prior biologic failure was 60% in the 45-mg upadacitinib group and 53% in the placebo group. Among those who failed a prior biologic, the rates were 67% in the 45-mg upadacitinib group and 66% in the placebo group.
The adverse events were “issues that have already been identified with JAK inhibitors, the biochemical abnormalities with CPK [creatine phosphokinase] elevations and transaminase elevations,” Dr. Feagan said.
There were no cases of herpes zoster among patients who received placebo compared with five cases in the 45-mg upadacitinib group without prior biologic failure and 10 cases in the prior biologic failure group.
“The zoster signal is there even at induction with the 45-mg dose versus placebo,” Dr. Feagan said.
‘Encouraging’ results
The study indicates that upadacitinib is effective in improving endoscopic outcomes for patients with Crohn’s disease, regardless of their prior biologic treatments, Robin L. Dalal, MD, assistant professor of medicine at Vanderbilt University in Nashville, Tenn., said when asked to comment on the study.
“This is important because, as the treatment landscape for Crohn’s disease has expanded, sequencing of therapies has become more complex,” added Dr. Dalal, who was not involved in the research. “For upadacitinib in Crohn’s disease, prior biologic use may not be a factor in endoscopic response rates.”
The findings are “very encouraging for physicians and practitioners who treat IBD [inflammatory bowel disease] patients,” Maithili Chitnavis, MD, of the inflammatory bowel disease section at Atrium Health Gastroenterology in Charlotte, N.C., said when asked for comment.
“We clearly care about how patients feel overall, but endoscopic and histologic outcomes are important to investigate because we want to ensure there is internal healing to prevent a lot of the longstanding complications of Crohn’s disease, such as malignancy, strictures, fistulizing/penetrating disease, and need for surgery,” said Dr. Chitnavis, who was not involved with the study.
Upadacitinib is an oral agent, which distinguishes it from the injectable or infusion-based biologic therapies for Crohn’s disease, Dr. Chitnavis noted.
The finding that the medication works in patients with or without prior biologic failure is important, she said.
“With its anticipated ... approval for Crohn’s disease [by the Food and Drug Administration], it is expected that patients will have had to have demonstrated a lack of or loss of response to another biologic, specifically in the anti-TNF category (for example, infliximab, adalimumab, certolizumab) prior to starting upadacitinib due to concerns of potential side effects associated with the class of medications to which it belongs,” Dr. Chitnavis said. “Therefore, it makes it even more relevant to know how patients who have failed a prior biologic respond to this therapy.”
Dr. Feagan has reported serving as a consultant and speaker for AbbVie. Dr. Dalal has reported being a consultant for AbbVie in 2021. Dr. Chitnavis has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings of this subanalysis come from two phase 3 induction trials (U-EXCEL and U-EXCEED) and one maintenance study (U-ENDURE) of upadacitinib in this patient population.
“Upadacitinib shows large differences relative to placebo in endoscopic response and remission ... in a difficult-to-treat population of patients, the majority of whom had failed an advanced therapy,” lead investigator Brian Feagan, MD, senior scientific director of the GI contract research firm Alimentiv in London, Ontario, said in an interview.
“The absolute magnitude of the finding was unanticipated – a greater treatment effect than might be anticipated for these outcomes compared with other advanced treatments for Crohn’s disease in these higher-risk patients,” he said.
Dr. Feagan presented the research at the annual congress of the European Crohn’s and Colitis Organisation.
Research methodology
At baseline, participants had an average daily stool frequency of 4 or more and/or an abdominal pain score of 2 or greater. They also had a Simple Endoscopic Score for Crohn’s disease of 6 or more, excluding a narrowing component, or a score of 4 or more for isolated ileal Crohn’s disease.
In the treatment induction phase, patients were randomly assigned 2:1, with 674 people receiving 45 mg upadacitinib and 347 taking a placebo once daily for 12 weeks.
Participants who experienced at least a 30% decrease in stool frequency and/or daily abdominal pain scores were enrolled in the maintenance phase of the study. For this phase, patients were randomly assigned again, with 168 receiving 30 mg upadacitinib, 169 receiving 15 mg upadacitinib, and 165 taking a placebo once daily for 52 weeks.
In each induction and maintenance cohort, more than 70% of patients had failed one prior biologic therapy, with failure defined as inadequate response or intolerance. Among those who failed a previous biologic in induction, 96% had also failed prior treatment with an anti–tumor necrosis factor (anti-TNF) inhibitor.
Participants’ mean age was 38-40 years, and 52%-55% were men. Patients who had not failed previous therapy had Crohn’s disease for a median of 6-7 years. In contrast, the prior-failure group had Crohn’s disease for a median of 9-10 years.
Key outcomes
At 12 weeks, endoscopic response among patients who had not failed a prior biologic was 52% in the treatment group versus 16% of the placebo group. In the prior-failure group, endoscopic response was observed in 36% and 5%, respectively.
Endoscopic remission at 12 weeks among patients who had not failed a prior biologic was 36% in the treatment group versus 10% in the placebo group. In the prior-failure group, endoscopic remission was 20% in the treatment group versus 3% in those who took placebo.
Participants in the treatment groups of the 52-week maintenance phase of the study experienced higher endoscopic response and endoscopic remission rates compared with those who received placebo.
Endoscopic response in the group without prior biologic failure was 44% in the 30-mg upadacitinib group, 40% in the 15-mg group, and 18% in the placebo group. Among those with prior biologic failure, endoscopic response was seen in 39% of the 30-mg upadacitinib group, 23% of the 15-mg group, and 4% of the placebo group.
There is a “very striking difference in endoscopic response rates between the high dose and placebo,” Dr. Feagan said. “That difference here is in the response rate. You see dose separation.”
Endoscopic remission among those without prior biologic failure was observed in 34% of the 30-mg upadacitinib group, 27% of the 15-mg group, and 16% of the placebo group. Among those with prior biologic failure, endoscopic remission was seen in 27% of the 30-mg upadacitinib group, 16% of the 15-mg group, and 2% of the placebo group.
The results show “a clear advantage for the 30-mg dose versus the 15-mg in the maintenance component, especially in patients who had failed an advanced therapy,” Dr. Feagan said.
Safety signals
Upadacitinib was well tolerated in the induction and maintenance phases, and no new safety risks were observed compared with the known safety profile of the drug, the researchers noted.
For example, during the induction studies, the rate of any adverse event among patients without prior biologic failure was 60% in the 45-mg upadacitinib group and 53% in the placebo group. Among those who failed a prior biologic, the rates were 67% in the 45-mg upadacitinib group and 66% in the placebo group.
The adverse events were “issues that have already been identified with JAK inhibitors, the biochemical abnormalities with CPK [creatine phosphokinase] elevations and transaminase elevations,” Dr. Feagan said.
There were no cases of herpes zoster among patients who received placebo compared with five cases in the 45-mg upadacitinib group without prior biologic failure and 10 cases in the prior biologic failure group.
“The zoster signal is there even at induction with the 45-mg dose versus placebo,” Dr. Feagan said.
‘Encouraging’ results
The study indicates that upadacitinib is effective in improving endoscopic outcomes for patients with Crohn’s disease, regardless of their prior biologic treatments, Robin L. Dalal, MD, assistant professor of medicine at Vanderbilt University in Nashville, Tenn., said when asked to comment on the study.
“This is important because, as the treatment landscape for Crohn’s disease has expanded, sequencing of therapies has become more complex,” added Dr. Dalal, who was not involved in the research. “For upadacitinib in Crohn’s disease, prior biologic use may not be a factor in endoscopic response rates.”
The findings are “very encouraging for physicians and practitioners who treat IBD [inflammatory bowel disease] patients,” Maithili Chitnavis, MD, of the inflammatory bowel disease section at Atrium Health Gastroenterology in Charlotte, N.C., said when asked for comment.
“We clearly care about how patients feel overall, but endoscopic and histologic outcomes are important to investigate because we want to ensure there is internal healing to prevent a lot of the longstanding complications of Crohn’s disease, such as malignancy, strictures, fistulizing/penetrating disease, and need for surgery,” said Dr. Chitnavis, who was not involved with the study.
Upadacitinib is an oral agent, which distinguishes it from the injectable or infusion-based biologic therapies for Crohn’s disease, Dr. Chitnavis noted.
The finding that the medication works in patients with or without prior biologic failure is important, she said.
“With its anticipated ... approval for Crohn’s disease [by the Food and Drug Administration], it is expected that patients will have had to have demonstrated a lack of or loss of response to another biologic, specifically in the anti-TNF category (for example, infliximab, adalimumab, certolizumab) prior to starting upadacitinib due to concerns of potential side effects associated with the class of medications to which it belongs,” Dr. Chitnavis said. “Therefore, it makes it even more relevant to know how patients who have failed a prior biologic respond to this therapy.”
Dr. Feagan has reported serving as a consultant and speaker for AbbVie. Dr. Dalal has reported being a consultant for AbbVie in 2021. Dr. Chitnavis has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings of this subanalysis come from two phase 3 induction trials (U-EXCEL and U-EXCEED) and one maintenance study (U-ENDURE) of upadacitinib in this patient population.
“Upadacitinib shows large differences relative to placebo in endoscopic response and remission ... in a difficult-to-treat population of patients, the majority of whom had failed an advanced therapy,” lead investigator Brian Feagan, MD, senior scientific director of the GI contract research firm Alimentiv in London, Ontario, said in an interview.
“The absolute magnitude of the finding was unanticipated – a greater treatment effect than might be anticipated for these outcomes compared with other advanced treatments for Crohn’s disease in these higher-risk patients,” he said.
Dr. Feagan presented the research at the annual congress of the European Crohn’s and Colitis Organisation.
Research methodology
At baseline, participants had an average daily stool frequency of 4 or more and/or an abdominal pain score of 2 or greater. They also had a Simple Endoscopic Score for Crohn’s disease of 6 or more, excluding a narrowing component, or a score of 4 or more for isolated ileal Crohn’s disease.
In the treatment induction phase, patients were randomly assigned 2:1, with 674 people receiving 45 mg upadacitinib and 347 taking a placebo once daily for 12 weeks.
Participants who experienced at least a 30% decrease in stool frequency and/or daily abdominal pain scores were enrolled in the maintenance phase of the study. For this phase, patients were randomly assigned again, with 168 receiving 30 mg upadacitinib, 169 receiving 15 mg upadacitinib, and 165 taking a placebo once daily for 52 weeks.
In each induction and maintenance cohort, more than 70% of patients had failed one prior biologic therapy, with failure defined as inadequate response or intolerance. Among those who failed a previous biologic in induction, 96% had also failed prior treatment with an anti–tumor necrosis factor (anti-TNF) inhibitor.
Participants’ mean age was 38-40 years, and 52%-55% were men. Patients who had not failed previous therapy had Crohn’s disease for a median of 6-7 years. In contrast, the prior-failure group had Crohn’s disease for a median of 9-10 years.
Key outcomes
At 12 weeks, endoscopic response among patients who had not failed a prior biologic was 52% in the treatment group versus 16% of the placebo group. In the prior-failure group, endoscopic response was observed in 36% and 5%, respectively.
Endoscopic remission at 12 weeks among patients who had not failed a prior biologic was 36% in the treatment group versus 10% in the placebo group. In the prior-failure group, endoscopic remission was 20% in the treatment group versus 3% in those who took placebo.
Participants in the treatment groups of the 52-week maintenance phase of the study experienced higher endoscopic response and endoscopic remission rates compared with those who received placebo.
Endoscopic response in the group without prior biologic failure was 44% in the 30-mg upadacitinib group, 40% in the 15-mg group, and 18% in the placebo group. Among those with prior biologic failure, endoscopic response was seen in 39% of the 30-mg upadacitinib group, 23% of the 15-mg group, and 4% of the placebo group.
There is a “very striking difference in endoscopic response rates between the high dose and placebo,” Dr. Feagan said. “That difference here is in the response rate. You see dose separation.”
Endoscopic remission among those without prior biologic failure was observed in 34% of the 30-mg upadacitinib group, 27% of the 15-mg group, and 16% of the placebo group. Among those with prior biologic failure, endoscopic remission was seen in 27% of the 30-mg upadacitinib group, 16% of the 15-mg group, and 2% of the placebo group.
The results show “a clear advantage for the 30-mg dose versus the 15-mg in the maintenance component, especially in patients who had failed an advanced therapy,” Dr. Feagan said.
Safety signals
Upadacitinib was well tolerated in the induction and maintenance phases, and no new safety risks were observed compared with the known safety profile of the drug, the researchers noted.
For example, during the induction studies, the rate of any adverse event among patients without prior biologic failure was 60% in the 45-mg upadacitinib group and 53% in the placebo group. Among those who failed a prior biologic, the rates were 67% in the 45-mg upadacitinib group and 66% in the placebo group.
The adverse events were “issues that have already been identified with JAK inhibitors, the biochemical abnormalities with CPK [creatine phosphokinase] elevations and transaminase elevations,” Dr. Feagan said.
There were no cases of herpes zoster among patients who received placebo compared with five cases in the 45-mg upadacitinib group without prior biologic failure and 10 cases in the prior biologic failure group.
“The zoster signal is there even at induction with the 45-mg dose versus placebo,” Dr. Feagan said.
‘Encouraging’ results
The study indicates that upadacitinib is effective in improving endoscopic outcomes for patients with Crohn’s disease, regardless of their prior biologic treatments, Robin L. Dalal, MD, assistant professor of medicine at Vanderbilt University in Nashville, Tenn., said when asked to comment on the study.
“This is important because, as the treatment landscape for Crohn’s disease has expanded, sequencing of therapies has become more complex,” added Dr. Dalal, who was not involved in the research. “For upadacitinib in Crohn’s disease, prior biologic use may not be a factor in endoscopic response rates.”
The findings are “very encouraging for physicians and practitioners who treat IBD [inflammatory bowel disease] patients,” Maithili Chitnavis, MD, of the inflammatory bowel disease section at Atrium Health Gastroenterology in Charlotte, N.C., said when asked for comment.
“We clearly care about how patients feel overall, but endoscopic and histologic outcomes are important to investigate because we want to ensure there is internal healing to prevent a lot of the longstanding complications of Crohn’s disease, such as malignancy, strictures, fistulizing/penetrating disease, and need for surgery,” said Dr. Chitnavis, who was not involved with the study.
Upadacitinib is an oral agent, which distinguishes it from the injectable or infusion-based biologic therapies for Crohn’s disease, Dr. Chitnavis noted.
The finding that the medication works in patients with or without prior biologic failure is important, she said.
“With its anticipated ... approval for Crohn’s disease [by the Food and Drug Administration], it is expected that patients will have had to have demonstrated a lack of or loss of response to another biologic, specifically in the anti-TNF category (for example, infliximab, adalimumab, certolizumab) prior to starting upadacitinib due to concerns of potential side effects associated with the class of medications to which it belongs,” Dr. Chitnavis said. “Therefore, it makes it even more relevant to know how patients who have failed a prior biologic respond to this therapy.”
Dr. Feagan has reported serving as a consultant and speaker for AbbVie. Dr. Dalal has reported being a consultant for AbbVie in 2021. Dr. Chitnavis has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ECCO 2023
Cutting calories could slow the pace of aging: Study
That may seem like a little benefit for a large cut in calories. But experts say it’s actually a pretty big deal.
“In other studies, that same difference in pace of aging had meaningful consequences for people’s risk of dying,” says senior study author Daniel W. Belsky, PhD, a researcher at the Butler Columbia Aging Center at Columbia University, New York.
Cutting calories by 25% slowed the pace of aging in young and middle-aged adults by a few percentage points, compared with those who continued eating normally, the new research reveals. This first-of-its-kind study in humans adds to evidence from animal studies that the rate of aging can be changed.
Compared with 75 people who ate normally, the 145 people randomly assigned to cut back their calories slowed their pace of aging by 2%-3% over 2 years in the randomized controlled trial.
For example, a similar slowdown in the pace of aging was associated with a 10%-15% lower risk of dying over 10-15 years in previous work, Dr. Belsky says. “So 2%-3% slower aging doesn’t sound like maybe that big of a deal – but 10%-15% reduction in risk of dying seems like a big deal.”
Results of the study were published in the journal Nature Aging.
Even though the pace of aging slowed, the researchers did not find significant changes on two biological aging measures in the study, suggesting more work is needed.
The findings “are intriguing in that caloric restriction seemed to show a slower pace of aging in healthy adults,” says Vandana Sheth, a registered dietitian-nutritionist and owner of a nutrition consulting firm in Los Angeles. “This can have a significant impact on population health. However, larger studies need to be done to follow up on these findings.”
‘Exciting result’
Asked if the findings imply aging could be slowed down in people, Dr. Belsky says. “That is the ... exciting result from the trial. These results suggest it may be possible to slow the pace of biological aging with a behavioral intervention.”
But not everyone is completely convinced.
“This is good suggestive evidence that caloric restriction can modify aspects of biological aging in humans, similar to what has been known in laboratory animals for many decades,” says Matt Kaeberlein, PhD, director of the Healthy Aging and Longevity Research Institute at the University of Washington, Seattle, and senior author of a 2021 review article in Science.
Part of his concern is that cutting your calories by a quarter may not be a sustainable long-term strategy.
“It’s important to keep in mind that these measurements only report on a portion of biological aging and are probably not an accurate overall measurement of biological age or the rate of biological aging,” Dr. Kaeberlein says. The findings might suggest that “at the population level, a 25% reduction in caloric intake is unlikely to have large effects on biological aging unless implemented over many years, which is likely not reasonable for most people.”
Insight into intermittent fasting?
Cutting back on calories is related to other dietary strategies, including intermittent fasting and time-restricted eating, Dr. Belsky says. “Intermittent fasting and time-restricted eating are nutritional interventions that have been developed, in part, because in experiments with animals, they have some of the same biological effects as calorie restriction.”
There remain many unanswered questions.
“There are people who would argue that the reason calorie restriction does what it does is because when people are calorie-restricted, they also tend to restrict the times when they eat,” Dr. Belsky says. “They tend to have these longer fasts during the day.”
A version of this article first appeared on WebMD.com.
That may seem like a little benefit for a large cut in calories. But experts say it’s actually a pretty big deal.
“In other studies, that same difference in pace of aging had meaningful consequences for people’s risk of dying,” says senior study author Daniel W. Belsky, PhD, a researcher at the Butler Columbia Aging Center at Columbia University, New York.
Cutting calories by 25% slowed the pace of aging in young and middle-aged adults by a few percentage points, compared with those who continued eating normally, the new research reveals. This first-of-its-kind study in humans adds to evidence from animal studies that the rate of aging can be changed.
Compared with 75 people who ate normally, the 145 people randomly assigned to cut back their calories slowed their pace of aging by 2%-3% over 2 years in the randomized controlled trial.
For example, a similar slowdown in the pace of aging was associated with a 10%-15% lower risk of dying over 10-15 years in previous work, Dr. Belsky says. “So 2%-3% slower aging doesn’t sound like maybe that big of a deal – but 10%-15% reduction in risk of dying seems like a big deal.”
Results of the study were published in the journal Nature Aging.
Even though the pace of aging slowed, the researchers did not find significant changes on two biological aging measures in the study, suggesting more work is needed.
The findings “are intriguing in that caloric restriction seemed to show a slower pace of aging in healthy adults,” says Vandana Sheth, a registered dietitian-nutritionist and owner of a nutrition consulting firm in Los Angeles. “This can have a significant impact on population health. However, larger studies need to be done to follow up on these findings.”
‘Exciting result’
Asked if the findings imply aging could be slowed down in people, Dr. Belsky says. “That is the ... exciting result from the trial. These results suggest it may be possible to slow the pace of biological aging with a behavioral intervention.”
But not everyone is completely convinced.
“This is good suggestive evidence that caloric restriction can modify aspects of biological aging in humans, similar to what has been known in laboratory animals for many decades,” says Matt Kaeberlein, PhD, director of the Healthy Aging and Longevity Research Institute at the University of Washington, Seattle, and senior author of a 2021 review article in Science.
Part of his concern is that cutting your calories by a quarter may not be a sustainable long-term strategy.
“It’s important to keep in mind that these measurements only report on a portion of biological aging and are probably not an accurate overall measurement of biological age or the rate of biological aging,” Dr. Kaeberlein says. The findings might suggest that “at the population level, a 25% reduction in caloric intake is unlikely to have large effects on biological aging unless implemented over many years, which is likely not reasonable for most people.”
Insight into intermittent fasting?
Cutting back on calories is related to other dietary strategies, including intermittent fasting and time-restricted eating, Dr. Belsky says. “Intermittent fasting and time-restricted eating are nutritional interventions that have been developed, in part, because in experiments with animals, they have some of the same biological effects as calorie restriction.”
There remain many unanswered questions.
“There are people who would argue that the reason calorie restriction does what it does is because when people are calorie-restricted, they also tend to restrict the times when they eat,” Dr. Belsky says. “They tend to have these longer fasts during the day.”
A version of this article first appeared on WebMD.com.
That may seem like a little benefit for a large cut in calories. But experts say it’s actually a pretty big deal.
“In other studies, that same difference in pace of aging had meaningful consequences for people’s risk of dying,” says senior study author Daniel W. Belsky, PhD, a researcher at the Butler Columbia Aging Center at Columbia University, New York.
Cutting calories by 25% slowed the pace of aging in young and middle-aged adults by a few percentage points, compared with those who continued eating normally, the new research reveals. This first-of-its-kind study in humans adds to evidence from animal studies that the rate of aging can be changed.
Compared with 75 people who ate normally, the 145 people randomly assigned to cut back their calories slowed their pace of aging by 2%-3% over 2 years in the randomized controlled trial.
For example, a similar slowdown in the pace of aging was associated with a 10%-15% lower risk of dying over 10-15 years in previous work, Dr. Belsky says. “So 2%-3% slower aging doesn’t sound like maybe that big of a deal – but 10%-15% reduction in risk of dying seems like a big deal.”
Results of the study were published in the journal Nature Aging.
Even though the pace of aging slowed, the researchers did not find significant changes on two biological aging measures in the study, suggesting more work is needed.
The findings “are intriguing in that caloric restriction seemed to show a slower pace of aging in healthy adults,” says Vandana Sheth, a registered dietitian-nutritionist and owner of a nutrition consulting firm in Los Angeles. “This can have a significant impact on population health. However, larger studies need to be done to follow up on these findings.”
‘Exciting result’
Asked if the findings imply aging could be slowed down in people, Dr. Belsky says. “That is the ... exciting result from the trial. These results suggest it may be possible to slow the pace of biological aging with a behavioral intervention.”
But not everyone is completely convinced.
“This is good suggestive evidence that caloric restriction can modify aspects of biological aging in humans, similar to what has been known in laboratory animals for many decades,” says Matt Kaeberlein, PhD, director of the Healthy Aging and Longevity Research Institute at the University of Washington, Seattle, and senior author of a 2021 review article in Science.
Part of his concern is that cutting your calories by a quarter may not be a sustainable long-term strategy.
“It’s important to keep in mind that these measurements only report on a portion of biological aging and are probably not an accurate overall measurement of biological age or the rate of biological aging,” Dr. Kaeberlein says. The findings might suggest that “at the population level, a 25% reduction in caloric intake is unlikely to have large effects on biological aging unless implemented over many years, which is likely not reasonable for most people.”
Insight into intermittent fasting?
Cutting back on calories is related to other dietary strategies, including intermittent fasting and time-restricted eating, Dr. Belsky says. “Intermittent fasting and time-restricted eating are nutritional interventions that have been developed, in part, because in experiments with animals, they have some of the same biological effects as calorie restriction.”
There remain many unanswered questions.
“There are people who would argue that the reason calorie restriction does what it does is because when people are calorie-restricted, they also tend to restrict the times when they eat,” Dr. Belsky says. “They tend to have these longer fasts during the day.”
A version of this article first appeared on WebMD.com.
FROM NATURE AGING
IVF-conceived children show strong developmental performance
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
FROM PLOS MEDICINE
More work needed to optimize STI screening in primary care settings
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People living with HIV are a model population for vaccination
TAMPA – People living with HIV (PLWH) were more likely than other populations to get vaccinated for flu and COVID-19, to seek reputable sources of information, and to be connected through essential community organizations that share essential health and wellness information, according to the results of a large survey.
PLWH, therefore, would have been an ideal model population for creating and disseminating effective messaging around COVID-19 immunizations earlier in the pandemic, said Kathleen Gallagher, MPH, an epidemiologist, researcher, and health services administrator at the Patient Advocate Foundation.
The PLWH community can still offer valuable insights into effective ways to reach out to people, to disseminate correct information, and to link people with resources, Ms. Gallagher said during a poster presentation at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Local, community-based organizations “are the people that these individuals trust, they are people entrenched in their community, and they have existing relationships with them in terms of getting vaccinated and listening to their concerns,” Ms. Gallagher said.
“It’s a missed opportunity.”
A highly compliant group
The July 2021 survey of 271 PLWH was part of a larger, longitudinal survey of 1,400 people with any chronic illness asked about attitudes and barriers to vaccination. The PLWH population was important to focus on, the researchers note, because they could be potentially high risk for more serious COVID-19 outcomes.
The PLWH group was 81% White and 90% male, and 83% were age 56 or older. In addition, 86% had an annual household income below $48,000.
Ninety-three percent of the PLWH group had had flu vaccination in the prior 3 years and received at least one COVID-19 vaccination.
Unable vs. unwilling to vaccinate
Ms. Gallagher and colleagues found 12 people (4%) in the PLWH group did not get vaccinated against COVID-19. It’s a small number, “so you have to take this with a grain of salt,” she said. “But we asked them why they were hesitant. They either were unable or unwilling – and the unable part is not surprising.”
Those who were unable to get vaccinated were either homebound or had concerns about being in a clinic where they could be exposed to COVID while waiting to get the vaccine.
“And then there were some who were just not willing” to get vaccinated, Ms. Gallagher said. She added most cited vaccine safety concerns and “a lot of the misinformation or confusing information around efficacy.”
Trusted information sources
Although people reported getting COVID-19 vaccine information from multiple sources, including online and from television, 64% or nearly two-thirds sought information from their doctors or health care teams.
In fact, doctors emerged as the most trusted source, as indicated by 72% of PLWH.
“I was a little surprised that doctors scored so highly because, sometimes in other cohorts that we looked at, it wasn’t the case,” Ms. Gallagher said. However, she added, a lot of PLWH “have a very strong trust bond with their provider because this is a very personal, very sensitive diagnosis.”
How did social media score? “A whopping 1%,” she said. “So at least this was a savvy group, and they realized that that was not the place to go for vaccination information.”
Overcoming barriers
A lack of vaccine availability at the time of their appointment was the number one barrier to immunization. Also, a small number of people said knowing someone who had an adverse reaction to COVID-19 vaccination was a barrier for them. Ms. Gallagher explained that, by definition in the survey, an adverse reaction to vaccination had to be serious enough to drive people to seek medical care.
When asked to comment on the poster, Andrew Komensky, RN, told this news organization that he found the results “interesting, because I’m an infection preventionist, in addition to being an HIV nurse.” He is director of infection prevention and control at CharterCARE Health Partners, Providence, R.I.
Mr. Komensky said he was surprised that a high proportion of PLWH cited their doctor – and not their nurse – as the most trusted source of information. “In my experience in COVID care ... it was a nursing staff who had most of the contact with patients, who did most of the education, and provided most of the information surrounding vaccination and potential side effects.”
It made sense to Mr. Komensky that the PLWH population would be compliant with vaccinations. “People who are living with HIV do everything they possibly can just to stay healthy.”
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV (PLWH) were more likely than other populations to get vaccinated for flu and COVID-19, to seek reputable sources of information, and to be connected through essential community organizations that share essential health and wellness information, according to the results of a large survey.
PLWH, therefore, would have been an ideal model population for creating and disseminating effective messaging around COVID-19 immunizations earlier in the pandemic, said Kathleen Gallagher, MPH, an epidemiologist, researcher, and health services administrator at the Patient Advocate Foundation.
The PLWH community can still offer valuable insights into effective ways to reach out to people, to disseminate correct information, and to link people with resources, Ms. Gallagher said during a poster presentation at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Local, community-based organizations “are the people that these individuals trust, they are people entrenched in their community, and they have existing relationships with them in terms of getting vaccinated and listening to their concerns,” Ms. Gallagher said.
“It’s a missed opportunity.”
A highly compliant group
The July 2021 survey of 271 PLWH was part of a larger, longitudinal survey of 1,400 people with any chronic illness asked about attitudes and barriers to vaccination. The PLWH population was important to focus on, the researchers note, because they could be potentially high risk for more serious COVID-19 outcomes.
The PLWH group was 81% White and 90% male, and 83% were age 56 or older. In addition, 86% had an annual household income below $48,000.
Ninety-three percent of the PLWH group had had flu vaccination in the prior 3 years and received at least one COVID-19 vaccination.
Unable vs. unwilling to vaccinate
Ms. Gallagher and colleagues found 12 people (4%) in the PLWH group did not get vaccinated against COVID-19. It’s a small number, “so you have to take this with a grain of salt,” she said. “But we asked them why they were hesitant. They either were unable or unwilling – and the unable part is not surprising.”
Those who were unable to get vaccinated were either homebound or had concerns about being in a clinic where they could be exposed to COVID while waiting to get the vaccine.
“And then there were some who were just not willing” to get vaccinated, Ms. Gallagher said. She added most cited vaccine safety concerns and “a lot of the misinformation or confusing information around efficacy.”
Trusted information sources
Although people reported getting COVID-19 vaccine information from multiple sources, including online and from television, 64% or nearly two-thirds sought information from their doctors or health care teams.
In fact, doctors emerged as the most trusted source, as indicated by 72% of PLWH.
“I was a little surprised that doctors scored so highly because, sometimes in other cohorts that we looked at, it wasn’t the case,” Ms. Gallagher said. However, she added, a lot of PLWH “have a very strong trust bond with their provider because this is a very personal, very sensitive diagnosis.”
How did social media score? “A whopping 1%,” she said. “So at least this was a savvy group, and they realized that that was not the place to go for vaccination information.”
Overcoming barriers
A lack of vaccine availability at the time of their appointment was the number one barrier to immunization. Also, a small number of people said knowing someone who had an adverse reaction to COVID-19 vaccination was a barrier for them. Ms. Gallagher explained that, by definition in the survey, an adverse reaction to vaccination had to be serious enough to drive people to seek medical care.
When asked to comment on the poster, Andrew Komensky, RN, told this news organization that he found the results “interesting, because I’m an infection preventionist, in addition to being an HIV nurse.” He is director of infection prevention and control at CharterCARE Health Partners, Providence, R.I.
Mr. Komensky said he was surprised that a high proportion of PLWH cited their doctor – and not their nurse – as the most trusted source of information. “In my experience in COVID care ... it was a nursing staff who had most of the contact with patients, who did most of the education, and provided most of the information surrounding vaccination and potential side effects.”
It made sense to Mr. Komensky that the PLWH population would be compliant with vaccinations. “People who are living with HIV do everything they possibly can just to stay healthy.”
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV (PLWH) were more likely than other populations to get vaccinated for flu and COVID-19, to seek reputable sources of information, and to be connected through essential community organizations that share essential health and wellness information, according to the results of a large survey.
PLWH, therefore, would have been an ideal model population for creating and disseminating effective messaging around COVID-19 immunizations earlier in the pandemic, said Kathleen Gallagher, MPH, an epidemiologist, researcher, and health services administrator at the Patient Advocate Foundation.
The PLWH community can still offer valuable insights into effective ways to reach out to people, to disseminate correct information, and to link people with resources, Ms. Gallagher said during a poster presentation at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Local, community-based organizations “are the people that these individuals trust, they are people entrenched in their community, and they have existing relationships with them in terms of getting vaccinated and listening to their concerns,” Ms. Gallagher said.
“It’s a missed opportunity.”
A highly compliant group
The July 2021 survey of 271 PLWH was part of a larger, longitudinal survey of 1,400 people with any chronic illness asked about attitudes and barriers to vaccination. The PLWH population was important to focus on, the researchers note, because they could be potentially high risk for more serious COVID-19 outcomes.
The PLWH group was 81% White and 90% male, and 83% were age 56 or older. In addition, 86% had an annual household income below $48,000.
Ninety-three percent of the PLWH group had had flu vaccination in the prior 3 years and received at least one COVID-19 vaccination.
Unable vs. unwilling to vaccinate
Ms. Gallagher and colleagues found 12 people (4%) in the PLWH group did not get vaccinated against COVID-19. It’s a small number, “so you have to take this with a grain of salt,” she said. “But we asked them why they were hesitant. They either were unable or unwilling – and the unable part is not surprising.”
Those who were unable to get vaccinated were either homebound or had concerns about being in a clinic where they could be exposed to COVID while waiting to get the vaccine.
“And then there were some who were just not willing” to get vaccinated, Ms. Gallagher said. She added most cited vaccine safety concerns and “a lot of the misinformation or confusing information around efficacy.”
Trusted information sources
Although people reported getting COVID-19 vaccine information from multiple sources, including online and from television, 64% or nearly two-thirds sought information from their doctors or health care teams.
In fact, doctors emerged as the most trusted source, as indicated by 72% of PLWH.
“I was a little surprised that doctors scored so highly because, sometimes in other cohorts that we looked at, it wasn’t the case,” Ms. Gallagher said. However, she added, a lot of PLWH “have a very strong trust bond with their provider because this is a very personal, very sensitive diagnosis.”
How did social media score? “A whopping 1%,” she said. “So at least this was a savvy group, and they realized that that was not the place to go for vaccination information.”
Overcoming barriers
A lack of vaccine availability at the time of their appointment was the number one barrier to immunization. Also, a small number of people said knowing someone who had an adverse reaction to COVID-19 vaccination was a barrier for them. Ms. Gallagher explained that, by definition in the survey, an adverse reaction to vaccination had to be serious enough to drive people to seek medical care.
When asked to comment on the poster, Andrew Komensky, RN, told this news organization that he found the results “interesting, because I’m an infection preventionist, in addition to being an HIV nurse.” He is director of infection prevention and control at CharterCARE Health Partners, Providence, R.I.
Mr. Komensky said he was surprised that a high proportion of PLWH cited their doctor – and not their nurse – as the most trusted source of information. “In my experience in COVID care ... it was a nursing staff who had most of the contact with patients, who did most of the education, and provided most of the information surrounding vaccination and potential side effects.”
It made sense to Mr. Komensky that the PLWH population would be compliant with vaccinations. “People who are living with HIV do everything they possibly can just to stay healthy.”
A version of this article first appeared on Medscape.com.
FROM ANAC 2022
PrEP education during STI testing could boost HIV protection
TAMPA –
It comes down to numbers, said Gabriela Brito, MSN, RN, ACRN, a researcher at nonprofit CAN Community Health, headquartered in Sarasota, Fla. More people seek screening for STIs compared with those who actively seek PrEP for HIV prevention.
“One out of five individuals got tested and were diagnosed with an STI in 2021, so we can capture a huge amount of people just from STI testing and direct them to PrEP programs,” Ms. Brito said in an interview during a poster presentation here at the annual meeting of the Association of Nurses in AIDS Care (ANAC). “So our initiative is pretty much about capturing people” at the point of care.
Ms. Brito reported that as of September 30, 2022, 2,174 patients were receiving PrEP services through one of 40 CAN Community Health clinics. Nearly one-third, 32%, were initially seen for free STI screening.
Striving for better adherence
In some cases, the issue is not starting people on PrEP, it’s keeping them on the regimen over time. The study revealed that 61% of the people were still taking the medication at 6 months.
This figure might have been even lower without CAN Community Health PrEP navigators. Of the 2,174 patients, 63% work with a “PrEP navigator.” These navigators help people access the medication and check in with them on a regular basis to address any questions or reasons behind a lack of adherence.
“If we’re seeing someone’s missing their appointments, our PrEP navigator will start reaching out to them to see what’s going on,” study coauthor Cheryl Netherly, BSW, LPN, ACLPN, said in an interview.
“It could be they moved to a different area or entered a mutually monogamous relationship. They don’t realize they can continue through telehealth if they need to, because sometimes it is hard to get off of work to go [see] the doctor,” Ms. Netherly added. “So we find ways to break those barriers.”
More education needed
Greater awareness around PrEP is another issue. “I think educating people and educating professionals, it’s really crucial. It can also help diminish the stigma around PrEP,” Ms. Brito said.
An analogy is when birth control pills first came out, and some providers would not prescribe them because they were concerned women would be promiscuous, Ms. Netherly said.
“When PrEP first came out, there was a lot of that same mindset,” Ms. Netherly added. “But PrEP does not change your behavior. It’s just adding a layer of protection to the behavior, so you can understand how to keep yourself healthy.”
A primary care tenet
The strategy of identifying potential PrEP candidates during STI screening is “extremely important,” Myra L. Rutland, CPN, DNP, FNP-BC, a family nurse practitioner and director for infectious disease and community outreach at Spectrum Community Health Center in Philadelphia, said when asked to comment. Ms. Rutland was not involved in the CAN Community Health study.
“This is primary care at its most generic level. Primary care means that you intervene before there’s a problem,” Ms. Rutland said.
“We have great medications. Now if patients are adherent to the medication, they are not just a little bit effective – they are between 95% and 99% effective at preventing HIV,” she added.
The goal is to increase awareness that “if you contract any type of sexual transmitted infection ... that means that perhaps you may have come in contact with HIV,” Ms. Rutland said. “So why not offer PrEP? I do that with all of my patients.”
The study was independently supported. Ms. Brito and Ms. Rutland report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA –
It comes down to numbers, said Gabriela Brito, MSN, RN, ACRN, a researcher at nonprofit CAN Community Health, headquartered in Sarasota, Fla. More people seek screening for STIs compared with those who actively seek PrEP for HIV prevention.
“One out of five individuals got tested and were diagnosed with an STI in 2021, so we can capture a huge amount of people just from STI testing and direct them to PrEP programs,” Ms. Brito said in an interview during a poster presentation here at the annual meeting of the Association of Nurses in AIDS Care (ANAC). “So our initiative is pretty much about capturing people” at the point of care.
Ms. Brito reported that as of September 30, 2022, 2,174 patients were receiving PrEP services through one of 40 CAN Community Health clinics. Nearly one-third, 32%, were initially seen for free STI screening.
Striving for better adherence
In some cases, the issue is not starting people on PrEP, it’s keeping them on the regimen over time. The study revealed that 61% of the people were still taking the medication at 6 months.
This figure might have been even lower without CAN Community Health PrEP navigators. Of the 2,174 patients, 63% work with a “PrEP navigator.” These navigators help people access the medication and check in with them on a regular basis to address any questions or reasons behind a lack of adherence.
“If we’re seeing someone’s missing their appointments, our PrEP navigator will start reaching out to them to see what’s going on,” study coauthor Cheryl Netherly, BSW, LPN, ACLPN, said in an interview.
“It could be they moved to a different area or entered a mutually monogamous relationship. They don’t realize they can continue through telehealth if they need to, because sometimes it is hard to get off of work to go [see] the doctor,” Ms. Netherly added. “So we find ways to break those barriers.”
More education needed
Greater awareness around PrEP is another issue. “I think educating people and educating professionals, it’s really crucial. It can also help diminish the stigma around PrEP,” Ms. Brito said.
An analogy is when birth control pills first came out, and some providers would not prescribe them because they were concerned women would be promiscuous, Ms. Netherly said.
“When PrEP first came out, there was a lot of that same mindset,” Ms. Netherly added. “But PrEP does not change your behavior. It’s just adding a layer of protection to the behavior, so you can understand how to keep yourself healthy.”
A primary care tenet
The strategy of identifying potential PrEP candidates during STI screening is “extremely important,” Myra L. Rutland, CPN, DNP, FNP-BC, a family nurse practitioner and director for infectious disease and community outreach at Spectrum Community Health Center in Philadelphia, said when asked to comment. Ms. Rutland was not involved in the CAN Community Health study.
“This is primary care at its most generic level. Primary care means that you intervene before there’s a problem,” Ms. Rutland said.
“We have great medications. Now if patients are adherent to the medication, they are not just a little bit effective – they are between 95% and 99% effective at preventing HIV,” she added.
The goal is to increase awareness that “if you contract any type of sexual transmitted infection ... that means that perhaps you may have come in contact with HIV,” Ms. Rutland said. “So why not offer PrEP? I do that with all of my patients.”
The study was independently supported. Ms. Brito and Ms. Rutland report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA –
It comes down to numbers, said Gabriela Brito, MSN, RN, ACRN, a researcher at nonprofit CAN Community Health, headquartered in Sarasota, Fla. More people seek screening for STIs compared with those who actively seek PrEP for HIV prevention.
“One out of five individuals got tested and were diagnosed with an STI in 2021, so we can capture a huge amount of people just from STI testing and direct them to PrEP programs,” Ms. Brito said in an interview during a poster presentation here at the annual meeting of the Association of Nurses in AIDS Care (ANAC). “So our initiative is pretty much about capturing people” at the point of care.
Ms. Brito reported that as of September 30, 2022, 2,174 patients were receiving PrEP services through one of 40 CAN Community Health clinics. Nearly one-third, 32%, were initially seen for free STI screening.
Striving for better adherence
In some cases, the issue is not starting people on PrEP, it’s keeping them on the regimen over time. The study revealed that 61% of the people were still taking the medication at 6 months.
This figure might have been even lower without CAN Community Health PrEP navigators. Of the 2,174 patients, 63% work with a “PrEP navigator.” These navigators help people access the medication and check in with them on a regular basis to address any questions or reasons behind a lack of adherence.
“If we’re seeing someone’s missing their appointments, our PrEP navigator will start reaching out to them to see what’s going on,” study coauthor Cheryl Netherly, BSW, LPN, ACLPN, said in an interview.
“It could be they moved to a different area or entered a mutually monogamous relationship. They don’t realize they can continue through telehealth if they need to, because sometimes it is hard to get off of work to go [see] the doctor,” Ms. Netherly added. “So we find ways to break those barriers.”
More education needed
Greater awareness around PrEP is another issue. “I think educating people and educating professionals, it’s really crucial. It can also help diminish the stigma around PrEP,” Ms. Brito said.
An analogy is when birth control pills first came out, and some providers would not prescribe them because they were concerned women would be promiscuous, Ms. Netherly said.
“When PrEP first came out, there was a lot of that same mindset,” Ms. Netherly added. “But PrEP does not change your behavior. It’s just adding a layer of protection to the behavior, so you can understand how to keep yourself healthy.”
A primary care tenet
The strategy of identifying potential PrEP candidates during STI screening is “extremely important,” Myra L. Rutland, CPN, DNP, FNP-BC, a family nurse practitioner and director for infectious disease and community outreach at Spectrum Community Health Center in Philadelphia, said when asked to comment. Ms. Rutland was not involved in the CAN Community Health study.
“This is primary care at its most generic level. Primary care means that you intervene before there’s a problem,” Ms. Rutland said.
“We have great medications. Now if patients are adherent to the medication, they are not just a little bit effective – they are between 95% and 99% effective at preventing HIV,” she added.
The goal is to increase awareness that “if you contract any type of sexual transmitted infection ... that means that perhaps you may have come in contact with HIV,” Ms. Rutland said. “So why not offer PrEP? I do that with all of my patients.”
The study was independently supported. Ms. Brito and Ms. Rutland report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ANAC 2022
HIV: Treating ‘symptom clusters’ could help improve QOL
TAMPA – People living with HIV experience many symptoms that can be grouped into “clusters” to help guide therapy and ideally treat more than one symptom at a time in an effort to improve quality of life, according to a study presented at the annual meeting of the Association of Nurses in AIDS Care.
Interestingly, these symptoms were more common among people living with HIV who are older than 45 years versus those who are younger, with one exception.
“In HIV, with exception of anxiety, we saw older people had more symptoms than younger ones,” said Natalie Wilson, PhD, assistant professor of community health systems at the University of California, San Francisco.
Dr. Wilson and colleagues performed a study that also suggests the older group experienced more distress from their symptoms than the younger cohort, again with the exception of anxiety.
Symptom clusters are two or more related symptoms that occur together with or without the same etiology. “Imagine you can’t sleep and the next day you’re tired and have no energy, you have trouble remembering someone’s name ... and then the next night you get so anxious about not getting sleep that it keeps you from sleeping.” That’s an example of a symptom cluster, Dr. Wilson said.
A high burden
“Why should we even evaluate symptom clusters?” Dr. Wilson asked. “The symptom burden is still high in people living with HIV. The medications got better but the symptoms remain.”
A high symptom burden also is linked to lower adherence to antiretrovirals. Also, considering groups of symptoms together could lead to targeted interventions that treat multiple symptoms, she said, “instead of treating one symptom at a time and increasing the pill burden for people living with HIV.”
Accelerated aging concerns
In addition, people living with HIV can experience accelerated aging, which is one reason Dr. Wilson and colleagues chose the 45-year-old cutoff in the study. Living with inflammation from HIV and the toxicity of earlier treatments likely contribute.
“Those over age 45 have higher rates of age-associated noncommunicable comorbidities developing at an earlier age than uninfected people with comparable lifestyles and demographics,” Dr. Wilson said.
In the full study, published in the Journal of Pain and Symptom Management, a total 2,000 people living with HIV completed the 20-item HIV Symptom Index. The participants reported their symptoms on their first visit to one of six national HIV Centers of Excellence. People were asked to report presence or absence of a particular symptom, and if they had it, how distressing it was on a scale of 1 “doesn’t bother me” to 4 “bothers me a lot.”
Younger people not only reported more anxiety but were also more distressed by it, Dr. Wilson said. The older group was more likely to be distressed by muscle aches and joint pain, trouble remembering things, and more.
The mean age in the younger group was 35 years, and it was 55 years in the older group. A total of 86% in the younger group and 87% in the older were men, and there were some differences by race, Dr. Wilson said.
More research needed
“These findings warrant further confirmation,” Dr. Wilson added. Future work could evaluate whether symptom clusters share etiology and how symptom clusters change over time. “We need to look at outcomes over time. Can we predict poorer outcomes, such as cardiac events, over time based on symptom clusters?”
Also, as part of HIV treatment success in recent years, “Our guidelines are moving people out further – if you’re undetectable sometimes you can come back at 6 months or 1 year.” The question, she said, is then: “Do we need to watch people with certain symptom clusters more closely?”
Limitations of the study include a lack of information on symptom causes and severity and its cross-sectional design.
‘Absolutely useful’
The study is “absolutely useful,” said session moderator Cheryl Netherly, an HIV nurse and clinical educator for CAN Community Health headquartered in Sarasota, Fla.
“One of the things that she mentioned was people with HIV, especially long-term HIV, they’re aging faster than the population without HIV. So, that is really important to look at.”
People living with HIV and dying from age-related comorbidities is something “we never thought would happen,” Ms. Netherly said. “Unfortunately, we’re now losing them to the different things like kidney issues, heart disease, and diabetes.”
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Wilson and Ms. Netherly disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV experience many symptoms that can be grouped into “clusters” to help guide therapy and ideally treat more than one symptom at a time in an effort to improve quality of life, according to a study presented at the annual meeting of the Association of Nurses in AIDS Care.
Interestingly, these symptoms were more common among people living with HIV who are older than 45 years versus those who are younger, with one exception.
“In HIV, with exception of anxiety, we saw older people had more symptoms than younger ones,” said Natalie Wilson, PhD, assistant professor of community health systems at the University of California, San Francisco.
Dr. Wilson and colleagues performed a study that also suggests the older group experienced more distress from their symptoms than the younger cohort, again with the exception of anxiety.
Symptom clusters are two or more related symptoms that occur together with or without the same etiology. “Imagine you can’t sleep and the next day you’re tired and have no energy, you have trouble remembering someone’s name ... and then the next night you get so anxious about not getting sleep that it keeps you from sleeping.” That’s an example of a symptom cluster, Dr. Wilson said.
A high burden
“Why should we even evaluate symptom clusters?” Dr. Wilson asked. “The symptom burden is still high in people living with HIV. The medications got better but the symptoms remain.”
A high symptom burden also is linked to lower adherence to antiretrovirals. Also, considering groups of symptoms together could lead to targeted interventions that treat multiple symptoms, she said, “instead of treating one symptom at a time and increasing the pill burden for people living with HIV.”
Accelerated aging concerns
In addition, people living with HIV can experience accelerated aging, which is one reason Dr. Wilson and colleagues chose the 45-year-old cutoff in the study. Living with inflammation from HIV and the toxicity of earlier treatments likely contribute.
“Those over age 45 have higher rates of age-associated noncommunicable comorbidities developing at an earlier age than uninfected people with comparable lifestyles and demographics,” Dr. Wilson said.
In the full study, published in the Journal of Pain and Symptom Management, a total 2,000 people living with HIV completed the 20-item HIV Symptom Index. The participants reported their symptoms on their first visit to one of six national HIV Centers of Excellence. People were asked to report presence or absence of a particular symptom, and if they had it, how distressing it was on a scale of 1 “doesn’t bother me” to 4 “bothers me a lot.”
Younger people not only reported more anxiety but were also more distressed by it, Dr. Wilson said. The older group was more likely to be distressed by muscle aches and joint pain, trouble remembering things, and more.
The mean age in the younger group was 35 years, and it was 55 years in the older group. A total of 86% in the younger group and 87% in the older were men, and there were some differences by race, Dr. Wilson said.
More research needed
“These findings warrant further confirmation,” Dr. Wilson added. Future work could evaluate whether symptom clusters share etiology and how symptom clusters change over time. “We need to look at outcomes over time. Can we predict poorer outcomes, such as cardiac events, over time based on symptom clusters?”
Also, as part of HIV treatment success in recent years, “Our guidelines are moving people out further – if you’re undetectable sometimes you can come back at 6 months or 1 year.” The question, she said, is then: “Do we need to watch people with certain symptom clusters more closely?”
Limitations of the study include a lack of information on symptom causes and severity and its cross-sectional design.
‘Absolutely useful’
The study is “absolutely useful,” said session moderator Cheryl Netherly, an HIV nurse and clinical educator for CAN Community Health headquartered in Sarasota, Fla.
“One of the things that she mentioned was people with HIV, especially long-term HIV, they’re aging faster than the population without HIV. So, that is really important to look at.”
People living with HIV and dying from age-related comorbidities is something “we never thought would happen,” Ms. Netherly said. “Unfortunately, we’re now losing them to the different things like kidney issues, heart disease, and diabetes.”
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Wilson and Ms. Netherly disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV experience many symptoms that can be grouped into “clusters” to help guide therapy and ideally treat more than one symptom at a time in an effort to improve quality of life, according to a study presented at the annual meeting of the Association of Nurses in AIDS Care.
Interestingly, these symptoms were more common among people living with HIV who are older than 45 years versus those who are younger, with one exception.
“In HIV, with exception of anxiety, we saw older people had more symptoms than younger ones,” said Natalie Wilson, PhD, assistant professor of community health systems at the University of California, San Francisco.
Dr. Wilson and colleagues performed a study that also suggests the older group experienced more distress from their symptoms than the younger cohort, again with the exception of anxiety.
Symptom clusters are two or more related symptoms that occur together with or without the same etiology. “Imagine you can’t sleep and the next day you’re tired and have no energy, you have trouble remembering someone’s name ... and then the next night you get so anxious about not getting sleep that it keeps you from sleeping.” That’s an example of a symptom cluster, Dr. Wilson said.
A high burden
“Why should we even evaluate symptom clusters?” Dr. Wilson asked. “The symptom burden is still high in people living with HIV. The medications got better but the symptoms remain.”
A high symptom burden also is linked to lower adherence to antiretrovirals. Also, considering groups of symptoms together could lead to targeted interventions that treat multiple symptoms, she said, “instead of treating one symptom at a time and increasing the pill burden for people living with HIV.”
Accelerated aging concerns
In addition, people living with HIV can experience accelerated aging, which is one reason Dr. Wilson and colleagues chose the 45-year-old cutoff in the study. Living with inflammation from HIV and the toxicity of earlier treatments likely contribute.
“Those over age 45 have higher rates of age-associated noncommunicable comorbidities developing at an earlier age than uninfected people with comparable lifestyles and demographics,” Dr. Wilson said.
In the full study, published in the Journal of Pain and Symptom Management, a total 2,000 people living with HIV completed the 20-item HIV Symptom Index. The participants reported their symptoms on their first visit to one of six national HIV Centers of Excellence. People were asked to report presence or absence of a particular symptom, and if they had it, how distressing it was on a scale of 1 “doesn’t bother me” to 4 “bothers me a lot.”
Younger people not only reported more anxiety but were also more distressed by it, Dr. Wilson said. The older group was more likely to be distressed by muscle aches and joint pain, trouble remembering things, and more.
The mean age in the younger group was 35 years, and it was 55 years in the older group. A total of 86% in the younger group and 87% in the older were men, and there were some differences by race, Dr. Wilson said.
More research needed
“These findings warrant further confirmation,” Dr. Wilson added. Future work could evaluate whether symptom clusters share etiology and how symptom clusters change over time. “We need to look at outcomes over time. Can we predict poorer outcomes, such as cardiac events, over time based on symptom clusters?”
Also, as part of HIV treatment success in recent years, “Our guidelines are moving people out further – if you’re undetectable sometimes you can come back at 6 months or 1 year.” The question, she said, is then: “Do we need to watch people with certain symptom clusters more closely?”
Limitations of the study include a lack of information on symptom causes and severity and its cross-sectional design.
‘Absolutely useful’
The study is “absolutely useful,” said session moderator Cheryl Netherly, an HIV nurse and clinical educator for CAN Community Health headquartered in Sarasota, Fla.
“One of the things that she mentioned was people with HIV, especially long-term HIV, they’re aging faster than the population without HIV. So, that is really important to look at.”
People living with HIV and dying from age-related comorbidities is something “we never thought would happen,” Ms. Netherly said. “Unfortunately, we’re now losing them to the different things like kidney issues, heart disease, and diabetes.”
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Wilson and Ms. Netherly disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.