User login
Racism and gynecologic surgery: A time to act
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).
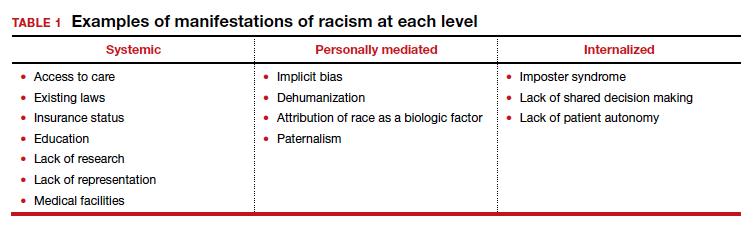
Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).

Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
Although recent events have spurred much discourse regarding systemic racism, the issue of racism is old, very old. Unfortunately, our gynecologic surgery history is rooted in racism, with numerous documented procedures performed on enslaved women without their consent. Over the years, racism has continued to permeate gynecologic surgery in so far as access to quality care, patient outcomes, and inclusion in research. While racial disparities with regard to stage at diagnosis and survival of gynecologic malignancy has been documented, this discussion is outside the scope of this article.
Racial disparities in gyn surgery: The evidence
More data exist with regard to hysterectomy and racism than with any other gynecologic surgery. Most notably, a minimally invasive approach to hysterectomy is less likely to occur for minority women, even in universally insured patient populations and when controlling for factors predisposing patients to an abdominal approach.
Minority women undergo MIS for hysterectomy less often
Ranjit and colleagues assessed hysterectomy data between 2006 and 2010 from National TRICARE Prime and Prime Plus data to evaluate if racial differences existed in a universally insured population of US Armed Services members and their dependents. African American patients were significantly less likely than White patients to undergo a total vaginal hysterectomy (relative risk ratio [RRR], 0.63; 95% confidence interval [CI], 0.58–0.69) or total laparoscopic hysterectomy (RRR, 0.65; 95% CI, 0.60–0.71) compared with abdominal hysterectomy. Asian patients were also less likely to receive the vaginal (RRR, 0.71; 95% CI, 0.60–0.84) or laparoscopic (RRR, 0.69; 95% CI, 0.58–0.83) approach to hysterectomy than White patients.1 These findings remained when controlled for surgery indication, suggesting that racial inequity was not attributed solely to preoperative patient factors. However, the authors could not control for specific patient factors such as body mass index and uterine weight.
Katon and colleagues reviewed data on patients who underwent hysterectomy for uterine fibroids at a Veterans Affairs hospital and found 99 excess abdominal hysterectomies were performed among Black women compared with White women. Despite controlling for predisposing factors related to abdominal surgery, facility, and geography (teaching hospital, higher volume hysterectomy), Black women were still less likely to undergo minimally invasive hysterectomy.2 The difference in approach between both groups remained largely unexplained.2
Pollack and colleagues reviewed hysterectomy data from Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project State Inpatient Database and State Ambulatory Surgery Databases between 2010 and 2014 from Colorado, Florida, Maryland, New Jersey, and New York. They found that African American and Hispanic women were less likely to undergo vaginal (adjusted standardized prevalence ratio [aPR], 0.93; 95% CI, 0.90–0.96 and aPR, 0.95; 95% CI, 0.93−0.97, respectively) and laparoscopic hysterectomy (aPR, 0.90; 95% CI, 0.87−0.94 and aPR, 0.95; 95% CI, 0.92−0.98, respectively) than White women. Asian/Pacific Islander women were less likely to undergo vaginal hysterectomy (aPR, 0.88; 95% CI, 0.81−0.96). They also found that hospitals providing care to more racial/ethnic minority women performed more abdominal and fewer vaginal procedures compared with other hospitals.3
Sanei-Moghaddam and colleagues reviewed data from University of Pittsburgh Medical Center–affiliated hospitals and found that European-American women had 0.47 times lower odds of undergoing abdominal hysterectomy compared with ethnic/race minority group women. Also, traditional Medicaid and Medicare enrollees had 2- to 4-times higher odds of having an abdominal hysterectomy compared with patients with commercial insurance.4 Evidently, insurance and payer status and hospital, along with race, were associated with abdominal hysterectomy.
Postop complications higher among Black women. One study of the National Surgical Quality Improvement Program 2015 hysterectomy database found that Black women were more likely to undergo open hysterectomy than White women despite controlling for patient factors associated with open hysterectomy, including uterine weight (adjusted odds ratio [aOR], 2.02; 95% CI, 1.85–2.20).5 Black women also were more likely to develop both minor and major postoperative complications despite controlling for route of hysterectomy (major complications aOR, 1.56; 95% CI, 1.25–1.95 and minor complications aOR, 1.27; 95% CI, 1.11–1.47). Their study was limited by inability to control for surgeon volume and experience and hospital-specific factors.5
Hospital size and surgeon volume found to play a role in disparities. In an effort to address hospital and surgeon factors and racial disparities in minimally invasive hysterectomy, Mehta and colleagues evaluated an all payer system in Maryland. Black (reference White; aOR, 0.70; 95% CI, 0.63–0.78) and Hispanic patients (aOR, 0.62; 95% CI, 0.48–0.80) were less likely to undergo minimally invasive hysterectomy. Patients who had surgery at small- and medium-sized hospitals or by medium-volume surgeons (medium vs high volume: OR, 0.78; 95% CI, 0.71–0.87) were also more likely to undergo open hysterectomy.6 The study authors suggest increased utilization of higher volume surgeons for referrals or to assist lower-volume surgeons as potential solutions to address racial disparities.6
Continue to: Surgical outcome disparities extend beyond hysterectomy route...
Surgical outcome disparities extend beyond hysterectomy route
While the bulk of data with regard to gynecologic surgery and racism addresses minimally invasive approach to treatment of fibroids and hysterectomy, limited data regarding ectopic pregnancy and adnexal surgery reveal similar findings. Hsu and colleagues reported that Black (adjusted risk ratio [aRR], 0.76; 95% CI, 0.69–0.85) and Hispanic (aRR, 0.80; 95% CI, 0.66–0.96) women treated surgically for ectopic pregnancy were less likely to undergo tubal-sparing procedures than White women.7 Their study did not control for human chorionic gonadotropin levels, ectopic size, or comorbidities as measured by the Elixhauser Comorbidity Index.
The data regarding gynecologic surgery and racial inequity are sparse but manifest differences that are unexplained entirely by patient payer status and individual patient factors. Studies do confirm hospital and surgeon characteristics play a part in provision of minimally invasive hysterectomy.
Forming a conceptual re-framework to achieve health equity
The centuries-long impact of racism on our field, and more specifically on gynecologic surgery, will take time and a conscious effort to overcome. In 2001, the Institute of Medicine outlined 6 domains for improvement, amongst them equitable care—“ensuring quality of care does not vary because of characteristics.”8 As highlighted above, some aspects of gynecologic surgery have proven to be inequitable, specifically in the provision of minimally invasive hysterectomy and treatment of ectopic pregnancy in Black women. The lack of studies on racism and gynecologic surgery as it pertains to other benign gynecologic conditions highlights the need for more research and measures that target each level of racism and, ultimately, achieve health equity.
Priority #1: Support and funding. In 2016, the Institute for Healthcare Improvement (IHI) published a white paper describing a framework to bring about health equity. First and foremost, institutions and individuals must prioritize health equity by obtaining leadership support and adequate funding.9 In August 2020, several leading obstetrics and gynecology organizations published a joint statement highlighting their initial plan of action to address racism and provide equitable care.10 As leading professional organizations prioritize equity, we can hope institutions and departments continue to do so as well.
Priority #2: Measuring the extent of the problem. Once adequate support and funding is established, the IHI recommends9:
- establishing structures and processes with an overseeing committee and dedicated budget
- deploying strategies with comprehensive data collection and pertinent metrics.
Continue to: Applying the levels of racism to a new framework...
Applying the levels of racism to a new framework
Given the numerous untouched areas of research and components contributing to racial disparities in gynecologic surgery, determining a starting point can prove overwhelming. We suggest employing a conceptual framework that considers the different levels of racism (TABLE 1).

Three different levels of racism have been described previously:
- systemic/institutionalized,
- personally mediated
- internalized.11,12
Systemic racism refers to differential access to services and goods in society and power within society, for example housing, education, medical care, and voting and representation.12 Systemic racism is arguably the overarching form of racism. The studies by Mehta and colleagues and Pollack et al specifically highlight a lack of adequate access to minimally invasive hysterectomy and a subsequent increase in complication rates in minority race groups.3,13 Access to care is only one example of systemic racism that requires action at multiple levels by professional organizations, hospitals, community organizations, and individual departments with multiple targeted solutions (TABLE 2).
Mediated racism. The second form of racism is personally mediated racism, in other words discrimination and prejudice formed by preconceived notions of a person based on their race.12 In the joint statement published by the leading obstetrics and gynecology organizations in August 2020, a recognition of race as a social construct without the biological weight we have long afforded it was made explicit. This realization can be applied in the day-to-day categorization of patients and, most notably, the formation of a diagnosis and treatment plan.
A concrete example of potentially biased treatment is illustrated when limiting management options to the “unreliable” patient. Exposure to stereotypes and misinformation can develop into implicit bias and subsequently make the most intelligent, compassionate provider show behavior with microaggressions. This subtle behavior can play a major role in patient-provider communication and in turn affect care satisfaction, provider trust, and shared decision making.14 The Implicit bias Association Test or MPathic-VR virtual human simulations can be used to identify provider-specific implicit bias.14,15
Internalized racism. Lastly, internalized racism refers to the individual’s acceptance of negative messages regarding their own abilities and worth,12 which is seen commonly in imposter syndrome. Imposter syndrome, which is a failure to internalize one’s own successes and persistent fear of being discovered as a fraud, a condition which has been more commonly seen in ethnic minority groups.16 A patient’s internalized racism can manifest as self-devaluation and helplessness which may make a patient less likely to question their treatment.12,17 Moreover, some evidence exists indicating that patients with diabetes identified physician discrimination and internalized racism as factors impeding shared decision making.18

The next steps first require recognition
Racial inequity has long infiltrated our medical field and the discussion surrounding the effects of racism on our patients and providers, and research, is long overdue. Although research continues to emerge regarding race inequity and gynecologic surgery, much remains to be done. In recognizing the levels of racism and the roles they play in our provision of good, equitable, patient-centered care, we—as individuals, departments, and organizations—can combat racism and strive for health equity. ●
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24:790-796.
- Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930-936.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2.
- Sanei-Moghaddam A, Kang C, Edwards RP, et al. Racial and socioeconomic disparities in hysterectomy route for benign conditions. J Racial Ethn Health Disparities. 2018;5:758-765.
- Alexander AL, Strohl AE, Rieder S, et al. Examining disparities in route of surgery and postoperative complications in black race and hysterectomy. Obstet Gynecol. 2019;133:6-12.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49. e1-49.e10.
- Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001.
- Wyatt R, Laderman M, Botwinick L, et al. Achieving Health Equity: A Guide for Health Care Organizations. Cambridge, MA: Institute for Healthcare Improvement; 2016.
- Joint Statement: Collective Action Addressing Racism. AAGL web site. https://www.aagl.org/aaglnews/joint-statement -collective-action-addressing-racism/. Released August 27, 2020. Accessed January 22, 2021.
- Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
- Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212-1215.
- Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497.e1-497.e10.
- Hagiwara N, Elston Lafata J, Mezuk B, et al. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102:1738-1743.
- Kron FW, Detters MD, Scerbo MW, et al. Using a computer simulation for teaching communication skills: A blinded multisite mixed methods randomized controlled trial. Patient Educ Couns. 2017;100:748-759.
- Bravata DM, Watts SA, Keefer AL, et al. Prevalence, predictors, and treatment of impostor syndrome: a systematic review. J Gen Intern Med. 2020;35:1252.
- Peek ME, Odoms-Young A, Quinn MT, et al. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med. 2010;71:13.
- Peek MA, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9.
Disparities in cervical cancer in African American women: What primary care physicians can do
African American, Hispanic, American Indian, and Alaskan Native women continue to be disproportionately affected by cervical cancer compared with white women. From 2006 to 2010, the incidence of cervical cancer in African American women was 10.3 per 100,000; in white women it was 7.2.1 The mortality rate from cervical cancer in African American women is twice that in white women.1 Although cervical cancer rates have decreased nationwide, significant racial health disparities persist.
As the first-line healthcare providers for many women, the primary care physician and the general obstetrician-gynecologist are optimally positioned to reduce these disparities.
Cervical cancer is the third most common gynecologic cancer, after uterine and ovarian cancer. Nearly 13,000 new cases are diagnosed each year in the United States, and more than 4,000 women die of it.2 Fortunately, cervical cancer can be significantly prevented with adequate screening and vaccination against human papillomavirus (HPV).
WHY ARE BLACK WOMEN MORE LIKELY TO DIE OF CERVICAL CANCER?
Later stage at diagnosis. African American women are more likely to present with advanced cervical cancer than non-Hispanic white women.3–6
Less-aggressive treatment. African American women are more likely to receive no treatment after a cancer diagnosis.6 Differences in treatment may be attributed to comorbid conditions, stage at cancer diagnosis, and patient refusal.5,7
Less access to care. A study from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute looked at 7,267 women (4,431 non-Hispanic white women, 1,830 Hispanic white women, and 1,006 non-Hispanic African American women) who were diagnosed with primary invasive cervical cancer from 1992 to 1996 and followed through 2000. African American women had a 19% higher mortality rate compared with non-Hispanic white women during follow-up despite adjusting for age, stage, histology, and time of first treatment.8
However, a later study from the same program found no such difference after 1995, when the data were adjusted for marital status, disease stage, age, treatment, grade, and histology.6
Equal access to healthcare may eliminate most of the disparity.7 A study in women with cervical cancer who sought treatment within the United States military healthcare system found no difference in treatment or 5- and 10-year survival rates between African American and white women.5 Equal access to comprehensive healthcare eliminated any disparity once cervical cancer was diagnosed.
CERVICAL CANCER SCREENING
The value of cervical cancer screening and prevention is well established. In 1941, Papanicolau reported that cervical cancer could be detected from vaginal smears.9 Since the development and widespread implementation of the “Pap” smear, cervical cancer rates have decreased dramatically in the United States.
Another major advance was the discovery that persistent infection with HPV is necessary for the development of cervical cancer, precancerous lesions, and genital warts.10
With advancing research, guidelines for cervical cancer screening have changed considerably over the years. Today, combined cervical cytologic and HPV testing is the mainstay. (Isolated HPV testing is generally not available outside clinical trials.)
Who should be screened?
Previous recommendations called for women to undergo Pap testing when they first became sexually active and then every year. However, cervical lesions are likely to regress in young women.11 One study found that 28% of cervical intimal neoplasia (CIN) grade 2 and 3 lesions spontaneously regressed by 15 weeks, although lesions associated with HPV 16 infection were less likely to regress than with other HPV types.12 A study of college women found that HPV infection persisted in only 9% of women after 24 months.13
To minimize unnecessary treatment of young women with dysplasia, the American Society for Colposcopy and Cervical Pathology in 2012 recommended cytologic screening for all women 21 years or older, regardless of age at first sexual encounter.14 Screening intervals were changed from every year to every 3 years until age 30, at which time cotesting with cytology and HPV testing is performed every 5 years. Routine cotesting is not recommended for women younger than 30, who have a high likelihood of HPV infection and spontaneous regression.
In 2014, the US Food and Drug Administration approved primary HPV screening (ie, testing for HPV first, and then performing cytology in samples that test positive) for women age 25 and older.15
Patients who need further evaluation and testing should be referred for colposcopy. The current guidelines for patients who have abnormal results on cervical cancer screening16 can be reviewed at www.asccp.org/asccp-guidelines.
As screening guidelines continue to evolve, primary care physicians will need to stay current and also help educate their patients. For example, many of our patients have undergone annual Pap screening for most of their lives and may not yet know about the new testing intervals.
Are there disparities in screening and follow-up?
Disparities in screening and follow-up may exist, but the evidence is not clear-cut.
In a 2013 National Health Interview Survey report, the rates of cervical cancer screening with Pap tests did not differ between African American and white women.17 However, the information on Pap testing was based on a single question asking participants if they had had a Pap test in the last 3 years. In our experience, patients may confuse Pap tests with speculum examinations.
Once women are screened, adequate and timely follow-up of abnormal results is key.
In a study from the National Breast and Cervical Cancer Early Detection Program,18 women who had cytology findings of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesions were to undergo repeat Pap testing every 4 to 6 months for 2 years. African American women were the least likely to have a follow-up Pap smear compared with other racial groups.
On the other hand, there was no difference related to race in follow-up rates of abnormal Pap tests in women ages 47 to 64 in the South Carolina Breast and Cervical Cancer Early Detection Program.19
In a study in an urban population (predominantly African African), the overall follow-up rate was only 26% at 12 months from an initial abnormal Pap smear. This study did not find any differences in follow-up according to race or ethnicity; however, it had insufficient power to detect a difference because only 15% of the study participants were white.20
What is in a genotype?
HPV is implicated in progression to both squamous cell carcinoma and adenocarcinoma of the cervix. Worldwide, HPV genotypes 16 and 18 are associated with 73% of cases of invasive cervical cancer; most of the remainder are associated with, in order of decreasing prevalence, genotypes 58, 33, 45, 31, 52, 35, 59, 39, 51, and 56.21
High-grade cervical lesions in African American women may less often be positive for HPV 16 and 18 than in white women.22,23 On the other hand, the proportion of non-Hispanic black women infected with HPV 35 and 58 was significantly higher than in non-Hispanic white women.22 Regardless, HPV screening is recommended for women of all races and ethnicities.
The 2-valent and 4-valent HPV vaccines do not cover HPV 35 or 58. The newer 9-valent vaccine covers HPV 58 (but not 35) and so may in theory decrease any potential disparity related to infection with a specific oncogenic subtype.
THE ROLE OF PREVENTION
HPV vaccination
The Females United to Unilaterally Reduce Endo/Ectocervical Disease study demonstrated that the 4-valent vaccine was highly effective against cervical intraepithelial neoplasia due to HPV 16 and 18.24 In another study, the 2-valent vaccine reduced the incidence of CIN 3 or higher by 87% in women who received all 3 doses and who had no evidence of HPV infection at baseline.25
HPV vaccination is expensive. Each shot costs about $130, plus the cost of administering it. Although the Vaccines for Children program covers the HPV vaccine for uninsured and underinsured children and adolescents under age 19, Medicaid coverage varies from state to state for adults over age 21.
The Advisory Committee on Immunization Practices (ACIP)26 recommends routine vaccination for:
- Males 11 or 12 years old
- Females ages 9 to 26.
In October 2016, the ACIP approved a 2-dose series given 6 to 12 months apart for patients starting vaccination at ages 9 through 14 years who are not immunocompromised. Others should receive a 3-dose series, with the second dose given 1 to 2 months after the first dose and the third dose given 6 months after the first dose.27 Previously, 3 doses were recommended for everyone.
Disparities in HPV vaccination rates
HPV vaccination rates among adolescents in the United States increased from 33.6% in 2013 to 41.7% in 2014.28 However, HPV vaccination rates continue to lag behind those of other routine vaccines, such as Tdap and meningococcal conjugate.
Reagan-Steiner et al28 reported that more black than white girls age 13 through 17 received at least 1 dose of a 3-dose HPV vaccination series, but more white girls received all 3 doses (70.6% vs 61.6%). In contrast, a meta-analysis by Fisher et al29 found African American and uninsured women generally less likely to initiate the HPV vaccination series. Kessels et al30 reported similar findings.
Barriers to HPV vaccination
Barriers to HPV vaccination can be provider-dependent, parental, or institutional.
Malo et al31 surveyed Florida Medicaid providers and found that those who participated in the Vaccines for Children program were less likely to cite lack of reimbursement as a barrier to vaccination.
Meites et al32 surveyed sexually transmitted disease clinics and found that common reasons for not offering HPV vaccine were cost, staff time, and difficulty coordinating follow-up visits to complete the series.
Providers report lack of urgency or lack of perception of cervical cancer as a true public health threat, safety concerns regarding the vaccine, and the inability to coadminister vaccines as barriers.33
Studies have shown that relatively few parents (up to 18%) of parents are concerned about the effect of the vaccine on sexual activity.34 Rather, they are most likely to cite lack of information regarding the vaccine, lack of physician recommendation, and not knowing where to receive the vaccine as barriers.35,36
Guerry et al37 determined that the single most important factor in vaccine initiation was physician recommendation, a finding reiterated in other studies.35,38 A study in North Carolina identified failure of physician recommendation as one of the missed opportunities for vaccination of young women.39
Therefore, the primary care physician, as the initial contact with the child or young adult, holds a responsibility to narrow this gap. In simply discussing and recommending the vaccine, physicians could increase vaccination rates.
REPRODUCTIVE HEALTH
Although 80% of women will be infected with HPV in their lifetime, only a small proportion will develop cervical cancer, suggesting there are other cofactors in the progression to cervical cancer.40
Given the infectious etiology of cervical cancer, other contributing reproductive health factors have been described. As expected, the number of sexual partners correlates with HPV infection.41,42 Younger age at first intercourse has been linked to development of cervical neoplasia, consistent with persistent infection leading to neoplasia.41,42
Primary care physicians should provide timely and comprehensive sexual education, including information on safe sexual practices and pregnancy prevention.
Human immunodeficiency virus
In 2010, the estimated rate of new human immunodeficiency virus (HIV) infections in African American women was nearly 20 times greater than in white women.43 Previous studies have shown a clear relationship between HIV and HPV-associated cancers, including cervical neoplasia and invasive cervical cancer.44,45
Women with HIV should receive screening for cervical cancer at the time of diagnosis, 6 months after the initial diagnosis, and annually thereafter.46
Conflicting evidence exists regarding the effect of highly active antiretroviral therapy on the incidence of HPV-related disease, so aggressive screening and management of cervical neoplasia is recommended for women with HIV, regardless of CD4+ levels or viral load.47–49
Additional infectious culprits
Coinfection with other sexually transmitted infections, specifically Chlamydia, herpes, and HIV, has been associated with cervical neoplasia and invasive cervical cancer. A positive linear association exists between the number of sexually transmitted infections and cervical neoplasia.50
C trachomatis is the most common sexually transmitted infection in the United States, with a 6-times higher rate in African American women.51 Women who are seropositive for C trachomatis are at twofold higher risk of developing squamous cell cervical cancer.52,53 Women who are seropositive for Chlamydia infection, herpes virus 2, or HPV are at markedly increased risk of invasive cervical cancer.50
Tobacco use
The negative impact of smoking on numerous other cancers resulted in investigation of its role in cervical cancer.
Early case-control studies found an association between cervical cancer and smoking,54 but because these studies did not account for HPV infection status, they could not establish causality. Subsequently, several studies did control for HPV infection; the risk of squamous cervical cancer was twice as high in women who had ever smoked.55 Furthermore, the more cigarettes smoked per day, the higher the risk of cervical neoplasia.41,56
According to the US Centers for Disease Control and Prevention in 2014, the highest prevalence of smoking was among American Indian and Alaskan Native women, 32.5% of whom said they smoked every day, compared with 17.2% of white women and 13.7% of African American women.57
HOW CAN PRIMARY CARE PHYSICIANS CLOSE THE GAP?
By promoting HPV vaccination to children and young adults, primary care physicians can help prevent cervical cancer. Moreover, primary care physicians will see most adolescents for a nonpreventive health visit, an optimal opportunity to discuss sexual activity practices and HPV vaccination.58 Including the HPV vaccine as routine with other vaccinations can close the gap.38
Screening and treatment of sexually transmitted infection during these visits can affect the risk that future HPV infection will progress to neoplasia or cancer. Persistent lifestyle modification counseling, especially smoking cessation through motivational interviewing, can lessen the risk of cervical cancer neoplasia progression.
Additionally, in light of recent changes in cervical cancer screening guidelines, the primary care physician’s role as educator is of utmost importance. In one study, although 99% of women had received a Pap test, 87% could not identify the purpose of the Pap test.59 The primary care physician’s role is perhaps the most influential in preventing disease and, as such, has the greatest impact on a patient’s disease process.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw 2015; 13:395-404.
- Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer 2007; 110:234–243.
- Farley JH, Hines JF, Taylor RR, et al. Equal care ensures equal survival for African-American women with cervical carcinoma. Cancer 2001; 91:869–873.
- Rauh-Hain JA, Clemmer JT, Bradford LS, et al. Racial disparities in cervical cancer survival over time. Cancer 2013; 119:3644–3652.
- Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014; 133:353–361.
- Patel DA, Barnholtz-Sloan JS, Patel MK, Malone JM Jr, Chuba PJ, Schwartz K. A population-based study of racial and ethnic differences in survival among women with invasive cervical cancer: analysis of surveillance, epidemiology, and end results data. Gynecol Oncol 2005; 97:550–558.
- Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. 1941. Arch Pathol Lab Med 1997; 121:211–224.
- Walboomers JM, Jacobs M V, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 2004; 364:1678–1683.
- Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 2005; 11:4717–4723.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423-428.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125:330–337.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013; 17(suppl 1):S1–S27.
- Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use—United States, 2013. MMWR 2015; 64:464–468.
- Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005; 105:1323–1328.
- Eggleston KS, Coker AL, Luchok KJ, Meyer TE. Adherence to recommendations for follow-up to abnormal Pap tests. Obstet Gynecol 2007; 109:1332–1341.
- Peterson NB, Han J, Freund KM. Inadequate follow-up for abnormal Pap smears in an urban population. J Natl Med Assoc 2003; 95:825–832.
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927–935.
- Hariri S, Unger ER, Powell SE, et al; HPV-IMPACT Working Group. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 2012; 206:1878–1886.
- Niccolai LM, Russ C, Julian PJ, et al. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions: associations with race, ethnicity, and poverty. Cancer 2013; 119:3052–3058.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1705–1708.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65:1405–1408.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:784–792.
- Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol 2013; 42:896–908.
- Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine 2012; 30:3546–3556.
- Malo TL, Hassani D, Staras SA, Shenkman EA, Giuliano AR, Vadaparampil ST. Do Florida Medicaid providers’ barriers to HPV vaccination vary based on VFC program participation? Matern Child Health J 2013; 17:609–615.
- Meites E, Llata E, Hariri S, et al. HPV vaccine implementation in STD clinics—STD Surveillance Network. Sex Transm Dis 2012; 39:32–34.
- Perkins RB, Clark JA. What affects human papillomavirus vaccination rates? A qualitative analysis of providers’ perceptions. Womens Health Issues 2012; 22:e379–e386.
- Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014; 168:76–82.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Bastani R, Glenn BA, Tsui J, et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev 2011; 20:1463–1472.
- Guerry SL, De Rosa CJ, Markowitz LE, et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine 2011; 29:2235–2241.
- Hull PC, Williams EA, Khabele D, Dean C, Bond B, Sanderson M. HPV vaccine use among African American girls: qualitative formative research using a participatory social marketing approach. Gynecol Oncol 2014; 132(suppl 1):S13–S20.
- Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011; 38:197–204.
- Wang SS, Zuna RE, Wentzensen N, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev 2009; 18:113–120.
- Deacon JM, Evans CD, Yule R, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case-control study nested within the Manchester cohort. Br J Cancer 2000; 83:1565–1572.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and sexual behavior: collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol Biomarkers Prev 2009; 18:1060–1069.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17(No. 4). https://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed September 12, 2017.
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000; 92:1500–1510.
- Schäfer A, Friedmann W, Mielke M, Schwartländer B, Koch MA. The increased frequency of cervical dysplasia-neoplasia in women infected with the human immunodeficiency virus is related to the degree of immunosuppression. Am J Obstet Gynecol 1991; 164:593–599.
- Phillips AA, Justman JE. Screening HIV-infected patients for non-AIDS-defining malignancies. Curr HIV/AIDS Rep 2009; 6:83–92.
- De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev 2008; 17:545–554.
- Palefsky JM. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol 2003; 15:382–388.
- Adler DH. The impact of HAART on HPV-related cervical disease. Curr HIV Res 2010; 8:493–497.
- Castellsagué X, Pawlita M, Roura E, et al. Prospective seroepidemiologic study on the role of human papillomavirus and other infections in cervical carcinogenesis: evidence from the EPIC cohort. Int J Cancer 2014; 135:440–452.
- Centers for Disease Control and Prevention (CDC). 2013 sexually transmitted disease surveillance. www.cdc.gov/std/stats13/exordium.htm. Accessed September 12, 2017.
- Smith JS, Bosetti C, Muñoz N, et al; IARC multicentric case-control study. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer 2004; 111:431–439.
- Koskela P, Anttila T, Bjørge T, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer 2000; 85:35–39.
- Office on Smoking and Health (US). Women and smoking: a report of the Surgeon General: Chapter 3. Health consequences of tobacco use among women. http://www.ncbi.nlm.nih.gov/books/NBK44312. Accessed September 12, 2017.
- Plummer M, Herrero R, Franceschi S, et al; IARC Multi-centre Cervical Cancer Study Group. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case—control study. Cancer Causes Control 2003; 14:805–814.
- Ho GY, Kadish AS, Burk RD, et al. HPV 16 and cigarette smoking as risk factors for high-grade cervical intra-epithelial neoplasia. Int J Cancer 1998; 78:281–285.
- Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep 2015; 64:1233–1240.
- Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med 2010; 8:511–516.
- Lindau ST, Tomori C, Lyons T, Langseth L, Bennett CL, Garcia P. The association of health literacy with cervical cancer prevention knowledge and health behaviors in a multiethnic cohort of women. Am J Obstet Gynecol 2002; 186:938–943.
African American, Hispanic, American Indian, and Alaskan Native women continue to be disproportionately affected by cervical cancer compared with white women. From 2006 to 2010, the incidence of cervical cancer in African American women was 10.3 per 100,000; in white women it was 7.2.1 The mortality rate from cervical cancer in African American women is twice that in white women.1 Although cervical cancer rates have decreased nationwide, significant racial health disparities persist.
As the first-line healthcare providers for many women, the primary care physician and the general obstetrician-gynecologist are optimally positioned to reduce these disparities.
Cervical cancer is the third most common gynecologic cancer, after uterine and ovarian cancer. Nearly 13,000 new cases are diagnosed each year in the United States, and more than 4,000 women die of it.2 Fortunately, cervical cancer can be significantly prevented with adequate screening and vaccination against human papillomavirus (HPV).
WHY ARE BLACK WOMEN MORE LIKELY TO DIE OF CERVICAL CANCER?
Later stage at diagnosis. African American women are more likely to present with advanced cervical cancer than non-Hispanic white women.3–6
Less-aggressive treatment. African American women are more likely to receive no treatment after a cancer diagnosis.6 Differences in treatment may be attributed to comorbid conditions, stage at cancer diagnosis, and patient refusal.5,7
Less access to care. A study from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute looked at 7,267 women (4,431 non-Hispanic white women, 1,830 Hispanic white women, and 1,006 non-Hispanic African American women) who were diagnosed with primary invasive cervical cancer from 1992 to 1996 and followed through 2000. African American women had a 19% higher mortality rate compared with non-Hispanic white women during follow-up despite adjusting for age, stage, histology, and time of first treatment.8
However, a later study from the same program found no such difference after 1995, when the data were adjusted for marital status, disease stage, age, treatment, grade, and histology.6
Equal access to healthcare may eliminate most of the disparity.7 A study in women with cervical cancer who sought treatment within the United States military healthcare system found no difference in treatment or 5- and 10-year survival rates between African American and white women.5 Equal access to comprehensive healthcare eliminated any disparity once cervical cancer was diagnosed.
CERVICAL CANCER SCREENING
The value of cervical cancer screening and prevention is well established. In 1941, Papanicolau reported that cervical cancer could be detected from vaginal smears.9 Since the development and widespread implementation of the “Pap” smear, cervical cancer rates have decreased dramatically in the United States.
Another major advance was the discovery that persistent infection with HPV is necessary for the development of cervical cancer, precancerous lesions, and genital warts.10
With advancing research, guidelines for cervical cancer screening have changed considerably over the years. Today, combined cervical cytologic and HPV testing is the mainstay. (Isolated HPV testing is generally not available outside clinical trials.)
Who should be screened?
Previous recommendations called for women to undergo Pap testing when they first became sexually active and then every year. However, cervical lesions are likely to regress in young women.11 One study found that 28% of cervical intimal neoplasia (CIN) grade 2 and 3 lesions spontaneously regressed by 15 weeks, although lesions associated with HPV 16 infection were less likely to regress than with other HPV types.12 A study of college women found that HPV infection persisted in only 9% of women after 24 months.13
To minimize unnecessary treatment of young women with dysplasia, the American Society for Colposcopy and Cervical Pathology in 2012 recommended cytologic screening for all women 21 years or older, regardless of age at first sexual encounter.14 Screening intervals were changed from every year to every 3 years until age 30, at which time cotesting with cytology and HPV testing is performed every 5 years. Routine cotesting is not recommended for women younger than 30, who have a high likelihood of HPV infection and spontaneous regression.
In 2014, the US Food and Drug Administration approved primary HPV screening (ie, testing for HPV first, and then performing cytology in samples that test positive) for women age 25 and older.15
Patients who need further evaluation and testing should be referred for colposcopy. The current guidelines for patients who have abnormal results on cervical cancer screening16 can be reviewed at www.asccp.org/asccp-guidelines.
As screening guidelines continue to evolve, primary care physicians will need to stay current and also help educate their patients. For example, many of our patients have undergone annual Pap screening for most of their lives and may not yet know about the new testing intervals.
Are there disparities in screening and follow-up?
Disparities in screening and follow-up may exist, but the evidence is not clear-cut.
In a 2013 National Health Interview Survey report, the rates of cervical cancer screening with Pap tests did not differ between African American and white women.17 However, the information on Pap testing was based on a single question asking participants if they had had a Pap test in the last 3 years. In our experience, patients may confuse Pap tests with speculum examinations.
Once women are screened, adequate and timely follow-up of abnormal results is key.
In a study from the National Breast and Cervical Cancer Early Detection Program,18 women who had cytology findings of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesions were to undergo repeat Pap testing every 4 to 6 months for 2 years. African American women were the least likely to have a follow-up Pap smear compared with other racial groups.
On the other hand, there was no difference related to race in follow-up rates of abnormal Pap tests in women ages 47 to 64 in the South Carolina Breast and Cervical Cancer Early Detection Program.19
In a study in an urban population (predominantly African African), the overall follow-up rate was only 26% at 12 months from an initial abnormal Pap smear. This study did not find any differences in follow-up according to race or ethnicity; however, it had insufficient power to detect a difference because only 15% of the study participants were white.20
What is in a genotype?
HPV is implicated in progression to both squamous cell carcinoma and adenocarcinoma of the cervix. Worldwide, HPV genotypes 16 and 18 are associated with 73% of cases of invasive cervical cancer; most of the remainder are associated with, in order of decreasing prevalence, genotypes 58, 33, 45, 31, 52, 35, 59, 39, 51, and 56.21
High-grade cervical lesions in African American women may less often be positive for HPV 16 and 18 than in white women.22,23 On the other hand, the proportion of non-Hispanic black women infected with HPV 35 and 58 was significantly higher than in non-Hispanic white women.22 Regardless, HPV screening is recommended for women of all races and ethnicities.
The 2-valent and 4-valent HPV vaccines do not cover HPV 35 or 58. The newer 9-valent vaccine covers HPV 58 (but not 35) and so may in theory decrease any potential disparity related to infection with a specific oncogenic subtype.
THE ROLE OF PREVENTION
HPV vaccination
The Females United to Unilaterally Reduce Endo/Ectocervical Disease study demonstrated that the 4-valent vaccine was highly effective against cervical intraepithelial neoplasia due to HPV 16 and 18.24 In another study, the 2-valent vaccine reduced the incidence of CIN 3 or higher by 87% in women who received all 3 doses and who had no evidence of HPV infection at baseline.25
HPV vaccination is expensive. Each shot costs about $130, plus the cost of administering it. Although the Vaccines for Children program covers the HPV vaccine for uninsured and underinsured children and adolescents under age 19, Medicaid coverage varies from state to state for adults over age 21.
The Advisory Committee on Immunization Practices (ACIP)26 recommends routine vaccination for:
- Males 11 or 12 years old
- Females ages 9 to 26.
In October 2016, the ACIP approved a 2-dose series given 6 to 12 months apart for patients starting vaccination at ages 9 through 14 years who are not immunocompromised. Others should receive a 3-dose series, with the second dose given 1 to 2 months after the first dose and the third dose given 6 months after the first dose.27 Previously, 3 doses were recommended for everyone.
Disparities in HPV vaccination rates
HPV vaccination rates among adolescents in the United States increased from 33.6% in 2013 to 41.7% in 2014.28 However, HPV vaccination rates continue to lag behind those of other routine vaccines, such as Tdap and meningococcal conjugate.
Reagan-Steiner et al28 reported that more black than white girls age 13 through 17 received at least 1 dose of a 3-dose HPV vaccination series, but more white girls received all 3 doses (70.6% vs 61.6%). In contrast, a meta-analysis by Fisher et al29 found African American and uninsured women generally less likely to initiate the HPV vaccination series. Kessels et al30 reported similar findings.
Barriers to HPV vaccination
Barriers to HPV vaccination can be provider-dependent, parental, or institutional.
Malo et al31 surveyed Florida Medicaid providers and found that those who participated in the Vaccines for Children program were less likely to cite lack of reimbursement as a barrier to vaccination.
Meites et al32 surveyed sexually transmitted disease clinics and found that common reasons for not offering HPV vaccine were cost, staff time, and difficulty coordinating follow-up visits to complete the series.
Providers report lack of urgency or lack of perception of cervical cancer as a true public health threat, safety concerns regarding the vaccine, and the inability to coadminister vaccines as barriers.33
Studies have shown that relatively few parents (up to 18%) of parents are concerned about the effect of the vaccine on sexual activity.34 Rather, they are most likely to cite lack of information regarding the vaccine, lack of physician recommendation, and not knowing where to receive the vaccine as barriers.35,36
Guerry et al37 determined that the single most important factor in vaccine initiation was physician recommendation, a finding reiterated in other studies.35,38 A study in North Carolina identified failure of physician recommendation as one of the missed opportunities for vaccination of young women.39
Therefore, the primary care physician, as the initial contact with the child or young adult, holds a responsibility to narrow this gap. In simply discussing and recommending the vaccine, physicians could increase vaccination rates.
REPRODUCTIVE HEALTH
Although 80% of women will be infected with HPV in their lifetime, only a small proportion will develop cervical cancer, suggesting there are other cofactors in the progression to cervical cancer.40
Given the infectious etiology of cervical cancer, other contributing reproductive health factors have been described. As expected, the number of sexual partners correlates with HPV infection.41,42 Younger age at first intercourse has been linked to development of cervical neoplasia, consistent with persistent infection leading to neoplasia.41,42
Primary care physicians should provide timely and comprehensive sexual education, including information on safe sexual practices and pregnancy prevention.
Human immunodeficiency virus
In 2010, the estimated rate of new human immunodeficiency virus (HIV) infections in African American women was nearly 20 times greater than in white women.43 Previous studies have shown a clear relationship between HIV and HPV-associated cancers, including cervical neoplasia and invasive cervical cancer.44,45
Women with HIV should receive screening for cervical cancer at the time of diagnosis, 6 months after the initial diagnosis, and annually thereafter.46
Conflicting evidence exists regarding the effect of highly active antiretroviral therapy on the incidence of HPV-related disease, so aggressive screening and management of cervical neoplasia is recommended for women with HIV, regardless of CD4+ levels or viral load.47–49
Additional infectious culprits
Coinfection with other sexually transmitted infections, specifically Chlamydia, herpes, and HIV, has been associated with cervical neoplasia and invasive cervical cancer. A positive linear association exists between the number of sexually transmitted infections and cervical neoplasia.50
C trachomatis is the most common sexually transmitted infection in the United States, with a 6-times higher rate in African American women.51 Women who are seropositive for C trachomatis are at twofold higher risk of developing squamous cell cervical cancer.52,53 Women who are seropositive for Chlamydia infection, herpes virus 2, or HPV are at markedly increased risk of invasive cervical cancer.50
Tobacco use
The negative impact of smoking on numerous other cancers resulted in investigation of its role in cervical cancer.
Early case-control studies found an association between cervical cancer and smoking,54 but because these studies did not account for HPV infection status, they could not establish causality. Subsequently, several studies did control for HPV infection; the risk of squamous cervical cancer was twice as high in women who had ever smoked.55 Furthermore, the more cigarettes smoked per day, the higher the risk of cervical neoplasia.41,56
According to the US Centers for Disease Control and Prevention in 2014, the highest prevalence of smoking was among American Indian and Alaskan Native women, 32.5% of whom said they smoked every day, compared with 17.2% of white women and 13.7% of African American women.57
HOW CAN PRIMARY CARE PHYSICIANS CLOSE THE GAP?
By promoting HPV vaccination to children and young adults, primary care physicians can help prevent cervical cancer. Moreover, primary care physicians will see most adolescents for a nonpreventive health visit, an optimal opportunity to discuss sexual activity practices and HPV vaccination.58 Including the HPV vaccine as routine with other vaccinations can close the gap.38
Screening and treatment of sexually transmitted infection during these visits can affect the risk that future HPV infection will progress to neoplasia or cancer. Persistent lifestyle modification counseling, especially smoking cessation through motivational interviewing, can lessen the risk of cervical cancer neoplasia progression.
Additionally, in light of recent changes in cervical cancer screening guidelines, the primary care physician’s role as educator is of utmost importance. In one study, although 99% of women had received a Pap test, 87% could not identify the purpose of the Pap test.59 The primary care physician’s role is perhaps the most influential in preventing disease and, as such, has the greatest impact on a patient’s disease process.
African American, Hispanic, American Indian, and Alaskan Native women continue to be disproportionately affected by cervical cancer compared with white women. From 2006 to 2010, the incidence of cervical cancer in African American women was 10.3 per 100,000; in white women it was 7.2.1 The mortality rate from cervical cancer in African American women is twice that in white women.1 Although cervical cancer rates have decreased nationwide, significant racial health disparities persist.
As the first-line healthcare providers for many women, the primary care physician and the general obstetrician-gynecologist are optimally positioned to reduce these disparities.
Cervical cancer is the third most common gynecologic cancer, after uterine and ovarian cancer. Nearly 13,000 new cases are diagnosed each year in the United States, and more than 4,000 women die of it.2 Fortunately, cervical cancer can be significantly prevented with adequate screening and vaccination against human papillomavirus (HPV).
WHY ARE BLACK WOMEN MORE LIKELY TO DIE OF CERVICAL CANCER?
Later stage at diagnosis. African American women are more likely to present with advanced cervical cancer than non-Hispanic white women.3–6
Less-aggressive treatment. African American women are more likely to receive no treatment after a cancer diagnosis.6 Differences in treatment may be attributed to comorbid conditions, stage at cancer diagnosis, and patient refusal.5,7
Less access to care. A study from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute looked at 7,267 women (4,431 non-Hispanic white women, 1,830 Hispanic white women, and 1,006 non-Hispanic African American women) who were diagnosed with primary invasive cervical cancer from 1992 to 1996 and followed through 2000. African American women had a 19% higher mortality rate compared with non-Hispanic white women during follow-up despite adjusting for age, stage, histology, and time of first treatment.8
However, a later study from the same program found no such difference after 1995, when the data were adjusted for marital status, disease stage, age, treatment, grade, and histology.6
Equal access to healthcare may eliminate most of the disparity.7 A study in women with cervical cancer who sought treatment within the United States military healthcare system found no difference in treatment or 5- and 10-year survival rates between African American and white women.5 Equal access to comprehensive healthcare eliminated any disparity once cervical cancer was diagnosed.
CERVICAL CANCER SCREENING
The value of cervical cancer screening and prevention is well established. In 1941, Papanicolau reported that cervical cancer could be detected from vaginal smears.9 Since the development and widespread implementation of the “Pap” smear, cervical cancer rates have decreased dramatically in the United States.
Another major advance was the discovery that persistent infection with HPV is necessary for the development of cervical cancer, precancerous lesions, and genital warts.10
With advancing research, guidelines for cervical cancer screening have changed considerably over the years. Today, combined cervical cytologic and HPV testing is the mainstay. (Isolated HPV testing is generally not available outside clinical trials.)
Who should be screened?
Previous recommendations called for women to undergo Pap testing when they first became sexually active and then every year. However, cervical lesions are likely to regress in young women.11 One study found that 28% of cervical intimal neoplasia (CIN) grade 2 and 3 lesions spontaneously regressed by 15 weeks, although lesions associated with HPV 16 infection were less likely to regress than with other HPV types.12 A study of college women found that HPV infection persisted in only 9% of women after 24 months.13
To minimize unnecessary treatment of young women with dysplasia, the American Society for Colposcopy and Cervical Pathology in 2012 recommended cytologic screening for all women 21 years or older, regardless of age at first sexual encounter.14 Screening intervals were changed from every year to every 3 years until age 30, at which time cotesting with cytology and HPV testing is performed every 5 years. Routine cotesting is not recommended for women younger than 30, who have a high likelihood of HPV infection and spontaneous regression.
In 2014, the US Food and Drug Administration approved primary HPV screening (ie, testing for HPV first, and then performing cytology in samples that test positive) for women age 25 and older.15
Patients who need further evaluation and testing should be referred for colposcopy. The current guidelines for patients who have abnormal results on cervical cancer screening16 can be reviewed at www.asccp.org/asccp-guidelines.
As screening guidelines continue to evolve, primary care physicians will need to stay current and also help educate their patients. For example, many of our patients have undergone annual Pap screening for most of their lives and may not yet know about the new testing intervals.
Are there disparities in screening and follow-up?
Disparities in screening and follow-up may exist, but the evidence is not clear-cut.
In a 2013 National Health Interview Survey report, the rates of cervical cancer screening with Pap tests did not differ between African American and white women.17 However, the information on Pap testing was based on a single question asking participants if they had had a Pap test in the last 3 years. In our experience, patients may confuse Pap tests with speculum examinations.
Once women are screened, adequate and timely follow-up of abnormal results is key.
In a study from the National Breast and Cervical Cancer Early Detection Program,18 women who had cytology findings of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesions were to undergo repeat Pap testing every 4 to 6 months for 2 years. African American women were the least likely to have a follow-up Pap smear compared with other racial groups.
On the other hand, there was no difference related to race in follow-up rates of abnormal Pap tests in women ages 47 to 64 in the South Carolina Breast and Cervical Cancer Early Detection Program.19
In a study in an urban population (predominantly African African), the overall follow-up rate was only 26% at 12 months from an initial abnormal Pap smear. This study did not find any differences in follow-up according to race or ethnicity; however, it had insufficient power to detect a difference because only 15% of the study participants were white.20
What is in a genotype?
HPV is implicated in progression to both squamous cell carcinoma and adenocarcinoma of the cervix. Worldwide, HPV genotypes 16 and 18 are associated with 73% of cases of invasive cervical cancer; most of the remainder are associated with, in order of decreasing prevalence, genotypes 58, 33, 45, 31, 52, 35, 59, 39, 51, and 56.21
High-grade cervical lesions in African American women may less often be positive for HPV 16 and 18 than in white women.22,23 On the other hand, the proportion of non-Hispanic black women infected with HPV 35 and 58 was significantly higher than in non-Hispanic white women.22 Regardless, HPV screening is recommended for women of all races and ethnicities.
The 2-valent and 4-valent HPV vaccines do not cover HPV 35 or 58. The newer 9-valent vaccine covers HPV 58 (but not 35) and so may in theory decrease any potential disparity related to infection with a specific oncogenic subtype.
THE ROLE OF PREVENTION
HPV vaccination
The Females United to Unilaterally Reduce Endo/Ectocervical Disease study demonstrated that the 4-valent vaccine was highly effective against cervical intraepithelial neoplasia due to HPV 16 and 18.24 In another study, the 2-valent vaccine reduced the incidence of CIN 3 or higher by 87% in women who received all 3 doses and who had no evidence of HPV infection at baseline.25
HPV vaccination is expensive. Each shot costs about $130, plus the cost of administering it. Although the Vaccines for Children program covers the HPV vaccine for uninsured and underinsured children and adolescents under age 19, Medicaid coverage varies from state to state for adults over age 21.
The Advisory Committee on Immunization Practices (ACIP)26 recommends routine vaccination for:
- Males 11 or 12 years old
- Females ages 9 to 26.
In October 2016, the ACIP approved a 2-dose series given 6 to 12 months apart for patients starting vaccination at ages 9 through 14 years who are not immunocompromised. Others should receive a 3-dose series, with the second dose given 1 to 2 months after the first dose and the third dose given 6 months after the first dose.27 Previously, 3 doses were recommended for everyone.
Disparities in HPV vaccination rates
HPV vaccination rates among adolescents in the United States increased from 33.6% in 2013 to 41.7% in 2014.28 However, HPV vaccination rates continue to lag behind those of other routine vaccines, such as Tdap and meningococcal conjugate.
Reagan-Steiner et al28 reported that more black than white girls age 13 through 17 received at least 1 dose of a 3-dose HPV vaccination series, but more white girls received all 3 doses (70.6% vs 61.6%). In contrast, a meta-analysis by Fisher et al29 found African American and uninsured women generally less likely to initiate the HPV vaccination series. Kessels et al30 reported similar findings.
Barriers to HPV vaccination
Barriers to HPV vaccination can be provider-dependent, parental, or institutional.
Malo et al31 surveyed Florida Medicaid providers and found that those who participated in the Vaccines for Children program were less likely to cite lack of reimbursement as a barrier to vaccination.
Meites et al32 surveyed sexually transmitted disease clinics and found that common reasons for not offering HPV vaccine were cost, staff time, and difficulty coordinating follow-up visits to complete the series.
Providers report lack of urgency or lack of perception of cervical cancer as a true public health threat, safety concerns regarding the vaccine, and the inability to coadminister vaccines as barriers.33
Studies have shown that relatively few parents (up to 18%) of parents are concerned about the effect of the vaccine on sexual activity.34 Rather, they are most likely to cite lack of information regarding the vaccine, lack of physician recommendation, and not knowing where to receive the vaccine as barriers.35,36
Guerry et al37 determined that the single most important factor in vaccine initiation was physician recommendation, a finding reiterated in other studies.35,38 A study in North Carolina identified failure of physician recommendation as one of the missed opportunities for vaccination of young women.39
Therefore, the primary care physician, as the initial contact with the child or young adult, holds a responsibility to narrow this gap. In simply discussing and recommending the vaccine, physicians could increase vaccination rates.
REPRODUCTIVE HEALTH
Although 80% of women will be infected with HPV in their lifetime, only a small proportion will develop cervical cancer, suggesting there are other cofactors in the progression to cervical cancer.40
Given the infectious etiology of cervical cancer, other contributing reproductive health factors have been described. As expected, the number of sexual partners correlates with HPV infection.41,42 Younger age at first intercourse has been linked to development of cervical neoplasia, consistent with persistent infection leading to neoplasia.41,42
Primary care physicians should provide timely and comprehensive sexual education, including information on safe sexual practices and pregnancy prevention.
Human immunodeficiency virus
In 2010, the estimated rate of new human immunodeficiency virus (HIV) infections in African American women was nearly 20 times greater than in white women.43 Previous studies have shown a clear relationship between HIV and HPV-associated cancers, including cervical neoplasia and invasive cervical cancer.44,45
Women with HIV should receive screening for cervical cancer at the time of diagnosis, 6 months after the initial diagnosis, and annually thereafter.46
Conflicting evidence exists regarding the effect of highly active antiretroviral therapy on the incidence of HPV-related disease, so aggressive screening and management of cervical neoplasia is recommended for women with HIV, regardless of CD4+ levels or viral load.47–49
Additional infectious culprits
Coinfection with other sexually transmitted infections, specifically Chlamydia, herpes, and HIV, has been associated with cervical neoplasia and invasive cervical cancer. A positive linear association exists between the number of sexually transmitted infections and cervical neoplasia.50
C trachomatis is the most common sexually transmitted infection in the United States, with a 6-times higher rate in African American women.51 Women who are seropositive for C trachomatis are at twofold higher risk of developing squamous cell cervical cancer.52,53 Women who are seropositive for Chlamydia infection, herpes virus 2, or HPV are at markedly increased risk of invasive cervical cancer.50
Tobacco use
The negative impact of smoking on numerous other cancers resulted in investigation of its role in cervical cancer.
Early case-control studies found an association between cervical cancer and smoking,54 but because these studies did not account for HPV infection status, they could not establish causality. Subsequently, several studies did control for HPV infection; the risk of squamous cervical cancer was twice as high in women who had ever smoked.55 Furthermore, the more cigarettes smoked per day, the higher the risk of cervical neoplasia.41,56
According to the US Centers for Disease Control and Prevention in 2014, the highest prevalence of smoking was among American Indian and Alaskan Native women, 32.5% of whom said they smoked every day, compared with 17.2% of white women and 13.7% of African American women.57
HOW CAN PRIMARY CARE PHYSICIANS CLOSE THE GAP?
By promoting HPV vaccination to children and young adults, primary care physicians can help prevent cervical cancer. Moreover, primary care physicians will see most adolescents for a nonpreventive health visit, an optimal opportunity to discuss sexual activity practices and HPV vaccination.58 Including the HPV vaccine as routine with other vaccinations can close the gap.38
Screening and treatment of sexually transmitted infection during these visits can affect the risk that future HPV infection will progress to neoplasia or cancer. Persistent lifestyle modification counseling, especially smoking cessation through motivational interviewing, can lessen the risk of cervical cancer neoplasia progression.
Additionally, in light of recent changes in cervical cancer screening guidelines, the primary care physician’s role as educator is of utmost importance. In one study, although 99% of women had received a Pap test, 87% could not identify the purpose of the Pap test.59 The primary care physician’s role is perhaps the most influential in preventing disease and, as such, has the greatest impact on a patient’s disease process.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw 2015; 13:395-404.
- Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer 2007; 110:234–243.
- Farley JH, Hines JF, Taylor RR, et al. Equal care ensures equal survival for African-American women with cervical carcinoma. Cancer 2001; 91:869–873.
- Rauh-Hain JA, Clemmer JT, Bradford LS, et al. Racial disparities in cervical cancer survival over time. Cancer 2013; 119:3644–3652.
- Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014; 133:353–361.
- Patel DA, Barnholtz-Sloan JS, Patel MK, Malone JM Jr, Chuba PJ, Schwartz K. A population-based study of racial and ethnic differences in survival among women with invasive cervical cancer: analysis of surveillance, epidemiology, and end results data. Gynecol Oncol 2005; 97:550–558.
- Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. 1941. Arch Pathol Lab Med 1997; 121:211–224.
- Walboomers JM, Jacobs M V, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 2004; 364:1678–1683.
- Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 2005; 11:4717–4723.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423-428.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125:330–337.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013; 17(suppl 1):S1–S27.
- Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use—United States, 2013. MMWR 2015; 64:464–468.
- Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005; 105:1323–1328.
- Eggleston KS, Coker AL, Luchok KJ, Meyer TE. Adherence to recommendations for follow-up to abnormal Pap tests. Obstet Gynecol 2007; 109:1332–1341.
- Peterson NB, Han J, Freund KM. Inadequate follow-up for abnormal Pap smears in an urban population. J Natl Med Assoc 2003; 95:825–832.
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927–935.
- Hariri S, Unger ER, Powell SE, et al; HPV-IMPACT Working Group. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 2012; 206:1878–1886.
- Niccolai LM, Russ C, Julian PJ, et al. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions: associations with race, ethnicity, and poverty. Cancer 2013; 119:3052–3058.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1705–1708.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65:1405–1408.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:784–792.
- Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol 2013; 42:896–908.
- Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine 2012; 30:3546–3556.
- Malo TL, Hassani D, Staras SA, Shenkman EA, Giuliano AR, Vadaparampil ST. Do Florida Medicaid providers’ barriers to HPV vaccination vary based on VFC program participation? Matern Child Health J 2013; 17:609–615.
- Meites E, Llata E, Hariri S, et al. HPV vaccine implementation in STD clinics—STD Surveillance Network. Sex Transm Dis 2012; 39:32–34.
- Perkins RB, Clark JA. What affects human papillomavirus vaccination rates? A qualitative analysis of providers’ perceptions. Womens Health Issues 2012; 22:e379–e386.
- Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014; 168:76–82.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Bastani R, Glenn BA, Tsui J, et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev 2011; 20:1463–1472.
- Guerry SL, De Rosa CJ, Markowitz LE, et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine 2011; 29:2235–2241.
- Hull PC, Williams EA, Khabele D, Dean C, Bond B, Sanderson M. HPV vaccine use among African American girls: qualitative formative research using a participatory social marketing approach. Gynecol Oncol 2014; 132(suppl 1):S13–S20.
- Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011; 38:197–204.
- Wang SS, Zuna RE, Wentzensen N, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev 2009; 18:113–120.
- Deacon JM, Evans CD, Yule R, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case-control study nested within the Manchester cohort. Br J Cancer 2000; 83:1565–1572.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and sexual behavior: collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol Biomarkers Prev 2009; 18:1060–1069.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17(No. 4). https://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed September 12, 2017.
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000; 92:1500–1510.
- Schäfer A, Friedmann W, Mielke M, Schwartländer B, Koch MA. The increased frequency of cervical dysplasia-neoplasia in women infected with the human immunodeficiency virus is related to the degree of immunosuppression. Am J Obstet Gynecol 1991; 164:593–599.
- Phillips AA, Justman JE. Screening HIV-infected patients for non-AIDS-defining malignancies. Curr HIV/AIDS Rep 2009; 6:83–92.
- De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev 2008; 17:545–554.
- Palefsky JM. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol 2003; 15:382–388.
- Adler DH. The impact of HAART on HPV-related cervical disease. Curr HIV Res 2010; 8:493–497.
- Castellsagué X, Pawlita M, Roura E, et al. Prospective seroepidemiologic study on the role of human papillomavirus and other infections in cervical carcinogenesis: evidence from the EPIC cohort. Int J Cancer 2014; 135:440–452.
- Centers for Disease Control and Prevention (CDC). 2013 sexually transmitted disease surveillance. www.cdc.gov/std/stats13/exordium.htm. Accessed September 12, 2017.
- Smith JS, Bosetti C, Muñoz N, et al; IARC multicentric case-control study. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer 2004; 111:431–439.
- Koskela P, Anttila T, Bjørge T, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer 2000; 85:35–39.
- Office on Smoking and Health (US). Women and smoking: a report of the Surgeon General: Chapter 3. Health consequences of tobacco use among women. http://www.ncbi.nlm.nih.gov/books/NBK44312. Accessed September 12, 2017.
- Plummer M, Herrero R, Franceschi S, et al; IARC Multi-centre Cervical Cancer Study Group. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case—control study. Cancer Causes Control 2003; 14:805–814.
- Ho GY, Kadish AS, Burk RD, et al. HPV 16 and cigarette smoking as risk factors for high-grade cervical intra-epithelial neoplasia. Int J Cancer 1998; 78:281–285.
- Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep 2015; 64:1233–1240.
- Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med 2010; 8:511–516.
- Lindau ST, Tomori C, Lyons T, Langseth L, Bennett CL, Garcia P. The association of health literacy with cervical cancer prevention knowledge and health behaviors in a multiethnic cohort of women. Am J Obstet Gynecol 2002; 186:938–943.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29.
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw 2015; 13:395-404.
- Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer 2007; 110:234–243.
- Farley JH, Hines JF, Taylor RR, et al. Equal care ensures equal survival for African-American women with cervical carcinoma. Cancer 2001; 91:869–873.
- Rauh-Hain JA, Clemmer JT, Bradford LS, et al. Racial disparities in cervical cancer survival over time. Cancer 2013; 119:3644–3652.
- Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014; 133:353–361.
- Patel DA, Barnholtz-Sloan JS, Patel MK, Malone JM Jr, Chuba PJ, Schwartz K. A population-based study of racial and ethnic differences in survival among women with invasive cervical cancer: analysis of surveillance, epidemiology, and end results data. Gynecol Oncol 2005; 97:550–558.
- Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. 1941. Arch Pathol Lab Med 1997; 121:211–224.
- Walboomers JM, Jacobs M V, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 2004; 364:1678–1683.
- Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 2005; 11:4717–4723.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423-428.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012; 62:147–172.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125:330–337.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013; 17(suppl 1):S1–S27.
- Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use—United States, 2013. MMWR 2015; 64:464–468.
- Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol 2005; 105:1323–1328.
- Eggleston KS, Coker AL, Luchok KJ, Meyer TE. Adherence to recommendations for follow-up to abnormal Pap tests. Obstet Gynecol 2007; 109:1332–1341.
- Peterson NB, Han J, Freund KM. Inadequate follow-up for abnormal Pap smears in an urban population. J Natl Med Assoc 2003; 95:825–832.
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927–935.
- Hariri S, Unger ER, Powell SE, et al; HPV-IMPACT Working Group. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 2012; 206:1878–1886.
- Niccolai LM, Russ C, Julian PJ, et al. Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions: associations with race, ethnicity, and poverty. Cancer 2013; 119:3052–3058.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1705–1708.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65:1405–1408.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:784–792.
- Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol 2013; 42:896–908.
- Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine 2012; 30:3546–3556.
- Malo TL, Hassani D, Staras SA, Shenkman EA, Giuliano AR, Vadaparampil ST. Do Florida Medicaid providers’ barriers to HPV vaccination vary based on VFC program participation? Matern Child Health J 2013; 17:609–615.
- Meites E, Llata E, Hariri S, et al. HPV vaccine implementation in STD clinics—STD Surveillance Network. Sex Transm Dis 2012; 39:32–34.
- Perkins RB, Clark JA. What affects human papillomavirus vaccination rates? A qualitative analysis of providers’ perceptions. Womens Health Issues 2012; 22:e379–e386.
- Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014; 168:76–82.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Bastani R, Glenn BA, Tsui J, et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev 2011; 20:1463–1472.
- Guerry SL, De Rosa CJ, Markowitz LE, et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine 2011; 29:2235–2241.
- Hull PC, Williams EA, Khabele D, Dean C, Bond B, Sanderson M. HPV vaccine use among African American girls: qualitative formative research using a participatory social marketing approach. Gynecol Oncol 2014; 132(suppl 1):S13–S20.
- Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011; 38:197–204.
- Wang SS, Zuna RE, Wentzensen N, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev 2009; 18:113–120.
- Deacon JM, Evans CD, Yule R, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case-control study nested within the Manchester cohort. Br J Cancer 2000; 83:1565–1572.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and sexual behavior: collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol Biomarkers Prev 2009; 18:1060–1069.
- Centers for Disease Control and Prevention (CDC). Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17(No. 4). https://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed September 12, 2017.
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000; 92:1500–1510.
- Schäfer A, Friedmann W, Mielke M, Schwartländer B, Koch MA. The increased frequency of cervical dysplasia-neoplasia in women infected with the human immunodeficiency virus is related to the degree of immunosuppression. Am J Obstet Gynecol 1991; 164:593–599.
- Phillips AA, Justman JE. Screening HIV-infected patients for non-AIDS-defining malignancies. Curr HIV/AIDS Rep 2009; 6:83–92.
- De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev 2008; 17:545–554.
- Palefsky JM. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol 2003; 15:382–388.
- Adler DH. The impact of HAART on HPV-related cervical disease. Curr HIV Res 2010; 8:493–497.
- Castellsagué X, Pawlita M, Roura E, et al. Prospective seroepidemiologic study on the role of human papillomavirus and other infections in cervical carcinogenesis: evidence from the EPIC cohort. Int J Cancer 2014; 135:440–452.
- Centers for Disease Control and Prevention (CDC). 2013 sexually transmitted disease surveillance. www.cdc.gov/std/stats13/exordium.htm. Accessed September 12, 2017.
- Smith JS, Bosetti C, Muñoz N, et al; IARC multicentric case-control study. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer 2004; 111:431–439.
- Koskela P, Anttila T, Bjørge T, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer 2000; 85:35–39.
- Office on Smoking and Health (US). Women and smoking: a report of the Surgeon General: Chapter 3. Health consequences of tobacco use among women. http://www.ncbi.nlm.nih.gov/books/NBK44312. Accessed September 12, 2017.
- Plummer M, Herrero R, Franceschi S, et al; IARC Multi-centre Cervical Cancer Study Group. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case—control study. Cancer Causes Control 2003; 14:805–814.
- Ho GY, Kadish AS, Burk RD, et al. HPV 16 and cigarette smoking as risk factors for high-grade cervical intra-epithelial neoplasia. Int J Cancer 1998; 78:281–285.
- Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep 2015; 64:1233–1240.
- Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med 2010; 8:511–516.
- Lindau ST, Tomori C, Lyons T, Langseth L, Bennett CL, Garcia P. The association of health literacy with cervical cancer prevention knowledge and health behaviors in a multiethnic cohort of women. Am J Obstet Gynecol 2002; 186:938–943.




