User login
Recovery of Hair in the Psoriatic Plaques of a Patient With Coexistent Alopecia Universalis
To the Editor:
Both alopecia areata (AA) and psoriasis vulgaris are chronic relapsing autoimmune diseases, with AA causing nonscarring hair loss in approximately 0.1% to 0.2%1 of the population with a lifetime risk of 1.7%,2 and psoriasis more broadly impacting 1.5% to 2% of the population.3 The helper T cell (TH1) cytokine milieu is pathogenic in both conditions.4-6 IFN-γ knockout mice, unlike their wild-type counterparts, do not exhibit AA.7 Psoriasis is notably improved by IL-10 injections, which dampen the TH1 response.8 Distinct from AA, TH17 and TH22 cells have been implicated as key players in psoriasis pathogenesis, along with the associated IL-17 and IL-22 cytokines.9-12
Few cases of patients with concurrent AA and psoriasis have been described. Interestingly, these cases document normal hair regrowth in the areas of psoriasis.13-16 These cases may offer unique insight into the immune factors driving each disease. We describe a case of a man with both alopecia universalis (AU) and psoriasis who developed hair regrowth in some of the psoriatic plaques.
A 34-year-old man with concurrent AU and psoriasis who had not used any systemic or topical medication for either condition in the last year presented to our clinic seeking treatment. The patient had a history of alopecia totalis as a toddler that completely resolved by 4 years of age with the use of squaric acid dibutylester (SADBE). At 31 years of age, the alopecia recurred and was localized to the scalp. It was partially responsive to intralesional triamcinolone acetonide. The patient’s alopecia worsened over the 2 years following recurrence, ultimately progressing to AU. Two months after the alopecia recurrence, he developed the first psoriatic plaques. As the plaque psoriasis progressed, systemic therapy was initiated, first methotrexate and then etanercept. Shortly after developing AU, he lost his health insurance and discontinued all therapy. The patient’s psoriasis began to recur approximately 3 months after stopping etanercept. He was not using any other psoriasis medications. At that time, he noted terminal hair regrowth within some of the psoriatic plaques. No terminal hairs grew outside of the psoriatic plaques, and all regions with growth had previously been without hair for an extended period of time. The patient presented to our clinic approximately 1 year later. He had no other medical conditions and no relevant family history.
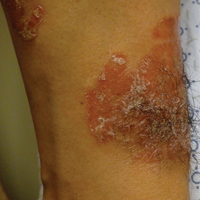
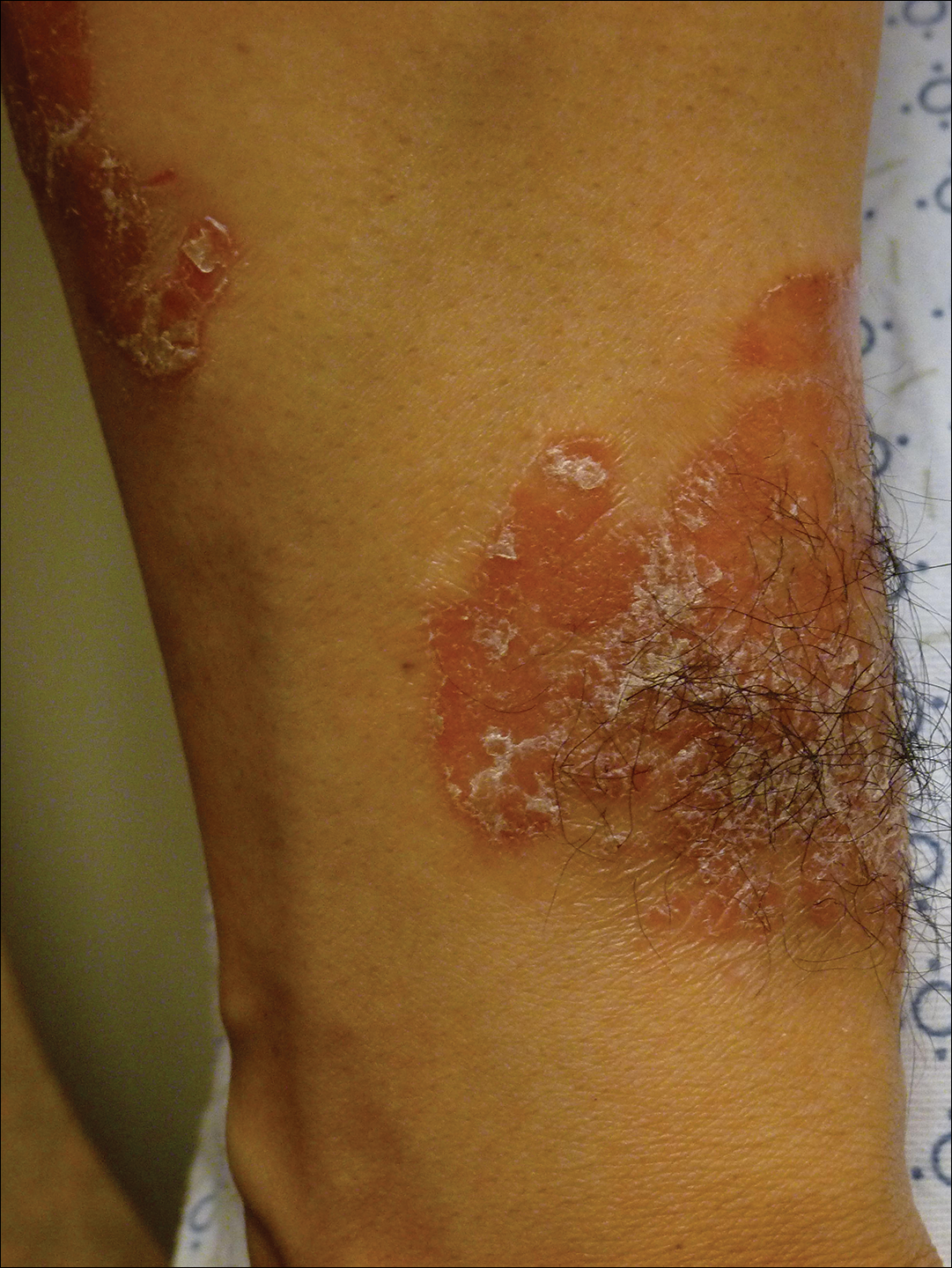
On initial physical examination, he had nonscarring hair loss involving nearly 100% of the body with psoriatic plaques on approximately 30% of the body surface area. Regions of terminal hair growth were confined to some but not all of the psoriatic plaques (Figure). Interestingly, the terminal hairs were primarily localized to the thickest central regions of the plaques. The patient’s psoriasis was treated with a combination of topical clobetasol and calcipotriene. In addition, he was started on tacrolimus ointment to the face and eyebrows for the AA. Maintenance of terminal hair within a region of topically treated psoriasis on the forearm persisted at the 2-month follow-up despite complete clearance of the corresponding psoriatic plaque. A small psoriatic plaque on the scalp cleared early with topical therapy without noticeable hair regrowth. The patient subsequently was started on contact immunotherapy with SADBE and intralesional triamcinolone acetonide for the scalp alopecia without satisfactory response. He decided to discontinue further attempts at treating the alopecia and requested to be restarted on etanercept therapy for recalcitrant psoriatic plaques. His psoriasis responded well to this therapy and he continues to be followed in our psoriasis clinic. One year after clearance of the treated psoriatic plaques, the corresponding terminal hairs persist.
Contact immunotherapy, most commonly with diphenylcyclopropenone or SADBE, is reported to have a 50% to 60% success rate in extensive AA, with a broad range of 9% to 87%17; however, randomized controlled trials testing the efficacy of contact immunotherapy are lacking. Although the mechanism of action of these topical sensitizers is not clearly delineated, it has been postulated that by inducing a new type of inflammatory response in the region, the immunologic milieu is changed, allowing the hair to grow. Some proposed mechanisms include promoting perifollicular lymphocyte apoptosis, preventing new recruitment of autoreactive lymphocytes, and allowing for the correction of aberrant major histocompatibility complex expression on the hair matrix epithelium to regain follicle immune privilege.18-20
Iatrogenic immunotherapy may work analogously to the natural immune system deviation demonstrated in our patient. Psoriasis and AA are believed to form competing immune cells and cytokine milieus, thus explaining how an individual with AA could regain normal hair growth in areas of psoriasis.15,16 The Renbök phenomenon, or reverse Köbner phenomenon, coined by Happle et al13 can be used to describe both the iatrogenic and natural cases of dermatologic disease improvement in response to secondary insults.14
A complex cascade of immune cells and cytokines coordinate AA pathogenesis. In the acute stage of AA, an inflammatory infiltrate of CD4+ T cells, CD8+ T cells, and antigen-presenting cells target anagen phase follicles, with a higher CD4+:CD8+ ratio in clinically active disease.21-23 Subcutaneous injections of either CD4+ or CD8+ lymphocyte subsets from mice with AA into normal-haired mice induces disease. However, CD8+ T cell injections rapidly produce apparent hair loss, whereas CD4+ T cells cause hair loss after several weeks, suggesting that CD8+ T cells directly modulate AA hair loss and CD4+ T cells act as an aide.24 The growth, differentiation, and survival of CD8+ T cells are stimulated by IL-2 and IFN-γ. Alopecia areata biopsies demonstrate a prevalence of TH1 cytokines, and patients with localized AA, alopecia totalis, and AU have notably higher serum IFN-γ levels compared to controls.25 In murine models, IL-1α and IL-1β increase during the catagen phase of the hair cycle and peak during the telogen phase.26 Excessive IL-1β expression is detected in the early stages of human disease, and certain IL-1β polymorphisms are associated with severe forms of AA.26 The role of tumor necrosis factor (TNF) α in AA is not well understood. In vitro studies show it inhibits hair growth, suggesting the cytokine may play a role in AA.27 However, anti–TNF-α therapy is not effective in AA, and case reports propose these therapies rarely induce AA.28-31
The TH1 response is likewise critical to psoriatic plaque development. IFN-γ and TNF-α are overexpressed in psoriatic plaques.32 IFN-γ has an antiproliferative and differentiation-inducing effect on normal keratinocytes, but psoriatic epithelial cells in vitro respond differently to the cytokine with a notably diminished growth inhibition.33,34 One explanation for the role of IFN-γ is that it stimulates dendritic cells to produce IL-1 and IL-23.35 IL-23 activates TH17 cells36; TH1 and TH17 conditions produce IL-22 whose serum level correlates with disease severity.37-39 IL-22 induces keratinocyte proliferation and migration and inhibits keratinocyte differentiation, helping account for hallmarks of the disease.40 Patients with psoriasis have increased levels of TH1, TH17, and TH22 cells, as well as their associated cytokines, in the skin and blood compared to controls.4,11,32,39,41
Alopecia areata and psoriasis are regulated by complex and still not entirely understood immune interactions. The fact that many of the same therapies are used to treat both diseases emphasizes both their overlapping characteristics and the lack of targeted therapy. It is unclear if and how the topical or systemic therapies used in our patient to treat one disease affected the natural history of the other condition. It is important to highlight, however, that the patient had not been treated for months when he developed the psoriatic plaques with hair regrowth. Other case reports also document hair regrowth in untreated plaques,13,16 making it unlikely to be a side effect of the medication regimen. For both psoriasis and AA, the immune cell composition and cytokine levels in the skin or serum vary throughout a patient’s disease course depending on severity of disease or response to treatment.6,39,42,43 Therefore, we hypothesize that the 2 conditions interact in a similarly distinct manner based on each disease’s stage and intensity in the patient. Both our patient’s course thus far and the various presentations described by other groups support this hypothesis. Our patient had a small region of psoriasis on the scalp that cleared without any terminal hair growth. He also had larger plaques on the forearms that developed hair growth most predominantly within the thicker regions of the plaques. His unique presentation highlights the fluidity of the immune factors driving psoriasis vulgaris and AA.
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York, NY: McGraw-Hill; 2009.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57-62.
- Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25:781-788.
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515-521.
- Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319-329.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022-1030.e395.
- Happle R, van der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köebner reaction observed in alopecia areata. Eur J Dermatol. 1991;2:39-40.
- Ito T, Hashizume H, Takigawa M. Contact immunotherapy-induced Renbök phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol. 2010;20:126-127.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbök phenomenon. Clin Exp Dermatol. 2007;32:320-321.
- Harris JE, Seykora JT, Lee RA. Renbök phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol. 2010;146:422-425.
- Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355-363.
- Herbst V, Zöller M, Kissling S, et al. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-542.
- Zöller M, Freyschmidt-Paul P, Vitacolonna M, et al. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135:398-408.
- Bröcker EB, Echternacht-Happle K, Hamm H, et al. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564-568.
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216-223.
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26-30.
- Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276:333-334.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8+ cells induces localized hair loss whereas CD4+/CD25– cells promote systemic alopecia areata and CD4+/CD25+ cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- Arca E, Muşabak U, Akar A, et al. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004;14:33-36.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235-238.
- Philpott MP, Sanders DA, Bowen J, et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942-948.
- Le Bidre E, Chaby G, Martin L, et al. Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol. 2011;138:285-293.
- Ferran M, Calvet J, Almirall M, et al. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol. 2011;25:479-484.
- Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17:127-129.
- Pelivani N, Hassan AS, Braathen LR, et al. Alopecia areata universalis elicited during treatment with adalimumab. Dermatology. 2008;216:320-323.
- Uyemura K, Yamamura M, Fivenson DF, et al. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701-705.
- Baker BS, Powles AV, Valdimarsson H, et al. An altered response by psoriatic keratinocytes to gamma interferon. Scan J Immunol. 1988;28:735-740.
- Jackson M, Howie SE, Weller R, et al. Psoriatic keratinocytes show reduced IRF-1 and STAT-1alpha activation in response to gamma-IFN. FASEB J. 1999;13:495-502.
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385-422.
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314-324.
- Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657.
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687.
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702.
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183.
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699-711.
- Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530-533.
To the Editor:
Both alopecia areata (AA) and psoriasis vulgaris are chronic relapsing autoimmune diseases, with AA causing nonscarring hair loss in approximately 0.1% to 0.2%1 of the population with a lifetime risk of 1.7%,2 and psoriasis more broadly impacting 1.5% to 2% of the population.3 The helper T cell (TH1) cytokine milieu is pathogenic in both conditions.4-6 IFN-γ knockout mice, unlike their wild-type counterparts, do not exhibit AA.7 Psoriasis is notably improved by IL-10 injections, which dampen the TH1 response.8 Distinct from AA, TH17 and TH22 cells have been implicated as key players in psoriasis pathogenesis, along with the associated IL-17 and IL-22 cytokines.9-12
Few cases of patients with concurrent AA and psoriasis have been described. Interestingly, these cases document normal hair regrowth in the areas of psoriasis.13-16 These cases may offer unique insight into the immune factors driving each disease. We describe a case of a man with both alopecia universalis (AU) and psoriasis who developed hair regrowth in some of the psoriatic plaques.
A 34-year-old man with concurrent AU and psoriasis who had not used any systemic or topical medication for either condition in the last year presented to our clinic seeking treatment. The patient had a history of alopecia totalis as a toddler that completely resolved by 4 years of age with the use of squaric acid dibutylester (SADBE). At 31 years of age, the alopecia recurred and was localized to the scalp. It was partially responsive to intralesional triamcinolone acetonide. The patient’s alopecia worsened over the 2 years following recurrence, ultimately progressing to AU. Two months after the alopecia recurrence, he developed the first psoriatic plaques. As the plaque psoriasis progressed, systemic therapy was initiated, first methotrexate and then etanercept. Shortly after developing AU, he lost his health insurance and discontinued all therapy. The patient’s psoriasis began to recur approximately 3 months after stopping etanercept. He was not using any other psoriasis medications. At that time, he noted terminal hair regrowth within some of the psoriatic plaques. No terminal hairs grew outside of the psoriatic plaques, and all regions with growth had previously been without hair for an extended period of time. The patient presented to our clinic approximately 1 year later. He had no other medical conditions and no relevant family history.
On initial physical examination, he had nonscarring hair loss involving nearly 100% of the body with psoriatic plaques on approximately 30% of the body surface area. Regions of terminal hair growth were confined to some but not all of the psoriatic plaques (Figure). Interestingly, the terminal hairs were primarily localized to the thickest central regions of the plaques. The patient’s psoriasis was treated with a combination of topical clobetasol and calcipotriene. In addition, he was started on tacrolimus ointment to the face and eyebrows for the AA. Maintenance of terminal hair within a region of topically treated psoriasis on the forearm persisted at the 2-month follow-up despite complete clearance of the corresponding psoriatic plaque. A small psoriatic plaque on the scalp cleared early with topical therapy without noticeable hair regrowth. The patient subsequently was started on contact immunotherapy with SADBE and intralesional triamcinolone acetonide for the scalp alopecia without satisfactory response. He decided to discontinue further attempts at treating the alopecia and requested to be restarted on etanercept therapy for recalcitrant psoriatic plaques. His psoriasis responded well to this therapy and he continues to be followed in our psoriasis clinic. One year after clearance of the treated psoriatic plaques, the corresponding terminal hairs persist.

Contact immunotherapy, most commonly with diphenylcyclopropenone or SADBE, is reported to have a 50% to 60% success rate in extensive AA, with a broad range of 9% to 87%17; however, randomized controlled trials testing the efficacy of contact immunotherapy are lacking. Although the mechanism of action of these topical sensitizers is not clearly delineated, it has been postulated that by inducing a new type of inflammatory response in the region, the immunologic milieu is changed, allowing the hair to grow. Some proposed mechanisms include promoting perifollicular lymphocyte apoptosis, preventing new recruitment of autoreactive lymphocytes, and allowing for the correction of aberrant major histocompatibility complex expression on the hair matrix epithelium to regain follicle immune privilege.18-20
Iatrogenic immunotherapy may work analogously to the natural immune system deviation demonstrated in our patient. Psoriasis and AA are believed to form competing immune cells and cytokine milieus, thus explaining how an individual with AA could regain normal hair growth in areas of psoriasis.15,16 The Renbök phenomenon, or reverse Köbner phenomenon, coined by Happle et al13 can be used to describe both the iatrogenic and natural cases of dermatologic disease improvement in response to secondary insults.14
A complex cascade of immune cells and cytokines coordinate AA pathogenesis. In the acute stage of AA, an inflammatory infiltrate of CD4+ T cells, CD8+ T cells, and antigen-presenting cells target anagen phase follicles, with a higher CD4+:CD8+ ratio in clinically active disease.21-23 Subcutaneous injections of either CD4+ or CD8+ lymphocyte subsets from mice with AA into normal-haired mice induces disease. However, CD8+ T cell injections rapidly produce apparent hair loss, whereas CD4+ T cells cause hair loss after several weeks, suggesting that CD8+ T cells directly modulate AA hair loss and CD4+ T cells act as an aide.24 The growth, differentiation, and survival of CD8+ T cells are stimulated by IL-2 and IFN-γ. Alopecia areata biopsies demonstrate a prevalence of TH1 cytokines, and patients with localized AA, alopecia totalis, and AU have notably higher serum IFN-γ levels compared to controls.25 In murine models, IL-1α and IL-1β increase during the catagen phase of the hair cycle and peak during the telogen phase.26 Excessive IL-1β expression is detected in the early stages of human disease, and certain IL-1β polymorphisms are associated with severe forms of AA.26 The role of tumor necrosis factor (TNF) α in AA is not well understood. In vitro studies show it inhibits hair growth, suggesting the cytokine may play a role in AA.27 However, anti–TNF-α therapy is not effective in AA, and case reports propose these therapies rarely induce AA.28-31
The TH1 response is likewise critical to psoriatic plaque development. IFN-γ and TNF-α are overexpressed in psoriatic plaques.32 IFN-γ has an antiproliferative and differentiation-inducing effect on normal keratinocytes, but psoriatic epithelial cells in vitro respond differently to the cytokine with a notably diminished growth inhibition.33,34 One explanation for the role of IFN-γ is that it stimulates dendritic cells to produce IL-1 and IL-23.35 IL-23 activates TH17 cells36; TH1 and TH17 conditions produce IL-22 whose serum level correlates with disease severity.37-39 IL-22 induces keratinocyte proliferation and migration and inhibits keratinocyte differentiation, helping account for hallmarks of the disease.40 Patients with psoriasis have increased levels of TH1, TH17, and TH22 cells, as well as their associated cytokines, in the skin and blood compared to controls.4,11,32,39,41
Alopecia areata and psoriasis are regulated by complex and still not entirely understood immune interactions. The fact that many of the same therapies are used to treat both diseases emphasizes both their overlapping characteristics and the lack of targeted therapy. It is unclear if and how the topical or systemic therapies used in our patient to treat one disease affected the natural history of the other condition. It is important to highlight, however, that the patient had not been treated for months when he developed the psoriatic plaques with hair regrowth. Other case reports also document hair regrowth in untreated plaques,13,16 making it unlikely to be a side effect of the medication regimen. For both psoriasis and AA, the immune cell composition and cytokine levels in the skin or serum vary throughout a patient’s disease course depending on severity of disease or response to treatment.6,39,42,43 Therefore, we hypothesize that the 2 conditions interact in a similarly distinct manner based on each disease’s stage and intensity in the patient. Both our patient’s course thus far and the various presentations described by other groups support this hypothesis. Our patient had a small region of psoriasis on the scalp that cleared without any terminal hair growth. He also had larger plaques on the forearms that developed hair growth most predominantly within the thicker regions of the plaques. His unique presentation highlights the fluidity of the immune factors driving psoriasis vulgaris and AA.
To the Editor:
Both alopecia areata (AA) and psoriasis vulgaris are chronic relapsing autoimmune diseases, with AA causing nonscarring hair loss in approximately 0.1% to 0.2%1 of the population with a lifetime risk of 1.7%,2 and psoriasis more broadly impacting 1.5% to 2% of the population.3 The helper T cell (TH1) cytokine milieu is pathogenic in both conditions.4-6 IFN-γ knockout mice, unlike their wild-type counterparts, do not exhibit AA.7 Psoriasis is notably improved by IL-10 injections, which dampen the TH1 response.8 Distinct from AA, TH17 and TH22 cells have been implicated as key players in psoriasis pathogenesis, along with the associated IL-17 and IL-22 cytokines.9-12
Few cases of patients with concurrent AA and psoriasis have been described. Interestingly, these cases document normal hair regrowth in the areas of psoriasis.13-16 These cases may offer unique insight into the immune factors driving each disease. We describe a case of a man with both alopecia universalis (AU) and psoriasis who developed hair regrowth in some of the psoriatic plaques.
A 34-year-old man with concurrent AU and psoriasis who had not used any systemic or topical medication for either condition in the last year presented to our clinic seeking treatment. The patient had a history of alopecia totalis as a toddler that completely resolved by 4 years of age with the use of squaric acid dibutylester (SADBE). At 31 years of age, the alopecia recurred and was localized to the scalp. It was partially responsive to intralesional triamcinolone acetonide. The patient’s alopecia worsened over the 2 years following recurrence, ultimately progressing to AU. Two months after the alopecia recurrence, he developed the first psoriatic plaques. As the plaque psoriasis progressed, systemic therapy was initiated, first methotrexate and then etanercept. Shortly after developing AU, he lost his health insurance and discontinued all therapy. The patient’s psoriasis began to recur approximately 3 months after stopping etanercept. He was not using any other psoriasis medications. At that time, he noted terminal hair regrowth within some of the psoriatic plaques. No terminal hairs grew outside of the psoriatic plaques, and all regions with growth had previously been without hair for an extended period of time. The patient presented to our clinic approximately 1 year later. He had no other medical conditions and no relevant family history.
On initial physical examination, he had nonscarring hair loss involving nearly 100% of the body with psoriatic plaques on approximately 30% of the body surface area. Regions of terminal hair growth were confined to some but not all of the psoriatic plaques (Figure). Interestingly, the terminal hairs were primarily localized to the thickest central regions of the plaques. The patient’s psoriasis was treated with a combination of topical clobetasol and calcipotriene. In addition, he was started on tacrolimus ointment to the face and eyebrows for the AA. Maintenance of terminal hair within a region of topically treated psoriasis on the forearm persisted at the 2-month follow-up despite complete clearance of the corresponding psoriatic plaque. A small psoriatic plaque on the scalp cleared early with topical therapy without noticeable hair regrowth. The patient subsequently was started on contact immunotherapy with SADBE and intralesional triamcinolone acetonide for the scalp alopecia without satisfactory response. He decided to discontinue further attempts at treating the alopecia and requested to be restarted on etanercept therapy for recalcitrant psoriatic plaques. His psoriasis responded well to this therapy and he continues to be followed in our psoriasis clinic. One year after clearance of the treated psoriatic plaques, the corresponding terminal hairs persist.

Contact immunotherapy, most commonly with diphenylcyclopropenone or SADBE, is reported to have a 50% to 60% success rate in extensive AA, with a broad range of 9% to 87%17; however, randomized controlled trials testing the efficacy of contact immunotherapy are lacking. Although the mechanism of action of these topical sensitizers is not clearly delineated, it has been postulated that by inducing a new type of inflammatory response in the region, the immunologic milieu is changed, allowing the hair to grow. Some proposed mechanisms include promoting perifollicular lymphocyte apoptosis, preventing new recruitment of autoreactive lymphocytes, and allowing for the correction of aberrant major histocompatibility complex expression on the hair matrix epithelium to regain follicle immune privilege.18-20
Iatrogenic immunotherapy may work analogously to the natural immune system deviation demonstrated in our patient. Psoriasis and AA are believed to form competing immune cells and cytokine milieus, thus explaining how an individual with AA could regain normal hair growth in areas of psoriasis.15,16 The Renbök phenomenon, or reverse Köbner phenomenon, coined by Happle et al13 can be used to describe both the iatrogenic and natural cases of dermatologic disease improvement in response to secondary insults.14
A complex cascade of immune cells and cytokines coordinate AA pathogenesis. In the acute stage of AA, an inflammatory infiltrate of CD4+ T cells, CD8+ T cells, and antigen-presenting cells target anagen phase follicles, with a higher CD4+:CD8+ ratio in clinically active disease.21-23 Subcutaneous injections of either CD4+ or CD8+ lymphocyte subsets from mice with AA into normal-haired mice induces disease. However, CD8+ T cell injections rapidly produce apparent hair loss, whereas CD4+ T cells cause hair loss after several weeks, suggesting that CD8+ T cells directly modulate AA hair loss and CD4+ T cells act as an aide.24 The growth, differentiation, and survival of CD8+ T cells are stimulated by IL-2 and IFN-γ. Alopecia areata biopsies demonstrate a prevalence of TH1 cytokines, and patients with localized AA, alopecia totalis, and AU have notably higher serum IFN-γ levels compared to controls.25 In murine models, IL-1α and IL-1β increase during the catagen phase of the hair cycle and peak during the telogen phase.26 Excessive IL-1β expression is detected in the early stages of human disease, and certain IL-1β polymorphisms are associated with severe forms of AA.26 The role of tumor necrosis factor (TNF) α in AA is not well understood. In vitro studies show it inhibits hair growth, suggesting the cytokine may play a role in AA.27 However, anti–TNF-α therapy is not effective in AA, and case reports propose these therapies rarely induce AA.28-31
The TH1 response is likewise critical to psoriatic plaque development. IFN-γ and TNF-α are overexpressed in psoriatic plaques.32 IFN-γ has an antiproliferative and differentiation-inducing effect on normal keratinocytes, but psoriatic epithelial cells in vitro respond differently to the cytokine with a notably diminished growth inhibition.33,34 One explanation for the role of IFN-γ is that it stimulates dendritic cells to produce IL-1 and IL-23.35 IL-23 activates TH17 cells36; TH1 and TH17 conditions produce IL-22 whose serum level correlates with disease severity.37-39 IL-22 induces keratinocyte proliferation and migration and inhibits keratinocyte differentiation, helping account for hallmarks of the disease.40 Patients with psoriasis have increased levels of TH1, TH17, and TH22 cells, as well as their associated cytokines, in the skin and blood compared to controls.4,11,32,39,41
Alopecia areata and psoriasis are regulated by complex and still not entirely understood immune interactions. The fact that many of the same therapies are used to treat both diseases emphasizes both their overlapping characteristics and the lack of targeted therapy. It is unclear if and how the topical or systemic therapies used in our patient to treat one disease affected the natural history of the other condition. It is important to highlight, however, that the patient had not been treated for months when he developed the psoriatic plaques with hair regrowth. Other case reports also document hair regrowth in untreated plaques,13,16 making it unlikely to be a side effect of the medication regimen. For both psoriasis and AA, the immune cell composition and cytokine levels in the skin or serum vary throughout a patient’s disease course depending on severity of disease or response to treatment.6,39,42,43 Therefore, we hypothesize that the 2 conditions interact in a similarly distinct manner based on each disease’s stage and intensity in the patient. Both our patient’s course thus far and the various presentations described by other groups support this hypothesis. Our patient had a small region of psoriasis on the scalp that cleared without any terminal hair growth. He also had larger plaques on the forearms that developed hair growth most predominantly within the thicker regions of the plaques. His unique presentation highlights the fluidity of the immune factors driving psoriasis vulgaris and AA.
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York, NY: McGraw-Hill; 2009.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57-62.
- Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25:781-788.
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515-521.
- Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319-329.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022-1030.e395.
- Happle R, van der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köebner reaction observed in alopecia areata. Eur J Dermatol. 1991;2:39-40.
- Ito T, Hashizume H, Takigawa M. Contact immunotherapy-induced Renbök phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol. 2010;20:126-127.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbök phenomenon. Clin Exp Dermatol. 2007;32:320-321.
- Harris JE, Seykora JT, Lee RA. Renbök phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol. 2010;146:422-425.
- Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355-363.
- Herbst V, Zöller M, Kissling S, et al. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-542.
- Zöller M, Freyschmidt-Paul P, Vitacolonna M, et al. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135:398-408.
- Bröcker EB, Echternacht-Happle K, Hamm H, et al. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564-568.
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216-223.
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26-30.
- Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276:333-334.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8+ cells induces localized hair loss whereas CD4+/CD25– cells promote systemic alopecia areata and CD4+/CD25+ cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- Arca E, Muşabak U, Akar A, et al. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004;14:33-36.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235-238.
- Philpott MP, Sanders DA, Bowen J, et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942-948.
- Le Bidre E, Chaby G, Martin L, et al. Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol. 2011;138:285-293.
- Ferran M, Calvet J, Almirall M, et al. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol. 2011;25:479-484.
- Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17:127-129.
- Pelivani N, Hassan AS, Braathen LR, et al. Alopecia areata universalis elicited during treatment with adalimumab. Dermatology. 2008;216:320-323.
- Uyemura K, Yamamura M, Fivenson DF, et al. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701-705.
- Baker BS, Powles AV, Valdimarsson H, et al. An altered response by psoriatic keratinocytes to gamma interferon. Scan J Immunol. 1988;28:735-740.
- Jackson M, Howie SE, Weller R, et al. Psoriatic keratinocytes show reduced IRF-1 and STAT-1alpha activation in response to gamma-IFN. FASEB J. 1999;13:495-502.
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385-422.
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314-324.
- Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657.
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687.
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702.
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183.
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699-711.
- Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530-533.
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York, NY: McGraw-Hill; 2009.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57-62.
- Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25:781-788.
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515-521.
- Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319-329.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022-1030.e395.
- Happle R, van der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köebner reaction observed in alopecia areata. Eur J Dermatol. 1991;2:39-40.
- Ito T, Hashizume H, Takigawa M. Contact immunotherapy-induced Renbök phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol. 2010;20:126-127.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbök phenomenon. Clin Exp Dermatol. 2007;32:320-321.
- Harris JE, Seykora JT, Lee RA. Renbök phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol. 2010;146:422-425.
- Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355-363.
- Herbst V, Zöller M, Kissling S, et al. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-542.
- Zöller M, Freyschmidt-Paul P, Vitacolonna M, et al. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135:398-408.
- Bröcker EB, Echternacht-Happle K, Hamm H, et al. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564-568.
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216-223.
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26-30.
- Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276:333-334.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8+ cells induces localized hair loss whereas CD4+/CD25– cells promote systemic alopecia areata and CD4+/CD25+ cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- Arca E, Muşabak U, Akar A, et al. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004;14:33-36.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235-238.
- Philpott MP, Sanders DA, Bowen J, et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942-948.
- Le Bidre E, Chaby G, Martin L, et al. Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol. 2011;138:285-293.
- Ferran M, Calvet J, Almirall M, et al. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol. 2011;25:479-484.
- Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17:127-129.
- Pelivani N, Hassan AS, Braathen LR, et al. Alopecia areata universalis elicited during treatment with adalimumab. Dermatology. 2008;216:320-323.
- Uyemura K, Yamamura M, Fivenson DF, et al. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701-705.
- Baker BS, Powles AV, Valdimarsson H, et al. An altered response by psoriatic keratinocytes to gamma interferon. Scan J Immunol. 1988;28:735-740.
- Jackson M, Howie SE, Weller R, et al. Psoriatic keratinocytes show reduced IRF-1 and STAT-1alpha activation in response to gamma-IFN. FASEB J. 1999;13:495-502.
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385-422.
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314-324.
- Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657.
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687.
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702.
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183.
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699-711.
- Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530-533.
Practice Points
- The Renbök phenomenon, or reverse Köbner phenomenon, describes cases where secondary insults improve dermatologic disease.
- Current evidence suggests that alopecia areata (AA) is driven by a helper T cell (TH1) response whereas psoriasis vulgaris is driven by TH1, TH17, and TH22.
- Patients with concurrent AA and psoriasis can develop normal hair regrowth confined to the psoriatic plaques. Developing methods to artificially alter the cytokine milieu in affected skin may lead to new therapeutic options for each condition.
Papules on the Face and Body
The Diagnosis: Lichen Spinulosus
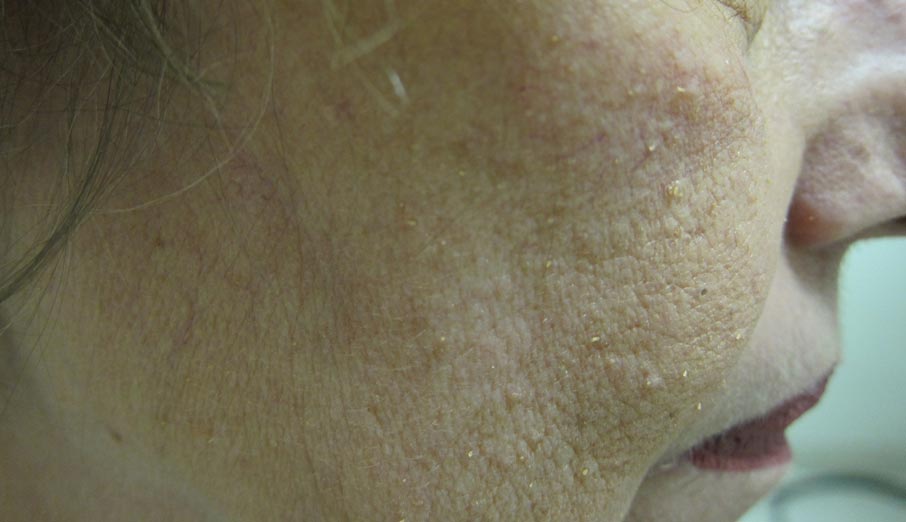
Lichen spinulosus, also referred to as keratosis spinulosa, is a disorder of keratinization characterized by grouped 1- to 3-mm papules with a horny spine localized to follicles (Figure).1 These lesions most commonly occur in the first through third decades of life, presenting as 2- to 6-cm patches on the neck, buttocks, thighs, abdomen, or extensor surfaces.1-4 Some patients report mild pruritus.1 The cause is unknown.1-3,5-7 Several proposed but unproven explanations include atopy,2,4 genetic predisposition,1,2 toxins,3,5 infection,5 abnormal immune response,8 and vitamin deficiency.1,6,7
Our patient’s presentation is atypical due to her age and the involvement of her face. Generalized lichen spinulosus in adults likely is rare. A few similar cases have been reported: a 61-year-old woman with Crohn disease and lichen spinulosus affecting the groin, inframammary region, and back8; 2 case reports linked to alcoholism-associated nutritional deficiency6,7; and generalized lichen spinulosus–like eruptions in 2 patients with human immuno-deficiency virus infection.9,10 Our patient’s medical history indicated an extensive smoking history; thiamine deficiency 5 years prior treated with vitamin B complex supplements, which she still takes; and a recent diagnosis of vitamin D deficiency. She had no evidence of immunodeficiency or systemic illness on routine screening.
The disorders of follicular keratinization are lichen spinulosus, keratosis pilaris, keratosis pilaris atrophicans, pityriasis rubra pilaris, lichen planopilaris, erythromelanosis follicularis faciei, and phrynoderma.11 The clinical differential diagnosis of lichen spinulosus includes keratosis pilaris, phrynoderma, pityriasis rubra pilaris, and frictional lichenoid eruption. Lichen spinulosus can be distinguished from keratosis pilaris by 4 factors1,11: (1) keratosis pilaris lesions develop slowly over time as opposed to the rapid onset in lichen spinulosus; (2) keratosis pilaris is preferentially located on the upper arms and legs; (3) keratosis pilaris does not develop in small clusters; (4) keratosis pilaris, unlike lichen spinulosus, often has a thin outline of perifollicular erythema. Histopathologically, lichen spinulosus is similar to keratosis pilaris, showing dilated hair follicles with a keratin plug and perifollicular and perivascular dermal lymphocytic infiltrate.1 A punch biopsy from our patient’s cheek demonstrated focal follicular hyperkeratosis with dermal perivascular inflammation. Periodic acid–Schiff with diastase stain was negative for pathogenic fungal organisms.
Treatment of lichen spinulosus is initiated to address cosmetic concerns. Traditionally, keratolytics and emollients are utilized. Success has been described with salicylic acid gel 6% without occlusion for 8 weeks12 or with occlusion for 2 weeks.13 Tar preparations and mid-potency topical corticosteroids may be used on lesions not located on the face.2,4,15 Topical vitamin A,2 lactic acid,4 and ammonium lactate lotion2 have been therapeutic in some cases. Facial lesions have been successfully treated with tacalcitol14 or tretinoin gel 0.04% in combination with hydroactive adhesive applications.15 In the case of lichen spinulosus accompanying alcoholism, oral vitamin supplementation has been sufficient for resolution.6,7
Our patient was initially prescribed ammonium lactate lotion twice daily and tretinoin cream 0.025% for facial application nightly. She only used the tretinoin briefly due to skin irritation, and she discontinued use of ammonium lactate due to lotion texture. Three months of vitamin A and vitamin B complex supplementation did not lead to any improvement. She believed the papules softened by scrubbing them with a loofah in the shower and then moisturizing. Malignancy workup, including a colonoscopy, mammography, chest radiograph, and basic blood tests, were negative. No remarkable change was noted by the patient at 1-year follow-up.
1. Friedman SJ. Lichen spinulosus. clinicopathologic review of thirty-five cases. J Am Acad Dermatol. 1990;22:261-264.
2. Boyd AS. Lichen spinulosus: case report and overview. Cutis. 1989;43:557-560.
3. Adamson H. Lichen pilaris, seu spinulosis. Br J Dermatol. 1905;17:39-54.
4. Strickling WA, Norton SA. Spiny eruption on the neck. diagnosis: lichen spinulosus (LS). Arch Dermatol. 2000;136:1165-1170.
5. Becker S. Lichen spinulosus following intradermal application of diphtheria toxin. Arch Dermatol Syph. 1930;21:839-840.
6. Irgang S. Lichen spinulosus responsive to ascorbic acid (vitamin C). case in an alcoholic adult. Skin. 1964;3:145-146.
7. Kabashima R, Sugita K, Kabashima K, et al. Lichen spinulosus in an alcoholic patient. Acta Derm Venereol. 2009;89:311-312.
8. Kano Y, Orihara M, Yagita A, et al. Lichen spinulosus in a patient with Crohn disease. Int J Dermatol. 1995;34:670-671.
9. Cohen SJ, Dicken CH. Generalized lichen spinulosus in an HIV-positive man. J Am Acad Dermatol. 1991;25:116-118.
10. Resnick SD, Murrell DF, Woosley J. Acne conglobata and a generalized lichen spinulosus-like eruption in a man seropositive for human immunodeficiency virus. J Am Acad Dermatol. 1992;26:1013-1014.
11. McMichael A, Curtis A, Guzman-Sanchez D, et al. Folliculitis and other follicular disorders. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 1. 3rd ed. New York, NY: Elsevier; 2012:571-586.
12. Tuyp E, McLeod WA, Boyko W. Lichen spinulosus with immunofluorescent studies. Cutis. 1984;33:197-200.
13. Maiocco KJ, Miller OF. Lichen spinulosus: response to therapy. Cutis. 1976;17:294-299.
14. Kim SH, Kang JH, Seo JK, et al. Successful treatment of lichen spinulosus with topical tacalcitol cream. Pediatr Dermatol. 2010;27:546-547.
15. Forman SB, Hudgins EM, Blaylock WK. Lichen spinulosus: excellent response to tretinoin gel and hydroactive adhesive applications. Arch Dermatol. 2007;143:122-123.
The Diagnosis: Lichen Spinulosus
Lichen spinulosus, also referred to as keratosis spinulosa, is a disorder of keratinization characterized by grouped 1- to 3-mm papules with a horny spine localized to follicles (Figure).1 These lesions most commonly occur in the first through third decades of life, presenting as 2- to 6-cm patches on the neck, buttocks, thighs, abdomen, or extensor surfaces.1-4 Some patients report mild pruritus.1 The cause is unknown.1-3,5-7 Several proposed but unproven explanations include atopy,2,4 genetic predisposition,1,2 toxins,3,5 infection,5 abnormal immune response,8 and vitamin deficiency.1,6,7
Our patient’s presentation is atypical due to her age and the involvement of her face. Generalized lichen spinulosus in adults likely is rare. A few similar cases have been reported: a 61-year-old woman with Crohn disease and lichen spinulosus affecting the groin, inframammary region, and back8; 2 case reports linked to alcoholism-associated nutritional deficiency6,7; and generalized lichen spinulosus–like eruptions in 2 patients with human immuno-deficiency virus infection.9,10 Our patient’s medical history indicated an extensive smoking history; thiamine deficiency 5 years prior treated with vitamin B complex supplements, which she still takes; and a recent diagnosis of vitamin D deficiency. She had no evidence of immunodeficiency or systemic illness on routine screening.
The disorders of follicular keratinization are lichen spinulosus, keratosis pilaris, keratosis pilaris atrophicans, pityriasis rubra pilaris, lichen planopilaris, erythromelanosis follicularis faciei, and phrynoderma.11 The clinical differential diagnosis of lichen spinulosus includes keratosis pilaris, phrynoderma, pityriasis rubra pilaris, and frictional lichenoid eruption. Lichen spinulosus can be distinguished from keratosis pilaris by 4 factors1,11: (1) keratosis pilaris lesions develop slowly over time as opposed to the rapid onset in lichen spinulosus; (2) keratosis pilaris is preferentially located on the upper arms and legs; (3) keratosis pilaris does not develop in small clusters; (4) keratosis pilaris, unlike lichen spinulosus, often has a thin outline of perifollicular erythema. Histopathologically, lichen spinulosus is similar to keratosis pilaris, showing dilated hair follicles with a keratin plug and perifollicular and perivascular dermal lymphocytic infiltrate.1 A punch biopsy from our patient’s cheek demonstrated focal follicular hyperkeratosis with dermal perivascular inflammation. Periodic acid–Schiff with diastase stain was negative for pathogenic fungal organisms.
Treatment of lichen spinulosus is initiated to address cosmetic concerns. Traditionally, keratolytics and emollients are utilized. Success has been described with salicylic acid gel 6% without occlusion for 8 weeks12 or with occlusion for 2 weeks.13 Tar preparations and mid-potency topical corticosteroids may be used on lesions not located on the face.2,4,15 Topical vitamin A,2 lactic acid,4 and ammonium lactate lotion2 have been therapeutic in some cases. Facial lesions have been successfully treated with tacalcitol14 or tretinoin gel 0.04% in combination with hydroactive adhesive applications.15 In the case of lichen spinulosus accompanying alcoholism, oral vitamin supplementation has been sufficient for resolution.6,7
Our patient was initially prescribed ammonium lactate lotion twice daily and tretinoin cream 0.025% for facial application nightly. She only used the tretinoin briefly due to skin irritation, and she discontinued use of ammonium lactate due to lotion texture. Three months of vitamin A and vitamin B complex supplementation did not lead to any improvement. She believed the papules softened by scrubbing them with a loofah in the shower and then moisturizing. Malignancy workup, including a colonoscopy, mammography, chest radiograph, and basic blood tests, were negative. No remarkable change was noted by the patient at 1-year follow-up.
The Diagnosis: Lichen Spinulosus
Lichen spinulosus, also referred to as keratosis spinulosa, is a disorder of keratinization characterized by grouped 1- to 3-mm papules with a horny spine localized to follicles (Figure).1 These lesions most commonly occur in the first through third decades of life, presenting as 2- to 6-cm patches on the neck, buttocks, thighs, abdomen, or extensor surfaces.1-4 Some patients report mild pruritus.1 The cause is unknown.1-3,5-7 Several proposed but unproven explanations include atopy,2,4 genetic predisposition,1,2 toxins,3,5 infection,5 abnormal immune response,8 and vitamin deficiency.1,6,7
Our patient’s presentation is atypical due to her age and the involvement of her face. Generalized lichen spinulosus in adults likely is rare. A few similar cases have been reported: a 61-year-old woman with Crohn disease and lichen spinulosus affecting the groin, inframammary region, and back8; 2 case reports linked to alcoholism-associated nutritional deficiency6,7; and generalized lichen spinulosus–like eruptions in 2 patients with human immuno-deficiency virus infection.9,10 Our patient’s medical history indicated an extensive smoking history; thiamine deficiency 5 years prior treated with vitamin B complex supplements, which she still takes; and a recent diagnosis of vitamin D deficiency. She had no evidence of immunodeficiency or systemic illness on routine screening.
The disorders of follicular keratinization are lichen spinulosus, keratosis pilaris, keratosis pilaris atrophicans, pityriasis rubra pilaris, lichen planopilaris, erythromelanosis follicularis faciei, and phrynoderma.11 The clinical differential diagnosis of lichen spinulosus includes keratosis pilaris, phrynoderma, pityriasis rubra pilaris, and frictional lichenoid eruption. Lichen spinulosus can be distinguished from keratosis pilaris by 4 factors1,11: (1) keratosis pilaris lesions develop slowly over time as opposed to the rapid onset in lichen spinulosus; (2) keratosis pilaris is preferentially located on the upper arms and legs; (3) keratosis pilaris does not develop in small clusters; (4) keratosis pilaris, unlike lichen spinulosus, often has a thin outline of perifollicular erythema. Histopathologically, lichen spinulosus is similar to keratosis pilaris, showing dilated hair follicles with a keratin plug and perifollicular and perivascular dermal lymphocytic infiltrate.1 A punch biopsy from our patient’s cheek demonstrated focal follicular hyperkeratosis with dermal perivascular inflammation. Periodic acid–Schiff with diastase stain was negative for pathogenic fungal organisms.
Treatment of lichen spinulosus is initiated to address cosmetic concerns. Traditionally, keratolytics and emollients are utilized. Success has been described with salicylic acid gel 6% without occlusion for 8 weeks12 or with occlusion for 2 weeks.13 Tar preparations and mid-potency topical corticosteroids may be used on lesions not located on the face.2,4,15 Topical vitamin A,2 lactic acid,4 and ammonium lactate lotion2 have been therapeutic in some cases. Facial lesions have been successfully treated with tacalcitol14 or tretinoin gel 0.04% in combination with hydroactive adhesive applications.15 In the case of lichen spinulosus accompanying alcoholism, oral vitamin supplementation has been sufficient for resolution.6,7
Our patient was initially prescribed ammonium lactate lotion twice daily and tretinoin cream 0.025% for facial application nightly. She only used the tretinoin briefly due to skin irritation, and she discontinued use of ammonium lactate due to lotion texture. Three months of vitamin A and vitamin B complex supplementation did not lead to any improvement. She believed the papules softened by scrubbing them with a loofah in the shower and then moisturizing. Malignancy workup, including a colonoscopy, mammography, chest radiograph, and basic blood tests, were negative. No remarkable change was noted by the patient at 1-year follow-up.
1. Friedman SJ. Lichen spinulosus. clinicopathologic review of thirty-five cases. J Am Acad Dermatol. 1990;22:261-264.
2. Boyd AS. Lichen spinulosus: case report and overview. Cutis. 1989;43:557-560.
3. Adamson H. Lichen pilaris, seu spinulosis. Br J Dermatol. 1905;17:39-54.
4. Strickling WA, Norton SA. Spiny eruption on the neck. diagnosis: lichen spinulosus (LS). Arch Dermatol. 2000;136:1165-1170.
5. Becker S. Lichen spinulosus following intradermal application of diphtheria toxin. Arch Dermatol Syph. 1930;21:839-840.
6. Irgang S. Lichen spinulosus responsive to ascorbic acid (vitamin C). case in an alcoholic adult. Skin. 1964;3:145-146.
7. Kabashima R, Sugita K, Kabashima K, et al. Lichen spinulosus in an alcoholic patient. Acta Derm Venereol. 2009;89:311-312.
8. Kano Y, Orihara M, Yagita A, et al. Lichen spinulosus in a patient with Crohn disease. Int J Dermatol. 1995;34:670-671.
9. Cohen SJ, Dicken CH. Generalized lichen spinulosus in an HIV-positive man. J Am Acad Dermatol. 1991;25:116-118.
10. Resnick SD, Murrell DF, Woosley J. Acne conglobata and a generalized lichen spinulosus-like eruption in a man seropositive for human immunodeficiency virus. J Am Acad Dermatol. 1992;26:1013-1014.
11. McMichael A, Curtis A, Guzman-Sanchez D, et al. Folliculitis and other follicular disorders. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 1. 3rd ed. New York, NY: Elsevier; 2012:571-586.
12. Tuyp E, McLeod WA, Boyko W. Lichen spinulosus with immunofluorescent studies. Cutis. 1984;33:197-200.
13. Maiocco KJ, Miller OF. Lichen spinulosus: response to therapy. Cutis. 1976;17:294-299.
14. Kim SH, Kang JH, Seo JK, et al. Successful treatment of lichen spinulosus with topical tacalcitol cream. Pediatr Dermatol. 2010;27:546-547.
15. Forman SB, Hudgins EM, Blaylock WK. Lichen spinulosus: excellent response to tretinoin gel and hydroactive adhesive applications. Arch Dermatol. 2007;143:122-123.
1. Friedman SJ. Lichen spinulosus. clinicopathologic review of thirty-five cases. J Am Acad Dermatol. 1990;22:261-264.
2. Boyd AS. Lichen spinulosus: case report and overview. Cutis. 1989;43:557-560.
3. Adamson H. Lichen pilaris, seu spinulosis. Br J Dermatol. 1905;17:39-54.
4. Strickling WA, Norton SA. Spiny eruption on the neck. diagnosis: lichen spinulosus (LS). Arch Dermatol. 2000;136:1165-1170.
5. Becker S. Lichen spinulosus following intradermal application of diphtheria toxin. Arch Dermatol Syph. 1930;21:839-840.
6. Irgang S. Lichen spinulosus responsive to ascorbic acid (vitamin C). case in an alcoholic adult. Skin. 1964;3:145-146.
7. Kabashima R, Sugita K, Kabashima K, et al. Lichen spinulosus in an alcoholic patient. Acta Derm Venereol. 2009;89:311-312.
8. Kano Y, Orihara M, Yagita A, et al. Lichen spinulosus in a patient with Crohn disease. Int J Dermatol. 1995;34:670-671.
9. Cohen SJ, Dicken CH. Generalized lichen spinulosus in an HIV-positive man. J Am Acad Dermatol. 1991;25:116-118.
10. Resnick SD, Murrell DF, Woosley J. Acne conglobata and a generalized lichen spinulosus-like eruption in a man seropositive for human immunodeficiency virus. J Am Acad Dermatol. 1992;26:1013-1014.
11. McMichael A, Curtis A, Guzman-Sanchez D, et al. Folliculitis and other follicular disorders. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 1. 3rd ed. New York, NY: Elsevier; 2012:571-586.
12. Tuyp E, McLeod WA, Boyko W. Lichen spinulosus with immunofluorescent studies. Cutis. 1984;33:197-200.
13. Maiocco KJ, Miller OF. Lichen spinulosus: response to therapy. Cutis. 1976;17:294-299.
14. Kim SH, Kang JH, Seo JK, et al. Successful treatment of lichen spinulosus with topical tacalcitol cream. Pediatr Dermatol. 2010;27:546-547.
15. Forman SB, Hudgins EM, Blaylock WK. Lichen spinulosus: excellent response to tretinoin gel and hydroactive adhesive applications. Arch Dermatol. 2007;143:122-123.
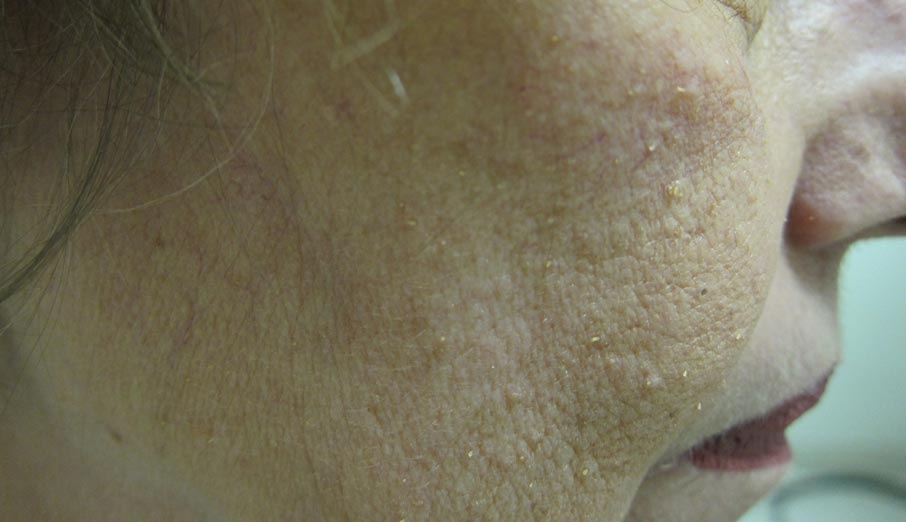
A 65-year-old woman presented for evaluation of papules on the face and body that had developed over a short period of time approximately 1.5 years prior. The papules were entirely asymptomatic. She had no prior treatment. On physical examination multiple flesh-colored papules with a central keratotic spicule were noted on the face, neck, arms, and legs.