User login
<i>Mycobacterium abscessus</i> Infection Following Home Dermabrasion
Case Report
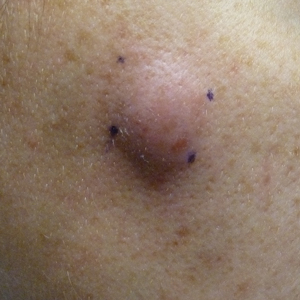
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).
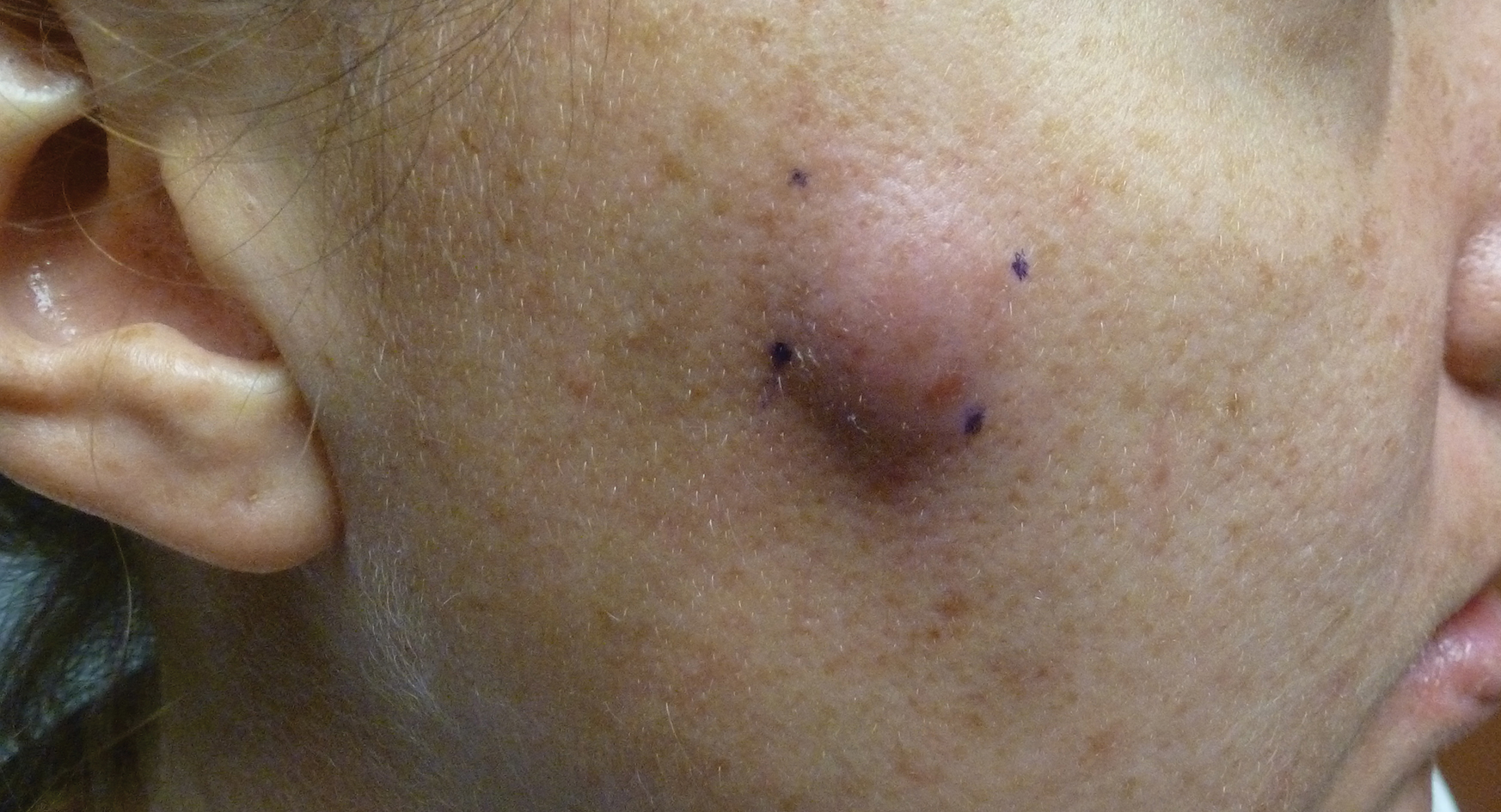
A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
Case Report
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).

A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
Case Report
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).

A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
Practice Points
- Atypical mycobacteria are included in the differential for cutaneous abscesses.
- At-home cosmetic treatments often carry unrecognized risks for adverse events.
- Obtain culture prior to initiation of empiric antibiotics.
Rash and fever in a 14-month-old girl
A 14-MONTH-OLD GIRL was brought to our medical center with a widespread pruritic eruption and fever that she’d had for 2 days. She had a temperature of 103.2° F, heart rate of 166 beats/min, respiratory rate of 32 breaths/min, and an oxygen saturation level of 100%.
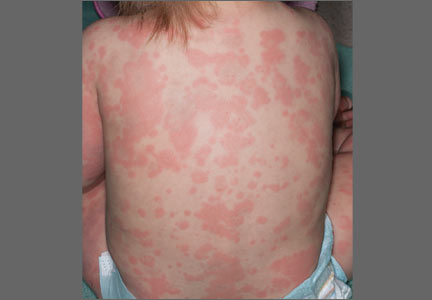
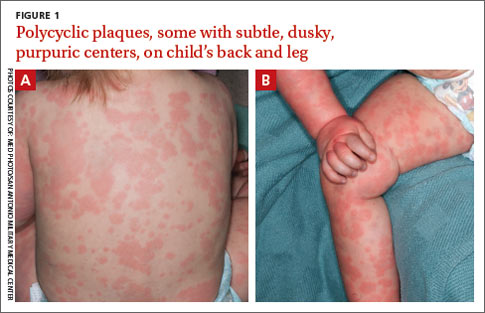
On physical examination, the toddler was active, appeared well-nourished, and was not in acute distress. She had ill-defined, polycyclic, urticarial plaques with subtle, purpuric, dusky-appearing changes distributed widely over her face, trunk, and extremities (FIGURE 1A AND 1B). She had no signs of arthritis and did not exhibit an antalgic gait. She was otherwise healthy and had no personal or family history of connective tissue disease.
Lab results showed a slightly elevated erythrocyte sedimentation rate (ESR) (16 mm/hr) and mild thrombocytopenia (platelet count: 109,000/mcL). Complete blood count, comprehensive metabolic panel, and uric acid tests were unremarkable. Nine days earlier, the toddler had been diagnosed with otitis media and prescribed oral amoxicillin 50 mg/kg/d taken in 2 doses.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
Based on the appearance of the plaques and the recent history of amoxicillin use, we diagnosed urticaria multiforme (UM) in this patient. UM is a benign, self-limited, cutaneous, histamine-dependent, hypersensitivity reaction that occurs predominantly among patients ages 4 months to 4 years.1
In UM, the eruption typically occurs one to 3 days after a viral infection or the administration of certain medications.2 A low-grade fever may or may not precede the eruption, and symptoms are usually limited to pruritus. Small urticarial papules and plaques initially appear and then coalesce to form blanching, erythematous, annular, polycyclic wheals.
Unlike “ordinary” acute urticaria, which is characterized by round or oval-shaped erythematous, edematous papules and plaques, UM plaques will have annular, gyrate, serpiginous, polycyclic, and/or target lesions with ecchymotic or dusky-appearing centers.1,3 Individual UM lesions typically last less than 24 hours, while the disease itself can persist for 2 to 12 days, until all lesions heal and the skin returns to normal.2
The diagnosis of UM is a clinical one. When a patient presents with urticarial lesions, ask about the timing of rash onset and the duration of individual lesions. Also ask whether the patient has had a fever, dermatographism, acral edema, or other symptoms, such as arthralgias and myalgias.2
Although a biopsy is typically not performed on this type of lesion, the histology will be the same as that seen in ordinary acute urticaria: dermal edema with some perivascular and interstitial infiltrates of eosinophils, neutrophils, and lymphocytes.3
Differential Dx includes serum sickness-like reaction, EM
Patients with serum sickness-like reaction (SSLR) will have a more severe clinical presentation than those with UM, characterized by a high-grade fever, lymphadenopathy, myalgia, and arthralgia.2 Also, the time between viral infection/medication administration and onset of eruption is greater in SSLR: 7 to 21 days, as opposed to one to 3 days in UM.1 In addition, the individual lesions of UM only last about a day, whereas the plaques of SSLR can last from a few days to a few weeks.
Patients with erythema multiforme (EM) will complain of pain and burning, rather than itching.2 Both mucosal surfaces and skin may be involved, with typical targetoid lesions often distributed acrally.2 Overlying blistering or necrosis of the epidermis is commonly seen in EM, and like SSLR, the plaques are fixed, rather than transient. Although the plaques of UM, SSLR, and EM can all contain a dusky center, the lesions of SSLR and EM usually resolve with postinflammatory hyperpigmentation, which is typically not seen in UM.1,2
Stop the offending drug, start an antihistamine
Treatment for UM involves discontinuing the offending medication and inhibiting the effects of histamine release. The combination of second-generation antihistamines (eg, cetirizine, fexofenadine, or loratadine) every morning and first-generation antihistamines (eg, diphenhydramine) at night for pruritus is the mainstay of treatment.1,2 Acetaminophen can be used for mild fever; however, aspirin and nonsteroidal anti-inflammatory drugs should be avoided because these medications may worsen the urticarial eruption.4
Our patient had already finished her course of amoxicillin when she first presented with the rash, so we prescribed an oral antihistamine—cetirizine 5 mg BID. Six days after rash onset, when mother and child returned for follow-up, the patient’s lesions had completely resolved. There was no residual postinflammatory hyperpigmentation, which confirmed the diagnosis of UM.
We advised the mother that her daughter was allergic to amoxicillin and told her to avoid it in the future.
CORRESPONDENCE
Casey Bowen, MD, Dermatology Clinic, 2200 Bergquist Dr, STE 1, JBSA-Lackland, TX 78236-9908; casey.bowen.2@us.af.mil
1. Mathur AN, Mathes TF. Urticaria mimickers in children. Dermatol Ther. 2013;26:467-475.
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Peroni A, Colato C, Schena D, et al. Urticarial lesions: if not urticaria, what else? The differential diagnosis of urticaria: part I. Cutaneous diseases. J Am Acad Dermatol. 2010;62:541-555.
4. Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, et al. Aspirin-exacerbated cutaneous disease (AECD) is a distinct subphenotype of chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2015;29:698-701.
A 14-MONTH-OLD GIRL was brought to our medical center with a widespread pruritic eruption and fever that she’d had for 2 days. She had a temperature of 103.2° F, heart rate of 166 beats/min, respiratory rate of 32 breaths/min, and an oxygen saturation level of 100%.
On physical examination, the toddler was active, appeared well-nourished, and was not in acute distress. She had ill-defined, polycyclic, urticarial plaques with subtle, purpuric, dusky-appearing changes distributed widely over her face, trunk, and extremities (FIGURE 1A AND 1B). She had no signs of arthritis and did not exhibit an antalgic gait. She was otherwise healthy and had no personal or family history of connective tissue disease.
Lab results showed a slightly elevated erythrocyte sedimentation rate (ESR) (16 mm/hr) and mild thrombocytopenia (platelet count: 109,000/mcL). Complete blood count, comprehensive metabolic panel, and uric acid tests were unremarkable. Nine days earlier, the toddler had been diagnosed with otitis media and prescribed oral amoxicillin 50 mg/kg/d taken in 2 doses.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
Based on the appearance of the plaques and the recent history of amoxicillin use, we diagnosed urticaria multiforme (UM) in this patient. UM is a benign, self-limited, cutaneous, histamine-dependent, hypersensitivity reaction that occurs predominantly among patients ages 4 months to 4 years.1
In UM, the eruption typically occurs one to 3 days after a viral infection or the administration of certain medications.2 A low-grade fever may or may not precede the eruption, and symptoms are usually limited to pruritus. Small urticarial papules and plaques initially appear and then coalesce to form blanching, erythematous, annular, polycyclic wheals.
Unlike “ordinary” acute urticaria, which is characterized by round or oval-shaped erythematous, edematous papules and plaques, UM plaques will have annular, gyrate, serpiginous, polycyclic, and/or target lesions with ecchymotic or dusky-appearing centers.1,3 Individual UM lesions typically last less than 24 hours, while the disease itself can persist for 2 to 12 days, until all lesions heal and the skin returns to normal.2
The diagnosis of UM is a clinical one. When a patient presents with urticarial lesions, ask about the timing of rash onset and the duration of individual lesions. Also ask whether the patient has had a fever, dermatographism, acral edema, or other symptoms, such as arthralgias and myalgias.2
Although a biopsy is typically not performed on this type of lesion, the histology will be the same as that seen in ordinary acute urticaria: dermal edema with some perivascular and interstitial infiltrates of eosinophils, neutrophils, and lymphocytes.3
Differential Dx includes serum sickness-like reaction, EM
Patients with serum sickness-like reaction (SSLR) will have a more severe clinical presentation than those with UM, characterized by a high-grade fever, lymphadenopathy, myalgia, and arthralgia.2 Also, the time between viral infection/medication administration and onset of eruption is greater in SSLR: 7 to 21 days, as opposed to one to 3 days in UM.1 In addition, the individual lesions of UM only last about a day, whereas the plaques of SSLR can last from a few days to a few weeks.
Patients with erythema multiforme (EM) will complain of pain and burning, rather than itching.2 Both mucosal surfaces and skin may be involved, with typical targetoid lesions often distributed acrally.2 Overlying blistering or necrosis of the epidermis is commonly seen in EM, and like SSLR, the plaques are fixed, rather than transient. Although the plaques of UM, SSLR, and EM can all contain a dusky center, the lesions of SSLR and EM usually resolve with postinflammatory hyperpigmentation, which is typically not seen in UM.1,2
Stop the offending drug, start an antihistamine
Treatment for UM involves discontinuing the offending medication and inhibiting the effects of histamine release. The combination of second-generation antihistamines (eg, cetirizine, fexofenadine, or loratadine) every morning and first-generation antihistamines (eg, diphenhydramine) at night for pruritus is the mainstay of treatment.1,2 Acetaminophen can be used for mild fever; however, aspirin and nonsteroidal anti-inflammatory drugs should be avoided because these medications may worsen the urticarial eruption.4
Our patient had already finished her course of amoxicillin when she first presented with the rash, so we prescribed an oral antihistamine—cetirizine 5 mg BID. Six days after rash onset, when mother and child returned for follow-up, the patient’s lesions had completely resolved. There was no residual postinflammatory hyperpigmentation, which confirmed the diagnosis of UM.
We advised the mother that her daughter was allergic to amoxicillin and told her to avoid it in the future.
CORRESPONDENCE
Casey Bowen, MD, Dermatology Clinic, 2200 Bergquist Dr, STE 1, JBSA-Lackland, TX 78236-9908; casey.bowen.2@us.af.mil
A 14-MONTH-OLD GIRL was brought to our medical center with a widespread pruritic eruption and fever that she’d had for 2 days. She had a temperature of 103.2° F, heart rate of 166 beats/min, respiratory rate of 32 breaths/min, and an oxygen saturation level of 100%.
On physical examination, the toddler was active, appeared well-nourished, and was not in acute distress. She had ill-defined, polycyclic, urticarial plaques with subtle, purpuric, dusky-appearing changes distributed widely over her face, trunk, and extremities (FIGURE 1A AND 1B). She had no signs of arthritis and did not exhibit an antalgic gait. She was otherwise healthy and had no personal or family history of connective tissue disease.
Lab results showed a slightly elevated erythrocyte sedimentation rate (ESR) (16 mm/hr) and mild thrombocytopenia (platelet count: 109,000/mcL). Complete blood count, comprehensive metabolic panel, and uric acid tests were unremarkable. Nine days earlier, the toddler had been diagnosed with otitis media and prescribed oral amoxicillin 50 mg/kg/d taken in 2 doses.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
Based on the appearance of the plaques and the recent history of amoxicillin use, we diagnosed urticaria multiforme (UM) in this patient. UM is a benign, self-limited, cutaneous, histamine-dependent, hypersensitivity reaction that occurs predominantly among patients ages 4 months to 4 years.1
In UM, the eruption typically occurs one to 3 days after a viral infection or the administration of certain medications.2 A low-grade fever may or may not precede the eruption, and symptoms are usually limited to pruritus. Small urticarial papules and plaques initially appear and then coalesce to form blanching, erythematous, annular, polycyclic wheals.
Unlike “ordinary” acute urticaria, which is characterized by round or oval-shaped erythematous, edematous papules and plaques, UM plaques will have annular, gyrate, serpiginous, polycyclic, and/or target lesions with ecchymotic or dusky-appearing centers.1,3 Individual UM lesions typically last less than 24 hours, while the disease itself can persist for 2 to 12 days, until all lesions heal and the skin returns to normal.2
The diagnosis of UM is a clinical one. When a patient presents with urticarial lesions, ask about the timing of rash onset and the duration of individual lesions. Also ask whether the patient has had a fever, dermatographism, acral edema, or other symptoms, such as arthralgias and myalgias.2
Although a biopsy is typically not performed on this type of lesion, the histology will be the same as that seen in ordinary acute urticaria: dermal edema with some perivascular and interstitial infiltrates of eosinophils, neutrophils, and lymphocytes.3
Differential Dx includes serum sickness-like reaction, EM
Patients with serum sickness-like reaction (SSLR) will have a more severe clinical presentation than those with UM, characterized by a high-grade fever, lymphadenopathy, myalgia, and arthralgia.2 Also, the time between viral infection/medication administration and onset of eruption is greater in SSLR: 7 to 21 days, as opposed to one to 3 days in UM.1 In addition, the individual lesions of UM only last about a day, whereas the plaques of SSLR can last from a few days to a few weeks.
Patients with erythema multiforme (EM) will complain of pain and burning, rather than itching.2 Both mucosal surfaces and skin may be involved, with typical targetoid lesions often distributed acrally.2 Overlying blistering or necrosis of the epidermis is commonly seen in EM, and like SSLR, the plaques are fixed, rather than transient. Although the plaques of UM, SSLR, and EM can all contain a dusky center, the lesions of SSLR and EM usually resolve with postinflammatory hyperpigmentation, which is typically not seen in UM.1,2
Stop the offending drug, start an antihistamine
Treatment for UM involves discontinuing the offending medication and inhibiting the effects of histamine release. The combination of second-generation antihistamines (eg, cetirizine, fexofenadine, or loratadine) every morning and first-generation antihistamines (eg, diphenhydramine) at night for pruritus is the mainstay of treatment.1,2 Acetaminophen can be used for mild fever; however, aspirin and nonsteroidal anti-inflammatory drugs should be avoided because these medications may worsen the urticarial eruption.4
Our patient had already finished her course of amoxicillin when she first presented with the rash, so we prescribed an oral antihistamine—cetirizine 5 mg BID. Six days after rash onset, when mother and child returned for follow-up, the patient’s lesions had completely resolved. There was no residual postinflammatory hyperpigmentation, which confirmed the diagnosis of UM.
We advised the mother that her daughter was allergic to amoxicillin and told her to avoid it in the future.
CORRESPONDENCE
Casey Bowen, MD, Dermatology Clinic, 2200 Bergquist Dr, STE 1, JBSA-Lackland, TX 78236-9908; casey.bowen.2@us.af.mil
1. Mathur AN, Mathes TF. Urticaria mimickers in children. Dermatol Ther. 2013;26:467-475.
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Peroni A, Colato C, Schena D, et al. Urticarial lesions: if not urticaria, what else? The differential diagnosis of urticaria: part I. Cutaneous diseases. J Am Acad Dermatol. 2010;62:541-555.
4. Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, et al. Aspirin-exacerbated cutaneous disease (AECD) is a distinct subphenotype of chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2015;29:698-701.
1. Mathur AN, Mathes TF. Urticaria mimickers in children. Dermatol Ther. 2013;26:467-475.
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Peroni A, Colato C, Schena D, et al. Urticarial lesions: if not urticaria, what else? The differential diagnosis of urticaria: part I. Cutaneous diseases. J Am Acad Dermatol. 2010;62:541-555.
4. Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, et al. Aspirin-exacerbated cutaneous disease (AECD) is a distinct subphenotype of chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2015;29:698-701.
Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
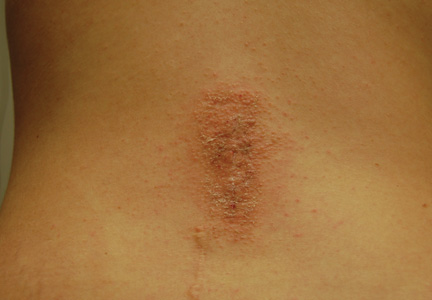
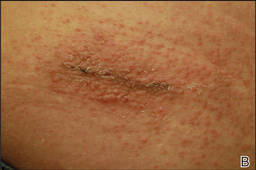
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
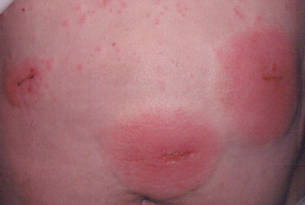
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
|
| Figure 2. Acute eczematous plaques at wound closures. |
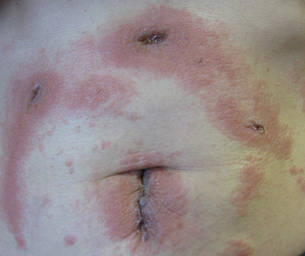
|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
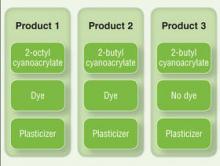
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Practice Points
- It is important for physicians to recognize that skin adhesives are a potential source of allergic contact dermatitis (ACD) in a postsurgical setting.
- There are 3 primary components of skin adhesives that are potential contactants, including a cyanoacrylate, a plasticizer, and a dye.
- Treatment of ACD to skin adhesives is straightforward, including removal of any remaining adhesive and applying topical steroids.