User login
Extreme Athlete, 18, With Worsening Cough
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
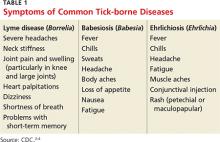
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
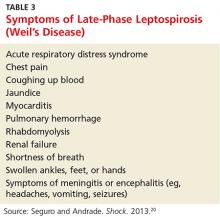
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291
IN THIS ARTICLE
- Adverse effects of ciprofloxacin
- Symptoms of common tick-borne diseases
- Symptoms of phase 1 and late-phase disease
- Additional resources
Jane, an 18-year-old college student, presents in early November with a three-week history of worsening cough and sinus congestion. Recently, the cough has been interrupting her sleep and yellow-green nasal drainage and sinus pressure have increased. Ordinarily very fit and athletic, she reports that since she arrived at college two months ago, her body has become “more fragile.”
Further questioning reveals that, over the past two months, the patient’s symptoms have included extreme fatigue, severe unremitting headache, blurred vision, shortness of breath, and a racing heart rate on exertion. Her symptoms make it impossible for her to maintain her demanding exercise routine, a development that compounds her frustration and sadness. She has also been forced to limit her participation in school activities, with significant academic decline as a result.
Aside from depression (well controlled with bupropion HCl extended release, 300 mg/d), Jane’s medical history is unremarkable. She reports having “excellent health” until she arrived at her mid-Atlantic urban college.
A complicated history
Born and raised in Connecticut, Jane is an avid runner who competes in extreme sports. This past summer, she trained for and participated in two “mud run” events (ie, endurance races of several miles with numerous challenges and obstacles) in Connecticut and New York. Training included endurance runs and sprints, as well as crawling through mud-laden fields and woods.
She also did a three-week summer internship on an oyster farm. There, she was required to shuck oysters and stand in brackish water for six-hour shifts to examine oyster beds. In the process, she sustained numerous cuts and bruises on her hands, arms, and legs.
A week or so after returning to college in late August, Jane developed blisters on both heels, which progressed to infected ulcerations. She was evaluated at the university hospital emergency department (ED) and treated with a 21-day course of ciprofloxacin. When left-sided unilateral knee swelling developed about two weeks later, she underwent arthrocentesis at the university health center, but joint aspirate was not sent for analysis. A two-week course of antibiotic therapy was initiated.
From October to her presentation in early November, Jane has experienced intermittent fevers and chills, with a temperature as high as 101°F. In addition, she complains of fasciculations and weakness in her lower limbs; dyspnea, tachycardia, and dizziness during or after any exertion; unremitting posterior neck pain; and a constant, severe headache located primarily in the bitemporal region. She developed bilateral conjunctivitis, which resolved spontaneously in about one week; persistent blurred vision; a transient petechial chest rash; recurring episodes of syncope; pyelonephritis; a persistent vaginal yeast infection; decreased appetite; and a 7-lb weight loss (5% of her total body weight).
Jane’s academic and athletic performance has been severely impaired. Once a long-distance runner, she can no longer walk any distance without frequent rest. In the four months since the mud runs, the patient reports, she has been seen in the student health center four times and in the ED twice. Additionally, she has undergone thorough examinations by clinicians specializing in infectious disease, pulmonology, neurology, and neuro-ophthalmology. She has undergone lab work, including
• Complete blood cell count with differential
• Comprehensive metabolic panel
• Urinalysis and urine culture
• Lyme antibody and blood polymerase chain reaction (PCR)
• HIV testing
• Rheumatoid factor
• Erythrocyte sedimentation rate (ESR)
• C-reactive protein (CRP)
• Epstein-Barr virus IgM
• Cytomegalovirus (CMV) IgM
• Human granulocytic ehrlichiosis (HGE) antibody and human anaplasma phagocytophilum (HGA)
• HGA PCR
• Rickettsia antibody panel
• Babesia microti antibodies
• Pregnancy testing
• Chest x-ray
• Lumbar puncture
All lab results were within normal range. In light of this, several clinicians have told Jane that her illness is “all in her head.”
Continue for the patient investigates >>
The patient investigates
In mid-December, after she has returned home from college, Jane’s symptoms abruptly worsen. She complains of feeling “shakier,” with weakness in her legs and what she calls “brain fog.” Her headache, blurred vision, and dizziness have worsened. Frightened and concerned, she returns to the ED. Results of a thorough evaluation, including lumbar puncture, reveal no abnormality.
Jane has become extremely frail. She is losing weight, her hair has lost its luster, and her nails are cracking and bleeding. She is unable to walk without concern for falling and cannot climb the 20 steps to her bedroom. Once a healthy and vibrant 18-year-old, she now spends most of her time in a lethargic state on a first-floor living room couch.
Frustrated by her unexplained declining health, she begins to research illnesses associated with extreme sports and prolonged marine exposure. She returns to ask about three possible explanations for her condition:
1. Adverse effects of ciprofloxacin use, which include fever or chills, dizziness, racing heartbeat, headache, and nausea.1
2. A tick-borne disease, possibly contracted during her practice runs in the Connecticut woods (see Table 1).2-4 Each year, she recalls, she has found and removed four or five embedded ticks. In the northeastern United States, the most common tick-borne diseases are borreliosis, babesiosis, and ehrlichiosis.5-7
3. Leptospirosis, contracted through the patient’s exposure to mud and brackish water during her summer activities. According to her research, more than 25 outbreaks and 600 cases of leptospirosis (between 1931 and 1998) have been associated with fresh pond, creek, or river water.8
Based on Jane’s symptoms and history, and in accord with her research, early-phase leptospirosis is identified as a diagnosis of exclusion (with a possible comorbid tick-borne zoonosis).
Continue for discussion >>
DISCUSSION
Leptospirosis develops when humans come into contact with animal urine infected by leptospires—that is, pathogenic spirochetes excreted via the renal tubules of infected host animals.9,10 While host animals include dogs, pigs, cattle, reptiles, and amphibians, the animal most commonly associated with human infection is the brown rat (Rattus norvegicus).11-15
Leptospires enter the human host through mucous membranes, cuts, or abrasions in the skin. Individuals at increased risk for infection include those whose work or other activities expose them “to animal reservoirs or contaminated environments”—including participants in water sports and similar recreation.11-14 As Mwachui et al explain, “recreational exposure to [Leptospira-]contaminated water has become more important for sport enthusiasts, swimmers and travellers from industrialized countries,” whereas flooding is usually involved in infection in undeveloped countries.16
The largest outbreak of leptospirosis reported in the US to date occurred in 1998, when heavy rains preceded a triathlon in Springfield, Illinois. When many participants became ill after the event, researchers from the National Center for Infectious Diseases were able to contact and test 834 of the 876 competing athletes; of these, 98 (12%) reported being ill and 52 (11%) tested positive for leptospirosis. Additionally, 14 of the 248 community residents who were sickened (6%) tested positive.17 According to CDC estimates, between 100 and 200 cases of leptospirosis develop annually in the US, with about half occurring in Hawaii.9
Onset of symptoms, which are described as protean and nonspecific, occurs two days to four weeks after exposure, making leptospirosis difficult to diagnosewithout a high degree of suspicion; zoonotic exposure (as with freshwater or mud sports) or a history of travel to Hawaii, Tahiti, Thailand, Indonesia, the Caribbean, and/or Costa Rica may raise suspicion.12-14,18 In early-phase leptospirosis, symptoms can mimic those of influenza, meningitis, malaria, dengue fever, scrub typhus, rickettsial disease, and typhoid fever (see Table 2).10 Thus, when a patient presents with these symptoms, it is imperative that the clinician consider leptospirosis.19Of note: Flu-like symptoms with conjunctival suffusion are considered pathognomonic for leptospirosis.18
About 10% of patients with early-phase leptospirosis will develop late-phase disease (ie, Weil’s disease), with severe symptoms that include jaundice, meningitis, pulmonary hemorrhage, and acute kidney injury (see Table 3 for a more detailed list).20 The case patient’s history and symptoms were consistent with a diagnosis of early-phase leptospirosis.
Epidemiology
In 2015, leptospirosis was estimated to affect more than 1 million persons worldwide, with 58,900 deaths attributed to the disease each year—making leptospirosis the leading cause of death attributable to zoonotic illness.11 Historically, leptospirosis-associated morbidity and mortality have been greatest in resource-poor countries with tropical climates (eg, southern and Southeast Asia, Central America and tropical Latin America, and East Sub-Saharan Africa).11,12
However, illness resulting from recreational exposures to contaminated water has been linked to increasing travel to exotic destinations, participation in adventure travel, and the growing popularity of extreme sports involving fresh water.9 Recreational mud run events, for example, involve swimming in potentially contaminated waters and crawling through flooded farm fields where animal urine can be present—an ideal environment for Leptospira to thrive and for participants to contract the disease.14,15
Continue for laboratory work-up >>
Laboratory work-up
Diagnosis of leptospirosis is challenging.21 Laboratory tests vary, depending on the timing and stage of infection, and are mostly unavailable in resource-poor countries. Test results for the patient with early-phase leptospirosis may demonstrate renal or hepatic abnormalities.18 However, laboratory confirmation of leptospirosis requires22
• A fourfold increase in antibody titer between acute and convalescent serum samples, as detected by microscopic agglutination testing (MAT) or
• A high MAT titer (> 1:400 to 1:800), in single or paired samples or
• Isolation of pathogenic Leptospira species from a normally sterile site or
• Detection of DNA from pathogenic Leptospira species by PCR
A positive laboratory result is, of course, confirmatory. However, negative laboratory findings must be viewed with healthy skepticism.12 A false-negative result may merely indicate the shortcoming of the testing method to accurately assess the presence of Leptospira.
Treatment options
The high mortality rate associated with severe leptospirosis makes early diagnosis and treatment essential.23 The World Health Organization warns that antibiotic treatment for leptospirosis must be instituted within five days of symptom onset.10
Treatment options for an ambulatory patient with mild symptoms and no organ involvement include oral doxycycline (100 mg bid for 5-7 d) or oral azithromycin (500 mg/d for 5-7 d). For patients with organ involvement, IV penicillin (1.5 million U every 6 h for 7 d), ceftriaxone (1 g/d for 7 d), or cefotaxime (1 g every 6 h for 7 d) may be considered.12,20
OUTCOME FOR THE CASE PATIENT
With leptospirosis as the diagnosis of exclusion, Jane was treated successfully with a 21-day course of oral doxycycline (100 mg bid). She has been symptom free since completing the regimen. After undergoing physical therapy and athletic training, she has been able to resume her full exercise regimen, and her recovery is considered complete.
CONCLUSION
The growing popularity of adventure travel and “extreme sports” events, particularly triathlons and mud runs, may precipitate an increase in associated infections with Leptospira and other zoonotic pathogens. For patients with flulike symptoms who routinely engage in such sports—especially those who present with conjunctival suffusion—leptospirosis should be considered in the differential diagnosis.
REFERENCES
1. Owens RC Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl 2):S144-S157.
2. CDC. Signs and symptoms of untreated Lyme disease (2015). www.cdc.gov/lyme/signs_symptoms/index.html. Accessed June 7, 2016.
3. CDC. Parasites: babesiosis (2014). www.cdc.gov/parasites/babesiosis/disease.html. Accessed June 7, 2016.
4. CDC. Ehrlichiosis: symptoms, diagnosis, and treatment (2013). www.cdc.gov/Ehrlichiosis/symptoms/index.html. Accessed June 7, 2016.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5. [Epub ahead of print]
6. Choi E, Pyzocha NJ, Maurer DM. Tick-borne illnesses. Curr Sports Med Rep. 2016;15(2):98-104.
7. Chomel B. Lyme disease. Rev Sci Tech. 2015;34(2):569-576.
8. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326.
9. CDC. Leptospirosis: signs and symptoms (2016). www.cdc.gov/leptospirosis/symptoms/index.html. Accessed June 7, 2016.
10. World Health Organization, International Leptospirosis Society. Human Leptospirosis: Guidance for Diagnosis, Surveillance, and Control (2003). http://apps.who.int/iris/bitstream/10665/42667/1/WHO_CDS_CSR_EPH_2002.23.pdf. Accessed June 7, 2016.
11. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
12. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97.
13. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine et Maladies Infectieuses. 2013;43(1):1-9.
14. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039.
15. Zavitsanou A, Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Sci J. 2008;2(2):75-82.
16. Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843.
17. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593-1599.
18. Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974-1998. Clin Infect Dis. 2001;33(11):1834-1841.
19. Yaakob Y, Rodrigues KF, John DV. Leptospirosis: recent incidents and available diagnostics—a review. Med J Malaysia. 2015;70(6):351-355.
20. Seguro AC, Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39(suppl 1):17-23.
21. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245-252.
22. Waggoner JJ, Balassiano I, Mohamed-Hadley A, et al. Reverse-transcriptase PCR detection of Leptospira: absence of agreement with single-specimen microscopic agglutination testing. PLoS One. 2015;10(7):e0132988.
23. Iwasaki H, Chagan-Yasutan H, Leano PS, et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn Microbiol Infect Dis. 2016;84(4):287-291