User login
Challenges and innovations in training gyn surgeons
Obstetrics and gynecology (ObGyn) is a surgical specialty, yet the training of ObGyn residents differs significantly from that of residents in other surgical specialties. In addition to attaining competency in both the distinct but related fields of obstetrics and gynecology, ObGyn residents have their training condensed into 4 years rather than the 5 years’ training of many other surgical specialties. This limits the time dedicated to gynecologic surgery, currently 18 to 20 months in most programs, and has been exacerbated by tighter duty-hour restrictions.1
Additionally, with increasing demand for minimally invasive procedures, residents are expected to attain competency in a growing breadth of gynecologic procedures in a patient population with increasing morbidity, and they may have less autonomy to do so in an increasingly litigious environment.2 Furthermore, annual hysterectomy cases are declining, from about 680,000 in 2002 to 430,000 in 2010,3 and these declining rates are seen in the low case numbers of recent graduates.4
Training time, procedure complexity
With less time to master a growing body of increasingly complex procedures, is the profession adequately training gynecologic surgeons? Many gynecologic surgeons are concerned that the answer is no and that significant shifts in resident training are needed to generate safe and competent gynecologic surgeons. These training deficits represent a deficiency in the quality of care for women specifically, and thus the inattention to training gynecologic surgeons should be considered a health care disparity.
The concern over insufficient attention to gynecologic surgical training is not new, nor are proposed solutions, with many physicians citing the above concerns.5-9 In 2018, the Accreditation Council for Graduate Medical Education (ACGME) case minimums for hysterectomy increased to 85 from 70 hysterectomies, with a shift toward minimally invasive hysterectomy.10 Otherwise, minimal national changes have been made in this century to training gynecologic surgeons.
Tracking as an option
Many critics of current ObGyn training argue that obstetrics and gynecology, while related, have significantly different pathologies, surgical approaches, and skill sets and thus warrant the option to track toward obstetrics or gynecology after attaining limited core skill set in residency. In 2010, the Carnegie Foundation for the Advancement of Teaching called for the need for increased individualization opportunities in graduate medical education, citing that minimal changes have been made to medical education since the Flexner Report a century prior.11
Notably, tracking has been implemented with success at Cleveland Clinic, where residents are given 5 to 10 weeks of time allotted to their specific fields of interest, while still meeting minimum ACGME requirements and, in some cases, exceeding hysterectomy minimums by as much as 500%.12 Tracking is viewed positively by a majority of program directors.13 See the box below for Dr. Ferrando’s experience on tracking at the Cleveland Clinic.
Simulation training
Other educators advocate for maximizing preparedness for the operating room by using high-fidelity simulation.14,15 Simulation allows for the acquisition of basic technical skills needed for surgery as well as for repetition not easily achieved in the current surgical environment. Additionally, it provides lower-level learners the opportunity to acquire basic skills in a safe setting, thereby enhancing the ability to participate meaningfully on arrival in the operating room.16
In 2018, the American Board of Obstetrics and Gynecology added the Fundamentals of Laparoscopic Surgery certification as a new requirement for board certification.17 Laparoscopic and robotic surgery simulators allow trainees to develop coordination and specific skills, like knot tying and suturing. Additionally, models are available with varying levels of fidelity for vaginal and abdominal hysterectomy.18-20 See the box below for Dr. Miyazaki’s experience in developing the Miya Model trainer for vaginal surgery simulation.
Structured feedback
Finally, if a resident has limited exposure to a specific procedure, maximizing the preparation and feedback for each procedure is paramount. However, surgeons receive minimal formal training in teaching trainees, which leads to inconsistent and underutilized feedback.21 Specific structured feedback models have been implemented with success in the general surgery literature, including the SHARP (Set learning objectives, How did it go, Address concerns, Review learning points, Plan ahead) and BID (Briefing, Intraoperative, Debriefing) models.22,23
Reimbursement reform
While surgical reimbursement is not directly tied to resident education, decreased reimbursement to women’s health pathology and procedures has the downstream effect of decreasing the funds available for ObGyn departments to invest in research and education. Additionally, “suboptimal mastery or maintenance of appropriate surgical skills results in procedural inefficiencies that compound surgical cost.”5 Providers and payors alike should therefore be motivated to improve funding in order to improve adequate training of gynecologic surgeons. Payment reform is necessary to equally value women’s health procedures but also can ensure that gynecologic surgeons have the funds needed to train a competent next generation of ObGyn physicians. ●
- Residents and fellows have significant constraints that limit adequate training in gynecologic surgery. In a panel discussion at the 48th annual meeting of the Society of Gynecologic Surgeons, Drs. Zimmerman, Ferrando, and Miyazaki spoke about potential solutions.
- Allowing residents to track toward obstetric or gynecologic subspecialties may improve surgical volume of trainees who aim for a future career in gynecologic surgery.
- Simulation has demonstrated efficacy in enabling residents to prepare and improve their technical skills for specific procedures prior to entering the operating room.
Cecile A. Ferrando, MD, MPH
In his 2013 presidential address at the opening ceremony of the 42nd AAGL Global Congress on Minimally Invasive Gynecology, Javier Magrina, MD, asked the audience, “Isn’t it time to separate the O from the G?”7 Since that address, this catchy question has been posed several times, and it continues to be a topic of interest to many ObGyn educators seeking to innovate the curriculum and to better train our next generation’s gynecologic surgeons.
Several concerns have been raised about the current traditional 4-year residency training program, which has been impacted by the reduction of training hours due to duty-hour rules in the setting of decreased surgical volume and new technologies used to perform surgery. While other surgical specialties have begun to innovate their pathways for trainees, ObGyn has been a little slower to make a significant transition in its approach to training.
In 2012, Cleveland Clinic decided to lead the way in innovation regarding residency training. At its inception, the curriculum was designed to allow “tracking blocks” through each academic year to allow residents to gain additional experience in their specialty of choice. The program was carefully designed to assure that residents would achieve all 28 of the core obstetrics and gynecology milestones while still allowing for curricular flexibility.
Currently, residents are given autonomy to design their own tracking blocks with an assigned mentor for the rotation. Allowing residents to spend more time in their specialty of choice permits them to fine-tune skills that a standard curriculum may not have afforded the opportunity to home in on. It also allows residents to gain exposure to specialties that are not part of the core program, such as vulvar health, breast health and surgery, and gender affirmation surgery.
The Cleveland Clinic experience has been successful thus far. Importantly, preliminary data show that the tracking program does not interfere with the overall case number necessary for graduation. Residents also have succeeded in their postgraduation pursuits, including those who chose to specialize in general obstetrics and gynecology.
Cleveland Clinic is no longer the only program to incorporate tracking into its curriculum. This innovation is likely to become more standard as medical education in ObGyn evolves. We have not yet “separated the O from the G” completely in our specialty. However, thought leaders in our field are recognizing the need to better prepare our trainees, and this flexibility in mindset is bound to lead to a paradigm that may become the new standard for our specialty.
Acknowledgments: John E. Jelovsek, MD, the first Program Director of the Cleveland Clinic Residency in Obstetrics & Gynecology, who was responsible for creating the tracking program; and Vicki Reed, MD, the current Program Director, who has continued to innovate the program.
The Miya Model (Miyazaki Enterprises LLC) is a multiprocedural vaginal surgery simulator born from the need for standardized, scalable training in response to reductions in the average surgical case volume per resident. The Miya Model supports various basic procedures, such as pelvic exams and dilation and curettage, as well as full surgical procedures, including anterior and posterior colporrhaphy, midurethral and retropubic slings, cystoscopy, and vaginal hysterectomy. Training with the Miya Model moves resident surgical education from the operating room to any simulation lab or office-based setting. With rapidly declining resident surgical case volumes, there is an even stronger need to provide additional training outside of the operating room theater. Creation and development of the Miya Model were fueled by a desire to create a safer and more efficient method to educate residents without the risk of patient harm.
Miyazaki Enterprises has taken the Miya Model from a vision on paper to a standardized, commercially available product to help support resident and physician education. The Miya Model has undergone numerous rounds of waterfall and agile development, validity testing, and the creation of internal and external processes to achieve this vision. It serves as an example that ideas originating from significant demonstrated market need can be successfully created and deployed by a physician.
- Espey E, Ogburn T, Puscheck E. Impact of duty hour limitations on resident and student education in obstetrics and gynecology. J Reprod Med. 2007;52:345-348.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395-398. doi: 10.1097/AOG.0b013e3181955011.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241. doi: 10.1097/AOG.0b013e318299a6cf.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Female Pelvic Med Reconstr Surg. 2021;27:382-387. doi: 10.1097/SPV.0000000000000879.
- Podratz KC. Gynecologic surgery: an imperiled ballet. Presidential address. Am J Obstet Gynecol. 1998;178:1229-1234. doi: 10.1016/ s0002-9378(98)70327-8.
- Bissonnette JM, Gabbe SG, Hammond CB, et al. Restructuring residency training in obstetrics and gynecology. Am J Obstet Gynecol. 1999;180(3 pt 1):516-518. doi: 10.1016/s0002-9378(99)70246-2.
- Magrina JF. Isn’t it time to separate the O from the G? J Minim Invasive Gynecol. 2014;21:501-503. doi: 10.1016/j.jmig.2014.01.022.
- Merrill JA. Needed changes in obstetric-gynecologic training. Obstet Gynecol Surv. 1994;49:1-2.
- Lauer JK, Advincula AP. The future of the gynecologic surgeon: rationale for and steps toward subspecialization of complex gynecologic surgery. J Minim Invasive Gynecol. 2021;28:726-729. doi: 10.1016/j.jmig.2020.12.031.
- Hall EF, Raker CA, Hampton BS. Variability in gynecologic case volume of obstetrician-gynecologist residents graduating from 2009 to 2017. Am J Obstet Gynecol. 2020;222:617.e1-617.e8. doi: 10.1016/j .ajog.2019.11.1258.
- Irby DM, Cooke M, O’Brien BC. Calls for reform of medical education by the Carnegie Foundation for the Advancement of Teaching: 1910 and 2010. Acad Med. 2010;85:220-227. doi: 10.1097 /ACM.0b013e3181c88449.
- Reed VR, Emery J, Farrell RM, et al. Tracking—a flexible obstetrics and gynecology residency curriculum. Obstet Gynecol. 2019;134(suppl 1):29s-33s. doi: 10.1097/AOG.0000000000003464.
- Hariton E, Freret TS, Nitecki R, et al. Program director perceptions of subspecialty tracking in obstetrics and gynecology residency. J Grad Med Educ. 2018;10:665-670. doi: 10.4300/JGME-D-18-00096.1.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasive Gynecol. 2021;28:490-495. doi: 10.1016/j .jmig.2020.12.001.
- Wohlrab K, Jelovsek JE, Myers D. Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol. 2017;217:522-526. doi: 10.1016/j.ajog.2017.05.017.
- Chen CC, Green IC, Colbert-Getz JM, et al. Warm-up on a simulator improves residents’ performance in laparoscopic surgery: a randomized trial. Int Urogynecol J. 2013;24:1615-1622. doi: 10.1007 /s00192-013-2066-2.
- Fundamentals of Laparoscopic Surgery. ABOG announces new eligibility requirement for board certification. January 23, 2018. Accessed May 12, 2022. https://www.flsprogram.org/news/abog -announces-new-eligibility-requirement-board-certification/.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1. doi: 10.1016/j .jmig.2020.12.006.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379. doi: 10.1097/SIH.0b013e318266d0c6.
- Stickrath E, Alston M. A novel abdominal hysterectomy simulator and its impact on obstetrics and gynecology residents’ surgical confidence. MedEdPORTAL. 2017;13:10636. doi: 10.15766/mep_2374-8265.10636.
- McKendy KM, Watanabe Y, Lee L, et al. Perioperative feedback in surgical training: a systematic review. Am J Surg. 2017;214:117-126. doi: 10.1016/j.amjsurg.2016.12.014.
- Ahmed M, Arora S, Russ S, et al. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258:958-963. doi: 10.1097/SLA.0b013e31828c88fc.
- Anderson CI, Gupta RN, Larson JR, et al. Impact of objectively assessing surgeons’ teaching on effective perioperative instructional behaviors. JAMA Surg. 2013;148:915-922. doi: 10.1001/jamasurg.2013.2144.
Obstetrics and gynecology (ObGyn) is a surgical specialty, yet the training of ObGyn residents differs significantly from that of residents in other surgical specialties. In addition to attaining competency in both the distinct but related fields of obstetrics and gynecology, ObGyn residents have their training condensed into 4 years rather than the 5 years’ training of many other surgical specialties. This limits the time dedicated to gynecologic surgery, currently 18 to 20 months in most programs, and has been exacerbated by tighter duty-hour restrictions.1
Additionally, with increasing demand for minimally invasive procedures, residents are expected to attain competency in a growing breadth of gynecologic procedures in a patient population with increasing morbidity, and they may have less autonomy to do so in an increasingly litigious environment.2 Furthermore, annual hysterectomy cases are declining, from about 680,000 in 2002 to 430,000 in 2010,3 and these declining rates are seen in the low case numbers of recent graduates.4
Training time, procedure complexity
With less time to master a growing body of increasingly complex procedures, is the profession adequately training gynecologic surgeons? Many gynecologic surgeons are concerned that the answer is no and that significant shifts in resident training are needed to generate safe and competent gynecologic surgeons. These training deficits represent a deficiency in the quality of care for women specifically, and thus the inattention to training gynecologic surgeons should be considered a health care disparity.
The concern over insufficient attention to gynecologic surgical training is not new, nor are proposed solutions, with many physicians citing the above concerns.5-9 In 2018, the Accreditation Council for Graduate Medical Education (ACGME) case minimums for hysterectomy increased to 85 from 70 hysterectomies, with a shift toward minimally invasive hysterectomy.10 Otherwise, minimal national changes have been made in this century to training gynecologic surgeons.
Tracking as an option
Many critics of current ObGyn training argue that obstetrics and gynecology, while related, have significantly different pathologies, surgical approaches, and skill sets and thus warrant the option to track toward obstetrics or gynecology after attaining limited core skill set in residency. In 2010, the Carnegie Foundation for the Advancement of Teaching called for the need for increased individualization opportunities in graduate medical education, citing that minimal changes have been made to medical education since the Flexner Report a century prior.11
Notably, tracking has been implemented with success at Cleveland Clinic, where residents are given 5 to 10 weeks of time allotted to their specific fields of interest, while still meeting minimum ACGME requirements and, in some cases, exceeding hysterectomy minimums by as much as 500%.12 Tracking is viewed positively by a majority of program directors.13 See the box below for Dr. Ferrando’s experience on tracking at the Cleveland Clinic.
Simulation training
Other educators advocate for maximizing preparedness for the operating room by using high-fidelity simulation.14,15 Simulation allows for the acquisition of basic technical skills needed for surgery as well as for repetition not easily achieved in the current surgical environment. Additionally, it provides lower-level learners the opportunity to acquire basic skills in a safe setting, thereby enhancing the ability to participate meaningfully on arrival in the operating room.16
In 2018, the American Board of Obstetrics and Gynecology added the Fundamentals of Laparoscopic Surgery certification as a new requirement for board certification.17 Laparoscopic and robotic surgery simulators allow trainees to develop coordination and specific skills, like knot tying and suturing. Additionally, models are available with varying levels of fidelity for vaginal and abdominal hysterectomy.18-20 See the box below for Dr. Miyazaki’s experience in developing the Miya Model trainer for vaginal surgery simulation.
Structured feedback
Finally, if a resident has limited exposure to a specific procedure, maximizing the preparation and feedback for each procedure is paramount. However, surgeons receive minimal formal training in teaching trainees, which leads to inconsistent and underutilized feedback.21 Specific structured feedback models have been implemented with success in the general surgery literature, including the SHARP (Set learning objectives, How did it go, Address concerns, Review learning points, Plan ahead) and BID (Briefing, Intraoperative, Debriefing) models.22,23
Reimbursement reform
While surgical reimbursement is not directly tied to resident education, decreased reimbursement to women’s health pathology and procedures has the downstream effect of decreasing the funds available for ObGyn departments to invest in research and education. Additionally, “suboptimal mastery or maintenance of appropriate surgical skills results in procedural inefficiencies that compound surgical cost.”5 Providers and payors alike should therefore be motivated to improve funding in order to improve adequate training of gynecologic surgeons. Payment reform is necessary to equally value women’s health procedures but also can ensure that gynecologic surgeons have the funds needed to train a competent next generation of ObGyn physicians. ●
- Residents and fellows have significant constraints that limit adequate training in gynecologic surgery. In a panel discussion at the 48th annual meeting of the Society of Gynecologic Surgeons, Drs. Zimmerman, Ferrando, and Miyazaki spoke about potential solutions.
- Allowing residents to track toward obstetric or gynecologic subspecialties may improve surgical volume of trainees who aim for a future career in gynecologic surgery.
- Simulation has demonstrated efficacy in enabling residents to prepare and improve their technical skills for specific procedures prior to entering the operating room.
Cecile A. Ferrando, MD, MPH
In his 2013 presidential address at the opening ceremony of the 42nd AAGL Global Congress on Minimally Invasive Gynecology, Javier Magrina, MD, asked the audience, “Isn’t it time to separate the O from the G?”7 Since that address, this catchy question has been posed several times, and it continues to be a topic of interest to many ObGyn educators seeking to innovate the curriculum and to better train our next generation’s gynecologic surgeons.
Several concerns have been raised about the current traditional 4-year residency training program, which has been impacted by the reduction of training hours due to duty-hour rules in the setting of decreased surgical volume and new technologies used to perform surgery. While other surgical specialties have begun to innovate their pathways for trainees, ObGyn has been a little slower to make a significant transition in its approach to training.
In 2012, Cleveland Clinic decided to lead the way in innovation regarding residency training. At its inception, the curriculum was designed to allow “tracking blocks” through each academic year to allow residents to gain additional experience in their specialty of choice. The program was carefully designed to assure that residents would achieve all 28 of the core obstetrics and gynecology milestones while still allowing for curricular flexibility.
Currently, residents are given autonomy to design their own tracking blocks with an assigned mentor for the rotation. Allowing residents to spend more time in their specialty of choice permits them to fine-tune skills that a standard curriculum may not have afforded the opportunity to home in on. It also allows residents to gain exposure to specialties that are not part of the core program, such as vulvar health, breast health and surgery, and gender affirmation surgery.
The Cleveland Clinic experience has been successful thus far. Importantly, preliminary data show that the tracking program does not interfere with the overall case number necessary for graduation. Residents also have succeeded in their postgraduation pursuits, including those who chose to specialize in general obstetrics and gynecology.
Cleveland Clinic is no longer the only program to incorporate tracking into its curriculum. This innovation is likely to become more standard as medical education in ObGyn evolves. We have not yet “separated the O from the G” completely in our specialty. However, thought leaders in our field are recognizing the need to better prepare our trainees, and this flexibility in mindset is bound to lead to a paradigm that may become the new standard for our specialty.
Acknowledgments: John E. Jelovsek, MD, the first Program Director of the Cleveland Clinic Residency in Obstetrics & Gynecology, who was responsible for creating the tracking program; and Vicki Reed, MD, the current Program Director, who has continued to innovate the program.
The Miya Model (Miyazaki Enterprises LLC) is a multiprocedural vaginal surgery simulator born from the need for standardized, scalable training in response to reductions in the average surgical case volume per resident. The Miya Model supports various basic procedures, such as pelvic exams and dilation and curettage, as well as full surgical procedures, including anterior and posterior colporrhaphy, midurethral and retropubic slings, cystoscopy, and vaginal hysterectomy. Training with the Miya Model moves resident surgical education from the operating room to any simulation lab or office-based setting. With rapidly declining resident surgical case volumes, there is an even stronger need to provide additional training outside of the operating room theater. Creation and development of the Miya Model were fueled by a desire to create a safer and more efficient method to educate residents without the risk of patient harm.
Miyazaki Enterprises has taken the Miya Model from a vision on paper to a standardized, commercially available product to help support resident and physician education. The Miya Model has undergone numerous rounds of waterfall and agile development, validity testing, and the creation of internal and external processes to achieve this vision. It serves as an example that ideas originating from significant demonstrated market need can be successfully created and deployed by a physician.
Obstetrics and gynecology (ObGyn) is a surgical specialty, yet the training of ObGyn residents differs significantly from that of residents in other surgical specialties. In addition to attaining competency in both the distinct but related fields of obstetrics and gynecology, ObGyn residents have their training condensed into 4 years rather than the 5 years’ training of many other surgical specialties. This limits the time dedicated to gynecologic surgery, currently 18 to 20 months in most programs, and has been exacerbated by tighter duty-hour restrictions.1
Additionally, with increasing demand for minimally invasive procedures, residents are expected to attain competency in a growing breadth of gynecologic procedures in a patient population with increasing morbidity, and they may have less autonomy to do so in an increasingly litigious environment.2 Furthermore, annual hysterectomy cases are declining, from about 680,000 in 2002 to 430,000 in 2010,3 and these declining rates are seen in the low case numbers of recent graduates.4
Training time, procedure complexity
With less time to master a growing body of increasingly complex procedures, is the profession adequately training gynecologic surgeons? Many gynecologic surgeons are concerned that the answer is no and that significant shifts in resident training are needed to generate safe and competent gynecologic surgeons. These training deficits represent a deficiency in the quality of care for women specifically, and thus the inattention to training gynecologic surgeons should be considered a health care disparity.
The concern over insufficient attention to gynecologic surgical training is not new, nor are proposed solutions, with many physicians citing the above concerns.5-9 In 2018, the Accreditation Council for Graduate Medical Education (ACGME) case minimums for hysterectomy increased to 85 from 70 hysterectomies, with a shift toward minimally invasive hysterectomy.10 Otherwise, minimal national changes have been made in this century to training gynecologic surgeons.
Tracking as an option
Many critics of current ObGyn training argue that obstetrics and gynecology, while related, have significantly different pathologies, surgical approaches, and skill sets and thus warrant the option to track toward obstetrics or gynecology after attaining limited core skill set in residency. In 2010, the Carnegie Foundation for the Advancement of Teaching called for the need for increased individualization opportunities in graduate medical education, citing that minimal changes have been made to medical education since the Flexner Report a century prior.11
Notably, tracking has been implemented with success at Cleveland Clinic, where residents are given 5 to 10 weeks of time allotted to their specific fields of interest, while still meeting minimum ACGME requirements and, in some cases, exceeding hysterectomy minimums by as much as 500%.12 Tracking is viewed positively by a majority of program directors.13 See the box below for Dr. Ferrando’s experience on tracking at the Cleveland Clinic.
Simulation training
Other educators advocate for maximizing preparedness for the operating room by using high-fidelity simulation.14,15 Simulation allows for the acquisition of basic technical skills needed for surgery as well as for repetition not easily achieved in the current surgical environment. Additionally, it provides lower-level learners the opportunity to acquire basic skills in a safe setting, thereby enhancing the ability to participate meaningfully on arrival in the operating room.16
In 2018, the American Board of Obstetrics and Gynecology added the Fundamentals of Laparoscopic Surgery certification as a new requirement for board certification.17 Laparoscopic and robotic surgery simulators allow trainees to develop coordination and specific skills, like knot tying and suturing. Additionally, models are available with varying levels of fidelity for vaginal and abdominal hysterectomy.18-20 See the box below for Dr. Miyazaki’s experience in developing the Miya Model trainer for vaginal surgery simulation.
Structured feedback
Finally, if a resident has limited exposure to a specific procedure, maximizing the preparation and feedback for each procedure is paramount. However, surgeons receive minimal formal training in teaching trainees, which leads to inconsistent and underutilized feedback.21 Specific structured feedback models have been implemented with success in the general surgery literature, including the SHARP (Set learning objectives, How did it go, Address concerns, Review learning points, Plan ahead) and BID (Briefing, Intraoperative, Debriefing) models.22,23
Reimbursement reform
While surgical reimbursement is not directly tied to resident education, decreased reimbursement to women’s health pathology and procedures has the downstream effect of decreasing the funds available for ObGyn departments to invest in research and education. Additionally, “suboptimal mastery or maintenance of appropriate surgical skills results in procedural inefficiencies that compound surgical cost.”5 Providers and payors alike should therefore be motivated to improve funding in order to improve adequate training of gynecologic surgeons. Payment reform is necessary to equally value women’s health procedures but also can ensure that gynecologic surgeons have the funds needed to train a competent next generation of ObGyn physicians. ●
- Residents and fellows have significant constraints that limit adequate training in gynecologic surgery. In a panel discussion at the 48th annual meeting of the Society of Gynecologic Surgeons, Drs. Zimmerman, Ferrando, and Miyazaki spoke about potential solutions.
- Allowing residents to track toward obstetric or gynecologic subspecialties may improve surgical volume of trainees who aim for a future career in gynecologic surgery.
- Simulation has demonstrated efficacy in enabling residents to prepare and improve their technical skills for specific procedures prior to entering the operating room.
Cecile A. Ferrando, MD, MPH
In his 2013 presidential address at the opening ceremony of the 42nd AAGL Global Congress on Minimally Invasive Gynecology, Javier Magrina, MD, asked the audience, “Isn’t it time to separate the O from the G?”7 Since that address, this catchy question has been posed several times, and it continues to be a topic of interest to many ObGyn educators seeking to innovate the curriculum and to better train our next generation’s gynecologic surgeons.
Several concerns have been raised about the current traditional 4-year residency training program, which has been impacted by the reduction of training hours due to duty-hour rules in the setting of decreased surgical volume and new technologies used to perform surgery. While other surgical specialties have begun to innovate their pathways for trainees, ObGyn has been a little slower to make a significant transition in its approach to training.
In 2012, Cleveland Clinic decided to lead the way in innovation regarding residency training. At its inception, the curriculum was designed to allow “tracking blocks” through each academic year to allow residents to gain additional experience in their specialty of choice. The program was carefully designed to assure that residents would achieve all 28 of the core obstetrics and gynecology milestones while still allowing for curricular flexibility.
Currently, residents are given autonomy to design their own tracking blocks with an assigned mentor for the rotation. Allowing residents to spend more time in their specialty of choice permits them to fine-tune skills that a standard curriculum may not have afforded the opportunity to home in on. It also allows residents to gain exposure to specialties that are not part of the core program, such as vulvar health, breast health and surgery, and gender affirmation surgery.
The Cleveland Clinic experience has been successful thus far. Importantly, preliminary data show that the tracking program does not interfere with the overall case number necessary for graduation. Residents also have succeeded in their postgraduation pursuits, including those who chose to specialize in general obstetrics and gynecology.
Cleveland Clinic is no longer the only program to incorporate tracking into its curriculum. This innovation is likely to become more standard as medical education in ObGyn evolves. We have not yet “separated the O from the G” completely in our specialty. However, thought leaders in our field are recognizing the need to better prepare our trainees, and this flexibility in mindset is bound to lead to a paradigm that may become the new standard for our specialty.
Acknowledgments: John E. Jelovsek, MD, the first Program Director of the Cleveland Clinic Residency in Obstetrics & Gynecology, who was responsible for creating the tracking program; and Vicki Reed, MD, the current Program Director, who has continued to innovate the program.
The Miya Model (Miyazaki Enterprises LLC) is a multiprocedural vaginal surgery simulator born from the need for standardized, scalable training in response to reductions in the average surgical case volume per resident. The Miya Model supports various basic procedures, such as pelvic exams and dilation and curettage, as well as full surgical procedures, including anterior and posterior colporrhaphy, midurethral and retropubic slings, cystoscopy, and vaginal hysterectomy. Training with the Miya Model moves resident surgical education from the operating room to any simulation lab or office-based setting. With rapidly declining resident surgical case volumes, there is an even stronger need to provide additional training outside of the operating room theater. Creation and development of the Miya Model were fueled by a desire to create a safer and more efficient method to educate residents without the risk of patient harm.
Miyazaki Enterprises has taken the Miya Model from a vision on paper to a standardized, commercially available product to help support resident and physician education. The Miya Model has undergone numerous rounds of waterfall and agile development, validity testing, and the creation of internal and external processes to achieve this vision. It serves as an example that ideas originating from significant demonstrated market need can be successfully created and deployed by a physician.
- Espey E, Ogburn T, Puscheck E. Impact of duty hour limitations on resident and student education in obstetrics and gynecology. J Reprod Med. 2007;52:345-348.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395-398. doi: 10.1097/AOG.0b013e3181955011.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241. doi: 10.1097/AOG.0b013e318299a6cf.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Female Pelvic Med Reconstr Surg. 2021;27:382-387. doi: 10.1097/SPV.0000000000000879.
- Podratz KC. Gynecologic surgery: an imperiled ballet. Presidential address. Am J Obstet Gynecol. 1998;178:1229-1234. doi: 10.1016/ s0002-9378(98)70327-8.
- Bissonnette JM, Gabbe SG, Hammond CB, et al. Restructuring residency training in obstetrics and gynecology. Am J Obstet Gynecol. 1999;180(3 pt 1):516-518. doi: 10.1016/s0002-9378(99)70246-2.
- Magrina JF. Isn’t it time to separate the O from the G? J Minim Invasive Gynecol. 2014;21:501-503. doi: 10.1016/j.jmig.2014.01.022.
- Merrill JA. Needed changes in obstetric-gynecologic training. Obstet Gynecol Surv. 1994;49:1-2.
- Lauer JK, Advincula AP. The future of the gynecologic surgeon: rationale for and steps toward subspecialization of complex gynecologic surgery. J Minim Invasive Gynecol. 2021;28:726-729. doi: 10.1016/j.jmig.2020.12.031.
- Hall EF, Raker CA, Hampton BS. Variability in gynecologic case volume of obstetrician-gynecologist residents graduating from 2009 to 2017. Am J Obstet Gynecol. 2020;222:617.e1-617.e8. doi: 10.1016/j .ajog.2019.11.1258.
- Irby DM, Cooke M, O’Brien BC. Calls for reform of medical education by the Carnegie Foundation for the Advancement of Teaching: 1910 and 2010. Acad Med. 2010;85:220-227. doi: 10.1097 /ACM.0b013e3181c88449.
- Reed VR, Emery J, Farrell RM, et al. Tracking—a flexible obstetrics and gynecology residency curriculum. Obstet Gynecol. 2019;134(suppl 1):29s-33s. doi: 10.1097/AOG.0000000000003464.
- Hariton E, Freret TS, Nitecki R, et al. Program director perceptions of subspecialty tracking in obstetrics and gynecology residency. J Grad Med Educ. 2018;10:665-670. doi: 10.4300/JGME-D-18-00096.1.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasive Gynecol. 2021;28:490-495. doi: 10.1016/j .jmig.2020.12.001.
- Wohlrab K, Jelovsek JE, Myers D. Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol. 2017;217:522-526. doi: 10.1016/j.ajog.2017.05.017.
- Chen CC, Green IC, Colbert-Getz JM, et al. Warm-up on a simulator improves residents’ performance in laparoscopic surgery: a randomized trial. Int Urogynecol J. 2013;24:1615-1622. doi: 10.1007 /s00192-013-2066-2.
- Fundamentals of Laparoscopic Surgery. ABOG announces new eligibility requirement for board certification. January 23, 2018. Accessed May 12, 2022. https://www.flsprogram.org/news/abog -announces-new-eligibility-requirement-board-certification/.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1. doi: 10.1016/j .jmig.2020.12.006.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379. doi: 10.1097/SIH.0b013e318266d0c6.
- Stickrath E, Alston M. A novel abdominal hysterectomy simulator and its impact on obstetrics and gynecology residents’ surgical confidence. MedEdPORTAL. 2017;13:10636. doi: 10.15766/mep_2374-8265.10636.
- McKendy KM, Watanabe Y, Lee L, et al. Perioperative feedback in surgical training: a systematic review. Am J Surg. 2017;214:117-126. doi: 10.1016/j.amjsurg.2016.12.014.
- Ahmed M, Arora S, Russ S, et al. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258:958-963. doi: 10.1097/SLA.0b013e31828c88fc.
- Anderson CI, Gupta RN, Larson JR, et al. Impact of objectively assessing surgeons’ teaching on effective perioperative instructional behaviors. JAMA Surg. 2013;148:915-922. doi: 10.1001/jamasurg.2013.2144.
- Espey E, Ogburn T, Puscheck E. Impact of duty hour limitations on resident and student education in obstetrics and gynecology. J Reprod Med. 2007;52:345-348.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395-398. doi: 10.1097/AOG.0b013e3181955011.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241. doi: 10.1097/AOG.0b013e318299a6cf.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Female Pelvic Med Reconstr Surg. 2021;27:382-387. doi: 10.1097/SPV.0000000000000879.
- Podratz KC. Gynecologic surgery: an imperiled ballet. Presidential address. Am J Obstet Gynecol. 1998;178:1229-1234. doi: 10.1016/ s0002-9378(98)70327-8.
- Bissonnette JM, Gabbe SG, Hammond CB, et al. Restructuring residency training in obstetrics and gynecology. Am J Obstet Gynecol. 1999;180(3 pt 1):516-518. doi: 10.1016/s0002-9378(99)70246-2.
- Magrina JF. Isn’t it time to separate the O from the G? J Minim Invasive Gynecol. 2014;21:501-503. doi: 10.1016/j.jmig.2014.01.022.
- Merrill JA. Needed changes in obstetric-gynecologic training. Obstet Gynecol Surv. 1994;49:1-2.
- Lauer JK, Advincula AP. The future of the gynecologic surgeon: rationale for and steps toward subspecialization of complex gynecologic surgery. J Minim Invasive Gynecol. 2021;28:726-729. doi: 10.1016/j.jmig.2020.12.031.
- Hall EF, Raker CA, Hampton BS. Variability in gynecologic case volume of obstetrician-gynecologist residents graduating from 2009 to 2017. Am J Obstet Gynecol. 2020;222:617.e1-617.e8. doi: 10.1016/j .ajog.2019.11.1258.
- Irby DM, Cooke M, O’Brien BC. Calls for reform of medical education by the Carnegie Foundation for the Advancement of Teaching: 1910 and 2010. Acad Med. 2010;85:220-227. doi: 10.1097 /ACM.0b013e3181c88449.
- Reed VR, Emery J, Farrell RM, et al. Tracking—a flexible obstetrics and gynecology residency curriculum. Obstet Gynecol. 2019;134(suppl 1):29s-33s. doi: 10.1097/AOG.0000000000003464.
- Hariton E, Freret TS, Nitecki R, et al. Program director perceptions of subspecialty tracking in obstetrics and gynecology residency. J Grad Med Educ. 2018;10:665-670. doi: 10.4300/JGME-D-18-00096.1.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasive Gynecol. 2021;28:490-495. doi: 10.1016/j .jmig.2020.12.001.
- Wohlrab K, Jelovsek JE, Myers D. Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol. 2017;217:522-526. doi: 10.1016/j.ajog.2017.05.017.
- Chen CC, Green IC, Colbert-Getz JM, et al. Warm-up on a simulator improves residents’ performance in laparoscopic surgery: a randomized trial. Int Urogynecol J. 2013;24:1615-1622. doi: 10.1007 /s00192-013-2066-2.
- Fundamentals of Laparoscopic Surgery. ABOG announces new eligibility requirement for board certification. January 23, 2018. Accessed May 12, 2022. https://www.flsprogram.org/news/abog -announces-new-eligibility-requirement-board-certification/.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1. doi: 10.1016/j .jmig.2020.12.006.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379. doi: 10.1097/SIH.0b013e318266d0c6.
- Stickrath E, Alston M. A novel abdominal hysterectomy simulator and its impact on obstetrics and gynecology residents’ surgical confidence. MedEdPORTAL. 2017;13:10636. doi: 10.15766/mep_2374-8265.10636.
- McKendy KM, Watanabe Y, Lee L, et al. Perioperative feedback in surgical training: a systematic review. Am J Surg. 2017;214:117-126. doi: 10.1016/j.amjsurg.2016.12.014.
- Ahmed M, Arora S, Russ S, et al. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258:958-963. doi: 10.1097/SLA.0b013e31828c88fc.
- Anderson CI, Gupta RN, Larson JR, et al. Impact of objectively assessing surgeons’ teaching on effective perioperative instructional behaviors. JAMA Surg. 2013;148:915-922. doi: 10.1001/jamasurg.2013.2144.
Vaginal hysterectomy: Is skill the limiting factor?
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
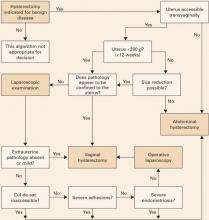
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
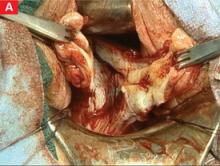
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
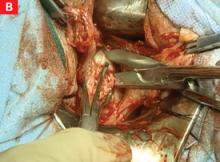
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
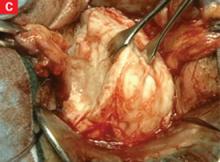
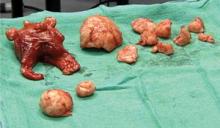
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.