User login
Dasatinib shows promise as a control agent for CAR T cells
The tyrosine kinase inhibitor dasatinib was found to transiently inhibit CAR T-cell function in mice, suggesting applicability as an on-off control for CAR T-cell therapy, investigators report.
“In this study, we focused our efforts on evaluating dasatinib as a control drug for CAR T cells,” wrote Katrin Mestermann, PhD, of the University of Würzburg in Germany and colleagues in Science Translational Medicine.
The researchers explored the pharmacological effects of dasatinib on CAR T-cell function using a mouse xenograft model. In this model, dasatinib was administered every 6 hours to sustain serum levels above a mandatory threshold to ensure human pharmacokinetic equivalence.
The team observed that dasatinib interferes with several cellular mechanisms that effect CAR T-cell activity, including inhibition of protein phosphorylation and cytokine secretion, among others.
Short-term administration of dasatinib was shown to not alter antilymphoma activity of CD19-CAR T cells, according to data from in vivo experiments.
In addition, upon cessation of dasatinib, CAR T-cell antitumor activity was rapidly restored, based on in vitro experiment data.
Dr. Mestermann and her colleagues also noted that the dose of dasatinib was titratable to attain either partial or complete inhibition of CAR T-cell activity. These effects could be maintained for several days without altering T-cell survival.
“A short treatment course of dasatinib, administered early after CAR T-cell infusion, protects a proportion of mice from otherwise fatal cytokine release syndrome,” the researchers wrote.
Cytokine release syndrome is the most frequently seen acute toxicity linked to CAR T-cell therapy, they explained.
“Further studies are warranted to determine whether dasatinib is also effective in clinical situations with established cytokine release syndrome,” they concluded.
The study was funded by the Cluster Biotechnologie Bayern and Free State of Bavaria, the German Cancer Aid, and the University of Würzburg. The authors reported financial affiliations with Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Takeda, and others.
SOURCE: Mestermann K et al. Sci Transl Med. 2019 Jul 3. doi: 10.1126/scitranslmed.aau5907.
The tyrosine kinase inhibitor dasatinib was found to transiently inhibit CAR T-cell function in mice, suggesting applicability as an on-off control for CAR T-cell therapy, investigators report.
“In this study, we focused our efforts on evaluating dasatinib as a control drug for CAR T cells,” wrote Katrin Mestermann, PhD, of the University of Würzburg in Germany and colleagues in Science Translational Medicine.
The researchers explored the pharmacological effects of dasatinib on CAR T-cell function using a mouse xenograft model. In this model, dasatinib was administered every 6 hours to sustain serum levels above a mandatory threshold to ensure human pharmacokinetic equivalence.
The team observed that dasatinib interferes with several cellular mechanisms that effect CAR T-cell activity, including inhibition of protein phosphorylation and cytokine secretion, among others.
Short-term administration of dasatinib was shown to not alter antilymphoma activity of CD19-CAR T cells, according to data from in vivo experiments.
In addition, upon cessation of dasatinib, CAR T-cell antitumor activity was rapidly restored, based on in vitro experiment data.
Dr. Mestermann and her colleagues also noted that the dose of dasatinib was titratable to attain either partial or complete inhibition of CAR T-cell activity. These effects could be maintained for several days without altering T-cell survival.
“A short treatment course of dasatinib, administered early after CAR T-cell infusion, protects a proportion of mice from otherwise fatal cytokine release syndrome,” the researchers wrote.
Cytokine release syndrome is the most frequently seen acute toxicity linked to CAR T-cell therapy, they explained.
“Further studies are warranted to determine whether dasatinib is also effective in clinical situations with established cytokine release syndrome,” they concluded.
The study was funded by the Cluster Biotechnologie Bayern and Free State of Bavaria, the German Cancer Aid, and the University of Würzburg. The authors reported financial affiliations with Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Takeda, and others.
SOURCE: Mestermann K et al. Sci Transl Med. 2019 Jul 3. doi: 10.1126/scitranslmed.aau5907.
The tyrosine kinase inhibitor dasatinib was found to transiently inhibit CAR T-cell function in mice, suggesting applicability as an on-off control for CAR T-cell therapy, investigators report.
“In this study, we focused our efforts on evaluating dasatinib as a control drug for CAR T cells,” wrote Katrin Mestermann, PhD, of the University of Würzburg in Germany and colleagues in Science Translational Medicine.
The researchers explored the pharmacological effects of dasatinib on CAR T-cell function using a mouse xenograft model. In this model, dasatinib was administered every 6 hours to sustain serum levels above a mandatory threshold to ensure human pharmacokinetic equivalence.
The team observed that dasatinib interferes with several cellular mechanisms that effect CAR T-cell activity, including inhibition of protein phosphorylation and cytokine secretion, among others.
Short-term administration of dasatinib was shown to not alter antilymphoma activity of CD19-CAR T cells, according to data from in vivo experiments.
In addition, upon cessation of dasatinib, CAR T-cell antitumor activity was rapidly restored, based on in vitro experiment data.
Dr. Mestermann and her colleagues also noted that the dose of dasatinib was titratable to attain either partial or complete inhibition of CAR T-cell activity. These effects could be maintained for several days without altering T-cell survival.
“A short treatment course of dasatinib, administered early after CAR T-cell infusion, protects a proportion of mice from otherwise fatal cytokine release syndrome,” the researchers wrote.
Cytokine release syndrome is the most frequently seen acute toxicity linked to CAR T-cell therapy, they explained.
“Further studies are warranted to determine whether dasatinib is also effective in clinical situations with established cytokine release syndrome,” they concluded.
The study was funded by the Cluster Biotechnologie Bayern and Free State of Bavaria, the German Cancer Aid, and the University of Würzburg. The authors reported financial affiliations with Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Takeda, and others.
SOURCE: Mestermann K et al. Sci Transl Med. 2019 Jul 3. doi: 10.1126/scitranslmed.aau5907.
FROM SCIENCE TRANSLATIONAL MEDICINE
Radiation bridging with axi-cel appears safe in DLBCL
according to results from a case series.
“Effective bridging strategies may be needed to provide patients with aggressive disease access to CAR T therapy,” wrote Austin J. Sim MD, JD, of Moffitt Cancer Center in Tampa and colleagues. The findings were reported in the International Journal of Radiation Oncology, Biology, Physics.
The study included a total of 12 patients planned to receive bridging treatment with radiation prior to axicabtagene ciloleucel infusion. The cohort consisted of patients with highly aggressive disease, including six with double-hit lymphoma and six with disease 10 cm or greater in diameter.
Study participants received a radiation dose between 2 and 4 Gy per fraction to a median of 20 Gy (range, 6-36.5 Gy) and half of the participants received 20 Gy in 5 fractions or 30 Gy in 10 fractions. Of the 12 patients, 7 were administered concomitant chemotherapy.
“One patient who underwent apheresis and radiation therapy ultimately did not proceed with CAR T infusion, but was still included in our analysis,” the researchers noted.
After analysis, the researchers reported that, during bridging radiation therapy, no patients had significant toxicities or in-field disease progression of disease prior to CAR T infusion.
Post CAR T infusion, 27% of patients experienced neurotoxicity or severe cytokine release syndrome.
At 30 days, the objective response rate was 81.8%, with 27% attaining complete response. At final follow-up, the best response rate was 81.8%, with complete response achieved in 45% of patients.
The researchers acknowledged that a key limitation of the study was the retrospective design. As a result, radiation dosing was not uniform and the optimal dose and fractionation remains unclear.
In addition, Dr. Sim and colleagues advised that caution should be taken if irradiation is initiated before T-cell apheresis, and if so, blood counts should be monitored closely.
“Future investigation is warranted to optimize the use of bridging radiation before CAR T therapy,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Atara Biotherapeutics, AstraZeneca, Celgene, GlaxoSmithKline, Novartis, Precision Biosciences, and several others.
SOURCE: Sim AJ et al. Int J Radiat Oncol Biol Phys. 2019 Jun 5. doi: 10.1016/j.ijrobp.2019.05.065.
according to results from a case series.
“Effective bridging strategies may be needed to provide patients with aggressive disease access to CAR T therapy,” wrote Austin J. Sim MD, JD, of Moffitt Cancer Center in Tampa and colleagues. The findings were reported in the International Journal of Radiation Oncology, Biology, Physics.
The study included a total of 12 patients planned to receive bridging treatment with radiation prior to axicabtagene ciloleucel infusion. The cohort consisted of patients with highly aggressive disease, including six with double-hit lymphoma and six with disease 10 cm or greater in diameter.
Study participants received a radiation dose between 2 and 4 Gy per fraction to a median of 20 Gy (range, 6-36.5 Gy) and half of the participants received 20 Gy in 5 fractions or 30 Gy in 10 fractions. Of the 12 patients, 7 were administered concomitant chemotherapy.
“One patient who underwent apheresis and radiation therapy ultimately did not proceed with CAR T infusion, but was still included in our analysis,” the researchers noted.
After analysis, the researchers reported that, during bridging radiation therapy, no patients had significant toxicities or in-field disease progression of disease prior to CAR T infusion.
Post CAR T infusion, 27% of patients experienced neurotoxicity or severe cytokine release syndrome.
At 30 days, the objective response rate was 81.8%, with 27% attaining complete response. At final follow-up, the best response rate was 81.8%, with complete response achieved in 45% of patients.
The researchers acknowledged that a key limitation of the study was the retrospective design. As a result, radiation dosing was not uniform and the optimal dose and fractionation remains unclear.
In addition, Dr. Sim and colleagues advised that caution should be taken if irradiation is initiated before T-cell apheresis, and if so, blood counts should be monitored closely.
“Future investigation is warranted to optimize the use of bridging radiation before CAR T therapy,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Atara Biotherapeutics, AstraZeneca, Celgene, GlaxoSmithKline, Novartis, Precision Biosciences, and several others.
SOURCE: Sim AJ et al. Int J Radiat Oncol Biol Phys. 2019 Jun 5. doi: 10.1016/j.ijrobp.2019.05.065.
according to results from a case series.
“Effective bridging strategies may be needed to provide patients with aggressive disease access to CAR T therapy,” wrote Austin J. Sim MD, JD, of Moffitt Cancer Center in Tampa and colleagues. The findings were reported in the International Journal of Radiation Oncology, Biology, Physics.
The study included a total of 12 patients planned to receive bridging treatment with radiation prior to axicabtagene ciloleucel infusion. The cohort consisted of patients with highly aggressive disease, including six with double-hit lymphoma and six with disease 10 cm or greater in diameter.
Study participants received a radiation dose between 2 and 4 Gy per fraction to a median of 20 Gy (range, 6-36.5 Gy) and half of the participants received 20 Gy in 5 fractions or 30 Gy in 10 fractions. Of the 12 patients, 7 were administered concomitant chemotherapy.
“One patient who underwent apheresis and radiation therapy ultimately did not proceed with CAR T infusion, but was still included in our analysis,” the researchers noted.
After analysis, the researchers reported that, during bridging radiation therapy, no patients had significant toxicities or in-field disease progression of disease prior to CAR T infusion.
Post CAR T infusion, 27% of patients experienced neurotoxicity or severe cytokine release syndrome.
At 30 days, the objective response rate was 81.8%, with 27% attaining complete response. At final follow-up, the best response rate was 81.8%, with complete response achieved in 45% of patients.
The researchers acknowledged that a key limitation of the study was the retrospective design. As a result, radiation dosing was not uniform and the optimal dose and fractionation remains unclear.
In addition, Dr. Sim and colleagues advised that caution should be taken if irradiation is initiated before T-cell apheresis, and if so, blood counts should be monitored closely.
“Future investigation is warranted to optimize the use of bridging radiation before CAR T therapy,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Atara Biotherapeutics, AstraZeneca, Celgene, GlaxoSmithKline, Novartis, Precision Biosciences, and several others.
SOURCE: Sim AJ et al. Int J Radiat Oncol Biol Phys. 2019 Jun 5. doi: 10.1016/j.ijrobp.2019.05.065.
FROM THE INTERNATIONAL JOURNAL OF RADIATION ONCOLOGY, BIOLOGY, PHYSICS
Polatuzumab vedotin combo shows promise in DLBCL
according to preliminary results from a phase 1b-2 trial.
Polatuzumab vedotin has already shown single-agent activity in relapsed/refractory diffuse large B-cell lymphoma, Hervé Tilly, MD, PhD, of the University of Rouen (France), and colleagues wrote in Lancet Oncology. “We explored the combination of polatuzumab vedotin with either rituximab, cyclophosphamide, doxorubicin, and prednisone [R-CHP] or obinutuzumab, cyclophosphamide, doxorubicin, and prednisone [G-CHP].”
With respect to polatuzumab vedotin dosing, the maximum investigated dose was 1.8 mg/kg. The novel agent was infused on day 2 of cycles 1 and 2 and subsequently on day 1 of each cycle thereafter for a total of 6-8 cycles, with each cycle lasting of 21 days.
The primary outcomes of this ongoing study were treatment safety and tolerability, as well as the establishment of recommended phase 2 dosing. Secondary endpoints included overall response rate, complete response=, among others.
A total of 82 patients were included in final analysis, 25 in the phase 1b dose escalation phase and 57 in the expansion phase.
After analysis, Dr. Tilly and his colleagues reported two dose-limiting toxicities: One patient experienced a grade 4 pulmonary embolism (1.8 mg/kg plus R-CHP) and another had grade 4 febrile neutropenia and grade 3 thrombocytopenia (1.4 mg/kg plus G-CHP).
At a median follow-up of 21.5 months, the overall response rate was 89% in study participants, including 77% of patients who achieved a complete response and 12% who had a partial response.
The recommended phase 2 dose of polatuzumab vedotin was 1.8 mg/kg. At this dose, the most common grade 3 or higher toxicities were neutropenia (30%), febrile neutropenia (18%), and thrombocytopenia (9%).
The researchers acknowledged a key limitation of the study was the nonrandomized design. As a result, dose and regimen comparisons could not be made.
The study was funded by F. Hoffmann-La Roche and Genentech. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Janssen, Merck, Pfizer, and others.
SOURCE: Tilly H et al. Lancet Oncol. 2019 May 14. doi: 10.1016/S1470-2045(19)30091-9.
according to preliminary results from a phase 1b-2 trial.
Polatuzumab vedotin has already shown single-agent activity in relapsed/refractory diffuse large B-cell lymphoma, Hervé Tilly, MD, PhD, of the University of Rouen (France), and colleagues wrote in Lancet Oncology. “We explored the combination of polatuzumab vedotin with either rituximab, cyclophosphamide, doxorubicin, and prednisone [R-CHP] or obinutuzumab, cyclophosphamide, doxorubicin, and prednisone [G-CHP].”
With respect to polatuzumab vedotin dosing, the maximum investigated dose was 1.8 mg/kg. The novel agent was infused on day 2 of cycles 1 and 2 and subsequently on day 1 of each cycle thereafter for a total of 6-8 cycles, with each cycle lasting of 21 days.
The primary outcomes of this ongoing study were treatment safety and tolerability, as well as the establishment of recommended phase 2 dosing. Secondary endpoints included overall response rate, complete response=, among others.
A total of 82 patients were included in final analysis, 25 in the phase 1b dose escalation phase and 57 in the expansion phase.
After analysis, Dr. Tilly and his colleagues reported two dose-limiting toxicities: One patient experienced a grade 4 pulmonary embolism (1.8 mg/kg plus R-CHP) and another had grade 4 febrile neutropenia and grade 3 thrombocytopenia (1.4 mg/kg plus G-CHP).
At a median follow-up of 21.5 months, the overall response rate was 89% in study participants, including 77% of patients who achieved a complete response and 12% who had a partial response.
The recommended phase 2 dose of polatuzumab vedotin was 1.8 mg/kg. At this dose, the most common grade 3 or higher toxicities were neutropenia (30%), febrile neutropenia (18%), and thrombocytopenia (9%).
The researchers acknowledged a key limitation of the study was the nonrandomized design. As a result, dose and regimen comparisons could not be made.
The study was funded by F. Hoffmann-La Roche and Genentech. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Janssen, Merck, Pfizer, and others.
SOURCE: Tilly H et al. Lancet Oncol. 2019 May 14. doi: 10.1016/S1470-2045(19)30091-9.
according to preliminary results from a phase 1b-2 trial.
Polatuzumab vedotin has already shown single-agent activity in relapsed/refractory diffuse large B-cell lymphoma, Hervé Tilly, MD, PhD, of the University of Rouen (France), and colleagues wrote in Lancet Oncology. “We explored the combination of polatuzumab vedotin with either rituximab, cyclophosphamide, doxorubicin, and prednisone [R-CHP] or obinutuzumab, cyclophosphamide, doxorubicin, and prednisone [G-CHP].”
With respect to polatuzumab vedotin dosing, the maximum investigated dose was 1.8 mg/kg. The novel agent was infused on day 2 of cycles 1 and 2 and subsequently on day 1 of each cycle thereafter for a total of 6-8 cycles, with each cycle lasting of 21 days.
The primary outcomes of this ongoing study were treatment safety and tolerability, as well as the establishment of recommended phase 2 dosing. Secondary endpoints included overall response rate, complete response=, among others.
A total of 82 patients were included in final analysis, 25 in the phase 1b dose escalation phase and 57 in the expansion phase.
After analysis, Dr. Tilly and his colleagues reported two dose-limiting toxicities: One patient experienced a grade 4 pulmonary embolism (1.8 mg/kg plus R-CHP) and another had grade 4 febrile neutropenia and grade 3 thrombocytopenia (1.4 mg/kg plus G-CHP).
At a median follow-up of 21.5 months, the overall response rate was 89% in study participants, including 77% of patients who achieved a complete response and 12% who had a partial response.
The recommended phase 2 dose of polatuzumab vedotin was 1.8 mg/kg. At this dose, the most common grade 3 or higher toxicities were neutropenia (30%), febrile neutropenia (18%), and thrombocytopenia (9%).
The researchers acknowledged a key limitation of the study was the nonrandomized design. As a result, dose and regimen comparisons could not be made.
The study was funded by F. Hoffmann-La Roche and Genentech. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Celgene, Gilead, Janssen, Merck, Pfizer, and others.
SOURCE: Tilly H et al. Lancet Oncol. 2019 May 14. doi: 10.1016/S1470-2045(19)30091-9.
FROM LANCET ONCOLOGY
Influenza vaccination status in DLBCL poorly documented
Annual influenza vaccination rates and documentation of vaccination status appear to be suboptimal among patients with newly diagnosed diffuse large B-cell lymphoma, based on a retrospective analysis of data in the state of Georgia.
The researchers reviewed medical records of patients with a new diagnosis of diffuse large B-cell lymphoma at three Georgia hospitals. Documentation related to vaccine administration, refusal, and patient counseling was collected between Feb. 1, 2015, and Oct. 31, 2017, Andres Chang, MD, PhD, of Emory University, Atlanta, and colleagues wrote. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
Vaccination status within 1 year of diagnosis was documented for 61 of 107 patients. Among the 61 patients with documentation of influenza vaccination status, 35 (57%) were vaccinated. No reason was documented for vaccine refusal, and there was no follow-up documentation on vaccine counseling by nursing staff, physicians, or advanced practice providers in any of these admitted patients.
Nearly all documentation of influenza vaccination status came from the nursing staff. Only 4 of the 61 patients had documentation provided by primary oncologists or advanced practice providers, and those patients also had documentation from the outpatient nursing staff who subsequently administered the influenza vaccine.
“Routine outpatient vaccination screening and strategies for sharing and linking patient vaccination status between providers in different health care systems at the state and national levels could improve vaccination documentation in patients with lymphoma and provide opportunities to improve compliance,” the researchers wrote.
A key limitation of the study was the lack of adequate documentation, they explained. As a result, the reported vaccination rates were not more rigorously evaluated.
The study was funded by the Winship Cancer Institute and the National Institutes of Health. The authors reported financial affiliations with AbbVie, Acerta Pharma, Celgene, Gilead, Janssen, Pharmacyclics, and others.
SOURCE: Chang A et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.018.
Annual influenza vaccination rates and documentation of vaccination status appear to be suboptimal among patients with newly diagnosed diffuse large B-cell lymphoma, based on a retrospective analysis of data in the state of Georgia.
The researchers reviewed medical records of patients with a new diagnosis of diffuse large B-cell lymphoma at three Georgia hospitals. Documentation related to vaccine administration, refusal, and patient counseling was collected between Feb. 1, 2015, and Oct. 31, 2017, Andres Chang, MD, PhD, of Emory University, Atlanta, and colleagues wrote. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
Vaccination status within 1 year of diagnosis was documented for 61 of 107 patients. Among the 61 patients with documentation of influenza vaccination status, 35 (57%) were vaccinated. No reason was documented for vaccine refusal, and there was no follow-up documentation on vaccine counseling by nursing staff, physicians, or advanced practice providers in any of these admitted patients.
Nearly all documentation of influenza vaccination status came from the nursing staff. Only 4 of the 61 patients had documentation provided by primary oncologists or advanced practice providers, and those patients also had documentation from the outpatient nursing staff who subsequently administered the influenza vaccine.
“Routine outpatient vaccination screening and strategies for sharing and linking patient vaccination status between providers in different health care systems at the state and national levels could improve vaccination documentation in patients with lymphoma and provide opportunities to improve compliance,” the researchers wrote.
A key limitation of the study was the lack of adequate documentation, they explained. As a result, the reported vaccination rates were not more rigorously evaluated.
The study was funded by the Winship Cancer Institute and the National Institutes of Health. The authors reported financial affiliations with AbbVie, Acerta Pharma, Celgene, Gilead, Janssen, Pharmacyclics, and others.
SOURCE: Chang A et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.018.
Annual influenza vaccination rates and documentation of vaccination status appear to be suboptimal among patients with newly diagnosed diffuse large B-cell lymphoma, based on a retrospective analysis of data in the state of Georgia.
The researchers reviewed medical records of patients with a new diagnosis of diffuse large B-cell lymphoma at three Georgia hospitals. Documentation related to vaccine administration, refusal, and patient counseling was collected between Feb. 1, 2015, and Oct. 31, 2017, Andres Chang, MD, PhD, of Emory University, Atlanta, and colleagues wrote. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
Vaccination status within 1 year of diagnosis was documented for 61 of 107 patients. Among the 61 patients with documentation of influenza vaccination status, 35 (57%) were vaccinated. No reason was documented for vaccine refusal, and there was no follow-up documentation on vaccine counseling by nursing staff, physicians, or advanced practice providers in any of these admitted patients.
Nearly all documentation of influenza vaccination status came from the nursing staff. Only 4 of the 61 patients had documentation provided by primary oncologists or advanced practice providers, and those patients also had documentation from the outpatient nursing staff who subsequently administered the influenza vaccine.
“Routine outpatient vaccination screening and strategies for sharing and linking patient vaccination status between providers in different health care systems at the state and national levels could improve vaccination documentation in patients with lymphoma and provide opportunities to improve compliance,” the researchers wrote.
A key limitation of the study was the lack of adequate documentation, they explained. As a result, the reported vaccination rates were not more rigorously evaluated.
The study was funded by the Winship Cancer Institute and the National Institutes of Health. The authors reported financial affiliations with AbbVie, Acerta Pharma, Celgene, Gilead, Janssen, Pharmacyclics, and others.
SOURCE: Chang A et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.018.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
rFVIII product shows better PK profile than rFVIIIFc
The recombinant factor VIII (rFVIII) product BAY 94‐9027 had a better pharmacokinetic (PK) profile than a recombinant factor VIII-Fc fusion protein (rFVIIIFc) in patients with hemophilia A, according to a recent report.
“The objective of the current study was to directly compare the PK profiles of BAY 94-9027 and rFVIIIFc,” wrote Anita Shah, lead author and an employee of Bayer, and colleagues. The findings were published in the Annals of Hematology.
In a two-way PK crossover study, adults (aged 18-65 years) with severe hemophilia A were randomized to receive a single intravenous dose (60 IU/kg) of BAY 94-9027 or rFVIIIFc. These infusions were followed by a crossover to a single infusion of the other product.
The maximum wash-out period between infusions was 28 days, with a greater than or equal to 7-day wash-out between doses. FVIII activity was analyzed using a single one-stage clotting assay.
After population PK modeling, the median time to achieve FVIII threshold levels (1 IU/dL) was found to be 13 hours longer for BAY 94-9027 than it was for rFVIIIFc following a single intravenous dose of 60 IU/kg.
In addition, the team reported that the geometric mean area under the curve from baseline to the last data point was significantly greater for BAY 94-9027 than it was for rFVIIIFc (coefficient of variation, 2,940 vs. 2,360 IU h/dL; P = .0001)
“This increase in the time above threshold may thereby provide improved bleeding protection,” the authors explained.
With respect to safety, no adverse events were reported among study participants.
The researchers acknowledged a key limitation of the study was the nonexistence of a two-stage chromogenic assay to measure FVIII activity of both products. As a result, a one-stage clotting assay was used to analyze FVIII activity for both treatments.
“Real-world data may provide an insight into whether these PK advantages provide additional bleeding protection,” they concluded.
The study was funded by Bayer AG. The authors reported financial affiliations with Bayer, LFB, Octapharma, Pfizer, Roche, Shire, and several others.
SOURCE: Shah A et al. Ann Hematol. 2019 Jun 24. doi: 10.1007/s00277-019-03747-2.
The recombinant factor VIII (rFVIII) product BAY 94‐9027 had a better pharmacokinetic (PK) profile than a recombinant factor VIII-Fc fusion protein (rFVIIIFc) in patients with hemophilia A, according to a recent report.
“The objective of the current study was to directly compare the PK profiles of BAY 94-9027 and rFVIIIFc,” wrote Anita Shah, lead author and an employee of Bayer, and colleagues. The findings were published in the Annals of Hematology.
In a two-way PK crossover study, adults (aged 18-65 years) with severe hemophilia A were randomized to receive a single intravenous dose (60 IU/kg) of BAY 94-9027 or rFVIIIFc. These infusions were followed by a crossover to a single infusion of the other product.
The maximum wash-out period between infusions was 28 days, with a greater than or equal to 7-day wash-out between doses. FVIII activity was analyzed using a single one-stage clotting assay.
After population PK modeling, the median time to achieve FVIII threshold levels (1 IU/dL) was found to be 13 hours longer for BAY 94-9027 than it was for rFVIIIFc following a single intravenous dose of 60 IU/kg.
In addition, the team reported that the geometric mean area under the curve from baseline to the last data point was significantly greater for BAY 94-9027 than it was for rFVIIIFc (coefficient of variation, 2,940 vs. 2,360 IU h/dL; P = .0001)
“This increase in the time above threshold may thereby provide improved bleeding protection,” the authors explained.
With respect to safety, no adverse events were reported among study participants.
The researchers acknowledged a key limitation of the study was the nonexistence of a two-stage chromogenic assay to measure FVIII activity of both products. As a result, a one-stage clotting assay was used to analyze FVIII activity for both treatments.
“Real-world data may provide an insight into whether these PK advantages provide additional bleeding protection,” they concluded.
The study was funded by Bayer AG. The authors reported financial affiliations with Bayer, LFB, Octapharma, Pfizer, Roche, Shire, and several others.
SOURCE: Shah A et al. Ann Hematol. 2019 Jun 24. doi: 10.1007/s00277-019-03747-2.
The recombinant factor VIII (rFVIII) product BAY 94‐9027 had a better pharmacokinetic (PK) profile than a recombinant factor VIII-Fc fusion protein (rFVIIIFc) in patients with hemophilia A, according to a recent report.
“The objective of the current study was to directly compare the PK profiles of BAY 94-9027 and rFVIIIFc,” wrote Anita Shah, lead author and an employee of Bayer, and colleagues. The findings were published in the Annals of Hematology.
In a two-way PK crossover study, adults (aged 18-65 years) with severe hemophilia A were randomized to receive a single intravenous dose (60 IU/kg) of BAY 94-9027 or rFVIIIFc. These infusions were followed by a crossover to a single infusion of the other product.
The maximum wash-out period between infusions was 28 days, with a greater than or equal to 7-day wash-out between doses. FVIII activity was analyzed using a single one-stage clotting assay.
After population PK modeling, the median time to achieve FVIII threshold levels (1 IU/dL) was found to be 13 hours longer for BAY 94-9027 than it was for rFVIIIFc following a single intravenous dose of 60 IU/kg.
In addition, the team reported that the geometric mean area under the curve from baseline to the last data point was significantly greater for BAY 94-9027 than it was for rFVIIIFc (coefficient of variation, 2,940 vs. 2,360 IU h/dL; P = .0001)
“This increase in the time above threshold may thereby provide improved bleeding protection,” the authors explained.
With respect to safety, no adverse events were reported among study participants.
The researchers acknowledged a key limitation of the study was the nonexistence of a two-stage chromogenic assay to measure FVIII activity of both products. As a result, a one-stage clotting assay was used to analyze FVIII activity for both treatments.
“Real-world data may provide an insight into whether these PK advantages provide additional bleeding protection,” they concluded.
The study was funded by Bayer AG. The authors reported financial affiliations with Bayer, LFB, Octapharma, Pfizer, Roche, Shire, and several others.
SOURCE: Shah A et al. Ann Hematol. 2019 Jun 24. doi: 10.1007/s00277-019-03747-2.
FROM ANNALS OF HEMATOLOGY
Day 75 is key threshold in FVIII inhibitor development
For previously untreated patients with severe hemophilia A, the risk of developing factor VIII (FVIII) inhibitors becomes marginal after 75 exposure days, according to an observational study of more than 1,000 infants on prophylaxis.
“Most inhibitors develop during the first 50 exposure days to FVIII, with 50% of inhibitors already present after 14-15 exposure days,” wrote H. Marijke van den Berg, MD, PhD, of the PedNet Haemophilia Research Foundation, Baarn, the Netherlands. The findings were published in Blood.
Dr. van den Berg and her colleagues aimed to characterize the risk of inhibitor development beyond 50 exposure days and to calculate the age when patients reach near-zero risk. The researchers followed 1,038 previously untreated patients with severe hemophilia A from first exposure to FVIII until inhibitor development, up to a maximum of 1,000 exposure days. Data was obtained from the PedNet Haemophilia Registry.
From the initial cohort, 943 patients (91%) were followed until 50 exposure days, and 899 (87%) were followed until 75 exposure days. Inhibitor development was defined by a minimum of two positive inhibitor titers in conjunction with reduced in-vivo FVIII recovery. The team also conducted a survival analysis to measure inhibitor incidence and reported median ages at initial exposure and at exposure day 75.
After analysis, the researchers found that 298 of 300 (99.3%) occurrences of inhibitor development ensued within 75 exposure days. No inhibitor development occurred between exposure day 75 and 150. The final two occurrences developed at exposure day 249 and 262, each with a low titer. The median age at first exposure was 1.1 years versus 2.3 years at exposure day 75.
“Our study shows that children on prophylaxis reach a near-zero risk plateau of inhibitor development at 75 [exposure days] only 1.2 years after the first [exposure day],” they wrote.
The researchers explained that these findings could impact the design of future clinical studies for previously untreated patients with severe hemophilia A. And they noted that these data are applicable to patients administered early prophylaxis, since the majority of study participants began prophylaxis early in life. “Frequent testing for inhibitors until 75 instead of 50 exposure days, therefore, is feasible and should be recommended for all [previously untreated patients],” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: van den Berg HM et al. Blood. 2019 Jun 11. doi: 10.1182/blood.2019000658.
For previously untreated patients with severe hemophilia A, the risk of developing factor VIII (FVIII) inhibitors becomes marginal after 75 exposure days, according to an observational study of more than 1,000 infants on prophylaxis.
“Most inhibitors develop during the first 50 exposure days to FVIII, with 50% of inhibitors already present after 14-15 exposure days,” wrote H. Marijke van den Berg, MD, PhD, of the PedNet Haemophilia Research Foundation, Baarn, the Netherlands. The findings were published in Blood.
Dr. van den Berg and her colleagues aimed to characterize the risk of inhibitor development beyond 50 exposure days and to calculate the age when patients reach near-zero risk. The researchers followed 1,038 previously untreated patients with severe hemophilia A from first exposure to FVIII until inhibitor development, up to a maximum of 1,000 exposure days. Data was obtained from the PedNet Haemophilia Registry.
From the initial cohort, 943 patients (91%) were followed until 50 exposure days, and 899 (87%) were followed until 75 exposure days. Inhibitor development was defined by a minimum of two positive inhibitor titers in conjunction with reduced in-vivo FVIII recovery. The team also conducted a survival analysis to measure inhibitor incidence and reported median ages at initial exposure and at exposure day 75.
After analysis, the researchers found that 298 of 300 (99.3%) occurrences of inhibitor development ensued within 75 exposure days. No inhibitor development occurred between exposure day 75 and 150. The final two occurrences developed at exposure day 249 and 262, each with a low titer. The median age at first exposure was 1.1 years versus 2.3 years at exposure day 75.
“Our study shows that children on prophylaxis reach a near-zero risk plateau of inhibitor development at 75 [exposure days] only 1.2 years after the first [exposure day],” they wrote.
The researchers explained that these findings could impact the design of future clinical studies for previously untreated patients with severe hemophilia A. And they noted that these data are applicable to patients administered early prophylaxis, since the majority of study participants began prophylaxis early in life. “Frequent testing for inhibitors until 75 instead of 50 exposure days, therefore, is feasible and should be recommended for all [previously untreated patients],” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: van den Berg HM et al. Blood. 2019 Jun 11. doi: 10.1182/blood.2019000658.
For previously untreated patients with severe hemophilia A, the risk of developing factor VIII (FVIII) inhibitors becomes marginal after 75 exposure days, according to an observational study of more than 1,000 infants on prophylaxis.
“Most inhibitors develop during the first 50 exposure days to FVIII, with 50% of inhibitors already present after 14-15 exposure days,” wrote H. Marijke van den Berg, MD, PhD, of the PedNet Haemophilia Research Foundation, Baarn, the Netherlands. The findings were published in Blood.
Dr. van den Berg and her colleagues aimed to characterize the risk of inhibitor development beyond 50 exposure days and to calculate the age when patients reach near-zero risk. The researchers followed 1,038 previously untreated patients with severe hemophilia A from first exposure to FVIII until inhibitor development, up to a maximum of 1,000 exposure days. Data was obtained from the PedNet Haemophilia Registry.
From the initial cohort, 943 patients (91%) were followed until 50 exposure days, and 899 (87%) were followed until 75 exposure days. Inhibitor development was defined by a minimum of two positive inhibitor titers in conjunction with reduced in-vivo FVIII recovery. The team also conducted a survival analysis to measure inhibitor incidence and reported median ages at initial exposure and at exposure day 75.
After analysis, the researchers found that 298 of 300 (99.3%) occurrences of inhibitor development ensued within 75 exposure days. No inhibitor development occurred between exposure day 75 and 150. The final two occurrences developed at exposure day 249 and 262, each with a low titer. The median age at first exposure was 1.1 years versus 2.3 years at exposure day 75.
“Our study shows that children on prophylaxis reach a near-zero risk plateau of inhibitor development at 75 [exposure days] only 1.2 years after the first [exposure day],” they wrote.
The researchers explained that these findings could impact the design of future clinical studies for previously untreated patients with severe hemophilia A. And they noted that these data are applicable to patients administered early prophylaxis, since the majority of study participants began prophylaxis early in life. “Frequent testing for inhibitors until 75 instead of 50 exposure days, therefore, is feasible and should be recommended for all [previously untreated patients],” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: van den Berg HM et al. Blood. 2019 Jun 11. doi: 10.1182/blood.2019000658.
FROM BLOOD
Anticholinergic drugs linked to dementia in older populations
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
FROM JAMA INTERNAL MEDICINE
Real-world data confirm nonacog alfa efficacy in hemophilia B
The recombinant factor IX product nonacog alfa appears safe and effective for patients with hemophilia B, according to results from a recent postmarketing study in Japan.
While nonacog alfa was approved in the United States and Europe in 1997, the product wasn’t approved in Japan until 2009. Since it was the first recombinant factor IX product available there, and only a small number of patients were enrolled in domestic clinical trials, the Japanese government required additional real-world studies.
“In the last couple of years, several extended half‐life blood coagulation factor products gained regulatory approval for the treatment of hemophilia B. However, access to this most advanced treatment option remains limited to developed countries, and the need for standard half‐life recombinant or plasma‐derived FIX products is still high,” wrote Katsuyuki Fukutake, MD, PhD, of Tokyo Medical University, Japan, and colleagues.
The researchers conducted an observational postmarketing surveillance study of 312 patients with hemophilia B who received nonacog alfa therapy from 2010 to 2014. The team evaluated the real-world safety, including the incidence of inhibitors and adverse events, and effectiveness of the recombinant product in Japan.
The findings were published in Haemophilia.
The study included both previously treated and untreated participants who were followed for 1 and 2 years, respectively, after starting recombinant factor IX therapy.
The primary safety outcome was the incidence and number of adverse drug reactions. Effectiveness was measured using clinical effectiveness rates and annualized bleeding rates (ABR).
After analysis, the researchers found that the effectiveness rates were 95.5% and 93.7% for patients who received routine prophylaxis and on-demand treatment, respectively.
The median ABR was lower during routine prophylaxis – 2.0 – versus the rest of the observation period – 8.3. “This difference was prominent among patients with severe haemophilia B or haemophilic arthropathy,” the researchers wrote.
With respect to safety, 11 adverse drug reactions were seen in seven previously treated patients. New inhibitor development was not observed in any participants, but recurrence was seen in one patient.
“Our results are consistent with those of previous studies where the incidence of inhibitor antibody development in hemophilia B has been reported as 1%-5%,” Dr. Fukutake and colleagues wrote.
The researchers acknowledged that one key limitation of the study was the observational design.
“The results suggest that nonacog alfa was well tolerated and appropriately used under routine clinical practice,” the authors concluded.
The study was funded and conducted by Pfizer Japan. The authors reported financial relationships with Pfizer and several other companies. One coauthor is an employee of Pfizer Japan.
SOURCE: Fukutake K et al. Haemophilia. 2019 Jun 6. doi: 10.1111/hae.13783.
The recombinant factor IX product nonacog alfa appears safe and effective for patients with hemophilia B, according to results from a recent postmarketing study in Japan.
While nonacog alfa was approved in the United States and Europe in 1997, the product wasn’t approved in Japan until 2009. Since it was the first recombinant factor IX product available there, and only a small number of patients were enrolled in domestic clinical trials, the Japanese government required additional real-world studies.
“In the last couple of years, several extended half‐life blood coagulation factor products gained regulatory approval for the treatment of hemophilia B. However, access to this most advanced treatment option remains limited to developed countries, and the need for standard half‐life recombinant or plasma‐derived FIX products is still high,” wrote Katsuyuki Fukutake, MD, PhD, of Tokyo Medical University, Japan, and colleagues.
The researchers conducted an observational postmarketing surveillance study of 312 patients with hemophilia B who received nonacog alfa therapy from 2010 to 2014. The team evaluated the real-world safety, including the incidence of inhibitors and adverse events, and effectiveness of the recombinant product in Japan.
The findings were published in Haemophilia.
The study included both previously treated and untreated participants who were followed for 1 and 2 years, respectively, after starting recombinant factor IX therapy.
The primary safety outcome was the incidence and number of adverse drug reactions. Effectiveness was measured using clinical effectiveness rates and annualized bleeding rates (ABR).
After analysis, the researchers found that the effectiveness rates were 95.5% and 93.7% for patients who received routine prophylaxis and on-demand treatment, respectively.
The median ABR was lower during routine prophylaxis – 2.0 – versus the rest of the observation period – 8.3. “This difference was prominent among patients with severe haemophilia B or haemophilic arthropathy,” the researchers wrote.
With respect to safety, 11 adverse drug reactions were seen in seven previously treated patients. New inhibitor development was not observed in any participants, but recurrence was seen in one patient.
“Our results are consistent with those of previous studies where the incidence of inhibitor antibody development in hemophilia B has been reported as 1%-5%,” Dr. Fukutake and colleagues wrote.
The researchers acknowledged that one key limitation of the study was the observational design.
“The results suggest that nonacog alfa was well tolerated and appropriately used under routine clinical practice,” the authors concluded.
The study was funded and conducted by Pfizer Japan. The authors reported financial relationships with Pfizer and several other companies. One coauthor is an employee of Pfizer Japan.
SOURCE: Fukutake K et al. Haemophilia. 2019 Jun 6. doi: 10.1111/hae.13783.
The recombinant factor IX product nonacog alfa appears safe and effective for patients with hemophilia B, according to results from a recent postmarketing study in Japan.
While nonacog alfa was approved in the United States and Europe in 1997, the product wasn’t approved in Japan until 2009. Since it was the first recombinant factor IX product available there, and only a small number of patients were enrolled in domestic clinical trials, the Japanese government required additional real-world studies.
“In the last couple of years, several extended half‐life blood coagulation factor products gained regulatory approval for the treatment of hemophilia B. However, access to this most advanced treatment option remains limited to developed countries, and the need for standard half‐life recombinant or plasma‐derived FIX products is still high,” wrote Katsuyuki Fukutake, MD, PhD, of Tokyo Medical University, Japan, and colleagues.
The researchers conducted an observational postmarketing surveillance study of 312 patients with hemophilia B who received nonacog alfa therapy from 2010 to 2014. The team evaluated the real-world safety, including the incidence of inhibitors and adverse events, and effectiveness of the recombinant product in Japan.
The findings were published in Haemophilia.
The study included both previously treated and untreated participants who were followed for 1 and 2 years, respectively, after starting recombinant factor IX therapy.
The primary safety outcome was the incidence and number of adverse drug reactions. Effectiveness was measured using clinical effectiveness rates and annualized bleeding rates (ABR).
After analysis, the researchers found that the effectiveness rates were 95.5% and 93.7% for patients who received routine prophylaxis and on-demand treatment, respectively.
The median ABR was lower during routine prophylaxis – 2.0 – versus the rest of the observation period – 8.3. “This difference was prominent among patients with severe haemophilia B or haemophilic arthropathy,” the researchers wrote.
With respect to safety, 11 adverse drug reactions were seen in seven previously treated patients. New inhibitor development was not observed in any participants, but recurrence was seen in one patient.
“Our results are consistent with those of previous studies where the incidence of inhibitor antibody development in hemophilia B has been reported as 1%-5%,” Dr. Fukutake and colleagues wrote.
The researchers acknowledged that one key limitation of the study was the observational design.
“The results suggest that nonacog alfa was well tolerated and appropriately used under routine clinical practice,” the authors concluded.
The study was funded and conducted by Pfizer Japan. The authors reported financial relationships with Pfizer and several other companies. One coauthor is an employee of Pfizer Japan.
SOURCE: Fukutake K et al. Haemophilia. 2019 Jun 6. doi: 10.1111/hae.13783.
FROM HAEMOPHILIA
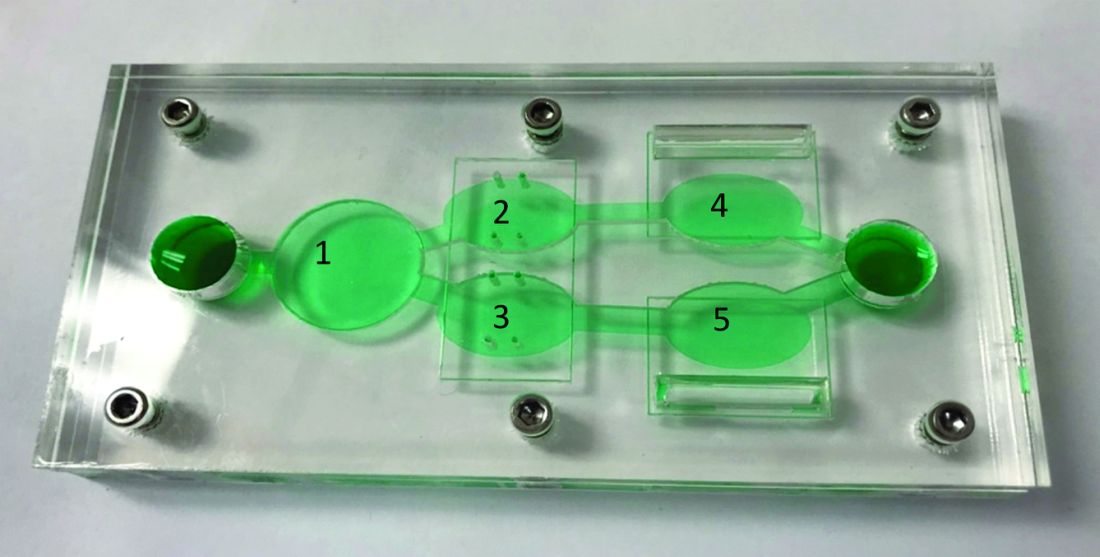
Novel chip system could improve preclinical drug studies
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of anticancer therapies.
Major finding: Overall, results support the utility of the system to assess both off-target toxicity and on-target efficacy for various anticancer drugs.
Study details: A study exploring the utility of a multi-organ-on-a-chip system to assess safety and effectiveness of anticancer therapies in the preclinical setting.
Disclosures: The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
Source: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
Chemoradiotherapy no better than chemo alone in endometrial carcinoma
Chemotherapy plus radiation therapy (chemoradiotherapy) was not associated with improved relapse-free survival versus chemotherapy alone in patients with stage III or IVA endometrial cancer, according to results from a phase 3 trial.
“This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known,” wrote Daniela Matei, MD, of Northwestern University, Chicago, and colleagues. The results were published in the New England Journal of Medicine.
The Gynecologic Oncology Group (GOG) 258 study included 736 patients with stage III or IVA endometrial carcinoma who were randomized in a 1:1 fashion to receive platinum-based chemotherapy plus volume–directed external-beam radiation therapy every 21 days for a total of four cycles or chemotherapy alone every 21 days for a total of six cycles.
The primary endpoint measured was relapse-free survival; secondary endpoints included safety, overall survival (OS), and quality of life.
At 60 months, the proportion of patients alive and relapse free was 59% (95% confidence interval, 53-65) and 58% (95% CI, 53-64) in the chemoradiotherapy and chemotherapy alone arms, respectively (hazard ratio, 0.90; 90% CI, 0.74-1.10).
“The data on overall survival are not sufficiently mature to allow comparison between the groups,” the researchers wrote.
With respect to safety, grade 3, 4, or 5 toxicities were reported in 58% and 63% of patients in the chemoradiotherapy and chemotherapy alone arms, respectively.
“Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation,” Dr. Matei and colleagues explained.
A major strength of the study was the broad inclusion criteria, which included patients with nonperitoneal, lymph-node, pelvic, and adnexal metastasis, the researchers noted.
“Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse,” they concluded.
The National Cancer Institute supported the study. The authors reported financial affiliations with AstraZeneca, Clovis, Genentech, the GOG Foundation, Tesaro, and several others.
SOURCE: Matei D et al. N Engl J Med. 2019 Jun 13. doi: 10.1056/NEJMoa1813181.
Chemotherapy plus radiation therapy (chemoradiotherapy) was not associated with improved relapse-free survival versus chemotherapy alone in patients with stage III or IVA endometrial cancer, according to results from a phase 3 trial.
“This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known,” wrote Daniela Matei, MD, of Northwestern University, Chicago, and colleagues. The results were published in the New England Journal of Medicine.
The Gynecologic Oncology Group (GOG) 258 study included 736 patients with stage III or IVA endometrial carcinoma who were randomized in a 1:1 fashion to receive platinum-based chemotherapy plus volume–directed external-beam radiation therapy every 21 days for a total of four cycles or chemotherapy alone every 21 days for a total of six cycles.
The primary endpoint measured was relapse-free survival; secondary endpoints included safety, overall survival (OS), and quality of life.
At 60 months, the proportion of patients alive and relapse free was 59% (95% confidence interval, 53-65) and 58% (95% CI, 53-64) in the chemoradiotherapy and chemotherapy alone arms, respectively (hazard ratio, 0.90; 90% CI, 0.74-1.10).
“The data on overall survival are not sufficiently mature to allow comparison between the groups,” the researchers wrote.
With respect to safety, grade 3, 4, or 5 toxicities were reported in 58% and 63% of patients in the chemoradiotherapy and chemotherapy alone arms, respectively.
“Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation,” Dr. Matei and colleagues explained.
A major strength of the study was the broad inclusion criteria, which included patients with nonperitoneal, lymph-node, pelvic, and adnexal metastasis, the researchers noted.
“Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse,” they concluded.
The National Cancer Institute supported the study. The authors reported financial affiliations with AstraZeneca, Clovis, Genentech, the GOG Foundation, Tesaro, and several others.
SOURCE: Matei D et al. N Engl J Med. 2019 Jun 13. doi: 10.1056/NEJMoa1813181.
Chemotherapy plus radiation therapy (chemoradiotherapy) was not associated with improved relapse-free survival versus chemotherapy alone in patients with stage III or IVA endometrial cancer, according to results from a phase 3 trial.
“This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known,” wrote Daniela Matei, MD, of Northwestern University, Chicago, and colleagues. The results were published in the New England Journal of Medicine.
The Gynecologic Oncology Group (GOG) 258 study included 736 patients with stage III or IVA endometrial carcinoma who were randomized in a 1:1 fashion to receive platinum-based chemotherapy plus volume–directed external-beam radiation therapy every 21 days for a total of four cycles or chemotherapy alone every 21 days for a total of six cycles.
The primary endpoint measured was relapse-free survival; secondary endpoints included safety, overall survival (OS), and quality of life.
At 60 months, the proportion of patients alive and relapse free was 59% (95% confidence interval, 53-65) and 58% (95% CI, 53-64) in the chemoradiotherapy and chemotherapy alone arms, respectively (hazard ratio, 0.90; 90% CI, 0.74-1.10).
“The data on overall survival are not sufficiently mature to allow comparison between the groups,” the researchers wrote.
With respect to safety, grade 3, 4, or 5 toxicities were reported in 58% and 63% of patients in the chemoradiotherapy and chemotherapy alone arms, respectively.
“Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation,” Dr. Matei and colleagues explained.
A major strength of the study was the broad inclusion criteria, which included patients with nonperitoneal, lymph-node, pelvic, and adnexal metastasis, the researchers noted.
“Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse,” they concluded.
The National Cancer Institute supported the study. The authors reported financial affiliations with AstraZeneca, Clovis, Genentech, the GOG Foundation, Tesaro, and several others.
SOURCE: Matei D et al. N Engl J Med. 2019 Jun 13. doi: 10.1056/NEJMoa1813181.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE