User login
CDC panel updates info on rare side effect after J&J vaccine
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Disturbing number of hospital workers still unvaccinated
Tim Oswalt had been in a Fort Worth, Texas, hospital for over a month, receiving treatment for a grapefruit-sized tumor in his chest that was pressing on his heart and lungs. It turned out to be stage 3 non-Hodgkin lymphoma.
Then one day in January, he was moved from his semi-private room to an isolated one with special ventilation. The staff explained he had been infected by the virus that was once again surging in many areas of the country, including Texas.
“How the hell did I catch COVID?” he asked the staff, who now approached him in full moon-suit personal protective equipment (PPE).
The hospital was locked down, and Mr. Oswalt hadn’t had any visitors in weeks. Neither of his two roommates tested positive. He’d been tested for COVID several times over the course of his nearly 5-week stay and was always negative.
“‘Well, you know, it’s easy to [catch it] in a hospital,’” Mr. Oswalt said he was told by hospital staff. “‘We’re having a bad outbreak. So you were just exposed somehow.’”
Officials at John Peter Smith Hospital, where Mr. Oswalt was treated, said they are puzzled by his case. According to their infection prevention team, none of his caregivers tested positive for COVID-19, nor did Mr. Oswalt share space with any other COVID-positive patients. And yet, local media reported a surge in cases among JPS hospital staff in December.
“Infection of any kind is a constant battle within hospitals and one that we all take seriously,” said Rob Stephenson, MD, chief quality officer at JPS Health Network. “Anyone in a vulnerable health condition at the height of the pandemic would have been at greater risk for contracting COVID-19 inside – or even more so, outside – the hospital.”
Mr. Oswalt was diagnosed with COVID in early January. JPS Hospital began vaccinating its health care workers about 2 weeks earlier, so there had not yet been enough time for any of them to develop full protection against catching or spreading the virus.
Today, the hospital said 74% of its staff – 5,300 of 7,200 workers – are now vaccinated.
against the SARS-CoV2 virus.
Refusing vaccinations
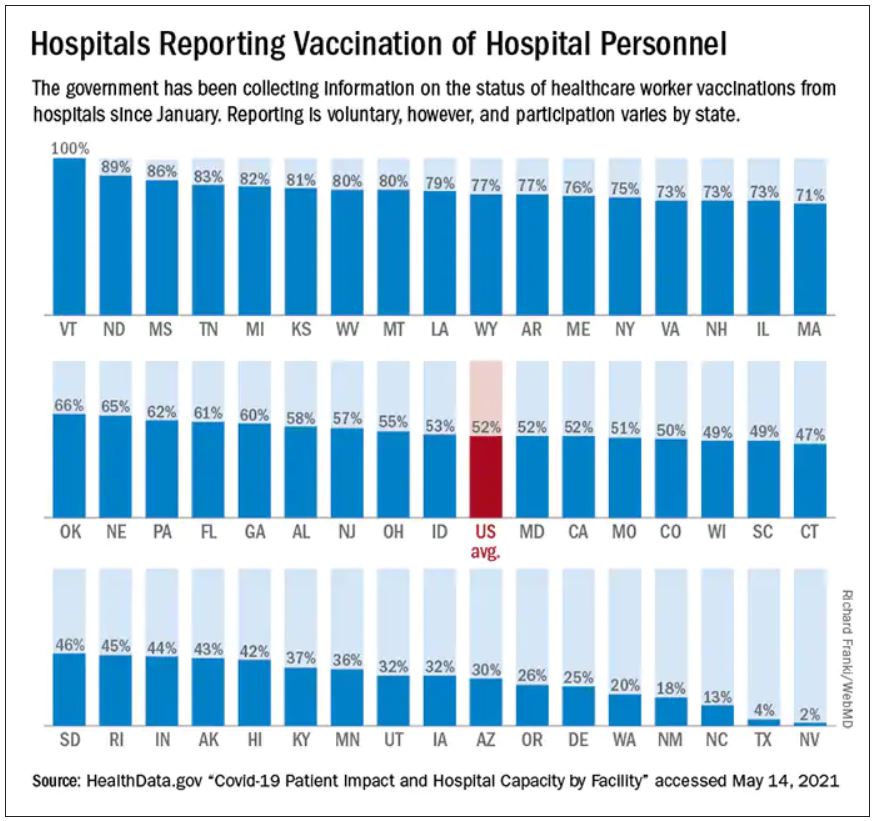
In fact, nationwide, 1 in 4 hospital workers who have direct contact with patients had not yet received a single dose of a COVID vaccine by the end of May, according to a WebMD and Medscape Medical News analysis of data collected by the U.S. Department of Health and Human Services (HHS) from 2,500 hospitals across the United States.
Among the nation’s 50 largest hospitals, the percentage of unvaccinated health care workers appears to be even larger, about 1 in 3. Vaccination rates range from a high of 99% at Houston Methodist Hospital, which was the first in the nation to mandate the shots for its workers, to a low between 30% and 40% at some hospitals in Florida.
Memorial Hermann Texas Medical Center in Houston has 1,180 beds and sits less than half a mile from Houston Methodist Hospital. But in terms of worker vaccinations, it is farther away.
Memorial Hermann reported to HHS that about 32% of its 28,000 workers haven’t been inoculated. The hospital’s PR office contests that figure, putting it closer to 25% unvaccinated across their health system. The hospital said it is boosting participation by offering a $300 “shot of hope” bonus to workers who start their vaccination series by the end of June.
Lakeland Regional Medical Center in Lakeland, Fla., reported to HHS that 63% of its health care personnel are still unvaccinated. The hospital did not return a call to verify that number.
To boost vaccination rates, more hospitals are starting to require the shots, after the Equal Employment Opportunity Commission gave its green light to mandates in May.
“It’s a real problem that you have such high levels of unvaccinated individuals in hospitals,” said Lawrence Gostin, JD, director of the O’Neill Institute for National and Global Health Law at Georgetown University, Washington.
“We have to protect our health workforce, and we have to protect our patients. Hospitals should be the safest places in the country, and the only way to make them safe is to have a fully vaccinated workforce,” Mr. Gostin said.
Is the data misleading?
The HHS system designed to amass hospital data was set up quickly, to respond to an emergency. For that reason, experts say the information hasn’t been as carefully collected or vetted as it normally would have been. Some hospitals may have misunderstood how to report their vaccination numbers.
In addition, reporting data on worker vaccinations is voluntary. Only about half of hospitals have chosen to share their numbers. In other cases, like Texas, states have blocked the public release of these statistics.
AdventHealth Orlando, a 1,300-bed hospital in Florida, reported to HHS that 56% of its staff have not started their shots. But spokesman Jeff Grainger said the figures probably overstate the number of unvaccinated workers because the hospital doesn’t always know when people get vaccinated outside of its campus, at a local pharmacy, for example.
For those reasons, the picture of health care worker vaccinations across the country is incomplete.
Where hospitals fall behind
Even if the data are flawed, the vaccination rates from hospitals mirror the general population. A May Gallup poll, for example, found 24% of Americans said they definitely won’t get the vaccine. Another 12% say they plan to get it but are waiting.
The data also align with recent studies. A review of 35 studies by researchers at New Mexico State University that assessed hesitancy in more than 76,000 health care workers around the world found about 23% of them were reluctant to get the shots.
An ongoing monthly survey of more than 1.9 million U.S. Facebook users led by researchers at Carnegie Mellon University, Pittsburgh recently looked at vaccine hesitancy by occupation. It revealed a spectrum of hesitancy among health care workers corresponding to income and education, ranging from a low of 9% among pharmacists to highs of 20%-23% among nursing aides and emergency medical technicians. About 12% of registered nurses and doctors admitted to being hesitant to get a shot.
“Health care workers are not monolithic,” said study author Jagdish Khubchandani, professor of public health sciences at New Mexico State.
“There’s a big divide between males, doctoral degree holders, older people and the younger low-income, low-education frontline, female, health care workers. They are the most hesitant,” he said. Support staff typically outnumbers doctors at hospitals about 3 to 1.
“There is outreach work to be done there,” said Robin Mejia, PhD, director of the Statistics and Human Rights Program at Carnegie Mellon, who is leading the study on Facebook’s survey data. “These are also high-contact professions. These are people who are seeing patients on a regular basis.”
That’s why, when the Centers for Disease Control and Prevention was planning the national vaccine rollout, they prioritized health care workers for the initially scarce first doses. The intent was to protect vulnerable workers and their patients who are at high risk of infection. But the CDC had another reason for putting health care workers first: After they were safely vaccinated, the hope was that they would encourage wary patients to do the same.
Hospitals were supposed to be hubs of education to help build trust within less confident communities. But not all hospitals have risen to that challenge.
Political affiliation seems to be one contributing factor in vaccine hesitancy. Take for example Calhoun, Ga., the seat of Gordon County, where residents voted for Donald Trump over Joe Biden by a 67-point margin in the 2020 general election. Studies have found that Republicans are more likely to decline vaccines than Democrats.
People who live in rural areas are less likely to be vaccinated than those who live in cities, and that’s true in Gordon County too. Vaccinations are lagging in this northwest corner of Georgia where factory jobs in chicken processing plants and carpet manufacturing energize the local economy. Just 24% of Gordon County residents are fully vaccinated, according to the Georgia Department of Public Health.
At AdventHealth Gordon, a 112-bed hospital in Calhoun, just 35% of the 1,723 workers that serve the hospital are at least partially vaccinated, according to data reported to HHS.
‘I am not vaccinated’
One reason some hospital staff say they are resisting COVID vaccination is because it’s so new and not yet fully approved by the FDA.
“I am not vaccinated,” said a social services worker for AdventHealth Gordon who asked that her name not be used because she was unauthorized to speak to this news organization and Georgia Health News (who collaborated on this project). “I just have not felt the need to do that at this time.”
The woman said she doesn’t have a problem with vaccines. She gets the flu shot every year. “I’ve been vaccinated all my life,” she said. But she doesn’t view COVID-19 vaccination in the same way.
“I want to see more testing done,” she said. “It took a long time to get a flu vaccine, and we made a COVID vaccine in 6 months. I want to know, before I start putting something into my body, that the testing is done.”
Staff at her hospital were given the option to be vaccinated or wear a mask. She chose the mask.
Many of her coworkers share her feelings, she said.
Mask expert Linsey Marr, PhD, a professor of civil and environmental engineering at Virginia Tech University, Blacksburg, Va., said N95 masks and vaccines are both highly effective, but the protection from the vaccine is superior because it is continuous.
“It’s hard to wear an N95 at all times. You have to take it off to eat, for example, in a break room in a hospital. I should point out that you can be exposed to the virus in other buildings besides a hospital – restaurants, stores, people’s homes – and because someone can be infected without symptoms, you could easily be around an infected person without knowing it,” she said.
Eventually, staff at AdventHealth Gordon may get a stronger nudge to get the shots. Chief Medical Officer Joseph Joyave, MD, said AdventHealth asks workers to get flu vaccines or provide the hospital with a reason why they won’t. He expects a similar policy will be adopted for COVID vaccines once they are fully licensed by the FDA.
In the meantime, he does not believe that the hospital is putting patients at risk with its low vaccination rate. “We continue to use PPE, masking in all clinical areas, and continue to screen daily all employees and visitors,” he said.
AdventHealth, the 12th largest hospital system in the nation with 49 hospitals, has at least 20 hospitals with vaccination rates lower than 50%, according to HHS data.
Other hospital systems have approached hesitation around the COVID vaccines differently.
When infectious disease experts at Vanderbilt Hospital in Nashville realized early on that many of their workers felt unsure about the vaccines, they set out to provide a wealth of information.
“There was a lot of hesitancy and skepticism,” said William Schaffner, MD, a professor of preventive medicine and infectious disease at Vanderbilt. So the infectious disease division put together a multifaceted program including Q&As, educational sessions, and one-on-one visits with employees “from the custodians all the way up to the C-Suite,” he said.
Today, HHS data shows the hospital is 83% vaccinated. Dr. Schaffner thinks the true number is probably higher, about 90%. “We’re very pleased with that,” he said.
In his experience with flu vaccinations, it was extremely difficult in the first year to get workers to take flu shots. The second year it was easier. By the third year it was humdrum, he said, because it had become a cultural norm.
Dr. Schaffner expects winning people over to the COVID vaccines will follow a similar course, but “we’re not there yet,” he said.
Protecting patients and caregivers
There is no question that health care workers carried a heavy load through the worst months of the pandemic. Many of them worked to the point of exhaustion and burnout. Some were the only conduits between isolated patients and their families, holding hands and mobile phones so distanced loved ones could video chat. Many were left inadequately protected because of shortages of masks, gowns, gloves, and other gear.
An investigation by Kaiser Health News and The Guardian recently revealed that more than 3,600 health care workers died in COVID’s first year in the United States.
Vaccination of health care workers is important to protect these frontline workers and their families who will continue to be at risk of coming into contact with the infection, even as the number of cases falls.
Hesitancy in health care is also dangerous because these clinicians and allied health workers – who may not show any symptoms – can also carry the virus to someone who wouldn’t survive an infection, including patients with organ transplants, those with autoimmune diseases, premature infants, and the elderly.
It is not known how often patients in the United States are infected with COVID in health care settings, but case reports reveal that hospitals are still experiencing outbreaks.
On June 1, Northern Lights A.R. Gould Hospital in Presque Isle, Maine, announced a COVID outbreak on its medical-surgical unit. As of June 22, 13 residents and staff have caught the virus, according to the Maine Centers for Disease Control, which is investigating. Four of the first five staff members to test positive had not been fully vaccinated.
According to HHS data, about 20% of the health care workers at that hospital are still unvaccinated.
Oregon Health & Science University experienced a COVID outbreak connected to the hospital’s cardiovascular care unit from April to mid-May of this year. According to hospital spokesperson Tracy Brawley, a patient visitor brought the infection to campus, where it ultimately spread to 14 others, including “patients, visitors, employees, and learners.”
In a written statement, the hospital said “nearly all” health care workers who tested positive were previously vaccinated and experienced no symptoms or only minor ones. The hospital said it hasn’t identified any onward transmission from health care workers to patients, and also stated: “It is not yet understood how transmission may have occurred between patients, visitors, and health care workers.”
In March, an unvaccinated health care worker in Kentucky carried a SARS-CoV-2 variant back to the nursing home where the person worked. Some 90% of the residents were fully vaccinated. Ultimately, 26 patients were infected; 18 of them were fully vaccinated. And 20 health care workers, four of whom were vaccinated, were infected.
Vaccines slowed the virus down and made infections less severe, but in this fragile population, they couldn’t stop it completely. One resident, who had survived a bout of COVID almost a year earlier, died. According to the CDC’s Morbidity and Mortality Weekly Report, 47% of the workers in that facility were unvaccinated.
In the United Kingdom, statistics collected through that country’s National Health Service also suggest a heavy toll. More than 32,300 patients caught COVID in English hospitals since March 2020. Up to 8,700 of them died, according to a recent analysis by The Guardian. The U.K. government recently made COVID vaccinations mandatory for health care workers.
COVID delays cancer care
When Mr. Oswalt, the Fort Worth, Texas man with non-Hodgkin lymphoma, contracted COVID-19, the virus took down his kidneys first. Toxins were building up in his blood, so doctors prescribed dialysis to support his body and buy his kidneys time to heal.
He was in one of these dialysis treatments when his lungs succumbed.
“Look, I can’t breathe,” he told the nurse who was supervising his treatment. The nurse gestured to an oxygen tank already hanging by his side, and said, “You should be OK.”
But he wasn’t.
“I can’t breathe,” Mr. Oswalt said again. Then the air hunger hit. Mr. Oswalt began gasping and couldn’t stop. Today, his voice breaks when he describes this moment. “A lot of it becomes a blur.”
When Mr. Oswalt, 61, regained consciousness, he was hooked up to a ventilator to ease his breathing.
For days, Mr. Oswalt clung to the edge of life. His wife, Molly, who wasn’t allowed to see him in the hospital, got a call that he might not make it through the night. She made frantic phone calls to her brother and sister and prayed.
Mr. Oswalt was on a ventilator for about a week. His kidneys and lungs healed enough so that he could restart his chemotherapy. He was eventually discharged home on January 22.
The last time he was scanned, the large tumor in his chest had shrunk from the size of a grapefruit to the size of a dime.
But having COVID on top of cancer has had a devastating effect on his life. Before he got sick, Molly said, he couldn’t stay still. He was busy all the time. After spending months in the hospital, his energy was depleted. He couldn’t keep his swimming pool installation business going.
He and Molly had to give up their house in Fort Worth and move in with family in Amarillo. He has had to pause his cancer treatments while doctors wait for his kidneys to heal. Relatives have been raising money on GoFundMe to pay their bills.
Months after moving across the state to Amarillo and hoping for better days, Tim said he got good news this week: He no longer needs dialysis. A new round of tests found no signs of cancer. His white blood cell count is back to normal. His lymph nodes are no longer swollen.
He goes back for another scan in a few weeks, but the doctor told him she isn’t going to recommend any further chemo at this point.
“It was shocking, to tell you the truth. It still is. When I talk about it, I get kind of emotional” about his recovery, he said.
Tim said he was really dreading more chemotherapy. His hair has just started growing back. He can finally taste food again. He wasn’t ready to face more side effects from the treatments, or the COVID – he no longer knows exactly which diagnosis led to his most debilitating symptoms.
He said his ordeal has left him with no patience for health care workers who don’t think they need to be vaccinated.
The way he sees it, it’s no different than the electrical training he had to get before he could wire the lights and pumps in a swimming pool.
“You know, if I don’t certify and keep my license, I can’t work on anything electrical. So, if I’ve made the choice not to go down and take the test and get a license, then I made the choice not to work on electrical stuff,” he said.
He supports the growing number of hospitals that have made vaccination mandatory for their workers.
“They don’t let electricians put people at risk. And they shouldn’t let health care workers for sure,” he said.
A version of this article first appeared on Medscape.com.
Tim Oswalt had been in a Fort Worth, Texas, hospital for over a month, receiving treatment for a grapefruit-sized tumor in his chest that was pressing on his heart and lungs. It turned out to be stage 3 non-Hodgkin lymphoma.
Then one day in January, he was moved from his semi-private room to an isolated one with special ventilation. The staff explained he had been infected by the virus that was once again surging in many areas of the country, including Texas.
“How the hell did I catch COVID?” he asked the staff, who now approached him in full moon-suit personal protective equipment (PPE).
The hospital was locked down, and Mr. Oswalt hadn’t had any visitors in weeks. Neither of his two roommates tested positive. He’d been tested for COVID several times over the course of his nearly 5-week stay and was always negative.
“‘Well, you know, it’s easy to [catch it] in a hospital,’” Mr. Oswalt said he was told by hospital staff. “‘We’re having a bad outbreak. So you were just exposed somehow.’”
Officials at John Peter Smith Hospital, where Mr. Oswalt was treated, said they are puzzled by his case. According to their infection prevention team, none of his caregivers tested positive for COVID-19, nor did Mr. Oswalt share space with any other COVID-positive patients. And yet, local media reported a surge in cases among JPS hospital staff in December.
“Infection of any kind is a constant battle within hospitals and one that we all take seriously,” said Rob Stephenson, MD, chief quality officer at JPS Health Network. “Anyone in a vulnerable health condition at the height of the pandemic would have been at greater risk for contracting COVID-19 inside – or even more so, outside – the hospital.”
Mr. Oswalt was diagnosed with COVID in early January. JPS Hospital began vaccinating its health care workers about 2 weeks earlier, so there had not yet been enough time for any of them to develop full protection against catching or spreading the virus.
Today, the hospital said 74% of its staff – 5,300 of 7,200 workers – are now vaccinated.
against the SARS-CoV2 virus.
Refusing vaccinations
In fact, nationwide, 1 in 4 hospital workers who have direct contact with patients had not yet received a single dose of a COVID vaccine by the end of May, according to a WebMD and Medscape Medical News analysis of data collected by the U.S. Department of Health and Human Services (HHS) from 2,500 hospitals across the United States.
Among the nation’s 50 largest hospitals, the percentage of unvaccinated health care workers appears to be even larger, about 1 in 3. Vaccination rates range from a high of 99% at Houston Methodist Hospital, which was the first in the nation to mandate the shots for its workers, to a low between 30% and 40% at some hospitals in Florida.
Memorial Hermann Texas Medical Center in Houston has 1,180 beds and sits less than half a mile from Houston Methodist Hospital. But in terms of worker vaccinations, it is farther away.
Memorial Hermann reported to HHS that about 32% of its 28,000 workers haven’t been inoculated. The hospital’s PR office contests that figure, putting it closer to 25% unvaccinated across their health system. The hospital said it is boosting participation by offering a $300 “shot of hope” bonus to workers who start their vaccination series by the end of June.
Lakeland Regional Medical Center in Lakeland, Fla., reported to HHS that 63% of its health care personnel are still unvaccinated. The hospital did not return a call to verify that number.
To boost vaccination rates, more hospitals are starting to require the shots, after the Equal Employment Opportunity Commission gave its green light to mandates in May.
“It’s a real problem that you have such high levels of unvaccinated individuals in hospitals,” said Lawrence Gostin, JD, director of the O’Neill Institute for National and Global Health Law at Georgetown University, Washington.
“We have to protect our health workforce, and we have to protect our patients. Hospitals should be the safest places in the country, and the only way to make them safe is to have a fully vaccinated workforce,” Mr. Gostin said.
Is the data misleading?
The HHS system designed to amass hospital data was set up quickly, to respond to an emergency. For that reason, experts say the information hasn’t been as carefully collected or vetted as it normally would have been. Some hospitals may have misunderstood how to report their vaccination numbers.
In addition, reporting data on worker vaccinations is voluntary. Only about half of hospitals have chosen to share their numbers. In other cases, like Texas, states have blocked the public release of these statistics.
AdventHealth Orlando, a 1,300-bed hospital in Florida, reported to HHS that 56% of its staff have not started their shots. But spokesman Jeff Grainger said the figures probably overstate the number of unvaccinated workers because the hospital doesn’t always know when people get vaccinated outside of its campus, at a local pharmacy, for example.
For those reasons, the picture of health care worker vaccinations across the country is incomplete.
Where hospitals fall behind
Even if the data are flawed, the vaccination rates from hospitals mirror the general population. A May Gallup poll, for example, found 24% of Americans said they definitely won’t get the vaccine. Another 12% say they plan to get it but are waiting.
The data also align with recent studies. A review of 35 studies by researchers at New Mexico State University that assessed hesitancy in more than 76,000 health care workers around the world found about 23% of them were reluctant to get the shots.
An ongoing monthly survey of more than 1.9 million U.S. Facebook users led by researchers at Carnegie Mellon University, Pittsburgh recently looked at vaccine hesitancy by occupation. It revealed a spectrum of hesitancy among health care workers corresponding to income and education, ranging from a low of 9% among pharmacists to highs of 20%-23% among nursing aides and emergency medical technicians. About 12% of registered nurses and doctors admitted to being hesitant to get a shot.
“Health care workers are not monolithic,” said study author Jagdish Khubchandani, professor of public health sciences at New Mexico State.
“There’s a big divide between males, doctoral degree holders, older people and the younger low-income, low-education frontline, female, health care workers. They are the most hesitant,” he said. Support staff typically outnumbers doctors at hospitals about 3 to 1.
“There is outreach work to be done there,” said Robin Mejia, PhD, director of the Statistics and Human Rights Program at Carnegie Mellon, who is leading the study on Facebook’s survey data. “These are also high-contact professions. These are people who are seeing patients on a regular basis.”
That’s why, when the Centers for Disease Control and Prevention was planning the national vaccine rollout, they prioritized health care workers for the initially scarce first doses. The intent was to protect vulnerable workers and their patients who are at high risk of infection. But the CDC had another reason for putting health care workers first: After they were safely vaccinated, the hope was that they would encourage wary patients to do the same.
Hospitals were supposed to be hubs of education to help build trust within less confident communities. But not all hospitals have risen to that challenge.
Political affiliation seems to be one contributing factor in vaccine hesitancy. Take for example Calhoun, Ga., the seat of Gordon County, where residents voted for Donald Trump over Joe Biden by a 67-point margin in the 2020 general election. Studies have found that Republicans are more likely to decline vaccines than Democrats.
People who live in rural areas are less likely to be vaccinated than those who live in cities, and that’s true in Gordon County too. Vaccinations are lagging in this northwest corner of Georgia where factory jobs in chicken processing plants and carpet manufacturing energize the local economy. Just 24% of Gordon County residents are fully vaccinated, according to the Georgia Department of Public Health.
At AdventHealth Gordon, a 112-bed hospital in Calhoun, just 35% of the 1,723 workers that serve the hospital are at least partially vaccinated, according to data reported to HHS.
‘I am not vaccinated’
One reason some hospital staff say they are resisting COVID vaccination is because it’s so new and not yet fully approved by the FDA.
“I am not vaccinated,” said a social services worker for AdventHealth Gordon who asked that her name not be used because she was unauthorized to speak to this news organization and Georgia Health News (who collaborated on this project). “I just have not felt the need to do that at this time.”
The woman said she doesn’t have a problem with vaccines. She gets the flu shot every year. “I’ve been vaccinated all my life,” she said. But she doesn’t view COVID-19 vaccination in the same way.
“I want to see more testing done,” she said. “It took a long time to get a flu vaccine, and we made a COVID vaccine in 6 months. I want to know, before I start putting something into my body, that the testing is done.”
Staff at her hospital were given the option to be vaccinated or wear a mask. She chose the mask.
Many of her coworkers share her feelings, she said.
Mask expert Linsey Marr, PhD, a professor of civil and environmental engineering at Virginia Tech University, Blacksburg, Va., said N95 masks and vaccines are both highly effective, but the protection from the vaccine is superior because it is continuous.
“It’s hard to wear an N95 at all times. You have to take it off to eat, for example, in a break room in a hospital. I should point out that you can be exposed to the virus in other buildings besides a hospital – restaurants, stores, people’s homes – and because someone can be infected without symptoms, you could easily be around an infected person without knowing it,” she said.
Eventually, staff at AdventHealth Gordon may get a stronger nudge to get the shots. Chief Medical Officer Joseph Joyave, MD, said AdventHealth asks workers to get flu vaccines or provide the hospital with a reason why they won’t. He expects a similar policy will be adopted for COVID vaccines once they are fully licensed by the FDA.
In the meantime, he does not believe that the hospital is putting patients at risk with its low vaccination rate. “We continue to use PPE, masking in all clinical areas, and continue to screen daily all employees and visitors,” he said.
AdventHealth, the 12th largest hospital system in the nation with 49 hospitals, has at least 20 hospitals with vaccination rates lower than 50%, according to HHS data.
Other hospital systems have approached hesitation around the COVID vaccines differently.
When infectious disease experts at Vanderbilt Hospital in Nashville realized early on that many of their workers felt unsure about the vaccines, they set out to provide a wealth of information.
“There was a lot of hesitancy and skepticism,” said William Schaffner, MD, a professor of preventive medicine and infectious disease at Vanderbilt. So the infectious disease division put together a multifaceted program including Q&As, educational sessions, and one-on-one visits with employees “from the custodians all the way up to the C-Suite,” he said.
Today, HHS data shows the hospital is 83% vaccinated. Dr. Schaffner thinks the true number is probably higher, about 90%. “We’re very pleased with that,” he said.
In his experience with flu vaccinations, it was extremely difficult in the first year to get workers to take flu shots. The second year it was easier. By the third year it was humdrum, he said, because it had become a cultural norm.
Dr. Schaffner expects winning people over to the COVID vaccines will follow a similar course, but “we’re not there yet,” he said.
Protecting patients and caregivers
There is no question that health care workers carried a heavy load through the worst months of the pandemic. Many of them worked to the point of exhaustion and burnout. Some were the only conduits between isolated patients and their families, holding hands and mobile phones so distanced loved ones could video chat. Many were left inadequately protected because of shortages of masks, gowns, gloves, and other gear.
An investigation by Kaiser Health News and The Guardian recently revealed that more than 3,600 health care workers died in COVID’s first year in the United States.
Vaccination of health care workers is important to protect these frontline workers and their families who will continue to be at risk of coming into contact with the infection, even as the number of cases falls.
Hesitancy in health care is also dangerous because these clinicians and allied health workers – who may not show any symptoms – can also carry the virus to someone who wouldn’t survive an infection, including patients with organ transplants, those with autoimmune diseases, premature infants, and the elderly.
It is not known how often patients in the United States are infected with COVID in health care settings, but case reports reveal that hospitals are still experiencing outbreaks.
On June 1, Northern Lights A.R. Gould Hospital in Presque Isle, Maine, announced a COVID outbreak on its medical-surgical unit. As of June 22, 13 residents and staff have caught the virus, according to the Maine Centers for Disease Control, which is investigating. Four of the first five staff members to test positive had not been fully vaccinated.
According to HHS data, about 20% of the health care workers at that hospital are still unvaccinated.
Oregon Health & Science University experienced a COVID outbreak connected to the hospital’s cardiovascular care unit from April to mid-May of this year. According to hospital spokesperson Tracy Brawley, a patient visitor brought the infection to campus, where it ultimately spread to 14 others, including “patients, visitors, employees, and learners.”
In a written statement, the hospital said “nearly all” health care workers who tested positive were previously vaccinated and experienced no symptoms or only minor ones. The hospital said it hasn’t identified any onward transmission from health care workers to patients, and also stated: “It is not yet understood how transmission may have occurred between patients, visitors, and health care workers.”
In March, an unvaccinated health care worker in Kentucky carried a SARS-CoV-2 variant back to the nursing home where the person worked. Some 90% of the residents were fully vaccinated. Ultimately, 26 patients were infected; 18 of them were fully vaccinated. And 20 health care workers, four of whom were vaccinated, were infected.
Vaccines slowed the virus down and made infections less severe, but in this fragile population, they couldn’t stop it completely. One resident, who had survived a bout of COVID almost a year earlier, died. According to the CDC’s Morbidity and Mortality Weekly Report, 47% of the workers in that facility were unvaccinated.
In the United Kingdom, statistics collected through that country’s National Health Service also suggest a heavy toll. More than 32,300 patients caught COVID in English hospitals since March 2020. Up to 8,700 of them died, according to a recent analysis by The Guardian. The U.K. government recently made COVID vaccinations mandatory for health care workers.
COVID delays cancer care
When Mr. Oswalt, the Fort Worth, Texas man with non-Hodgkin lymphoma, contracted COVID-19, the virus took down his kidneys first. Toxins were building up in his blood, so doctors prescribed dialysis to support his body and buy his kidneys time to heal.
He was in one of these dialysis treatments when his lungs succumbed.
“Look, I can’t breathe,” he told the nurse who was supervising his treatment. The nurse gestured to an oxygen tank already hanging by his side, and said, “You should be OK.”
But he wasn’t.
“I can’t breathe,” Mr. Oswalt said again. Then the air hunger hit. Mr. Oswalt began gasping and couldn’t stop. Today, his voice breaks when he describes this moment. “A lot of it becomes a blur.”
When Mr. Oswalt, 61, regained consciousness, he was hooked up to a ventilator to ease his breathing.
For days, Mr. Oswalt clung to the edge of life. His wife, Molly, who wasn’t allowed to see him in the hospital, got a call that he might not make it through the night. She made frantic phone calls to her brother and sister and prayed.
Mr. Oswalt was on a ventilator for about a week. His kidneys and lungs healed enough so that he could restart his chemotherapy. He was eventually discharged home on January 22.
The last time he was scanned, the large tumor in his chest had shrunk from the size of a grapefruit to the size of a dime.
But having COVID on top of cancer has had a devastating effect on his life. Before he got sick, Molly said, he couldn’t stay still. He was busy all the time. After spending months in the hospital, his energy was depleted. He couldn’t keep his swimming pool installation business going.
He and Molly had to give up their house in Fort Worth and move in with family in Amarillo. He has had to pause his cancer treatments while doctors wait for his kidneys to heal. Relatives have been raising money on GoFundMe to pay their bills.
Months after moving across the state to Amarillo and hoping for better days, Tim said he got good news this week: He no longer needs dialysis. A new round of tests found no signs of cancer. His white blood cell count is back to normal. His lymph nodes are no longer swollen.
He goes back for another scan in a few weeks, but the doctor told him she isn’t going to recommend any further chemo at this point.
“It was shocking, to tell you the truth. It still is. When I talk about it, I get kind of emotional” about his recovery, he said.
Tim said he was really dreading more chemotherapy. His hair has just started growing back. He can finally taste food again. He wasn’t ready to face more side effects from the treatments, or the COVID – he no longer knows exactly which diagnosis led to his most debilitating symptoms.
He said his ordeal has left him with no patience for health care workers who don’t think they need to be vaccinated.
The way he sees it, it’s no different than the electrical training he had to get before he could wire the lights and pumps in a swimming pool.
“You know, if I don’t certify and keep my license, I can’t work on anything electrical. So, if I’ve made the choice not to go down and take the test and get a license, then I made the choice not to work on electrical stuff,” he said.
He supports the growing number of hospitals that have made vaccination mandatory for their workers.
“They don’t let electricians put people at risk. And they shouldn’t let health care workers for sure,” he said.
A version of this article first appeared on Medscape.com.
Tim Oswalt had been in a Fort Worth, Texas, hospital for over a month, receiving treatment for a grapefruit-sized tumor in his chest that was pressing on his heart and lungs. It turned out to be stage 3 non-Hodgkin lymphoma.
Then one day in January, he was moved from his semi-private room to an isolated one with special ventilation. The staff explained he had been infected by the virus that was once again surging in many areas of the country, including Texas.
“How the hell did I catch COVID?” he asked the staff, who now approached him in full moon-suit personal protective equipment (PPE).
The hospital was locked down, and Mr. Oswalt hadn’t had any visitors in weeks. Neither of his two roommates tested positive. He’d been tested for COVID several times over the course of his nearly 5-week stay and was always negative.
“‘Well, you know, it’s easy to [catch it] in a hospital,’” Mr. Oswalt said he was told by hospital staff. “‘We’re having a bad outbreak. So you were just exposed somehow.’”
Officials at John Peter Smith Hospital, where Mr. Oswalt was treated, said they are puzzled by his case. According to their infection prevention team, none of his caregivers tested positive for COVID-19, nor did Mr. Oswalt share space with any other COVID-positive patients. And yet, local media reported a surge in cases among JPS hospital staff in December.
“Infection of any kind is a constant battle within hospitals and one that we all take seriously,” said Rob Stephenson, MD, chief quality officer at JPS Health Network. “Anyone in a vulnerable health condition at the height of the pandemic would have been at greater risk for contracting COVID-19 inside – or even more so, outside – the hospital.”
Mr. Oswalt was diagnosed with COVID in early January. JPS Hospital began vaccinating its health care workers about 2 weeks earlier, so there had not yet been enough time for any of them to develop full protection against catching or spreading the virus.
Today, the hospital said 74% of its staff – 5,300 of 7,200 workers – are now vaccinated.
against the SARS-CoV2 virus.
Refusing vaccinations
In fact, nationwide, 1 in 4 hospital workers who have direct contact with patients had not yet received a single dose of a COVID vaccine by the end of May, according to a WebMD and Medscape Medical News analysis of data collected by the U.S. Department of Health and Human Services (HHS) from 2,500 hospitals across the United States.
Among the nation’s 50 largest hospitals, the percentage of unvaccinated health care workers appears to be even larger, about 1 in 3. Vaccination rates range from a high of 99% at Houston Methodist Hospital, which was the first in the nation to mandate the shots for its workers, to a low between 30% and 40% at some hospitals in Florida.
Memorial Hermann Texas Medical Center in Houston has 1,180 beds and sits less than half a mile from Houston Methodist Hospital. But in terms of worker vaccinations, it is farther away.
Memorial Hermann reported to HHS that about 32% of its 28,000 workers haven’t been inoculated. The hospital’s PR office contests that figure, putting it closer to 25% unvaccinated across their health system. The hospital said it is boosting participation by offering a $300 “shot of hope” bonus to workers who start their vaccination series by the end of June.
Lakeland Regional Medical Center in Lakeland, Fla., reported to HHS that 63% of its health care personnel are still unvaccinated. The hospital did not return a call to verify that number.
To boost vaccination rates, more hospitals are starting to require the shots, after the Equal Employment Opportunity Commission gave its green light to mandates in May.
“It’s a real problem that you have such high levels of unvaccinated individuals in hospitals,” said Lawrence Gostin, JD, director of the O’Neill Institute for National and Global Health Law at Georgetown University, Washington.
“We have to protect our health workforce, and we have to protect our patients. Hospitals should be the safest places in the country, and the only way to make them safe is to have a fully vaccinated workforce,” Mr. Gostin said.
Is the data misleading?
The HHS system designed to amass hospital data was set up quickly, to respond to an emergency. For that reason, experts say the information hasn’t been as carefully collected or vetted as it normally would have been. Some hospitals may have misunderstood how to report their vaccination numbers.
In addition, reporting data on worker vaccinations is voluntary. Only about half of hospitals have chosen to share their numbers. In other cases, like Texas, states have blocked the public release of these statistics.
AdventHealth Orlando, a 1,300-bed hospital in Florida, reported to HHS that 56% of its staff have not started their shots. But spokesman Jeff Grainger said the figures probably overstate the number of unvaccinated workers because the hospital doesn’t always know when people get vaccinated outside of its campus, at a local pharmacy, for example.
For those reasons, the picture of health care worker vaccinations across the country is incomplete.
Where hospitals fall behind
Even if the data are flawed, the vaccination rates from hospitals mirror the general population. A May Gallup poll, for example, found 24% of Americans said they definitely won’t get the vaccine. Another 12% say they plan to get it but are waiting.
The data also align with recent studies. A review of 35 studies by researchers at New Mexico State University that assessed hesitancy in more than 76,000 health care workers around the world found about 23% of them were reluctant to get the shots.
An ongoing monthly survey of more than 1.9 million U.S. Facebook users led by researchers at Carnegie Mellon University, Pittsburgh recently looked at vaccine hesitancy by occupation. It revealed a spectrum of hesitancy among health care workers corresponding to income and education, ranging from a low of 9% among pharmacists to highs of 20%-23% among nursing aides and emergency medical technicians. About 12% of registered nurses and doctors admitted to being hesitant to get a shot.
“Health care workers are not monolithic,” said study author Jagdish Khubchandani, professor of public health sciences at New Mexico State.
“There’s a big divide between males, doctoral degree holders, older people and the younger low-income, low-education frontline, female, health care workers. They are the most hesitant,” he said. Support staff typically outnumbers doctors at hospitals about 3 to 1.
“There is outreach work to be done there,” said Robin Mejia, PhD, director of the Statistics and Human Rights Program at Carnegie Mellon, who is leading the study on Facebook’s survey data. “These are also high-contact professions. These are people who are seeing patients on a regular basis.”
That’s why, when the Centers for Disease Control and Prevention was planning the national vaccine rollout, they prioritized health care workers for the initially scarce first doses. The intent was to protect vulnerable workers and their patients who are at high risk of infection. But the CDC had another reason for putting health care workers first: After they were safely vaccinated, the hope was that they would encourage wary patients to do the same.
Hospitals were supposed to be hubs of education to help build trust within less confident communities. But not all hospitals have risen to that challenge.
Political affiliation seems to be one contributing factor in vaccine hesitancy. Take for example Calhoun, Ga., the seat of Gordon County, where residents voted for Donald Trump over Joe Biden by a 67-point margin in the 2020 general election. Studies have found that Republicans are more likely to decline vaccines than Democrats.
People who live in rural areas are less likely to be vaccinated than those who live in cities, and that’s true in Gordon County too. Vaccinations are lagging in this northwest corner of Georgia where factory jobs in chicken processing plants and carpet manufacturing energize the local economy. Just 24% of Gordon County residents are fully vaccinated, according to the Georgia Department of Public Health.
At AdventHealth Gordon, a 112-bed hospital in Calhoun, just 35% of the 1,723 workers that serve the hospital are at least partially vaccinated, according to data reported to HHS.
‘I am not vaccinated’
One reason some hospital staff say they are resisting COVID vaccination is because it’s so new and not yet fully approved by the FDA.
“I am not vaccinated,” said a social services worker for AdventHealth Gordon who asked that her name not be used because she was unauthorized to speak to this news organization and Georgia Health News (who collaborated on this project). “I just have not felt the need to do that at this time.”
The woman said she doesn’t have a problem with vaccines. She gets the flu shot every year. “I’ve been vaccinated all my life,” she said. But she doesn’t view COVID-19 vaccination in the same way.
“I want to see more testing done,” she said. “It took a long time to get a flu vaccine, and we made a COVID vaccine in 6 months. I want to know, before I start putting something into my body, that the testing is done.”
Staff at her hospital were given the option to be vaccinated or wear a mask. She chose the mask.
Many of her coworkers share her feelings, she said.
Mask expert Linsey Marr, PhD, a professor of civil and environmental engineering at Virginia Tech University, Blacksburg, Va., said N95 masks and vaccines are both highly effective, but the protection from the vaccine is superior because it is continuous.
“It’s hard to wear an N95 at all times. You have to take it off to eat, for example, in a break room in a hospital. I should point out that you can be exposed to the virus in other buildings besides a hospital – restaurants, stores, people’s homes – and because someone can be infected without symptoms, you could easily be around an infected person without knowing it,” she said.
Eventually, staff at AdventHealth Gordon may get a stronger nudge to get the shots. Chief Medical Officer Joseph Joyave, MD, said AdventHealth asks workers to get flu vaccines or provide the hospital with a reason why they won’t. He expects a similar policy will be adopted for COVID vaccines once they are fully licensed by the FDA.
In the meantime, he does not believe that the hospital is putting patients at risk with its low vaccination rate. “We continue to use PPE, masking in all clinical areas, and continue to screen daily all employees and visitors,” he said.
AdventHealth, the 12th largest hospital system in the nation with 49 hospitals, has at least 20 hospitals with vaccination rates lower than 50%, according to HHS data.
Other hospital systems have approached hesitation around the COVID vaccines differently.
When infectious disease experts at Vanderbilt Hospital in Nashville realized early on that many of their workers felt unsure about the vaccines, they set out to provide a wealth of information.
“There was a lot of hesitancy and skepticism,” said William Schaffner, MD, a professor of preventive medicine and infectious disease at Vanderbilt. So the infectious disease division put together a multifaceted program including Q&As, educational sessions, and one-on-one visits with employees “from the custodians all the way up to the C-Suite,” he said.
Today, HHS data shows the hospital is 83% vaccinated. Dr. Schaffner thinks the true number is probably higher, about 90%. “We’re very pleased with that,” he said.
In his experience with flu vaccinations, it was extremely difficult in the first year to get workers to take flu shots. The second year it was easier. By the third year it was humdrum, he said, because it had become a cultural norm.
Dr. Schaffner expects winning people over to the COVID vaccines will follow a similar course, but “we’re not there yet,” he said.
Protecting patients and caregivers
There is no question that health care workers carried a heavy load through the worst months of the pandemic. Many of them worked to the point of exhaustion and burnout. Some were the only conduits between isolated patients and their families, holding hands and mobile phones so distanced loved ones could video chat. Many were left inadequately protected because of shortages of masks, gowns, gloves, and other gear.
An investigation by Kaiser Health News and The Guardian recently revealed that more than 3,600 health care workers died in COVID’s first year in the United States.
Vaccination of health care workers is important to protect these frontline workers and their families who will continue to be at risk of coming into contact with the infection, even as the number of cases falls.
Hesitancy in health care is also dangerous because these clinicians and allied health workers – who may not show any symptoms – can also carry the virus to someone who wouldn’t survive an infection, including patients with organ transplants, those with autoimmune diseases, premature infants, and the elderly.
It is not known how often patients in the United States are infected with COVID in health care settings, but case reports reveal that hospitals are still experiencing outbreaks.
On June 1, Northern Lights A.R. Gould Hospital in Presque Isle, Maine, announced a COVID outbreak on its medical-surgical unit. As of June 22, 13 residents and staff have caught the virus, according to the Maine Centers for Disease Control, which is investigating. Four of the first five staff members to test positive had not been fully vaccinated.
According to HHS data, about 20% of the health care workers at that hospital are still unvaccinated.
Oregon Health & Science University experienced a COVID outbreak connected to the hospital’s cardiovascular care unit from April to mid-May of this year. According to hospital spokesperson Tracy Brawley, a patient visitor brought the infection to campus, where it ultimately spread to 14 others, including “patients, visitors, employees, and learners.”
In a written statement, the hospital said “nearly all” health care workers who tested positive were previously vaccinated and experienced no symptoms or only minor ones. The hospital said it hasn’t identified any onward transmission from health care workers to patients, and also stated: “It is not yet understood how transmission may have occurred between patients, visitors, and health care workers.”
In March, an unvaccinated health care worker in Kentucky carried a SARS-CoV-2 variant back to the nursing home where the person worked. Some 90% of the residents were fully vaccinated. Ultimately, 26 patients were infected; 18 of them were fully vaccinated. And 20 health care workers, four of whom were vaccinated, were infected.
Vaccines slowed the virus down and made infections less severe, but in this fragile population, they couldn’t stop it completely. One resident, who had survived a bout of COVID almost a year earlier, died. According to the CDC’s Morbidity and Mortality Weekly Report, 47% of the workers in that facility were unvaccinated.
In the United Kingdom, statistics collected through that country’s National Health Service also suggest a heavy toll. More than 32,300 patients caught COVID in English hospitals since March 2020. Up to 8,700 of them died, according to a recent analysis by The Guardian. The U.K. government recently made COVID vaccinations mandatory for health care workers.
COVID delays cancer care
When Mr. Oswalt, the Fort Worth, Texas man with non-Hodgkin lymphoma, contracted COVID-19, the virus took down his kidneys first. Toxins were building up in his blood, so doctors prescribed dialysis to support his body and buy his kidneys time to heal.
He was in one of these dialysis treatments when his lungs succumbed.
“Look, I can’t breathe,” he told the nurse who was supervising his treatment. The nurse gestured to an oxygen tank already hanging by his side, and said, “You should be OK.”
But he wasn’t.
“I can’t breathe,” Mr. Oswalt said again. Then the air hunger hit. Mr. Oswalt began gasping and couldn’t stop. Today, his voice breaks when he describes this moment. “A lot of it becomes a blur.”
When Mr. Oswalt, 61, regained consciousness, he was hooked up to a ventilator to ease his breathing.
For days, Mr. Oswalt clung to the edge of life. His wife, Molly, who wasn’t allowed to see him in the hospital, got a call that he might not make it through the night. She made frantic phone calls to her brother and sister and prayed.
Mr. Oswalt was on a ventilator for about a week. His kidneys and lungs healed enough so that he could restart his chemotherapy. He was eventually discharged home on January 22.
The last time he was scanned, the large tumor in his chest had shrunk from the size of a grapefruit to the size of a dime.
But having COVID on top of cancer has had a devastating effect on his life. Before he got sick, Molly said, he couldn’t stay still. He was busy all the time. After spending months in the hospital, his energy was depleted. He couldn’t keep his swimming pool installation business going.
He and Molly had to give up their house in Fort Worth and move in with family in Amarillo. He has had to pause his cancer treatments while doctors wait for his kidneys to heal. Relatives have been raising money on GoFundMe to pay their bills.
Months after moving across the state to Amarillo and hoping for better days, Tim said he got good news this week: He no longer needs dialysis. A new round of tests found no signs of cancer. His white blood cell count is back to normal. His lymph nodes are no longer swollen.
He goes back for another scan in a few weeks, but the doctor told him she isn’t going to recommend any further chemo at this point.
“It was shocking, to tell you the truth. It still is. When I talk about it, I get kind of emotional” about his recovery, he said.
Tim said he was really dreading more chemotherapy. His hair has just started growing back. He can finally taste food again. He wasn’t ready to face more side effects from the treatments, or the COVID – he no longer knows exactly which diagnosis led to his most debilitating symptoms.
He said his ordeal has left him with no patience for health care workers who don’t think they need to be vaccinated.
The way he sees it, it’s no different than the electrical training he had to get before he could wire the lights and pumps in a swimming pool.
“You know, if I don’t certify and keep my license, I can’t work on anything electrical. So, if I’ve made the choice not to go down and take the test and get a license, then I made the choice not to work on electrical stuff,” he said.
He supports the growing number of hospitals that have made vaccination mandatory for their workers.
“They don’t let electricians put people at risk. And they shouldn’t let health care workers for sure,” he said.
A version of this article first appeared on Medscape.com.
Why getting a COVID-19 vaccine to children could take time
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
More evidence links COVID vaccines to rare cases of myocarditis in youth
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
Texas hospital workers sue over vaccine mandates
objecting to its policy of requiring employees and contractors to be vaccinated against COVID-19 or risk losing their jobs.
Plaintiffs include Jennifer Bridges, RN, a medical-surgical nurse at the hospital who has become the public face and voice of health care workers who object to mandatory vaccination, as well as Bob Nevens, the hospital’s director of corporate risk.
Mr. Nevens said the hospital was requiring him to be vaccinated even though he doesn’t treat patients and has been working from home for most of the past year.
“My civil rights and liberties have been trampled on,” he said in comments posted on an online petition. “My right to protect myself from unknown side effects of these vaccines has been placed below the optics of ‘leading medicine,’ “ he said.
Mr. Nevens says in his comments that he was fired on April 15, although the lawsuit says he is currently employed by the hospital’s corporate office.
The Texas attorney who filed the lawsuit, Jared Woodfill, is known to champion conservative causes. In March 2020, he challenged Harris County’s stay-at-home order, charging that it violated religious liberty. He was chairman of the Harris County Republican Party for more than a decade. His website says he is a frequent guest on the local Fox News affiliate.
The lawsuit hinges on a section of the federal law that authorizes emergency use of medical products – US Code 360bbb-3.
That law says that individuals to whom the product is administered should be informed “of the option to accept or refuse administration of the product, of the consequence, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.”
Legal experts are split as to what the provision means for vaccination mandates. Courts have not yet weighed in with their interpretations of the law.
The petition also repeats a popular antivaccination argument that likens requiring a vaccine approved for emergency use to the kind of medical experimentation performed by Nazi doctors on Jewish prisoners in concentration camps. It says forcing people to choose between an experimental vaccine and a job is a violation of the Nuremberg Code, which says that people must voluntarily and knowingly consent to participating in research.
The vaccines have already been tested in clinical trials. People who are getting them now are not part of those studies, though vaccine manufacturers, regulators, and safety experts are still watching closely for any sign of problems tied to the new shots.
It is true, however, that the emergency use authorization granted by the U.S. Food and Drug Administraiton sped up the process of getting the vaccines onto market. Vaccine manufacturers are currently completing the process of submitting documentation required for a full biologics license application, the mechanism the FDA uses for full approval.
Houston Methodist sent an email to employees in April notifying them that they had until June 7 to start the vaccination process or apply for a medical or religious exemption. Those who decide not to will be terminated.
Marc Boom, MD, the health care system’s president and CEO, has explained that the policy is in place to protect patients and that it was the first hospital in the United States to require it. Since then, other hospitals, including the University of Pennsylvania Health System, have required COVID vaccines.
A version of this article first appeared on Medscape.com.
objecting to its policy of requiring employees and contractors to be vaccinated against COVID-19 or risk losing their jobs.
Plaintiffs include Jennifer Bridges, RN, a medical-surgical nurse at the hospital who has become the public face and voice of health care workers who object to mandatory vaccination, as well as Bob Nevens, the hospital’s director of corporate risk.
Mr. Nevens said the hospital was requiring him to be vaccinated even though he doesn’t treat patients and has been working from home for most of the past year.
“My civil rights and liberties have been trampled on,” he said in comments posted on an online petition. “My right to protect myself from unknown side effects of these vaccines has been placed below the optics of ‘leading medicine,’ “ he said.
Mr. Nevens says in his comments that he was fired on April 15, although the lawsuit says he is currently employed by the hospital’s corporate office.
The Texas attorney who filed the lawsuit, Jared Woodfill, is known to champion conservative causes. In March 2020, he challenged Harris County’s stay-at-home order, charging that it violated religious liberty. He was chairman of the Harris County Republican Party for more than a decade. His website says he is a frequent guest on the local Fox News affiliate.
The lawsuit hinges on a section of the federal law that authorizes emergency use of medical products – US Code 360bbb-3.
That law says that individuals to whom the product is administered should be informed “of the option to accept or refuse administration of the product, of the consequence, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.”
Legal experts are split as to what the provision means for vaccination mandates. Courts have not yet weighed in with their interpretations of the law.
The petition also repeats a popular antivaccination argument that likens requiring a vaccine approved for emergency use to the kind of medical experimentation performed by Nazi doctors on Jewish prisoners in concentration camps. It says forcing people to choose between an experimental vaccine and a job is a violation of the Nuremberg Code, which says that people must voluntarily and knowingly consent to participating in research.
The vaccines have already been tested in clinical trials. People who are getting them now are not part of those studies, though vaccine manufacturers, regulators, and safety experts are still watching closely for any sign of problems tied to the new shots.
It is true, however, that the emergency use authorization granted by the U.S. Food and Drug Administraiton sped up the process of getting the vaccines onto market. Vaccine manufacturers are currently completing the process of submitting documentation required for a full biologics license application, the mechanism the FDA uses for full approval.
Houston Methodist sent an email to employees in April notifying them that they had until June 7 to start the vaccination process or apply for a medical or religious exemption. Those who decide not to will be terminated.
Marc Boom, MD, the health care system’s president and CEO, has explained that the policy is in place to protect patients and that it was the first hospital in the United States to require it. Since then, other hospitals, including the University of Pennsylvania Health System, have required COVID vaccines.
A version of this article first appeared on Medscape.com.
objecting to its policy of requiring employees and contractors to be vaccinated against COVID-19 or risk losing their jobs.
Plaintiffs include Jennifer Bridges, RN, a medical-surgical nurse at the hospital who has become the public face and voice of health care workers who object to mandatory vaccination, as well as Bob Nevens, the hospital’s director of corporate risk.
Mr. Nevens said the hospital was requiring him to be vaccinated even though he doesn’t treat patients and has been working from home for most of the past year.
“My civil rights and liberties have been trampled on,” he said in comments posted on an online petition. “My right to protect myself from unknown side effects of these vaccines has been placed below the optics of ‘leading medicine,’ “ he said.
Mr. Nevens says in his comments that he was fired on April 15, although the lawsuit says he is currently employed by the hospital’s corporate office.
The Texas attorney who filed the lawsuit, Jared Woodfill, is known to champion conservative causes. In March 2020, he challenged Harris County’s stay-at-home order, charging that it violated religious liberty. He was chairman of the Harris County Republican Party for more than a decade. His website says he is a frequent guest on the local Fox News affiliate.
The lawsuit hinges on a section of the federal law that authorizes emergency use of medical products – US Code 360bbb-3.
That law says that individuals to whom the product is administered should be informed “of the option to accept or refuse administration of the product, of the consequence, if any, of refusing administration of the product, and of the alternatives to the product that are available and of their benefits and risks.”
Legal experts are split as to what the provision means for vaccination mandates. Courts have not yet weighed in with their interpretations of the law.
The petition also repeats a popular antivaccination argument that likens requiring a vaccine approved for emergency use to the kind of medical experimentation performed by Nazi doctors on Jewish prisoners in concentration camps. It says forcing people to choose between an experimental vaccine and a job is a violation of the Nuremberg Code, which says that people must voluntarily and knowingly consent to participating in research.
The vaccines have already been tested in clinical trials. People who are getting them now are not part of those studies, though vaccine manufacturers, regulators, and safety experts are still watching closely for any sign of problems tied to the new shots.
It is true, however, that the emergency use authorization granted by the U.S. Food and Drug Administraiton sped up the process of getting the vaccines onto market. Vaccine manufacturers are currently completing the process of submitting documentation required for a full biologics license application, the mechanism the FDA uses for full approval.
Houston Methodist sent an email to employees in April notifying them that they had until June 7 to start the vaccination process or apply for a medical or religious exemption. Those who decide not to will be terminated.
Marc Boom, MD, the health care system’s president and CEO, has explained that the policy is in place to protect patients and that it was the first hospital in the United States to require it. Since then, other hospitals, including the University of Pennsylvania Health System, have required COVID vaccines.
A version of this article first appeared on Medscape.com.
CDC guidelines coming on long COVID
The Centers for Disease Control and Prevention is finalizing new guidelines to help clinicians diagnose and manage long COVID, or postacute sequelae of SARS-CoV-2 infection.
In a day-long congressional hearing on April 28, John Brooks, MD, a medical epidemiologist at the CDC’s division of HIV/AIDS prevention, testified that the guidelines were going through the clearance process at the agency, but would be forthcoming.
“They should be coming out very shortly,” Dr. Brooks said.
The guidelines, which were developed in collaboration with newly established long-COVID clinics and patient advocacy groups, will “illustrate how to diagnose and begin to pull together what we know about management,” of the complex condition, he said.
For many doctors and patients who are struggling to understand symptoms that persist for months after the initial viral infection, the guidelines can’t come soon enough.
National Institutes of Health Director Francis Collins, MD, PhD, who also testified at the hearing, estimated that as many as 3 million people could be left with chronic health problems after even mild COVID infections.
“I can’t overstate how serious this issue is for the health of our nation,” he said.
Dr. Collins said his estimate was based on studies showing that roughly 10% of people who get COVID could be affected by this and whose “long-term course is uncertain,” he said. So far, more than 32 million Americans are known to have been infected with the new coronavirus.
“We need to make sure we put our arms around them and bring answers and care to them,” said Rep. Anna Eshoo (D-Calif.), chairwoman of the Subcommittee on Health.
Jennifer Possick, MD, who directs the post-COVID recovery program at Yale New Haven (Conn.) Hospital, testified that the tidal wave of patients she and her colleagues were seeing was overwhelming.
“We are a well-resourced program at an academic medical center, but we are swamped by the need in our community. This year, we have seen more patients with post COVID-19 conditions in our clinic alone than we have new cases of asthma and COPD combined,” she said. “The magnitude of the challenge is daunting.”
Dr. Possick estimated that there are “over 60” clinics in the United States that have started to treat long-COVID patients, but said they are grassroots efforts and all very different from each other.
“Whoever had the resources, had the time, [and] was able to take the initiative and forge to the relationships because most of them are multidisciplinary, did so,” she said.
Patients testify
Several representatives shared moving personal stories of loved ones or staffers who remained ill months after a COVID diagnosis.
Rep. Ann Kuster, from New Hampshire, talked about her 34-year-old niece, a member of the U.S. Ski Team, who had COVID just over a year ago and “continues to struggle with everything, even the simplest activities of daily living” she said. “She has to choose between taking a shower or making dinner. I’m so proud of her for hanging in there.”
Long-COVID patients invited to testify by the subcommittee described months of disability that left them with soaring medical bills and no ability to work to pay them.
“I am now a poor, Black, disabled woman, living with long COVID,” said Chimere Smith, who said she had been a school teacher in Baltimore. “Saying it aloud makes it no more easy to accept.”
She said COVID had affected her ability to think clearly and caused debilitating fatigue, which prevented her from working. She said she lost her vision for almost 5 months because doctors misdiagnosed a cataract caused by long COVID as dry eye.
“If I did not have a loving family, I [would] be speaking to you today [from] my car, the only property I now own.”
Ms. Smith said that long-COVID clinics, which are mostly housed within academic medical centers, were not going to be accessible for all long-haulers, who are disproportionately women of color. She has started a clinic, based out of her church, to help other patients from her community.
“No one wants to hear that long COVID has decimated my life or the lives of other black women in less than a year,” Ms. Smith said. “We’ve just been waiting and hoping for compassionate doctors and politicians who would acknowledge us.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is finalizing new guidelines to help clinicians diagnose and manage long COVID, or postacute sequelae of SARS-CoV-2 infection.
In a day-long congressional hearing on April 28, John Brooks, MD, a medical epidemiologist at the CDC’s division of HIV/AIDS prevention, testified that the guidelines were going through the clearance process at the agency, but would be forthcoming.
“They should be coming out very shortly,” Dr. Brooks said.
The guidelines, which were developed in collaboration with newly established long-COVID clinics and patient advocacy groups, will “illustrate how to diagnose and begin to pull together what we know about management,” of the complex condition, he said.
For many doctors and patients who are struggling to understand symptoms that persist for months after the initial viral infection, the guidelines can’t come soon enough.
National Institutes of Health Director Francis Collins, MD, PhD, who also testified at the hearing, estimated that as many as 3 million people could be left with chronic health problems after even mild COVID infections.
“I can’t overstate how serious this issue is for the health of our nation,” he said.
Dr. Collins said his estimate was based on studies showing that roughly 10% of people who get COVID could be affected by this and whose “long-term course is uncertain,” he said. So far, more than 32 million Americans are known to have been infected with the new coronavirus.
“We need to make sure we put our arms around them and bring answers and care to them,” said Rep. Anna Eshoo (D-Calif.), chairwoman of the Subcommittee on Health.
Jennifer Possick, MD, who directs the post-COVID recovery program at Yale New Haven (Conn.) Hospital, testified that the tidal wave of patients she and her colleagues were seeing was overwhelming.
“We are a well-resourced program at an academic medical center, but we are swamped by the need in our community. This year, we have seen more patients with post COVID-19 conditions in our clinic alone than we have new cases of asthma and COPD combined,” she said. “The magnitude of the challenge is daunting.”
Dr. Possick estimated that there are “over 60” clinics in the United States that have started to treat long-COVID patients, but said they are grassroots efforts and all very different from each other.
“Whoever had the resources, had the time, [and] was able to take the initiative and forge to the relationships because most of them are multidisciplinary, did so,” she said.
Patients testify
Several representatives shared moving personal stories of loved ones or staffers who remained ill months after a COVID diagnosis.
Rep. Ann Kuster, from New Hampshire, talked about her 34-year-old niece, a member of the U.S. Ski Team, who had COVID just over a year ago and “continues to struggle with everything, even the simplest activities of daily living” she said. “She has to choose between taking a shower or making dinner. I’m so proud of her for hanging in there.”
Long-COVID patients invited to testify by the subcommittee described months of disability that left them with soaring medical bills and no ability to work to pay them.
“I am now a poor, Black, disabled woman, living with long COVID,” said Chimere Smith, who said she had been a school teacher in Baltimore. “Saying it aloud makes it no more easy to accept.”
She said COVID had affected her ability to think clearly and caused debilitating fatigue, which prevented her from working. She said she lost her vision for almost 5 months because doctors misdiagnosed a cataract caused by long COVID as dry eye.
“If I did not have a loving family, I [would] be speaking to you today [from] my car, the only property I now own.”
Ms. Smith said that long-COVID clinics, which are mostly housed within academic medical centers, were not going to be accessible for all long-haulers, who are disproportionately women of color. She has started a clinic, based out of her church, to help other patients from her community.
“No one wants to hear that long COVID has decimated my life or the lives of other black women in less than a year,” Ms. Smith said. “We’ve just been waiting and hoping for compassionate doctors and politicians who would acknowledge us.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is finalizing new guidelines to help clinicians diagnose and manage long COVID, or postacute sequelae of SARS-CoV-2 infection.
In a day-long congressional hearing on April 28, John Brooks, MD, a medical epidemiologist at the CDC’s division of HIV/AIDS prevention, testified that the guidelines were going through the clearance process at the agency, but would be forthcoming.
“They should be coming out very shortly,” Dr. Brooks said.
The guidelines, which were developed in collaboration with newly established long-COVID clinics and patient advocacy groups, will “illustrate how to diagnose and begin to pull together what we know about management,” of the complex condition, he said.
For many doctors and patients who are struggling to understand symptoms that persist for months after the initial viral infection, the guidelines can’t come soon enough.
National Institutes of Health Director Francis Collins, MD, PhD, who also testified at the hearing, estimated that as many as 3 million people could be left with chronic health problems after even mild COVID infections.
“I can’t overstate how serious this issue is for the health of our nation,” he said.
Dr. Collins said his estimate was based on studies showing that roughly 10% of people who get COVID could be affected by this and whose “long-term course is uncertain,” he said. So far, more than 32 million Americans are known to have been infected with the new coronavirus.
“We need to make sure we put our arms around them and bring answers and care to them,” said Rep. Anna Eshoo (D-Calif.), chairwoman of the Subcommittee on Health.
Jennifer Possick, MD, who directs the post-COVID recovery program at Yale New Haven (Conn.) Hospital, testified that the tidal wave of patients she and her colleagues were seeing was overwhelming.
“We are a well-resourced program at an academic medical center, but we are swamped by the need in our community. This year, we have seen more patients with post COVID-19 conditions in our clinic alone than we have new cases of asthma and COPD combined,” she said. “The magnitude of the challenge is daunting.”
Dr. Possick estimated that there are “over 60” clinics in the United States that have started to treat long-COVID patients, but said they are grassroots efforts and all very different from each other.
“Whoever had the resources, had the time, [and] was able to take the initiative and forge to the relationships because most of them are multidisciplinary, did so,” she said.
Patients testify
Several representatives shared moving personal stories of loved ones or staffers who remained ill months after a COVID diagnosis.
Rep. Ann Kuster, from New Hampshire, talked about her 34-year-old niece, a member of the U.S. Ski Team, who had COVID just over a year ago and “continues to struggle with everything, even the simplest activities of daily living” she said. “She has to choose between taking a shower or making dinner. I’m so proud of her for hanging in there.”
Long-COVID patients invited to testify by the subcommittee described months of disability that left them with soaring medical bills and no ability to work to pay them.
“I am now a poor, Black, disabled woman, living with long COVID,” said Chimere Smith, who said she had been a school teacher in Baltimore. “Saying it aloud makes it no more easy to accept.”
She said COVID had affected her ability to think clearly and caused debilitating fatigue, which prevented her from working. She said she lost her vision for almost 5 months because doctors misdiagnosed a cataract caused by long COVID as dry eye.
“If I did not have a loving family, I [would] be speaking to you today [from] my car, the only property I now own.”
Ms. Smith said that long-COVID clinics, which are mostly housed within academic medical centers, were not going to be accessible for all long-haulers, who are disproportionately women of color. She has started a clinic, based out of her church, to help other patients from her community.
“No one wants to hear that long COVID has decimated my life or the lives of other black women in less than a year,” Ms. Smith said. “We’ve just been waiting and hoping for compassionate doctors and politicians who would acknowledge us.”
A version of this article first appeared on Medscape.com.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
U.S. finally hits its stride with COVID-19 vaccination rollouts
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Children likely the ‘leading edge’ in spread of COVID-19 variants
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
COVID-19 variants now detected in more animals, may find hosts in mice
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.