User login
My Kidney Is Fine, Can’t You Cystatin C?
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
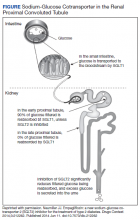
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
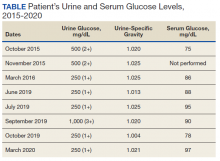
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Not All Pulmonary Nodules in Smokers are Lung Cancer
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
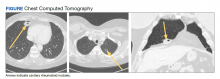
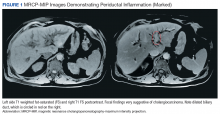
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
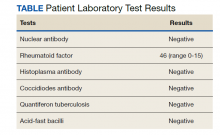
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
Glucosuria Is Not Always Due to Diabetes
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
An Imposter Twice Over: A Case of IgG4-Related Disease
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.