User login
Imiquimod Cream 2.5% and 3.75% Applied Once Daily to Treat External Genital Warts in Men
External genital warts (EGWs), which are caused by infection with select types of human papillomavirus (HPV), are one of the most prevalent and fastest growing sexually transmitted infections.1 External genital warts affect approximately 1% of sexually active adults in the United States and Europe, with another 15% having subclinical infections; more than 1 million new cases of EGWs are diagnosed annually.2-4 Although the condition is not life threatening, lesions can cause symptoms, such as burning, itching, bleeding, pain and dyspareunia, and potential urethral or rectal obstruction. External genital warts also have been associated with adverse psychological effects.5-8
The time between exposure to HPV and development of EGWs can vary from a few weeks to several months or years (median, 2.9 months).9 Many HPV infections are mild and transient, resolving spontaneously.10 As many as 30% of EGWs will regress over 4 months and approximately 90% clear within 2 years.11,12 However, even with treatment, the median time to resolution is 5.9 months.9
Imiquimod cream 5%, which has been successfully used to treat EGWs since it was approved by the US Food and Drug Administration in 1997, is applied to lesions 3 times weekly at bedtime until clearance is achieved or for a maximum of 16 weeks.13 In clinical studies, complete clearance has been reported in 35% to 75% of participants.14-21 However, it is important to note that not all anogenital regions with warts were required to be treated in these studies,14-21 and newly arising warts were not included in the analysis.17 Reported clearance rates were higher and median clearance time was shorter in women.17 Relatively low recurrence rates (6%–26%) have been reported after successful clearance of EGWs.16,17,20,21
Long treatment durations are always a concern for patient adherence. Although increasing the dosing frequency with imiquimod cream 5% might be considered an attractive option to reduce the length of the treatment course, it has resulted in greater incidence and severity of local adverse events (AEs) in some studies without improved efficacy.18,22,23 Thus lower concentrations of imiquimod (ie, 2.5% and 3.75% formulations) were developed to potentially decrease treatment duration and provide a daily dosing regimen.
We report the results of 2 identical, placebo-controlled, phase 3 studies evaluating the safety and efficacy of imiquimod cream 2.5% and 3.75% in treating EGWs in men. Pooled results from a female subgroup previously have been reported.24 Although the percentage of women who reported ever being diagnosed with EGWs was higher than in men (7.2% vs 4%) in one survey,25 other assessments have found a similar prevalence of EGWs among both genders.26-28 We provide important insights herein by reporting efficacy and tolerability data for imiquimod cream 2.5% and 3.75% in the treatment of EGWs in males.
Methods
Study Design
Male patients aged 12 years and older with 2 to 30 EGWs in the inguinal, perineal, and/or perianal areas as well as on the glans penis, penile shaft, scrotum, and/or foreskin were enrolled in 2 identical, multicenter, randomized, parallel-group, double-blind, placebo-controlled studies. Participants were randomized (2:2:1) to self-treatment with imiquimod cream 3.75% or 2.5% or placebo once daily until complete clearance was achieved or for a maximum of 8 weeks (end of treatment [EOT]). There was a follow-up period of up to 8 weeks (end of study [EOS]) in participants who did not achieve complete clearance by EOT. All participants who achieved complete clearance by EOS entered a 12-week observational follow-up period to assess recurrence.
Primary and Secondary Efficacy Criteria
The primary efficacy end point was complete clearance rate, which was defined as the proportion of participants by the EOS visit with zero EGWs (that either existed at baseline and any warts developing during the study) in all anogenital anatomic areas. It is important to note that this primary efficacy end point was very conservative in that it included any new warts occurring during the study that may not have received a full treatment course. Lesions were counted in all assessed anatomic areas without distinction between those that were identified at baseline or those that were newly identified during the study period. If new EGWs appeared during the study in new anatomic areas, such lesions were treated with the study medication as they appeared. Therefore, any newly arising EGWs received less than the full course of treatment, as therapy was not extended beyond the 8-week study period. Participants were evaluated for the presence of any EGWs in all anatomic areas without distinction between lesions that were present at baseline and newly arising EGWs. Therefore, development of new EGWs during the study period could potentially lower clearance rates.
Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
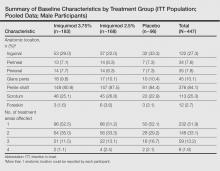
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
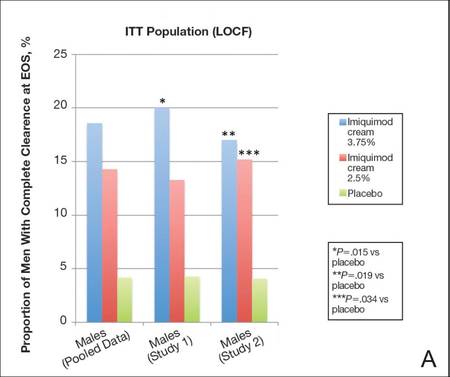
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).
|
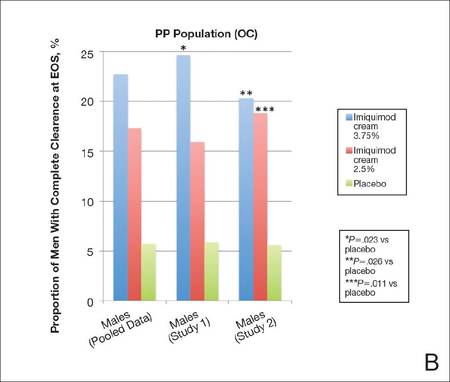
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).
The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.
Acknowledgments—The authors thank Christina Cognata Smith, PharmD, and Mandeep Kaur, MD (both previously of Valeant Pharmaceuticals North America, LLC, Bridgewater, New Jersey), as well as Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for assistance with the preparation of the manuscript. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis.
1. Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10.
2. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
3. Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3-8.
4. Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447-1454.
5. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161-166.
6. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10:113.
7. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychsom Obstet Gynaecol. 2010;31:16-23.
8. Lawrence S, Walzman M, Sheppard S, et al. The psychological impact caused by genital warts: has the Department of Health’s choice of vaccination missed the opportunity to prevent such morbidity? Int J STD AIDS. 2009;20:696-700.
9. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
10. Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; April 2007.
11. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458-476.
12. Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
13. Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2010.
14. Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of genital warts. Sex Transm Dis. 2011;38:216-218.
15. Beutner KR, Tyring SK, Trofatter KF Jr, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789-794.
16. Beutner KR, Spruance SL, Hougham AJ, et al. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230-239.
17. Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch Dermatol. 1998;134:25-30.
18. Fife KH, Ferenczy A, Douglas JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis. 2001;28:226-231.
19. Garland SM, Waddell R, Mindel A, et al. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17:448-452.
20. Schofer H, Van Ophoven A, Henke U, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642-648.
21. Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627-631.
22. Gollnick H, Barasso R, Jappe U, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22-28.
23. Trofatter KF Jr, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynecol Obstet. 2002;76:191-193.
24. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies [published online ahead of print August 24, 2011]. Infect Dis Obstet Gynecol. 2011;2011:806105.
25. Dinh TH, Sternberg M, Dunne EF, et al. Genital warts among 18- to 59-year-olds in the US, National Health and Nutrition Examination Survey, 1999-2004. Sex Transm Dis. 2008;35:357-360.
26. Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107-1122.
27. Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748-752.
28. Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114-120.
External genital warts (EGWs), which are caused by infection with select types of human papillomavirus (HPV), are one of the most prevalent and fastest growing sexually transmitted infections.1 External genital warts affect approximately 1% of sexually active adults in the United States and Europe, with another 15% having subclinical infections; more than 1 million new cases of EGWs are diagnosed annually.2-4 Although the condition is not life threatening, lesions can cause symptoms, such as burning, itching, bleeding, pain and dyspareunia, and potential urethral or rectal obstruction. External genital warts also have been associated with adverse psychological effects.5-8
The time between exposure to HPV and development of EGWs can vary from a few weeks to several months or years (median, 2.9 months).9 Many HPV infections are mild and transient, resolving spontaneously.10 As many as 30% of EGWs will regress over 4 months and approximately 90% clear within 2 years.11,12 However, even with treatment, the median time to resolution is 5.9 months.9
Imiquimod cream 5%, which has been successfully used to treat EGWs since it was approved by the US Food and Drug Administration in 1997, is applied to lesions 3 times weekly at bedtime until clearance is achieved or for a maximum of 16 weeks.13 In clinical studies, complete clearance has been reported in 35% to 75% of participants.14-21 However, it is important to note that not all anogenital regions with warts were required to be treated in these studies,14-21 and newly arising warts were not included in the analysis.17 Reported clearance rates were higher and median clearance time was shorter in women.17 Relatively low recurrence rates (6%–26%) have been reported after successful clearance of EGWs.16,17,20,21
Long treatment durations are always a concern for patient adherence. Although increasing the dosing frequency with imiquimod cream 5% might be considered an attractive option to reduce the length of the treatment course, it has resulted in greater incidence and severity of local adverse events (AEs) in some studies without improved efficacy.18,22,23 Thus lower concentrations of imiquimod (ie, 2.5% and 3.75% formulations) were developed to potentially decrease treatment duration and provide a daily dosing regimen.
We report the results of 2 identical, placebo-controlled, phase 3 studies evaluating the safety and efficacy of imiquimod cream 2.5% and 3.75% in treating EGWs in men. Pooled results from a female subgroup previously have been reported.24 Although the percentage of women who reported ever being diagnosed with EGWs was higher than in men (7.2% vs 4%) in one survey,25 other assessments have found a similar prevalence of EGWs among both genders.26-28 We provide important insights herein by reporting efficacy and tolerability data for imiquimod cream 2.5% and 3.75% in the treatment of EGWs in males.
Methods
Study Design
Male patients aged 12 years and older with 2 to 30 EGWs in the inguinal, perineal, and/or perianal areas as well as on the glans penis, penile shaft, scrotum, and/or foreskin were enrolled in 2 identical, multicenter, randomized, parallel-group, double-blind, placebo-controlled studies. Participants were randomized (2:2:1) to self-treatment with imiquimod cream 3.75% or 2.5% or placebo once daily until complete clearance was achieved or for a maximum of 8 weeks (end of treatment [EOT]). There was a follow-up period of up to 8 weeks (end of study [EOS]) in participants who did not achieve complete clearance by EOT. All participants who achieved complete clearance by EOS entered a 12-week observational follow-up period to assess recurrence.
Primary and Secondary Efficacy Criteria
The primary efficacy end point was complete clearance rate, which was defined as the proportion of participants by the EOS visit with zero EGWs (that either existed at baseline and any warts developing during the study) in all anogenital anatomic areas. It is important to note that this primary efficacy end point was very conservative in that it included any new warts occurring during the study that may not have received a full treatment course. Lesions were counted in all assessed anatomic areas without distinction between those that were identified at baseline or those that were newly identified during the study period. If new EGWs appeared during the study in new anatomic areas, such lesions were treated with the study medication as they appeared. Therefore, any newly arising EGWs received less than the full course of treatment, as therapy was not extended beyond the 8-week study period. Participants were evaluated for the presence of any EGWs in all anatomic areas without distinction between lesions that were present at baseline and newly arising EGWs. Therefore, development of new EGWs during the study period could potentially lower clearance rates.
Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).


|
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).
The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.
Acknowledgments—The authors thank Christina Cognata Smith, PharmD, and Mandeep Kaur, MD (both previously of Valeant Pharmaceuticals North America, LLC, Bridgewater, New Jersey), as well as Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for assistance with the preparation of the manuscript. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis.
External genital warts (EGWs), which are caused by infection with select types of human papillomavirus (HPV), are one of the most prevalent and fastest growing sexually transmitted infections.1 External genital warts affect approximately 1% of sexually active adults in the United States and Europe, with another 15% having subclinical infections; more than 1 million new cases of EGWs are diagnosed annually.2-4 Although the condition is not life threatening, lesions can cause symptoms, such as burning, itching, bleeding, pain and dyspareunia, and potential urethral or rectal obstruction. External genital warts also have been associated with adverse psychological effects.5-8
The time between exposure to HPV and development of EGWs can vary from a few weeks to several months or years (median, 2.9 months).9 Many HPV infections are mild and transient, resolving spontaneously.10 As many as 30% of EGWs will regress over 4 months and approximately 90% clear within 2 years.11,12 However, even with treatment, the median time to resolution is 5.9 months.9
Imiquimod cream 5%, which has been successfully used to treat EGWs since it was approved by the US Food and Drug Administration in 1997, is applied to lesions 3 times weekly at bedtime until clearance is achieved or for a maximum of 16 weeks.13 In clinical studies, complete clearance has been reported in 35% to 75% of participants.14-21 However, it is important to note that not all anogenital regions with warts were required to be treated in these studies,14-21 and newly arising warts were not included in the analysis.17 Reported clearance rates were higher and median clearance time was shorter in women.17 Relatively low recurrence rates (6%–26%) have been reported after successful clearance of EGWs.16,17,20,21
Long treatment durations are always a concern for patient adherence. Although increasing the dosing frequency with imiquimod cream 5% might be considered an attractive option to reduce the length of the treatment course, it has resulted in greater incidence and severity of local adverse events (AEs) in some studies without improved efficacy.18,22,23 Thus lower concentrations of imiquimod (ie, 2.5% and 3.75% formulations) were developed to potentially decrease treatment duration and provide a daily dosing regimen.
We report the results of 2 identical, placebo-controlled, phase 3 studies evaluating the safety and efficacy of imiquimod cream 2.5% and 3.75% in treating EGWs in men. Pooled results from a female subgroup previously have been reported.24 Although the percentage of women who reported ever being diagnosed with EGWs was higher than in men (7.2% vs 4%) in one survey,25 other assessments have found a similar prevalence of EGWs among both genders.26-28 We provide important insights herein by reporting efficacy and tolerability data for imiquimod cream 2.5% and 3.75% in the treatment of EGWs in males.
Methods
Study Design
Male patients aged 12 years and older with 2 to 30 EGWs in the inguinal, perineal, and/or perianal areas as well as on the glans penis, penile shaft, scrotum, and/or foreskin were enrolled in 2 identical, multicenter, randomized, parallel-group, double-blind, placebo-controlled studies. Participants were randomized (2:2:1) to self-treatment with imiquimod cream 3.75% or 2.5% or placebo once daily until complete clearance was achieved or for a maximum of 8 weeks (end of treatment [EOT]). There was a follow-up period of up to 8 weeks (end of study [EOS]) in participants who did not achieve complete clearance by EOT. All participants who achieved complete clearance by EOS entered a 12-week observational follow-up period to assess recurrence.
Primary and Secondary Efficacy Criteria
The primary efficacy end point was complete clearance rate, which was defined as the proportion of participants by the EOS visit with zero EGWs (that either existed at baseline and any warts developing during the study) in all anogenital anatomic areas. It is important to note that this primary efficacy end point was very conservative in that it included any new warts occurring during the study that may not have received a full treatment course. Lesions were counted in all assessed anatomic areas without distinction between those that were identified at baseline or those that were newly identified during the study period. If new EGWs appeared during the study in new anatomic areas, such lesions were treated with the study medication as they appeared. Therefore, any newly arising EGWs received less than the full course of treatment, as therapy was not extended beyond the 8-week study period. Participants were evaluated for the presence of any EGWs in all anatomic areas without distinction between lesions that were present at baseline and newly arising EGWs. Therefore, development of new EGWs during the study period could potentially lower clearance rates.
Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).


|
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).
The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.
Acknowledgments—The authors thank Christina Cognata Smith, PharmD, and Mandeep Kaur, MD (both previously of Valeant Pharmaceuticals North America, LLC, Bridgewater, New Jersey), as well as Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for assistance with the preparation of the manuscript. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis.
1. Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10.
2. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
3. Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3-8.
4. Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447-1454.
5. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161-166.
6. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10:113.
7. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychsom Obstet Gynaecol. 2010;31:16-23.
8. Lawrence S, Walzman M, Sheppard S, et al. The psychological impact caused by genital warts: has the Department of Health’s choice of vaccination missed the opportunity to prevent such morbidity? Int J STD AIDS. 2009;20:696-700.
9. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
10. Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; April 2007.
11. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458-476.
12. Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
13. Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2010.
14. Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of genital warts. Sex Transm Dis. 2011;38:216-218.
15. Beutner KR, Tyring SK, Trofatter KF Jr, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789-794.
16. Beutner KR, Spruance SL, Hougham AJ, et al. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230-239.
17. Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch Dermatol. 1998;134:25-30.
18. Fife KH, Ferenczy A, Douglas JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis. 2001;28:226-231.
19. Garland SM, Waddell R, Mindel A, et al. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17:448-452.
20. Schofer H, Van Ophoven A, Henke U, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642-648.
21. Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627-631.
22. Gollnick H, Barasso R, Jappe U, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22-28.
23. Trofatter KF Jr, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynecol Obstet. 2002;76:191-193.
24. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies [published online ahead of print August 24, 2011]. Infect Dis Obstet Gynecol. 2011;2011:806105.
25. Dinh TH, Sternberg M, Dunne EF, et al. Genital warts among 18- to 59-year-olds in the US, National Health and Nutrition Examination Survey, 1999-2004. Sex Transm Dis. 2008;35:357-360.
26. Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107-1122.
27. Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748-752.
28. Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114-120.
1. Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10.
2. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
3. Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3-8.
4. Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447-1454.
5. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161-166.
6. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10:113.
7. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychsom Obstet Gynaecol. 2010;31:16-23.
8. Lawrence S, Walzman M, Sheppard S, et al. The psychological impact caused by genital warts: has the Department of Health’s choice of vaccination missed the opportunity to prevent such morbidity? Int J STD AIDS. 2009;20:696-700.
9. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
10. Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; April 2007.
11. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458-476.
12. Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
13. Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2010.
14. Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of genital warts. Sex Transm Dis. 2011;38:216-218.
15. Beutner KR, Tyring SK, Trofatter KF Jr, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789-794.
16. Beutner KR, Spruance SL, Hougham AJ, et al. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230-239.
17. Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch Dermatol. 1998;134:25-30.
18. Fife KH, Ferenczy A, Douglas JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis. 2001;28:226-231.
19. Garland SM, Waddell R, Mindel A, et al. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17:448-452.
20. Schofer H, Van Ophoven A, Henke U, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642-648.
21. Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627-631.
22. Gollnick H, Barasso R, Jappe U, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22-28.
23. Trofatter KF Jr, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynecol Obstet. 2002;76:191-193.
24. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies [published online ahead of print August 24, 2011]. Infect Dis Obstet Gynecol. 2011;2011:806105.
25. Dinh TH, Sternberg M, Dunne EF, et al. Genital warts among 18- to 59-year-olds in the US, National Health and Nutrition Examination Survey, 1999-2004. Sex Transm Dis. 2008;35:357-360.
26. Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107-1122.
27. Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748-752.
28. Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114-120.
Practice Points
- Imiquimod cream, both 2.5% and 3.75% concentrations, is more effective than placebo in treating external genital warts (EGWs) in men.
- Imiquimod cream, in both concentrations tested, is somewhat less effective in men than in women in the same protocol.
- Imiquimod cream treatment of EGWs is better tolerated in men than in women in the same protocol.