User login
Given Time, the First Biologic Is Likely to Work
NEW YORK — Prescribing errors, such as premature withdrawal of a biologic agent once remission is achieved and hasty switching of agents, can undermine optimum results with biologics in the management of rheumatoid arthritis, according to Dr. Yusuf Yazici.
For instance, results from the BEST (Behandel Strategieen) trial (Ann. Rheum. Dis. 2009;68[suppl. 3]:544) showed that if patients who achieved remission with biologic therapy stopped that therapy, within 2 years 54% (62/115) stayed in drug-free remission, said Dr. Yazici, a rheumatologist who is director of the Seligman Center for Advanced Therapeutics and Behcet's Syndrome Evaluation, Treatment and Research Center at the New York University Hospital for Joint Diseases. The remaining patients saw their disease flare, but while about three-quarters of those (39/53 or 34% of the original group) were brought back into remission within 6 months, about one-quarter (14/53 or 12% of the original group of 115) did not achieve remission again. “That number is too large,” said Dr. Yazici.
“Just as we would not consider stopping treatment for diabetes or hypertension, this chronic disease treatment approach should also be considered in patients with RA,” said Dr. Yazici. He advised against tapering or stopping the combination of medications that was required to achieve remission unless there is a safety concern.
Another problem that Dr. Yazici has noticed is failure to allow enough time for one tumor necrosis factor–inhibiting (TNFi) biologic to take effect before switching to another biologic. Common reasons for switching cited are inefficacy or adverse events.
In a retrospective analysis of an insurance claims database of 9,075 patients with RA who started a TNFi agent during the period 2000-2005, Dr. Yazici saw more frequent changes among different TNFi agents and shorter duration of treatment before change, as time progressed. The use of a first-prescribed biologic medication dropped by about 45% after the first year and 70% after the second year; by 3 years, only a small percentage remained on the same therapy. In this study, infliximab had the highest duration of continuation, about 50% at 2 years. After adalimumab was introduced into the market, a dramatic drop in time to switch was observed, from a mean of 454 days to 237 days among TNFi agents (J. Rheumatol. 2009;36:907-13). “The more biologics we have, the faster we switch, it seems,” commented Dr. Yazici.
Dr. Yazici also cited data from the DANBIO registry, a nationwide Danish registry of patients with RA, in which 2,326 patients were observed after initiation of biologic therapy.
After 4 years, 56% were still taking etanercept, 52% were still on adalimumab, and 41% remained on infliximab. Drug withdrawal was primarily attributed to adverse effects and secondarily to lack of efficacy (Arthritis Rheum. 2010;62:22-32).
Published data on etanercept, adalimumab, infliximab, and abatacept suggest no real differences in efficacy in most patients who use them, said Dr. Yazici. Data from registries tend to show no preference for one over another. He suggested that physicians allow at least a 3- to 6-month trial period before switching biologic agents.
Dr. Yazici serves as a consultant to Bristol-Myers Squibb, Celgene, Genentech, Roche, and UCB.
NEW YORK — Prescribing errors, such as premature withdrawal of a biologic agent once remission is achieved and hasty switching of agents, can undermine optimum results with biologics in the management of rheumatoid arthritis, according to Dr. Yusuf Yazici.
For instance, results from the BEST (Behandel Strategieen) trial (Ann. Rheum. Dis. 2009;68[suppl. 3]:544) showed that if patients who achieved remission with biologic therapy stopped that therapy, within 2 years 54% (62/115) stayed in drug-free remission, said Dr. Yazici, a rheumatologist who is director of the Seligman Center for Advanced Therapeutics and Behcet's Syndrome Evaluation, Treatment and Research Center at the New York University Hospital for Joint Diseases. The remaining patients saw their disease flare, but while about three-quarters of those (39/53 or 34% of the original group) were brought back into remission within 6 months, about one-quarter (14/53 or 12% of the original group of 115) did not achieve remission again. “That number is too large,” said Dr. Yazici.
“Just as we would not consider stopping treatment for diabetes or hypertension, this chronic disease treatment approach should also be considered in patients with RA,” said Dr. Yazici. He advised against tapering or stopping the combination of medications that was required to achieve remission unless there is a safety concern.
Another problem that Dr. Yazici has noticed is failure to allow enough time for one tumor necrosis factor–inhibiting (TNFi) biologic to take effect before switching to another biologic. Common reasons for switching cited are inefficacy or adverse events.
In a retrospective analysis of an insurance claims database of 9,075 patients with RA who started a TNFi agent during the period 2000-2005, Dr. Yazici saw more frequent changes among different TNFi agents and shorter duration of treatment before change, as time progressed. The use of a first-prescribed biologic medication dropped by about 45% after the first year and 70% after the second year; by 3 years, only a small percentage remained on the same therapy. In this study, infliximab had the highest duration of continuation, about 50% at 2 years. After adalimumab was introduced into the market, a dramatic drop in time to switch was observed, from a mean of 454 days to 237 days among TNFi agents (J. Rheumatol. 2009;36:907-13). “The more biologics we have, the faster we switch, it seems,” commented Dr. Yazici.
Dr. Yazici also cited data from the DANBIO registry, a nationwide Danish registry of patients with RA, in which 2,326 patients were observed after initiation of biologic therapy.
After 4 years, 56% were still taking etanercept, 52% were still on adalimumab, and 41% remained on infliximab. Drug withdrawal was primarily attributed to adverse effects and secondarily to lack of efficacy (Arthritis Rheum. 2010;62:22-32).
Published data on etanercept, adalimumab, infliximab, and abatacept suggest no real differences in efficacy in most patients who use them, said Dr. Yazici. Data from registries tend to show no preference for one over another. He suggested that physicians allow at least a 3- to 6-month trial period before switching biologic agents.
Dr. Yazici serves as a consultant to Bristol-Myers Squibb, Celgene, Genentech, Roche, and UCB.
NEW YORK — Prescribing errors, such as premature withdrawal of a biologic agent once remission is achieved and hasty switching of agents, can undermine optimum results with biologics in the management of rheumatoid arthritis, according to Dr. Yusuf Yazici.
For instance, results from the BEST (Behandel Strategieen) trial (Ann. Rheum. Dis. 2009;68[suppl. 3]:544) showed that if patients who achieved remission with biologic therapy stopped that therapy, within 2 years 54% (62/115) stayed in drug-free remission, said Dr. Yazici, a rheumatologist who is director of the Seligman Center for Advanced Therapeutics and Behcet's Syndrome Evaluation, Treatment and Research Center at the New York University Hospital for Joint Diseases. The remaining patients saw their disease flare, but while about three-quarters of those (39/53 or 34% of the original group) were brought back into remission within 6 months, about one-quarter (14/53 or 12% of the original group of 115) did not achieve remission again. “That number is too large,” said Dr. Yazici.
“Just as we would not consider stopping treatment for diabetes or hypertension, this chronic disease treatment approach should also be considered in patients with RA,” said Dr. Yazici. He advised against tapering or stopping the combination of medications that was required to achieve remission unless there is a safety concern.
Another problem that Dr. Yazici has noticed is failure to allow enough time for one tumor necrosis factor–inhibiting (TNFi) biologic to take effect before switching to another biologic. Common reasons for switching cited are inefficacy or adverse events.
In a retrospective analysis of an insurance claims database of 9,075 patients with RA who started a TNFi agent during the period 2000-2005, Dr. Yazici saw more frequent changes among different TNFi agents and shorter duration of treatment before change, as time progressed. The use of a first-prescribed biologic medication dropped by about 45% after the first year and 70% after the second year; by 3 years, only a small percentage remained on the same therapy. In this study, infliximab had the highest duration of continuation, about 50% at 2 years. After adalimumab was introduced into the market, a dramatic drop in time to switch was observed, from a mean of 454 days to 237 days among TNFi agents (J. Rheumatol. 2009;36:907-13). “The more biologics we have, the faster we switch, it seems,” commented Dr. Yazici.
Dr. Yazici also cited data from the DANBIO registry, a nationwide Danish registry of patients with RA, in which 2,326 patients were observed after initiation of biologic therapy.
After 4 years, 56% were still taking etanercept, 52% were still on adalimumab, and 41% remained on infliximab. Drug withdrawal was primarily attributed to adverse effects and secondarily to lack of efficacy (Arthritis Rheum. 2010;62:22-32).
Published data on etanercept, adalimumab, infliximab, and abatacept suggest no real differences in efficacy in most patients who use them, said Dr. Yazici. Data from registries tend to show no preference for one over another. He suggested that physicians allow at least a 3- to 6-month trial period before switching biologic agents.
Dr. Yazici serves as a consultant to Bristol-Myers Squibb, Celgene, Genentech, Roche, and UCB.
Ten-Point Evidence-Based Guide To the Rheumatology Visit
NEW YORK — Despite clinical advances, most rheumatology patient encounters are conducted much as they were 40 years ago, according to Dr. Theodore Pincus, who spoke at both the New York University Hospital for Joint Diseases meeting on Evidence-Based RA Therapy and the Fifth Annual Clinical Research Methodology Course.
And the patient loses out as a result.
Laboratory tests that are usually performed are not necessarily diagnostic, as 30%-40% of patients with rheumatoid arthritis have normal values of many measures (erythrocyte-sedimentation rate, C-reactive protein, and presence of rheumatoid factor and/or anti–cyclic citrullinated peptide antibodies). In addition, radiography and formal joint counts have significant clinical limitations, said Dr Pincus.
There is underuse of patient self-assessment tools such as the HAQ (Health Assessment Questionnaire) or MDHAQ (Multidimensional Health Assessment Questionnaire), both of which predict work disability, costs, and death from RA more precisely than do radiographs or laboratory tests, he said.
“I believe the MDHAQ-RAPID3 [Routine Assessment of Patient Index Data 3] should be incorporated into your infrastructure of care,” said Dr. Pincus, a clinical professor of medicine at New York University.
He described a 10-point checklist for all visits with patients who have rheumatic disease that is based on evidence and that relies more upon patient self-assessment and physician global assessment than it does on findings from joint counts, laboratory tests, or radiography.
Dr. Pincus proposed that physicians follow the 10-measure checklist during every clinical encounter to document patient status and quantify patient progress. (See box.) The checklist includes six self-report measures from the MDHAQ self-report questionnaire, including evaluation of function, pain, fatigue, and other symptoms; a patient global estimate of status; and the RAPID3 score. The four physician global measures include assessment of inflammation, damage, and changes that are noninflammatory, as well as a physician global estimate of status.
The MDHAQ is a version of the HAQ, which was the only patient self-assessment tool actually developed in the clinic, said Dr. Pincus. The MDHAQ has been modified to reflect escalating standards of rheumatology care, so currently patients are asked if they can walk 2 miles or participate in recreational activities or sports. Queries about sleep, anxiety, and depression have also been added. In addition, the MDHAQ provides a review of systems and recent medical history information.
According to Dr. Pincus, the HAQ and MDHAQ are better predictors than are joint count, laboratory tests, or radiographs of functional status, work disability, joint replacement surgery, or cost.
Dr. Pincus reported having no relevant financial disclosures.
Visit Checklist
In the 10-point checklist, the patient MDHAQ self-report questionnaire measures include the following:
▸ Function.
▸ Pain.
▸ Patient global estimate of status.
▸ RAPID3.
▸ Fatigue.
The physician global measures include the following:
▸ Physician global estimate of status.
▸ Inflammation.
▸ Damage.
▸ Noninflammatory/nondamage.
Source: Dr. Pincus
NEW YORK — Despite clinical advances, most rheumatology patient encounters are conducted much as they were 40 years ago, according to Dr. Theodore Pincus, who spoke at both the New York University Hospital for Joint Diseases meeting on Evidence-Based RA Therapy and the Fifth Annual Clinical Research Methodology Course.
And the patient loses out as a result.
Laboratory tests that are usually performed are not necessarily diagnostic, as 30%-40% of patients with rheumatoid arthritis have normal values of many measures (erythrocyte-sedimentation rate, C-reactive protein, and presence of rheumatoid factor and/or anti–cyclic citrullinated peptide antibodies). In addition, radiography and formal joint counts have significant clinical limitations, said Dr Pincus.
There is underuse of patient self-assessment tools such as the HAQ (Health Assessment Questionnaire) or MDHAQ (Multidimensional Health Assessment Questionnaire), both of which predict work disability, costs, and death from RA more precisely than do radiographs or laboratory tests, he said.
“I believe the MDHAQ-RAPID3 [Routine Assessment of Patient Index Data 3] should be incorporated into your infrastructure of care,” said Dr. Pincus, a clinical professor of medicine at New York University.
He described a 10-point checklist for all visits with patients who have rheumatic disease that is based on evidence and that relies more upon patient self-assessment and physician global assessment than it does on findings from joint counts, laboratory tests, or radiography.
Dr. Pincus proposed that physicians follow the 10-measure checklist during every clinical encounter to document patient status and quantify patient progress. (See box.) The checklist includes six self-report measures from the MDHAQ self-report questionnaire, including evaluation of function, pain, fatigue, and other symptoms; a patient global estimate of status; and the RAPID3 score. The four physician global measures include assessment of inflammation, damage, and changes that are noninflammatory, as well as a physician global estimate of status.
The MDHAQ is a version of the HAQ, which was the only patient self-assessment tool actually developed in the clinic, said Dr. Pincus. The MDHAQ has been modified to reflect escalating standards of rheumatology care, so currently patients are asked if they can walk 2 miles or participate in recreational activities or sports. Queries about sleep, anxiety, and depression have also been added. In addition, the MDHAQ provides a review of systems and recent medical history information.
According to Dr. Pincus, the HAQ and MDHAQ are better predictors than are joint count, laboratory tests, or radiographs of functional status, work disability, joint replacement surgery, or cost.
Dr. Pincus reported having no relevant financial disclosures.
Visit Checklist
In the 10-point checklist, the patient MDHAQ self-report questionnaire measures include the following:
▸ Function.
▸ Pain.
▸ Patient global estimate of status.
▸ RAPID3.
▸ Fatigue.
The physician global measures include the following:
▸ Physician global estimate of status.
▸ Inflammation.
▸ Damage.
▸ Noninflammatory/nondamage.
Source: Dr. Pincus
NEW YORK — Despite clinical advances, most rheumatology patient encounters are conducted much as they were 40 years ago, according to Dr. Theodore Pincus, who spoke at both the New York University Hospital for Joint Diseases meeting on Evidence-Based RA Therapy and the Fifth Annual Clinical Research Methodology Course.
And the patient loses out as a result.
Laboratory tests that are usually performed are not necessarily diagnostic, as 30%-40% of patients with rheumatoid arthritis have normal values of many measures (erythrocyte-sedimentation rate, C-reactive protein, and presence of rheumatoid factor and/or anti–cyclic citrullinated peptide antibodies). In addition, radiography and formal joint counts have significant clinical limitations, said Dr Pincus.
There is underuse of patient self-assessment tools such as the HAQ (Health Assessment Questionnaire) or MDHAQ (Multidimensional Health Assessment Questionnaire), both of which predict work disability, costs, and death from RA more precisely than do radiographs or laboratory tests, he said.
“I believe the MDHAQ-RAPID3 [Routine Assessment of Patient Index Data 3] should be incorporated into your infrastructure of care,” said Dr. Pincus, a clinical professor of medicine at New York University.
He described a 10-point checklist for all visits with patients who have rheumatic disease that is based on evidence and that relies more upon patient self-assessment and physician global assessment than it does on findings from joint counts, laboratory tests, or radiography.
Dr. Pincus proposed that physicians follow the 10-measure checklist during every clinical encounter to document patient status and quantify patient progress. (See box.) The checklist includes six self-report measures from the MDHAQ self-report questionnaire, including evaluation of function, pain, fatigue, and other symptoms; a patient global estimate of status; and the RAPID3 score. The four physician global measures include assessment of inflammation, damage, and changes that are noninflammatory, as well as a physician global estimate of status.
The MDHAQ is a version of the HAQ, which was the only patient self-assessment tool actually developed in the clinic, said Dr. Pincus. The MDHAQ has been modified to reflect escalating standards of rheumatology care, so currently patients are asked if they can walk 2 miles or participate in recreational activities or sports. Queries about sleep, anxiety, and depression have also been added. In addition, the MDHAQ provides a review of systems and recent medical history information.
According to Dr. Pincus, the HAQ and MDHAQ are better predictors than are joint count, laboratory tests, or radiographs of functional status, work disability, joint replacement surgery, or cost.
Dr. Pincus reported having no relevant financial disclosures.
Visit Checklist
In the 10-point checklist, the patient MDHAQ self-report questionnaire measures include the following:
▸ Function.
▸ Pain.
▸ Patient global estimate of status.
▸ RAPID3.
▸ Fatigue.
The physician global measures include the following:
▸ Physician global estimate of status.
▸ Inflammation.
▸ Damage.
▸ Noninflammatory/nondamage.
Source: Dr. Pincus
New Methods, Funding Propel Neurogenetics
Within the last 2 years, new DNA sequencing technologies, dubbed NextGen (“next generation”), have been introduced that are revolutionizing the field of neurogenetics. As these advances gain recognition within and outside the medical community, the pace of progress is quickening, with the help of support from broad-based advocacy groups and more funding options. These technologies are already bringing greater diagnostic opportunities to practice, although the time might not yet be ripe for translating the results into information useful for the clinical management of patients.
“For rare diseases, this is a real game-changer type of technology,” commented Matthew J. Huentelman, Ph.D., a neurogeneticist affiliated with the Translational Genomics Research Institute in Phoenix. “We have had some significant leaps in what we can do with the human genome over the past couple of years, especially in the mechanics and chemistry of how sequencing is done. Several companies have built machines that sequence DNA in fundamentally different ways than we did 5–10 years ago.” Since 2007, Dr. Huentelman has been applying NextGen technology to the study of Alzheimer's disease and autism.
Whole exome DNA sequencing is one of the NextGen technologies that are being applied to neurologic disorders. Unlike whole genome sequencing, whole exome DNA sequencing focuses on the small portion of the genome (less than 2%) that contains protein-coding bits of DNA, known as the exome. For example, Dr. Murat Gunel, chief of the Neurovascular Surgery Program and codirector of the Program on Neurogenetics at Yale University, New Haven, Conn., recently used whole exome sequencing to determine that several distinct types of malformations of cortical development, including microcephaly, pachygyria with cortical thickening, and hypoplasia of the corpus callosum, were all associated with recessive mutations in a single gene, WDR62 (Nature 2010;467:207–10). This discovery was made by analyzing the DNA of several children with microcephaly among 30 interrelated Turkish families.
The last few months have brought other reports that used whole exome sequencing to identify causative gene mutations for Perrault syndrome (Am. J. Hum. Genet. 2010;87:282–8), Joubert's syndrome (Am. J. Hum. Genet. 2010;86:93–7), and Fowler syndrome (Human Mutation 2010;31:918–23).
One advantage of exome sequencing studies is that researchers can use smaller sample sets – with an “n” as small as 1 – in a comprehensive fashion, Dr. Huentelman said. “This is great for rare diseases or to gain a foothold in a disease that has been hard to understand at the population level. For instance, we can analyze the differences between twins discordant for autism.”
Advantages of exome sequencing include its speed and cost-effectiveness. Dr. Gunel reports that in his laboratory, whole genome sequencing might take several weeks and cost about $50,000 while he can receive the results of whole exome sequencing within 9 days, at a cost of about $3,500.
“These tests are still pricey,” said Dr. Andrea Gropman, a neurogeneticist at the Children's National Medical Center and George Washington University, both in Washington. For sequencing genes related to autism, she said, costs might range from $1,000 to $5,800, which insurance companies might or might not cover. “For some families, that means they must pay out of pocket.”
New Funding Opportunities
Advances in the field might be attributable, in part, to greater funding opportunities. Dr. Gunel's research was supported in part by a $2.9 million “stimulus grant” from the National Institute of Neurological Disorders and Stroke, part of the $8.2 billion provided to the National Institutes of Health from the American Recovery and Reinvestment Act. He plans to use his stimulus money to extend whole exome sequencing to hundreds of other families affected by malformations of cortical development.
Another significant source of funds has been distributed through the NIH's Office of Rare Diseases Research (ORDR), which allocated almost $70 million in 2003 toward the creation of 10 clinical consortia and one Data and Technology Coordinating Center.
“In the late 1990s and early 2000, we felt we didn't have a good infrastructure in place to do research in rare diseases – of which we estimated there were at least 6,800,” said Steve C. Groft, Pharm.D., director of the ORDR. “We hoped the funds we provided could go toward establishing that infrastructure.”
Recently, a second round of funding of approximately $125 million was awarded to 19 consortia, including 6 of the 10 original groups. Nine of the consortia are related to neurologic disease, including those focusing on Angelman, Rett, and Prader-Willi syndromes; autonomic rare diseases; neurologic channelopathies; spinocerebellar ataxias; dystonias; lysosomal diseases; inherited neuropathies; brain vascular malformations; and mitochondrial diseases. Consortia have grown as they have become successful in establishing partners from other branches of NIH and industry, Dr. Groft said.
One of the most unique aspects of the Rare Disease Clinical Research Grants is the mandate to collaborate with a patient advocacy group. “We have noticed that, in order to have a successful research program for rare diseases, we need the involvement of patient advocacy groups. These groups are essential to help with patient recruitment and to act as liaisons between researchers and the group's constituency of patients and their families,” Dr. Groft said.
In fact, a Coalition of Patient Advocacy Groups has been formed as an arm of the Rare Disease Clinical Research Network. “The role of advocacy groups has really matured over the last several years,” Dr. Gropman said. “I work closely with an amazing advocacy group in the Urea Cycle Disorders Consortium. They have gone from a grassroots support of families to major partners in research.”
Dr. Gropman credits the group with raising important clinical questions that only those living with affected individuals might be aware of, educating patients and their families about the importance of participation in clinical trials, as well providing financial support and finding philanthropic partners. “Patient advocates have a vested interest in moving the clinical research process along,” she said.
Another new initiative aimed at facilitating funding for rare diseases is the R.A.R.E. (Rare Disease Advocacy, Research, and Education) Project's Global Genes Fund. The organization's Web site, which is in development, aims to serve as a clearinghouse for rare disease philanthropy.
“As long as the rare disease community works in their separate disease silos, we can only get so far. … If we can bring the community together and not focus on an individual disease, we can create a unifying campaign and rare diseases then becomes a huge public health issue, larger than the U.S. AIDS community and equal to that of breast cancer,” said Nicole Boice, founder and president of R.A.R.E.
One part of the Global Genes Fund, which is set to launch in the second quarter of 2011, will showcase innovative scientific research projects that individuals or businesses can contribute to, in donations as small as $25. Initially, Ms. Boice expects to focus on 10–20 research projects. “As the platform grows and is successful at securing funding, this will catalyze this type of innovative funding.”
Using Neurogenetics in Practice
Genetic testing can be helpful for diagnosing some neurologic disorders, but a “bewildering” maze of tests is available, Dr. Gropman said. Vendors have put together predetermined panels for whole exome sequencing of some disorders, such as spinal cerebellar ataxia or disorders associated with mental disabilities.
“Using a panel, you can test for a number of different genes simultaneously, but you cannot separate them,” she said.
Also coming to market are “designer” panels that allow clinicians to pick and choose which genes to target, but they require knowing a priori which mutation to focus on.
“While the technology has exploded, knowing which test to order can be baffling for many clinicians who do not have extensive backgrounds in genetics. If we find a variant but don't know its significance, it opens up a diagnostic conundrum,” Dr. Gropman said.
The field is still in flux. So many other questions must be resolved, such as how to deal with difficult or unanticipated results and whether technology and interpretation need to be standardized.
Genotyping is not just a black-and-white issue, according to Dr. Huentelman. In addition to the knowledge of a patient's mutation status, genotyping results must also take into consideration the risk of developing clinical symptoms, and the ethical and pragmatic issues that knowledge raises for patients and physicians.
As a first step, neurologists should begin educating themselves about the new technologies, potential applications, and shortcomings, by attending sessions on neurogenetics at annual professional meetings or more specialized conferences.
Creating a professional relationship with a geneticist or genetic counselor might provide access to the most up-to-date information and options.
“You need someone fully entrenched in the field of human genetics,” said Dr. Huentelman, who advocates creating a genetics team to sort through the maze of data that can be generated by a genetic analysis.
None of the sources contacted for this article had any relevant financial disclosures.
DNA sequencing 'is great for rare diseases or to gain a foothold in a disease that has been hard to understand….'
Source DR. HUENTELMAN
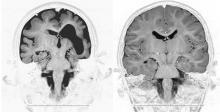
Brain MRI scans of a child with microcephaly, polymicrogyria, and schizencephaly (left) and brain scans of a healthy child (right).
Source Courtesy Dr. Murat Gunel
Within the last 2 years, new DNA sequencing technologies, dubbed NextGen (“next generation”), have been introduced that are revolutionizing the field of neurogenetics. As these advances gain recognition within and outside the medical community, the pace of progress is quickening, with the help of support from broad-based advocacy groups and more funding options. These technologies are already bringing greater diagnostic opportunities to practice, although the time might not yet be ripe for translating the results into information useful for the clinical management of patients.
“For rare diseases, this is a real game-changer type of technology,” commented Matthew J. Huentelman, Ph.D., a neurogeneticist affiliated with the Translational Genomics Research Institute in Phoenix. “We have had some significant leaps in what we can do with the human genome over the past couple of years, especially in the mechanics and chemistry of how sequencing is done. Several companies have built machines that sequence DNA in fundamentally different ways than we did 5–10 years ago.” Since 2007, Dr. Huentelman has been applying NextGen technology to the study of Alzheimer's disease and autism.
Whole exome DNA sequencing is one of the NextGen technologies that are being applied to neurologic disorders. Unlike whole genome sequencing, whole exome DNA sequencing focuses on the small portion of the genome (less than 2%) that contains protein-coding bits of DNA, known as the exome. For example, Dr. Murat Gunel, chief of the Neurovascular Surgery Program and codirector of the Program on Neurogenetics at Yale University, New Haven, Conn., recently used whole exome sequencing to determine that several distinct types of malformations of cortical development, including microcephaly, pachygyria with cortical thickening, and hypoplasia of the corpus callosum, were all associated with recessive mutations in a single gene, WDR62 (Nature 2010;467:207–10). This discovery was made by analyzing the DNA of several children with microcephaly among 30 interrelated Turkish families.
The last few months have brought other reports that used whole exome sequencing to identify causative gene mutations for Perrault syndrome (Am. J. Hum. Genet. 2010;87:282–8), Joubert's syndrome (Am. J. Hum. Genet. 2010;86:93–7), and Fowler syndrome (Human Mutation 2010;31:918–23).
One advantage of exome sequencing studies is that researchers can use smaller sample sets – with an “n” as small as 1 – in a comprehensive fashion, Dr. Huentelman said. “This is great for rare diseases or to gain a foothold in a disease that has been hard to understand at the population level. For instance, we can analyze the differences between twins discordant for autism.”
Advantages of exome sequencing include its speed and cost-effectiveness. Dr. Gunel reports that in his laboratory, whole genome sequencing might take several weeks and cost about $50,000 while he can receive the results of whole exome sequencing within 9 days, at a cost of about $3,500.
“These tests are still pricey,” said Dr. Andrea Gropman, a neurogeneticist at the Children's National Medical Center and George Washington University, both in Washington. For sequencing genes related to autism, she said, costs might range from $1,000 to $5,800, which insurance companies might or might not cover. “For some families, that means they must pay out of pocket.”
New Funding Opportunities
Advances in the field might be attributable, in part, to greater funding opportunities. Dr. Gunel's research was supported in part by a $2.9 million “stimulus grant” from the National Institute of Neurological Disorders and Stroke, part of the $8.2 billion provided to the National Institutes of Health from the American Recovery and Reinvestment Act. He plans to use his stimulus money to extend whole exome sequencing to hundreds of other families affected by malformations of cortical development.
Another significant source of funds has been distributed through the NIH's Office of Rare Diseases Research (ORDR), which allocated almost $70 million in 2003 toward the creation of 10 clinical consortia and one Data and Technology Coordinating Center.
“In the late 1990s and early 2000, we felt we didn't have a good infrastructure in place to do research in rare diseases – of which we estimated there were at least 6,800,” said Steve C. Groft, Pharm.D., director of the ORDR. “We hoped the funds we provided could go toward establishing that infrastructure.”
Recently, a second round of funding of approximately $125 million was awarded to 19 consortia, including 6 of the 10 original groups. Nine of the consortia are related to neurologic disease, including those focusing on Angelman, Rett, and Prader-Willi syndromes; autonomic rare diseases; neurologic channelopathies; spinocerebellar ataxias; dystonias; lysosomal diseases; inherited neuropathies; brain vascular malformations; and mitochondrial diseases. Consortia have grown as they have become successful in establishing partners from other branches of NIH and industry, Dr. Groft said.
One of the most unique aspects of the Rare Disease Clinical Research Grants is the mandate to collaborate with a patient advocacy group. “We have noticed that, in order to have a successful research program for rare diseases, we need the involvement of patient advocacy groups. These groups are essential to help with patient recruitment and to act as liaisons between researchers and the group's constituency of patients and their families,” Dr. Groft said.
In fact, a Coalition of Patient Advocacy Groups has been formed as an arm of the Rare Disease Clinical Research Network. “The role of advocacy groups has really matured over the last several years,” Dr. Gropman said. “I work closely with an amazing advocacy group in the Urea Cycle Disorders Consortium. They have gone from a grassroots support of families to major partners in research.”
Dr. Gropman credits the group with raising important clinical questions that only those living with affected individuals might be aware of, educating patients and their families about the importance of participation in clinical trials, as well providing financial support and finding philanthropic partners. “Patient advocates have a vested interest in moving the clinical research process along,” she said.
Another new initiative aimed at facilitating funding for rare diseases is the R.A.R.E. (Rare Disease Advocacy, Research, and Education) Project's Global Genes Fund. The organization's Web site, which is in development, aims to serve as a clearinghouse for rare disease philanthropy.
“As long as the rare disease community works in their separate disease silos, we can only get so far. … If we can bring the community together and not focus on an individual disease, we can create a unifying campaign and rare diseases then becomes a huge public health issue, larger than the U.S. AIDS community and equal to that of breast cancer,” said Nicole Boice, founder and president of R.A.R.E.
One part of the Global Genes Fund, which is set to launch in the second quarter of 2011, will showcase innovative scientific research projects that individuals or businesses can contribute to, in donations as small as $25. Initially, Ms. Boice expects to focus on 10–20 research projects. “As the platform grows and is successful at securing funding, this will catalyze this type of innovative funding.”
Using Neurogenetics in Practice
Genetic testing can be helpful for diagnosing some neurologic disorders, but a “bewildering” maze of tests is available, Dr. Gropman said. Vendors have put together predetermined panels for whole exome sequencing of some disorders, such as spinal cerebellar ataxia or disorders associated with mental disabilities.
“Using a panel, you can test for a number of different genes simultaneously, but you cannot separate them,” she said.
Also coming to market are “designer” panels that allow clinicians to pick and choose which genes to target, but they require knowing a priori which mutation to focus on.
“While the technology has exploded, knowing which test to order can be baffling for many clinicians who do not have extensive backgrounds in genetics. If we find a variant but don't know its significance, it opens up a diagnostic conundrum,” Dr. Gropman said.
The field is still in flux. So many other questions must be resolved, such as how to deal with difficult or unanticipated results and whether technology and interpretation need to be standardized.
Genotyping is not just a black-and-white issue, according to Dr. Huentelman. In addition to the knowledge of a patient's mutation status, genotyping results must also take into consideration the risk of developing clinical symptoms, and the ethical and pragmatic issues that knowledge raises for patients and physicians.
As a first step, neurologists should begin educating themselves about the new technologies, potential applications, and shortcomings, by attending sessions on neurogenetics at annual professional meetings or more specialized conferences.
Creating a professional relationship with a geneticist or genetic counselor might provide access to the most up-to-date information and options.
“You need someone fully entrenched in the field of human genetics,” said Dr. Huentelman, who advocates creating a genetics team to sort through the maze of data that can be generated by a genetic analysis.
None of the sources contacted for this article had any relevant financial disclosures.
DNA sequencing 'is great for rare diseases or to gain a foothold in a disease that has been hard to understand….'
Source DR. HUENTELMAN
Brain MRI scans of a child with microcephaly, polymicrogyria, and schizencephaly (left) and brain scans of a healthy child (right).
Source Courtesy Dr. Murat Gunel
Within the last 2 years, new DNA sequencing technologies, dubbed NextGen (“next generation”), have been introduced that are revolutionizing the field of neurogenetics. As these advances gain recognition within and outside the medical community, the pace of progress is quickening, with the help of support from broad-based advocacy groups and more funding options. These technologies are already bringing greater diagnostic opportunities to practice, although the time might not yet be ripe for translating the results into information useful for the clinical management of patients.
“For rare diseases, this is a real game-changer type of technology,” commented Matthew J. Huentelman, Ph.D., a neurogeneticist affiliated with the Translational Genomics Research Institute in Phoenix. “We have had some significant leaps in what we can do with the human genome over the past couple of years, especially in the mechanics and chemistry of how sequencing is done. Several companies have built machines that sequence DNA in fundamentally different ways than we did 5–10 years ago.” Since 2007, Dr. Huentelman has been applying NextGen technology to the study of Alzheimer's disease and autism.
Whole exome DNA sequencing is one of the NextGen technologies that are being applied to neurologic disorders. Unlike whole genome sequencing, whole exome DNA sequencing focuses on the small portion of the genome (less than 2%) that contains protein-coding bits of DNA, known as the exome. For example, Dr. Murat Gunel, chief of the Neurovascular Surgery Program and codirector of the Program on Neurogenetics at Yale University, New Haven, Conn., recently used whole exome sequencing to determine that several distinct types of malformations of cortical development, including microcephaly, pachygyria with cortical thickening, and hypoplasia of the corpus callosum, were all associated with recessive mutations in a single gene, WDR62 (Nature 2010;467:207–10). This discovery was made by analyzing the DNA of several children with microcephaly among 30 interrelated Turkish families.
The last few months have brought other reports that used whole exome sequencing to identify causative gene mutations for Perrault syndrome (Am. J. Hum. Genet. 2010;87:282–8), Joubert's syndrome (Am. J. Hum. Genet. 2010;86:93–7), and Fowler syndrome (Human Mutation 2010;31:918–23).
One advantage of exome sequencing studies is that researchers can use smaller sample sets – with an “n” as small as 1 – in a comprehensive fashion, Dr. Huentelman said. “This is great for rare diseases or to gain a foothold in a disease that has been hard to understand at the population level. For instance, we can analyze the differences between twins discordant for autism.”
Advantages of exome sequencing include its speed and cost-effectiveness. Dr. Gunel reports that in his laboratory, whole genome sequencing might take several weeks and cost about $50,000 while he can receive the results of whole exome sequencing within 9 days, at a cost of about $3,500.
“These tests are still pricey,” said Dr. Andrea Gropman, a neurogeneticist at the Children's National Medical Center and George Washington University, both in Washington. For sequencing genes related to autism, she said, costs might range from $1,000 to $5,800, which insurance companies might or might not cover. “For some families, that means they must pay out of pocket.”
New Funding Opportunities
Advances in the field might be attributable, in part, to greater funding opportunities. Dr. Gunel's research was supported in part by a $2.9 million “stimulus grant” from the National Institute of Neurological Disorders and Stroke, part of the $8.2 billion provided to the National Institutes of Health from the American Recovery and Reinvestment Act. He plans to use his stimulus money to extend whole exome sequencing to hundreds of other families affected by malformations of cortical development.
Another significant source of funds has been distributed through the NIH's Office of Rare Diseases Research (ORDR), which allocated almost $70 million in 2003 toward the creation of 10 clinical consortia and one Data and Technology Coordinating Center.
“In the late 1990s and early 2000, we felt we didn't have a good infrastructure in place to do research in rare diseases – of which we estimated there were at least 6,800,” said Steve C. Groft, Pharm.D., director of the ORDR. “We hoped the funds we provided could go toward establishing that infrastructure.”
Recently, a second round of funding of approximately $125 million was awarded to 19 consortia, including 6 of the 10 original groups. Nine of the consortia are related to neurologic disease, including those focusing on Angelman, Rett, and Prader-Willi syndromes; autonomic rare diseases; neurologic channelopathies; spinocerebellar ataxias; dystonias; lysosomal diseases; inherited neuropathies; brain vascular malformations; and mitochondrial diseases. Consortia have grown as they have become successful in establishing partners from other branches of NIH and industry, Dr. Groft said.
One of the most unique aspects of the Rare Disease Clinical Research Grants is the mandate to collaborate with a patient advocacy group. “We have noticed that, in order to have a successful research program for rare diseases, we need the involvement of patient advocacy groups. These groups are essential to help with patient recruitment and to act as liaisons between researchers and the group's constituency of patients and their families,” Dr. Groft said.
In fact, a Coalition of Patient Advocacy Groups has been formed as an arm of the Rare Disease Clinical Research Network. “The role of advocacy groups has really matured over the last several years,” Dr. Gropman said. “I work closely with an amazing advocacy group in the Urea Cycle Disorders Consortium. They have gone from a grassroots support of families to major partners in research.”
Dr. Gropman credits the group with raising important clinical questions that only those living with affected individuals might be aware of, educating patients and their families about the importance of participation in clinical trials, as well providing financial support and finding philanthropic partners. “Patient advocates have a vested interest in moving the clinical research process along,” she said.
Another new initiative aimed at facilitating funding for rare diseases is the R.A.R.E. (Rare Disease Advocacy, Research, and Education) Project's Global Genes Fund. The organization's Web site, which is in development, aims to serve as a clearinghouse for rare disease philanthropy.
“As long as the rare disease community works in their separate disease silos, we can only get so far. … If we can bring the community together and not focus on an individual disease, we can create a unifying campaign and rare diseases then becomes a huge public health issue, larger than the U.S. AIDS community and equal to that of breast cancer,” said Nicole Boice, founder and president of R.A.R.E.
One part of the Global Genes Fund, which is set to launch in the second quarter of 2011, will showcase innovative scientific research projects that individuals or businesses can contribute to, in donations as small as $25. Initially, Ms. Boice expects to focus on 10–20 research projects. “As the platform grows and is successful at securing funding, this will catalyze this type of innovative funding.”
Using Neurogenetics in Practice
Genetic testing can be helpful for diagnosing some neurologic disorders, but a “bewildering” maze of tests is available, Dr. Gropman said. Vendors have put together predetermined panels for whole exome sequencing of some disorders, such as spinal cerebellar ataxia or disorders associated with mental disabilities.
“Using a panel, you can test for a number of different genes simultaneously, but you cannot separate them,” she said.
Also coming to market are “designer” panels that allow clinicians to pick and choose which genes to target, but they require knowing a priori which mutation to focus on.
“While the technology has exploded, knowing which test to order can be baffling for many clinicians who do not have extensive backgrounds in genetics. If we find a variant but don't know its significance, it opens up a diagnostic conundrum,” Dr. Gropman said.
The field is still in flux. So many other questions must be resolved, such as how to deal with difficult or unanticipated results and whether technology and interpretation need to be standardized.
Genotyping is not just a black-and-white issue, according to Dr. Huentelman. In addition to the knowledge of a patient's mutation status, genotyping results must also take into consideration the risk of developing clinical symptoms, and the ethical and pragmatic issues that knowledge raises for patients and physicians.
As a first step, neurologists should begin educating themselves about the new technologies, potential applications, and shortcomings, by attending sessions on neurogenetics at annual professional meetings or more specialized conferences.
Creating a professional relationship with a geneticist or genetic counselor might provide access to the most up-to-date information and options.
“You need someone fully entrenched in the field of human genetics,” said Dr. Huentelman, who advocates creating a genetics team to sort through the maze of data that can be generated by a genetic analysis.
None of the sources contacted for this article had any relevant financial disclosures.
DNA sequencing 'is great for rare diseases or to gain a foothold in a disease that has been hard to understand….'
Source DR. HUENTELMAN
Brain MRI scans of a child with microcephaly, polymicrogyria, and schizencephaly (left) and brain scans of a healthy child (right).
Source Courtesy Dr. Murat Gunel
New Methods, Funding Propel Neurogenetics Closer to Practice
Within the last 2 years, new DNA sequencing technologies, dubbed NextGen (“next generation”), have been introduced that are revolutionizing the field of neurogenetics. As these advances gain recognition within and outside the medical community, the pace of progress is quickening, with the help of support from broad-based advocacy groups and more funding options. These technologies are already bringing greater diagnostic opportunities to practice, although the time may not yet be ripe for translating the results into information useful for the clinical management of patients.
“For rare diseases, this is a real game-changer type of technology,” commented Matthew J. Huentelman, Ph.D., a neurogeneticist affiliated with the Translational Genomics Research Institute in Phoenix. “We have had some significant leaps in what we can do with the human genome over the past couple of years, especially in the mechanics and chemistry of how sequencing is done. Several companies have built machines that sequence DNA in fundamentally different ways than we did 5-10 years ago.” Since 2007, Dr. Huentelman has been applying NextGen technology to the study of Alzheimer’s disease and autism.
Whole exome DNA sequencing is one of the NextGen technologies that are being applied to neurologic disorders. Unlike whole genome sequencing, whole exome DNA sequencing focuses on the small portion of the genome (less than 2%) that contains protein-coding bits of DNA, known as the exome. For example, Dr. Murat Gunel, chief of the Neurovascular Surgery Program and codirector of the Program on Neurogenetics at Yale University, New Haven, Conn., recently used whole exome sequencing to determine that several distinct types of malformations of cortical development, including microcephaly, pachygyria with cortical thickening, and hypoplasia of the corpus callosum were all associated with recessive mutations in a single gene, WDR62 (Nature 2010;467:207-10). This discovery was made by analyzing the DNA of several children with microcephaly among 30 interrelated Turkish families.
The last few months have brought other reports that used whole exome sequencing to identify causative gene mutations for Perrault syndrome (Am. J. Hum. Genet. 2010;87:282-8), Joubert’s syndrome (Am. J. Hum. Genet. 2010;86:93-7), and Fowler syndrome (Human Mutation 2010;31:918-23).
One advantage of exome sequencing studies is that researchers can use smaller sample sets – with an “n” as small as 1 – in a comprehensive fashion, Dr. Huentelman said. “This is great for rare diseases or to gain a foothold in a disease that has been hard to understand at the population level. For instance, we can analyze the differences between twins discordant for autism.”
Advantages of exome sequencing include its speed and cost effectiveness. Dr. Gunel reports that in his laboratory, whole genome sequencing might take several weeks and cost about $50,000 while he can receive the results of whole exome sequencing within 9 days, at a cost of about $3,500.
“These tests are still pricey,” said Dr. Andrea Gropman, a neurogeneticist at the Children’s National Medical Center and George Washington University, both in Washington. For sequencing genes related to autism, she said that costs may range from $1,000 to $5,800, which insurance companies may or may not cover. “For some families, that means they must pay out of pocket.”
New Funding Opportunities
Advances in the field may be due, in part, to greater funding opportunities. Dr. Gunel’s research was supported in part by a $2.9 million “stimulus grant” from the National Institute of Neurological Disorders and Stroke, part of the $8.2 billion provided to the National Institutes of Health from the American Recovery and Reinvestment Act. He plans to use his stimulus money to extend whole exome sequencing to hundreds of other families affected by malformations of cortical development.
Another significant source of funds has been distributed through the NIH’s Office of Rare Diseases Research (ORDR), which allocated almost $70 million in 2003 toward the creation of 10 clinical consortia and one Data and Technology Coordinating Center.
“In the late 1990s and early 2000, we felt we didn’t have a good infrastructure in place to do research in rare diseases – of which we estimated there were at least 6,800,” said Steve C. Groft, Pharm.D., director of the ORDR. “We hoped the funds we provided could go toward establishing that infrastructure.”
Recently, a second round of funding of approximately $125 million was awarded to 19 consortia, including 6 of the 10 original groups. Nine of the consortia are related to neurologic disease, including those focusing on Angelman, Rett, and Prader-Willi syndromes; autonomic rare diseases; neurologic channelopathies; spinocerebellar ataxias; dystonias; lysosomal diseases; inherited neuropathies; brain vascular malformations; and mitochondrial diseases. Consortia have grown as they have become successful in establishing partners from other branches of NIH and industry, Dr. Groft said.
One of the most unique aspects of the Rare Disease Clinical Research Grants is the mandate to collaborate with a patient advocacy group. “We have noticed that, in order to have a successful research program for rare diseases, we need the involvement of patient advocacy groups. These groups are essential to help with patient recruitment and to act as liaisons between researchers and the group’s constituency of patients and their families,” Dr. Groft said.
In fact, a Coalition of Patient Advocacy Groups has been formed as an arm of the Rare Disease Clinical Research Network.
“The role of advocacy groups has really matured over the last several years,” Dr. Gropman said. “I work closely with an amazing advocacy group in the Urea Cycle Disorders Consortium. They have gone from a grassroots support of families to major partners in research.”
Dr. Gropman credits the group with raising important clinical questions that only those living with affected individuals might be aware of, educating patients and their families about the importance of participation in clinical trials, as well providing financial support and finding philanthropic partners.
“Patient advocates have a vested interest in moving the clinical research process along,” she said.
Another new initiative to facilitate funding for rare diseases is the R.A.R.E. (Rare Disease Advocacy, Research, and Education) Project’s Global Genes Fund, a Web site in development that aims to serve as a clearinghouse for rare disease philanthropy.
“As long as the rare disease community works in their separate disease silos, we can only get so far. ... If we can bring the community together and not focus on an individual disease, we can create a unifying campaign and rare diseases then becomes a huge public health issue, larger than the U.S. AIDS community and equal to that of breast cancer,” said Nicole Boice, founder and president of R.A.R.E.
One part of the Global Genes Fund, which is set to launch in the second quarter of 2011, will showcase innovative scientific research projects that individuals or businesses can contribute to, in donations as small as $25. Initially, Ms. Boice expects to focus on 10-20 research projects.
“As the platform grows and is successful at securing funding, this will catalyze this type of innovative funding,” she said.
Using Neurogenetics in Practice
Genetic testing can be helpful for diagnosing some neurologic disorders, but there is a “bewildering” maze of tests available, Dr. Gropman said. Vendors have put together pre-determined panels for whole exome sequencing of some disorders, such as spinal cerebellar ataxia or disorders associated with mental disabilities.
“Using a panel, you can test for a number of different genes simultaneously, but you cannot separate them,” she said.
Also coming to market are “designer” panels that allow clinicians to pick and choose which genes to target, but they require knowing a priori which mutation to focus on.
“While the technology has exploded, knowing which test to order can be baffling for many clinicians who do not have extensive backgrounds in genetics. If we find a variant but don’t know its significance, it opens up a diagnostic conundrum,” Dr. Gropman said. The field is still in flux, so many other questions must be resolved, such as how to deal with difficult or unanticipated results and whether technology and interpretation need to be standardized.
Genotyping is not just a black-and-white issue, according to Dr. Huentelman. In addition to the knowledge of a patient’s mutation status, genotyping results must also take into consideration the risk of developing clinical symptoms and the ethical and pragmatic issues that knowledge raises for patients and physicians.
As a first step, neurologists should begin educating themselves about the new technologies, potential applications, and shortcomings, by attending sessions on neurogenetics at annual professional meetings or more specialized conferences. While commercial vendors may provide varying degrees of advice about test selection, creating a professional relationship with a geneticist or genetic counselor might provide access to the most up-to-date information and options.
“You need someone fully entrenched in the field of human genetics,” said Dr. Huentelman, who advocates creating a genetics team to sort through the maze of data that can be generated by a genetic analysis.
As a specialist in translational medicine, Dr. Huentelman focuses on understanding the genetic cause of disease and then translating this information into new diagnostic tests and therapeutics. While new DNA sequencing methods are quickly making the first goal a reality, accomplishing the second goal still remains “hopes and dreams,” he said. The ultimate goal of genetic mapping is to personalize medicine by shifting clinical practice from that based on clinical symptoms to that tailored to an individual’s genetic make-up, but broad gaps in knowledge remain. The hope is to develop pharmaceuticals to correct the deficiency caused by a particular mutation or to use genetic testing to preselect patients who might benefit from a particular therapy, similar to the ongoing work on apolipoprotein E genotype status and Alzheimer’s disease.
“We are reaching the critical mass level” in neurogenetic research, Dr. Groft said. As increasing numbers of patients with genetic disorders are followed, clinicians and researchers are developing the clinical skills and diagnostic tools to advance the understanding of disease pathogenesis. At the same time, these accomplishments are piquing interest and collaboration from others in the medical community, spurred by the increasing availability of funds and non-monetary support from the government, private foundations, industry, and patient advocacy groups. It is anticipated that neurogenetics is on the cusp of major breakthroughs for clinical neurology.
Disclosures: None of the sources contacted for this article had any relevant financial disclosures.
Within the last 2 years, new DNA sequencing technologies, dubbed NextGen (“next generation”), have been introduced that are revolutionizing the field of neurogenetics. As these advances gain recognition within and outside the medical community, the pace of progress is quickening, with the help of support from broad-based advocacy groups and more funding options. These technologies are already bringing greater diagnostic opportunities to practice, although the time may not yet be ripe for translating the results into information useful for the clinical management of patients.
“For rare diseases, this is a real game-changer type of technology,” commented Matthew J. Huentelman, Ph.D., a neurogeneticist affiliated with the Translational Genomics Research Institute in Phoenix. “We have had some significant leaps in what we can do with the human genome over the past couple of years, especially in the mechanics and chemistry of how sequencing is done. Several companies have built machines that sequence DNA in fundamentally different ways than we did 5-10 years ago.” Since 2007, Dr. Huentelman has been applying NextGen technology to the study of Alzheimer’s disease and autism.
Whole exome DNA sequencing is one of the NextGen technologies that are being applied to neurologic disorders. Unlike whole genome sequencing, whole exome DNA sequencing focuses on the small portion of the genome (less than 2%) that contains protein-coding bits of DNA, known as the exome. For example, Dr. Murat Gunel, chief of the Neurovascular Surgery Program and codirector of the Program on Neurogenetics at Yale University, New Haven, Conn., recently used whole exome sequencing to determine that several distinct types of malformations of cortical development, including microcephaly, pachygyria with cortical thickening, and hypoplasia of the corpus callosum were all associated with recessive mutations in a single gene, WDR62 (Nature 2010;467:207-10). This discovery was made by analyzing the DNA of several children with microcephaly among 30 interrelated Turkish families.
The last few months have brought other reports that used whole exome sequencing to identify causative gene mutations for Perrault syndrome (Am. J. Hum. Genet. 2010;87:282-8), Joubert’s syndrome (Am. J. Hum. Genet. 2010;86:93-7), and Fowler syndrome (Human Mutation 2010;31:918-23).
One advantage of exome sequencing studies is that researchers can use smaller sample sets – with an “n” as small as 1 – in a comprehensive fashion, Dr. Huentelman said. “This is great for rare diseases or to gain a foothold in a disease that has been hard to understand at the population level. For instance, we can analyze the differences between twins discordant for autism.”
Advantages of exome sequencing include its speed and cost effectiveness. Dr. Gunel reports that in his laboratory, whole genome sequencing might take several weeks and cost about $50,000 while he can receive the results of whole exome sequencing within 9 days, at a cost of about $3,500.
“These tests are still pricey,” said Dr. Andrea Gropman, a neurogeneticist at the Children’s National Medical Center and George Washington University, both in Washington. For sequencing genes related to autism, she said that costs may range from $1,000 to $5,800, which insurance companies may or may not cover. “For some families, that means they must pay out of pocket.”
New Funding Opportunities
Advances in the field may be due, in part, to greater funding opportunities. Dr. Gunel’s research was supported in part by a $2.9 million “stimulus grant” from the National Institute of Neurological Disorders and Stroke, part of the $8.2 billion provided to the National Institutes of Health from the American Recovery and Reinvestment Act. He plans to use his stimulus money to extend whole exome sequencing to hundreds of other families affected by malformations of cortical development.
Another significant source of funds has been distributed through the NIH’s Office of Rare Diseases Research (ORDR), which allocated almost $70 million in 2003 toward the creation of 10 clinical consortia and one Data and Technology Coordinating Center.
“In the late 1990s and early 2000, we felt we didn’t have a good infrastructure in place to do research in rare diseases – of which we estimated there were at least 6,800,” said Steve C. Groft, Pharm.D., director of the ORDR. “We hoped the funds we provided could go toward establishing that infrastructure.”
Recently, a second round of funding of approximately $125 million was awarded to 19 consortia, including 6 of the 10 original groups. Nine of the consortia are related to neurologic disease, including those focusing on Angelman, Rett, and Prader-Willi syndromes; autonomic rare diseases; neurologic channelopathies; spinocerebellar ataxias; dystonias; lysosomal diseases; inherited neuropathies; brain vascular malformations; and mitochondrial diseases. Consortia have grown as they have become successful in establishing partners from other branches of NIH and industry, Dr. Groft said.
One of the most unique aspects of the Rare Disease Clinical Research Grants is the mandate to collaborate with a patient advocacy group. “We have noticed that, in order to have a successful research program for rare diseases, we need the involvement of patient advocacy groups. These groups are essential to help with patient recruitment and to act as liaisons between researchers and the group’s constituency of patients and their families,” Dr. Groft said.
In fact, a Coalition of Patient Advocacy Groups has been formed as an arm of the Rare Disease Clinical Research Network.
“The role of advocacy groups has really matured over the last several years,” Dr. Gropman said. “I work closely with an amazing advocacy group in the Urea Cycle Disorders Consortium. They have gone from a grassroots support of families to major partners in research.”
Dr. Gropman credits the group with raising important clinical questions that only those living with affected individuals might be aware of, educating patients and their families about the importance of participation in clinical trials, as well providing financial support and finding philanthropic partners.
“Patient advocates have a vested interest in moving the clinical research process along,” she said.
Another new initiative to facilitate funding for rare diseases is the R.A.R.E. (Rare Disease Advocacy, Research, and Education) Project’s Global Genes Fund, a Web site in development that aims to serve as a clearinghouse for rare disease philanthropy.
“As long as the rare disease community works in their separate disease silos, we can only get so far. ... If we can bring the community together and not focus on an individual disease, we can create a unifying campaign and rare diseases then becomes a huge public health issue, larger than the U.S. AIDS community and equal to that of breast cancer,” said Nicole Boice, founder and president of R.A.R.E.
One part of the Global Genes Fund, which is set to launch in the second quarter of 2011, will showcase innovative scientific research projects that individuals or businesses can contribute to, in donations as small as $25. Initially, Ms. Boice expects to focus on 10-20 research projects.
“As the platform grows and is successful at securing funding, this will catalyze this type of innovative funding,” she said.
Using Neurogenetics in Practice
Genetic testing can be helpful for diagnosing some neurologic disorders, but there is a “bewildering” maze of tests available, Dr. Gropman said. Vendors have put together pre-determined panels for whole exome sequencing of some disorders, such as spinal cerebellar ataxia or disorders associated with mental disabilities.
“Using a panel, you can test for a number of different genes simultaneously, but you cannot separate them,” she said.
Also coming to market are “designer” panels that allow clinicians to pick and choose which genes to target, but they require knowing a priori which mutation to focus on.
“While the technology has exploded, knowing which test to order can be baffling for many clinicians who do not have extensive backgrounds in genetics. If we find a variant but don’t know its significance, it opens up a diagnostic conundrum,” Dr. Gropman said. The field is still in flux, so many other questions must be resolved, such as how to deal with difficult or unanticipated results and whether technology and interpretation need to be standardized.
Genotyping is not just a black-and-white issue, according to Dr. Huentelman. In addition to the knowledge of a patient’s mutation status, genotyping results must also take into consideration the risk of developing clinical symptoms and the ethical and pragmatic issues that knowledge raises for patients and physicians.
As a first step, neurologists should begin educating themselves about the new technologies, potential applications, and shortcomings, by attending sessions on neurogenetics at annual professional meetings or more specialized conferences. While commercial vendors may provide varying degrees of advice about test selection, creating a professional relationship with a geneticist or genetic counselor might provide access to the most up-to-date information and options.
“You need someone fully entrenched in the field of human genetics,” said Dr. Huentelman, who advocates creating a genetics team to sort through the maze of data that can be generated by a genetic analysis.
As a specialist in translational medicine, Dr. Huentelman focuses on understanding the genetic cause of disease and then translating this information into new diagnostic tests and therapeutics. While new DNA sequencing methods are quickly making the first goal a reality, accomplishing the second goal still remains “hopes and dreams,” he said. The ultimate goal of genetic mapping is to personalize medicine by shifting clinical practice from that based on clinical symptoms to that tailored to an individual’s genetic make-up, but broad gaps in knowledge remain. The hope is to develop pharmaceuticals to correct the deficiency caused by a particular mutation or to use genetic testing to preselect patients who might benefit from a particular therapy, similar to the ongoing work on apolipoprotein E genotype status and Alzheimer’s disease.
“We are reaching the critical mass level” in neurogenetic research, Dr. Groft said. As increasing numbers of patients with genetic disorders are followed, clinicians and researchers are developing the clinical skills and diagnostic tools to advance the understanding of disease pathogenesis. At the same time, these accomplishments are piquing interest and collaboration from others in the medical community, spurred by the increasing availability of funds and non-monetary support from the government, private foundations, industry, and patient advocacy groups. It is anticipated that neurogenetics is on the cusp of major breakthroughs for clinical neurology.
Disclosures: None of the sources contacted for this article had any relevant financial disclosures.
Within the last 2 years, new DNA sequencing technologies, dubbed NextGen (“next generation”), have been introduced that are revolutionizing the field of neurogenetics. As these advances gain recognition within and outside the medical community, the pace of progress is quickening, with the help of support from broad-based advocacy groups and more funding options. These technologies are already bringing greater diagnostic opportunities to practice, although the time may not yet be ripe for translating the results into information useful for the clinical management of patients.
“For rare diseases, this is a real game-changer type of technology,” commented Matthew J. Huentelman, Ph.D., a neurogeneticist affiliated with the Translational Genomics Research Institute in Phoenix. “We have had some significant leaps in what we can do with the human genome over the past couple of years, especially in the mechanics and chemistry of how sequencing is done. Several companies have built machines that sequence DNA in fundamentally different ways than we did 5-10 years ago.” Since 2007, Dr. Huentelman has been applying NextGen technology to the study of Alzheimer’s disease and autism.
Whole exome DNA sequencing is one of the NextGen technologies that are being applied to neurologic disorders. Unlike whole genome sequencing, whole exome DNA sequencing focuses on the small portion of the genome (less than 2%) that contains protein-coding bits of DNA, known as the exome. For example, Dr. Murat Gunel, chief of the Neurovascular Surgery Program and codirector of the Program on Neurogenetics at Yale University, New Haven, Conn., recently used whole exome sequencing to determine that several distinct types of malformations of cortical development, including microcephaly, pachygyria with cortical thickening, and hypoplasia of the corpus callosum were all associated with recessive mutations in a single gene, WDR62 (Nature 2010;467:207-10). This discovery was made by analyzing the DNA of several children with microcephaly among 30 interrelated Turkish families.
The last few months have brought other reports that used whole exome sequencing to identify causative gene mutations for Perrault syndrome (Am. J. Hum. Genet. 2010;87:282-8), Joubert’s syndrome (Am. J. Hum. Genet. 2010;86:93-7), and Fowler syndrome (Human Mutation 2010;31:918-23).
One advantage of exome sequencing studies is that researchers can use smaller sample sets – with an “n” as small as 1 – in a comprehensive fashion, Dr. Huentelman said. “This is great for rare diseases or to gain a foothold in a disease that has been hard to understand at the population level. For instance, we can analyze the differences between twins discordant for autism.”
Advantages of exome sequencing include its speed and cost effectiveness. Dr. Gunel reports that in his laboratory, whole genome sequencing might take several weeks and cost about $50,000 while he can receive the results of whole exome sequencing within 9 days, at a cost of about $3,500.
“These tests are still pricey,” said Dr. Andrea Gropman, a neurogeneticist at the Children’s National Medical Center and George Washington University, both in Washington. For sequencing genes related to autism, she said that costs may range from $1,000 to $5,800, which insurance companies may or may not cover. “For some families, that means they must pay out of pocket.”
New Funding Opportunities
Advances in the field may be due, in part, to greater funding opportunities. Dr. Gunel’s research was supported in part by a $2.9 million “stimulus grant” from the National Institute of Neurological Disorders and Stroke, part of the $8.2 billion provided to the National Institutes of Health from the American Recovery and Reinvestment Act. He plans to use his stimulus money to extend whole exome sequencing to hundreds of other families affected by malformations of cortical development.
Another significant source of funds has been distributed through the NIH’s Office of Rare Diseases Research (ORDR), which allocated almost $70 million in 2003 toward the creation of 10 clinical consortia and one Data and Technology Coordinating Center.
“In the late 1990s and early 2000, we felt we didn’t have a good infrastructure in place to do research in rare diseases – of which we estimated there were at least 6,800,” said Steve C. Groft, Pharm.D., director of the ORDR. “We hoped the funds we provided could go toward establishing that infrastructure.”
Recently, a second round of funding of approximately $125 million was awarded to 19 consortia, including 6 of the 10 original groups. Nine of the consortia are related to neurologic disease, including those focusing on Angelman, Rett, and Prader-Willi syndromes; autonomic rare diseases; neurologic channelopathies; spinocerebellar ataxias; dystonias; lysosomal diseases; inherited neuropathies; brain vascular malformations; and mitochondrial diseases. Consortia have grown as they have become successful in establishing partners from other branches of NIH and industry, Dr. Groft said.
One of the most unique aspects of the Rare Disease Clinical Research Grants is the mandate to collaborate with a patient advocacy group. “We have noticed that, in order to have a successful research program for rare diseases, we need the involvement of patient advocacy groups. These groups are essential to help with patient recruitment and to act as liaisons between researchers and the group’s constituency of patients and their families,” Dr. Groft said.
In fact, a Coalition of Patient Advocacy Groups has been formed as an arm of the Rare Disease Clinical Research Network.
“The role of advocacy groups has really matured over the last several years,” Dr. Gropman said. “I work closely with an amazing advocacy group in the Urea Cycle Disorders Consortium. They have gone from a grassroots support of families to major partners in research.”
Dr. Gropman credits the group with raising important clinical questions that only those living with affected individuals might be aware of, educating patients and their families about the importance of participation in clinical trials, as well providing financial support and finding philanthropic partners.
“Patient advocates have a vested interest in moving the clinical research process along,” she said.
Another new initiative to facilitate funding for rare diseases is the R.A.R.E. (Rare Disease Advocacy, Research, and Education) Project’s Global Genes Fund, a Web site in development that aims to serve as a clearinghouse for rare disease philanthropy.
“As long as the rare disease community works in their separate disease silos, we can only get so far. ... If we can bring the community together and not focus on an individual disease, we can create a unifying campaign and rare diseases then becomes a huge public health issue, larger than the U.S. AIDS community and equal to that of breast cancer,” said Nicole Boice, founder and president of R.A.R.E.
One part of the Global Genes Fund, which is set to launch in the second quarter of 2011, will showcase innovative scientific research projects that individuals or businesses can contribute to, in donations as small as $25. Initially, Ms. Boice expects to focus on 10-20 research projects.
“As the platform grows and is successful at securing funding, this will catalyze this type of innovative funding,” she said.
Using Neurogenetics in Practice
Genetic testing can be helpful for diagnosing some neurologic disorders, but there is a “bewildering” maze of tests available, Dr. Gropman said. Vendors have put together pre-determined panels for whole exome sequencing of some disorders, such as spinal cerebellar ataxia or disorders associated with mental disabilities.
“Using a panel, you can test for a number of different genes simultaneously, but you cannot separate them,” she said.
Also coming to market are “designer” panels that allow clinicians to pick and choose which genes to target, but they require knowing a priori which mutation to focus on.
“While the technology has exploded, knowing which test to order can be baffling for many clinicians who do not have extensive backgrounds in genetics. If we find a variant but don’t know its significance, it opens up a diagnostic conundrum,” Dr. Gropman said. The field is still in flux, so many other questions must be resolved, such as how to deal with difficult or unanticipated results and whether technology and interpretation need to be standardized.
Genotyping is not just a black-and-white issue, according to Dr. Huentelman. In addition to the knowledge of a patient’s mutation status, genotyping results must also take into consideration the risk of developing clinical symptoms and the ethical and pragmatic issues that knowledge raises for patients and physicians.
As a first step, neurologists should begin educating themselves about the new technologies, potential applications, and shortcomings, by attending sessions on neurogenetics at annual professional meetings or more specialized conferences. While commercial vendors may provide varying degrees of advice about test selection, creating a professional relationship with a geneticist or genetic counselor might provide access to the most up-to-date information and options.
“You need someone fully entrenched in the field of human genetics,” said Dr. Huentelman, who advocates creating a genetics team to sort through the maze of data that can be generated by a genetic analysis.
As a specialist in translational medicine, Dr. Huentelman focuses on understanding the genetic cause of disease and then translating this information into new diagnostic tests and therapeutics. While new DNA sequencing methods are quickly making the first goal a reality, accomplishing the second goal still remains “hopes and dreams,” he said. The ultimate goal of genetic mapping is to personalize medicine by shifting clinical practice from that based on clinical symptoms to that tailored to an individual’s genetic make-up, but broad gaps in knowledge remain. The hope is to develop pharmaceuticals to correct the deficiency caused by a particular mutation or to use genetic testing to preselect patients who might benefit from a particular therapy, similar to the ongoing work on apolipoprotein E genotype status and Alzheimer’s disease.
“We are reaching the critical mass level” in neurogenetic research, Dr. Groft said. As increasing numbers of patients with genetic disorders are followed, clinicians and researchers are developing the clinical skills and diagnostic tools to advance the understanding of disease pathogenesis. At the same time, these accomplishments are piquing interest and collaboration from others in the medical community, spurred by the increasing availability of funds and non-monetary support from the government, private foundations, industry, and patient advocacy groups. It is anticipated that neurogenetics is on the cusp of major breakthroughs for clinical neurology.
Disclosures: None of the sources contacted for this article had any relevant financial disclosures.
Estrogen May Mediate Brain Aneurysm Risk
Major Finding: Compared with a control group matched for age and educational levels, a significantly lower percentage of postmenopausal women with cerebral aneurysms reported lifetime use of oral contraceptives (77.6% vs. 60) and hormone therapy (44.8% vs. 23.7%).
Data Source: Retrospective case-control study of 60 women with ruptured and unruptured aneurysms seen by one physician at a single center and a control group of 420 patients from a publicly available data set.
Disclosures: Dr. Chen reported no relevant disclosures.
CARLSBAD, CALIF. — Women with cerebral aneurysms were less likely to have used oral contraceptives or hormone replacement therapy in their lifetime than controls, according to the results of a retrospective case-control study of 60 women with aneurysms.
“These findings suggest that our case group may have had not only lower levels of estrogen, but also more severe physiologic fluctuations in their estrogen level as compared to population averages. To take this one step further, therapies that mitigate the effects of low estrogen level and fluctuations may decrease the risk for cerebral aneurysms,” Dr. Michael Chen said at the meeting.
The study included 21 women with ruptured aneurysms and 39 with unruptured aneurysms who were under the care of a single physician during 2008-2010. Their median age was 52 years, with a range of 31-80 years. Dr. Chen and his associates conducted telephone interviews with the patients to obtain detailed medical, social, family, and gynecologic histories, including information about lifetime use and duration of use of OCs and hormone replacement therapy, as well as menstrual and reproductive history.
For the control group, the investigators spoke with 4,682 random American women from a publicly available data set (Ann. Epidemiol. 2002;12:213-22) that was generated as part of a National Institutes of Health–funded study exploring the use of OCs and hormone replacement therapy in breast cancer.
For each case, seven control patients matched for age and educational levels were selected, giving 420 control patients. Measures such as median age, body mass index (BMI), age of menarche, nulliparity, and age of first pregnancy were comparable between the case and control patients.
Significantly fewer women with an aneurysm reported lifetime use of OCs than did controls (60% vs. 77.6%, P = .003) as well as use of hormone replacement therapy (23.7% vs. 44.8%, P = .002). The median age of menopause for women with aneurysms was more than 2 years earlier than for the control group (44.5 vs. 46.9 years, P = .53), and 50% of cases had menopause before age 45, compared with 34% of controls (P = .18). “The patient group also had a high rate of early hysterectomies,” noted Dr. Chen, an interventional vascular neurologist affiliated with Rush University Medical Center, Chicago.
One of the limitations of the study is that the vasculature of the control group had not been evaluated, although it is assumed that 3%-4% of the general population harbors an unruptured cerebral aneurysm. Another limitation is that the control group data were generated during 1994-1998, before the use of hormone replacement therapy decreased substantially in 2002 following the publication of the Women's Health Initiative Study on breast cancer, Dr. Chen said.
He said he hopes these findings will guide future research.
“Understanding more about the effects of estrogen at the receptor level may allow us to find therapies that target that receptor in order to provide some protection for women at risk of developing aneurysms or harboring unruptured aneurysms.”
Major Finding: Compared with a control group matched for age and educational levels, a significantly lower percentage of postmenopausal women with cerebral aneurysms reported lifetime use of oral contraceptives (77.6% vs. 60) and hormone therapy (44.8% vs. 23.7%).
Data Source: Retrospective case-control study of 60 women with ruptured and unruptured aneurysms seen by one physician at a single center and a control group of 420 patients from a publicly available data set.
Disclosures: Dr. Chen reported no relevant disclosures.
CARLSBAD, CALIF. — Women with cerebral aneurysms were less likely to have used oral contraceptives or hormone replacement therapy in their lifetime than controls, according to the results of a retrospective case-control study of 60 women with aneurysms.
“These findings suggest that our case group may have had not only lower levels of estrogen, but also more severe physiologic fluctuations in their estrogen level as compared to population averages. To take this one step further, therapies that mitigate the effects of low estrogen level and fluctuations may decrease the risk for cerebral aneurysms,” Dr. Michael Chen said at the meeting.
The study included 21 women with ruptured aneurysms and 39 with unruptured aneurysms who were under the care of a single physician during 2008-2010. Their median age was 52 years, with a range of 31-80 years. Dr. Chen and his associates conducted telephone interviews with the patients to obtain detailed medical, social, family, and gynecologic histories, including information about lifetime use and duration of use of OCs and hormone replacement therapy, as well as menstrual and reproductive history.
For the control group, the investigators spoke with 4,682 random American women from a publicly available data set (Ann. Epidemiol. 2002;12:213-22) that was generated as part of a National Institutes of Health–funded study exploring the use of OCs and hormone replacement therapy in breast cancer.
For each case, seven control patients matched for age and educational levels were selected, giving 420 control patients. Measures such as median age, body mass index (BMI), age of menarche, nulliparity, and age of first pregnancy were comparable between the case and control patients.
Significantly fewer women with an aneurysm reported lifetime use of OCs than did controls (60% vs. 77.6%, P = .003) as well as use of hormone replacement therapy (23.7% vs. 44.8%, P = .002). The median age of menopause for women with aneurysms was more than 2 years earlier than for the control group (44.5 vs. 46.9 years, P = .53), and 50% of cases had menopause before age 45, compared with 34% of controls (P = .18). “The patient group also had a high rate of early hysterectomies,” noted Dr. Chen, an interventional vascular neurologist affiliated with Rush University Medical Center, Chicago.
One of the limitations of the study is that the vasculature of the control group had not been evaluated, although it is assumed that 3%-4% of the general population harbors an unruptured cerebral aneurysm. Another limitation is that the control group data were generated during 1994-1998, before the use of hormone replacement therapy decreased substantially in 2002 following the publication of the Women's Health Initiative Study on breast cancer, Dr. Chen said.
He said he hopes these findings will guide future research.
“Understanding more about the effects of estrogen at the receptor level may allow us to find therapies that target that receptor in order to provide some protection for women at risk of developing aneurysms or harboring unruptured aneurysms.”
Major Finding: Compared with a control group matched for age and educational levels, a significantly lower percentage of postmenopausal women with cerebral aneurysms reported lifetime use of oral contraceptives (77.6% vs. 60) and hormone therapy (44.8% vs. 23.7%).
Data Source: Retrospective case-control study of 60 women with ruptured and unruptured aneurysms seen by one physician at a single center and a control group of 420 patients from a publicly available data set.
Disclosures: Dr. Chen reported no relevant disclosures.
CARLSBAD, CALIF. — Women with cerebral aneurysms were less likely to have used oral contraceptives or hormone replacement therapy in their lifetime than controls, according to the results of a retrospective case-control study of 60 women with aneurysms.
“These findings suggest that our case group may have had not only lower levels of estrogen, but also more severe physiologic fluctuations in their estrogen level as compared to population averages. To take this one step further, therapies that mitigate the effects of low estrogen level and fluctuations may decrease the risk for cerebral aneurysms,” Dr. Michael Chen said at the meeting.
The study included 21 women with ruptured aneurysms and 39 with unruptured aneurysms who were under the care of a single physician during 2008-2010. Their median age was 52 years, with a range of 31-80 years. Dr. Chen and his associates conducted telephone interviews with the patients to obtain detailed medical, social, family, and gynecologic histories, including information about lifetime use and duration of use of OCs and hormone replacement therapy, as well as menstrual and reproductive history.
For the control group, the investigators spoke with 4,682 random American women from a publicly available data set (Ann. Epidemiol. 2002;12:213-22) that was generated as part of a National Institutes of Health–funded study exploring the use of OCs and hormone replacement therapy in breast cancer.
For each case, seven control patients matched for age and educational levels were selected, giving 420 control patients. Measures such as median age, body mass index (BMI), age of menarche, nulliparity, and age of first pregnancy were comparable between the case and control patients.
Significantly fewer women with an aneurysm reported lifetime use of OCs than did controls (60% vs. 77.6%, P = .003) as well as use of hormone replacement therapy (23.7% vs. 44.8%, P = .002). The median age of menopause for women with aneurysms was more than 2 years earlier than for the control group (44.5 vs. 46.9 years, P = .53), and 50% of cases had menopause before age 45, compared with 34% of controls (P = .18). “The patient group also had a high rate of early hysterectomies,” noted Dr. Chen, an interventional vascular neurologist affiliated with Rush University Medical Center, Chicago.
One of the limitations of the study is that the vasculature of the control group had not been evaluated, although it is assumed that 3%-4% of the general population harbors an unruptured cerebral aneurysm. Another limitation is that the control group data were generated during 1994-1998, before the use of hormone replacement therapy decreased substantially in 2002 following the publication of the Women's Health Initiative Study on breast cancer, Dr. Chen said.
He said he hopes these findings will guide future research.
“Understanding more about the effects of estrogen at the receptor level may allow us to find therapies that target that receptor in order to provide some protection for women at risk of developing aneurysms or harboring unruptured aneurysms.”
From the annual meeting of the Society of NeuroInterventional Surgery
Antiplatelets Needed After Aneurysm Stenting
CARLSBAD, CALIF. — Delayed thromboses have been observed after the cessation of double antiplatelet therapy in patients who have received an Enterprise stent for an intracranial aneurysm.
Seven of 213 patients in the Interstate Collaboration of the Enterprise Stent Coiling (ICES) Multicenter Registry experienced a thrombotic event 2-24 weeks after placement of the stent and stopping aspirin and clopidogrel, Dr. J. Mocco reported at the annual meeting of the Society of Neurointerventional Surgery. Another two patients experienced acute cases of stent thrombosis.
Ten U.S. sites participate in the registry to rapidly provide large-volume, real-world results regarding experience in using the Enterprise Vascular Reconstruction Device and Delivery System (J. Neurosurg. 2009;110:35-9) for the treatment of intracranial aneurysms. The stent system is designed to assist in the embolic coiling of wide-neck aneurysms.
In the seven cases, the patients either did not comply with the drug regimen or stopped taking the antiplatelets on the order of the physician.
The data suggest that early cessation of double antiplatelet therapy is not ideal; but it is currently unclear what the optimum time course should be, said Dr. Mocco, an endovascular neurosurgeon at the University of Florida, Gainesville.
Use of the Enterprise stent was linked with 90% or greater occlusion in 88% of aneurysms. Permanent morbidity (all minor) occurred in 1.4% of patients.
None of the thrombotic events were associated with coil prolapse or parent vessel tortuosity, said Dr. Mocco. Three events had no angiographic corollary, and two were diagnosed based on symptoms.
Dr. Mocco gave his presentation at a session sponsored by Codman Neurovascular, maker of the Enterprise stent. He said that he received no direct financial support from Codman, and the data were generated independent of commercial influence, although the University of Florida receives an educational grant and consulting fees from Codman.
CARLSBAD, CALIF. — Delayed thromboses have been observed after the cessation of double antiplatelet therapy in patients who have received an Enterprise stent for an intracranial aneurysm.
Seven of 213 patients in the Interstate Collaboration of the Enterprise Stent Coiling (ICES) Multicenter Registry experienced a thrombotic event 2-24 weeks after placement of the stent and stopping aspirin and clopidogrel, Dr. J. Mocco reported at the annual meeting of the Society of Neurointerventional Surgery. Another two patients experienced acute cases of stent thrombosis.
Ten U.S. sites participate in the registry to rapidly provide large-volume, real-world results regarding experience in using the Enterprise Vascular Reconstruction Device and Delivery System (J. Neurosurg. 2009;110:35-9) for the treatment of intracranial aneurysms. The stent system is designed to assist in the embolic coiling of wide-neck aneurysms.
In the seven cases, the patients either did not comply with the drug regimen or stopped taking the antiplatelets on the order of the physician.
The data suggest that early cessation of double antiplatelet therapy is not ideal; but it is currently unclear what the optimum time course should be, said Dr. Mocco, an endovascular neurosurgeon at the University of Florida, Gainesville.
Use of the Enterprise stent was linked with 90% or greater occlusion in 88% of aneurysms. Permanent morbidity (all minor) occurred in 1.4% of patients.
None of the thrombotic events were associated with coil prolapse or parent vessel tortuosity, said Dr. Mocco. Three events had no angiographic corollary, and two were diagnosed based on symptoms.
Dr. Mocco gave his presentation at a session sponsored by Codman Neurovascular, maker of the Enterprise stent. He said that he received no direct financial support from Codman, and the data were generated independent of commercial influence, although the University of Florida receives an educational grant and consulting fees from Codman.
CARLSBAD, CALIF. — Delayed thromboses have been observed after the cessation of double antiplatelet therapy in patients who have received an Enterprise stent for an intracranial aneurysm.
Seven of 213 patients in the Interstate Collaboration of the Enterprise Stent Coiling (ICES) Multicenter Registry experienced a thrombotic event 2-24 weeks after placement of the stent and stopping aspirin and clopidogrel, Dr. J. Mocco reported at the annual meeting of the Society of Neurointerventional Surgery. Another two patients experienced acute cases of stent thrombosis.
Ten U.S. sites participate in the registry to rapidly provide large-volume, real-world results regarding experience in using the Enterprise Vascular Reconstruction Device and Delivery System (J. Neurosurg. 2009;110:35-9) for the treatment of intracranial aneurysms. The stent system is designed to assist in the embolic coiling of wide-neck aneurysms.
In the seven cases, the patients either did not comply with the drug regimen or stopped taking the antiplatelets on the order of the physician.
The data suggest that early cessation of double antiplatelet therapy is not ideal; but it is currently unclear what the optimum time course should be, said Dr. Mocco, an endovascular neurosurgeon at the University of Florida, Gainesville.
Use of the Enterprise stent was linked with 90% or greater occlusion in 88% of aneurysms. Permanent morbidity (all minor) occurred in 1.4% of patients.
None of the thrombotic events were associated with coil prolapse or parent vessel tortuosity, said Dr. Mocco. Three events had no angiographic corollary, and two were diagnosed based on symptoms.
Dr. Mocco gave his presentation at a session sponsored by Codman Neurovascular, maker of the Enterprise stent. He said that he received no direct financial support from Codman, and the data were generated independent of commercial influence, although the University of Florida receives an educational grant and consulting fees from Codman.
Estrogen Stabilization May Prevent Aneurysms
Major Finding: Compared with a control group matched for age and educational levels, a significantly lower percentage of postmenopausal women with cerebral aneurysms reported lifetime use of oral contraceptives (77.6% vs. 60%, respectively) and hormone therapy (44.8% vs. 23.7%).
Data Source: Retrospective case-control study of 60 women with ruptured and unruptured aneurysms seen by one physician at a single center and a control group of 420 patients from a publicly available data set.
Disclosures: Dr. Chen reported no relevant disclosures.
Carlsbad, Calif. — Women with cerebral aneurysms were less likely to have used oral contraceptives or hormone replacement therapy in their lifetimes than controls, according to the results of a retrospective case-control study of 60 women with aneurysms.
“These findings suggest that our case group may have had not only lower levels of estrogen, but also more severe physiologic fluctuations in their estrogen level as compared to population averages. To take this one step further, therapies that mitigate the effects of low estrogen level and fluctuations may decrease the risk for cerebral aneurysms,” Dr. Michael Chen said
The study included 21 women with ruptured aneurysms and 39 with unruptured aneurysms who were under the care of a single physician during 2008-2010. Their median age was 52 years, with a range of 31-80 years. Dr. Chen and his associates conducted telephone interviews with the patients to obtain detailed medical, social, family, and gynecologic histories, including information about lifetime use and duration of use of oral contraceptives and hormone replacement therapy, as well as menstrual and reproductive history.
For the control group, the investigators spoke with 4,682 random American women from a publicly available data set (Ann. Epidemiol. 2002;12:213-22) that was generated as part of a National Institutes of Health–funded study exploring the use of oral contraceptives and hormone replacement therapy in breast cancer.
For each case, seven control patients matched for age and educational levels were selected, giving 420 control patients. Measures such as median age, body mass index (BMI), age of menarche, nulliparity, and age of first pregnancy were comparable between the case and control patients.
Significantly fewer women with an aneurysm reported lifetime use of oral contraceptives than did controls (60% vs. 77.6%, P = .003) as well as use of hormone replacement therapy (23.7% vs. 44.8%, P = .002). The median age of menopause for women with aneurysms was more than 2 years earlier than for the control group (44.5 vs. 46.9 years, P = .53) and 50% of cases had menopause before age 45, compared with 34% of controls (P = .18). “The patient group also had a high rate of early hysterectomies,” noted Dr. Chen, an interventional vascular neurologist affiliated with Rush University Medical Center, Chicago.
One of the limitations of the study is that the vasculature of the control group had not been evaluated, although it is assumed that 3%-4% of the general population harbors an unruptured cerebral aneurysm. Another limitation is that the control group data were generated during 1994-1998, before the use of hormone replacement therapy decreased substantially in 2002, Dr. Chen said.
He hopes these findings will guide future research not only on the role of estrogen in aneurysm development, but also the role of the estrogen receptor specific to cerebral vasculature.
“Understanding more about the effects of estrogen at the receptor level may allow us to find therapies that target that receptor in order to provide some protection for women at risk of developing aneurysms or harboring unruptured aneurysms,” Dr. Chen concluded.
Major Finding: Compared with a control group matched for age and educational levels, a significantly lower percentage of postmenopausal women with cerebral aneurysms reported lifetime use of oral contraceptives (77.6% vs. 60%, respectively) and hormone therapy (44.8% vs. 23.7%).
Data Source: Retrospective case-control study of 60 women with ruptured and unruptured aneurysms seen by one physician at a single center and a control group of 420 patients from a publicly available data set.
Disclosures: Dr. Chen reported no relevant disclosures.
Carlsbad, Calif. — Women with cerebral aneurysms were less likely to have used oral contraceptives or hormone replacement therapy in their lifetimes than controls, according to the results of a retrospective case-control study of 60 women with aneurysms.
“These findings suggest that our case group may have had not only lower levels of estrogen, but also more severe physiologic fluctuations in their estrogen level as compared to population averages. To take this one step further, therapies that mitigate the effects of low estrogen level and fluctuations may decrease the risk for cerebral aneurysms,” Dr. Michael Chen said
The study included 21 women with ruptured aneurysms and 39 with unruptured aneurysms who were under the care of a single physician during 2008-2010. Their median age was 52 years, with a range of 31-80 years. Dr. Chen and his associates conducted telephone interviews with the patients to obtain detailed medical, social, family, and gynecologic histories, including information about lifetime use and duration of use of oral contraceptives and hormone replacement therapy, as well as menstrual and reproductive history.
For the control group, the investigators spoke with 4,682 random American women from a publicly available data set (Ann. Epidemiol. 2002;12:213-22) that was generated as part of a National Institutes of Health–funded study exploring the use of oral contraceptives and hormone replacement therapy in breast cancer.
For each case, seven control patients matched for age and educational levels were selected, giving 420 control patients. Measures such as median age, body mass index (BMI), age of menarche, nulliparity, and age of first pregnancy were comparable between the case and control patients.
Significantly fewer women with an aneurysm reported lifetime use of oral contraceptives than did controls (60% vs. 77.6%, P = .003) as well as use of hormone replacement therapy (23.7% vs. 44.8%, P = .002). The median age of menopause for women with aneurysms was more than 2 years earlier than for the control group (44.5 vs. 46.9 years, P = .53) and 50% of cases had menopause before age 45, compared with 34% of controls (P = .18). “The patient group also had a high rate of early hysterectomies,” noted Dr. Chen, an interventional vascular neurologist affiliated with Rush University Medical Center, Chicago.
One of the limitations of the study is that the vasculature of the control group had not been evaluated, although it is assumed that 3%-4% of the general population harbors an unruptured cerebral aneurysm. Another limitation is that the control group data were generated during 1994-1998, before the use of hormone replacement therapy decreased substantially in 2002, Dr. Chen said.
He hopes these findings will guide future research not only on the role of estrogen in aneurysm development, but also the role of the estrogen receptor specific to cerebral vasculature.
“Understanding more about the effects of estrogen at the receptor level may allow us to find therapies that target that receptor in order to provide some protection for women at risk of developing aneurysms or harboring unruptured aneurysms,” Dr. Chen concluded.
Major Finding: Compared with a control group matched for age and educational levels, a significantly lower percentage of postmenopausal women with cerebral aneurysms reported lifetime use of oral contraceptives (77.6% vs. 60%, respectively) and hormone therapy (44.8% vs. 23.7%).
Data Source: Retrospective case-control study of 60 women with ruptured and unruptured aneurysms seen by one physician at a single center and a control group of 420 patients from a publicly available data set.
Disclosures: Dr. Chen reported no relevant disclosures.
Carlsbad, Calif. — Women with cerebral aneurysms were less likely to have used oral contraceptives or hormone replacement therapy in their lifetimes than controls, according to the results of a retrospective case-control study of 60 women with aneurysms.
“These findings suggest that our case group may have had not only lower levels of estrogen, but also more severe physiologic fluctuations in their estrogen level as compared to population averages. To take this one step further, therapies that mitigate the effects of low estrogen level and fluctuations may decrease the risk for cerebral aneurysms,” Dr. Michael Chen said
The study included 21 women with ruptured aneurysms and 39 with unruptured aneurysms who were under the care of a single physician during 2008-2010. Their median age was 52 years, with a range of 31-80 years. Dr. Chen and his associates conducted telephone interviews with the patients to obtain detailed medical, social, family, and gynecologic histories, including information about lifetime use and duration of use of oral contraceptives and hormone replacement therapy, as well as menstrual and reproductive history.
For the control group, the investigators spoke with 4,682 random American women from a publicly available data set (Ann. Epidemiol. 2002;12:213-22) that was generated as part of a National Institutes of Health–funded study exploring the use of oral contraceptives and hormone replacement therapy in breast cancer.
For each case, seven control patients matched for age and educational levels were selected, giving 420 control patients. Measures such as median age, body mass index (BMI), age of menarche, nulliparity, and age of first pregnancy were comparable between the case and control patients.
Significantly fewer women with an aneurysm reported lifetime use of oral contraceptives than did controls (60% vs. 77.6%, P = .003) as well as use of hormone replacement therapy (23.7% vs. 44.8%, P = .002). The median age of menopause for women with aneurysms was more than 2 years earlier than for the control group (44.5 vs. 46.9 years, P = .53) and 50% of cases had menopause before age 45, compared with 34% of controls (P = .18). “The patient group also had a high rate of early hysterectomies,” noted Dr. Chen, an interventional vascular neurologist affiliated with Rush University Medical Center, Chicago.
One of the limitations of the study is that the vasculature of the control group had not been evaluated, although it is assumed that 3%-4% of the general population harbors an unruptured cerebral aneurysm. Another limitation is that the control group data were generated during 1994-1998, before the use of hormone replacement therapy decreased substantially in 2002, Dr. Chen said.
He hopes these findings will guide future research not only on the role of estrogen in aneurysm development, but also the role of the estrogen receptor specific to cerebral vasculature.
“Understanding more about the effects of estrogen at the receptor level may allow us to find therapies that target that receptor in order to provide some protection for women at risk of developing aneurysms or harboring unruptured aneurysms,” Dr. Chen concluded.
Aneurysmal Coiling Often Leads to Acute Headache
Major Finding: Postprocedural headaches occurred in 72% of patients who underwent intracranial endovascular aneurysmal coiling.
Data Source: A retrospective chart review of 263 patients who underwent intracranial endovascular aneurysmal coiling.
Disclosures: Dr. Baron had no relevant disclosures.
CARLSBAD, CALIF. – Nearly three quarters of patients had an acute headache after endovascular coiling of cerebral aneurysms in a review of a 3-year period at a single center.
The postprocedural headaches occurred significantly more often in women, smokers, and patients with a preprocedural history of headache or anxiety and depression, according to Dr. Eric P. Baron.
“Optimized risk-factor management prior to coiling may help decrease the occurrence of postcoiling headache.
“The presence of these risk factors may also help predict those more likely to complain of postcoiling headache and help guide clinical decisions of neuroimaging or other testing.
However, good clinical judgment should always supersede in these decisions,” said Dr. Baron, a neurologist who is affiliated with the Center for Headache and Pain at the Cleveland Clinic Neurological Institute.
Although urgent diagnostic procedures to evaluate postcoiling headaches proved unhelpful, Dr. Baron and his colleagues found that triptans or dihydroergotamine (DHE) safely treated both pre- and postcoiling headaches in a small group of migraineurs.
Headache was also common in aneurysm patients before coiling, both for those who underwent emergent and elective coiling. Coiling resolved headaches in a small proportion of these patients, he noted.
“Triptans and DHE may not necessarily be a contraindication in all migraineurs with pre- and postcoiling headache, and aneurysmal coiling may actually resolve preexisting headaches in a select group of patients, but at this time, predicting that group of patients is unclear.
“Ultimately, further prospective studies are necessary to better evaluate all of these trends,” Dr. Baron said.
The investigators reviewed the records of 263 adult patients (200 women and 63 men) who underwent either emergent or elective intracranial endovascular coiling for aneurysm treatment between July 2006 and June 2009.
Patients with skull defects, ventricular shunt placement, cranial trauma, extracranial procedures, and intracranial neoplasms or infections were excluded.
Most (76%) of the aneurysms were located in the anterior circulation; 24% were in the posterior circulation. A headache developed following coiling in 189 (72%) patients.
A significantly greater percentage of patients with headaches were women (81%).
More women overall also developed postcoiling headache than did men (77% vs. 57%).
Smoking was a significant risk factor for postprocedural headache. A majority of patients (56%) with postcoiling headaches were smokers, and 85% of all smokers developed postcoiling headache.
The incidence of postcoiling headache was higher in women who smoked than it was in men who smoked (90% vs. 70%, respectively).
Postcoiling headaches also affected 86% of patients with either anxiety or depression.
Postprocedural headaches were significantly more likely to occur among patients who experienced headaches prior to undergoing endovascular coiling, regardless of the length of time they had had them, the review found.
Headache complaints spurred 118 urgent diagnostic procedures, including 69 noncontrast CTs, 7 CT angiograms, 29 MR scans (including angiography and venography), 5 cerebral angiograms, and 8 lumbar punctures. All were negative for an acute process that was felt to be the cause of the headache.
“Excessive diagnostic testing is often obtained in patients with prior intracranial endovascular coiling. Results are frequently low yield and may lead to unnecessary risks and costs,” Dr. Baron said.
Pre- and postcoiled aneurysms often are considered a contraindication for the use of triptans or ergots such as DHE to treat headaches in migraineurs, according to Dr. Baron. But in this cohort, triptans were used without incident in 10 cases before coiling and in 10 cases after coiling; DHE was used for one patient after coiling.
Headaches resolved after coiling in a small proportion of patients, including 27% of patients who underwent emergency coiling, 16% of patients who had headaches for less than 1 year before elective coiling, and 11% of patients who had headaches for 1 year or longer before elective coiling.
Major Finding: Postprocedural headaches occurred in 72% of patients who underwent intracranial endovascular aneurysmal coiling.
Data Source: A retrospective chart review of 263 patients who underwent intracranial endovascular aneurysmal coiling.
Disclosures: Dr. Baron had no relevant disclosures.
CARLSBAD, CALIF. – Nearly three quarters of patients had an acute headache after endovascular coiling of cerebral aneurysms in a review of a 3-year period at a single center.
The postprocedural headaches occurred significantly more often in women, smokers, and patients with a preprocedural history of headache or anxiety and depression, according to Dr. Eric P. Baron.
“Optimized risk-factor management prior to coiling may help decrease the occurrence of postcoiling headache.
“The presence of these risk factors may also help predict those more likely to complain of postcoiling headache and help guide clinical decisions of neuroimaging or other testing.
However, good clinical judgment should always supersede in these decisions,” said Dr. Baron, a neurologist who is affiliated with the Center for Headache and Pain at the Cleveland Clinic Neurological Institute.
Although urgent diagnostic procedures to evaluate postcoiling headaches proved unhelpful, Dr. Baron and his colleagues found that triptans or dihydroergotamine (DHE) safely treated both pre- and postcoiling headaches in a small group of migraineurs.
Headache was also common in aneurysm patients before coiling, both for those who underwent emergent and elective coiling. Coiling resolved headaches in a small proportion of these patients, he noted.
“Triptans and DHE may not necessarily be a contraindication in all migraineurs with pre- and postcoiling headache, and aneurysmal coiling may actually resolve preexisting headaches in a select group of patients, but at this time, predicting that group of patients is unclear.
“Ultimately, further prospective studies are necessary to better evaluate all of these trends,” Dr. Baron said.
The investigators reviewed the records of 263 adult patients (200 women and 63 men) who underwent either emergent or elective intracranial endovascular coiling for aneurysm treatment between July 2006 and June 2009.
Patients with skull defects, ventricular shunt placement, cranial trauma, extracranial procedures, and intracranial neoplasms or infections were excluded.
Most (76%) of the aneurysms were located in the anterior circulation; 24% were in the posterior circulation. A headache developed following coiling in 189 (72%) patients.
A significantly greater percentage of patients with headaches were women (81%).
More women overall also developed postcoiling headache than did men (77% vs. 57%).
Smoking was a significant risk factor for postprocedural headache. A majority of patients (56%) with postcoiling headaches were smokers, and 85% of all smokers developed postcoiling headache.
The incidence of postcoiling headache was higher in women who smoked than it was in men who smoked (90% vs. 70%, respectively).
Postcoiling headaches also affected 86% of patients with either anxiety or depression.
Postprocedural headaches were significantly more likely to occur among patients who experienced headaches prior to undergoing endovascular coiling, regardless of the length of time they had had them, the review found.
Headache complaints spurred 118 urgent diagnostic procedures, including 69 noncontrast CTs, 7 CT angiograms, 29 MR scans (including angiography and venography), 5 cerebral angiograms, and 8 lumbar punctures. All were negative for an acute process that was felt to be the cause of the headache.
“Excessive diagnostic testing is often obtained in patients with prior intracranial endovascular coiling. Results are frequently low yield and may lead to unnecessary risks and costs,” Dr. Baron said.
Pre- and postcoiled aneurysms often are considered a contraindication for the use of triptans or ergots such as DHE to treat headaches in migraineurs, according to Dr. Baron. But in this cohort, triptans were used without incident in 10 cases before coiling and in 10 cases after coiling; DHE was used for one patient after coiling.
Headaches resolved after coiling in a small proportion of patients, including 27% of patients who underwent emergency coiling, 16% of patients who had headaches for less than 1 year before elective coiling, and 11% of patients who had headaches for 1 year or longer before elective coiling.
Major Finding: Postprocedural headaches occurred in 72% of patients who underwent intracranial endovascular aneurysmal coiling.
Data Source: A retrospective chart review of 263 patients who underwent intracranial endovascular aneurysmal coiling.
Disclosures: Dr. Baron had no relevant disclosures.
CARLSBAD, CALIF. – Nearly three quarters of patients had an acute headache after endovascular coiling of cerebral aneurysms in a review of a 3-year period at a single center.
The postprocedural headaches occurred significantly more often in women, smokers, and patients with a preprocedural history of headache or anxiety and depression, according to Dr. Eric P. Baron.
“Optimized risk-factor management prior to coiling may help decrease the occurrence of postcoiling headache.
“The presence of these risk factors may also help predict those more likely to complain of postcoiling headache and help guide clinical decisions of neuroimaging or other testing.
However, good clinical judgment should always supersede in these decisions,” said Dr. Baron, a neurologist who is affiliated with the Center for Headache and Pain at the Cleveland Clinic Neurological Institute.
Although urgent diagnostic procedures to evaluate postcoiling headaches proved unhelpful, Dr. Baron and his colleagues found that triptans or dihydroergotamine (DHE) safely treated both pre- and postcoiling headaches in a small group of migraineurs.
Headache was also common in aneurysm patients before coiling, both for those who underwent emergent and elective coiling. Coiling resolved headaches in a small proportion of these patients, he noted.
“Triptans and DHE may not necessarily be a contraindication in all migraineurs with pre- and postcoiling headache, and aneurysmal coiling may actually resolve preexisting headaches in a select group of patients, but at this time, predicting that group of patients is unclear.
“Ultimately, further prospective studies are necessary to better evaluate all of these trends,” Dr. Baron said.
The investigators reviewed the records of 263 adult patients (200 women and 63 men) who underwent either emergent or elective intracranial endovascular coiling for aneurysm treatment between July 2006 and June 2009.
Patients with skull defects, ventricular shunt placement, cranial trauma, extracranial procedures, and intracranial neoplasms or infections were excluded.
Most (76%) of the aneurysms were located in the anterior circulation; 24% were in the posterior circulation. A headache developed following coiling in 189 (72%) patients.
A significantly greater percentage of patients with headaches were women (81%).
More women overall also developed postcoiling headache than did men (77% vs. 57%).
Smoking was a significant risk factor for postprocedural headache. A majority of patients (56%) with postcoiling headaches were smokers, and 85% of all smokers developed postcoiling headache.
The incidence of postcoiling headache was higher in women who smoked than it was in men who smoked (90% vs. 70%, respectively).
Postcoiling headaches also affected 86% of patients with either anxiety or depression.
Postprocedural headaches were significantly more likely to occur among patients who experienced headaches prior to undergoing endovascular coiling, regardless of the length of time they had had them, the review found.
Headache complaints spurred 118 urgent diagnostic procedures, including 69 noncontrast CTs, 7 CT angiograms, 29 MR scans (including angiography and venography), 5 cerebral angiograms, and 8 lumbar punctures. All were negative for an acute process that was felt to be the cause of the headache.
“Excessive diagnostic testing is often obtained in patients with prior intracranial endovascular coiling. Results are frequently low yield and may lead to unnecessary risks and costs,” Dr. Baron said.
Pre- and postcoiled aneurysms often are considered a contraindication for the use of triptans or ergots such as DHE to treat headaches in migraineurs, according to Dr. Baron. But in this cohort, triptans were used without incident in 10 cases before coiling and in 10 cases after coiling; DHE was used for one patient after coiling.
Headaches resolved after coiling in a small proportion of patients, including 27% of patients who underwent emergency coiling, 16% of patients who had headaches for less than 1 year before elective coiling, and 11% of patients who had headaches for 1 year or longer before elective coiling.
Experts Debate Impact of AAN’s New Guidelines for Acute Ischemic Stroke Diagnosis
CARLSBAD, Calif. – Time will tell if current practice for stroke diagnosis will change following issuance of a new guideline from the American Academy of Neurology that recommends diffusion weighted MRI for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.
“Not a lot of people are using MRI,” Dr. A. Greg Sorensen, a professor in radiology at Harvard Medical School, Boston, and member of the guideline subcommittee, said at the annual meeting of the Society of NeuroInterventional Surgery. An informal poll he conducted at the meeting of about 300 physicians on the front line of stroke treatment indicated that 24% were using diffusion-weighted MR imaging (DWI) for patients who present within 4.5 hours of symptom onset and 38% were using it after 4.5 hours of symptom onset.
The guideline was issued by a panel of neurologists, neuroradiologists, and radiologists who performed a literature search in which they identified relevant abstracts published from 1966 through January 2008 that were related to the diagnostic and prognostic value of DWI and perfusion-weighted imaging (PWI).
The panelists concluded that there was strong (level A) evidence that DWI should be considered superior to non–contrast CT for the diagnosis of acute ischemic stroke in patients presenting within 12 hours of symptom onset, with an estimated sensitivity of 80%-90%. However, there was insufficient evidence to support or refute the value of PWI for acute ischemic stroke diagnosis.
They reported that although non–contrast CT is the current diagnostic standard for acute stroke, it has limited sensitivity, especially in the initial hours (Neurology 2010;75:177-85).
In addition, they found level B evidence to support the use of DWI volume in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation strokes. Baseline PWI volume also was found to be useful in predicting baseline clinical stroke severity, although the evidence for that was weak (level C).
Weak evidence was available to support the use of DWI volume in predicting clinical outcome, as measured by the National Institutes of Health Stroke Scale and Barthel Index for daily functioning, and the panelists determined that baseline DWI was not useful in predicting baseline NIHSS score in posterior-circulation strokes.
“This information is not a revelation. We have known for some time that DWI is superior to non–contrast CT for the diagnosis of ischemic stroke,” commented Dr. Ansaar T. Rai in an interview. He had spoken at the meeting on the use of CT perfusion for acute stroke imaging. “Non–contrast CT of the brain has been around since the early 1970s – it’s an old technology. But this study did not compare DWI/PWI with CT perfusion. I think a more appropriate issue is to identify the best advanced imaging study that can be efficiently used to diagnose acute ischemic stroke and triage stroke patients.
“If treatment for ischemic stroke is going to gain ground from its current dismal state, in which only 1%-2% of strokes are being treated, then stroke treatment must be disseminated widely, not just in big centers,” said Dr. Rai, director of interventional neuroradiology at West Virginia University Health Sciences Center, Morgantown.
He pointed out that CT is three times more available than MRI nationwide. In addition, CT scans are generally available 24 hours a day, 7 days a week in almost every hospital, unlike MRIs which are generally not available after 5 p.m. or require that a technician to be called in, a situation that is expensive and wastes valuable time.
For these reasons, Dr. Rai said he advocates the use of a stroke CT protocol – including a non–contrast CT, CT angiogram, and CT perfusion – as the diagnostic test of choice for suspected acute ischemic stroke. “The whole test takes 30 seconds to perform and within 5 minutes we have the information of exactly where the clot is and what part of the brain is affected.”
He put advanced CT imaging on a par with DWI/PWI for diagnosis of stroke and triaging patients for treatment, especially endovascular therapy. “At our institution, based on our experience, we are going to continue to [use] the ‘stroke CT’ for triage of acute stroke patients.”
At the University of California, Los Angeles, Stroke Center, a major clinical and research center, Dr. David S. Liebeskind, the director of stroke imaging, has state-of-the-art imaging tools readily available. Like Dr. Rai, he agreed with the AAN’s findings that DWI is preferred over non–contrast CT, but also said that the more appropriate comparison would have been DWI/PWI to equivalent CT techniques including perfusion imaging.
Dr. Liebeskind said in an interview that the importance of the AAN guideline lay in the fact that it is “one of the first changes in the recommended use of imaging modalities for ischemic stroke since CT was introduced 35 years ago.”
At UCLA, DWI is the preferred imaging modality for diagnosis of acute ischemic stroke. However, he said that in 2010, all imaging modalities should be used as appropriate to refine diagnosis and guide treatment. “At centers that have access to both CT and MRI, it is important to get the most information as fast as possible. Although DWI is more sensitive than non–contrast CT and may be preferred, if the CT scanner is available and MRI is not, go with the CT,” he said.
CT perfusion can give the same information as MR perfusion, Dr. Liebeskind asserted. “I’ve come to see that there is often a routine preference for a given modality at each stroke center, and it varies across sites. Centers seem to excel in the use of the particular imaging modality used most often – CT or MRI.”
He emphasized that imaging studies are an essential component of stroke patient evaluation, and clinicians, whether they are neurologists or not, must quickly interpret and apply the information in real time to make rapid decisions about treatment. “Imaging studies can tell us if it is too late or too risky to institute a particular therapy or guide us to do something fairly aggressive during early phases to reverse any potential neurological injury,” he said.
Dr. Rai serves as a consultant to Boston Scientific Neurovascular and Concentric Medical Inc., which makes neurointerventional products. Dr. Liebeskind is a consultant for Concentric Medical and CoAxia Inc., maker of a perfusion augmentation device. Information about Dr. Sorensen’s disclosures was not available at press time.
CARLSBAD, Calif. – Time will tell if current practice for stroke diagnosis will change following issuance of a new guideline from the American Academy of Neurology that recommends diffusion weighted MRI for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.
“Not a lot of people are using MRI,” Dr. A. Greg Sorensen, a professor in radiology at Harvard Medical School, Boston, and member of the guideline subcommittee, said at the annual meeting of the Society of NeuroInterventional Surgery. An informal poll he conducted at the meeting of about 300 physicians on the front line of stroke treatment indicated that 24% were using diffusion-weighted MR imaging (DWI) for patients who present within 4.5 hours of symptom onset and 38% were using it after 4.5 hours of symptom onset.
The guideline was issued by a panel of neurologists, neuroradiologists, and radiologists who performed a literature search in which they identified relevant abstracts published from 1966 through January 2008 that were related to the diagnostic and prognostic value of DWI and perfusion-weighted imaging (PWI).
The panelists concluded that there was strong (level A) evidence that DWI should be considered superior to non–contrast CT for the diagnosis of acute ischemic stroke in patients presenting within 12 hours of symptom onset, with an estimated sensitivity of 80%-90%. However, there was insufficient evidence to support or refute the value of PWI for acute ischemic stroke diagnosis.
They reported that although non–contrast CT is the current diagnostic standard for acute stroke, it has limited sensitivity, especially in the initial hours (Neurology 2010;75:177-85).
In addition, they found level B evidence to support the use of DWI volume in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation strokes. Baseline PWI volume also was found to be useful in predicting baseline clinical stroke severity, although the evidence for that was weak (level C).
Weak evidence was available to support the use of DWI volume in predicting clinical outcome, as measured by the National Institutes of Health Stroke Scale and Barthel Index for daily functioning, and the panelists determined that baseline DWI was not useful in predicting baseline NIHSS score in posterior-circulation strokes.
“This information is not a revelation. We have known for some time that DWI is superior to non–contrast CT for the diagnosis of ischemic stroke,” commented Dr. Ansaar T. Rai in an interview. He had spoken at the meeting on the use of CT perfusion for acute stroke imaging. “Non–contrast CT of the brain has been around since the early 1970s – it’s an old technology. But this study did not compare DWI/PWI with CT perfusion. I think a more appropriate issue is to identify the best advanced imaging study that can be efficiently used to diagnose acute ischemic stroke and triage stroke patients.
“If treatment for ischemic stroke is going to gain ground from its current dismal state, in which only 1%-2% of strokes are being treated, then stroke treatment must be disseminated widely, not just in big centers,” said Dr. Rai, director of interventional neuroradiology at West Virginia University Health Sciences Center, Morgantown.
He pointed out that CT is three times more available than MRI nationwide. In addition, CT scans are generally available 24 hours a day, 7 days a week in almost every hospital, unlike MRIs which are generally not available after 5 p.m. or require that a technician to be called in, a situation that is expensive and wastes valuable time.
For these reasons, Dr. Rai said he advocates the use of a stroke CT protocol – including a non–contrast CT, CT angiogram, and CT perfusion – as the diagnostic test of choice for suspected acute ischemic stroke. “The whole test takes 30 seconds to perform and within 5 minutes we have the information of exactly where the clot is and what part of the brain is affected.”
He put advanced CT imaging on a par with DWI/PWI for diagnosis of stroke and triaging patients for treatment, especially endovascular therapy. “At our institution, based on our experience, we are going to continue to [use] the ‘stroke CT’ for triage of acute stroke patients.”
At the University of California, Los Angeles, Stroke Center, a major clinical and research center, Dr. David S. Liebeskind, the director of stroke imaging, has state-of-the-art imaging tools readily available. Like Dr. Rai, he agreed with the AAN’s findings that DWI is preferred over non–contrast CT, but also said that the more appropriate comparison would have been DWI/PWI to equivalent CT techniques including perfusion imaging.
Dr. Liebeskind said in an interview that the importance of the AAN guideline lay in the fact that it is “one of the first changes in the recommended use of imaging modalities for ischemic stroke since CT was introduced 35 years ago.”
At UCLA, DWI is the preferred imaging modality for diagnosis of acute ischemic stroke. However, he said that in 2010, all imaging modalities should be used as appropriate to refine diagnosis and guide treatment. “At centers that have access to both CT and MRI, it is important to get the most information as fast as possible. Although DWI is more sensitive than non–contrast CT and may be preferred, if the CT scanner is available and MRI is not, go with the CT,” he said.
CT perfusion can give the same information as MR perfusion, Dr. Liebeskind asserted. “I’ve come to see that there is often a routine preference for a given modality at each stroke center, and it varies across sites. Centers seem to excel in the use of the particular imaging modality used most often – CT or MRI.”
He emphasized that imaging studies are an essential component of stroke patient evaluation, and clinicians, whether they are neurologists or not, must quickly interpret and apply the information in real time to make rapid decisions about treatment. “Imaging studies can tell us if it is too late or too risky to institute a particular therapy or guide us to do something fairly aggressive during early phases to reverse any potential neurological injury,” he said.
Dr. Rai serves as a consultant to Boston Scientific Neurovascular and Concentric Medical Inc., which makes neurointerventional products. Dr. Liebeskind is a consultant for Concentric Medical and CoAxia Inc., maker of a perfusion augmentation device. Information about Dr. Sorensen’s disclosures was not available at press time.
CARLSBAD, Calif. – Time will tell if current practice for stroke diagnosis will change following issuance of a new guideline from the American Academy of Neurology that recommends diffusion weighted MRI for diagnosing acute ischemic stroke in patients presenting within 12 hours of symptom onset.
“Not a lot of people are using MRI,” Dr. A. Greg Sorensen, a professor in radiology at Harvard Medical School, Boston, and member of the guideline subcommittee, said at the annual meeting of the Society of NeuroInterventional Surgery. An informal poll he conducted at the meeting of about 300 physicians on the front line of stroke treatment indicated that 24% were using diffusion-weighted MR imaging (DWI) for patients who present within 4.5 hours of symptom onset and 38% were using it after 4.5 hours of symptom onset.
The guideline was issued by a panel of neurologists, neuroradiologists, and radiologists who performed a literature search in which they identified relevant abstracts published from 1966 through January 2008 that were related to the diagnostic and prognostic value of DWI and perfusion-weighted imaging (PWI).
The panelists concluded that there was strong (level A) evidence that DWI should be considered superior to non–contrast CT for the diagnosis of acute ischemic stroke in patients presenting within 12 hours of symptom onset, with an estimated sensitivity of 80%-90%. However, there was insufficient evidence to support or refute the value of PWI for acute ischemic stroke diagnosis.
They reported that although non–contrast CT is the current diagnostic standard for acute stroke, it has limited sensitivity, especially in the initial hours (Neurology 2010;75:177-85).
In addition, they found level B evidence to support the use of DWI volume in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation strokes. Baseline PWI volume also was found to be useful in predicting baseline clinical stroke severity, although the evidence for that was weak (level C).
Weak evidence was available to support the use of DWI volume in predicting clinical outcome, as measured by the National Institutes of Health Stroke Scale and Barthel Index for daily functioning, and the panelists determined that baseline DWI was not useful in predicting baseline NIHSS score in posterior-circulation strokes.
“This information is not a revelation. We have known for some time that DWI is superior to non–contrast CT for the diagnosis of ischemic stroke,” commented Dr. Ansaar T. Rai in an interview. He had spoken at the meeting on the use of CT perfusion for acute stroke imaging. “Non–contrast CT of the brain has been around since the early 1970s – it’s an old technology. But this study did not compare DWI/PWI with CT perfusion. I think a more appropriate issue is to identify the best advanced imaging study that can be efficiently used to diagnose acute ischemic stroke and triage stroke patients.
“If treatment for ischemic stroke is going to gain ground from its current dismal state, in which only 1%-2% of strokes are being treated, then stroke treatment must be disseminated widely, not just in big centers,” said Dr. Rai, director of interventional neuroradiology at West Virginia University Health Sciences Center, Morgantown.
He pointed out that CT is three times more available than MRI nationwide. In addition, CT scans are generally available 24 hours a day, 7 days a week in almost every hospital, unlike MRIs which are generally not available after 5 p.m. or require that a technician to be called in, a situation that is expensive and wastes valuable time.
For these reasons, Dr. Rai said he advocates the use of a stroke CT protocol – including a non–contrast CT, CT angiogram, and CT perfusion – as the diagnostic test of choice for suspected acute ischemic stroke. “The whole test takes 30 seconds to perform and within 5 minutes we have the information of exactly where the clot is and what part of the brain is affected.”
He put advanced CT imaging on a par with DWI/PWI for diagnosis of stroke and triaging patients for treatment, especially endovascular therapy. “At our institution, based on our experience, we are going to continue to [use] the ‘stroke CT’ for triage of acute stroke patients.”
At the University of California, Los Angeles, Stroke Center, a major clinical and research center, Dr. David S. Liebeskind, the director of stroke imaging, has state-of-the-art imaging tools readily available. Like Dr. Rai, he agreed with the AAN’s findings that DWI is preferred over non–contrast CT, but also said that the more appropriate comparison would have been DWI/PWI to equivalent CT techniques including perfusion imaging.
Dr. Liebeskind said in an interview that the importance of the AAN guideline lay in the fact that it is “one of the first changes in the recommended use of imaging modalities for ischemic stroke since CT was introduced 35 years ago.”
At UCLA, DWI is the preferred imaging modality for diagnosis of acute ischemic stroke. However, he said that in 2010, all imaging modalities should be used as appropriate to refine diagnosis and guide treatment. “At centers that have access to both CT and MRI, it is important to get the most information as fast as possible. Although DWI is more sensitive than non–contrast CT and may be preferred, if the CT scanner is available and MRI is not, go with the CT,” he said.
CT perfusion can give the same information as MR perfusion, Dr. Liebeskind asserted. “I’ve come to see that there is often a routine preference for a given modality at each stroke center, and it varies across sites. Centers seem to excel in the use of the particular imaging modality used most often – CT or MRI.”
He emphasized that imaging studies are an essential component of stroke patient evaluation, and clinicians, whether they are neurologists or not, must quickly interpret and apply the information in real time to make rapid decisions about treatment. “Imaging studies can tell us if it is too late or too risky to institute a particular therapy or guide us to do something fairly aggressive during early phases to reverse any potential neurological injury,” he said.
Dr. Rai serves as a consultant to Boston Scientific Neurovascular and Concentric Medical Inc., which makes neurointerventional products. Dr. Liebeskind is a consultant for Concentric Medical and CoAxia Inc., maker of a perfusion augmentation device. Information about Dr. Sorensen’s disclosures was not available at press time.
Stopping Antiplatelets May Risk Thrombosis After Aneurysm Stenting
CARLSBAD, Calif. – Delayed thrombotic events have been observed following the cessation of double antiplatelet therapy in patients who have received an Enterprise stent for an intracranial aneurysm.
Seven of 213 patients in the Interstate Collaboration of the Enterprise Stent Coiling (ICES) Multicenter Registry experienced a thrombotic event 2-24 weeks after placement of the stent and stopping aspirin and clopidogrel, Dr. J. Mocco reported at the annual meeting of the Society of NeuroInterventional Surgery. Another two patients experienced acute cases of stent thrombosis.
Ten sites in the United States and Puerto Rico participate in the registry to rapidly provide large-volume, real-world results regarding experience in using the Enterprise Vascular Reconstruction Device and Delivery System (J. Neurosurg. 2009;110:35-9) for the treatment of intracranial aneurysms. The stent system is designed to assist in the embolic coiling of wide-neck aneurysms.
In the seven cases, the patients either did not comply with the drug regimen or stopped taking the antiplatelets on the order of the physician.
The data suggest that early cessation of double antiplatelet therapy is not ideal, although it is currently unclear what the optimum time course should be, said Dr. Mocco, an endovascular neurosurgeon affiliated with the University of Florida, Gainesville.
Use of the Enterprise stent to assist with aneurysm coiling was associated with 90% or greater occlusion in 88% of aneurysms. Permanent morbidity (all minor) occurred in 1.4% of patients.
None of the thrombotic events were associated with coil prolapse or parent vessel tortuosity, said Dr. Mocco. Three events had no angiographic corollary and two were diagnosed based on symptoms.
Dr. Mocco gave his presentation during a session sponsored by Codman Neurovascular, the manufacturer of the Enterprise stent. He disclosed that he received no direct financial support from Codman and the data were generated independent of commercial influence, although the University of Florida receives an educational grant and consulting fees from Codman. He is a consultant to several other companies that manufacture cerebrovascular therapies.
CARLSBAD, Calif. – Delayed thrombotic events have been observed following the cessation of double antiplatelet therapy in patients who have received an Enterprise stent for an intracranial aneurysm.
Seven of 213 patients in the Interstate Collaboration of the Enterprise Stent Coiling (ICES) Multicenter Registry experienced a thrombotic event 2-24 weeks after placement of the stent and stopping aspirin and clopidogrel, Dr. J. Mocco reported at the annual meeting of the Society of NeuroInterventional Surgery. Another two patients experienced acute cases of stent thrombosis.
Ten sites in the United States and Puerto Rico participate in the registry to rapidly provide large-volume, real-world results regarding experience in using the Enterprise Vascular Reconstruction Device and Delivery System (J. Neurosurg. 2009;110:35-9) for the treatment of intracranial aneurysms. The stent system is designed to assist in the embolic coiling of wide-neck aneurysms.
In the seven cases, the patients either did not comply with the drug regimen or stopped taking the antiplatelets on the order of the physician.
The data suggest that early cessation of double antiplatelet therapy is not ideal, although it is currently unclear what the optimum time course should be, said Dr. Mocco, an endovascular neurosurgeon affiliated with the University of Florida, Gainesville.
Use of the Enterprise stent to assist with aneurysm coiling was associated with 90% or greater occlusion in 88% of aneurysms. Permanent morbidity (all minor) occurred in 1.4% of patients.
None of the thrombotic events were associated with coil prolapse or parent vessel tortuosity, said Dr. Mocco. Three events had no angiographic corollary and two were diagnosed based on symptoms.
Dr. Mocco gave his presentation during a session sponsored by Codman Neurovascular, the manufacturer of the Enterprise stent. He disclosed that he received no direct financial support from Codman and the data were generated independent of commercial influence, although the University of Florida receives an educational grant and consulting fees from Codman. He is a consultant to several other companies that manufacture cerebrovascular therapies.
CARLSBAD, Calif. – Delayed thrombotic events have been observed following the cessation of double antiplatelet therapy in patients who have received an Enterprise stent for an intracranial aneurysm.
Seven of 213 patients in the Interstate Collaboration of the Enterprise Stent Coiling (ICES) Multicenter Registry experienced a thrombotic event 2-24 weeks after placement of the stent and stopping aspirin and clopidogrel, Dr. J. Mocco reported at the annual meeting of the Society of NeuroInterventional Surgery. Another two patients experienced acute cases of stent thrombosis.
Ten sites in the United States and Puerto Rico participate in the registry to rapidly provide large-volume, real-world results regarding experience in using the Enterprise Vascular Reconstruction Device and Delivery System (J. Neurosurg. 2009;110:35-9) for the treatment of intracranial aneurysms. The stent system is designed to assist in the embolic coiling of wide-neck aneurysms.
In the seven cases, the patients either did not comply with the drug regimen or stopped taking the antiplatelets on the order of the physician.
The data suggest that early cessation of double antiplatelet therapy is not ideal, although it is currently unclear what the optimum time course should be, said Dr. Mocco, an endovascular neurosurgeon affiliated with the University of Florida, Gainesville.
Use of the Enterprise stent to assist with aneurysm coiling was associated with 90% or greater occlusion in 88% of aneurysms. Permanent morbidity (all minor) occurred in 1.4% of patients.
None of the thrombotic events were associated with coil prolapse or parent vessel tortuosity, said Dr. Mocco. Three events had no angiographic corollary and two were diagnosed based on symptoms.
Dr. Mocco gave his presentation during a session sponsored by Codman Neurovascular, the manufacturer of the Enterprise stent. He disclosed that he received no direct financial support from Codman and the data were generated independent of commercial influence, although the University of Florida receives an educational grant and consulting fees from Codman. He is a consultant to several other companies that manufacture cerebrovascular therapies.