User login
Dyspnea and Hyperinflation in Chronic Obstructive Pulmonary Disease: Impact on Physical Activity
Introduction
Dyspnea, the sensation of difficult or labored breathing, is the most common symptom in chronic obstructive pulmonary disease (COPD) and the primary symptom that limits physical activity in more advanced disease.1 According to the American Thoracic Society, dyspnea may be measured according to 3 domains2:
- what breathing feels like for the patient
- how distressed the patient feels when breathing
- how dyspnea affects functional ability, employment, health-related quality of life, or health status.
As disease severity increases, breathlessness becomes more disabling at lower activity levels. These changes further impact the quality of life of patients, and can lead to anxiety and depression.11
Physical inactivity is often considered to be a major contributor to the progression of COPD,6 and is linked to hospitalizations and increased all-cause mortality.12 There is therefore a need to recognize symptoms early and treat them accordingly.
CASE STUDY:
KD, a 64-year-old woman, presented to her primary care physician’s office for a routine visit. Upon assessment, KD revealed that she used to enjoy going on walks with her neighbor, but she cannot walk up the hills in her neighborhood anymore without feeling “incredibly breathless.” She has become increasingly concerned that she is “having trouble getting a full breath.” KD informed her doctor that these symptoms had worsened since her last visit, and so she had stopped going on neighborhood walks. She was diagnosed with COPD 4 years ago, and is currently using a long-acting muscarinic antagonist (LAMA) bronchodilator. KD has a 40 pack-year smoking history, and has previously been advised to stop smoking, but has relapsed several times. She has a medical history of hypertension and depression, and a notable family history of emphysema, breast cancer, and diabetes.
The relationship between lung hyperinflation and dyspnea in COPD
In COPD, pathologic changes give rise to physiologic abnormalities such as mucus hypersecretion and ciliary dysfunction, gas exchange abnormalities, pulmonary hypertension, and airflow limitation and lung hyperinflation.13 Lung hyperinflation, an increase in resting functional residual volume above a normal level, represents a mechanical link between the characteristic expiratory airflow impairment, dyspnea, and physical activity limitation in COPD.1
Although patients can compensate for several of the negative consequences of hyperinflation (eg, altering the chest wall due to overdistended lungs), such compensatory mechanisms are unable to cope with large increases in ventilation, such as those that occur during exercise.1 Air trapping, together with ineffectiveness of respiratory muscle function, leads to increased ventilation requirements and dynamic pulmonary hyperinflation, resulting in dyspnea.1
Patients with COPD describe a sensation of “air hunger,” reporting “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” and a feeling that they “cannot get a deep breath,”18 whereas, in fact, they are limited in their ability to fully exhale. Verbal descriptors (eg, “air hunger” or “chest tightness”) are important tools in understanding a patient’s experience with dyspnea, and a patient’s choice of descriptor may be related to dyspnea severity, and the level of distress that dyspnea causes a given patient.19 Air hunger in turn encourages faster breathing, leading to further shortness of breath and more dynamic hyperinflation.1,20
To deflate the lungs of patients with COPD, physiologic, pharmacologic, and possibly surgical interventions are required:
- Controlled breathing techniques (eg, purse-lipped breathing) that encourage slow and deep breathing can correct abnormal chest wall motion, decrease the work of breathing, increase breathing efficiency, and improve the distribution of ventilation to empty the lungs.21
- Bronchodilators can help to achieve lung deflation by improving ventilatory mechanics, as shown by increases in inspiratory capacity and vital capacity.22
- Lung volume reduction surgery can also be considered to treat severe hyperinflation in emphysematous patients5; bronchoscopic interventions that lower lung volumes are also in development.23
The impact of lung hyperinflation and dyspnea on physical activity in COPD
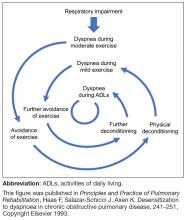
Dyspnea and hyperinflation are closely interrelated with physical activity limitation,16,29,30 and so can be viewed as significant contributors to patient disability. During an acute exacerbation, patients with COPD will experience worsening airway obstruction, dynamic hyperinflation, and dyspnea.31 Patients with a greater number of comorbid conditions may also have greater shortness of breath.32 In addition, patients with COPD and hyperinflation perform less physical activity than individuals without hyperinflation, regardless of COPD severity, as assessed using the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging (stage I, mild; stage II, moderate; stage III, severe; stage IV, very severe) and BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) index.33 These patients also exhibit increases in dyspnea perception during commonly performed ADLs, which may limit physical activity and worsen lung hyperinflation.33 More limited physical activity also contributes to higher dyspnea scores during ADLs.8
Furthermore, the ability to perform typical ADLs may be significantly altered or eliminated altogether in patients with COPD.11 Leisure activities are often the first to be dropped by patients, as they generally require greater effort than simpler tasks, and are not critical to daily life.11 Eventually, these activities become progressively more difficult, and most patients with moderate or severe COPD can struggle to complete even the most basic daily activities.11
In addition to the morbidity burden and impact on ADLs, lower levels of physical activity in patients with COPD have also been shown to increase the risk of mortality and exacerbations, and elevate the risk of comorbidities such as heart disease and metabolic disease.34 In light of these observations, improving exercise capacity should be a key goal in COPD management.
Assessment and measurement of dyspnea and hyperinflation
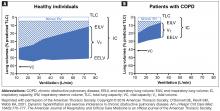
Reducing hyperinflation and dyspnea is essential for improving physical activity endurance and overall physical activity in patients with COPD; therefore, measuring the degree of impairment is important.22 Clinicians should be aware that some patients may have relief of dyspnea due to improvements in hyperinflation, despite relatively mild changes in FEV1.35 Lung volume measures, including total lung capacity, residual volume and functional residual capacity, are valuable tools in the assessment of lung hyperinflation in COPD, and therefore constitute a key component of pulmonary function testing.36 However, expanded pulmonary function testing may be required for patients with severe dyspnea that does not correspond to spirometric findings, or cases in which diagnosis is uncertain.37
Lung volumes are evaluated primarily by body plethysmography, during which a patient sits inside an airtight “body box” equipped to measure pressure and volume changes.14,38 Helium dilution and nitrogen washing can also be used to measure functional residual capacity in patients with COPD,14 but body plethysmography is considered to be a more accurate method of lung volume evaluation in patients with severe airflow obstruction.14,38 Radiographic techniques can also be used, but due to a lack of standardization, they are not typically utilized in clinical practice.14 Measurement of IC may complement other lung volume measures as part of assessment of hyperinflation.16 This can be measured using either spirometry or body plethysmography.39,40
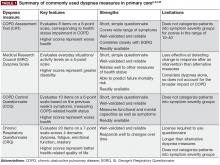
In addition to evaluating hyperinflation, ADLs, physical activity, exercise capacity, and dyspnea should all be assessed in patients with COPD in primary care. It is known that patients may self-limit ADLs to avoid symptoms of COPD; in doing so, worsening symptoms may be underappreciated, and subsequently underreported, by the patient. Thus, it is essential that physicians ask patients with COPD, as well as individuals at risk of COPD, questions about changes in their physical activity or ability to perform common tasks. There are a number of methods to measure functional performance, but for a simple assessment of ADLs, clinicians can ask the patient or caregiver questions related to basic daily tasks.11 In early COPD, patients who experience mild dyspnea during exercise should be able to perform most productive activities. Patients with stable COPD and moderate dyspnea during exercise should be able to carry out most of the higher functioning ADLs, whereas patients with severe COPD may struggle to complete basic ADLs without assistance.11 It should be noted, however, that patients may experience dyspnea with fairly routine activities, and even reduce physical activity at relatively early stages of airflow limitation.41,42
Other tests may be useful in assessing the impact of an intervention, be it pharmacologic or nonpharmacologic, on dyspnea severity. For example, increases in the 6-minute-walk distance (6MWD) have been shown to correlate with improvements in dyspnea.46 The 6MWD has also been shown to be an important predictor of hospitalization and mortality in patients with COPD.47 However, it is important to note that improvements in 6MWD show only a very weak correlation with patient-reported outcomes,48 and may be a less sensitive measure for patients with less disability than those with more profound functional limitation.49 Moreover, 6MWD can be affected by a patient’s psychologic motivation,6,50 as well as other comorbidities observed in patients with COPD, such as osteoporosis, heart failure, and peripheral vascular disease.46,51 Although not used for COPD diagnosis or evaluation of dyspnea or physical activity limitation, a chest X-ray can also be a useful tool for excluding alternative diagnoses, as well as for detecting significant comorbidities in patients with COPD, such as concomitant respiratory, cardiac, and skeletal diseases.5
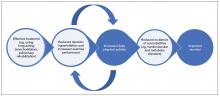
Management of dyspnea and hyperinflation in primary care
Pulmonary rehabilitation is a tailored intervention that encompasses exercise training, education, and self-management support for people with chronic respiratory disease, based on detailed assessment of their exercise capacity and symptoms.52 Pulmonary rehabilitation is as important as medication in COPD management, providing a cost-effective intervention with minimal adverse effects.53 Moreover, pulmonary rehabilitation has been shown to benefit patients with mild to severe dyspnea (as classified according to the Medical Research Council dyspnea scale), demonstrating the value of successful execution of these programs in patients with COPD, irrespective of disease severity.54 Although the most significant improvements in patient quality of life are observed when a multimodality approach is used, exercise and proper pulmonary rehabilitation programs have been shown to improve quality of life more than medication alone.5,55 Notably, there are few supporting data for the use of supplemental oxygen in patients experiencing dyspnea without hypoxemia. Oxygen supplementation is only of minimal benefit to relieving the sensation of dyspnea.56,57
The relationship between the impact of pulmonary rehabilitation in patients with COPD and frailty scores has also been evaluated. Frailty scores are calculated based on an individual’s level of physical activity, and other key criteria that are indicative of their ability to self-manage their medical condition.58 These scores are particularly relevant in the context of COPD, given the high prevalence of the condition in older people.58 Although frailty is a strong independent predictor of noncompletion of pulmonary rehabilitation, completion of a pulmonary rehabilitation program in patients who are frail has been shown to reverse their frailty in the short term.58 It is therefore important that physicians guide and encourage these patients for the duration of a pulmonary rehabilitation program, from initiation through to completion, to ensure that those who are likely to derive the greatest benefit from pulmonary rehabilitation are supported to do so.
In addition to pulmonary rehabilitation, other nonpharmacologic interventions have emerged in recent years that may help to relieve dyspnea in patients with COPD. Airway clearance devices, such as acapella (Smiths Medical; Minneapolis, MN), Flutter (Allergan; Dublin, Ireland), Lung Flute (Medical Acoustics; Buffalo, NY), Quake (Thayer Medical; Tucson, AZ), and Aerobika (Monaghan Medical; Plattsburgh, NY) promote the clearance of sputum through the application of positive expiratory pressure, possibly allowing medicines to penetrate the lungs more effectively, and improving diffuse airflow obstruction.59-61 Incorporating an airway clearance device into a bronchodilator therapy regimen has been shown to improve dyspnea scores, both before and after exercise, compared with bronchodilator therapy combined with a nonfunctional control device in patients with severe COPD.59 In addition, noninvasive forms of ventilation, such as continuous positive airway pressure and bi-level positive airway pressure (BiPAP), have been shown to effectively reduce dyspnea in patients with COPD.62,63 In a 24-month study in patients with severe COPD, resting dyspnea improved significantly in patients using the BiPAP Auto-Trak (Philips Respironics, Best, The Netherlands) in conjunction with their regular bronchodilator therapy, compared with those receiving long-term oxygen therapy in addition to their typical therapeutic regimen.63 Further studies are required to establish the impact of these devices in the management of dyspnea and other symptoms of COPD.
These nonpharmacologic interventions can be supplemented with pharmacologic treatments to help patients achieve their treatment goals of improved dyspnea and increased exercise performance. Bronchodilators, which form the basis of various COPD treatment options, include5:
- short-acting muscarinic antagonists (SAMAs), such as ipratropium
- short-acting β2-agonists (SABAs), such as albuterol, levalbuterol, and terbutaline
- SAMA/SABA combinations
- LAMAs, such as aclidinium, glycopyrrolate, tiotropium, and umeclidinium
- long-acting β 2-agonists (LABAs), such as arformoterol, indacaterol, formoterol, olodaterol, salmeterol, and vilanterol
- LAMA/LABA combinations (umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, glycopyrrolate/indacaterol)
Inhaled corticosteroids can also be used in a fixed-dose combination with a LABA, which can be combined with a LAMA, in select patients5; however, these combination products may have minimal value in treating dyspnea unless asthma is concomitantly present.5,64 Further discussion of the different treatment options available for patients with COPD can be found in the final article of this supplement.
In addition to improving quality of life, long-acting bronchodilators, such as LAMAs, LABAs, and LAMA/LABA combinations, increase expiratory flow, reduce dynamic hyperinflation, and improve exercise capacity of patients.65-67 As disease severity worsens, physicians may opt for long-acting bronchodilator options that have twice-daily dosing, which may confer a benefit in improving night-time symptom control.68
As well as active pharmacologic and nonpharmacologic interventions, physicians should always encourage smoking cessation in patients with COPD, as this has the greatest capacity to influence the natural course of the disease.5 It is essential that health care providers continually deliver smoking cessation messages to patients with COPD; patients can also be supported to stop smoking by using nicotine replacement therapy, pharmacologic interventions, attending smoking cessation programs, and counseling.5
Lung volume reduction surgery may also be considered as a strategy for the management of dyspnea in severe, refractory COPD.69 Similarly, nonsurgical bronchoscopic interventions are being developed that look to achieve similar results to lung volume reduction surgery, including endobronchial one-way valves, lung volume reduction coils, airway bypasses, adhesives, and vapor therapy.23
CASE STUDY:
The primary care physician assessed KD’s dyspnea using the CAT and ordered a chest X-ray to identify any significant comorbidities, such as concomitant respiratory, skeletal, or cardiac diseases. As KD’s CAT score was 17, and her symptoms were uncontrolled on LAMA monotherapy, her physician prescribed a long-acting LAMA/LABA combination, along with pulmonary rehabilitation. The physician also counseled KD on the importance of smoking cessation, and referred her to a local smoking cessation program.
Conclusions
Dyspnea, the most common symptom of COPD and the primary consequence of the condition’s characteristic lung hyperinflation, is a heavy burden on the lives of patients. The impact of dyspnea is perhaps most apparent in the context of physical activity, with activity limitation observed frequently in patients with COPD, regardless of disease stage. This can affect patients’ quality of life significantly, and has long-term consequences on disease progression. Improving dyspnea and increasing exercise endurance should therefore be a key goal for COPD management, which should encompass both nonpharmacologic interventions, such as pulmonary rehabilitation, and pharmacologic interventions, such as use of bronchodilator therapy.
- O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180-184.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272.
- Agusti A, Hedner J, Marin J, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://gold.copd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed November 27, 2017.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577-588.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-1394.
- Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131-137.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977.
- Haas F, Salazar-Schicci J, Axen K. Desensitization to dyspnoea in chronic obstructive pulmonary disease. In: Casaburi R, Petty TL, eds. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: W.B. Saunders; 1993:241-251.
- Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268-278.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187-201.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176-179.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
- Dubé BP, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1.
- Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385.
- Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259-264.
- Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101-111.
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003;40(5 Suppl 2):25-33.
- O’Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Pract. 2015;1:4.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(Suppl 4):S407-S415.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
- O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-549.
- Light RW. Mechanics of respiration. In: George RB, ed. Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:24-38.
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591-597.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753-762.
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181-188.
- Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506-512.
- Di Marco F, Santus P, Sotgiu G, Blasi F, Centanni S. Does improving exercise capacity and daily activity represent the holistic perspective of a new COPD approach? COPD. 2015;12(5):575-581.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042-1050.
- Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745-752.
- Burkhardt R, Pankow W. The diagnosis of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2014;111(49):834-845, quiz 846.
- O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108-1115.
- Criée CP, Sorichter S, Smith HJ, et al; Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959-971.
- Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir Med. 2017;12:3.
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834-840.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005-1011.
- Calverley PMA, Georgopoulos D. Symptoms and signs of COPD. In: Siafakas NM, ed. Management of Chronic Obstructive Pulmonary Disease: European Respiratory Society Journals; 2006.
- Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447-456.
- Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD—revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725-738.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382-386.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225.
- Grant A, Moore L. Pulmonary rehabilitation. In: Blackler L, Jones C, Mooney C, eds. Managing Chronic Obstructive Pulmonary Disease. West Sussex, England: John Wiley & Sons; 2007.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042-1048.
- Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
- Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103(7):1070-1075.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179-187.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429.
- Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988-995.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA Jr. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest. 2002;121(3):702-707.
- Chatburn RL. High-frequency assisted airway clearance. Respir Care. 2007;52(9):1224-1235; discussion 1235-1227.
- Clini E. Positive expiratory pressure techniques in respiratory patients: old evidence and new insights. Breathe. 2009;6(2):153-159.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(2):281-289.
- Clini E, Sturani C, Rossi A, et al; Rehabilitation and Chronic Care Study Group; Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529-538.
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(6):477-482.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288-1296.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
- O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients [published correction appears in Respir Res. 2017;18(1):35]. Respir Res. 2017;18(1):19.
- Shah AA, D’Amico TA. Lung volume reduction surgery for the management of refractory dyspnea in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3(2):107-111.
Introduction
Dyspnea, the sensation of difficult or labored breathing, is the most common symptom in chronic obstructive pulmonary disease (COPD) and the primary symptom that limits physical activity in more advanced disease.1 According to the American Thoracic Society, dyspnea may be measured according to 3 domains2:
- what breathing feels like for the patient
- how distressed the patient feels when breathing
- how dyspnea affects functional ability, employment, health-related quality of life, or health status.
As disease severity increases, breathlessness becomes more disabling at lower activity levels. These changes further impact the quality of life of patients, and can lead to anxiety and depression.11
Physical inactivity is often considered to be a major contributor to the progression of COPD,6 and is linked to hospitalizations and increased all-cause mortality.12 There is therefore a need to recognize symptoms early and treat them accordingly.
CASE STUDY:
KD, a 64-year-old woman, presented to her primary care physician’s office for a routine visit. Upon assessment, KD revealed that she used to enjoy going on walks with her neighbor, but she cannot walk up the hills in her neighborhood anymore without feeling “incredibly breathless.” She has become increasingly concerned that she is “having trouble getting a full breath.” KD informed her doctor that these symptoms had worsened since her last visit, and so she had stopped going on neighborhood walks. She was diagnosed with COPD 4 years ago, and is currently using a long-acting muscarinic antagonist (LAMA) bronchodilator. KD has a 40 pack-year smoking history, and has previously been advised to stop smoking, but has relapsed several times. She has a medical history of hypertension and depression, and a notable family history of emphysema, breast cancer, and diabetes.
The relationship between lung hyperinflation and dyspnea in COPD
In COPD, pathologic changes give rise to physiologic abnormalities such as mucus hypersecretion and ciliary dysfunction, gas exchange abnormalities, pulmonary hypertension, and airflow limitation and lung hyperinflation.13 Lung hyperinflation, an increase in resting functional residual volume above a normal level, represents a mechanical link between the characteristic expiratory airflow impairment, dyspnea, and physical activity limitation in COPD.1
Although patients can compensate for several of the negative consequences of hyperinflation (eg, altering the chest wall due to overdistended lungs), such compensatory mechanisms are unable to cope with large increases in ventilation, such as those that occur during exercise.1 Air trapping, together with ineffectiveness of respiratory muscle function, leads to increased ventilation requirements and dynamic pulmonary hyperinflation, resulting in dyspnea.1
Patients with COPD describe a sensation of “air hunger,” reporting “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” and a feeling that they “cannot get a deep breath,”18 whereas, in fact, they are limited in their ability to fully exhale. Verbal descriptors (eg, “air hunger” or “chest tightness”) are important tools in understanding a patient’s experience with dyspnea, and a patient’s choice of descriptor may be related to dyspnea severity, and the level of distress that dyspnea causes a given patient.19 Air hunger in turn encourages faster breathing, leading to further shortness of breath and more dynamic hyperinflation.1,20
To deflate the lungs of patients with COPD, physiologic, pharmacologic, and possibly surgical interventions are required:
- Controlled breathing techniques (eg, purse-lipped breathing) that encourage slow and deep breathing can correct abnormal chest wall motion, decrease the work of breathing, increase breathing efficiency, and improve the distribution of ventilation to empty the lungs.21
- Bronchodilators can help to achieve lung deflation by improving ventilatory mechanics, as shown by increases in inspiratory capacity and vital capacity.22
- Lung volume reduction surgery can also be considered to treat severe hyperinflation in emphysematous patients5; bronchoscopic interventions that lower lung volumes are also in development.23
The impact of lung hyperinflation and dyspnea on physical activity in COPD
Dyspnea and hyperinflation are closely interrelated with physical activity limitation,16,29,30 and so can be viewed as significant contributors to patient disability. During an acute exacerbation, patients with COPD will experience worsening airway obstruction, dynamic hyperinflation, and dyspnea.31 Patients with a greater number of comorbid conditions may also have greater shortness of breath.32 In addition, patients with COPD and hyperinflation perform less physical activity than individuals without hyperinflation, regardless of COPD severity, as assessed using the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging (stage I, mild; stage II, moderate; stage III, severe; stage IV, very severe) and BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) index.33 These patients also exhibit increases in dyspnea perception during commonly performed ADLs, which may limit physical activity and worsen lung hyperinflation.33 More limited physical activity also contributes to higher dyspnea scores during ADLs.8
Furthermore, the ability to perform typical ADLs may be significantly altered or eliminated altogether in patients with COPD.11 Leisure activities are often the first to be dropped by patients, as they generally require greater effort than simpler tasks, and are not critical to daily life.11 Eventually, these activities become progressively more difficult, and most patients with moderate or severe COPD can struggle to complete even the most basic daily activities.11
In addition to the morbidity burden and impact on ADLs, lower levels of physical activity in patients with COPD have also been shown to increase the risk of mortality and exacerbations, and elevate the risk of comorbidities such as heart disease and metabolic disease.34 In light of these observations, improving exercise capacity should be a key goal in COPD management.
Assessment and measurement of dyspnea and hyperinflation
Reducing hyperinflation and dyspnea is essential for improving physical activity endurance and overall physical activity in patients with COPD; therefore, measuring the degree of impairment is important.22 Clinicians should be aware that some patients may have relief of dyspnea due to improvements in hyperinflation, despite relatively mild changes in FEV1.35 Lung volume measures, including total lung capacity, residual volume and functional residual capacity, are valuable tools in the assessment of lung hyperinflation in COPD, and therefore constitute a key component of pulmonary function testing.36 However, expanded pulmonary function testing may be required for patients with severe dyspnea that does not correspond to spirometric findings, or cases in which diagnosis is uncertain.37
Lung volumes are evaluated primarily by body plethysmography, during which a patient sits inside an airtight “body box” equipped to measure pressure and volume changes.14,38 Helium dilution and nitrogen washing can also be used to measure functional residual capacity in patients with COPD,14 but body plethysmography is considered to be a more accurate method of lung volume evaluation in patients with severe airflow obstruction.14,38 Radiographic techniques can also be used, but due to a lack of standardization, they are not typically utilized in clinical practice.14 Measurement of IC may complement other lung volume measures as part of assessment of hyperinflation.16 This can be measured using either spirometry or body plethysmography.39,40
In addition to evaluating hyperinflation, ADLs, physical activity, exercise capacity, and dyspnea should all be assessed in patients with COPD in primary care. It is known that patients may self-limit ADLs to avoid symptoms of COPD; in doing so, worsening symptoms may be underappreciated, and subsequently underreported, by the patient. Thus, it is essential that physicians ask patients with COPD, as well as individuals at risk of COPD, questions about changes in their physical activity or ability to perform common tasks. There are a number of methods to measure functional performance, but for a simple assessment of ADLs, clinicians can ask the patient or caregiver questions related to basic daily tasks.11 In early COPD, patients who experience mild dyspnea during exercise should be able to perform most productive activities. Patients with stable COPD and moderate dyspnea during exercise should be able to carry out most of the higher functioning ADLs, whereas patients with severe COPD may struggle to complete basic ADLs without assistance.11 It should be noted, however, that patients may experience dyspnea with fairly routine activities, and even reduce physical activity at relatively early stages of airflow limitation.41,42
Other tests may be useful in assessing the impact of an intervention, be it pharmacologic or nonpharmacologic, on dyspnea severity. For example, increases in the 6-minute-walk distance (6MWD) have been shown to correlate with improvements in dyspnea.46 The 6MWD has also been shown to be an important predictor of hospitalization and mortality in patients with COPD.47 However, it is important to note that improvements in 6MWD show only a very weak correlation with patient-reported outcomes,48 and may be a less sensitive measure for patients with less disability than those with more profound functional limitation.49 Moreover, 6MWD can be affected by a patient’s psychologic motivation,6,50 as well as other comorbidities observed in patients with COPD, such as osteoporosis, heart failure, and peripheral vascular disease.46,51 Although not used for COPD diagnosis or evaluation of dyspnea or physical activity limitation, a chest X-ray can also be a useful tool for excluding alternative diagnoses, as well as for detecting significant comorbidities in patients with COPD, such as concomitant respiratory, cardiac, and skeletal diseases.5
Management of dyspnea and hyperinflation in primary care
Pulmonary rehabilitation is a tailored intervention that encompasses exercise training, education, and self-management support for people with chronic respiratory disease, based on detailed assessment of their exercise capacity and symptoms.52 Pulmonary rehabilitation is as important as medication in COPD management, providing a cost-effective intervention with minimal adverse effects.53 Moreover, pulmonary rehabilitation has been shown to benefit patients with mild to severe dyspnea (as classified according to the Medical Research Council dyspnea scale), demonstrating the value of successful execution of these programs in patients with COPD, irrespective of disease severity.54 Although the most significant improvements in patient quality of life are observed when a multimodality approach is used, exercise and proper pulmonary rehabilitation programs have been shown to improve quality of life more than medication alone.5,55 Notably, there are few supporting data for the use of supplemental oxygen in patients experiencing dyspnea without hypoxemia. Oxygen supplementation is only of minimal benefit to relieving the sensation of dyspnea.56,57
The relationship between the impact of pulmonary rehabilitation in patients with COPD and frailty scores has also been evaluated. Frailty scores are calculated based on an individual’s level of physical activity, and other key criteria that are indicative of their ability to self-manage their medical condition.58 These scores are particularly relevant in the context of COPD, given the high prevalence of the condition in older people.58 Although frailty is a strong independent predictor of noncompletion of pulmonary rehabilitation, completion of a pulmonary rehabilitation program in patients who are frail has been shown to reverse their frailty in the short term.58 It is therefore important that physicians guide and encourage these patients for the duration of a pulmonary rehabilitation program, from initiation through to completion, to ensure that those who are likely to derive the greatest benefit from pulmonary rehabilitation are supported to do so.
In addition to pulmonary rehabilitation, other nonpharmacologic interventions have emerged in recent years that may help to relieve dyspnea in patients with COPD. Airway clearance devices, such as acapella (Smiths Medical; Minneapolis, MN), Flutter (Allergan; Dublin, Ireland), Lung Flute (Medical Acoustics; Buffalo, NY), Quake (Thayer Medical; Tucson, AZ), and Aerobika (Monaghan Medical; Plattsburgh, NY) promote the clearance of sputum through the application of positive expiratory pressure, possibly allowing medicines to penetrate the lungs more effectively, and improving diffuse airflow obstruction.59-61 Incorporating an airway clearance device into a bronchodilator therapy regimen has been shown to improve dyspnea scores, both before and after exercise, compared with bronchodilator therapy combined with a nonfunctional control device in patients with severe COPD.59 In addition, noninvasive forms of ventilation, such as continuous positive airway pressure and bi-level positive airway pressure (BiPAP), have been shown to effectively reduce dyspnea in patients with COPD.62,63 In a 24-month study in patients with severe COPD, resting dyspnea improved significantly in patients using the BiPAP Auto-Trak (Philips Respironics, Best, The Netherlands) in conjunction with their regular bronchodilator therapy, compared with those receiving long-term oxygen therapy in addition to their typical therapeutic regimen.63 Further studies are required to establish the impact of these devices in the management of dyspnea and other symptoms of COPD.
These nonpharmacologic interventions can be supplemented with pharmacologic treatments to help patients achieve their treatment goals of improved dyspnea and increased exercise performance. Bronchodilators, which form the basis of various COPD treatment options, include5:
- short-acting muscarinic antagonists (SAMAs), such as ipratropium
- short-acting β2-agonists (SABAs), such as albuterol, levalbuterol, and terbutaline
- SAMA/SABA combinations
- LAMAs, such as aclidinium, glycopyrrolate, tiotropium, and umeclidinium
- long-acting β 2-agonists (LABAs), such as arformoterol, indacaterol, formoterol, olodaterol, salmeterol, and vilanterol
- LAMA/LABA combinations (umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, glycopyrrolate/indacaterol)
Inhaled corticosteroids can also be used in a fixed-dose combination with a LABA, which can be combined with a LAMA, in select patients5; however, these combination products may have minimal value in treating dyspnea unless asthma is concomitantly present.5,64 Further discussion of the different treatment options available for patients with COPD can be found in the final article of this supplement.
In addition to improving quality of life, long-acting bronchodilators, such as LAMAs, LABAs, and LAMA/LABA combinations, increase expiratory flow, reduce dynamic hyperinflation, and improve exercise capacity of patients.65-67 As disease severity worsens, physicians may opt for long-acting bronchodilator options that have twice-daily dosing, which may confer a benefit in improving night-time symptom control.68
As well as active pharmacologic and nonpharmacologic interventions, physicians should always encourage smoking cessation in patients with COPD, as this has the greatest capacity to influence the natural course of the disease.5 It is essential that health care providers continually deliver smoking cessation messages to patients with COPD; patients can also be supported to stop smoking by using nicotine replacement therapy, pharmacologic interventions, attending smoking cessation programs, and counseling.5
Lung volume reduction surgery may also be considered as a strategy for the management of dyspnea in severe, refractory COPD.69 Similarly, nonsurgical bronchoscopic interventions are being developed that look to achieve similar results to lung volume reduction surgery, including endobronchial one-way valves, lung volume reduction coils, airway bypasses, adhesives, and vapor therapy.23
CASE STUDY:
The primary care physician assessed KD’s dyspnea using the CAT and ordered a chest X-ray to identify any significant comorbidities, such as concomitant respiratory, skeletal, or cardiac diseases. As KD’s CAT score was 17, and her symptoms were uncontrolled on LAMA monotherapy, her physician prescribed a long-acting LAMA/LABA combination, along with pulmonary rehabilitation. The physician also counseled KD on the importance of smoking cessation, and referred her to a local smoking cessation program.
Conclusions
Dyspnea, the most common symptom of COPD and the primary consequence of the condition’s characteristic lung hyperinflation, is a heavy burden on the lives of patients. The impact of dyspnea is perhaps most apparent in the context of physical activity, with activity limitation observed frequently in patients with COPD, regardless of disease stage. This can affect patients’ quality of life significantly, and has long-term consequences on disease progression. Improving dyspnea and increasing exercise endurance should therefore be a key goal for COPD management, which should encompass both nonpharmacologic interventions, such as pulmonary rehabilitation, and pharmacologic interventions, such as use of bronchodilator therapy.
Introduction
Dyspnea, the sensation of difficult or labored breathing, is the most common symptom in chronic obstructive pulmonary disease (COPD) and the primary symptom that limits physical activity in more advanced disease.1 According to the American Thoracic Society, dyspnea may be measured according to 3 domains2:
- what breathing feels like for the patient
- how distressed the patient feels when breathing
- how dyspnea affects functional ability, employment, health-related quality of life, or health status.
As disease severity increases, breathlessness becomes more disabling at lower activity levels. These changes further impact the quality of life of patients, and can lead to anxiety and depression.11
Physical inactivity is often considered to be a major contributor to the progression of COPD,6 and is linked to hospitalizations and increased all-cause mortality.12 There is therefore a need to recognize symptoms early and treat them accordingly.
CASE STUDY:
KD, a 64-year-old woman, presented to her primary care physician’s office for a routine visit. Upon assessment, KD revealed that she used to enjoy going on walks with her neighbor, but she cannot walk up the hills in her neighborhood anymore without feeling “incredibly breathless.” She has become increasingly concerned that she is “having trouble getting a full breath.” KD informed her doctor that these symptoms had worsened since her last visit, and so she had stopped going on neighborhood walks. She was diagnosed with COPD 4 years ago, and is currently using a long-acting muscarinic antagonist (LAMA) bronchodilator. KD has a 40 pack-year smoking history, and has previously been advised to stop smoking, but has relapsed several times. She has a medical history of hypertension and depression, and a notable family history of emphysema, breast cancer, and diabetes.
The relationship between lung hyperinflation and dyspnea in COPD
In COPD, pathologic changes give rise to physiologic abnormalities such as mucus hypersecretion and ciliary dysfunction, gas exchange abnormalities, pulmonary hypertension, and airflow limitation and lung hyperinflation.13 Lung hyperinflation, an increase in resting functional residual volume above a normal level, represents a mechanical link between the characteristic expiratory airflow impairment, dyspnea, and physical activity limitation in COPD.1
Although patients can compensate for several of the negative consequences of hyperinflation (eg, altering the chest wall due to overdistended lungs), such compensatory mechanisms are unable to cope with large increases in ventilation, such as those that occur during exercise.1 Air trapping, together with ineffectiveness of respiratory muscle function, leads to increased ventilation requirements and dynamic pulmonary hyperinflation, resulting in dyspnea.1
Patients with COPD describe a sensation of “air hunger,” reporting “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” and a feeling that they “cannot get a deep breath,”18 whereas, in fact, they are limited in their ability to fully exhale. Verbal descriptors (eg, “air hunger” or “chest tightness”) are important tools in understanding a patient’s experience with dyspnea, and a patient’s choice of descriptor may be related to dyspnea severity, and the level of distress that dyspnea causes a given patient.19 Air hunger in turn encourages faster breathing, leading to further shortness of breath and more dynamic hyperinflation.1,20
To deflate the lungs of patients with COPD, physiologic, pharmacologic, and possibly surgical interventions are required:
- Controlled breathing techniques (eg, purse-lipped breathing) that encourage slow and deep breathing can correct abnormal chest wall motion, decrease the work of breathing, increase breathing efficiency, and improve the distribution of ventilation to empty the lungs.21
- Bronchodilators can help to achieve lung deflation by improving ventilatory mechanics, as shown by increases in inspiratory capacity and vital capacity.22
- Lung volume reduction surgery can also be considered to treat severe hyperinflation in emphysematous patients5; bronchoscopic interventions that lower lung volumes are also in development.23
The impact of lung hyperinflation and dyspnea on physical activity in COPD
Dyspnea and hyperinflation are closely interrelated with physical activity limitation,16,29,30 and so can be viewed as significant contributors to patient disability. During an acute exacerbation, patients with COPD will experience worsening airway obstruction, dynamic hyperinflation, and dyspnea.31 Patients with a greater number of comorbid conditions may also have greater shortness of breath.32 In addition, patients with COPD and hyperinflation perform less physical activity than individuals without hyperinflation, regardless of COPD severity, as assessed using the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging (stage I, mild; stage II, moderate; stage III, severe; stage IV, very severe) and BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) index.33 These patients also exhibit increases in dyspnea perception during commonly performed ADLs, which may limit physical activity and worsen lung hyperinflation.33 More limited physical activity also contributes to higher dyspnea scores during ADLs.8
Furthermore, the ability to perform typical ADLs may be significantly altered or eliminated altogether in patients with COPD.11 Leisure activities are often the first to be dropped by patients, as they generally require greater effort than simpler tasks, and are not critical to daily life.11 Eventually, these activities become progressively more difficult, and most patients with moderate or severe COPD can struggle to complete even the most basic daily activities.11
In addition to the morbidity burden and impact on ADLs, lower levels of physical activity in patients with COPD have also been shown to increase the risk of mortality and exacerbations, and elevate the risk of comorbidities such as heart disease and metabolic disease.34 In light of these observations, improving exercise capacity should be a key goal in COPD management.
Assessment and measurement of dyspnea and hyperinflation
Reducing hyperinflation and dyspnea is essential for improving physical activity endurance and overall physical activity in patients with COPD; therefore, measuring the degree of impairment is important.22 Clinicians should be aware that some patients may have relief of dyspnea due to improvements in hyperinflation, despite relatively mild changes in FEV1.35 Lung volume measures, including total lung capacity, residual volume and functional residual capacity, are valuable tools in the assessment of lung hyperinflation in COPD, and therefore constitute a key component of pulmonary function testing.36 However, expanded pulmonary function testing may be required for patients with severe dyspnea that does not correspond to spirometric findings, or cases in which diagnosis is uncertain.37
Lung volumes are evaluated primarily by body plethysmography, during which a patient sits inside an airtight “body box” equipped to measure pressure and volume changes.14,38 Helium dilution and nitrogen washing can also be used to measure functional residual capacity in patients with COPD,14 but body plethysmography is considered to be a more accurate method of lung volume evaluation in patients with severe airflow obstruction.14,38 Radiographic techniques can also be used, but due to a lack of standardization, they are not typically utilized in clinical practice.14 Measurement of IC may complement other lung volume measures as part of assessment of hyperinflation.16 This can be measured using either spirometry or body plethysmography.39,40
In addition to evaluating hyperinflation, ADLs, physical activity, exercise capacity, and dyspnea should all be assessed in patients with COPD in primary care. It is known that patients may self-limit ADLs to avoid symptoms of COPD; in doing so, worsening symptoms may be underappreciated, and subsequently underreported, by the patient. Thus, it is essential that physicians ask patients with COPD, as well as individuals at risk of COPD, questions about changes in their physical activity or ability to perform common tasks. There are a number of methods to measure functional performance, but for a simple assessment of ADLs, clinicians can ask the patient or caregiver questions related to basic daily tasks.11 In early COPD, patients who experience mild dyspnea during exercise should be able to perform most productive activities. Patients with stable COPD and moderate dyspnea during exercise should be able to carry out most of the higher functioning ADLs, whereas patients with severe COPD may struggle to complete basic ADLs without assistance.11 It should be noted, however, that patients may experience dyspnea with fairly routine activities, and even reduce physical activity at relatively early stages of airflow limitation.41,42
Other tests may be useful in assessing the impact of an intervention, be it pharmacologic or nonpharmacologic, on dyspnea severity. For example, increases in the 6-minute-walk distance (6MWD) have been shown to correlate with improvements in dyspnea.46 The 6MWD has also been shown to be an important predictor of hospitalization and mortality in patients with COPD.47 However, it is important to note that improvements in 6MWD show only a very weak correlation with patient-reported outcomes,48 and may be a less sensitive measure for patients with less disability than those with more profound functional limitation.49 Moreover, 6MWD can be affected by a patient’s psychologic motivation,6,50 as well as other comorbidities observed in patients with COPD, such as osteoporosis, heart failure, and peripheral vascular disease.46,51 Although not used for COPD diagnosis or evaluation of dyspnea or physical activity limitation, a chest X-ray can also be a useful tool for excluding alternative diagnoses, as well as for detecting significant comorbidities in patients with COPD, such as concomitant respiratory, cardiac, and skeletal diseases.5
Management of dyspnea and hyperinflation in primary care
Pulmonary rehabilitation is a tailored intervention that encompasses exercise training, education, and self-management support for people with chronic respiratory disease, based on detailed assessment of their exercise capacity and symptoms.52 Pulmonary rehabilitation is as important as medication in COPD management, providing a cost-effective intervention with minimal adverse effects.53 Moreover, pulmonary rehabilitation has been shown to benefit patients with mild to severe dyspnea (as classified according to the Medical Research Council dyspnea scale), demonstrating the value of successful execution of these programs in patients with COPD, irrespective of disease severity.54 Although the most significant improvements in patient quality of life are observed when a multimodality approach is used, exercise and proper pulmonary rehabilitation programs have been shown to improve quality of life more than medication alone.5,55 Notably, there are few supporting data for the use of supplemental oxygen in patients experiencing dyspnea without hypoxemia. Oxygen supplementation is only of minimal benefit to relieving the sensation of dyspnea.56,57
The relationship between the impact of pulmonary rehabilitation in patients with COPD and frailty scores has also been evaluated. Frailty scores are calculated based on an individual’s level of physical activity, and other key criteria that are indicative of their ability to self-manage their medical condition.58 These scores are particularly relevant in the context of COPD, given the high prevalence of the condition in older people.58 Although frailty is a strong independent predictor of noncompletion of pulmonary rehabilitation, completion of a pulmonary rehabilitation program in patients who are frail has been shown to reverse their frailty in the short term.58 It is therefore important that physicians guide and encourage these patients for the duration of a pulmonary rehabilitation program, from initiation through to completion, to ensure that those who are likely to derive the greatest benefit from pulmonary rehabilitation are supported to do so.
In addition to pulmonary rehabilitation, other nonpharmacologic interventions have emerged in recent years that may help to relieve dyspnea in patients with COPD. Airway clearance devices, such as acapella (Smiths Medical; Minneapolis, MN), Flutter (Allergan; Dublin, Ireland), Lung Flute (Medical Acoustics; Buffalo, NY), Quake (Thayer Medical; Tucson, AZ), and Aerobika (Monaghan Medical; Plattsburgh, NY) promote the clearance of sputum through the application of positive expiratory pressure, possibly allowing medicines to penetrate the lungs more effectively, and improving diffuse airflow obstruction.59-61 Incorporating an airway clearance device into a bronchodilator therapy regimen has been shown to improve dyspnea scores, both before and after exercise, compared with bronchodilator therapy combined with a nonfunctional control device in patients with severe COPD.59 In addition, noninvasive forms of ventilation, such as continuous positive airway pressure and bi-level positive airway pressure (BiPAP), have been shown to effectively reduce dyspnea in patients with COPD.62,63 In a 24-month study in patients with severe COPD, resting dyspnea improved significantly in patients using the BiPAP Auto-Trak (Philips Respironics, Best, The Netherlands) in conjunction with their regular bronchodilator therapy, compared with those receiving long-term oxygen therapy in addition to their typical therapeutic regimen.63 Further studies are required to establish the impact of these devices in the management of dyspnea and other symptoms of COPD.
These nonpharmacologic interventions can be supplemented with pharmacologic treatments to help patients achieve their treatment goals of improved dyspnea and increased exercise performance. Bronchodilators, which form the basis of various COPD treatment options, include5:
- short-acting muscarinic antagonists (SAMAs), such as ipratropium
- short-acting β2-agonists (SABAs), such as albuterol, levalbuterol, and terbutaline
- SAMA/SABA combinations
- LAMAs, such as aclidinium, glycopyrrolate, tiotropium, and umeclidinium
- long-acting β 2-agonists (LABAs), such as arformoterol, indacaterol, formoterol, olodaterol, salmeterol, and vilanterol
- LAMA/LABA combinations (umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, glycopyrrolate/indacaterol)
Inhaled corticosteroids can also be used in a fixed-dose combination with a LABA, which can be combined with a LAMA, in select patients5; however, these combination products may have minimal value in treating dyspnea unless asthma is concomitantly present.5,64 Further discussion of the different treatment options available for patients with COPD can be found in the final article of this supplement.
In addition to improving quality of life, long-acting bronchodilators, such as LAMAs, LABAs, and LAMA/LABA combinations, increase expiratory flow, reduce dynamic hyperinflation, and improve exercise capacity of patients.65-67 As disease severity worsens, physicians may opt for long-acting bronchodilator options that have twice-daily dosing, which may confer a benefit in improving night-time symptom control.68
As well as active pharmacologic and nonpharmacologic interventions, physicians should always encourage smoking cessation in patients with COPD, as this has the greatest capacity to influence the natural course of the disease.5 It is essential that health care providers continually deliver smoking cessation messages to patients with COPD; patients can also be supported to stop smoking by using nicotine replacement therapy, pharmacologic interventions, attending smoking cessation programs, and counseling.5
Lung volume reduction surgery may also be considered as a strategy for the management of dyspnea in severe, refractory COPD.69 Similarly, nonsurgical bronchoscopic interventions are being developed that look to achieve similar results to lung volume reduction surgery, including endobronchial one-way valves, lung volume reduction coils, airway bypasses, adhesives, and vapor therapy.23
CASE STUDY:
The primary care physician assessed KD’s dyspnea using the CAT and ordered a chest X-ray to identify any significant comorbidities, such as concomitant respiratory, skeletal, or cardiac diseases. As KD’s CAT score was 17, and her symptoms were uncontrolled on LAMA monotherapy, her physician prescribed a long-acting LAMA/LABA combination, along with pulmonary rehabilitation. The physician also counseled KD on the importance of smoking cessation, and referred her to a local smoking cessation program.
Conclusions
Dyspnea, the most common symptom of COPD and the primary consequence of the condition’s characteristic lung hyperinflation, is a heavy burden on the lives of patients. The impact of dyspnea is perhaps most apparent in the context of physical activity, with activity limitation observed frequently in patients with COPD, regardless of disease stage. This can affect patients’ quality of life significantly, and has long-term consequences on disease progression. Improving dyspnea and increasing exercise endurance should therefore be a key goal for COPD management, which should encompass both nonpharmacologic interventions, such as pulmonary rehabilitation, and pharmacologic interventions, such as use of bronchodilator therapy.
- O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180-184.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272.
- Agusti A, Hedner J, Marin J, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://gold.copd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed November 27, 2017.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577-588.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-1394.
- Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131-137.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977.
- Haas F, Salazar-Schicci J, Axen K. Desensitization to dyspnoea in chronic obstructive pulmonary disease. In: Casaburi R, Petty TL, eds. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: W.B. Saunders; 1993:241-251.
- Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268-278.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187-201.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176-179.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
- Dubé BP, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1.
- Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385.
- Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259-264.
- Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101-111.
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003;40(5 Suppl 2):25-33.
- O’Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Pract. 2015;1:4.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(Suppl 4):S407-S415.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
- O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-549.
- Light RW. Mechanics of respiration. In: George RB, ed. Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:24-38.
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591-597.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753-762.
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181-188.
- Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506-512.
- Di Marco F, Santus P, Sotgiu G, Blasi F, Centanni S. Does improving exercise capacity and daily activity represent the holistic perspective of a new COPD approach? COPD. 2015;12(5):575-581.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042-1050.
- Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745-752.
- Burkhardt R, Pankow W. The diagnosis of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2014;111(49):834-845, quiz 846.
- O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108-1115.
- Criée CP, Sorichter S, Smith HJ, et al; Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959-971.
- Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir Med. 2017;12:3.
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834-840.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005-1011.
- Calverley PMA, Georgopoulos D. Symptoms and signs of COPD. In: Siafakas NM, ed. Management of Chronic Obstructive Pulmonary Disease: European Respiratory Society Journals; 2006.
- Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447-456.
- Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD—revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725-738.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382-386.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225.
- Grant A, Moore L. Pulmonary rehabilitation. In: Blackler L, Jones C, Mooney C, eds. Managing Chronic Obstructive Pulmonary Disease. West Sussex, England: John Wiley & Sons; 2007.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042-1048.
- Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
- Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103(7):1070-1075.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179-187.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429.
- Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988-995.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA Jr. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest. 2002;121(3):702-707.
- Chatburn RL. High-frequency assisted airway clearance. Respir Care. 2007;52(9):1224-1235; discussion 1235-1227.
- Clini E. Positive expiratory pressure techniques in respiratory patients: old evidence and new insights. Breathe. 2009;6(2):153-159.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(2):281-289.
- Clini E, Sturani C, Rossi A, et al; Rehabilitation and Chronic Care Study Group; Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529-538.
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(6):477-482.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288-1296.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
- O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients [published correction appears in Respir Res. 2017;18(1):35]. Respir Res. 2017;18(1):19.
- Shah AA, D’Amico TA. Lung volume reduction surgery for the management of refractory dyspnea in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3(2):107-111.
- O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180-184.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272.
- Agusti A, Hedner J, Marin J, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://gold.copd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed November 27, 2017.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577-588.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-1394.
- Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131-137.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977.
- Haas F, Salazar-Schicci J, Axen K. Desensitization to dyspnoea in chronic obstructive pulmonary disease. In: Casaburi R, Petty TL, eds. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: W.B. Saunders; 1993:241-251.
- Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268-278.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187-201.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176-179.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
- Dubé BP, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1.
- Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385.
- Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259-264.
- Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101-111.
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003;40(5 Suppl 2):25-33.
- O’Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Pract. 2015;1:4.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(Suppl 4):S407-S415.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
- O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-549.
- Light RW. Mechanics of respiration. In: George RB, ed. Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:24-38.
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591-597.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753-762.
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181-188.
- Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506-512.
- Di Marco F, Santus P, Sotgiu G, Blasi F, Centanni S. Does improving exercise capacity and daily activity represent the holistic perspective of a new COPD approach? COPD. 2015;12(5):575-581.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042-1050.
- Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745-752.
- Burkhardt R, Pankow W. The diagnosis of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2014;111(49):834-845, quiz 846.
- O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108-1115.
- Criée CP, Sorichter S, Smith HJ, et al; Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959-971.
- Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir Med. 2017;12:3.
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834-840.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005-1011.
- Calverley PMA, Georgopoulos D. Symptoms and signs of COPD. In: Siafakas NM, ed. Management of Chronic Obstructive Pulmonary Disease: European Respiratory Society Journals; 2006.
- Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447-456.
- Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD—revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725-738.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382-386.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225.
- Grant A, Moore L. Pulmonary rehabilitation. In: Blackler L, Jones C, Mooney C, eds. Managing Chronic Obstructive Pulmonary Disease. West Sussex, England: John Wiley & Sons; 2007.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042-1048.
- Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
- Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103(7):1070-1075.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179-187.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429.
- Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988-995.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA Jr. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest. 2002;121(3):702-707.
- Chatburn RL. High-frequency assisted airway clearance. Respir Care. 2007;52(9):1224-1235; discussion 1235-1227.
- Clini E. Positive expiratory pressure techniques in respiratory patients: old evidence and new insights. Breathe. 2009;6(2):153-159.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(2):281-289.
- Clini E, Sturani C, Rossi A, et al; Rehabilitation and Chronic Care Study Group; Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529-538.
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(6):477-482.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288-1296.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
- O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients [published correction appears in Respir Res. 2017;18(1):35]. Respir Res. 2017;18(1):19.
- Shah AA, D’Amico TA. Lung volume reduction surgery for the management of refractory dyspnea in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3(2):107-111.
Anxiety and Depression in Chronic Obstructive Pulmonary Disease: Recognition and Management
Introduction
Anxiety and depression are common in patients with chronic obstructive pulmonary disease (COPD), occurring more frequently than in the general population1-4 or patients with other chronic diseases such as hypertension, diabetes, cancer, or musculoskeletal disorders.5,6 Their presence is associated with worse outcomes of COPD, and increased morbidity, mortality, disability, and health care expenditure.6-8 In spite of this, both anxiety and depression are frequently overlooked and undertreated in patients with COPD,9 and symptoms of anxiety and depression can overlap significantly, as well as overlap with COPD symptoms.7,10
Comorbid depressive disorders that may occur in patients with COPD include major depressive disorder, dysthymias (chronic depressive symptoms of mild severity), and minor depression.11 Depressive disorders are characterized by feelings of sadness, emptiness, and/or irritability, along with cognitive and somatic symptoms, which have a detrimental effect on the patient’s ability to function.11 Anxiety disorders include generalized anxiety disorder (GAD), phobias, and panic disorders.11 The main features of anxiety disorders, such as excessive fear and anxiety, may be accompanied by behavioral disturbances related to these symptoms, such as panic attacks and avoidance.11,12
The reported prevalence of depression in COPD varies widely between studies, owing to differences in sampling methods and degrees of illness severity used in assessment of depression6; rates have been reported to range from 10% to 42% in patients with stable COPD,6,13 and from 10% to 86% in patients with acute COPD exacerbation.14 Individuals with severe COPD are twice as likely to develop depression than patients with mild COPD.10
Prevalence rates for clinical anxiety in COPD range from 13% to 46% in outpatients and 10% to 55% among inpatients. GAD, panic disorders, and specific phobias are reported most frequently.15 Patients with COPD are 85% more likely to develop anxiety disorders compared with matched controls without COPD,4 and panic disorder is reported with a prevalence that is up to 10-fold higher than in the general population.16
Global prevalence rates of anxiety and depression are 1.8- and 1.4-fold higher in women than men, respectively17; the same gender difference is observed in patients with COPD.6 The higher prevalence rates of anxiety and depression in women are thought to be a result of sex differences in brain structure, function, and stress responses, as well as differences in exposure to reproductive hormones, social constraints, and experiences between women and men.18 However, psychologic comorbidity is an issue for both men and women with COPD, so it is important that clinicians are vigilant in recognizing anxiety and depression in both sexes, and are careful not to underestimate the burden in the male patient population.
It is also important to note that depression and anxiety often occur simultaneously in patients with COPD, with prevalence estimates of 26% to 43%.9,19,20 COPD patients with both depression and anxiety are at a heightened risk of suicidal ideation, increased physical disability, and chronic depressive symptoms versus those with either disorder alone.10,15 It is therefore important that comorbid anxiety and depression is not overlooked in patients with COPD.
Ensuring that anxiety and depression are recognized and treated effectively in patients with COPD is essential for optimizing outcomes. Primary care practitioners are well placed to diagnose anxiety and depression, and to ensure these conditions are suitably managed alongside treatments of COPD.
Potential mechanisms of anxiety and depression in COPD
Growing evidence suggests that the relationship between mood disorders—particularly depression—and COPD is bidirectional, meaning that mood disorders adversely impact prognosis in COPD, whereas COPD increases the risk of developing depression.21 For example, in a study of
60 stable patients with COPD, elevated dyspnea and reduced exercise capacity were the predominant mechanisms leading to anxiety and depression symptoms associated with the condition.22 In addition, the risk of new-onset depression was increased in COPD patients with moderate-to-severe dyspnea in a 3-year follow-up study.23 Conversely, depression has been shown to be a significant risk factor for disabling dyspnea (modified Medical Research Council score ≥2) in patients with COPD.24
COPD can lead to feelings of hopelessness, social isolation, reduced physical functioning, and sedentary lifestyle, all of which are associated with an increased level of depressive symptoms.25 Similarly, inadequate social support increases the risk of anxiety in patients with COPD.26 Therefore, ensuring that patients with COPD have high-quality support is very important for reducing anxiety and depressive symptoms.27
The exact mechanisms for the association between mood disorders and COPD remain unclear.7,10 Research to date indicates that the relationship between depression and impaired pulmonary function may be partly mediated by chronic inflammation7,10; systemic inflammation has been associated with other comorbidities of COPD (eg, muscle wasting and osteoporosis),28 and emerging data appear to show that proinflammatory cytokines partly mediate the association between depressive symptoms and pulmonary function.29 Smoking and hypoxemia may also influence the prevalence of depression in COPD, but symptom severity and impaired quality of life remain the most important determinants.6,30
Clinical studies have demonstrated that a number of patient-related factors, including female gender, younger age, current smoking, greater severity of airflow limitation, and lower socioeconomic status, are associated with a higher prevalence and/or increased risk of depression and/or anxiety in COPD.3,4,30,31 Frequent episodes of rehospitalization, and comorbidities such as hypertension, arthritis, cancer, and heart disease, have been found to increase the risk of anxiety and depression in patients with COPD.3,32 Risk of anxiety has been shown to increase with greater dyspnea severity.4 Pain, a frequently overlooked symptom in COPD, has been shown to be associated with symptoms of both anxiety and depression in patients with COPD.33 This is driven by worsened quality of life and sleep quality, decreased physical activity, and an increased fear of movement that occur as a result of pain.34
The impact of anxiety and depression in COPD
Comorbid anxiety and depression have a significant detrimental impact on morbidity and mortality in patients with COPD. Both disorders have been associated with an increased risk of death in COPD.13,35-37 Indeed, of 12 comorbidities proposed to be predictors of mortality in a cohort of 187 female outpatients with COPD, anxiety was associated with the highest risk of death.35,36
In addition, patients with COPD and anxiety and/or depression have a higher risk of COPD exacerbations,4,8,23,36,38-40 hospitalization,41,42 rehospitalization,14,36,43 longer hospital stays,37,41,44 and mortality after exacerbations,14,36,41 compared with patients without these comorbidities. Patients with COPD who have elevated anxiety symptoms also often experience their first hospitalization earlier in the natural course of COPD than those without anxiety.36
Psychologic comorbidities are also associated with worse lung function, dyspnea, and respiratory symptom burden in patients with COPD.37,40 Patients with COPD and anxiety are more likely to experience greater dyspnea at an earlier stage of disease than those without anxiety.36 Persistent smoking at 6 months after hospitalization for an acute exacerbation of COPD is also more likely to be seen in patients with depression.37
Patient-centered outcomes are worse in COPD patients with mood disorders. Both anxiety and depression have been shown to correlate with significantly reduced health-related quality of life (HRQoL), poorer physical health status, functional limitations, and reduced exercise capacity.4,23,37,40,45 The presence of either anxiety or depression at baseline has been shown to correlate with reduced HRQoL at 1-year follow-up, but depression appears to be the stronger predictor of low future HRQoL than anxiety.45
Additionally, mood disorders—particularly depression—reduce physical activity in patients with COPD.46,47 Emotional responses to COPD symptoms, such as dyspnea, can further decrease activity and worsen deconditioning, resulting in a downward spiral of reduced inactivity, social isolation, fear, anxiety, and depression.48
COPD patients with any comorbidity exhibit lower rates of medication adherence than those without comorbidities.49-51 Clinical studies have demonstrated that anxiety and depression are significant predictors of poor adherence to COPD interventions, including pulmonary rehabilitation (PR).51-55 Nonadherence to COPD therapies is associated with poor clinical outcomes, including higher hospitalization rates and increased emergency department visits, and increased costs.56,57 Health care expenditure, in terms of both specific COPD-related costs and general “all-cause” costs, is significantly higher in COPD patients with anxiety and/or depression than in those without.8
Diagnosis of anxiety and depression in patients with COPD
The underdiagnosis and undertreatment of anxiety and depression in this population is common and can adversely affect patient outcomes.6,7,9,10,58 Hence, it is crucial that anxiety and depression are identified and more effectively managed in clinical practice.10
Primary care practitioners are the main point of contact for many patients with COPD,6,59,60 and so can play a key role in screening for and early identification of anxiety and depression. However, detection of mood disorders by primary care practitioners is challenging for several reasons. These include the lack of a standardized approach in diagnosis, and inadequate knowledge or confidence in assessing psychological status (particularly given the number of strategies available for screening patients for mood disorders),6 as well as factors associated with time constraints, such as competing agendas, duration of visits, and high patient load.6,61 Furthermore, system-level barriers, such as lack of electronic medical records and adequate health insurance, as well as any communication gaps between primary care and mental health care, may hinder the detection and management of anxiety and depression.6 In addition, patients themselves may have a limited understanding of these comorbidities, or may be hesitant to discuss symptoms of anxiety or depression with their primary care practitioner owing to stigma around mental illness.6
Patients with COPD should be screened and assessed for anxiety and depression, and the United States Preventive Services Task Force recommends that clinicians screen for depression in all adults.6,62 There are several validated screening tools suitable for clinical use:
- Anxiety Inventory for Respiratory (AIR) Disease scale: a brief, easy-to-use tool for screening and measuring anxiety in COPD.63,64 It is a self-administered scale, and takes approximately 2 minutes to complete. The AIR scale is responsive to PR.64
- COPD Anxiety Questionnaire (CAF): a reliable tool for early identification of COPD-related anxiety.65
- Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire (PHQ; available at: http://www.phqscreeners.com/select-screener/): the PRIME-MD comprises 26 yes/no questions on the 5 most common psychiatric disorders, including depression and anxiety.66,67 This is not a diagnostic tool, but a high number of positive responses from a patient in any given module indicates that they require further clinical evaluation.
- PHQ-2 and PHQ-9 (Table 1; PHQ-9 available at http://www.phqscreeners.com/select-screener/): widely-used self-administered 2- and 9-item versions of the PRIME-MD, specific to depression; similarly, the 3-item PHQ-3 is available for anxiety assessment (Table 2).6,67,68 In a study investigating tools used by family physicians in England to assess depression, over 75% used PHQ-9.69
- Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire-version 20 (GHQ-20): both can be used to screen for psychologic distress in patients with COPD.71
- The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI): two 21-item self-report questionnaires that are widely used in the United States to evaluate anxiety and depression.72
In addition to specific anxiety and depression questionnaires (Tables 1 and 2), more general COPD assessments tools, such as the COPD Assessment Test and the COPD Clinical Questionnaire, also incorporate questions that may be indicative of symptoms of these comorbidities in patients with COPD.73
Management of anxiety and depression in COPD
Even though anxiety and depression are among the most common and burdensome comorbid conditions in COPD, less than one-third of patients with these comorbidities receive effective intervention.6,10 Primary care providers have an excellent opportunity to impact this care gap.
As in non-COPD patients, comorbid depression and anxiety may be treated with nonpharmacologic and/or pharmacologic interventions (Figure 1).76
Nonpharmacologic interventions
Evidence to date suggests that nonpharmacologic interventions such as behavioral therapy are as effective as antidepressants, and may be preferred by patients with mood disorders.12
Cognitive behavioral therapy (CBT), which is typically administered by psychologists/psychiatrists, may be effective in treating COPD-related anxiety and depression, especially in conjunction with exercise and education.12,76,77 Individualized or group CBT is the treatment of choice for addressing thinking patterns that contribute to anxiety and depression to change a patient’s behavior and emotional state.76 PR programs involve several components, including aerobic exercise, lung function training, and psycho-education.62,76 PR is suitable for most patients with COPD, and provides multiple benefits, including reduced hospitalizations in patients who have had a recent exacerbation, and improved dyspnea, exercise tolerance, and health status in patients with stable disease,62 as well as clinically and statistically significant improvements in depression and anxiety, irrespective of age.7,78,79 Exercise-based forms of PR appear to be the most effective for reducing mood symptoms,12,76 and incorporating psychotherapy may also improve psychologic outcomes.80 Stress reduction (relaxation) therapy aims to reduce anxiety-related physiologic changes, and includes a variety of techniques (eg, breathing exercises, sequential muscle relaxation, hypnosis, mindfulness meditation), some of which may be included in PR or used alongside other treatments (eg, CBT).76 Limited data indicate that such therapy may be beneficial for reducing anxiety and depression, as well as respiratory symptoms and dyspnea, in patients with COPD.12,76
Self-management techniques improve clinical outcomes in patients with COPD, but data on the management of depression or anxiety are inconclusive.7,12 A minimal, home-based, nurse-led, psycho-educational intervention was designed to encourage more open-ended, descriptive discussions of thoughts, emotions, behaviors, and bodily sensations in patients with COPD.81 The intervention, which involved nurses attending a 1-hour face-to-face session in the patients’ homes with a 15-minute telephone “booster” session 2 weeks later, helped patients with advanced COPD to self-manage their condition and provide relief from anxiety.81,82 However, it should be noted that there is currently a lack of high-quality data evaluating psychologic interventions in the COPD population.83
In addition, it is important that caregivers are supported in the management of patients with COPD and comorbid anxiety and/or depression; areas in which caregivers can be assisted in their role may include disease education and counseling, where appropriate.84
Given that smoking cessation is a key recommendation for patients with COPD,44,62 practitioners should be aware that patients with comorbid depression and anxiety may experience greater difficulty in smoking cessation, and worsened mood during nicotine withdrawal.44 Clinicians should therefore carefully monitor current smokers with COPD and comorbid depression/anxiety (using the tools described previously63,68,70,71) when they are attempting to quit smoking.
Pharmacologic interventions
Pharmacologic therapy of anxiety and depression has so far only been investigated in patients with COPD in small studies.76 However, the available evidence does not indicate that COPD patients with anxiety and depression should be managed any differently from individuals without COPD.62 As such, pharmacologic interventions are particularly important for patients with acute or severe anxiety or depression.
Antidepressant agents are categorized according to their mechanism of action, and most commonly include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors, bupropion (a norepinephrine- and dopamine-reuptake inhibitor; also approved for smoking cessation85), and mirtazapine (a norepinephrine and serotonin modulator), among others.86 SSRIs are the current firstline drug treatment for depression, and have been shown to significantly improve depression and anxiety in patients with COPD in some, but not all, trials published to date.76 However, it is important to note that a diagnosis of bipolar disorder must be ruled out before initiating standard antidepressant therapy.87 In addition to antidepressants, atypical antipsychotics have also been shown to be useful for treating anxiety, either as monotherapy or combination therapy, and possibly as an adjunctive therapy for the management of depression.88,89
Primary care practitioners can refer to existing guidelines on the management of anxiety and depression in patients with COPD,86,90 while taking certain factors into consideration. Any pharmacologic management strategy for the treatment of COPD may increase the risk of drug–drug or drug–disease interactions.76 For example, it is important to avoid medications that cause respiratory depression (eg, benzodiazepines [unless used with extreme caution], particularly in patients who are already CO2 retainers) or sedation; chosen drugs should have minimal other adverse effects.76 Moreover, SSRIs may also be associated with troublesome adverse effects during treatment initiation, such as gastrointestinal upset, headache, tremor, psychomotor activation, and sedation76; in addition, dry mouth is an adverse effect associated with both SSRI treatment and several inhaled therapies, so may be particularly problematic in patients with COPD.91,92 Currently, data are particularly scarce for the management of anxiety in patients with COPD, with inconclusive or contradictory findings reported for SSRIs, azapirones (including buspirone), and tricyclic antidepressants.76
In addition to monitoring adherence to COPD therapies, primary care practitioners should carefully monitor patients treated with antidepressants and anxiolytics for adherence. A meta-analysis of 18,245 individuals with chronic diseases showed that depressed patients had a 76% significantly higher risk of nonadherence to medication compared with those without depressive symptoms.93
Targeting dyspnea is key to the management of anxiety and depression in COPD, as breathlessness is frequently associated with the onset of both comorbidities.21,22 Therapeutic approaches to alleviating dyspnea include PR, optimizing respiratory mechanics and muscle function (with bronchodilator therapy), and reducing central neural drive to respiratory muscles with supplemental oxygen or opioid medication.94
Although bronchodilator therapy for COPD has not been shown to have significant direct effects on depression or anxiety,95 it can be assumed that the beneficial effects on dyspnea are likely to alleviate associated emotional and mood symptoms.
Further research into effective screening, diagnosis, and management of comorbid anxiety and depressive disorders in COPD is warranted, including evaluation of a broad range of nonpharmacologic and drug-based interventions, alone and in combination.76
Conclusions
Anxiety and depression are common, yet frequently overlooked, comorbidities in COPD. The impact of these psychologic comorbidities is significant; their consequences are evident in morbidity and mortality data, as well as in patient-reported outcomes. As key points of contact for patients with COPD, it is essential that primary care practitioners are vigilant in monitoring for anxiety and depression in their patients with COPD, making the most of the available tools that can support them in doing so, and maintain an ongoing line of communication with other members of the multidisciplinary team. Treatment of anxiety and depression in COPD should adopt a holistic approach that incorporates both nonpharmacologic and pharmacologic interventions. However, the impact of effective screening, diagnosis, and management of anxiety and depression on COPD burden in patients requires further investigation.
- Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016;33(2):174-178.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217-223.
- Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976.
- Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229-234.
- Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246-256.
- Maurer J, Rebbapragada V, Borson S, et al; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S-56S.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293-299.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- American Psychiatric Association. Depressive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
- Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33-39.
- Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):pii: 170026.
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858-866.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med. 2010;104(9):1246-1253.
- World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland; World Health Organization: 2017.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330.
- Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res. 2017;11(2):OC24-OC27.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15(12):1090-1096.
- Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766-777.
- Tetikkurt C, Ozdemir I, Tetikkurt S, Yilmaz N, Ertan T, Bayar N. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226-231.
- Yohannes AM, Müllerová H, Hanania NA, et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916-926.
- Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805-2812.
- Kirkil G, Deveci F, Deveci SE, Atmaca M. Anxiety and depression symptoms in patients with chronic obstructive pulmonary disease. Bulletin Clin Psychopharmacol. 2015;25(2):151-161.
- Fuller-Thomson E, Lacombe-Duncan A. Understanding the association between chronic obstructive pulmonary disease and current anxiety: a population-based study. COPD. 2016;13(5):622-631.
- Yohannes AM. Is it quality or quantity of social support needed for patients with chronic medical illness? J Psychosom Res. 2013;74(2):87-88.
- Fabbri LM, Luppi F, Beghe B, Rabe KF. The multiple components of COPD. In: Hanania NA, Sharafkhaneh A, eds. COPD: a guide to diagnosis and clinical management. Humana Press; 2010.
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53.
- Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604-611.
- Zhang Q, Liao J, Liao X, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2014;14:92.
- Jose AK, Chelangara DP, Shaji KS. Factors associated with anxiety and depression in chronic obstructive pulmonary disease. Int J Res Med Sci. 2016;4(4):1074-1079.
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898.
- Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246-1258.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95-109.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60-67.
- Montserrat-Capdevila J, Godoy P, Marsal JR, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung. 2017;195(1):77-85.
- Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918-923.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335-343.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122(5):1633-1637.
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414-419.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65-70.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501-512.
- Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287-1295.
- Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park HK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease [published online ahead of print June 7, 2017]. Korean J Intern Med. 2017;doi: 10.3904/kjim.2016.090.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667-677.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204.
- Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043.
- Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365-1373.
- Busch AM, Scott-Sheldon LA, Pierce J, et al. Depressed mood predicts pulmonary rehabilitation completion among women, but not men. Respir Med. 2014;108(7):1007-1013.
- Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout? J Cardiopulm Rehabil Prev. 2013;33(6):419-426.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105-116.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- Dury R. COPD and emotional distress: not always noticed and therefore untreated. Br J Community Nurs. 2016;21(3):138-141.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5(2):115-128.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed June 2017.
- Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the anxiety inventory for respiratory disease. Chest. 2013;144(5):1587-1596.
- Yohannes AM, Dryden S, Hanania NA. The responsiveness of the anxiety inventory for respiratory disease scale following pulmonary rehabilitation. Chest. 2016;150(1):188-195.
- Kühl K, Kuhn C, Kenn K, Rief W. [The COPD-Anxiety-Questionnaire (CAF): a new instrument to assess illness specific anxiety in COPD patients]. Psychother Psychosom Med Psychol. 2011;61(1):e1-e9. German.
- Tamburrino MB, Lynch DJ, Nagel RW, Smith MK. Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry. 2009;11(6):339-343.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348-353.
- Yohannes AM, Hann M, Sibbald B. The management of depressive symptoms in patients with COPD: a postal survey of general practitioners. Prim Health Care Res Dev. 2011;12(3):237-244.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
- Bratås O, Grønning K, Forbord T. Psychometric properties of the Hospital Anxiety and Depression Scale and The General Health Questionnaire-20 in COPD inpatients. Scand J Caring Sci. 2014;28(2):413-420.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343.
- Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57-65.
- Cantor L, Jacobson R. COPD: How to manage comorbid depression and anxiety. Curr Psychiatry. 2003;2(11):45-54.
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2007;5:17.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297-328.
- Doyle C, Bhar S, Fearn M, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol. 2017;22(3):542-556.
- Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491-496.
- Bennett D, Bowen B, McCarthy P, Subramaniam A, O’Connor M, Henry MT. Outcomes of pulmonary rehabilitation for COPD in older patients: a comparative study. COPD. 2017;14(2):170-175.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open. 2015;5(7):e008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med. 2016;121:109-116.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3: CD010673. doi:10.002/14651858.CD010673.pub2.
- Cafarella P, Effing T, Frith P. An evaluation of the needs of carers of people with COPD. EurResp J. 2012;40(Suppl 56).
- Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zyban/pdf/ZYBAN-PI-MG.PDF. Accessed June 2017.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(10):1.
- Pary R, Matuschka PR, Lewis S, Lippmann S. Managing bipolar depression. Psychiatry (Edgmont). 2006;3(2):30-41.
- Blier P. Atypical antipsychotics for mood and anxiety disorders: safe and effective adjuncts? J Psychiatry Neurosci. 2005;30(4):232-233.
- Vulink NC, Figee M, Denys D. Review of atypical antipsychotics in anxiety. Eur Neuropsychopharmacol. 2011;21(6):429-449.
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi:10.1002/14651858.CD010844.pub2.
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175-1182.
- O’Donnell DE, Webb KA, Harle I, Neder JA. Pharmacological management of breathlessness in COPD: recent advances and hopes for the future. Expert Rev Respir Med. 2016;10(7):823-834.
- Hyun MK, Lee NR, Jang EJ, Yim JJ, Lee CH. Effect of inhaled drugs on anxiety and depression in patients with chronic obstructive pulmonary disease: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:747-754.
Introduction
Anxiety and depression are common in patients with chronic obstructive pulmonary disease (COPD), occurring more frequently than in the general population1-4 or patients with other chronic diseases such as hypertension, diabetes, cancer, or musculoskeletal disorders.5,6 Their presence is associated with worse outcomes of COPD, and increased morbidity, mortality, disability, and health care expenditure.6-8 In spite of this, both anxiety and depression are frequently overlooked and undertreated in patients with COPD,9 and symptoms of anxiety and depression can overlap significantly, as well as overlap with COPD symptoms.7,10
Comorbid depressive disorders that may occur in patients with COPD include major depressive disorder, dysthymias (chronic depressive symptoms of mild severity), and minor depression.11 Depressive disorders are characterized by feelings of sadness, emptiness, and/or irritability, along with cognitive and somatic symptoms, which have a detrimental effect on the patient’s ability to function.11 Anxiety disorders include generalized anxiety disorder (GAD), phobias, and panic disorders.11 The main features of anxiety disorders, such as excessive fear and anxiety, may be accompanied by behavioral disturbances related to these symptoms, such as panic attacks and avoidance.11,12
The reported prevalence of depression in COPD varies widely between studies, owing to differences in sampling methods and degrees of illness severity used in assessment of depression6; rates have been reported to range from 10% to 42% in patients with stable COPD,6,13 and from 10% to 86% in patients with acute COPD exacerbation.14 Individuals with severe COPD are twice as likely to develop depression than patients with mild COPD.10
Prevalence rates for clinical anxiety in COPD range from 13% to 46% in outpatients and 10% to 55% among inpatients. GAD, panic disorders, and specific phobias are reported most frequently.15 Patients with COPD are 85% more likely to develop anxiety disorders compared with matched controls without COPD,4 and panic disorder is reported with a prevalence that is up to 10-fold higher than in the general population.16
Global prevalence rates of anxiety and depression are 1.8- and 1.4-fold higher in women than men, respectively17; the same gender difference is observed in patients with COPD.6 The higher prevalence rates of anxiety and depression in women are thought to be a result of sex differences in brain structure, function, and stress responses, as well as differences in exposure to reproductive hormones, social constraints, and experiences between women and men.18 However, psychologic comorbidity is an issue for both men and women with COPD, so it is important that clinicians are vigilant in recognizing anxiety and depression in both sexes, and are careful not to underestimate the burden in the male patient population.
It is also important to note that depression and anxiety often occur simultaneously in patients with COPD, with prevalence estimates of 26% to 43%.9,19,20 COPD patients with both depression and anxiety are at a heightened risk of suicidal ideation, increased physical disability, and chronic depressive symptoms versus those with either disorder alone.10,15 It is therefore important that comorbid anxiety and depression is not overlooked in patients with COPD.
Ensuring that anxiety and depression are recognized and treated effectively in patients with COPD is essential for optimizing outcomes. Primary care practitioners are well placed to diagnose anxiety and depression, and to ensure these conditions are suitably managed alongside treatments of COPD.
Potential mechanisms of anxiety and depression in COPD
Growing evidence suggests that the relationship between mood disorders—particularly depression—and COPD is bidirectional, meaning that mood disorders adversely impact prognosis in COPD, whereas COPD increases the risk of developing depression.21 For example, in a study of
60 stable patients with COPD, elevated dyspnea and reduced exercise capacity were the predominant mechanisms leading to anxiety and depression symptoms associated with the condition.22 In addition, the risk of new-onset depression was increased in COPD patients with moderate-to-severe dyspnea in a 3-year follow-up study.23 Conversely, depression has been shown to be a significant risk factor for disabling dyspnea (modified Medical Research Council score ≥2) in patients with COPD.24
COPD can lead to feelings of hopelessness, social isolation, reduced physical functioning, and sedentary lifestyle, all of which are associated with an increased level of depressive symptoms.25 Similarly, inadequate social support increases the risk of anxiety in patients with COPD.26 Therefore, ensuring that patients with COPD have high-quality support is very important for reducing anxiety and depressive symptoms.27
The exact mechanisms for the association between mood disorders and COPD remain unclear.7,10 Research to date indicates that the relationship between depression and impaired pulmonary function may be partly mediated by chronic inflammation7,10; systemic inflammation has been associated with other comorbidities of COPD (eg, muscle wasting and osteoporosis),28 and emerging data appear to show that proinflammatory cytokines partly mediate the association between depressive symptoms and pulmonary function.29 Smoking and hypoxemia may also influence the prevalence of depression in COPD, but symptom severity and impaired quality of life remain the most important determinants.6,30
Clinical studies have demonstrated that a number of patient-related factors, including female gender, younger age, current smoking, greater severity of airflow limitation, and lower socioeconomic status, are associated with a higher prevalence and/or increased risk of depression and/or anxiety in COPD.3,4,30,31 Frequent episodes of rehospitalization, and comorbidities such as hypertension, arthritis, cancer, and heart disease, have been found to increase the risk of anxiety and depression in patients with COPD.3,32 Risk of anxiety has been shown to increase with greater dyspnea severity.4 Pain, a frequently overlooked symptom in COPD, has been shown to be associated with symptoms of both anxiety and depression in patients with COPD.33 This is driven by worsened quality of life and sleep quality, decreased physical activity, and an increased fear of movement that occur as a result of pain.34
The impact of anxiety and depression in COPD
Comorbid anxiety and depression have a significant detrimental impact on morbidity and mortality in patients with COPD. Both disorders have been associated with an increased risk of death in COPD.13,35-37 Indeed, of 12 comorbidities proposed to be predictors of mortality in a cohort of 187 female outpatients with COPD, anxiety was associated with the highest risk of death.35,36
In addition, patients with COPD and anxiety and/or depression have a higher risk of COPD exacerbations,4,8,23,36,38-40 hospitalization,41,42 rehospitalization,14,36,43 longer hospital stays,37,41,44 and mortality after exacerbations,14,36,41 compared with patients without these comorbidities. Patients with COPD who have elevated anxiety symptoms also often experience their first hospitalization earlier in the natural course of COPD than those without anxiety.36
Psychologic comorbidities are also associated with worse lung function, dyspnea, and respiratory symptom burden in patients with COPD.37,40 Patients with COPD and anxiety are more likely to experience greater dyspnea at an earlier stage of disease than those without anxiety.36 Persistent smoking at 6 months after hospitalization for an acute exacerbation of COPD is also more likely to be seen in patients with depression.37
Patient-centered outcomes are worse in COPD patients with mood disorders. Both anxiety and depression have been shown to correlate with significantly reduced health-related quality of life (HRQoL), poorer physical health status, functional limitations, and reduced exercise capacity.4,23,37,40,45 The presence of either anxiety or depression at baseline has been shown to correlate with reduced HRQoL at 1-year follow-up, but depression appears to be the stronger predictor of low future HRQoL than anxiety.45
Additionally, mood disorders—particularly depression—reduce physical activity in patients with COPD.46,47 Emotional responses to COPD symptoms, such as dyspnea, can further decrease activity and worsen deconditioning, resulting in a downward spiral of reduced inactivity, social isolation, fear, anxiety, and depression.48
COPD patients with any comorbidity exhibit lower rates of medication adherence than those without comorbidities.49-51 Clinical studies have demonstrated that anxiety and depression are significant predictors of poor adherence to COPD interventions, including pulmonary rehabilitation (PR).51-55 Nonadherence to COPD therapies is associated with poor clinical outcomes, including higher hospitalization rates and increased emergency department visits, and increased costs.56,57 Health care expenditure, in terms of both specific COPD-related costs and general “all-cause” costs, is significantly higher in COPD patients with anxiety and/or depression than in those without.8
Diagnosis of anxiety and depression in patients with COPD
The underdiagnosis and undertreatment of anxiety and depression in this population is common and can adversely affect patient outcomes.6,7,9,10,58 Hence, it is crucial that anxiety and depression are identified and more effectively managed in clinical practice.10
Primary care practitioners are the main point of contact for many patients with COPD,6,59,60 and so can play a key role in screening for and early identification of anxiety and depression. However, detection of mood disorders by primary care practitioners is challenging for several reasons. These include the lack of a standardized approach in diagnosis, and inadequate knowledge or confidence in assessing psychological status (particularly given the number of strategies available for screening patients for mood disorders),6 as well as factors associated with time constraints, such as competing agendas, duration of visits, and high patient load.6,61 Furthermore, system-level barriers, such as lack of electronic medical records and adequate health insurance, as well as any communication gaps between primary care and mental health care, may hinder the detection and management of anxiety and depression.6 In addition, patients themselves may have a limited understanding of these comorbidities, or may be hesitant to discuss symptoms of anxiety or depression with their primary care practitioner owing to stigma around mental illness.6
Patients with COPD should be screened and assessed for anxiety and depression, and the United States Preventive Services Task Force recommends that clinicians screen for depression in all adults.6,62 There are several validated screening tools suitable for clinical use:
- Anxiety Inventory for Respiratory (AIR) Disease scale: a brief, easy-to-use tool for screening and measuring anxiety in COPD.63,64 It is a self-administered scale, and takes approximately 2 minutes to complete. The AIR scale is responsive to PR.64
- COPD Anxiety Questionnaire (CAF): a reliable tool for early identification of COPD-related anxiety.65
- Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire (PHQ; available at: http://www.phqscreeners.com/select-screener/): the PRIME-MD comprises 26 yes/no questions on the 5 most common psychiatric disorders, including depression and anxiety.66,67 This is not a diagnostic tool, but a high number of positive responses from a patient in any given module indicates that they require further clinical evaluation.
- PHQ-2 and PHQ-9 (Table 1; PHQ-9 available at http://www.phqscreeners.com/select-screener/): widely-used self-administered 2- and 9-item versions of the PRIME-MD, specific to depression; similarly, the 3-item PHQ-3 is available for anxiety assessment (Table 2).6,67,68 In a study investigating tools used by family physicians in England to assess depression, over 75% used PHQ-9.69
- Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire-version 20 (GHQ-20): both can be used to screen for psychologic distress in patients with COPD.71
- The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI): two 21-item self-report questionnaires that are widely used in the United States to evaluate anxiety and depression.72
In addition to specific anxiety and depression questionnaires (Tables 1 and 2), more general COPD assessments tools, such as the COPD Assessment Test and the COPD Clinical Questionnaire, also incorporate questions that may be indicative of symptoms of these comorbidities in patients with COPD.73
Management of anxiety and depression in COPD
Even though anxiety and depression are among the most common and burdensome comorbid conditions in COPD, less than one-third of patients with these comorbidities receive effective intervention.6,10 Primary care providers have an excellent opportunity to impact this care gap.
As in non-COPD patients, comorbid depression and anxiety may be treated with nonpharmacologic and/or pharmacologic interventions (Figure 1).76
Nonpharmacologic interventions
Evidence to date suggests that nonpharmacologic interventions such as behavioral therapy are as effective as antidepressants, and may be preferred by patients with mood disorders.12
Cognitive behavioral therapy (CBT), which is typically administered by psychologists/psychiatrists, may be effective in treating COPD-related anxiety and depression, especially in conjunction with exercise and education.12,76,77 Individualized or group CBT is the treatment of choice for addressing thinking patterns that contribute to anxiety and depression to change a patient’s behavior and emotional state.76 PR programs involve several components, including aerobic exercise, lung function training, and psycho-education.62,76 PR is suitable for most patients with COPD, and provides multiple benefits, including reduced hospitalizations in patients who have had a recent exacerbation, and improved dyspnea, exercise tolerance, and health status in patients with stable disease,62 as well as clinically and statistically significant improvements in depression and anxiety, irrespective of age.7,78,79 Exercise-based forms of PR appear to be the most effective for reducing mood symptoms,12,76 and incorporating psychotherapy may also improve psychologic outcomes.80 Stress reduction (relaxation) therapy aims to reduce anxiety-related physiologic changes, and includes a variety of techniques (eg, breathing exercises, sequential muscle relaxation, hypnosis, mindfulness meditation), some of which may be included in PR or used alongside other treatments (eg, CBT).76 Limited data indicate that such therapy may be beneficial for reducing anxiety and depression, as well as respiratory symptoms and dyspnea, in patients with COPD.12,76
Self-management techniques improve clinical outcomes in patients with COPD, but data on the management of depression or anxiety are inconclusive.7,12 A minimal, home-based, nurse-led, psycho-educational intervention was designed to encourage more open-ended, descriptive discussions of thoughts, emotions, behaviors, and bodily sensations in patients with COPD.81 The intervention, which involved nurses attending a 1-hour face-to-face session in the patients’ homes with a 15-minute telephone “booster” session 2 weeks later, helped patients with advanced COPD to self-manage their condition and provide relief from anxiety.81,82 However, it should be noted that there is currently a lack of high-quality data evaluating psychologic interventions in the COPD population.83
In addition, it is important that caregivers are supported in the management of patients with COPD and comorbid anxiety and/or depression; areas in which caregivers can be assisted in their role may include disease education and counseling, where appropriate.84
Given that smoking cessation is a key recommendation for patients with COPD,44,62 practitioners should be aware that patients with comorbid depression and anxiety may experience greater difficulty in smoking cessation, and worsened mood during nicotine withdrawal.44 Clinicians should therefore carefully monitor current smokers with COPD and comorbid depression/anxiety (using the tools described previously63,68,70,71) when they are attempting to quit smoking.
Pharmacologic interventions
Pharmacologic therapy of anxiety and depression has so far only been investigated in patients with COPD in small studies.76 However, the available evidence does not indicate that COPD patients with anxiety and depression should be managed any differently from individuals without COPD.62 As such, pharmacologic interventions are particularly important for patients with acute or severe anxiety or depression.
Antidepressant agents are categorized according to their mechanism of action, and most commonly include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors, bupropion (a norepinephrine- and dopamine-reuptake inhibitor; also approved for smoking cessation85), and mirtazapine (a norepinephrine and serotonin modulator), among others.86 SSRIs are the current firstline drug treatment for depression, and have been shown to significantly improve depression and anxiety in patients with COPD in some, but not all, trials published to date.76 However, it is important to note that a diagnosis of bipolar disorder must be ruled out before initiating standard antidepressant therapy.87 In addition to antidepressants, atypical antipsychotics have also been shown to be useful for treating anxiety, either as monotherapy or combination therapy, and possibly as an adjunctive therapy for the management of depression.88,89
Primary care practitioners can refer to existing guidelines on the management of anxiety and depression in patients with COPD,86,90 while taking certain factors into consideration. Any pharmacologic management strategy for the treatment of COPD may increase the risk of drug–drug or drug–disease interactions.76 For example, it is important to avoid medications that cause respiratory depression (eg, benzodiazepines [unless used with extreme caution], particularly in patients who are already CO2 retainers) or sedation; chosen drugs should have minimal other adverse effects.76 Moreover, SSRIs may also be associated with troublesome adverse effects during treatment initiation, such as gastrointestinal upset, headache, tremor, psychomotor activation, and sedation76; in addition, dry mouth is an adverse effect associated with both SSRI treatment and several inhaled therapies, so may be particularly problematic in patients with COPD.91,92 Currently, data are particularly scarce for the management of anxiety in patients with COPD, with inconclusive or contradictory findings reported for SSRIs, azapirones (including buspirone), and tricyclic antidepressants.76
In addition to monitoring adherence to COPD therapies, primary care practitioners should carefully monitor patients treated with antidepressants and anxiolytics for adherence. A meta-analysis of 18,245 individuals with chronic diseases showed that depressed patients had a 76% significantly higher risk of nonadherence to medication compared with those without depressive symptoms.93
Targeting dyspnea is key to the management of anxiety and depression in COPD, as breathlessness is frequently associated with the onset of both comorbidities.21,22 Therapeutic approaches to alleviating dyspnea include PR, optimizing respiratory mechanics and muscle function (with bronchodilator therapy), and reducing central neural drive to respiratory muscles with supplemental oxygen or opioid medication.94
Although bronchodilator therapy for COPD has not been shown to have significant direct effects on depression or anxiety,95 it can be assumed that the beneficial effects on dyspnea are likely to alleviate associated emotional and mood symptoms.
Further research into effective screening, diagnosis, and management of comorbid anxiety and depressive disorders in COPD is warranted, including evaluation of a broad range of nonpharmacologic and drug-based interventions, alone and in combination.76
Conclusions
Anxiety and depression are common, yet frequently overlooked, comorbidities in COPD. The impact of these psychologic comorbidities is significant; their consequences are evident in morbidity and mortality data, as well as in patient-reported outcomes. As key points of contact for patients with COPD, it is essential that primary care practitioners are vigilant in monitoring for anxiety and depression in their patients with COPD, making the most of the available tools that can support them in doing so, and maintain an ongoing line of communication with other members of the multidisciplinary team. Treatment of anxiety and depression in COPD should adopt a holistic approach that incorporates both nonpharmacologic and pharmacologic interventions. However, the impact of effective screening, diagnosis, and management of anxiety and depression on COPD burden in patients requires further investigation.
Introduction
Anxiety and depression are common in patients with chronic obstructive pulmonary disease (COPD), occurring more frequently than in the general population1-4 or patients with other chronic diseases such as hypertension, diabetes, cancer, or musculoskeletal disorders.5,6 Their presence is associated with worse outcomes of COPD, and increased morbidity, mortality, disability, and health care expenditure.6-8 In spite of this, both anxiety and depression are frequently overlooked and undertreated in patients with COPD,9 and symptoms of anxiety and depression can overlap significantly, as well as overlap with COPD symptoms.7,10
Comorbid depressive disorders that may occur in patients with COPD include major depressive disorder, dysthymias (chronic depressive symptoms of mild severity), and minor depression.11 Depressive disorders are characterized by feelings of sadness, emptiness, and/or irritability, along with cognitive and somatic symptoms, which have a detrimental effect on the patient’s ability to function.11 Anxiety disorders include generalized anxiety disorder (GAD), phobias, and panic disorders.11 The main features of anxiety disorders, such as excessive fear and anxiety, may be accompanied by behavioral disturbances related to these symptoms, such as panic attacks and avoidance.11,12
The reported prevalence of depression in COPD varies widely between studies, owing to differences in sampling methods and degrees of illness severity used in assessment of depression6; rates have been reported to range from 10% to 42% in patients with stable COPD,6,13 and from 10% to 86% in patients with acute COPD exacerbation.14 Individuals with severe COPD are twice as likely to develop depression than patients with mild COPD.10
Prevalence rates for clinical anxiety in COPD range from 13% to 46% in outpatients and 10% to 55% among inpatients. GAD, panic disorders, and specific phobias are reported most frequently.15 Patients with COPD are 85% more likely to develop anxiety disorders compared with matched controls without COPD,4 and panic disorder is reported with a prevalence that is up to 10-fold higher than in the general population.16
Global prevalence rates of anxiety and depression are 1.8- and 1.4-fold higher in women than men, respectively17; the same gender difference is observed in patients with COPD.6 The higher prevalence rates of anxiety and depression in women are thought to be a result of sex differences in brain structure, function, and stress responses, as well as differences in exposure to reproductive hormones, social constraints, and experiences between women and men.18 However, psychologic comorbidity is an issue for both men and women with COPD, so it is important that clinicians are vigilant in recognizing anxiety and depression in both sexes, and are careful not to underestimate the burden in the male patient population.
It is also important to note that depression and anxiety often occur simultaneously in patients with COPD, with prevalence estimates of 26% to 43%.9,19,20 COPD patients with both depression and anxiety are at a heightened risk of suicidal ideation, increased physical disability, and chronic depressive symptoms versus those with either disorder alone.10,15 It is therefore important that comorbid anxiety and depression is not overlooked in patients with COPD.
Ensuring that anxiety and depression are recognized and treated effectively in patients with COPD is essential for optimizing outcomes. Primary care practitioners are well placed to diagnose anxiety and depression, and to ensure these conditions are suitably managed alongside treatments of COPD.
Potential mechanisms of anxiety and depression in COPD
Growing evidence suggests that the relationship between mood disorders—particularly depression—and COPD is bidirectional, meaning that mood disorders adversely impact prognosis in COPD, whereas COPD increases the risk of developing depression.21 For example, in a study of
60 stable patients with COPD, elevated dyspnea and reduced exercise capacity were the predominant mechanisms leading to anxiety and depression symptoms associated with the condition.22 In addition, the risk of new-onset depression was increased in COPD patients with moderate-to-severe dyspnea in a 3-year follow-up study.23 Conversely, depression has been shown to be a significant risk factor for disabling dyspnea (modified Medical Research Council score ≥2) in patients with COPD.24
COPD can lead to feelings of hopelessness, social isolation, reduced physical functioning, and sedentary lifestyle, all of which are associated with an increased level of depressive symptoms.25 Similarly, inadequate social support increases the risk of anxiety in patients with COPD.26 Therefore, ensuring that patients with COPD have high-quality support is very important for reducing anxiety and depressive symptoms.27
The exact mechanisms for the association between mood disorders and COPD remain unclear.7,10 Research to date indicates that the relationship between depression and impaired pulmonary function may be partly mediated by chronic inflammation7,10; systemic inflammation has been associated with other comorbidities of COPD (eg, muscle wasting and osteoporosis),28 and emerging data appear to show that proinflammatory cytokines partly mediate the association between depressive symptoms and pulmonary function.29 Smoking and hypoxemia may also influence the prevalence of depression in COPD, but symptom severity and impaired quality of life remain the most important determinants.6,30
Clinical studies have demonstrated that a number of patient-related factors, including female gender, younger age, current smoking, greater severity of airflow limitation, and lower socioeconomic status, are associated with a higher prevalence and/or increased risk of depression and/or anxiety in COPD.3,4,30,31 Frequent episodes of rehospitalization, and comorbidities such as hypertension, arthritis, cancer, and heart disease, have been found to increase the risk of anxiety and depression in patients with COPD.3,32 Risk of anxiety has been shown to increase with greater dyspnea severity.4 Pain, a frequently overlooked symptom in COPD, has been shown to be associated with symptoms of both anxiety and depression in patients with COPD.33 This is driven by worsened quality of life and sleep quality, decreased physical activity, and an increased fear of movement that occur as a result of pain.34
The impact of anxiety and depression in COPD
Comorbid anxiety and depression have a significant detrimental impact on morbidity and mortality in patients with COPD. Both disorders have been associated with an increased risk of death in COPD.13,35-37 Indeed, of 12 comorbidities proposed to be predictors of mortality in a cohort of 187 female outpatients with COPD, anxiety was associated with the highest risk of death.35,36
In addition, patients with COPD and anxiety and/or depression have a higher risk of COPD exacerbations,4,8,23,36,38-40 hospitalization,41,42 rehospitalization,14,36,43 longer hospital stays,37,41,44 and mortality after exacerbations,14,36,41 compared with patients without these comorbidities. Patients with COPD who have elevated anxiety symptoms also often experience their first hospitalization earlier in the natural course of COPD than those without anxiety.36
Psychologic comorbidities are also associated with worse lung function, dyspnea, and respiratory symptom burden in patients with COPD.37,40 Patients with COPD and anxiety are more likely to experience greater dyspnea at an earlier stage of disease than those without anxiety.36 Persistent smoking at 6 months after hospitalization for an acute exacerbation of COPD is also more likely to be seen in patients with depression.37
Patient-centered outcomes are worse in COPD patients with mood disorders. Both anxiety and depression have been shown to correlate with significantly reduced health-related quality of life (HRQoL), poorer physical health status, functional limitations, and reduced exercise capacity.4,23,37,40,45 The presence of either anxiety or depression at baseline has been shown to correlate with reduced HRQoL at 1-year follow-up, but depression appears to be the stronger predictor of low future HRQoL than anxiety.45
Additionally, mood disorders—particularly depression—reduce physical activity in patients with COPD.46,47 Emotional responses to COPD symptoms, such as dyspnea, can further decrease activity and worsen deconditioning, resulting in a downward spiral of reduced inactivity, social isolation, fear, anxiety, and depression.48
COPD patients with any comorbidity exhibit lower rates of medication adherence than those without comorbidities.49-51 Clinical studies have demonstrated that anxiety and depression are significant predictors of poor adherence to COPD interventions, including pulmonary rehabilitation (PR).51-55 Nonadherence to COPD therapies is associated with poor clinical outcomes, including higher hospitalization rates and increased emergency department visits, and increased costs.56,57 Health care expenditure, in terms of both specific COPD-related costs and general “all-cause” costs, is significantly higher in COPD patients with anxiety and/or depression than in those without.8
Diagnosis of anxiety and depression in patients with COPD
The underdiagnosis and undertreatment of anxiety and depression in this population is common and can adversely affect patient outcomes.6,7,9,10,58 Hence, it is crucial that anxiety and depression are identified and more effectively managed in clinical practice.10
Primary care practitioners are the main point of contact for many patients with COPD,6,59,60 and so can play a key role in screening for and early identification of anxiety and depression. However, detection of mood disorders by primary care practitioners is challenging for several reasons. These include the lack of a standardized approach in diagnosis, and inadequate knowledge or confidence in assessing psychological status (particularly given the number of strategies available for screening patients for mood disorders),6 as well as factors associated with time constraints, such as competing agendas, duration of visits, and high patient load.6,61 Furthermore, system-level barriers, such as lack of electronic medical records and adequate health insurance, as well as any communication gaps between primary care and mental health care, may hinder the detection and management of anxiety and depression.6 In addition, patients themselves may have a limited understanding of these comorbidities, or may be hesitant to discuss symptoms of anxiety or depression with their primary care practitioner owing to stigma around mental illness.6
Patients with COPD should be screened and assessed for anxiety and depression, and the United States Preventive Services Task Force recommends that clinicians screen for depression in all adults.6,62 There are several validated screening tools suitable for clinical use:
- Anxiety Inventory for Respiratory (AIR) Disease scale: a brief, easy-to-use tool for screening and measuring anxiety in COPD.63,64 It is a self-administered scale, and takes approximately 2 minutes to complete. The AIR scale is responsive to PR.64
- COPD Anxiety Questionnaire (CAF): a reliable tool for early identification of COPD-related anxiety.65
- Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire (PHQ; available at: http://www.phqscreeners.com/select-screener/): the PRIME-MD comprises 26 yes/no questions on the 5 most common psychiatric disorders, including depression and anxiety.66,67 This is not a diagnostic tool, but a high number of positive responses from a patient in any given module indicates that they require further clinical evaluation.
- PHQ-2 and PHQ-9 (Table 1; PHQ-9 available at http://www.phqscreeners.com/select-screener/): widely-used self-administered 2- and 9-item versions of the PRIME-MD, specific to depression; similarly, the 3-item PHQ-3 is available for anxiety assessment (Table 2).6,67,68 In a study investigating tools used by family physicians in England to assess depression, over 75% used PHQ-9.69
- Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire-version 20 (GHQ-20): both can be used to screen for psychologic distress in patients with COPD.71
- The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI): two 21-item self-report questionnaires that are widely used in the United States to evaluate anxiety and depression.72
In addition to specific anxiety and depression questionnaires (Tables 1 and 2), more general COPD assessments tools, such as the COPD Assessment Test and the COPD Clinical Questionnaire, also incorporate questions that may be indicative of symptoms of these comorbidities in patients with COPD.73
Management of anxiety and depression in COPD
Even though anxiety and depression are among the most common and burdensome comorbid conditions in COPD, less than one-third of patients with these comorbidities receive effective intervention.6,10 Primary care providers have an excellent opportunity to impact this care gap.
As in non-COPD patients, comorbid depression and anxiety may be treated with nonpharmacologic and/or pharmacologic interventions (Figure 1).76
Nonpharmacologic interventions
Evidence to date suggests that nonpharmacologic interventions such as behavioral therapy are as effective as antidepressants, and may be preferred by patients with mood disorders.12
Cognitive behavioral therapy (CBT), which is typically administered by psychologists/psychiatrists, may be effective in treating COPD-related anxiety and depression, especially in conjunction with exercise and education.12,76,77 Individualized or group CBT is the treatment of choice for addressing thinking patterns that contribute to anxiety and depression to change a patient’s behavior and emotional state.76 PR programs involve several components, including aerobic exercise, lung function training, and psycho-education.62,76 PR is suitable for most patients with COPD, and provides multiple benefits, including reduced hospitalizations in patients who have had a recent exacerbation, and improved dyspnea, exercise tolerance, and health status in patients with stable disease,62 as well as clinically and statistically significant improvements in depression and anxiety, irrespective of age.7,78,79 Exercise-based forms of PR appear to be the most effective for reducing mood symptoms,12,76 and incorporating psychotherapy may also improve psychologic outcomes.80 Stress reduction (relaxation) therapy aims to reduce anxiety-related physiologic changes, and includes a variety of techniques (eg, breathing exercises, sequential muscle relaxation, hypnosis, mindfulness meditation), some of which may be included in PR or used alongside other treatments (eg, CBT).76 Limited data indicate that such therapy may be beneficial for reducing anxiety and depression, as well as respiratory symptoms and dyspnea, in patients with COPD.12,76
Self-management techniques improve clinical outcomes in patients with COPD, but data on the management of depression or anxiety are inconclusive.7,12 A minimal, home-based, nurse-led, psycho-educational intervention was designed to encourage more open-ended, descriptive discussions of thoughts, emotions, behaviors, and bodily sensations in patients with COPD.81 The intervention, which involved nurses attending a 1-hour face-to-face session in the patients’ homes with a 15-minute telephone “booster” session 2 weeks later, helped patients with advanced COPD to self-manage their condition and provide relief from anxiety.81,82 However, it should be noted that there is currently a lack of high-quality data evaluating psychologic interventions in the COPD population.83
In addition, it is important that caregivers are supported in the management of patients with COPD and comorbid anxiety and/or depression; areas in which caregivers can be assisted in their role may include disease education and counseling, where appropriate.84
Given that smoking cessation is a key recommendation for patients with COPD,44,62 practitioners should be aware that patients with comorbid depression and anxiety may experience greater difficulty in smoking cessation, and worsened mood during nicotine withdrawal.44 Clinicians should therefore carefully monitor current smokers with COPD and comorbid depression/anxiety (using the tools described previously63,68,70,71) when they are attempting to quit smoking.
Pharmacologic interventions
Pharmacologic therapy of anxiety and depression has so far only been investigated in patients with COPD in small studies.76 However, the available evidence does not indicate that COPD patients with anxiety and depression should be managed any differently from individuals without COPD.62 As such, pharmacologic interventions are particularly important for patients with acute or severe anxiety or depression.
Antidepressant agents are categorized according to their mechanism of action, and most commonly include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors, bupropion (a norepinephrine- and dopamine-reuptake inhibitor; also approved for smoking cessation85), and mirtazapine (a norepinephrine and serotonin modulator), among others.86 SSRIs are the current firstline drug treatment for depression, and have been shown to significantly improve depression and anxiety in patients with COPD in some, but not all, trials published to date.76 However, it is important to note that a diagnosis of bipolar disorder must be ruled out before initiating standard antidepressant therapy.87 In addition to antidepressants, atypical antipsychotics have also been shown to be useful for treating anxiety, either as monotherapy or combination therapy, and possibly as an adjunctive therapy for the management of depression.88,89
Primary care practitioners can refer to existing guidelines on the management of anxiety and depression in patients with COPD,86,90 while taking certain factors into consideration. Any pharmacologic management strategy for the treatment of COPD may increase the risk of drug–drug or drug–disease interactions.76 For example, it is important to avoid medications that cause respiratory depression (eg, benzodiazepines [unless used with extreme caution], particularly in patients who are already CO2 retainers) or sedation; chosen drugs should have minimal other adverse effects.76 Moreover, SSRIs may also be associated with troublesome adverse effects during treatment initiation, such as gastrointestinal upset, headache, tremor, psychomotor activation, and sedation76; in addition, dry mouth is an adverse effect associated with both SSRI treatment and several inhaled therapies, so may be particularly problematic in patients with COPD.91,92 Currently, data are particularly scarce for the management of anxiety in patients with COPD, with inconclusive or contradictory findings reported for SSRIs, azapirones (including buspirone), and tricyclic antidepressants.76
In addition to monitoring adherence to COPD therapies, primary care practitioners should carefully monitor patients treated with antidepressants and anxiolytics for adherence. A meta-analysis of 18,245 individuals with chronic diseases showed that depressed patients had a 76% significantly higher risk of nonadherence to medication compared with those without depressive symptoms.93
Targeting dyspnea is key to the management of anxiety and depression in COPD, as breathlessness is frequently associated with the onset of both comorbidities.21,22 Therapeutic approaches to alleviating dyspnea include PR, optimizing respiratory mechanics and muscle function (with bronchodilator therapy), and reducing central neural drive to respiratory muscles with supplemental oxygen or opioid medication.94
Although bronchodilator therapy for COPD has not been shown to have significant direct effects on depression or anxiety,95 it can be assumed that the beneficial effects on dyspnea are likely to alleviate associated emotional and mood symptoms.
Further research into effective screening, diagnosis, and management of comorbid anxiety and depressive disorders in COPD is warranted, including evaluation of a broad range of nonpharmacologic and drug-based interventions, alone and in combination.76
Conclusions
Anxiety and depression are common, yet frequently overlooked, comorbidities in COPD. The impact of these psychologic comorbidities is significant; their consequences are evident in morbidity and mortality data, as well as in patient-reported outcomes. As key points of contact for patients with COPD, it is essential that primary care practitioners are vigilant in monitoring for anxiety and depression in their patients with COPD, making the most of the available tools that can support them in doing so, and maintain an ongoing line of communication with other members of the multidisciplinary team. Treatment of anxiety and depression in COPD should adopt a holistic approach that incorporates both nonpharmacologic and pharmacologic interventions. However, the impact of effective screening, diagnosis, and management of anxiety and depression on COPD burden in patients requires further investigation.
- Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016;33(2):174-178.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217-223.
- Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976.
- Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229-234.
- Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246-256.
- Maurer J, Rebbapragada V, Borson S, et al; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S-56S.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293-299.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- American Psychiatric Association. Depressive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
- Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33-39.
- Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):pii: 170026.
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858-866.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med. 2010;104(9):1246-1253.
- World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland; World Health Organization: 2017.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330.
- Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res. 2017;11(2):OC24-OC27.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15(12):1090-1096.
- Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766-777.
- Tetikkurt C, Ozdemir I, Tetikkurt S, Yilmaz N, Ertan T, Bayar N. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226-231.
- Yohannes AM, Müllerová H, Hanania NA, et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916-926.
- Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805-2812.
- Kirkil G, Deveci F, Deveci SE, Atmaca M. Anxiety and depression symptoms in patients with chronic obstructive pulmonary disease. Bulletin Clin Psychopharmacol. 2015;25(2):151-161.
- Fuller-Thomson E, Lacombe-Duncan A. Understanding the association between chronic obstructive pulmonary disease and current anxiety: a population-based study. COPD. 2016;13(5):622-631.
- Yohannes AM. Is it quality or quantity of social support needed for patients with chronic medical illness? J Psychosom Res. 2013;74(2):87-88.
- Fabbri LM, Luppi F, Beghe B, Rabe KF. The multiple components of COPD. In: Hanania NA, Sharafkhaneh A, eds. COPD: a guide to diagnosis and clinical management. Humana Press; 2010.
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53.
- Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604-611.
- Zhang Q, Liao J, Liao X, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2014;14:92.
- Jose AK, Chelangara DP, Shaji KS. Factors associated with anxiety and depression in chronic obstructive pulmonary disease. Int J Res Med Sci. 2016;4(4):1074-1079.
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898.
- Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246-1258.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95-109.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60-67.
- Montserrat-Capdevila J, Godoy P, Marsal JR, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung. 2017;195(1):77-85.
- Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918-923.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335-343.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122(5):1633-1637.
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414-419.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65-70.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501-512.
- Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287-1295.
- Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park HK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease [published online ahead of print June 7, 2017]. Korean J Intern Med. 2017;doi: 10.3904/kjim.2016.090.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667-677.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204.
- Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043.
- Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365-1373.
- Busch AM, Scott-Sheldon LA, Pierce J, et al. Depressed mood predicts pulmonary rehabilitation completion among women, but not men. Respir Med. 2014;108(7):1007-1013.
- Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout? J Cardiopulm Rehabil Prev. 2013;33(6):419-426.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105-116.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- Dury R. COPD and emotional distress: not always noticed and therefore untreated. Br J Community Nurs. 2016;21(3):138-141.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5(2):115-128.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed June 2017.
- Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the anxiety inventory for respiratory disease. Chest. 2013;144(5):1587-1596.
- Yohannes AM, Dryden S, Hanania NA. The responsiveness of the anxiety inventory for respiratory disease scale following pulmonary rehabilitation. Chest. 2016;150(1):188-195.
- Kühl K, Kuhn C, Kenn K, Rief W. [The COPD-Anxiety-Questionnaire (CAF): a new instrument to assess illness specific anxiety in COPD patients]. Psychother Psychosom Med Psychol. 2011;61(1):e1-e9. German.
- Tamburrino MB, Lynch DJ, Nagel RW, Smith MK. Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry. 2009;11(6):339-343.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348-353.
- Yohannes AM, Hann M, Sibbald B. The management of depressive symptoms in patients with COPD: a postal survey of general practitioners. Prim Health Care Res Dev. 2011;12(3):237-244.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
- Bratås O, Grønning K, Forbord T. Psychometric properties of the Hospital Anxiety and Depression Scale and The General Health Questionnaire-20 in COPD inpatients. Scand J Caring Sci. 2014;28(2):413-420.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343.
- Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57-65.
- Cantor L, Jacobson R. COPD: How to manage comorbid depression and anxiety. Curr Psychiatry. 2003;2(11):45-54.
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2007;5:17.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297-328.
- Doyle C, Bhar S, Fearn M, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol. 2017;22(3):542-556.
- Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491-496.
- Bennett D, Bowen B, McCarthy P, Subramaniam A, O’Connor M, Henry MT. Outcomes of pulmonary rehabilitation for COPD in older patients: a comparative study. COPD. 2017;14(2):170-175.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open. 2015;5(7):e008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med. 2016;121:109-116.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3: CD010673. doi:10.002/14651858.CD010673.pub2.
- Cafarella P, Effing T, Frith P. An evaluation of the needs of carers of people with COPD. EurResp J. 2012;40(Suppl 56).
- Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zyban/pdf/ZYBAN-PI-MG.PDF. Accessed June 2017.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(10):1.
- Pary R, Matuschka PR, Lewis S, Lippmann S. Managing bipolar depression. Psychiatry (Edgmont). 2006;3(2):30-41.
- Blier P. Atypical antipsychotics for mood and anxiety disorders: safe and effective adjuncts? J Psychiatry Neurosci. 2005;30(4):232-233.
- Vulink NC, Figee M, Denys D. Review of atypical antipsychotics in anxiety. Eur Neuropsychopharmacol. 2011;21(6):429-449.
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi:10.1002/14651858.CD010844.pub2.
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175-1182.
- O’Donnell DE, Webb KA, Harle I, Neder JA. Pharmacological management of breathlessness in COPD: recent advances and hopes for the future. Expert Rev Respir Med. 2016;10(7):823-834.
- Hyun MK, Lee NR, Jang EJ, Yim JJ, Lee CH. Effect of inhaled drugs on anxiety and depression in patients with chronic obstructive pulmonary disease: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:747-754.
- Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016;33(2):174-178.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217-223.
- Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976.
- Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229-234.
- Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246-256.
- Maurer J, Rebbapragada V, Borson S, et al; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S-56S.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293-299.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- American Psychiatric Association. Depressive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
- Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33-39.
- Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):pii: 170026.
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858-866.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med. 2010;104(9):1246-1253.
- World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland; World Health Organization: 2017.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330.
- Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res. 2017;11(2):OC24-OC27.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15(12):1090-1096.
- Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766-777.
- Tetikkurt C, Ozdemir I, Tetikkurt S, Yilmaz N, Ertan T, Bayar N. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226-231.
- Yohannes AM, Müllerová H, Hanania NA, et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916-926.
- Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805-2812.
- Kirkil G, Deveci F, Deveci SE, Atmaca M. Anxiety and depression symptoms in patients with chronic obstructive pulmonary disease. Bulletin Clin Psychopharmacol. 2015;25(2):151-161.
- Fuller-Thomson E, Lacombe-Duncan A. Understanding the association between chronic obstructive pulmonary disease and current anxiety: a population-based study. COPD. 2016;13(5):622-631.
- Yohannes AM. Is it quality or quantity of social support needed for patients with chronic medical illness? J Psychosom Res. 2013;74(2):87-88.
- Fabbri LM, Luppi F, Beghe B, Rabe KF. The multiple components of COPD. In: Hanania NA, Sharafkhaneh A, eds. COPD: a guide to diagnosis and clinical management. Humana Press; 2010.
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53.
- Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604-611.
- Zhang Q, Liao J, Liao X, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2014;14:92.
- Jose AK, Chelangara DP, Shaji KS. Factors associated with anxiety and depression in chronic obstructive pulmonary disease. Int J Res Med Sci. 2016;4(4):1074-1079.
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898.
- Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246-1258.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95-109.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60-67.
- Montserrat-Capdevila J, Godoy P, Marsal JR, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung. 2017;195(1):77-85.
- Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918-923.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335-343.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122(5):1633-1637.
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414-419.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65-70.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501-512.
- Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287-1295.
- Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park HK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease [published online ahead of print June 7, 2017]. Korean J Intern Med. 2017;doi: 10.3904/kjim.2016.090.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667-677.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204.
- Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043.
- Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365-1373.
- Busch AM, Scott-Sheldon LA, Pierce J, et al. Depressed mood predicts pulmonary rehabilitation completion among women, but not men. Respir Med. 2014;108(7):1007-1013.
- Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout? J Cardiopulm Rehabil Prev. 2013;33(6):419-426.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105-116.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- Dury R. COPD and emotional distress: not always noticed and therefore untreated. Br J Community Nurs. 2016;21(3):138-141.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5(2):115-128.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed June 2017.
- Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the anxiety inventory for respiratory disease. Chest. 2013;144(5):1587-1596.
- Yohannes AM, Dryden S, Hanania NA. The responsiveness of the anxiety inventory for respiratory disease scale following pulmonary rehabilitation. Chest. 2016;150(1):188-195.
- Kühl K, Kuhn C, Kenn K, Rief W. [The COPD-Anxiety-Questionnaire (CAF): a new instrument to assess illness specific anxiety in COPD patients]. Psychother Psychosom Med Psychol. 2011;61(1):e1-e9. German.
- Tamburrino MB, Lynch DJ, Nagel RW, Smith MK. Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry. 2009;11(6):339-343.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348-353.
- Yohannes AM, Hann M, Sibbald B. The management of depressive symptoms in patients with COPD: a postal survey of general practitioners. Prim Health Care Res Dev. 2011;12(3):237-244.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
- Bratås O, Grønning K, Forbord T. Psychometric properties of the Hospital Anxiety and Depression Scale and The General Health Questionnaire-20 in COPD inpatients. Scand J Caring Sci. 2014;28(2):413-420.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343.
- Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57-65.
- Cantor L, Jacobson R. COPD: How to manage comorbid depression and anxiety. Curr Psychiatry. 2003;2(11):45-54.
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2007;5:17.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297-328.
- Doyle C, Bhar S, Fearn M, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol. 2017;22(3):542-556.
- Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491-496.
- Bennett D, Bowen B, McCarthy P, Subramaniam A, O’Connor M, Henry MT. Outcomes of pulmonary rehabilitation for COPD in older patients: a comparative study. COPD. 2017;14(2):170-175.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open. 2015;5(7):e008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med. 2016;121:109-116.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3: CD010673. doi:10.002/14651858.CD010673.pub2.
- Cafarella P, Effing T, Frith P. An evaluation of the needs of carers of people with COPD. EurResp J. 2012;40(Suppl 56).
- Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zyban/pdf/ZYBAN-PI-MG.PDF. Accessed June 2017.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(10):1.
- Pary R, Matuschka PR, Lewis S, Lippmann S. Managing bipolar depression. Psychiatry (Edgmont). 2006;3(2):30-41.
- Blier P. Atypical antipsychotics for mood and anxiety disorders: safe and effective adjuncts? J Psychiatry Neurosci. 2005;30(4):232-233.
- Vulink NC, Figee M, Denys D. Review of atypical antipsychotics in anxiety. Eur Neuropsychopharmacol. 2011;21(6):429-449.
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi:10.1002/14651858.CD010844.pub2.
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175-1182.
- O’Donnell DE, Webb KA, Harle I, Neder JA. Pharmacological management of breathlessness in COPD: recent advances and hopes for the future. Expert Rev Respir Med. 2016;10(7):823-834.
- Hyun MK, Lee NR, Jang EJ, Yim JJ, Lee CH. Effect of inhaled drugs on anxiety and depression in patients with chronic obstructive pulmonary disease: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:747-754.