User login
Things We Do for No Reason™: Ova and Parasite Testing in Patients With Acute Diarrhea Arising During Hospitalization
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
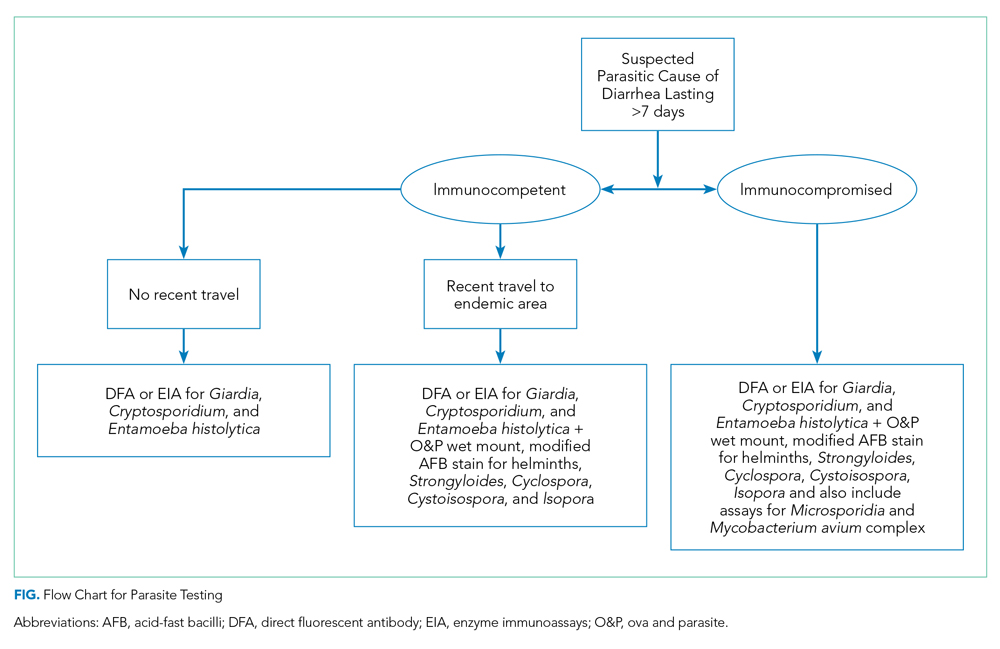
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.
RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing TWDFNR@hospitalmedicine.org .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing TWDFNR@hospitalmedicine.org .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3 Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing TWDFNR@hospitalmedicine.org .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
© 2021 Society of Hospital Medicine