User login
Phenytoin-Induced DRESS Syndrome: Clinical and Laboratory Characteristics
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
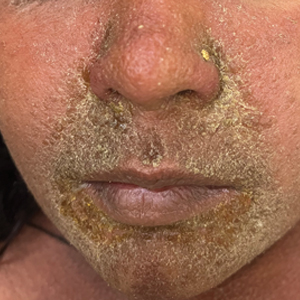
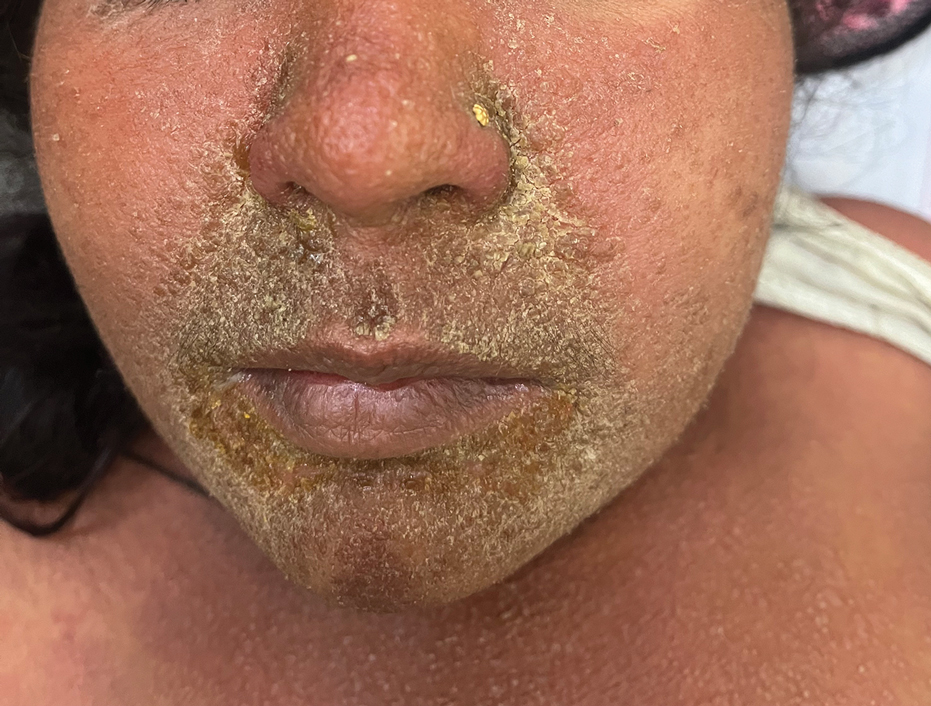
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.
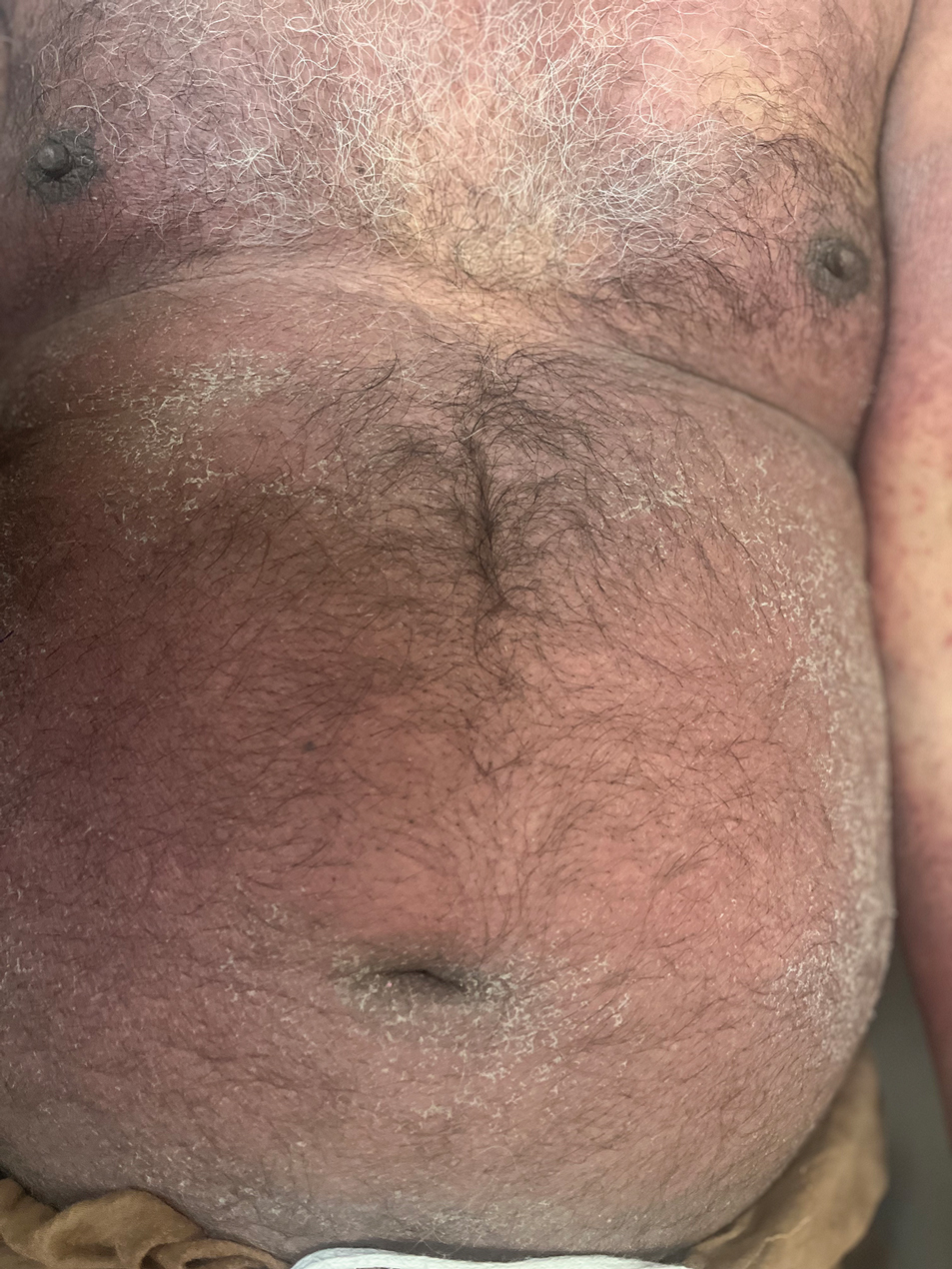
Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.
Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. doi:10.1016/s1085-5629(96)80038-1
- Patocka J, Wu Q, Nepovimova E, et al. Phenytoin—an anti-seizure drug: overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. doi:10.1016/j.fct.2020.111393
- Sasidharanpillai S, Chathoth AT, Khader A, et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol. 2019;85:266-275. doi:10.4103/ijdvl.IJDVL_482_17
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Brit J Dermatol. 2013;169:1071-1080.
- Morán-Mariños C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg. 2022;77:177-185. doi:10.1080/17843286.2020.1767459
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.

Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.

Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.
To the Editor:
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome—a severe cutaneous adverse drug reaction—is characterized by a cutaneous rash and systemic upset in the form of various internal organ and hematologic disturbances. This delayed and idiosyncratic syndrome went by several names, including anticonvulsant hypersensitivity syndrome, before Bocquet et al1 proposed the term DRESS syndrome.
Phenytoin, a hydantoin derivative used in neurology, was implicated in 41% of cases of DRESS syndrome in a study of 100 patients conducted in southern India.2,3 While DRESS syndrome is a newer name, the clinical picture of DRESS secondary to phenytoin use remains similar in that it manifests with a morbilliform rash and systemic upset. We sought to describe the clinical and laboratory characteristics of phenytoin-induced DRESS syndrome in this case series.
The analysis included 23 patients with DRESS syndrome secondary to phenytoin use who presented to a tertiary care institution in North India between July 2021 and December 2022, satisfied the European Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) criteria,4 and achieved a DRESS diagnostic score of more than 1. The mean age of the patients was 44 years (range, 14–74 years). There was a slight female predominance with a male to female ratio of 0.9:1. More than half of the patients (52.2% [12/23]) presented directly to the dermatology outpatient department; the remaining patients were referred from other departments (47.8% [11/23]). Patients primarily were receiving phenytoin for neurologic indications. Specific reasons included antiseizure prophylaxis following a traffic accident (34.8% [8/23]); epilepsy (26.1% [6/23]); and neoplastic (17.4% [4/23]), vascular (17.4% [4/23]), and infectious (4.3% [1/23]) causes. The mean latency period from drug intake to symptom onset was 29 days (range, 6–62 days), and the mean illness duration was 9 days (range, 1–45 days).
The majority of patients experienced pruritus (91.3% [21/23]) and fever (74.0% [17/23]), and all initially had a rash. Maculopapular morphology was seen in all patients. Erythema multiforme–like (17.4% [4/23]), erythrodermic (17.4% [4/23]), and vesicular (13.0% [3/23]) rashes also were documented (Figure 1). The trunk (100% [23/23]) and extremities (95.7% [22/23]) were involved most often, followed by the palms and soles (56.5% [13/23]). The mean total body surface area affected was 73.65%. Only 7 patients (30.4%) had mucosal involvement; nonhemorrhagic cheilitis was the most common manifestation.

Facial edema, a hallmark feature of DRESS syndrome, was noted in 69.6% (16/23) of patients (Figure 2). Lymphadenopathy was present in 43.5% (10/23) of patients; of those cases, the inguinal (40.0%; n=4) and cervical (30%; n=3) nodes most commonly were involved. Although DRESS syndrome can affect internal organs, this was an issue for only 2 (8.7%) patients who experienced mild hepatomegaly.

Laboratory investigations revealed a mean differential eosinophil percentage of 10.3% (reference range, 1%–4%), while the mean absolute eosinophil count was 1.0634×109/L (reference range, 0.02–0.5×109/L). Other hematologic findings included the mean percentages of neutrophils (60%; reference range, 50%–60%), lymphocytes (19.95%; reference range, 20%–50%), and monocytes (8.70%; reference range, 2%–8%).
Liver function tests revealed transaminitis5 as the most common finding, with mean aspartate aminotransferase levels of 109 U/L (reference range, 8–33 U/L), mean alanine aminotransferase of 97.9 U/L (reference range, 7–56 U/L), and mean alkaline phosphatase levels of 211.35 U/L (reference range, 44–147 U/L). Half of the patients had notable (>2 times the upper limit of normal) transaminitis.
Renal blood workup revealed slightly elevated blood urea nitrogen levels with a mean value of 28.4 mg/dL (reference range, 6–24 mg/dL), and mean serum creatinine was 0.78 mg/dL (reference range for men, 0.7–1.3 mg/dL; for women, 0.6–1.1 mg/dL).
All patients were treated with oral steroids (prednisolone 1 mg/kg/d) before tapering slowly over the following 6 to 8 weeks. The culprit drug (phenytoin) was stopped on the day of presentation. Resolution of rash and itching was seen in all patients by 3 weeks after presentation without any relapse by follow-up at 6 weeks from presentation to the hospital.
Our case series seeks to discuss the clinical and laboratory features of phenytoin-induced DRESS syndrome. Our patients had more erythrodermic and erythema multiforme–like morphologies, less mucosal involvement, more hepatic involvement, and earlier resolution.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. doi:10.1016/s1085-5629(96)80038-1
- Patocka J, Wu Q, Nepovimova E, et al. Phenytoin—an anti-seizure drug: overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. doi:10.1016/j.fct.2020.111393
- Sasidharanpillai S, Chathoth AT, Khader A, et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol. 2019;85:266-275. doi:10.4103/ijdvl.IJDVL_482_17
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Brit J Dermatol. 2013;169:1071-1080.
- Morán-Mariños C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg. 2022;77:177-185. doi:10.1080/17843286.2020.1767459
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250-257. doi:10.1016/s1085-5629(96)80038-1
- Patocka J, Wu Q, Nepovimova E, et al. Phenytoin—an anti-seizure drug: overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. doi:10.1016/j.fct.2020.111393
- Sasidharanpillai S, Chathoth AT, Khader A, et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol. 2019;85:266-275. doi:10.4103/ijdvl.IJDVL_482_17
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Brit J Dermatol. 2013;169:1071-1080.
- Morán-Mariños C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg. 2022;77:177-185. doi:10.1080/17843286.2020.1767459
Practice Points
- Phenytoin has been implicated in drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and common symptoms include rash, pruritus, and fever.
- Transaminitis may occur in patients with DRESS syndrome secondary to phenytoin use.
Spontaneously Draining Axillary Tumors in a Young Woman
THE DIAGNOSIS: Ectopic (Accessory) Breast Tissue
Ectopic (accessory) breast tissue (EBT) is a phenomenon caused by failed regression of one or more components of the embryonic mammary ridges— paired ectodermal thickenings that eventually develop into definitive breast tissue including the nipples, areolae, and parenchyma. Ectopic breast tissue is more common in women than men and is believed to be sporadic, although an autosomal-dominant inheritance mechanism with incomplete penetrance has been proposed for some cases.1 The reported incidence of EBT varies greatly among racial and ethnic groups but is most common in individuals of Asian descent. The incidence across all types of EBT is estimated at 0.25% to 6% in the general population.2
Observed clinical variations of EBT range from simple polythelia (additional nipple[s] without associated parenchyma) to complete polymastia (organized and differentiated accessory breasts). Some types of EBT are rarer than others: One report of gynecologic cancer screenings in 1660 patients found polymastia and polythelia incidences of 0.12% and 5.48%, respectively.3 Of the symptomatic variations, isolated parenchymal EBT without a nipple or areolar complex is the most common and may manifest clinically as unilateral or bilateral tender, mildly erythematous nodules or masses often located in the axillae. Ectopic breast tissue generally is observed along the milk line, a developmental regional designation corresponding to the embryologic mammary ridge and extending linearly from the anterior axilla to the inguinal fold on both sides of the body; however, there have been rare reports of EBT manifesting in areas outside the milk line, such as the face, neck, back, vulva, and extremities.2,3
Given that the underlying elements of EBT usually are hormone responsive (as with normal breast tissue), the initial symptom onset and subsequent manifestation frequently coincide with pubertal milestones, pregnancy, or lactation. Furthermore, some patients with EBT may experience symptom fluctuations in concordance with monthly menstrual phases. Many cases of EBT are selflimited and resolve within weeks to months after the end of a pregnancy or lactation, but some cases may persist. Continued observation and follow-up are advisable in all patients, as EBT symptoms often recur and the tissue is susceptible to the same disease processes that affect normal breasts, the most concerning of which is malignancy.4 Although the true incidence is limited by available data, primary ectopic breast malignancy has been estimated to account for 0.3% to 3.8% of diagnosed breast malignancies.2 Cases of malignancy arising from EBT often are of higher grade and poorer prognosis, a finding that may be attributable to diagnostic delays caused by oversight or misdiagnosis of EBT rather than inherent differences in the biologic profile of the tumors.2,4 Patients with a documented history of EBT may benefit from having their routine breast cancer screenings expanded to include areas with EBT foci.
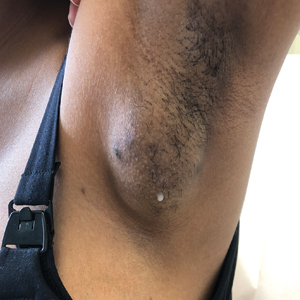
Potential misdiagnoses for EBT include subcutaneous lipoma, axillary lymphadenopathy, abscess, hidradenitis suppurativa, or malignancy. Features that are suggestive of EBT include symptom association with hormone fluctuations (eg, menstrual phases), absence of fever, and lactescent rather than purulent drainage. Among reported EBT cases, spontaneous lactation rarely is described and, if present, often is associated with a history of prior trauma (eg, core needle biopsy or local abscess formation).5 This trauma creates an aberrant connection known as a milk fistula between the underlying parenchyma and the skin surface. Interestingly, our patient denied any history of axillary trauma, but she was noted to be lactating from an apparent milk fistula rather than an organized secretory duct system.
Though a patient history and clinical examination may be sufficient to diagnose EBT cases that are more physically apparent and well correlated with hormone fluctuations, many cases require additional diagnostic studies for confirmation. Of the tools available, ultrasonography generally is considered first-line due to its noninvasive nature, low cost, minimal risk, and high diagnostic value.2 Ultrasonography quickly differentiates between abscesses and cystlike processes, which may appear as discrete areas of decreased echogenicity, and breast tissue, which manifests with fibroglandular tissue and lobules of fat.2,6 Additionally, ultrasonography may demonstrate the secretion of milk through ducts or fistulae, if present. Should examination with ultrasonography prove inconclusive, follow-up studies using conventional radiographic mammography or magnetic resonance imaging may be warranted. Biopsy of EBT foci generally is not indicated unless first-line noninvasive studies fail to yield a conclusive diagnosis; however, biopsy also may be warranted if initial imaging is suggestive of malignancy arising from EBT.2
Management of EBT generally is conservative, and symptoms often resolve without intervention.4 Symptomatic relief may be achieved through techniques such as application of warm/cold compresses, avoidance of mechanical stimulation, and use of over-the-counter pain medicine. In cases that are persistent, frequently recurrent, or associated with severe symptoms or that cause considerable cosmetic impact, management with surgical excision and/or liposuction may be warranted.7 In our patient, the symptoms were not bothersome enough to warrant surgical intervention, so she was managed conservatively and did not return for follow-up.
- Leung AK. Familial supernumerary nipples. Am J Med Genet. 1988;31:631-635. doi:10.1002/ajmg.1320310318
- Visconti G, Eltahir Y, Van Ginkel RJ, et al. Approach and management of primary ectopic breast carcinoma in the axilla: where are we? a comprehensive historical literature review. J Plast Reconstr Aesthet Surg. 2011;64:E1-E11. doi:10.1016/j.bjps.2010.08.015
- Göttlicher S. Incidence and location of polythelias, polymastias and mammae aberratae. a prospective one year study of 1,660 patients of a gynecologic practice. Article in German. Geburtshilfe Frauenheilkd. 1986;46:697-699. doi:10.1055/s-2008-1035944
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Firat D, Idiz O, Isik A, et al. Spontaneous milk fistula from an accessory breast: an extremely rare case. Breast J. 2015;21:554-555. doi:10.1111/tbj.12452
- Lim HS, Kim SJ, Baek JM, et al. Sonographic findings of accessory breast tissue in axilla and related diseases. J Ultrasound Med. 2017;36:1469-1478. doi:10.7863/ultra.16.06056
- Gentile P, Izzo V, Cervelli V. Fibroadenoma in the bilateral accessory axillary breast. Aesthetic Plast Surg. 2010;34:657-659. doi:10.1007/ s00266-010-9505-y
THE DIAGNOSIS: Ectopic (Accessory) Breast Tissue
Ectopic (accessory) breast tissue (EBT) is a phenomenon caused by failed regression of one or more components of the embryonic mammary ridges— paired ectodermal thickenings that eventually develop into definitive breast tissue including the nipples, areolae, and parenchyma. Ectopic breast tissue is more common in women than men and is believed to be sporadic, although an autosomal-dominant inheritance mechanism with incomplete penetrance has been proposed for some cases.1 The reported incidence of EBT varies greatly among racial and ethnic groups but is most common in individuals of Asian descent. The incidence across all types of EBT is estimated at 0.25% to 6% in the general population.2
Observed clinical variations of EBT range from simple polythelia (additional nipple[s] without associated parenchyma) to complete polymastia (organized and differentiated accessory breasts). Some types of EBT are rarer than others: One report of gynecologic cancer screenings in 1660 patients found polymastia and polythelia incidences of 0.12% and 5.48%, respectively.3 Of the symptomatic variations, isolated parenchymal EBT without a nipple or areolar complex is the most common and may manifest clinically as unilateral or bilateral tender, mildly erythematous nodules or masses often located in the axillae. Ectopic breast tissue generally is observed along the milk line, a developmental regional designation corresponding to the embryologic mammary ridge and extending linearly from the anterior axilla to the inguinal fold on both sides of the body; however, there have been rare reports of EBT manifesting in areas outside the milk line, such as the face, neck, back, vulva, and extremities.2,3
Given that the underlying elements of EBT usually are hormone responsive (as with normal breast tissue), the initial symptom onset and subsequent manifestation frequently coincide with pubertal milestones, pregnancy, or lactation. Furthermore, some patients with EBT may experience symptom fluctuations in concordance with monthly menstrual phases. Many cases of EBT are selflimited and resolve within weeks to months after the end of a pregnancy or lactation, but some cases may persist. Continued observation and follow-up are advisable in all patients, as EBT symptoms often recur and the tissue is susceptible to the same disease processes that affect normal breasts, the most concerning of which is malignancy.4 Although the true incidence is limited by available data, primary ectopic breast malignancy has been estimated to account for 0.3% to 3.8% of diagnosed breast malignancies.2 Cases of malignancy arising from EBT often are of higher grade and poorer prognosis, a finding that may be attributable to diagnostic delays caused by oversight or misdiagnosis of EBT rather than inherent differences in the biologic profile of the tumors.2,4 Patients with a documented history of EBT may benefit from having their routine breast cancer screenings expanded to include areas with EBT foci.
Potential misdiagnoses for EBT include subcutaneous lipoma, axillary lymphadenopathy, abscess, hidradenitis suppurativa, or malignancy. Features that are suggestive of EBT include symptom association with hormone fluctuations (eg, menstrual phases), absence of fever, and lactescent rather than purulent drainage. Among reported EBT cases, spontaneous lactation rarely is described and, if present, often is associated with a history of prior trauma (eg, core needle biopsy or local abscess formation).5 This trauma creates an aberrant connection known as a milk fistula between the underlying parenchyma and the skin surface. Interestingly, our patient denied any history of axillary trauma, but she was noted to be lactating from an apparent milk fistula rather than an organized secretory duct system.
Though a patient history and clinical examination may be sufficient to diagnose EBT cases that are more physically apparent and well correlated with hormone fluctuations, many cases require additional diagnostic studies for confirmation. Of the tools available, ultrasonography generally is considered first-line due to its noninvasive nature, low cost, minimal risk, and high diagnostic value.2 Ultrasonography quickly differentiates between abscesses and cystlike processes, which may appear as discrete areas of decreased echogenicity, and breast tissue, which manifests with fibroglandular tissue and lobules of fat.2,6 Additionally, ultrasonography may demonstrate the secretion of milk through ducts or fistulae, if present. Should examination with ultrasonography prove inconclusive, follow-up studies using conventional radiographic mammography or magnetic resonance imaging may be warranted. Biopsy of EBT foci generally is not indicated unless first-line noninvasive studies fail to yield a conclusive diagnosis; however, biopsy also may be warranted if initial imaging is suggestive of malignancy arising from EBT.2
Management of EBT generally is conservative, and symptoms often resolve without intervention.4 Symptomatic relief may be achieved through techniques such as application of warm/cold compresses, avoidance of mechanical stimulation, and use of over-the-counter pain medicine. In cases that are persistent, frequently recurrent, or associated with severe symptoms or that cause considerable cosmetic impact, management with surgical excision and/or liposuction may be warranted.7 In our patient, the symptoms were not bothersome enough to warrant surgical intervention, so she was managed conservatively and did not return for follow-up.
THE DIAGNOSIS: Ectopic (Accessory) Breast Tissue
Ectopic (accessory) breast tissue (EBT) is a phenomenon caused by failed regression of one or more components of the embryonic mammary ridges— paired ectodermal thickenings that eventually develop into definitive breast tissue including the nipples, areolae, and parenchyma. Ectopic breast tissue is more common in women than men and is believed to be sporadic, although an autosomal-dominant inheritance mechanism with incomplete penetrance has been proposed for some cases.1 The reported incidence of EBT varies greatly among racial and ethnic groups but is most common in individuals of Asian descent. The incidence across all types of EBT is estimated at 0.25% to 6% in the general population.2
Observed clinical variations of EBT range from simple polythelia (additional nipple[s] without associated parenchyma) to complete polymastia (organized and differentiated accessory breasts). Some types of EBT are rarer than others: One report of gynecologic cancer screenings in 1660 patients found polymastia and polythelia incidences of 0.12% and 5.48%, respectively.3 Of the symptomatic variations, isolated parenchymal EBT without a nipple or areolar complex is the most common and may manifest clinically as unilateral or bilateral tender, mildly erythematous nodules or masses often located in the axillae. Ectopic breast tissue generally is observed along the milk line, a developmental regional designation corresponding to the embryologic mammary ridge and extending linearly from the anterior axilla to the inguinal fold on both sides of the body; however, there have been rare reports of EBT manifesting in areas outside the milk line, such as the face, neck, back, vulva, and extremities.2,3
Given that the underlying elements of EBT usually are hormone responsive (as with normal breast tissue), the initial symptom onset and subsequent manifestation frequently coincide with pubertal milestones, pregnancy, or lactation. Furthermore, some patients with EBT may experience symptom fluctuations in concordance with monthly menstrual phases. Many cases of EBT are selflimited and resolve within weeks to months after the end of a pregnancy or lactation, but some cases may persist. Continued observation and follow-up are advisable in all patients, as EBT symptoms often recur and the tissue is susceptible to the same disease processes that affect normal breasts, the most concerning of which is malignancy.4 Although the true incidence is limited by available data, primary ectopic breast malignancy has been estimated to account for 0.3% to 3.8% of diagnosed breast malignancies.2 Cases of malignancy arising from EBT often are of higher grade and poorer prognosis, a finding that may be attributable to diagnostic delays caused by oversight or misdiagnosis of EBT rather than inherent differences in the biologic profile of the tumors.2,4 Patients with a documented history of EBT may benefit from having their routine breast cancer screenings expanded to include areas with EBT foci.
Potential misdiagnoses for EBT include subcutaneous lipoma, axillary lymphadenopathy, abscess, hidradenitis suppurativa, or malignancy. Features that are suggestive of EBT include symptom association with hormone fluctuations (eg, menstrual phases), absence of fever, and lactescent rather than purulent drainage. Among reported EBT cases, spontaneous lactation rarely is described and, if present, often is associated with a history of prior trauma (eg, core needle biopsy or local abscess formation).5 This trauma creates an aberrant connection known as a milk fistula between the underlying parenchyma and the skin surface. Interestingly, our patient denied any history of axillary trauma, but she was noted to be lactating from an apparent milk fistula rather than an organized secretory duct system.
Though a patient history and clinical examination may be sufficient to diagnose EBT cases that are more physically apparent and well correlated with hormone fluctuations, many cases require additional diagnostic studies for confirmation. Of the tools available, ultrasonography generally is considered first-line due to its noninvasive nature, low cost, minimal risk, and high diagnostic value.2 Ultrasonography quickly differentiates between abscesses and cystlike processes, which may appear as discrete areas of decreased echogenicity, and breast tissue, which manifests with fibroglandular tissue and lobules of fat.2,6 Additionally, ultrasonography may demonstrate the secretion of milk through ducts or fistulae, if present. Should examination with ultrasonography prove inconclusive, follow-up studies using conventional radiographic mammography or magnetic resonance imaging may be warranted. Biopsy of EBT foci generally is not indicated unless first-line noninvasive studies fail to yield a conclusive diagnosis; however, biopsy also may be warranted if initial imaging is suggestive of malignancy arising from EBT.2
Management of EBT generally is conservative, and symptoms often resolve without intervention.4 Symptomatic relief may be achieved through techniques such as application of warm/cold compresses, avoidance of mechanical stimulation, and use of over-the-counter pain medicine. In cases that are persistent, frequently recurrent, or associated with severe symptoms or that cause considerable cosmetic impact, management with surgical excision and/or liposuction may be warranted.7 In our patient, the symptoms were not bothersome enough to warrant surgical intervention, so she was managed conservatively and did not return for follow-up.
- Leung AK. Familial supernumerary nipples. Am J Med Genet. 1988;31:631-635. doi:10.1002/ajmg.1320310318
- Visconti G, Eltahir Y, Van Ginkel RJ, et al. Approach and management of primary ectopic breast carcinoma in the axilla: where are we? a comprehensive historical literature review. J Plast Reconstr Aesthet Surg. 2011;64:E1-E11. doi:10.1016/j.bjps.2010.08.015
- Göttlicher S. Incidence and location of polythelias, polymastias and mammae aberratae. a prospective one year study of 1,660 patients of a gynecologic practice. Article in German. Geburtshilfe Frauenheilkd. 1986;46:697-699. doi:10.1055/s-2008-1035944
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Firat D, Idiz O, Isik A, et al. Spontaneous milk fistula from an accessory breast: an extremely rare case. Breast J. 2015;21:554-555. doi:10.1111/tbj.12452
- Lim HS, Kim SJ, Baek JM, et al. Sonographic findings of accessory breast tissue in axilla and related diseases. J Ultrasound Med. 2017;36:1469-1478. doi:10.7863/ultra.16.06056
- Gentile P, Izzo V, Cervelli V. Fibroadenoma in the bilateral accessory axillary breast. Aesthetic Plast Surg. 2010;34:657-659. doi:10.1007/ s00266-010-9505-y
- Leung AK. Familial supernumerary nipples. Am J Med Genet. 1988;31:631-635. doi:10.1002/ajmg.1320310318
- Visconti G, Eltahir Y, Van Ginkel RJ, et al. Approach and management of primary ectopic breast carcinoma in the axilla: where are we? a comprehensive historical literature review. J Plast Reconstr Aesthet Surg. 2011;64:E1-E11. doi:10.1016/j.bjps.2010.08.015
- Göttlicher S. Incidence and location of polythelias, polymastias and mammae aberratae. a prospective one year study of 1,660 patients of a gynecologic practice. Article in German. Geburtshilfe Frauenheilkd. 1986;46:697-699. doi:10.1055/s-2008-1035944
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Firat D, Idiz O, Isik A, et al. Spontaneous milk fistula from an accessory breast: an extremely rare case. Breast J. 2015;21:554-555. doi:10.1111/tbj.12452
- Lim HS, Kim SJ, Baek JM, et al. Sonographic findings of accessory breast tissue in axilla and related diseases. J Ultrasound Med. 2017;36:1469-1478. doi:10.7863/ultra.16.06056
- Gentile P, Izzo V, Cervelli V. Fibroadenoma in the bilateral accessory axillary breast. Aesthetic Plast Surg. 2010;34:657-659. doi:10.1007/ s00266-010-9505-y
A 19-year-old G1P1A0 woman presented to the dermatology clinic for evaluation of bilateral axillary swelling, pain, and spontaneous drainage of approximately 2 weeks’ duration. The patient, who was 2 weeks postpartum, reported that the symptoms were associated with lactation when breastfeeding. She denied any personal or family history of hidradenitis suppurativa or other formally diagnosed dermatologic condition. Physical examination revealed a soft, mildly tender, well-circumscribed, nonfluctuant mobile mass in each axilla. Both lesions had a single central sinus tract with thin lactescent discharge that spontaneously drained and was expressible. A single thin hyperpigmented papule was noted on the anterior aspect of each mass.

Dactylitis Represents More Active and Severe PsA Phenotype
Key clinical point: The presence of clinical or subclinical dactylitis represented a more active and severe form of psoriatic arthritis (PsA), characterized by increased disease activity, swollen joint counts (SJCs), and tender joint counts (TJCs).
Major finding: PsA with dactylitis (clinical or subclinical) vs without dactylitis was associated with higher median disease activity index in PsA (DAPSA) scores (25.5 vs 16.1; P < .01), SJCs (4 vs 2; P < .001), and TJCs (4 vs 3; P < .01). PsA with subclinical dactylitis vs without dactylitis was associated with even higher DAPSA scores (27.2 vs 16.1; P < .05), SJCs (4.5 vs 2; P < .01), and TJCs (5 vs 3; P < .05).
Study details: This case-control study included 223 patients with PsA who were stratified on the basis of the presence of dactylitis (clinical or subclinical) or its absence at baseline.
Disclosures: This study was supported by the Youth Clinical Research Project of Peking University First Hospital and other sources. No conflicts of interest were reported.
Source: Song Z, Geng Y, Zhang X, Deng X, Zhang Z. Subclinical dactylitis represents a more active phenotype of psoriatic arthritis. Joint Bone Spine. Published online September 24, 2024. Source
Key clinical point: The presence of clinical or subclinical dactylitis represented a more active and severe form of psoriatic arthritis (PsA), characterized by increased disease activity, swollen joint counts (SJCs), and tender joint counts (TJCs).
Major finding: PsA with dactylitis (clinical or subclinical) vs without dactylitis was associated with higher median disease activity index in PsA (DAPSA) scores (25.5 vs 16.1; P < .01), SJCs (4 vs 2; P < .001), and TJCs (4 vs 3; P < .01). PsA with subclinical dactylitis vs without dactylitis was associated with even higher DAPSA scores (27.2 vs 16.1; P < .05), SJCs (4.5 vs 2; P < .01), and TJCs (5 vs 3; P < .05).
Study details: This case-control study included 223 patients with PsA who were stratified on the basis of the presence of dactylitis (clinical or subclinical) or its absence at baseline.
Disclosures: This study was supported by the Youth Clinical Research Project of Peking University First Hospital and other sources. No conflicts of interest were reported.
Source: Song Z, Geng Y, Zhang X, Deng X, Zhang Z. Subclinical dactylitis represents a more active phenotype of psoriatic arthritis. Joint Bone Spine. Published online September 24, 2024. Source
Key clinical point: The presence of clinical or subclinical dactylitis represented a more active and severe form of psoriatic arthritis (PsA), characterized by increased disease activity, swollen joint counts (SJCs), and tender joint counts (TJCs).
Major finding: PsA with dactylitis (clinical or subclinical) vs without dactylitis was associated with higher median disease activity index in PsA (DAPSA) scores (25.5 vs 16.1; P < .01), SJCs (4 vs 2; P < .001), and TJCs (4 vs 3; P < .01). PsA with subclinical dactylitis vs without dactylitis was associated with even higher DAPSA scores (27.2 vs 16.1; P < .05), SJCs (4.5 vs 2; P < .01), and TJCs (5 vs 3; P < .05).
Study details: This case-control study included 223 patients with PsA who were stratified on the basis of the presence of dactylitis (clinical or subclinical) or its absence at baseline.
Disclosures: This study was supported by the Youth Clinical Research Project of Peking University First Hospital and other sources. No conflicts of interest were reported.
Source: Song Z, Geng Y, Zhang X, Deng X, Zhang Z. Subclinical dactylitis represents a more active phenotype of psoriatic arthritis. Joint Bone Spine. Published online September 24, 2024. Source
Guselkumab Demonstrates Sustained Efficacy and Safety in PsA
Key clinical point: Guselkumab administered every 4 weeks (Q4W) or every 8 weeks (Q8W) yielded better clinical outcomes than placebo in patients with psoriatic arthritis (PsA), without any new safety concerns.
Major findings: At week 24, a higher proportion of patients receiving guselkumab Q4W (60%) and Q8W (51%) vs placebo (30%) achieved a ≥ 20% improvement in the American College of Rheumatology (ACR)20 response. The response rates increased through week 52 (69%-78) and were consistent at week 100 (76%-80%) across all the groups. Similar trends were observed for ACR50, with no new safety signals.
Study details: This post hoc analysis of the phase 3 trials DISCOVER-1 and DISCOVER-2 included 1002 biologic-naive patients with PsA who received 100 mg guselkumab Q4W, 100 mg guselkumab at weeks 0 and 4 and then Q8W, or placebo through week 24 with crossover to 100 mg guselkumab Q4W.
Disclosure: The study was sponsored by Janssen-Cilag Ltd. Four authors were employees of Johnson & Johnson. Several authors received research grants, consulting fees, or had ties with various sources.
Source: Mease P, Korotaeva T, Shesternya P, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis in Russia: A post hoc analysis of the DISCOVER-1 and -2 randomized clinical trials. Rheumatol Ther. Published online September 25, 2024. Source
Key clinical point: Guselkumab administered every 4 weeks (Q4W) or every 8 weeks (Q8W) yielded better clinical outcomes than placebo in patients with psoriatic arthritis (PsA), without any new safety concerns.
Major findings: At week 24, a higher proportion of patients receiving guselkumab Q4W (60%) and Q8W (51%) vs placebo (30%) achieved a ≥ 20% improvement in the American College of Rheumatology (ACR)20 response. The response rates increased through week 52 (69%-78) and were consistent at week 100 (76%-80%) across all the groups. Similar trends were observed for ACR50, with no new safety signals.
Study details: This post hoc analysis of the phase 3 trials DISCOVER-1 and DISCOVER-2 included 1002 biologic-naive patients with PsA who received 100 mg guselkumab Q4W, 100 mg guselkumab at weeks 0 and 4 and then Q8W, or placebo through week 24 with crossover to 100 mg guselkumab Q4W.
Disclosure: The study was sponsored by Janssen-Cilag Ltd. Four authors were employees of Johnson & Johnson. Several authors received research grants, consulting fees, or had ties with various sources.
Source: Mease P, Korotaeva T, Shesternya P, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis in Russia: A post hoc analysis of the DISCOVER-1 and -2 randomized clinical trials. Rheumatol Ther. Published online September 25, 2024. Source
Key clinical point: Guselkumab administered every 4 weeks (Q4W) or every 8 weeks (Q8W) yielded better clinical outcomes than placebo in patients with psoriatic arthritis (PsA), without any new safety concerns.
Major findings: At week 24, a higher proportion of patients receiving guselkumab Q4W (60%) and Q8W (51%) vs placebo (30%) achieved a ≥ 20% improvement in the American College of Rheumatology (ACR)20 response. The response rates increased through week 52 (69%-78) and were consistent at week 100 (76%-80%) across all the groups. Similar trends were observed for ACR50, with no new safety signals.
Study details: This post hoc analysis of the phase 3 trials DISCOVER-1 and DISCOVER-2 included 1002 biologic-naive patients with PsA who received 100 mg guselkumab Q4W, 100 mg guselkumab at weeks 0 and 4 and then Q8W, or placebo through week 24 with crossover to 100 mg guselkumab Q4W.
Disclosure: The study was sponsored by Janssen-Cilag Ltd. Four authors were employees of Johnson & Johnson. Several authors received research grants, consulting fees, or had ties with various sources.
Source: Mease P, Korotaeva T, Shesternya P, et al. Guselkumab in biologic-naïve patients with active psoriatic arthritis in Russia: A post hoc analysis of the DISCOVER-1 and -2 randomized clinical trials. Rheumatol Ther. Published online September 25, 2024. Source
Impact of Smoking on Treatment Outcomes of Tofacitinib in PsA
Key clinical point: In patients with psoriatic arthritis (PsA), tofacitinib demonstrated higher efficacy than placebo, regardless of smoking status; however, current or past smokers experienced increased rates of adverse events.
Major finding: At 3 months, 10 mg tofacitinib showed higher rates of a ≥ 50% improvement in the American College of Rheumatology response than placebo in current or past smokers (odds ratio [OR], 3.01; 95% CI, 1.43-6.33) and never smokers (OR, 6.53; 95% CI, 3.46-12.33). The incidence rates of treatment-emergent adverse events were higher in current or past smokers vs never smokers (adjusted incidence rate, 263.2 vs 208.9); 5 mg tofacitinib had comparable outcomes.
Study details: This post hoc analysis pooled data from phase 2 and 3 trials and a long-term extension study, involving 914 patients with PsA and 372 patients with ankylosing spondylitis who received tofacitinib (5 or 10 mg twice daily) or placebo, while considering their smoking status.
Disclosures: This study was sponsored by Pfizer. Four authors declared being current or former employees or shareholders of Pfizer. Other authors declared having ties with various sources.
Source: Ogdie A, Kristensen LE, Soriano ER, et al. Efficacy and safety of tofacitinib in patients with psoriatic arthritis or ankylosing spondylitis by cigarette smoking status. Rheumatol Ther. Published online September 25, 2024. Source
Key clinical point: In patients with psoriatic arthritis (PsA), tofacitinib demonstrated higher efficacy than placebo, regardless of smoking status; however, current or past smokers experienced increased rates of adverse events.
Major finding: At 3 months, 10 mg tofacitinib showed higher rates of a ≥ 50% improvement in the American College of Rheumatology response than placebo in current or past smokers (odds ratio [OR], 3.01; 95% CI, 1.43-6.33) and never smokers (OR, 6.53; 95% CI, 3.46-12.33). The incidence rates of treatment-emergent adverse events were higher in current or past smokers vs never smokers (adjusted incidence rate, 263.2 vs 208.9); 5 mg tofacitinib had comparable outcomes.
Study details: This post hoc analysis pooled data from phase 2 and 3 trials and a long-term extension study, involving 914 patients with PsA and 372 patients with ankylosing spondylitis who received tofacitinib (5 or 10 mg twice daily) or placebo, while considering their smoking status.
Disclosures: This study was sponsored by Pfizer. Four authors declared being current or former employees or shareholders of Pfizer. Other authors declared having ties with various sources.
Source: Ogdie A, Kristensen LE, Soriano ER, et al. Efficacy and safety of tofacitinib in patients with psoriatic arthritis or ankylosing spondylitis by cigarette smoking status. Rheumatol Ther. Published online September 25, 2024. Source
Key clinical point: In patients with psoriatic arthritis (PsA), tofacitinib demonstrated higher efficacy than placebo, regardless of smoking status; however, current or past smokers experienced increased rates of adverse events.
Major finding: At 3 months, 10 mg tofacitinib showed higher rates of a ≥ 50% improvement in the American College of Rheumatology response than placebo in current or past smokers (odds ratio [OR], 3.01; 95% CI, 1.43-6.33) and never smokers (OR, 6.53; 95% CI, 3.46-12.33). The incidence rates of treatment-emergent adverse events were higher in current or past smokers vs never smokers (adjusted incidence rate, 263.2 vs 208.9); 5 mg tofacitinib had comparable outcomes.
Study details: This post hoc analysis pooled data from phase 2 and 3 trials and a long-term extension study, involving 914 patients with PsA and 372 patients with ankylosing spondylitis who received tofacitinib (5 or 10 mg twice daily) or placebo, while considering their smoking status.
Disclosures: This study was sponsored by Pfizer. Four authors declared being current or former employees or shareholders of Pfizer. Other authors declared having ties with various sources.
Source: Ogdie A, Kristensen LE, Soriano ER, et al. Efficacy and safety of tofacitinib in patients with psoriatic arthritis or ankylosing spondylitis by cigarette smoking status. Rheumatol Ther. Published online September 25, 2024. Source
Intravenous Secukinumab Effective and Safe in PsA
Key clinical point: In patients with active psoriatic arthritis (PsA), intravenous secukinumab every 4 weeks demonstrated rapid and sustained efficacy, along with a safety profile consistent with that of subcutaneous secukinumab.
Major finding: At week 16, intravenous secukinumab vs placebo significantly improved the American College of Rheumatology 50 response rate (31.4% vs 6.3%; adjusted P < .0001). Improvements were observed as early as week 4 (P < .0005) and were sustained through week 52, regardless of whether patients continued intravenous secukinumab (58.0%) or switched from placebo to intravenous secukinumab (64.0%). No new safety signals were reported.
Study details: In this phase 3 INVIGORATE-2 trial, 381 patients with active PsA and either plaque psoriasis or nail psoriasis were randomly assigned to receive intravenous secukinumab or placebo with crossover to intravenous secukinumab at week 16.
Disclosures: This study was supported by Novartis. Five authors declared being employees of and owning stocks in Novartis. Several authors declared having ties with various sources, including Novartis.
Source: Kivitz A, Sedova L, Churchill M, et al. Efficacy and safety of intravenous secukinumab for the treatment of active psoriatic arthritis: Results from the randomized, placebo-controlled phase III INVIGORATE-2 study. Arthritis Rheumatol. Published online September 19, 2024. Source
Key clinical point: In patients with active psoriatic arthritis (PsA), intravenous secukinumab every 4 weeks demonstrated rapid and sustained efficacy, along with a safety profile consistent with that of subcutaneous secukinumab.
Major finding: At week 16, intravenous secukinumab vs placebo significantly improved the American College of Rheumatology 50 response rate (31.4% vs 6.3%; adjusted P < .0001). Improvements were observed as early as week 4 (P < .0005) and were sustained through week 52, regardless of whether patients continued intravenous secukinumab (58.0%) or switched from placebo to intravenous secukinumab (64.0%). No new safety signals were reported.
Study details: In this phase 3 INVIGORATE-2 trial, 381 patients with active PsA and either plaque psoriasis or nail psoriasis were randomly assigned to receive intravenous secukinumab or placebo with crossover to intravenous secukinumab at week 16.
Disclosures: This study was supported by Novartis. Five authors declared being employees of and owning stocks in Novartis. Several authors declared having ties with various sources, including Novartis.
Source: Kivitz A, Sedova L, Churchill M, et al. Efficacy and safety of intravenous secukinumab for the treatment of active psoriatic arthritis: Results from the randomized, placebo-controlled phase III INVIGORATE-2 study. Arthritis Rheumatol. Published online September 19, 2024. Source
Key clinical point: In patients with active psoriatic arthritis (PsA), intravenous secukinumab every 4 weeks demonstrated rapid and sustained efficacy, along with a safety profile consistent with that of subcutaneous secukinumab.
Major finding: At week 16, intravenous secukinumab vs placebo significantly improved the American College of Rheumatology 50 response rate (31.4% vs 6.3%; adjusted P < .0001). Improvements were observed as early as week 4 (P < .0005) and were sustained through week 52, regardless of whether patients continued intravenous secukinumab (58.0%) or switched from placebo to intravenous secukinumab (64.0%). No new safety signals were reported.
Study details: In this phase 3 INVIGORATE-2 trial, 381 patients with active PsA and either plaque psoriasis or nail psoriasis were randomly assigned to receive intravenous secukinumab or placebo with crossover to intravenous secukinumab at week 16.
Disclosures: This study was supported by Novartis. Five authors declared being employees of and owning stocks in Novartis. Several authors declared having ties with various sources, including Novartis.
Source: Kivitz A, Sedova L, Churchill M, et al. Efficacy and safety of intravenous secukinumab for the treatment of active psoriatic arthritis: Results from the randomized, placebo-controlled phase III INVIGORATE-2 study. Arthritis Rheumatol. Published online September 19, 2024. Source
Guselkumab Shows Persistent Effects in PsA
Key clinical point: In biologic-naive patients with active psoriatic arthritis (PsA), guselkumab administered every 8 weeks (Q8W) led to consistent patient-level improvements in joint disease activity, which persisted for 2 years.
Major finding: Guselkumab maintained high rates of minimal clinically important improvements (MCII) in the clinical Disease Activity Index for PsA (cDAPSA; 94%-99%), with improvements of ≥ 5.7 from baseline through week 52 with a dosing of 100 mg guselkumab Q8W. Among those achieving MCII by week 24, maintenance rates were 69.2% for the cDAPSA and 89.0% for the Psoriatic Arthritis Disease Activity Score at 100 weeks post-achievement.
Study details: This post hoc analysis of the phase 3 DISCOVER-2 trial included 248 biologic-naive patients with active PsA who received 100 mg guselkumab Q8W for 100 weeks.
Disclosures: This study was supported by Janssen Research & Development, LLC. Several authors disclosed holding stock or stock options; receiving grants, payment, honoraria, or consulting fees; or having other ties with various sources, including Janssen.
Source: Mease PJ, Baraliakos X, Chandran V, et al. Persistent patient-level effect of guselkumab at consecutive 8-week dosing visits and over time in patients with active psoriatic arthritis: Post hoc analysis of a 2-year, phase 3, randomized, controlled study. ACR Open Rheumatol. Published online October 4, 2024. Source
Key clinical point: In biologic-naive patients with active psoriatic arthritis (PsA), guselkumab administered every 8 weeks (Q8W) led to consistent patient-level improvements in joint disease activity, which persisted for 2 years.
Major finding: Guselkumab maintained high rates of minimal clinically important improvements (MCII) in the clinical Disease Activity Index for PsA (cDAPSA; 94%-99%), with improvements of ≥ 5.7 from baseline through week 52 with a dosing of 100 mg guselkumab Q8W. Among those achieving MCII by week 24, maintenance rates were 69.2% for the cDAPSA and 89.0% for the Psoriatic Arthritis Disease Activity Score at 100 weeks post-achievement.
Study details: This post hoc analysis of the phase 3 DISCOVER-2 trial included 248 biologic-naive patients with active PsA who received 100 mg guselkumab Q8W for 100 weeks.
Disclosures: This study was supported by Janssen Research & Development, LLC. Several authors disclosed holding stock or stock options; receiving grants, payment, honoraria, or consulting fees; or having other ties with various sources, including Janssen.
Source: Mease PJ, Baraliakos X, Chandran V, et al. Persistent patient-level effect of guselkumab at consecutive 8-week dosing visits and over time in patients with active psoriatic arthritis: Post hoc analysis of a 2-year, phase 3, randomized, controlled study. ACR Open Rheumatol. Published online October 4, 2024. Source
Key clinical point: In biologic-naive patients with active psoriatic arthritis (PsA), guselkumab administered every 8 weeks (Q8W) led to consistent patient-level improvements in joint disease activity, which persisted for 2 years.
Major finding: Guselkumab maintained high rates of minimal clinically important improvements (MCII) in the clinical Disease Activity Index for PsA (cDAPSA; 94%-99%), with improvements of ≥ 5.7 from baseline through week 52 with a dosing of 100 mg guselkumab Q8W. Among those achieving MCII by week 24, maintenance rates were 69.2% for the cDAPSA and 89.0% for the Psoriatic Arthritis Disease Activity Score at 100 weeks post-achievement.
Study details: This post hoc analysis of the phase 3 DISCOVER-2 trial included 248 biologic-naive patients with active PsA who received 100 mg guselkumab Q8W for 100 weeks.
Disclosures: This study was supported by Janssen Research & Development, LLC. Several authors disclosed holding stock or stock options; receiving grants, payment, honoraria, or consulting fees; or having other ties with various sources, including Janssen.
Source: Mease PJ, Baraliakos X, Chandran V, et al. Persistent patient-level effect of guselkumab at consecutive 8-week dosing visits and over time in patients with active psoriatic arthritis: Post hoc analysis of a 2-year, phase 3, randomized, controlled study. ACR Open Rheumatol. Published online October 4, 2024. Source
Secukinumab Promotes Long-Term Disease Control in PsA
Key clinical point: Secukinumab led to clinical improvements across all psoriatic arthritis (PsA) domains, achieving minimal disease activity and demonstrating a favorable 4-year safety profile in biologic-naive patients, those with previous treatment failure, and those with or without comorbidities.
Major finding: During the 48-month follow-up, secukinumab significantly reduced the proportion of patients with active tender joints, swollen joints, enthesitis, and dactylitis (all P < .01). Overall, 50% of patients achieved remission or low disease activity in PsA, with higher rates of minimal disease activity in biologic-naive vs non-naive patients (76.9% vs 66.2%; P < .01) and in those without comorbidities vs those with over three comorbidities (78.8% vs 48.7%; P < .001). Only 5.9% of patients discontinued treatment due to adverse events.
Study details: This 4-year prospective observational study included 685 patients with PsA who received secukinumab; 32.9% were biologic-naive and 74.2% had at least one comorbidity.
Disclosures: This study did not receive financial support from any pharmaceutical company. The authors declared no conflicts of interest.
Source: Ramonda R, Lorenzin M, Chimenti MS, et al. Four-year effectiveness, safety and drug retention rate of secukinumab in psoriatic arthritis: A real-life Italian multicenter cohort. Arthritis Res Ther. 2024;26:172. Source
Key clinical point: Secukinumab led to clinical improvements across all psoriatic arthritis (PsA) domains, achieving minimal disease activity and demonstrating a favorable 4-year safety profile in biologic-naive patients, those with previous treatment failure, and those with or without comorbidities.
Major finding: During the 48-month follow-up, secukinumab significantly reduced the proportion of patients with active tender joints, swollen joints, enthesitis, and dactylitis (all P < .01). Overall, 50% of patients achieved remission or low disease activity in PsA, with higher rates of minimal disease activity in biologic-naive vs non-naive patients (76.9% vs 66.2%; P < .01) and in those without comorbidities vs those with over three comorbidities (78.8% vs 48.7%; P < .001). Only 5.9% of patients discontinued treatment due to adverse events.
Study details: This 4-year prospective observational study included 685 patients with PsA who received secukinumab; 32.9% were biologic-naive and 74.2% had at least one comorbidity.
Disclosures: This study did not receive financial support from any pharmaceutical company. The authors declared no conflicts of interest.
Source: Ramonda R, Lorenzin M, Chimenti MS, et al. Four-year effectiveness, safety and drug retention rate of secukinumab in psoriatic arthritis: A real-life Italian multicenter cohort. Arthritis Res Ther. 2024;26:172. Source
Key clinical point: Secukinumab led to clinical improvements across all psoriatic arthritis (PsA) domains, achieving minimal disease activity and demonstrating a favorable 4-year safety profile in biologic-naive patients, those with previous treatment failure, and those with or without comorbidities.
Major finding: During the 48-month follow-up, secukinumab significantly reduced the proportion of patients with active tender joints, swollen joints, enthesitis, and dactylitis (all P < .01). Overall, 50% of patients achieved remission or low disease activity in PsA, with higher rates of minimal disease activity in biologic-naive vs non-naive patients (76.9% vs 66.2%; P < .01) and in those without comorbidities vs those with over three comorbidities (78.8% vs 48.7%; P < .001). Only 5.9% of patients discontinued treatment due to adverse events.
Study details: This 4-year prospective observational study included 685 patients with PsA who received secukinumab; 32.9% were biologic-naive and 74.2% had at least one comorbidity.
Disclosures: This study did not receive financial support from any pharmaceutical company. The authors declared no conflicts of interest.
Source: Ramonda R, Lorenzin M, Chimenti MS, et al. Four-year effectiveness, safety and drug retention rate of secukinumab in psoriatic arthritis: A real-life Italian multicenter cohort. Arthritis Res Ther. 2024;26:172. Source
Comparative Performance of Adalimumab and Secukinumab in PsA
Key clinical point: Adalimumab and secukinumab showed comparable efficacy in patients with psoriatic arthritis (PsA); however, secukinumab was more effective for skin improvement and adalimumab was superior in alleviating ultrasound-confirmed synovitis.
Major finding: At week 12, there was no significant difference between adalimumab and secukinumab in achieving a ≥ 20% improvement in the American College of Rheumatology response (odds ratio [OR], 0.59; P = .22). Secukinumab showed higher efficacy than adalimumab in achieving a 90% improvement in the Psoriasis Area and Severity Index (OR, 2.25; P = .03). At week 52, adalimumab achieved greater improvements in the ultrasound synovitis count (β, 0.94; P = .03) and synovitis power Doppler signal (β = 0.20; P = .02) than secukinumab.
Study details: This prospective real-world study included 116 patients with PsA (age, ≥ 18 years) who received secukinumab or adalimumab (both n = 58).
Disclosures: This study did not receive any specific funding. No conflicts of interest were reported.
Source: Wang Y, Xiao Y, Zhang L, et al. Superior effect of adalimumab versus secukinumab on ultrasound-confirmed synovitis in psoriatic arthritis: Comprehensive evidence from musculoskeletal ultrasound and clinical assessments. J Dermatolog Treat. 2024;35:2411849. Source
Key clinical point: Adalimumab and secukinumab showed comparable efficacy in patients with psoriatic arthritis (PsA); however, secukinumab was more effective for skin improvement and adalimumab was superior in alleviating ultrasound-confirmed synovitis.
Major finding: At week 12, there was no significant difference between adalimumab and secukinumab in achieving a ≥ 20% improvement in the American College of Rheumatology response (odds ratio [OR], 0.59; P = .22). Secukinumab showed higher efficacy than adalimumab in achieving a 90% improvement in the Psoriasis Area and Severity Index (OR, 2.25; P = .03). At week 52, adalimumab achieved greater improvements in the ultrasound synovitis count (β, 0.94; P = .03) and synovitis power Doppler signal (β = 0.20; P = .02) than secukinumab.
Study details: This prospective real-world study included 116 patients with PsA (age, ≥ 18 years) who received secukinumab or adalimumab (both n = 58).
Disclosures: This study did not receive any specific funding. No conflicts of interest were reported.
Source: Wang Y, Xiao Y, Zhang L, et al. Superior effect of adalimumab versus secukinumab on ultrasound-confirmed synovitis in psoriatic arthritis: Comprehensive evidence from musculoskeletal ultrasound and clinical assessments. J Dermatolog Treat. 2024;35:2411849. Source
Key clinical point: Adalimumab and secukinumab showed comparable efficacy in patients with psoriatic arthritis (PsA); however, secukinumab was more effective for skin improvement and adalimumab was superior in alleviating ultrasound-confirmed synovitis.
Major finding: At week 12, there was no significant difference between adalimumab and secukinumab in achieving a ≥ 20% improvement in the American College of Rheumatology response (odds ratio [OR], 0.59; P = .22). Secukinumab showed higher efficacy than adalimumab in achieving a 90% improvement in the Psoriasis Area and Severity Index (OR, 2.25; P = .03). At week 52, adalimumab achieved greater improvements in the ultrasound synovitis count (β, 0.94; P = .03) and synovitis power Doppler signal (β = 0.20; P = .02) than secukinumab.
Study details: This prospective real-world study included 116 patients with PsA (age, ≥ 18 years) who received secukinumab or adalimumab (both n = 58).
Disclosures: This study did not receive any specific funding. No conflicts of interest were reported.
Source: Wang Y, Xiao Y, Zhang L, et al. Superior effect of adalimumab versus secukinumab on ultrasound-confirmed synovitis in psoriatic arthritis: Comprehensive evidence from musculoskeletal ultrasound and clinical assessments. J Dermatolog Treat. 2024;35:2411849. Source
Weather Has a Limited Effect on PsA Symptoms
Key clinical point: In patients with psoriatic arthritis (PsA), disease activity was significantly lower in winter than in summer; however, the correlation between patient-reported outcomes (PROs) and weather-related factors lacked clinical significance.
Major finding: Disease activity scores, including the Clinical Disease Activity Index (mean, 8.2 vs 8.8; P < .001) and Simplified Disease Activity Index (mean, 8.6 vs 9.5; P < .001) scores, were significantly lower in winter than in summer. However, the association between weather-related factors and various PROs, including pain and fatigue measures, was not clinically significant (Pearson correlation coefficient, < 0.7).
Study details: In this study, 2665 PROs from 858 patients with PsA were analyzed and hourly measurements of temperature, relative humidity, and pressure were matched with disease activity and PROs in winter and summer.
Disclosures: The lead author received funding through the Canadian Association of Psoriasis Patients and the Canadian Institute of Health Research Institute of Musculoskeletal Health and Arthritis for this study. Some authors declared having ties with various sources.
Source: Joly-Chevrier M, Coupal L, Sauvageau LC, Movahedi M, Choquette D. A real-world analysis on weather variation disease activity and patient reported outcomes in psoriatic arthritis. J Rheumatol. Published online September 15, 2024. Source
Key clinical point: In patients with psoriatic arthritis (PsA), disease activity was significantly lower in winter than in summer; however, the correlation between patient-reported outcomes (PROs) and weather-related factors lacked clinical significance.
Major finding: Disease activity scores, including the Clinical Disease Activity Index (mean, 8.2 vs 8.8; P < .001) and Simplified Disease Activity Index (mean, 8.6 vs 9.5; P < .001) scores, were significantly lower in winter than in summer. However, the association between weather-related factors and various PROs, including pain and fatigue measures, was not clinically significant (Pearson correlation coefficient, < 0.7).
Study details: In this study, 2665 PROs from 858 patients with PsA were analyzed and hourly measurements of temperature, relative humidity, and pressure were matched with disease activity and PROs in winter and summer.
Disclosures: The lead author received funding through the Canadian Association of Psoriasis Patients and the Canadian Institute of Health Research Institute of Musculoskeletal Health and Arthritis for this study. Some authors declared having ties with various sources.
Source: Joly-Chevrier M, Coupal L, Sauvageau LC, Movahedi M, Choquette D. A real-world analysis on weather variation disease activity and patient reported outcomes in psoriatic arthritis. J Rheumatol. Published online September 15, 2024. Source
Key clinical point: In patients with psoriatic arthritis (PsA), disease activity was significantly lower in winter than in summer; however, the correlation between patient-reported outcomes (PROs) and weather-related factors lacked clinical significance.
Major finding: Disease activity scores, including the Clinical Disease Activity Index (mean, 8.2 vs 8.8; P < .001) and Simplified Disease Activity Index (mean, 8.6 vs 9.5; P < .001) scores, were significantly lower in winter than in summer. However, the association between weather-related factors and various PROs, including pain and fatigue measures, was not clinically significant (Pearson correlation coefficient, < 0.7).
Study details: In this study, 2665 PROs from 858 patients with PsA were analyzed and hourly measurements of temperature, relative humidity, and pressure were matched with disease activity and PROs in winter and summer.
Disclosures: The lead author received funding through the Canadian Association of Psoriasis Patients and the Canadian Institute of Health Research Institute of Musculoskeletal Health and Arthritis for this study. Some authors declared having ties with various sources.
Source: Joly-Chevrier M, Coupal L, Sauvageau LC, Movahedi M, Choquette D. A real-world analysis on weather variation disease activity and patient reported outcomes in psoriatic arthritis. J Rheumatol. Published online September 15, 2024. Source