User login
Flurbiprofen-Induced Unilateral Eyelid Angioedema
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
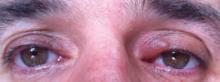
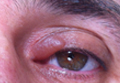
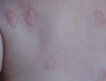
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
Successful Treatment of Schnitzler Syndrome With Canakinumab
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
Practice Question Answers: Medications in Dermatology, Part 2
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate