User login
More data support heart donation after circulatory death
TOPLINE:
There are no significant differences in 1-year mortality, survival to hospital discharge, severe primary graft dysfunction (PGD), and other outcomes post heart transplant between patients who receive a heart obtained by donation after circulatory death (DCD) and patients who receive a heart by donation after brain death (DBD), a new study has shown.
METHODOLOGY:
- The retrospective review included 385 patients (median age, 57.4 years; 26% women; 72.5% White) who underwent a heart transplant at Vanderbilt University Medical Center from January 2020 to January 2023. Of these, 263 received DBD hearts, and 122 received DCD hearts.
- In the DCD group, 17% of hearts were recovered by use of ex vivo machine perfusion (EVP), and 83% by use of normothermic regional perfusion followed by static cold storage; 4% of DBD hearts were recovered by use of EVP, and 96% by use of static cold storage.
- The primary outcome was survival at 1 year after transplantation; key secondary outcomes included survival to hospital discharge, survival at 30 days and 6 months after transplantation, and severe PGD.
TAKEAWAY:
- (hazard ratio, 0.77; 95% confidence interval, 0.32-1.81; P = .54), a finding that was unchanged when adjusted for recipient age.
- There were no significant differences in survival to hospital discharge (93.4% DBD vs. 94.5% DCD; HR, 0.72; 95% CI, 0.26-1.99; P = .53), to 30 days (95.1% DBD vs. 96.7% DCD; HR, 0.67; 95% CI, 0.22-2.05; P = .48), or to 6 months (92.8% DBD vs. 94.3% DCD; HR, 0.68; 95% CI, 0.25-1.85; P = .45) after transplantation.
- The incidence of severe PGD was similar between groups (5.7% DCD vs. 5.7% DBD; HR, 1.00; 95% CI, 0.41-2.4; P = .99).
- There were no significant between-group differences in other outcomes, including incidence of treated rejection and cases of cardiac allograft vasculopathy of grade 1 or greater on the International Society for scale at 1 year.
IN PRACTICE:
“Our findings add to the growing body of evidence in support of DCD heart transplantation,” the authors write, potentially expanding the heart donor pool. They note that outcomes remained similar between groups despite higher-risk patients being overrepresented in the DCD cohort.
In an accompanying editorial, Sean P. Pinney, MD, Center for Cardiovascular Health, Icahn School of Medicine at Mount Sinai, New York, and a colleague called the results “impressive” and “encouraging,” although there are still “important unknowns,” including longer-term outcomes, the financial impact of DCD, and whether results can be replicated in other centers.
“These results provide confidence that DCD can be safely and effectively performed without compromising outcomes, at least in a large-volume center of excellence,” and help provide evidence “to support the spreading acceptance of DCD among heart transplant programs.”
SOURCE:
The study was conducted by Hasan K. Siddiqi, MD, department of medicine, Vanderbilt University Medical Center, Nashville, Tenn., and colleagues. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
The study was conducted at a single center and had a retrospective design and a modest sample size that prevented adjustment for all potentially confounding variables. Meaningful differences among DCD recipients could not be explored with regard to organ recovery technique, and small but statistically meaningful differences in outcomes could not be detected, the authors note. Follow-up was limited to 1 year after transplantation.
DISCLOSURES:
The authors report no relevant conflicts of interest. Dr. Pinney has received consulting fees from Abbott, ADI, Ancora, CareDx, ImpulseDynamics, Medtronic, Nuwellis, Procyrion, Restore Medical, Transmedics, and Valgen Medtech.
A version of this article first appeared on Medscape.com.
TOPLINE:
There are no significant differences in 1-year mortality, survival to hospital discharge, severe primary graft dysfunction (PGD), and other outcomes post heart transplant between patients who receive a heart obtained by donation after circulatory death (DCD) and patients who receive a heart by donation after brain death (DBD), a new study has shown.
METHODOLOGY:
- The retrospective review included 385 patients (median age, 57.4 years; 26% women; 72.5% White) who underwent a heart transplant at Vanderbilt University Medical Center from January 2020 to January 2023. Of these, 263 received DBD hearts, and 122 received DCD hearts.
- In the DCD group, 17% of hearts were recovered by use of ex vivo machine perfusion (EVP), and 83% by use of normothermic regional perfusion followed by static cold storage; 4% of DBD hearts were recovered by use of EVP, and 96% by use of static cold storage.
- The primary outcome was survival at 1 year after transplantation; key secondary outcomes included survival to hospital discharge, survival at 30 days and 6 months after transplantation, and severe PGD.
TAKEAWAY:
- (hazard ratio, 0.77; 95% confidence interval, 0.32-1.81; P = .54), a finding that was unchanged when adjusted for recipient age.
- There were no significant differences in survival to hospital discharge (93.4% DBD vs. 94.5% DCD; HR, 0.72; 95% CI, 0.26-1.99; P = .53), to 30 days (95.1% DBD vs. 96.7% DCD; HR, 0.67; 95% CI, 0.22-2.05; P = .48), or to 6 months (92.8% DBD vs. 94.3% DCD; HR, 0.68; 95% CI, 0.25-1.85; P = .45) after transplantation.
- The incidence of severe PGD was similar between groups (5.7% DCD vs. 5.7% DBD; HR, 1.00; 95% CI, 0.41-2.4; P = .99).
- There were no significant between-group differences in other outcomes, including incidence of treated rejection and cases of cardiac allograft vasculopathy of grade 1 or greater on the International Society for scale at 1 year.
IN PRACTICE:
“Our findings add to the growing body of evidence in support of DCD heart transplantation,” the authors write, potentially expanding the heart donor pool. They note that outcomes remained similar between groups despite higher-risk patients being overrepresented in the DCD cohort.
In an accompanying editorial, Sean P. Pinney, MD, Center for Cardiovascular Health, Icahn School of Medicine at Mount Sinai, New York, and a colleague called the results “impressive” and “encouraging,” although there are still “important unknowns,” including longer-term outcomes, the financial impact of DCD, and whether results can be replicated in other centers.
“These results provide confidence that DCD can be safely and effectively performed without compromising outcomes, at least in a large-volume center of excellence,” and help provide evidence “to support the spreading acceptance of DCD among heart transplant programs.”
SOURCE:
The study was conducted by Hasan K. Siddiqi, MD, department of medicine, Vanderbilt University Medical Center, Nashville, Tenn., and colleagues. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
The study was conducted at a single center and had a retrospective design and a modest sample size that prevented adjustment for all potentially confounding variables. Meaningful differences among DCD recipients could not be explored with regard to organ recovery technique, and small but statistically meaningful differences in outcomes could not be detected, the authors note. Follow-up was limited to 1 year after transplantation.
DISCLOSURES:
The authors report no relevant conflicts of interest. Dr. Pinney has received consulting fees from Abbott, ADI, Ancora, CareDx, ImpulseDynamics, Medtronic, Nuwellis, Procyrion, Restore Medical, Transmedics, and Valgen Medtech.
A version of this article first appeared on Medscape.com.
TOPLINE:
There are no significant differences in 1-year mortality, survival to hospital discharge, severe primary graft dysfunction (PGD), and other outcomes post heart transplant between patients who receive a heart obtained by donation after circulatory death (DCD) and patients who receive a heart by donation after brain death (DBD), a new study has shown.
METHODOLOGY:
- The retrospective review included 385 patients (median age, 57.4 years; 26% women; 72.5% White) who underwent a heart transplant at Vanderbilt University Medical Center from January 2020 to January 2023. Of these, 263 received DBD hearts, and 122 received DCD hearts.
- In the DCD group, 17% of hearts were recovered by use of ex vivo machine perfusion (EVP), and 83% by use of normothermic regional perfusion followed by static cold storage; 4% of DBD hearts were recovered by use of EVP, and 96% by use of static cold storage.
- The primary outcome was survival at 1 year after transplantation; key secondary outcomes included survival to hospital discharge, survival at 30 days and 6 months after transplantation, and severe PGD.
TAKEAWAY:
- (hazard ratio, 0.77; 95% confidence interval, 0.32-1.81; P = .54), a finding that was unchanged when adjusted for recipient age.
- There were no significant differences in survival to hospital discharge (93.4% DBD vs. 94.5% DCD; HR, 0.72; 95% CI, 0.26-1.99; P = .53), to 30 days (95.1% DBD vs. 96.7% DCD; HR, 0.67; 95% CI, 0.22-2.05; P = .48), or to 6 months (92.8% DBD vs. 94.3% DCD; HR, 0.68; 95% CI, 0.25-1.85; P = .45) after transplantation.
- The incidence of severe PGD was similar between groups (5.7% DCD vs. 5.7% DBD; HR, 1.00; 95% CI, 0.41-2.4; P = .99).
- There were no significant between-group differences in other outcomes, including incidence of treated rejection and cases of cardiac allograft vasculopathy of grade 1 or greater on the International Society for scale at 1 year.
IN PRACTICE:
“Our findings add to the growing body of evidence in support of DCD heart transplantation,” the authors write, potentially expanding the heart donor pool. They note that outcomes remained similar between groups despite higher-risk patients being overrepresented in the DCD cohort.
In an accompanying editorial, Sean P. Pinney, MD, Center for Cardiovascular Health, Icahn School of Medicine at Mount Sinai, New York, and a colleague called the results “impressive” and “encouraging,” although there are still “important unknowns,” including longer-term outcomes, the financial impact of DCD, and whether results can be replicated in other centers.
“These results provide confidence that DCD can be safely and effectively performed without compromising outcomes, at least in a large-volume center of excellence,” and help provide evidence “to support the spreading acceptance of DCD among heart transplant programs.”
SOURCE:
The study was conducted by Hasan K. Siddiqi, MD, department of medicine, Vanderbilt University Medical Center, Nashville, Tenn., and colleagues. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
The study was conducted at a single center and had a retrospective design and a modest sample size that prevented adjustment for all potentially confounding variables. Meaningful differences among DCD recipients could not be explored with regard to organ recovery technique, and small but statistically meaningful differences in outcomes could not be detected, the authors note. Follow-up was limited to 1 year after transplantation.
DISCLOSURES:
The authors report no relevant conflicts of interest. Dr. Pinney has received consulting fees from Abbott, ADI, Ancora, CareDx, ImpulseDynamics, Medtronic, Nuwellis, Procyrion, Restore Medical, Transmedics, and Valgen Medtech.
A version of this article first appeared on Medscape.com.
Second pig-heart transplant patient at UM faring well
The organ passed an early test by avoiding hyperacute rejection.
Physicians for the patient, a 58-year-old former lab tech repeatedly turned down for standard allograft transplantation, say they are making good use of lessons from last year’s case of David Bennett, who survived in hospital with difficulty for 2 months after receiving the first such heart at the center in January 2022.
Mr. Bennett’s clinical course had been promising at first but grew turbulent with repeated bouts of infection followed by adjustments to his aggressive immunosuppressant regimen and other complications.
It was also learned weeks after the xenotransplant operation that the heart from the genetically modified donor pig had carried a porcine cytomegalovirus to Mr. Bennett’s body, although there was never evidence that the virus infected other organs or played a major role in his death.
The new xenotransplant recipient, Lawrence Faucette of Frederick, Md., is benefiting from that experience, which was documented in journal reports.
Mr. Faucette had been turned down by UMMC “and several other leading transplant hospitals due to his pre-existing peripheral vascular disease and complications with internal bleeding,” notes a UMMC press release describing his procedure.
The patient “is currently breathing on his own, and his heart is functioning well without any assistance from supportive devices,” says the statement.
Despite a few setbacks, Mr. Faucette is “on the right track,” said Muhammad M. Mohiuddin, MBBS, surgeon and xenotransplantation program director at the University of Maryland, Baltimore, in an interview.
“We’re taking one day at a time. His immune system is still intact, despite the heavy immune suppression,” he told this news organization. His heart didn’t carry a virus and “has not shown any signs of rejection so far.”
The University of Maryland team, Dr. Mohiuddin said, “is very hopeful that we will be able to at least mobilize the patient, and he can be discharged. But it’s a little too early to call.”
Mr. Faucette, as part of his immunosuppressant regimen, is receiving tegoprubart (Eledon Pharmaceuticals), an investigational antibody that blocks CD40 ligand. His predecessor Mr. Bennett, in contrast, had received a blocker of the CD40 receptor (Kiniksa Pharmaceuticals) along with other more familiar immunosuppressants.
The new anti–CD40-ligand blocker, Eledon said, is in phase 1 studies looking at efficacy in patients with conventional kidney transplants.
A version of this article appeared on Medscape.com.
The organ passed an early test by avoiding hyperacute rejection.
Physicians for the patient, a 58-year-old former lab tech repeatedly turned down for standard allograft transplantation, say they are making good use of lessons from last year’s case of David Bennett, who survived in hospital with difficulty for 2 months after receiving the first such heart at the center in January 2022.
Mr. Bennett’s clinical course had been promising at first but grew turbulent with repeated bouts of infection followed by adjustments to his aggressive immunosuppressant regimen and other complications.
It was also learned weeks after the xenotransplant operation that the heart from the genetically modified donor pig had carried a porcine cytomegalovirus to Mr. Bennett’s body, although there was never evidence that the virus infected other organs or played a major role in his death.
The new xenotransplant recipient, Lawrence Faucette of Frederick, Md., is benefiting from that experience, which was documented in journal reports.
Mr. Faucette had been turned down by UMMC “and several other leading transplant hospitals due to his pre-existing peripheral vascular disease and complications with internal bleeding,” notes a UMMC press release describing his procedure.
The patient “is currently breathing on his own, and his heart is functioning well without any assistance from supportive devices,” says the statement.
Despite a few setbacks, Mr. Faucette is “on the right track,” said Muhammad M. Mohiuddin, MBBS, surgeon and xenotransplantation program director at the University of Maryland, Baltimore, in an interview.
“We’re taking one day at a time. His immune system is still intact, despite the heavy immune suppression,” he told this news organization. His heart didn’t carry a virus and “has not shown any signs of rejection so far.”
The University of Maryland team, Dr. Mohiuddin said, “is very hopeful that we will be able to at least mobilize the patient, and he can be discharged. But it’s a little too early to call.”
Mr. Faucette, as part of his immunosuppressant regimen, is receiving tegoprubart (Eledon Pharmaceuticals), an investigational antibody that blocks CD40 ligand. His predecessor Mr. Bennett, in contrast, had received a blocker of the CD40 receptor (Kiniksa Pharmaceuticals) along with other more familiar immunosuppressants.
The new anti–CD40-ligand blocker, Eledon said, is in phase 1 studies looking at efficacy in patients with conventional kidney transplants.
A version of this article appeared on Medscape.com.
The organ passed an early test by avoiding hyperacute rejection.
Physicians for the patient, a 58-year-old former lab tech repeatedly turned down for standard allograft transplantation, say they are making good use of lessons from last year’s case of David Bennett, who survived in hospital with difficulty for 2 months after receiving the first such heart at the center in January 2022.
Mr. Bennett’s clinical course had been promising at first but grew turbulent with repeated bouts of infection followed by adjustments to his aggressive immunosuppressant regimen and other complications.
It was also learned weeks after the xenotransplant operation that the heart from the genetically modified donor pig had carried a porcine cytomegalovirus to Mr. Bennett’s body, although there was never evidence that the virus infected other organs or played a major role in his death.
The new xenotransplant recipient, Lawrence Faucette of Frederick, Md., is benefiting from that experience, which was documented in journal reports.
Mr. Faucette had been turned down by UMMC “and several other leading transplant hospitals due to his pre-existing peripheral vascular disease and complications with internal bleeding,” notes a UMMC press release describing his procedure.
The patient “is currently breathing on his own, and his heart is functioning well without any assistance from supportive devices,” says the statement.
Despite a few setbacks, Mr. Faucette is “on the right track,” said Muhammad M. Mohiuddin, MBBS, surgeon and xenotransplantation program director at the University of Maryland, Baltimore, in an interview.
“We’re taking one day at a time. His immune system is still intact, despite the heavy immune suppression,” he told this news organization. His heart didn’t carry a virus and “has not shown any signs of rejection so far.”
The University of Maryland team, Dr. Mohiuddin said, “is very hopeful that we will be able to at least mobilize the patient, and he can be discharged. But it’s a little too early to call.”
Mr. Faucette, as part of his immunosuppressant regimen, is receiving tegoprubart (Eledon Pharmaceuticals), an investigational antibody that blocks CD40 ligand. His predecessor Mr. Bennett, in contrast, had received a blocker of the CD40 receptor (Kiniksa Pharmaceuticals) along with other more familiar immunosuppressants.
The new anti–CD40-ligand blocker, Eledon said, is in phase 1 studies looking at efficacy in patients with conventional kidney transplants.
A version of this article appeared on Medscape.com.
STEMI trial fails to support post-PCI anticoagulation
The first randomized trial to evaluate postprocedural anticoagulation (PPA) in patients undergoing a primary percutaneous coronary intervention (PCI) for an ST-segment elevation myocardial infarction (STEMI) did not associate significant benefit – or significant harm – with any of the three tested regimens relative to placebo.
There has been a signal from nonrandomized studies that PPA reduces the risk for ischemic events, but no controlled prospective trials have evaluated the risk-benefit relationship in STEMI patients, said Yan Yan, MD, a researcher in Beijing Anzhen Hospital.
The results of the randomized trial, called RIGHT, were presented at the annual congress of the European Society of Cardiology by Dr. Yan, on behalf of a team of coinvestigators led by Nie Shaoping, MD, PhD, a cardiologist affiliated with Capital Medical University, Beijing.
The bottom line is that Dr. Yan concluded.
Objective study
In her presentation, Dr. Yan explained that an objective study has been needed to validate the common use of empirically administered PPA. According to Dr. Yan, PPA is being offered to up to 40% of STEMI patients in Europe, with even higher rates in China.
In the investigator-initiated RIGHT trial, 2,856 STEMI patients undergoing PCI were randomized to PPA or placebo in a 1:1 ratio. In the PPA arm, patients received one of three low-dose anticoagulation regimens over 48 hours or until discharge if this was longer: 0.2 mg/kg per hour of bivalirudin administered intravenously; 40 mg of enoxaparin administered subcutaneously; or 10 U/kg per hour of unfractionated heparin (UFH) to maintain an activated coagulation time between 150 and 200 seconds.
Each of the 53 participating Chinese centers selected one of the anticoagulation regimens. Matching placebos were employed in the double-blind design. All received bivalirudin anticoagulation during PCI. Exclusion criteria included unstable disease, such as cardiogenic shock, prior coronary artery bypass grafting, or an indication for anticoagulation other than PPA.
For the composite primary endpoint of all-cause death, nonfatal MI, nonfatal stroke, stent thrombosis, or urgent revascularization at 30 days, there was no difference between PPA and placebo. The event rate in both arms was 2.5%.
There were also no significant differences between PPA and placebo for any of the secondary ischemic endpoints, which included the individual components of the primary endpoint and cardiovascular death.
For the primary safety endpoint of Bleeding Academic Research Consortium (BARC) grade 3-5 bleeding, the slight increase in events among those in the placebo group did not approach statistical significance (P = .551). On other definitions of bleeding, which were secondary endpoints, PPA and placebo also did not differ significantly.
Compared for safety, the three anticoagulation regimens performed similarly with no significant interaction for the primary endpoint (P = .679).
For efficacy, the differences did range sufficiently to produce a significant interaction (P = .01) with enoxaparin appearing to be more effective, UFH less effective, and bivalirudin falling in between. This led Dr. Yan to speculate that the three anticoagulants “may not be equivalent,” although she said larger trials are needed to explore potential differences.
Design flawed?
The ESC-invited discussant, Pascal Vranckx, MD, PhD, medical director, cardiac critical care services, Hartcentrum Hasselt, Belgium, liked the question being asked in the study, but concluded that the design was flawed.
“There are a variety of anticoagulants employed in a variety of doses [for PPA] but we have very limited data. The research question is totally appropriate,” he said. However, he asked, “What went wrong? Was it the drugs, the trial, or both?”
The problem, he thinks, is the dose. Much of the design of RIGHT was based on the 2015 MATRIX trial, which did show a benefit from a single dose of bivalirudin following PCI relative to two other comparators. In that study, STEMI patients randomized to bivalirudin received a bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg per hour for at least 4 hours. The comparators were UFH or a control arm of low-molecular-weight heparin with optional glycoprotein IIb/IIIa inhibitors.
At 30 days, bivalirudin was associated with a 40% reduction (hazard ratio, 0.60; P = .001) relative to control for the composite primary endpoint of death or bleeding. Dr. Vranckx pointed out that MATRIX was a trial of a single-dose prolongation of PPA, whereas RIGHT was “a prolongation of a prolongation,” but he believes MATRIX data support higher doses of anticoagulation, particularly of bivalirudin.
“Perhaps low dose bivalirudin is not the way to go,” he speculated.
He further advised the authors to reevaluate the expected benefit from PPA following STEMI. In MATRIX, the risk for events was highly concentrated in the immediate period after PCI, suggesting that the opportunity to reduce risk is much lower as anticoagulation is prolonged. He suggested that the low number of events in RIGHT are consistent with the diminishing risk for events over time.
Nevertheless, Dr. Vranckx praised the authors for addressing a research question that is “timely and highly relevant.” He called the data “important” by drawing attention to a potential target for risk reduction, and encouraged additional trials to determine what PPA strategy, if any, can further reduce early ischemic events after PCI.
Dr. Yan and colleagues report financial relationships with Abbott, Boston Scientific, East China Pharmaceuticals, Saniju Medical and Pharmaceuticals, and Jiangsu Hengrui Pharmaceuticals, which provided funding for this study. Dr. Vranckx reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
The first randomized trial to evaluate postprocedural anticoagulation (PPA) in patients undergoing a primary percutaneous coronary intervention (PCI) for an ST-segment elevation myocardial infarction (STEMI) did not associate significant benefit – or significant harm – with any of the three tested regimens relative to placebo.
There has been a signal from nonrandomized studies that PPA reduces the risk for ischemic events, but no controlled prospective trials have evaluated the risk-benefit relationship in STEMI patients, said Yan Yan, MD, a researcher in Beijing Anzhen Hospital.
The results of the randomized trial, called RIGHT, were presented at the annual congress of the European Society of Cardiology by Dr. Yan, on behalf of a team of coinvestigators led by Nie Shaoping, MD, PhD, a cardiologist affiliated with Capital Medical University, Beijing.
The bottom line is that Dr. Yan concluded.
Objective study
In her presentation, Dr. Yan explained that an objective study has been needed to validate the common use of empirically administered PPA. According to Dr. Yan, PPA is being offered to up to 40% of STEMI patients in Europe, with even higher rates in China.
In the investigator-initiated RIGHT trial, 2,856 STEMI patients undergoing PCI were randomized to PPA or placebo in a 1:1 ratio. In the PPA arm, patients received one of three low-dose anticoagulation regimens over 48 hours or until discharge if this was longer: 0.2 mg/kg per hour of bivalirudin administered intravenously; 40 mg of enoxaparin administered subcutaneously; or 10 U/kg per hour of unfractionated heparin (UFH) to maintain an activated coagulation time between 150 and 200 seconds.
Each of the 53 participating Chinese centers selected one of the anticoagulation regimens. Matching placebos were employed in the double-blind design. All received bivalirudin anticoagulation during PCI. Exclusion criteria included unstable disease, such as cardiogenic shock, prior coronary artery bypass grafting, or an indication for anticoagulation other than PPA.
For the composite primary endpoint of all-cause death, nonfatal MI, nonfatal stroke, stent thrombosis, or urgent revascularization at 30 days, there was no difference between PPA and placebo. The event rate in both arms was 2.5%.
There were also no significant differences between PPA and placebo for any of the secondary ischemic endpoints, which included the individual components of the primary endpoint and cardiovascular death.
For the primary safety endpoint of Bleeding Academic Research Consortium (BARC) grade 3-5 bleeding, the slight increase in events among those in the placebo group did not approach statistical significance (P = .551). On other definitions of bleeding, which were secondary endpoints, PPA and placebo also did not differ significantly.
Compared for safety, the three anticoagulation regimens performed similarly with no significant interaction for the primary endpoint (P = .679).
For efficacy, the differences did range sufficiently to produce a significant interaction (P = .01) with enoxaparin appearing to be more effective, UFH less effective, and bivalirudin falling in between. This led Dr. Yan to speculate that the three anticoagulants “may not be equivalent,” although she said larger trials are needed to explore potential differences.
Design flawed?
The ESC-invited discussant, Pascal Vranckx, MD, PhD, medical director, cardiac critical care services, Hartcentrum Hasselt, Belgium, liked the question being asked in the study, but concluded that the design was flawed.
“There are a variety of anticoagulants employed in a variety of doses [for PPA] but we have very limited data. The research question is totally appropriate,” he said. However, he asked, “What went wrong? Was it the drugs, the trial, or both?”
The problem, he thinks, is the dose. Much of the design of RIGHT was based on the 2015 MATRIX trial, which did show a benefit from a single dose of bivalirudin following PCI relative to two other comparators. In that study, STEMI patients randomized to bivalirudin received a bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg per hour for at least 4 hours. The comparators were UFH or a control arm of low-molecular-weight heparin with optional glycoprotein IIb/IIIa inhibitors.
At 30 days, bivalirudin was associated with a 40% reduction (hazard ratio, 0.60; P = .001) relative to control for the composite primary endpoint of death or bleeding. Dr. Vranckx pointed out that MATRIX was a trial of a single-dose prolongation of PPA, whereas RIGHT was “a prolongation of a prolongation,” but he believes MATRIX data support higher doses of anticoagulation, particularly of bivalirudin.
“Perhaps low dose bivalirudin is not the way to go,” he speculated.
He further advised the authors to reevaluate the expected benefit from PPA following STEMI. In MATRIX, the risk for events was highly concentrated in the immediate period after PCI, suggesting that the opportunity to reduce risk is much lower as anticoagulation is prolonged. He suggested that the low number of events in RIGHT are consistent with the diminishing risk for events over time.
Nevertheless, Dr. Vranckx praised the authors for addressing a research question that is “timely and highly relevant.” He called the data “important” by drawing attention to a potential target for risk reduction, and encouraged additional trials to determine what PPA strategy, if any, can further reduce early ischemic events after PCI.
Dr. Yan and colleagues report financial relationships with Abbott, Boston Scientific, East China Pharmaceuticals, Saniju Medical and Pharmaceuticals, and Jiangsu Hengrui Pharmaceuticals, which provided funding for this study. Dr. Vranckx reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
The first randomized trial to evaluate postprocedural anticoagulation (PPA) in patients undergoing a primary percutaneous coronary intervention (PCI) for an ST-segment elevation myocardial infarction (STEMI) did not associate significant benefit – or significant harm – with any of the three tested regimens relative to placebo.
There has been a signal from nonrandomized studies that PPA reduces the risk for ischemic events, but no controlled prospective trials have evaluated the risk-benefit relationship in STEMI patients, said Yan Yan, MD, a researcher in Beijing Anzhen Hospital.
The results of the randomized trial, called RIGHT, were presented at the annual congress of the European Society of Cardiology by Dr. Yan, on behalf of a team of coinvestigators led by Nie Shaoping, MD, PhD, a cardiologist affiliated with Capital Medical University, Beijing.
The bottom line is that Dr. Yan concluded.
Objective study
In her presentation, Dr. Yan explained that an objective study has been needed to validate the common use of empirically administered PPA. According to Dr. Yan, PPA is being offered to up to 40% of STEMI patients in Europe, with even higher rates in China.
In the investigator-initiated RIGHT trial, 2,856 STEMI patients undergoing PCI were randomized to PPA or placebo in a 1:1 ratio. In the PPA arm, patients received one of three low-dose anticoagulation regimens over 48 hours or until discharge if this was longer: 0.2 mg/kg per hour of bivalirudin administered intravenously; 40 mg of enoxaparin administered subcutaneously; or 10 U/kg per hour of unfractionated heparin (UFH) to maintain an activated coagulation time between 150 and 200 seconds.
Each of the 53 participating Chinese centers selected one of the anticoagulation regimens. Matching placebos were employed in the double-blind design. All received bivalirudin anticoagulation during PCI. Exclusion criteria included unstable disease, such as cardiogenic shock, prior coronary artery bypass grafting, or an indication for anticoagulation other than PPA.
For the composite primary endpoint of all-cause death, nonfatal MI, nonfatal stroke, stent thrombosis, or urgent revascularization at 30 days, there was no difference between PPA and placebo. The event rate in both arms was 2.5%.
There were also no significant differences between PPA and placebo for any of the secondary ischemic endpoints, which included the individual components of the primary endpoint and cardiovascular death.
For the primary safety endpoint of Bleeding Academic Research Consortium (BARC) grade 3-5 bleeding, the slight increase in events among those in the placebo group did not approach statistical significance (P = .551). On other definitions of bleeding, which were secondary endpoints, PPA and placebo also did not differ significantly.
Compared for safety, the three anticoagulation regimens performed similarly with no significant interaction for the primary endpoint (P = .679).
For efficacy, the differences did range sufficiently to produce a significant interaction (P = .01) with enoxaparin appearing to be more effective, UFH less effective, and bivalirudin falling in between. This led Dr. Yan to speculate that the three anticoagulants “may not be equivalent,” although she said larger trials are needed to explore potential differences.
Design flawed?
The ESC-invited discussant, Pascal Vranckx, MD, PhD, medical director, cardiac critical care services, Hartcentrum Hasselt, Belgium, liked the question being asked in the study, but concluded that the design was flawed.
“There are a variety of anticoagulants employed in a variety of doses [for PPA] but we have very limited data. The research question is totally appropriate,” he said. However, he asked, “What went wrong? Was it the drugs, the trial, or both?”
The problem, he thinks, is the dose. Much of the design of RIGHT was based on the 2015 MATRIX trial, which did show a benefit from a single dose of bivalirudin following PCI relative to two other comparators. In that study, STEMI patients randomized to bivalirudin received a bolus of 0.75 mg/kg followed by an infusion of 1.75 mg/kg per hour for at least 4 hours. The comparators were UFH or a control arm of low-molecular-weight heparin with optional glycoprotein IIb/IIIa inhibitors.
At 30 days, bivalirudin was associated with a 40% reduction (hazard ratio, 0.60; P = .001) relative to control for the composite primary endpoint of death or bleeding. Dr. Vranckx pointed out that MATRIX was a trial of a single-dose prolongation of PPA, whereas RIGHT was “a prolongation of a prolongation,” but he believes MATRIX data support higher doses of anticoagulation, particularly of bivalirudin.
“Perhaps low dose bivalirudin is not the way to go,” he speculated.
He further advised the authors to reevaluate the expected benefit from PPA following STEMI. In MATRIX, the risk for events was highly concentrated in the immediate period after PCI, suggesting that the opportunity to reduce risk is much lower as anticoagulation is prolonged. He suggested that the low number of events in RIGHT are consistent with the diminishing risk for events over time.
Nevertheless, Dr. Vranckx praised the authors for addressing a research question that is “timely and highly relevant.” He called the data “important” by drawing attention to a potential target for risk reduction, and encouraged additional trials to determine what PPA strategy, if any, can further reduce early ischemic events after PCI.
Dr. Yan and colleagues report financial relationships with Abbott, Boston Scientific, East China Pharmaceuticals, Saniju Medical and Pharmaceuticals, and Jiangsu Hengrui Pharmaceuticals, which provided funding for this study. Dr. Vranckx reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Should intravascular imaging be almost routine in PCI?
A routine role for intravascular imaging (IVI) guidance for percutaneous coronary intervention (PCI) has long been favored by many of the technology’s researchers and enthusiasts. Now evidence from large, randomized trials may be catching up with such aspirations, though not without caveats.
One way IVI guidance may achieve that, the research suggests, albeit more speculatively, is by cutting risk for stent thrombosis, compared with the risk associated with angiography-only PCI.
The new studies, two large randomized IVI trials plus a meta-analysis of 20 such studies, were presented at the annual congress of the European Society of Cardiology.
In one, called ILUMIEN-4, PCI guided by optical coherence tomography (OCT) was associated with fewer procedural complications and better acute results – that is, larger post-PCI minimum stent area (MSA) – than in angiography-only procedures (P < .001). Poststenting MSA, an established predictor of clinical outcomes, was the primary imaging endpoint of the trial with almost 2,500 patients.
Yet the OCT group’s greater post-PCI MSA did not translate to reduced risk for the primary clinical endpoint of 2-year target-vessel failure. Among secondary endpoints, however, stent thrombosis at some point during the follow-up was 64% less likely (P = .02) with OCT guidance than angiography-only PCI.
ILUMIEN-4, despite its neutral clinical result, still “strongly advocates” for PCI guidance by OCT, at least among patients like those in the trial, said principal investigator Ziad Ali, MD, DPhil. He based that largely on the strategy’s greater postprocedure lumen areas in the trials, which are among “the strongest independent predictors for long term outcomes,” said Dr. Ali, of St. Francis Hospital & Heart Center, Roslyn, N.Y., at a press conference on IVI trials during the ESC Congress.
Selected complex lesion type
In contrast, the OCTOBER trial, presented at the sessions back to back with ILUMIEN-4, saw OCT guidance lead to better clinical outcomes than angiography alone after PCI of bifurcation lesions, which normally can be a special challenge for operators.
In the trial, which entered about 1,200 patients with such complex lesions, the 2-year risk for major adverse cardiac events (MACE) fell 60% after OCT-guided PCI, compared with angiography-only procedures (P = .035).
The finding is novel for showing that OCT guidance in bifurcation PCI can make a significant clinical difference, said OCTOBER investigator Niels R. Holm, MD, at the same media presentation on IVI trials.
“Multiple studies have shown that OCT allows for optimization of bifurcation PCI, and our results confirm that such optimization may improve the patient’s prognosis,” said Dr. Holm of Aarhus (Denmark) University Hospital.
ILUMIEN-4 and OCTOBER, both of which prespecified the Xience (Abbott) everolimus-eluting stent for the procedures, were published in the New England Journal of Medicine in tandem with their respective presentations at the ESC sessions.
Covering the spectrum
A meta-analysis presented at the same ESC session compared IVI using either OCT or intravascular ultrasound (IVUS) with angiography-only PCI across 20 randomized trials with a total of more than 12,000 patients.
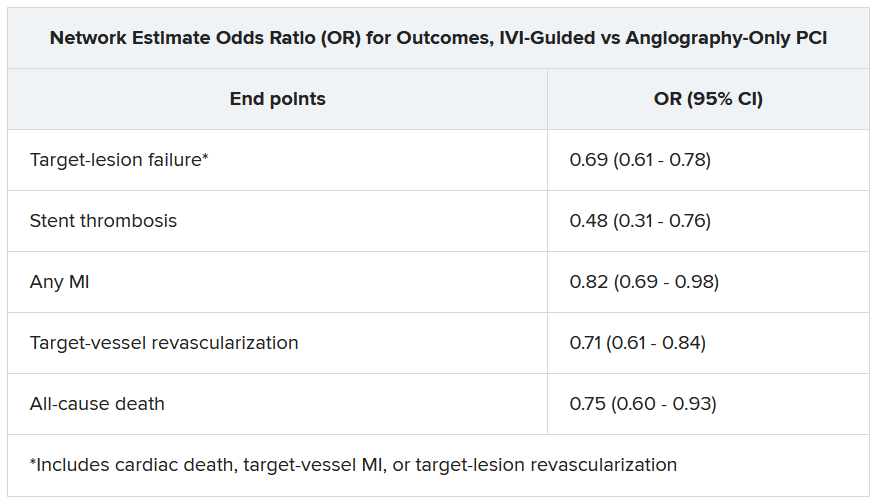
Significant outcomes for IVI guidance versus angiography alone included a 31% drop in risk for target-lesion failure, the primary endpoint. And this study, as well, showed a steep 52% reduction in risk for in-stent thrombosis with the IVI-guided approach.
And “for the first time” in IVI studies, “we demonstrated reductions in all-myocardial-infarction and all-cause death, the latter by 25%,” Gregg Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in presenting the meta-analysis. Dr. Stone is also the ILUMIEN-4 study chairperson.
“The routine use of OCT or IVUS to guide most PCI procedures will substantially improve patient event-free survival,” he predicted, “enhancing both the long-term safety and effectiveness of the procedure.”
Dr. Stone said that IVI guidance “should be standard of care, if not in all patients, then in most patients.” Part of the rationale: PCI is unlikely to be improved much further by incremental gains in drug-eluting stent design. “That technology has almost plateaued.” But there’s yet room for “substantially improved outcomes” from adjunctive treatments and techniques such as IVI guidance.
The 20 studies in the meta-analysis encompassed an array of patients and lesions both complex and noncomplex, Dr. Stone observed, including bifurcation lesions, chronic total occlusions, left-main coronary stenoses, and MI culprit lesions.
“They really covered the spectrum of PCI,” he said. “I’m not recommending that intravascular imaging be used in every single case. But I do think it should be used in the majority of patients” and be standard of care for PCI in left-main lesions and “complex coronary disease, high-risk patients, and high-risk lesions.”
Unique advantage
The IVI-guidance groups in both ILUMIEN-4 and the meta-analysis showed a significant drop in risk for stent thrombosis – that is, abrupt thrombotic vessel closure, which typically occurs in 1% or fewer PCI cases but can trigger an MI and pose a mortality risk up to 45%.
Those risk reductions are consistent with a unique IVI advantage: the ability to guide optimization of stent deployments. When formally presenting ILUMIEN-4 at the ESC sessions, Ali observed that IVUS and OCT imaging allows operators to identify and often correct less-than-ideal results of an initial stent delivery – such as residual gaps between stent struts and vessel wall – that may encroach on the lumen, with possible clinical consequences.
Such imaging, said Dr. Ali, “lets you identify tissue protrusions, malappositions, dissections, and untreated reference-segment disease” that may potentially trigger thrombosis. That makes a strong argument for giving IVI guidance a more common, perhaps even routine role in PCI procedures.
Selling routine IVI-guided PCI in practice
“I think the study results are quite clear,” said Deepak L. Bhatt, MD, MPH, as session comoderator following the OCTOBER presentation. “The challenge, though, will be convincing the average interventional cardiologist worldwide that it was specifically the imaging and not the extra care that the patient getting OCT also inherently receives.”
Did OCT’s better trial outcomes stem from IVI itself or from greater operator attentiveness to procedural results – such as, for example, more high-pressure expansions to optimize stent placement, “the sort of thing that tends to occur when invasive imaging is added on to just plain old angiography?” Dr. Bhatt asked of Lene N. Andreasen, MD, who had just presented the OCTOBER trial. “There’s no way of uncoupling the two things.”
What can be said, “at this point, to convince interventional cardiologists that the extra time, energy, expense, is truly indicated,” that the data are “sufficient to change global practice?” asked Dr. Bhatt, Mount Sinai Hospital and Icahn School of Medicine at Mount Sinai.
That remains an open question,” acknowledged Dr. Andreasen of Aarhus University Hospital. The best argument in favor of selective IVI-guided PCI is that “we actually see a clinical benefit” in the trials. “But of course, it comes with a cost. It comes with longer procedures and more contrast.” How clinical practice responds to the new data remains to be seen, she proposed.
ILUMIEN-4 and OCTOBER in detail
Conducted at 80 centers in 18 countries, ILUMIEN-4 randomly assigned patients with diabetes or complex coronary lesions to undergo PCI guided by OCT or using standard angiography only, 1,233 and 1,254 patients, respectively.
Post-PCI MSA averaged 5.72 mm2 with OCT guidance and 5.36 mm2 in the angiography-only group (P < .001).
Their rates of target-vessel failure at 2 years were not significantly different at 7.4% and 8.2%, respectively. The 2-year composite endpoint included cardiac death, target vessel–related MI, or ischemia-driven target-vessel revascularization.
Definite or probable stent thrombosis was observed over 2 years in 0.5% of the OCT group and 1.4% of those with angiography-only PCI (hazard ratio, 0.36; 95% confidence interval, 0.14-0.91; P = .02) favoring OCT.
The OCTOBER trial, conducted at 38 centers in Europe, entered 1201 patients with stable angina or acute coronary syndromes and angiographically identified complex bifurcation lesions. They involved the left-main coronary artery in about one-fifth of cases.
Patients were randomly assigned to bifurcation PCI guided by OCT or under standard angiography, 600 and 601 patients, respectively. Rates for procedure-related complications were similar at 6.8% and 5.7%, respectively.
Over a median of 2 years, 10.1% of the OCT group and 14.1% of angiography-only patients developed a MACE event, including cardiac death, target-lesion MI, or ischemia-driven target-lesion revascularization. The adjusted HR was 0.71 (95% CI, 0.51-0.98; P = .035) in favor of OCT.
Meta-analysis, trials to date
The meta-analysis presented by Dr. Stone included ILUMIEN-4, OCTOBER, and 18 earlier outcomes trials comparing PCI guided by IVI, either OCT or IVUS, and angiography-only PCI. It covered 12,428 patients with chronic or acute coronary disease and followed them a mean of 26 months; the longest follow-up was 5 years. They were assigned to IVI-guided or angiography-only PCI, 7,038 and 5,390 patients, respectively.
Dr. Stone and colleagues conducted a network meta-analysis of the 20 studies, that is, a combined analysis that allowed both direct and indirect comparisons of standard angiography-only procedures to each of the other studied comparator interventions including OCT, IVUS, and either OCT or IVUS. They then derived network-estimate odds ratios for IVI-guided PCI vs angiography-only procedures.
“Hopefully, this will impact the guidelines,” Dr. Stone said of the meta-analysis. Procedures guided by IVI might become more common in clinical practice if they were to garner a Class-I guideline recommendation, the strongest recommendation category.
“That would make a difference, but we’d also need to work to remove impediments to increasing intravascular imaging guidance” for most patients undergoing PCI, he said, referring to challenges in obtaining reimbursement for IVI-guided PCI and training enough operators to handle the projected demand.
ILUMIEN-4 was funded by Abbott. OCTOBER was supported by grants from Abbott Vascular, St. Jude Medical, and Aarhus University. The network meta-analysis received statistical support from Abbott. Dr. Ali disclosed institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems, Medtronic, the National Institutes of Health, Opsens Medical, Philips, and Teleflex; consulting fees from Astra Zeneca, Philips, Shockwave; and holding equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr. Holm and Dr. Bhatt reported numerous conflicts of interest. Dr. Andreasen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A routine role for intravascular imaging (IVI) guidance for percutaneous coronary intervention (PCI) has long been favored by many of the technology’s researchers and enthusiasts. Now evidence from large, randomized trials may be catching up with such aspirations, though not without caveats.
One way IVI guidance may achieve that, the research suggests, albeit more speculatively, is by cutting risk for stent thrombosis, compared with the risk associated with angiography-only PCI.
The new studies, two large randomized IVI trials plus a meta-analysis of 20 such studies, were presented at the annual congress of the European Society of Cardiology.
In one, called ILUMIEN-4, PCI guided by optical coherence tomography (OCT) was associated with fewer procedural complications and better acute results – that is, larger post-PCI minimum stent area (MSA) – than in angiography-only procedures (P < .001). Poststenting MSA, an established predictor of clinical outcomes, was the primary imaging endpoint of the trial with almost 2,500 patients.
Yet the OCT group’s greater post-PCI MSA did not translate to reduced risk for the primary clinical endpoint of 2-year target-vessel failure. Among secondary endpoints, however, stent thrombosis at some point during the follow-up was 64% less likely (P = .02) with OCT guidance than angiography-only PCI.
ILUMIEN-4, despite its neutral clinical result, still “strongly advocates” for PCI guidance by OCT, at least among patients like those in the trial, said principal investigator Ziad Ali, MD, DPhil. He based that largely on the strategy’s greater postprocedure lumen areas in the trials, which are among “the strongest independent predictors for long term outcomes,” said Dr. Ali, of St. Francis Hospital & Heart Center, Roslyn, N.Y., at a press conference on IVI trials during the ESC Congress.
Selected complex lesion type
In contrast, the OCTOBER trial, presented at the sessions back to back with ILUMIEN-4, saw OCT guidance lead to better clinical outcomes than angiography alone after PCI of bifurcation lesions, which normally can be a special challenge for operators.
In the trial, which entered about 1,200 patients with such complex lesions, the 2-year risk for major adverse cardiac events (MACE) fell 60% after OCT-guided PCI, compared with angiography-only procedures (P = .035).
The finding is novel for showing that OCT guidance in bifurcation PCI can make a significant clinical difference, said OCTOBER investigator Niels R. Holm, MD, at the same media presentation on IVI trials.
“Multiple studies have shown that OCT allows for optimization of bifurcation PCI, and our results confirm that such optimization may improve the patient’s prognosis,” said Dr. Holm of Aarhus (Denmark) University Hospital.
ILUMIEN-4 and OCTOBER, both of which prespecified the Xience (Abbott) everolimus-eluting stent for the procedures, were published in the New England Journal of Medicine in tandem with their respective presentations at the ESC sessions.
Covering the spectrum
A meta-analysis presented at the same ESC session compared IVI using either OCT or intravascular ultrasound (IVUS) with angiography-only PCI across 20 randomized trials with a total of more than 12,000 patients.
Significant outcomes for IVI guidance versus angiography alone included a 31% drop in risk for target-lesion failure, the primary endpoint. And this study, as well, showed a steep 52% reduction in risk for in-stent thrombosis with the IVI-guided approach.
And “for the first time” in IVI studies, “we demonstrated reductions in all-myocardial-infarction and all-cause death, the latter by 25%,” Gregg Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in presenting the meta-analysis. Dr. Stone is also the ILUMIEN-4 study chairperson.
“The routine use of OCT or IVUS to guide most PCI procedures will substantially improve patient event-free survival,” he predicted, “enhancing both the long-term safety and effectiveness of the procedure.”
Dr. Stone said that IVI guidance “should be standard of care, if not in all patients, then in most patients.” Part of the rationale: PCI is unlikely to be improved much further by incremental gains in drug-eluting stent design. “That technology has almost plateaued.” But there’s yet room for “substantially improved outcomes” from adjunctive treatments and techniques such as IVI guidance.
The 20 studies in the meta-analysis encompassed an array of patients and lesions both complex and noncomplex, Dr. Stone observed, including bifurcation lesions, chronic total occlusions, left-main coronary stenoses, and MI culprit lesions.
“They really covered the spectrum of PCI,” he said. “I’m not recommending that intravascular imaging be used in every single case. But I do think it should be used in the majority of patients” and be standard of care for PCI in left-main lesions and “complex coronary disease, high-risk patients, and high-risk lesions.”
Unique advantage
The IVI-guidance groups in both ILUMIEN-4 and the meta-analysis showed a significant drop in risk for stent thrombosis – that is, abrupt thrombotic vessel closure, which typically occurs in 1% or fewer PCI cases but can trigger an MI and pose a mortality risk up to 45%.
Those risk reductions are consistent with a unique IVI advantage: the ability to guide optimization of stent deployments. When formally presenting ILUMIEN-4 at the ESC sessions, Ali observed that IVUS and OCT imaging allows operators to identify and often correct less-than-ideal results of an initial stent delivery – such as residual gaps between stent struts and vessel wall – that may encroach on the lumen, with possible clinical consequences.
Such imaging, said Dr. Ali, “lets you identify tissue protrusions, malappositions, dissections, and untreated reference-segment disease” that may potentially trigger thrombosis. That makes a strong argument for giving IVI guidance a more common, perhaps even routine role in PCI procedures.
Selling routine IVI-guided PCI in practice
“I think the study results are quite clear,” said Deepak L. Bhatt, MD, MPH, as session comoderator following the OCTOBER presentation. “The challenge, though, will be convincing the average interventional cardiologist worldwide that it was specifically the imaging and not the extra care that the patient getting OCT also inherently receives.”
Did OCT’s better trial outcomes stem from IVI itself or from greater operator attentiveness to procedural results – such as, for example, more high-pressure expansions to optimize stent placement, “the sort of thing that tends to occur when invasive imaging is added on to just plain old angiography?” Dr. Bhatt asked of Lene N. Andreasen, MD, who had just presented the OCTOBER trial. “There’s no way of uncoupling the two things.”
What can be said, “at this point, to convince interventional cardiologists that the extra time, energy, expense, is truly indicated,” that the data are “sufficient to change global practice?” asked Dr. Bhatt, Mount Sinai Hospital and Icahn School of Medicine at Mount Sinai.
That remains an open question,” acknowledged Dr. Andreasen of Aarhus University Hospital. The best argument in favor of selective IVI-guided PCI is that “we actually see a clinical benefit” in the trials. “But of course, it comes with a cost. It comes with longer procedures and more contrast.” How clinical practice responds to the new data remains to be seen, she proposed.
ILUMIEN-4 and OCTOBER in detail
Conducted at 80 centers in 18 countries, ILUMIEN-4 randomly assigned patients with diabetes or complex coronary lesions to undergo PCI guided by OCT or using standard angiography only, 1,233 and 1,254 patients, respectively.
Post-PCI MSA averaged 5.72 mm2 with OCT guidance and 5.36 mm2 in the angiography-only group (P < .001).
Their rates of target-vessel failure at 2 years were not significantly different at 7.4% and 8.2%, respectively. The 2-year composite endpoint included cardiac death, target vessel–related MI, or ischemia-driven target-vessel revascularization.
Definite or probable stent thrombosis was observed over 2 years in 0.5% of the OCT group and 1.4% of those with angiography-only PCI (hazard ratio, 0.36; 95% confidence interval, 0.14-0.91; P = .02) favoring OCT.
The OCTOBER trial, conducted at 38 centers in Europe, entered 1201 patients with stable angina or acute coronary syndromes and angiographically identified complex bifurcation lesions. They involved the left-main coronary artery in about one-fifth of cases.
Patients were randomly assigned to bifurcation PCI guided by OCT or under standard angiography, 600 and 601 patients, respectively. Rates for procedure-related complications were similar at 6.8% and 5.7%, respectively.
Over a median of 2 years, 10.1% of the OCT group and 14.1% of angiography-only patients developed a MACE event, including cardiac death, target-lesion MI, or ischemia-driven target-lesion revascularization. The adjusted HR was 0.71 (95% CI, 0.51-0.98; P = .035) in favor of OCT.
Meta-analysis, trials to date
The meta-analysis presented by Dr. Stone included ILUMIEN-4, OCTOBER, and 18 earlier outcomes trials comparing PCI guided by IVI, either OCT or IVUS, and angiography-only PCI. It covered 12,428 patients with chronic or acute coronary disease and followed them a mean of 26 months; the longest follow-up was 5 years. They were assigned to IVI-guided or angiography-only PCI, 7,038 and 5,390 patients, respectively.
Dr. Stone and colleagues conducted a network meta-analysis of the 20 studies, that is, a combined analysis that allowed both direct and indirect comparisons of standard angiography-only procedures to each of the other studied comparator interventions including OCT, IVUS, and either OCT or IVUS. They then derived network-estimate odds ratios for IVI-guided PCI vs angiography-only procedures.
“Hopefully, this will impact the guidelines,” Dr. Stone said of the meta-analysis. Procedures guided by IVI might become more common in clinical practice if they were to garner a Class-I guideline recommendation, the strongest recommendation category.
“That would make a difference, but we’d also need to work to remove impediments to increasing intravascular imaging guidance” for most patients undergoing PCI, he said, referring to challenges in obtaining reimbursement for IVI-guided PCI and training enough operators to handle the projected demand.
ILUMIEN-4 was funded by Abbott. OCTOBER was supported by grants from Abbott Vascular, St. Jude Medical, and Aarhus University. The network meta-analysis received statistical support from Abbott. Dr. Ali disclosed institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems, Medtronic, the National Institutes of Health, Opsens Medical, Philips, and Teleflex; consulting fees from Astra Zeneca, Philips, Shockwave; and holding equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr. Holm and Dr. Bhatt reported numerous conflicts of interest. Dr. Andreasen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A routine role for intravascular imaging (IVI) guidance for percutaneous coronary intervention (PCI) has long been favored by many of the technology’s researchers and enthusiasts. Now evidence from large, randomized trials may be catching up with such aspirations, though not without caveats.
One way IVI guidance may achieve that, the research suggests, albeit more speculatively, is by cutting risk for stent thrombosis, compared with the risk associated with angiography-only PCI.
The new studies, two large randomized IVI trials plus a meta-analysis of 20 such studies, were presented at the annual congress of the European Society of Cardiology.
In one, called ILUMIEN-4, PCI guided by optical coherence tomography (OCT) was associated with fewer procedural complications and better acute results – that is, larger post-PCI minimum stent area (MSA) – than in angiography-only procedures (P < .001). Poststenting MSA, an established predictor of clinical outcomes, was the primary imaging endpoint of the trial with almost 2,500 patients.
Yet the OCT group’s greater post-PCI MSA did not translate to reduced risk for the primary clinical endpoint of 2-year target-vessel failure. Among secondary endpoints, however, stent thrombosis at some point during the follow-up was 64% less likely (P = .02) with OCT guidance than angiography-only PCI.
ILUMIEN-4, despite its neutral clinical result, still “strongly advocates” for PCI guidance by OCT, at least among patients like those in the trial, said principal investigator Ziad Ali, MD, DPhil. He based that largely on the strategy’s greater postprocedure lumen areas in the trials, which are among “the strongest independent predictors for long term outcomes,” said Dr. Ali, of St. Francis Hospital & Heart Center, Roslyn, N.Y., at a press conference on IVI trials during the ESC Congress.
Selected complex lesion type
In contrast, the OCTOBER trial, presented at the sessions back to back with ILUMIEN-4, saw OCT guidance lead to better clinical outcomes than angiography alone after PCI of bifurcation lesions, which normally can be a special challenge for operators.
In the trial, which entered about 1,200 patients with such complex lesions, the 2-year risk for major adverse cardiac events (MACE) fell 60% after OCT-guided PCI, compared with angiography-only procedures (P = .035).
The finding is novel for showing that OCT guidance in bifurcation PCI can make a significant clinical difference, said OCTOBER investigator Niels R. Holm, MD, at the same media presentation on IVI trials.
“Multiple studies have shown that OCT allows for optimization of bifurcation PCI, and our results confirm that such optimization may improve the patient’s prognosis,” said Dr. Holm of Aarhus (Denmark) University Hospital.
ILUMIEN-4 and OCTOBER, both of which prespecified the Xience (Abbott) everolimus-eluting stent for the procedures, were published in the New England Journal of Medicine in tandem with their respective presentations at the ESC sessions.
Covering the spectrum
A meta-analysis presented at the same ESC session compared IVI using either OCT or intravascular ultrasound (IVUS) with angiography-only PCI across 20 randomized trials with a total of more than 12,000 patients.
Significant outcomes for IVI guidance versus angiography alone included a 31% drop in risk for target-lesion failure, the primary endpoint. And this study, as well, showed a steep 52% reduction in risk for in-stent thrombosis with the IVI-guided approach.
And “for the first time” in IVI studies, “we demonstrated reductions in all-myocardial-infarction and all-cause death, the latter by 25%,” Gregg Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in presenting the meta-analysis. Dr. Stone is also the ILUMIEN-4 study chairperson.
“The routine use of OCT or IVUS to guide most PCI procedures will substantially improve patient event-free survival,” he predicted, “enhancing both the long-term safety and effectiveness of the procedure.”
Dr. Stone said that IVI guidance “should be standard of care, if not in all patients, then in most patients.” Part of the rationale: PCI is unlikely to be improved much further by incremental gains in drug-eluting stent design. “That technology has almost plateaued.” But there’s yet room for “substantially improved outcomes” from adjunctive treatments and techniques such as IVI guidance.
The 20 studies in the meta-analysis encompassed an array of patients and lesions both complex and noncomplex, Dr. Stone observed, including bifurcation lesions, chronic total occlusions, left-main coronary stenoses, and MI culprit lesions.
“They really covered the spectrum of PCI,” he said. “I’m not recommending that intravascular imaging be used in every single case. But I do think it should be used in the majority of patients” and be standard of care for PCI in left-main lesions and “complex coronary disease, high-risk patients, and high-risk lesions.”
Unique advantage
The IVI-guidance groups in both ILUMIEN-4 and the meta-analysis showed a significant drop in risk for stent thrombosis – that is, abrupt thrombotic vessel closure, which typically occurs in 1% or fewer PCI cases but can trigger an MI and pose a mortality risk up to 45%.
Those risk reductions are consistent with a unique IVI advantage: the ability to guide optimization of stent deployments. When formally presenting ILUMIEN-4 at the ESC sessions, Ali observed that IVUS and OCT imaging allows operators to identify and often correct less-than-ideal results of an initial stent delivery – such as residual gaps between stent struts and vessel wall – that may encroach on the lumen, with possible clinical consequences.
Such imaging, said Dr. Ali, “lets you identify tissue protrusions, malappositions, dissections, and untreated reference-segment disease” that may potentially trigger thrombosis. That makes a strong argument for giving IVI guidance a more common, perhaps even routine role in PCI procedures.
Selling routine IVI-guided PCI in practice
“I think the study results are quite clear,” said Deepak L. Bhatt, MD, MPH, as session comoderator following the OCTOBER presentation. “The challenge, though, will be convincing the average interventional cardiologist worldwide that it was specifically the imaging and not the extra care that the patient getting OCT also inherently receives.”
Did OCT’s better trial outcomes stem from IVI itself or from greater operator attentiveness to procedural results – such as, for example, more high-pressure expansions to optimize stent placement, “the sort of thing that tends to occur when invasive imaging is added on to just plain old angiography?” Dr. Bhatt asked of Lene N. Andreasen, MD, who had just presented the OCTOBER trial. “There’s no way of uncoupling the two things.”
What can be said, “at this point, to convince interventional cardiologists that the extra time, energy, expense, is truly indicated,” that the data are “sufficient to change global practice?” asked Dr. Bhatt, Mount Sinai Hospital and Icahn School of Medicine at Mount Sinai.
That remains an open question,” acknowledged Dr. Andreasen of Aarhus University Hospital. The best argument in favor of selective IVI-guided PCI is that “we actually see a clinical benefit” in the trials. “But of course, it comes with a cost. It comes with longer procedures and more contrast.” How clinical practice responds to the new data remains to be seen, she proposed.
ILUMIEN-4 and OCTOBER in detail
Conducted at 80 centers in 18 countries, ILUMIEN-4 randomly assigned patients with diabetes or complex coronary lesions to undergo PCI guided by OCT or using standard angiography only, 1,233 and 1,254 patients, respectively.
Post-PCI MSA averaged 5.72 mm2 with OCT guidance and 5.36 mm2 in the angiography-only group (P < .001).
Their rates of target-vessel failure at 2 years were not significantly different at 7.4% and 8.2%, respectively. The 2-year composite endpoint included cardiac death, target vessel–related MI, or ischemia-driven target-vessel revascularization.
Definite or probable stent thrombosis was observed over 2 years in 0.5% of the OCT group and 1.4% of those with angiography-only PCI (hazard ratio, 0.36; 95% confidence interval, 0.14-0.91; P = .02) favoring OCT.
The OCTOBER trial, conducted at 38 centers in Europe, entered 1201 patients with stable angina or acute coronary syndromes and angiographically identified complex bifurcation lesions. They involved the left-main coronary artery in about one-fifth of cases.
Patients were randomly assigned to bifurcation PCI guided by OCT or under standard angiography, 600 and 601 patients, respectively. Rates for procedure-related complications were similar at 6.8% and 5.7%, respectively.
Over a median of 2 years, 10.1% of the OCT group and 14.1% of angiography-only patients developed a MACE event, including cardiac death, target-lesion MI, or ischemia-driven target-lesion revascularization. The adjusted HR was 0.71 (95% CI, 0.51-0.98; P = .035) in favor of OCT.
Meta-analysis, trials to date
The meta-analysis presented by Dr. Stone included ILUMIEN-4, OCTOBER, and 18 earlier outcomes trials comparing PCI guided by IVI, either OCT or IVUS, and angiography-only PCI. It covered 12,428 patients with chronic or acute coronary disease and followed them a mean of 26 months; the longest follow-up was 5 years. They were assigned to IVI-guided or angiography-only PCI, 7,038 and 5,390 patients, respectively.
Dr. Stone and colleagues conducted a network meta-analysis of the 20 studies, that is, a combined analysis that allowed both direct and indirect comparisons of standard angiography-only procedures to each of the other studied comparator interventions including OCT, IVUS, and either OCT or IVUS. They then derived network-estimate odds ratios for IVI-guided PCI vs angiography-only procedures.
“Hopefully, this will impact the guidelines,” Dr. Stone said of the meta-analysis. Procedures guided by IVI might become more common in clinical practice if they were to garner a Class-I guideline recommendation, the strongest recommendation category.
“That would make a difference, but we’d also need to work to remove impediments to increasing intravascular imaging guidance” for most patients undergoing PCI, he said, referring to challenges in obtaining reimbursement for IVI-guided PCI and training enough operators to handle the projected demand.
ILUMIEN-4 was funded by Abbott. OCTOBER was supported by grants from Abbott Vascular, St. Jude Medical, and Aarhus University. The network meta-analysis received statistical support from Abbott. Dr. Ali disclosed institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems, Medtronic, the National Institutes of Health, Opsens Medical, Philips, and Teleflex; consulting fees from Astra Zeneca, Philips, Shockwave; and holding equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr. Holm and Dr. Bhatt reported numerous conflicts of interest. Dr. Andreasen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Is complete revascularization now compulsory? MULTISTARS-AMI and FIRE in context
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: Hi. This is Dr. Michelle O’Donoghue reporting for Medscape. Joining me today is Dr. Sahil Parikh, who’s a cardiologist and an interventionalist at Columbia University. He’s an associate professor of medicine.
We’ll be discussing two interesting trials that were presented at the ESC Congress here in Amsterdam. They do have the potential to be very practice-changing, so I think it’s worth talking about.
The FIRE trial
The first trial we’ll be talking about is the FIRE trial. Perhaps setting the stage, Sahil, I’d love to get your thoughts. We’ve had data in this space to suggest that, for patients with STEMI [ST-segment elevation myocardial infarction], a strategy of complete revascularization – and not only treating the culprit lesion but also treating additional lesions – may be of benefit. Where does that lead us in terms of what we didn’t know?
Sahil A. Parikh, MD: I think that the practice has moved, at least in the United States, over the past two decades, from staging percutaneous coronary interventions over 30 days from index to intervention to now trying to do patients in the same hospitalization whenever possible to achieve complete revascularization.
I think these data support not only that complete revascularization is compulsory now in these patients, but also doing it sooner rather than later, and that the benefit applies to most of the patients that we see in clinical practice. In the earlier data, the patients were relatively youthful – under Medicare age, less than 65 – and now this dataset has a median age of 80. This is more like the real-world clinical practice that most of us are encountering, and it extends the benefit, perhaps, greater than we’ve ever seen before.
O’Donoghue: The FIRE trial is interesting. As you say, it enrolled patients who were over the age of 75, where I think that some proceduralists are probably a little bit hesitant to think about complete revascularization due to concerns about any additional contrast load on their kidneys and other types of comorbidities. Of course, for any trial, there’s going to be some patient selection.
I think it’s very reassuring that even in this older patient group, a strategy of treating all the lesions – and not only in STEMI but also in non-STEMI patients – reduced cardiovascular events and mortality. I was really quite impressed by the mortality benefit.
Parikh: The mortality curve is almost surprising to me. On the other hand, it emboldens us now that we can treat these patients more completely and earlier in their clinical presentation. Certainly, we worried about contrast exposure and the duration of procedures in this older population, but it seems that the benefit that’s derived, which we saw in younger patients where we had a natural inclination to be more aggressive, extends also to this older population.
MULTISTARS AMI
O’Donoghue: To the question of timing, as you mentioned, prior to this, we had a study presented earlier this year, the BIOVASC trial, which also was suggestive that maybe earlier complete revascularization was better. But it wasn’t a significant difference, at least for the primary outcome. Now we have MULTISTARS AMI, which is very supportive of what we saw earlier this year, suggesting that complete revascularization really at the time that you’re treating the culprit may be the way to go.
Parikh: All of us, as interventionalists, are circumspect about what we might do in the middle of the night versus what we would do in the light of day. Certainly it seems clear, particularly if it’s straightforward anatomy, that taking care of it in the index procedure is not only saving contrast and fluoroscopy time, but it’s also providing a clinical benefit to the patients. That’s something that will also impact how clinicians interpret these data. Previously, there was always a question about whether we should just do it in the same hospitalization or do it at the same time. I think now, increasingly, we’re emboldened to do more in the index procedure.
O’Donoghue: When you’re thinking about nonculprit lesions and which ones to treat, do you always make that determination based on physiologic guidance of some kind? Are you using instantaneous wave-free ratio? What’s your practice?
Parikh: In the acute setting, imaging is superior for at least the assessment of which is a culprit. If you see a ruptured atherothrombotic situation on optical coherence tomography, for example, that’s fairly convincing and definitive. In the absence of that physiology, we are taught to avoid in the infarct-related artery because of potential spuriously false-negative findings.
In this situation, certainly, an imaging subgroup probably would be helpful because some of the benefit is almost certainly derived from identifying the infarct-related artery by accident – in other words, doing what you thought was the nonculprit artery, which is, in fact, the culprit. I think that probably is part of this. As somebody who uses imaging in the overwhelming number of my cases, I think that imaging would be an important surrogate to this.
Index procedure versus staged
O’Donoghue: For the operator who is coming in to do their STEMI case at 2:00 in the morning, would these data now push you toward doing complete revascularization at that time of night, or do you think that there is wiggle room in terms of interpreting these results regarding timing, where as long as you were doing it before hospital discharge and not, let’s say, 30 days out, that you may be able to derive the same benefit? What are some of the pros and cons?
Parikh: There’s definitely a fatigue factor in the middle of the night if it’s a particularly arduous intervention for the index infarct-related artery. I think there’s a human element where it may make sense just to stop and then bring the patient back in the same hospitalization. It’s clear, though, that doing complete revascularization is better and doing it sooner is better. How soon one actually does it is a judgment call, as ever.
In our practice, we’ve been pushing ourselves to get most of the patients done in their index hospitalization. If you have a left-sided culprit, the left anterior descending artery, for example, and there’s a high-grade stenosis in the circumflex, it may make sense to take care of that in the same index procedure. If, on the other hand, it’s in the right coronary artery where you have to put a new guide in and spend more time, that may be a patient whom you stage. I think those nuances will come up as interventionalists look at the subgroup analysis data more carefully.
O’Donoghue: Those are great points, and I think they also underscore that we always need to think about what type of patient was enrolled in these studies. Certainly, if you have somebody with renal dysfunction, there might be more concern about giving them a large contrast load all in one sitting, albeit hard to know whether they do or not. But spacing that out by just a couple of days would really have a big impact.
Parikh: Very often in the STEMI patient, you don’t have the benefit of knowing the creatinine. The patient will come in immediately, if not directly from the ambulance to the cath lab, and there are no laboratories at all to work with. If the patient has never been seen in the system before, you won’t know. Again, in those situations, one may have pause, particularly if it’s an older patient. I think what’s reassuring, though, is that the data are supportive of being more aggressive earlier, and certainly this is the dataset that we were looking for.
O’Donoghue: To summarize, the two key takeaways are that, one, we now have more data to support a complete revascularization strategy and even extending that now to non-STEMI patients. Two, sooner appears to be better, so ideally, all done at the time of the index procedure. I think this is very interesting science and we’ll see how it changes practice.
Thanks for joining me today. Signing off for Medscape, this is Dr. Michelle O’Donoghue.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: Hi. This is Dr. Michelle O’Donoghue reporting for Medscape. Joining me today is Dr. Sahil Parikh, who’s a cardiologist and an interventionalist at Columbia University. He’s an associate professor of medicine.
We’ll be discussing two interesting trials that were presented at the ESC Congress here in Amsterdam. They do have the potential to be very practice-changing, so I think it’s worth talking about.
The FIRE trial
The first trial we’ll be talking about is the FIRE trial. Perhaps setting the stage, Sahil, I’d love to get your thoughts. We’ve had data in this space to suggest that, for patients with STEMI [ST-segment elevation myocardial infarction], a strategy of complete revascularization – and not only treating the culprit lesion but also treating additional lesions – may be of benefit. Where does that lead us in terms of what we didn’t know?
Sahil A. Parikh, MD: I think that the practice has moved, at least in the United States, over the past two decades, from staging percutaneous coronary interventions over 30 days from index to intervention to now trying to do patients in the same hospitalization whenever possible to achieve complete revascularization.
I think these data support not only that complete revascularization is compulsory now in these patients, but also doing it sooner rather than later, and that the benefit applies to most of the patients that we see in clinical practice. In the earlier data, the patients were relatively youthful – under Medicare age, less than 65 – and now this dataset has a median age of 80. This is more like the real-world clinical practice that most of us are encountering, and it extends the benefit, perhaps, greater than we’ve ever seen before.
O’Donoghue: The FIRE trial is interesting. As you say, it enrolled patients who were over the age of 75, where I think that some proceduralists are probably a little bit hesitant to think about complete revascularization due to concerns about any additional contrast load on their kidneys and other types of comorbidities. Of course, for any trial, there’s going to be some patient selection.
I think it’s very reassuring that even in this older patient group, a strategy of treating all the lesions – and not only in STEMI but also in non-STEMI patients – reduced cardiovascular events and mortality. I was really quite impressed by the mortality benefit.
Parikh: The mortality curve is almost surprising to me. On the other hand, it emboldens us now that we can treat these patients more completely and earlier in their clinical presentation. Certainly, we worried about contrast exposure and the duration of procedures in this older population, but it seems that the benefit that’s derived, which we saw in younger patients where we had a natural inclination to be more aggressive, extends also to this older population.
MULTISTARS AMI
O’Donoghue: To the question of timing, as you mentioned, prior to this, we had a study presented earlier this year, the BIOVASC trial, which also was suggestive that maybe earlier complete revascularization was better. But it wasn’t a significant difference, at least for the primary outcome. Now we have MULTISTARS AMI, which is very supportive of what we saw earlier this year, suggesting that complete revascularization really at the time that you’re treating the culprit may be the way to go.
Parikh: All of us, as interventionalists, are circumspect about what we might do in the middle of the night versus what we would do in the light of day. Certainly it seems clear, particularly if it’s straightforward anatomy, that taking care of it in the index procedure is not only saving contrast and fluoroscopy time, but it’s also providing a clinical benefit to the patients. That’s something that will also impact how clinicians interpret these data. Previously, there was always a question about whether we should just do it in the same hospitalization or do it at the same time. I think now, increasingly, we’re emboldened to do more in the index procedure.
O’Donoghue: When you’re thinking about nonculprit lesions and which ones to treat, do you always make that determination based on physiologic guidance of some kind? Are you using instantaneous wave-free ratio? What’s your practice?
Parikh: In the acute setting, imaging is superior for at least the assessment of which is a culprit. If you see a ruptured atherothrombotic situation on optical coherence tomography, for example, that’s fairly convincing and definitive. In the absence of that physiology, we are taught to avoid in the infarct-related artery because of potential spuriously false-negative findings.
In this situation, certainly, an imaging subgroup probably would be helpful because some of the benefit is almost certainly derived from identifying the infarct-related artery by accident – in other words, doing what you thought was the nonculprit artery, which is, in fact, the culprit. I think that probably is part of this. As somebody who uses imaging in the overwhelming number of my cases, I think that imaging would be an important surrogate to this.
Index procedure versus staged
O’Donoghue: For the operator who is coming in to do their STEMI case at 2:00 in the morning, would these data now push you toward doing complete revascularization at that time of night, or do you think that there is wiggle room in terms of interpreting these results regarding timing, where as long as you were doing it before hospital discharge and not, let’s say, 30 days out, that you may be able to derive the same benefit? What are some of the pros and cons?
Parikh: There’s definitely a fatigue factor in the middle of the night if it’s a particularly arduous intervention for the index infarct-related artery. I think there’s a human element where it may make sense just to stop and then bring the patient back in the same hospitalization. It’s clear, though, that doing complete revascularization is better and doing it sooner is better. How soon one actually does it is a judgment call, as ever.
In our practice, we’ve been pushing ourselves to get most of the patients done in their index hospitalization. If you have a left-sided culprit, the left anterior descending artery, for example, and there’s a high-grade stenosis in the circumflex, it may make sense to take care of that in the same index procedure. If, on the other hand, it’s in the right coronary artery where you have to put a new guide in and spend more time, that may be a patient whom you stage. I think those nuances will come up as interventionalists look at the subgroup analysis data more carefully.
O’Donoghue: Those are great points, and I think they also underscore that we always need to think about what type of patient was enrolled in these studies. Certainly, if you have somebody with renal dysfunction, there might be more concern about giving them a large contrast load all in one sitting, albeit hard to know whether they do or not. But spacing that out by just a couple of days would really have a big impact.
Parikh: Very often in the STEMI patient, you don’t have the benefit of knowing the creatinine. The patient will come in immediately, if not directly from the ambulance to the cath lab, and there are no laboratories at all to work with. If the patient has never been seen in the system before, you won’t know. Again, in those situations, one may have pause, particularly if it’s an older patient. I think what’s reassuring, though, is that the data are supportive of being more aggressive earlier, and certainly this is the dataset that we were looking for.
O’Donoghue: To summarize, the two key takeaways are that, one, we now have more data to support a complete revascularization strategy and even extending that now to non-STEMI patients. Two, sooner appears to be better, so ideally, all done at the time of the index procedure. I think this is very interesting science and we’ll see how it changes practice.
Thanks for joining me today. Signing off for Medscape, this is Dr. Michelle O’Donoghue.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: Hi. This is Dr. Michelle O’Donoghue reporting for Medscape. Joining me today is Dr. Sahil Parikh, who’s a cardiologist and an interventionalist at Columbia University. He’s an associate professor of medicine.
We’ll be discussing two interesting trials that were presented at the ESC Congress here in Amsterdam. They do have the potential to be very practice-changing, so I think it’s worth talking about.
The FIRE trial
The first trial we’ll be talking about is the FIRE trial. Perhaps setting the stage, Sahil, I’d love to get your thoughts. We’ve had data in this space to suggest that, for patients with STEMI [ST-segment elevation myocardial infarction], a strategy of complete revascularization – and not only treating the culprit lesion but also treating additional lesions – may be of benefit. Where does that lead us in terms of what we didn’t know?
Sahil A. Parikh, MD: I think that the practice has moved, at least in the United States, over the past two decades, from staging percutaneous coronary interventions over 30 days from index to intervention to now trying to do patients in the same hospitalization whenever possible to achieve complete revascularization.
I think these data support not only that complete revascularization is compulsory now in these patients, but also doing it sooner rather than later, and that the benefit applies to most of the patients that we see in clinical practice. In the earlier data, the patients were relatively youthful – under Medicare age, less than 65 – and now this dataset has a median age of 80. This is more like the real-world clinical practice that most of us are encountering, and it extends the benefit, perhaps, greater than we’ve ever seen before.
O’Donoghue: The FIRE trial is interesting. As you say, it enrolled patients who were over the age of 75, where I think that some proceduralists are probably a little bit hesitant to think about complete revascularization due to concerns about any additional contrast load on their kidneys and other types of comorbidities. Of course, for any trial, there’s going to be some patient selection.
I think it’s very reassuring that even in this older patient group, a strategy of treating all the lesions – and not only in STEMI but also in non-STEMI patients – reduced cardiovascular events and mortality. I was really quite impressed by the mortality benefit.
Parikh: The mortality curve is almost surprising to me. On the other hand, it emboldens us now that we can treat these patients more completely and earlier in their clinical presentation. Certainly, we worried about contrast exposure and the duration of procedures in this older population, but it seems that the benefit that’s derived, which we saw in younger patients where we had a natural inclination to be more aggressive, extends also to this older population.
MULTISTARS AMI
O’Donoghue: To the question of timing, as you mentioned, prior to this, we had a study presented earlier this year, the BIOVASC trial, which also was suggestive that maybe earlier complete revascularization was better. But it wasn’t a significant difference, at least for the primary outcome. Now we have MULTISTARS AMI, which is very supportive of what we saw earlier this year, suggesting that complete revascularization really at the time that you’re treating the culprit may be the way to go.
Parikh: All of us, as interventionalists, are circumspect about what we might do in the middle of the night versus what we would do in the light of day. Certainly it seems clear, particularly if it’s straightforward anatomy, that taking care of it in the index procedure is not only saving contrast and fluoroscopy time, but it’s also providing a clinical benefit to the patients. That’s something that will also impact how clinicians interpret these data. Previously, there was always a question about whether we should just do it in the same hospitalization or do it at the same time. I think now, increasingly, we’re emboldened to do more in the index procedure.
O’Donoghue: When you’re thinking about nonculprit lesions and which ones to treat, do you always make that determination based on physiologic guidance of some kind? Are you using instantaneous wave-free ratio? What’s your practice?
Parikh: In the acute setting, imaging is superior for at least the assessment of which is a culprit. If you see a ruptured atherothrombotic situation on optical coherence tomography, for example, that’s fairly convincing and definitive. In the absence of that physiology, we are taught to avoid in the infarct-related artery because of potential spuriously false-negative findings.
In this situation, certainly, an imaging subgroup probably would be helpful because some of the benefit is almost certainly derived from identifying the infarct-related artery by accident – in other words, doing what you thought was the nonculprit artery, which is, in fact, the culprit. I think that probably is part of this. As somebody who uses imaging in the overwhelming number of my cases, I think that imaging would be an important surrogate to this.
Index procedure versus staged
O’Donoghue: For the operator who is coming in to do their STEMI case at 2:00 in the morning, would these data now push you toward doing complete revascularization at that time of night, or do you think that there is wiggle room in terms of interpreting these results regarding timing, where as long as you were doing it before hospital discharge and not, let’s say, 30 days out, that you may be able to derive the same benefit? What are some of the pros and cons?
Parikh: There’s definitely a fatigue factor in the middle of the night if it’s a particularly arduous intervention for the index infarct-related artery. I think there’s a human element where it may make sense just to stop and then bring the patient back in the same hospitalization. It’s clear, though, that doing complete revascularization is better and doing it sooner is better. How soon one actually does it is a judgment call, as ever.
In our practice, we’ve been pushing ourselves to get most of the patients done in their index hospitalization. If you have a left-sided culprit, the left anterior descending artery, for example, and there’s a high-grade stenosis in the circumflex, it may make sense to take care of that in the same index procedure. If, on the other hand, it’s in the right coronary artery where you have to put a new guide in and spend more time, that may be a patient whom you stage. I think those nuances will come up as interventionalists look at the subgroup analysis data more carefully.
O’Donoghue: Those are great points, and I think they also underscore that we always need to think about what type of patient was enrolled in these studies. Certainly, if you have somebody with renal dysfunction, there might be more concern about giving them a large contrast load all in one sitting, albeit hard to know whether they do or not. But spacing that out by just a couple of days would really have a big impact.
Parikh: Very often in the STEMI patient, you don’t have the benefit of knowing the creatinine. The patient will come in immediately, if not directly from the ambulance to the cath lab, and there are no laboratories at all to work with. If the patient has never been seen in the system before, you won’t know. Again, in those situations, one may have pause, particularly if it’s an older patient. I think what’s reassuring, though, is that the data are supportive of being more aggressive earlier, and certainly this is the dataset that we were looking for.
O’Donoghue: To summarize, the two key takeaways are that, one, we now have more data to support a complete revascularization strategy and even extending that now to non-STEMI patients. Two, sooner appears to be better, so ideally, all done at the time of the index procedure. I think this is very interesting science and we’ll see how it changes practice.
Thanks for joining me today. Signing off for Medscape, this is Dr. Michelle O’Donoghue.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group.
A version of this article first appeared on Medscape.com.
Will AI replace cardiologists and turn them into managers?
AMSTERDAM – At the Radical Health Festival Helsinki this past June, Gerhard Hindricks, MD, PhD, was challenged by a young man when he dared to look into the crystal ball. “In the middle of my presentation, a maybe 25-year-old man stood up and said, ‘Dr. Hindricks, in 10 years, we will no longer need you!’ ” Dr. Hindricks noted at the great debate event “Will Artificial Intelligence Replace Cardiologists?” held at the annual congress of the European Society of Cardiology. He subsequently had an interesting discussion with the man. In his opinion, the future role of the physician is “an unavoidable discussion for cardiovascular medicine.”
Dr. Hindricks, from the University of Leipzig (Germany), considered artificial intelligence in cardiology to be “potentially the most important topic of the congress” and suggested that “we have to be more open to introducing new technologies into our practice. I sometimes have the impression that we are neither quick nor open enough to introducing new technologies, to leaving the old and to letting the new, better technology be effective in our patients.”
Asset or threat?
AI is dramatically changing the field and the role of the physician – but it is not making cardiologists superfluous. In this respect, Dr. Hindricks; Folkert Asselbergs, MD, PhD, professor of cardiology at the Amsterdam Heart Center; and Harriette Van Spall, MD, associate professor of medicine at McMaster University in Hamilton, Ont., were unanimous: They agreed, although they assess the opportunities and risks posed by AI differently.
Dr. Asselbergs saw AI as less of a threat and more of an asset. In his opinion, a cardiology-specific speech model could be used to the advantage of both patient and physician. A medical chatbot could offer patients information and suggested readings, and it could create the patient’s self-reported medical history and medical summaries for laypersons.
For physicians, a medical chatbot could be beneficial in the creation of patient reports, the selection of relevant literature, the creation of automated laboratory orders, the review of clinical discharge reports, for consultations, and for processing the consultations, as well as for complying with guidelines.
Dr. Asselbergs considered AI’s primary advantage to be the time that it saves, which can then be used “for complex interventions, palliative care, and acute treatment.”
The advantages of AI, he said, include the following:
- Efficiency and scale of AI in data analysis
- Automation
- AI does not get tired and is not biased
- Proactive health care provision and early intervention
- Reduction in health care costs
- Remains up to date with the latest knowledge.
He sees the following disadvantages:
- Lack of human contact, empathy, and the physician-patient relationship
- Ethical implications and challenges
- The potential for AI to make incorrect diagnoses or to be influenced by bias in the training data.
Medical supervision needed
For Dr. Van Spall, AI is primarily a tool. A generative AI could create useful materials such as images, videos, text, sound, 3D models, virtual environments, notes for clinical visits, medical summaries, and answers to clinical queries. But “the use of AI can lead to misinformation and expose the patient to risk, and there are no laws regulating liability.”
Dr. Van Spall stressed that AI could greatly increase efficiency. For example, in echocardiography, chamber volumes and function can be quantified automatically. ECGs can be interpreted automatically. “Even the workload associated with reading off of screens can be reduced, compared with unsupported reading.” However, she maintained that the use of AI requires medical supervision. “AI cannot function without cardiologists,” since it has “enormous limitations.” Dr. Van Spall does not see “any way to close the gaps that cardiologists may leave in terms of knowledge, service, and communication.”
According to the American College of Cardiology, 26% of the 32,000 cardiologists in the United States are older than 61 years. “That is a net loss of 546 cardiologists per year. We must use AI to support cardiologists, not to replace them,” said Dr. Van Spall.
Cardiologists becoming supervisors?
Dr. Asselbergs saw AI as a means of creating more equality. “Nearly everyone now has a smartphone. Let’s take ultrasound via AI as an example. There are rural areas that have no access to health care. If nurses or dietitians there create an ultrasound based on AI and send pictures for medical analysis, it will really help people.”
Dr. Hindricks hypothesized that machine learning and AI will make a huge difference in the field of rare diseases. Rare diseases are massively underdiagnosed simply because they are so rare and it requires a lot of experience to recognize them. “Digital elements can significantly support this,” said Dr. Hindricks.
For Dr. Van Spall, AI could make care and treatment safer. There will be more digital tools and virtual models available during training too. “I believe that the cardiologist will continue to occupy an important role, in terms of communication and processes. I do not see this role disappearing,” she said. Efficiency and precision are so important. “To make good decisions, we also want to get in touch with the person we trust.”
For Dr. Asselbergs, the role of cardiologists will change to one of a supervisor. “More joint decision-making, more discussion with our patients: I think this is the direction we’re heading in.”
This article was translated from the Medscape German Edition.
A version of this article first appeared on Medscape.com.
AMSTERDAM – At the Radical Health Festival Helsinki this past June, Gerhard Hindricks, MD, PhD, was challenged by a young man when he dared to look into the crystal ball. “In the middle of my presentation, a maybe 25-year-old man stood up and said, ‘Dr. Hindricks, in 10 years, we will no longer need you!’ ” Dr. Hindricks noted at the great debate event “Will Artificial Intelligence Replace Cardiologists?” held at the annual congress of the European Society of Cardiology. He subsequently had an interesting discussion with the man. In his opinion, the future role of the physician is “an unavoidable discussion for cardiovascular medicine.”
Dr. Hindricks, from the University of Leipzig (Germany), considered artificial intelligence in cardiology to be “potentially the most important topic of the congress” and suggested that “we have to be more open to introducing new technologies into our practice. I sometimes have the impression that we are neither quick nor open enough to introducing new technologies, to leaving the old and to letting the new, better technology be effective in our patients.”
Asset or threat?
AI is dramatically changing the field and the role of the physician – but it is not making cardiologists superfluous. In this respect, Dr. Hindricks; Folkert Asselbergs, MD, PhD, professor of cardiology at the Amsterdam Heart Center; and Harriette Van Spall, MD, associate professor of medicine at McMaster University in Hamilton, Ont., were unanimous: They agreed, although they assess the opportunities and risks posed by AI differently.
Dr. Asselbergs saw AI as less of a threat and more of an asset. In his opinion, a cardiology-specific speech model could be used to the advantage of both patient and physician. A medical chatbot could offer patients information and suggested readings, and it could create the patient’s self-reported medical history and medical summaries for laypersons.
For physicians, a medical chatbot could be beneficial in the creation of patient reports, the selection of relevant literature, the creation of automated laboratory orders, the review of clinical discharge reports, for consultations, and for processing the consultations, as well as for complying with guidelines.
Dr. Asselbergs considered AI’s primary advantage to be the time that it saves, which can then be used “for complex interventions, palliative care, and acute treatment.”
The advantages of AI, he said, include the following:
- Efficiency and scale of AI in data analysis
- Automation
- AI does not get tired and is not biased
- Proactive health care provision and early intervention
- Reduction in health care costs
- Remains up to date with the latest knowledge.
He sees the following disadvantages:
- Lack of human contact, empathy, and the physician-patient relationship
- Ethical implications and challenges
- The potential for AI to make incorrect diagnoses or to be influenced by bias in the training data.
Medical supervision needed
For Dr. Van Spall, AI is primarily a tool. A generative AI could create useful materials such as images, videos, text, sound, 3D models, virtual environments, notes for clinical visits, medical summaries, and answers to clinical queries. But “the use of AI can lead to misinformation and expose the patient to risk, and there are no laws regulating liability.”
Dr. Van Spall stressed that AI could greatly increase efficiency. For example, in echocardiography, chamber volumes and function can be quantified automatically. ECGs can be interpreted automatically. “Even the workload associated with reading off of screens can be reduced, compared with unsupported reading.” However, she maintained that the use of AI requires medical supervision. “AI cannot function without cardiologists,” since it has “enormous limitations.” Dr. Van Spall does not see “any way to close the gaps that cardiologists may leave in terms of knowledge, service, and communication.”
According to the American College of Cardiology, 26% of the 32,000 cardiologists in the United States are older than 61 years. “That is a net loss of 546 cardiologists per year. We must use AI to support cardiologists, not to replace them,” said Dr. Van Spall.
Cardiologists becoming supervisors?
Dr. Asselbergs saw AI as a means of creating more equality. “Nearly everyone now has a smartphone. Let’s take ultrasound via AI as an example. There are rural areas that have no access to health care. If nurses or dietitians there create an ultrasound based on AI and send pictures for medical analysis, it will really help people.”
Dr. Hindricks hypothesized that machine learning and AI will make a huge difference in the field of rare diseases. Rare diseases are massively underdiagnosed simply because they are so rare and it requires a lot of experience to recognize them. “Digital elements can significantly support this,” said Dr. Hindricks.
For Dr. Van Spall, AI could make care and treatment safer. There will be more digital tools and virtual models available during training too. “I believe that the cardiologist will continue to occupy an important role, in terms of communication and processes. I do not see this role disappearing,” she said. Efficiency and precision are so important. “To make good decisions, we also want to get in touch with the person we trust.”
For Dr. Asselbergs, the role of cardiologists will change to one of a supervisor. “More joint decision-making, more discussion with our patients: I think this is the direction we’re heading in.”
This article was translated from the Medscape German Edition.
A version of this article first appeared on Medscape.com.
AMSTERDAM – At the Radical Health Festival Helsinki this past June, Gerhard Hindricks, MD, PhD, was challenged by a young man when he dared to look into the crystal ball. “In the middle of my presentation, a maybe 25-year-old man stood up and said, ‘Dr. Hindricks, in 10 years, we will no longer need you!’ ” Dr. Hindricks noted at the great debate event “Will Artificial Intelligence Replace Cardiologists?” held at the annual congress of the European Society of Cardiology. He subsequently had an interesting discussion with the man. In his opinion, the future role of the physician is “an unavoidable discussion for cardiovascular medicine.”
Dr. Hindricks, from the University of Leipzig (Germany), considered artificial intelligence in cardiology to be “potentially the most important topic of the congress” and suggested that “we have to be more open to introducing new technologies into our practice. I sometimes have the impression that we are neither quick nor open enough to introducing new technologies, to leaving the old and to letting the new, better technology be effective in our patients.”
Asset or threat?
AI is dramatically changing the field and the role of the physician – but it is not making cardiologists superfluous. In this respect, Dr. Hindricks; Folkert Asselbergs, MD, PhD, professor of cardiology at the Amsterdam Heart Center; and Harriette Van Spall, MD, associate professor of medicine at McMaster University in Hamilton, Ont., were unanimous: They agreed, although they assess the opportunities and risks posed by AI differently.
Dr. Asselbergs saw AI as less of a threat and more of an asset. In his opinion, a cardiology-specific speech model could be used to the advantage of both patient and physician. A medical chatbot could offer patients information and suggested readings, and it could create the patient’s self-reported medical history and medical summaries for laypersons.
For physicians, a medical chatbot could be beneficial in the creation of patient reports, the selection of relevant literature, the creation of automated laboratory orders, the review of clinical discharge reports, for consultations, and for processing the consultations, as well as for complying with guidelines.
Dr. Asselbergs considered AI’s primary advantage to be the time that it saves, which can then be used “for complex interventions, palliative care, and acute treatment.”
The advantages of AI, he said, include the following:
- Efficiency and scale of AI in data analysis
- Automation
- AI does not get tired and is not biased
- Proactive health care provision and early intervention
- Reduction in health care costs
- Remains up to date with the latest knowledge.
He sees the following disadvantages:
- Lack of human contact, empathy, and the physician-patient relationship
- Ethical implications and challenges
- The potential for AI to make incorrect diagnoses or to be influenced by bias in the training data.
Medical supervision needed
For Dr. Van Spall, AI is primarily a tool. A generative AI could create useful materials such as images, videos, text, sound, 3D models, virtual environments, notes for clinical visits, medical summaries, and answers to clinical queries. But “the use of AI can lead to misinformation and expose the patient to risk, and there are no laws regulating liability.”
Dr. Van Spall stressed that AI could greatly increase efficiency. For example, in echocardiography, chamber volumes and function can be quantified automatically. ECGs can be interpreted automatically. “Even the workload associated with reading off of screens can be reduced, compared with unsupported reading.” However, she maintained that the use of AI requires medical supervision. “AI cannot function without cardiologists,” since it has “enormous limitations.” Dr. Van Spall does not see “any way to close the gaps that cardiologists may leave in terms of knowledge, service, and communication.”
According to the American College of Cardiology, 26% of the 32,000 cardiologists in the United States are older than 61 years. “That is a net loss of 546 cardiologists per year. We must use AI to support cardiologists, not to replace them,” said Dr. Van Spall.
Cardiologists becoming supervisors?
Dr. Asselbergs saw AI as a means of creating more equality. “Nearly everyone now has a smartphone. Let’s take ultrasound via AI as an example. There are rural areas that have no access to health care. If nurses or dietitians there create an ultrasound based on AI and send pictures for medical analysis, it will really help people.”
Dr. Hindricks hypothesized that machine learning and AI will make a huge difference in the field of rare diseases. Rare diseases are massively underdiagnosed simply because they are so rare and it requires a lot of experience to recognize them. “Digital elements can significantly support this,” said Dr. Hindricks.
For Dr. Van Spall, AI could make care and treatment safer. There will be more digital tools and virtual models available during training too. “I believe that the cardiologist will continue to occupy an important role, in terms of communication and processes. I do not see this role disappearing,” she said. Efficiency and precision are so important. “To make good decisions, we also want to get in touch with the person we trust.”
For Dr. Asselbergs, the role of cardiologists will change to one of a supervisor. “More joint decision-making, more discussion with our patients: I think this is the direction we’re heading in.”
This article was translated from the Medscape German Edition.
A version of this article first appeared on Medscape.com.
AT ESC CONGRESS 2023
Aspirin still needed in first month after PCI: STOPDAPT-3
AMSTERDAM – Dropping aspirin and using low-dose prasugrel (Effient) alone in the initial month of treatment after percutaneous coronary intervention (PCI) failed to lower bleeding risk, compared with dual antiplatelet therapy (DAPT), and there was a signal of possible harm in terms of increased subacute stent thrombosis, in the STOPDAPT-3 trial.
“Therefore, dual antiplatelet therapy with aspirin and a P2Y12 inhibitor should still remain the standard strategy at least for 1 month after PCI,” said the trial’s lead investigator Masahiro Natsuaki, MD, Saga (Japan) University.
The STOPDAPT-3 trial was presented at the recent annual congress of the European Society of Cardiology.
Designated discussant Marco Valgimigli, MD, Cardiocentro Ticino Foundation, Lugano, Switzerland, explained that the current wisdom before this study was that aspirin withdrawal in the postacute phase after PCI (after 1 month of DAPT onwards) is associated with lower bleeding risk without affecting ischemic risk, but this STOPDAPT-3 trial is the first to look at the idea of not giving aspirin at all.
“This study is a well-designed, well-conducted trial, and the results are very clear: Dr. Valgimigli said.
He pointed out that the possible harm was not related to the coprimary cardiovascular composite endpoint, which did fulfill noninferiority, although he acknowledged the “generous” noninferiority margin.
Rather, the possible harm was related to an increase in subacute stent thrombosis, which was three times higher in the nonaspirin group (0.58% vs. 0.17%).
“While these absolute event rates are extremely low, they are unquestionably higher in the nonaspirin group,” he added.
In his presentation, Dr. Natsuaki explained that very short durations (1-3 months) of DAPT followed by P2Y12 inhibitor monotherapy has been shown to reduce bleeding events without increasing cardiovascular events, compared with longer durations of DAPT after PCI using drug-eluting stents.
However, the incidence of major bleeding events within the 1-month mandatory DAPT period after PCI remains high in clinical practice, particularly in patients with ACS or high bleeding risk.
In single-arm studies, use of prasugrel or ticagrelor (Brilinta) alone following new-generation drug-eluting stent implantation was not associated with any stent thrombosis in selected low-risk patients with or without ACS, and it is thought that removing aspirin from the DAPT regimen might reduce bleeding events early after PCI without compromising the risk of cardiovascular events. However, the efficacy and safety of this strategy has not been proven in randomized trials.
STOPDAPT-3 trial
STOPDAPT-3 investigated the efficacy and safety of prasugrel monotherapy compared with 1-month DAPT with aspirin and prasugrel in Japanese patients with ACS or high bleeding risk undergoing PCI with cobalt-chromium everolimus-eluting stents.
The study enrolled 6,002 patients with ACS or high bleeding risk who were randomly assigned to prasugrel monotherapy (3.75 mg/day; the licensed dose in Japan) or to DAPT with aspirin (81-100 mg/day) and prasugrel after a loading dose of prasugrel 20 mg in both groups.
There were two primary endpoints: major bleeding events (defined as BARC type 3 or 5) at 1 month for superiority and cardiovascular events (a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or stroke) at 1 month for noninferiority.
The major secondary endpoint was a composite of the coprimary bleeding and cardiovascular endpoints (cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, or major bleeding) at 1 month representing net clinical benefit.
Results showed that, at 1 month, the no-aspirin strategy was not superior to DAPT for the coprimary bleeding endpoint, with major bleeding events occurring in 4.47% of the prasugrel monotherapy group versus 4.71% of those on DAPT (hazard ratio, 0.95; 95% confidence interval, 0.75-1.20).
The prasugrel monotherapy strategy was noninferior to DAPT, although there was a relative 50% margin for the coprimary cardiovascular endpoint. Cardiovascular endpoints occurred in 4.12% of prasugrel monotherapy group versus 3.69% of the DAPT patients (HR, 1.12; 95% CI, 0.87-1.45; P for noninferiority = .01).
The major secondary net clinical benefit endpoint occurred in 7.14% patients in the prasugrel monotherapy group and 7.38% patients in the DAPT group, with no between-group difference, indicating a similar effect on net clinical benefit for both groups.
However, there was an excess of any coronary revascularization (1.15% vs. 0.57%) and definite or probable stent thrombosis (0.71% vs. 0.44%) in the prasugrel monotherapy group compared with the DAPT group, while definite stent thrombosis was not different between the two groups (0.47% vs. 0.37%).
In a subgroup analysis stratified by ACS and non-ACS, the excess risk for cardiovascular events in the no-aspirin group, compared with the DAPT group, was seen in patients with ACS, but not in those without ACS.
Future: Focus on dose and timing
In his discussion, Dr. Valgimigli said the implications of this trial for clinical practice were very clear: “Aspirin remains a cornerstone treatment in the periprocedural and acute phase of PCI in patients without indications for oral anticoagulation.”
However, he added that the study opens several important points for subsequent discussion.
These include the role of type and dose of P2Y12 inhibitor therapy used; specifically, he questioned whether the 3.75-mg dose of prasugrel was enough.
Dr. Valgimigli also pointed out that this study did not include a purely high bleeding risk population, and he said there was still potential to investigate periprocedure versus postprocedure aspirin administration.
The STOPDAPT-3 trial was funded by Abbott Medical Japan. Dr. Natsuaki reported receiving honoraria from Abbott Medical Japan, Daiichi Sankyo, and Bayer.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Dropping aspirin and using low-dose prasugrel (Effient) alone in the initial month of treatment after percutaneous coronary intervention (PCI) failed to lower bleeding risk, compared with dual antiplatelet therapy (DAPT), and there was a signal of possible harm in terms of increased subacute stent thrombosis, in the STOPDAPT-3 trial.
“Therefore, dual antiplatelet therapy with aspirin and a P2Y12 inhibitor should still remain the standard strategy at least for 1 month after PCI,” said the trial’s lead investigator Masahiro Natsuaki, MD, Saga (Japan) University.
The STOPDAPT-3 trial was presented at the recent annual congress of the European Society of Cardiology.
Designated discussant Marco Valgimigli, MD, Cardiocentro Ticino Foundation, Lugano, Switzerland, explained that the current wisdom before this study was that aspirin withdrawal in the postacute phase after PCI (after 1 month of DAPT onwards) is associated with lower bleeding risk without affecting ischemic risk, but this STOPDAPT-3 trial is the first to look at the idea of not giving aspirin at all.
“This study is a well-designed, well-conducted trial, and the results are very clear: Dr. Valgimigli said.
He pointed out that the possible harm was not related to the coprimary cardiovascular composite endpoint, which did fulfill noninferiority, although he acknowledged the “generous” noninferiority margin.
Rather, the possible harm was related to an increase in subacute stent thrombosis, which was three times higher in the nonaspirin group (0.58% vs. 0.17%).
“While these absolute event rates are extremely low, they are unquestionably higher in the nonaspirin group,” he added.
In his presentation, Dr. Natsuaki explained that very short durations (1-3 months) of DAPT followed by P2Y12 inhibitor monotherapy has been shown to reduce bleeding events without increasing cardiovascular events, compared with longer durations of DAPT after PCI using drug-eluting stents.
However, the incidence of major bleeding events within the 1-month mandatory DAPT period after PCI remains high in clinical practice, particularly in patients with ACS or high bleeding risk.
In single-arm studies, use of prasugrel or ticagrelor (Brilinta) alone following new-generation drug-eluting stent implantation was not associated with any stent thrombosis in selected low-risk patients with or without ACS, and it is thought that removing aspirin from the DAPT regimen might reduce bleeding events early after PCI without compromising the risk of cardiovascular events. However, the efficacy and safety of this strategy has not been proven in randomized trials.
STOPDAPT-3 trial
STOPDAPT-3 investigated the efficacy and safety of prasugrel monotherapy compared with 1-month DAPT with aspirin and prasugrel in Japanese patients with ACS or high bleeding risk undergoing PCI with cobalt-chromium everolimus-eluting stents.
The study enrolled 6,002 patients with ACS or high bleeding risk who were randomly assigned to prasugrel monotherapy (3.75 mg/day; the licensed dose in Japan) or to DAPT with aspirin (81-100 mg/day) and prasugrel after a loading dose of prasugrel 20 mg in both groups.
There were two primary endpoints: major bleeding events (defined as BARC type 3 or 5) at 1 month for superiority and cardiovascular events (a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or stroke) at 1 month for noninferiority.
The major secondary endpoint was a composite of the coprimary bleeding and cardiovascular endpoints (cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, or major bleeding) at 1 month representing net clinical benefit.
Results showed that, at 1 month, the no-aspirin strategy was not superior to DAPT for the coprimary bleeding endpoint, with major bleeding events occurring in 4.47% of the prasugrel monotherapy group versus 4.71% of those on DAPT (hazard ratio, 0.95; 95% confidence interval, 0.75-1.20).
The prasugrel monotherapy strategy was noninferior to DAPT, although there was a relative 50% margin for the coprimary cardiovascular endpoint. Cardiovascular endpoints occurred in 4.12% of prasugrel monotherapy group versus 3.69% of the DAPT patients (HR, 1.12; 95% CI, 0.87-1.45; P for noninferiority = .01).
The major secondary net clinical benefit endpoint occurred in 7.14% patients in the prasugrel monotherapy group and 7.38% patients in the DAPT group, with no between-group difference, indicating a similar effect on net clinical benefit for both groups.
However, there was an excess of any coronary revascularization (1.15% vs. 0.57%) and definite or probable stent thrombosis (0.71% vs. 0.44%) in the prasugrel monotherapy group compared with the DAPT group, while definite stent thrombosis was not different between the two groups (0.47% vs. 0.37%).
In a subgroup analysis stratified by ACS and non-ACS, the excess risk for cardiovascular events in the no-aspirin group, compared with the DAPT group, was seen in patients with ACS, but not in those without ACS.
Future: Focus on dose and timing
In his discussion, Dr. Valgimigli said the implications of this trial for clinical practice were very clear: “Aspirin remains a cornerstone treatment in the periprocedural and acute phase of PCI in patients without indications for oral anticoagulation.”
However, he added that the study opens several important points for subsequent discussion.
These include the role of type and dose of P2Y12 inhibitor therapy used; specifically, he questioned whether the 3.75-mg dose of prasugrel was enough.
Dr. Valgimigli also pointed out that this study did not include a purely high bleeding risk population, and he said there was still potential to investigate periprocedure versus postprocedure aspirin administration.
The STOPDAPT-3 trial was funded by Abbott Medical Japan. Dr. Natsuaki reported receiving honoraria from Abbott Medical Japan, Daiichi Sankyo, and Bayer.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Dropping aspirin and using low-dose prasugrel (Effient) alone in the initial month of treatment after percutaneous coronary intervention (PCI) failed to lower bleeding risk, compared with dual antiplatelet therapy (DAPT), and there was a signal of possible harm in terms of increased subacute stent thrombosis, in the STOPDAPT-3 trial.
“Therefore, dual antiplatelet therapy with aspirin and a P2Y12 inhibitor should still remain the standard strategy at least for 1 month after PCI,” said the trial’s lead investigator Masahiro Natsuaki, MD, Saga (Japan) University.
The STOPDAPT-3 trial was presented at the recent annual congress of the European Society of Cardiology.
Designated discussant Marco Valgimigli, MD, Cardiocentro Ticino Foundation, Lugano, Switzerland, explained that the current wisdom before this study was that aspirin withdrawal in the postacute phase after PCI (after 1 month of DAPT onwards) is associated with lower bleeding risk without affecting ischemic risk, but this STOPDAPT-3 trial is the first to look at the idea of not giving aspirin at all.
“This study is a well-designed, well-conducted trial, and the results are very clear: Dr. Valgimigli said.
He pointed out that the possible harm was not related to the coprimary cardiovascular composite endpoint, which did fulfill noninferiority, although he acknowledged the “generous” noninferiority margin.
Rather, the possible harm was related to an increase in subacute stent thrombosis, which was three times higher in the nonaspirin group (0.58% vs. 0.17%).
“While these absolute event rates are extremely low, they are unquestionably higher in the nonaspirin group,” he added.
In his presentation, Dr. Natsuaki explained that very short durations (1-3 months) of DAPT followed by P2Y12 inhibitor monotherapy has been shown to reduce bleeding events without increasing cardiovascular events, compared with longer durations of DAPT after PCI using drug-eluting stents.
However, the incidence of major bleeding events within the 1-month mandatory DAPT period after PCI remains high in clinical practice, particularly in patients with ACS or high bleeding risk.
In single-arm studies, use of prasugrel or ticagrelor (Brilinta) alone following new-generation drug-eluting stent implantation was not associated with any stent thrombosis in selected low-risk patients with or without ACS, and it is thought that removing aspirin from the DAPT regimen might reduce bleeding events early after PCI without compromising the risk of cardiovascular events. However, the efficacy and safety of this strategy has not been proven in randomized trials.
STOPDAPT-3 trial
STOPDAPT-3 investigated the efficacy and safety of prasugrel monotherapy compared with 1-month DAPT with aspirin and prasugrel in Japanese patients with ACS or high bleeding risk undergoing PCI with cobalt-chromium everolimus-eluting stents.
The study enrolled 6,002 patients with ACS or high bleeding risk who were randomly assigned to prasugrel monotherapy (3.75 mg/day; the licensed dose in Japan) or to DAPT with aspirin (81-100 mg/day) and prasugrel after a loading dose of prasugrel 20 mg in both groups.
There were two primary endpoints: major bleeding events (defined as BARC type 3 or 5) at 1 month for superiority and cardiovascular events (a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or stroke) at 1 month for noninferiority.
The major secondary endpoint was a composite of the coprimary bleeding and cardiovascular endpoints (cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, or major bleeding) at 1 month representing net clinical benefit.
Results showed that, at 1 month, the no-aspirin strategy was not superior to DAPT for the coprimary bleeding endpoint, with major bleeding events occurring in 4.47% of the prasugrel monotherapy group versus 4.71% of those on DAPT (hazard ratio, 0.95; 95% confidence interval, 0.75-1.20).
The prasugrel monotherapy strategy was noninferior to DAPT, although there was a relative 50% margin for the coprimary cardiovascular endpoint. Cardiovascular endpoints occurred in 4.12% of prasugrel monotherapy group versus 3.69% of the DAPT patients (HR, 1.12; 95% CI, 0.87-1.45; P for noninferiority = .01).
The major secondary net clinical benefit endpoint occurred in 7.14% patients in the prasugrel monotherapy group and 7.38% patients in the DAPT group, with no between-group difference, indicating a similar effect on net clinical benefit for both groups.
However, there was an excess of any coronary revascularization (1.15% vs. 0.57%) and definite or probable stent thrombosis (0.71% vs. 0.44%) in the prasugrel monotherapy group compared with the DAPT group, while definite stent thrombosis was not different between the two groups (0.47% vs. 0.37%).
In a subgroup analysis stratified by ACS and non-ACS, the excess risk for cardiovascular events in the no-aspirin group, compared with the DAPT group, was seen in patients with ACS, but not in those without ACS.
Future: Focus on dose and timing
In his discussion, Dr. Valgimigli said the implications of this trial for clinical practice were very clear: “Aspirin remains a cornerstone treatment in the periprocedural and acute phase of PCI in patients without indications for oral anticoagulation.”
However, he added that the study opens several important points for subsequent discussion.
These include the role of type and dose of P2Y12 inhibitor therapy used; specifically, he questioned whether the 3.75-mg dose of prasugrel was enough.
Dr. Valgimigli also pointed out that this study did not include a purely high bleeding risk population, and he said there was still potential to investigate periprocedure versus postprocedure aspirin administration.
The STOPDAPT-3 trial was funded by Abbott Medical Japan. Dr. Natsuaki reported receiving honoraria from Abbott Medical Japan, Daiichi Sankyo, and Bayer.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Parity for prompt and staged STEMI complete revascularization: MULTISTARS-AMI
The conclusion comes from a randomized outcomes trial of 840 patients that compared prompt same-session CR with a staged procedure carried out weeks later.
That CR is a worthy goal in such cases is largely settled, unlike the question of when to pursue revascularization of nonculprit lesions for best results. So the timing varies in practice, often depending on the patient’s clinical stability, risk status, or practical issues like cath lab resources or personnel.
The new trial, MULTISTARS-AMI, supports that kind of flexibility as safe in practice. Immediate, same-session CR led to a 48% drop in risk for a broad composite primary endpoint at 1 year, compared with staged CR an average of 37 days later. The finding was significant for noninferiority in the study’s primary analysis and secondarily, was significant for superiority (P < .001 in both cases).
The composite endpoint included death from any cause, MI, stroke, unplanned ischemia-driven revascularization, or heart failure (HF) hospitalization.
CR’s immediate effect on outcomes was numerically pronounced, but because it was prespecified as a noninferiority trial, MULTISTARS-AMI could show only that the strategy is comparable to staged CR, emphasized Barbara E. Stähli, MD, MPH, MBA, at a press conference.
Still, it appears to be the first trial of its kind to show that operators and patients can safely choose either percutaneous coronary intervention (PCI) approach and attain similar outcomes, said Dr. Stähli, University Hospital Zurich, MULTISTARS-AMI’s principal investigator.
Not only were the two approaches similar with respect to safety, she noted, but immediate CR seemed to require less use of contrast agent and fluoroscopy. “And you have only one procedure, so there’s only one arterial puncture.”
Dr. Stähli formally presented MULTISTARS-AMI at the annual congress of the European Society of Cardiology, held in Amsterdam. She is also lead author on its publication in the New England Journal of Medicine.
After her presentation, invited discussant Robert A. Byrne, PhD, MD, MB, BCh, said that the trial’s central message is that STEMI patients with MVD having primary PCI “should undergo complete revascularization within the first 45 days, with the timing of the non–infarct-related artery procedure individualized according to clinical risk and logistical considerations.”
Still, the trial “provides evidence, but not strong evidence, of benefits with routine immediate PCI during the index procedure as compared with staged outpatient PCI,” said Dr. Byrne, Mater Private Hospital and RCSI University of Medicine and Health Sciences, Dublin.
MULTISTARS-AMI randomly assigned patients at 37 European sites to undergo same-session CR (418 patients) or CR staged 19-45 days later (422 patients).
Rates for the primary endpoint at 1 year were 8.5% and 16.3%, respectively, for a risk ratio of 0.52 (95% confidence interval, 0.38-0.72). The difference was driven by fewer instances of nonfatal MI, 0.36 (95% CI, 0.16-0.80), and unplanned ischemia-driven revascularization, 0.42 (95% CI, 0.24-0.74), in the immediate-CR group.
The wee hours
Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health System, New York, said that, in general at his center, patients who are stable with a good primary PCI outcome and whose lesions aren’t very high risk are often discharged to return later for the staged CR procedure.
But after the new insights from MULTISTARS-AMI, Dr. Rao, who is not connected to the study, said in an interview that immediate same-session CR is indeed likely preferable to staged CR performed weeks later.
Same-session CR, however, may not always be practical or wise, he observed. For example, some patients with STEMI can be pretty sick with MVD that involves very complex lesions that might be better handled later.
“The wee hours of the night may not be the best time to tackle such complex lesions,” Dr. Rao observed. If confronted with, for example, a bifurcation that requires a complex procedure, “2 o’clock in the morning is probably not the best time to address something like that.”
So the trial’s more realistic translational message, he proposed, may be to perform CR after the primary PCI but during the same hospitalization, “unless there are some real mitigating circumstances.”
The trial was supported by Boston Scientific. Dr. Stähli had no disclosures. Dr. Byrne discloses research funding to his institution from Abbott Vascular, Translumina, Biosensors, and Boston Scientific. Dr. Rao had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The conclusion comes from a randomized outcomes trial of 840 patients that compared prompt same-session CR with a staged procedure carried out weeks later.
That CR is a worthy goal in such cases is largely settled, unlike the question of when to pursue revascularization of nonculprit lesions for best results. So the timing varies in practice, often depending on the patient’s clinical stability, risk status, or practical issues like cath lab resources or personnel.
The new trial, MULTISTARS-AMI, supports that kind of flexibility as safe in practice. Immediate, same-session CR led to a 48% drop in risk for a broad composite primary endpoint at 1 year, compared with staged CR an average of 37 days later. The finding was significant for noninferiority in the study’s primary analysis and secondarily, was significant for superiority (P < .001 in both cases).
The composite endpoint included death from any cause, MI, stroke, unplanned ischemia-driven revascularization, or heart failure (HF) hospitalization.
CR’s immediate effect on outcomes was numerically pronounced, but because it was prespecified as a noninferiority trial, MULTISTARS-AMI could show only that the strategy is comparable to staged CR, emphasized Barbara E. Stähli, MD, MPH, MBA, at a press conference.
Still, it appears to be the first trial of its kind to show that operators and patients can safely choose either percutaneous coronary intervention (PCI) approach and attain similar outcomes, said Dr. Stähli, University Hospital Zurich, MULTISTARS-AMI’s principal investigator.
Not only were the two approaches similar with respect to safety, she noted, but immediate CR seemed to require less use of contrast agent and fluoroscopy. “And you have only one procedure, so there’s only one arterial puncture.”
Dr. Stähli formally presented MULTISTARS-AMI at the annual congress of the European Society of Cardiology, held in Amsterdam. She is also lead author on its publication in the New England Journal of Medicine.
After her presentation, invited discussant Robert A. Byrne, PhD, MD, MB, BCh, said that the trial’s central message is that STEMI patients with MVD having primary PCI “should undergo complete revascularization within the first 45 days, with the timing of the non–infarct-related artery procedure individualized according to clinical risk and logistical considerations.”
Still, the trial “provides evidence, but not strong evidence, of benefits with routine immediate PCI during the index procedure as compared with staged outpatient PCI,” said Dr. Byrne, Mater Private Hospital and RCSI University of Medicine and Health Sciences, Dublin.
MULTISTARS-AMI randomly assigned patients at 37 European sites to undergo same-session CR (418 patients) or CR staged 19-45 days later (422 patients).
Rates for the primary endpoint at 1 year were 8.5% and 16.3%, respectively, for a risk ratio of 0.52 (95% confidence interval, 0.38-0.72). The difference was driven by fewer instances of nonfatal MI, 0.36 (95% CI, 0.16-0.80), and unplanned ischemia-driven revascularization, 0.42 (95% CI, 0.24-0.74), in the immediate-CR group.
The wee hours
Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health System, New York, said that, in general at his center, patients who are stable with a good primary PCI outcome and whose lesions aren’t very high risk are often discharged to return later for the staged CR procedure.
But after the new insights from MULTISTARS-AMI, Dr. Rao, who is not connected to the study, said in an interview that immediate same-session CR is indeed likely preferable to staged CR performed weeks later.
Same-session CR, however, may not always be practical or wise, he observed. For example, some patients with STEMI can be pretty sick with MVD that involves very complex lesions that might be better handled later.
“The wee hours of the night may not be the best time to tackle such complex lesions,” Dr. Rao observed. If confronted with, for example, a bifurcation that requires a complex procedure, “2 o’clock in the morning is probably not the best time to address something like that.”
So the trial’s more realistic translational message, he proposed, may be to perform CR after the primary PCI but during the same hospitalization, “unless there are some real mitigating circumstances.”
The trial was supported by Boston Scientific. Dr. Stähli had no disclosures. Dr. Byrne discloses research funding to his institution from Abbott Vascular, Translumina, Biosensors, and Boston Scientific. Dr. Rao had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The conclusion comes from a randomized outcomes trial of 840 patients that compared prompt same-session CR with a staged procedure carried out weeks later.
That CR is a worthy goal in such cases is largely settled, unlike the question of when to pursue revascularization of nonculprit lesions for best results. So the timing varies in practice, often depending on the patient’s clinical stability, risk status, or practical issues like cath lab resources or personnel.
The new trial, MULTISTARS-AMI, supports that kind of flexibility as safe in practice. Immediate, same-session CR led to a 48% drop in risk for a broad composite primary endpoint at 1 year, compared with staged CR an average of 37 days later. The finding was significant for noninferiority in the study’s primary analysis and secondarily, was significant for superiority (P < .001 in both cases).
The composite endpoint included death from any cause, MI, stroke, unplanned ischemia-driven revascularization, or heart failure (HF) hospitalization.
CR’s immediate effect on outcomes was numerically pronounced, but because it was prespecified as a noninferiority trial, MULTISTARS-AMI could show only that the strategy is comparable to staged CR, emphasized Barbara E. Stähli, MD, MPH, MBA, at a press conference.
Still, it appears to be the first trial of its kind to show that operators and patients can safely choose either percutaneous coronary intervention (PCI) approach and attain similar outcomes, said Dr. Stähli, University Hospital Zurich, MULTISTARS-AMI’s principal investigator.
Not only were the two approaches similar with respect to safety, she noted, but immediate CR seemed to require less use of contrast agent and fluoroscopy. “And you have only one procedure, so there’s only one arterial puncture.”
Dr. Stähli formally presented MULTISTARS-AMI at the annual congress of the European Society of Cardiology, held in Amsterdam. She is also lead author on its publication in the New England Journal of Medicine.
After her presentation, invited discussant Robert A. Byrne, PhD, MD, MB, BCh, said that the trial’s central message is that STEMI patients with MVD having primary PCI “should undergo complete revascularization within the first 45 days, with the timing of the non–infarct-related artery procedure individualized according to clinical risk and logistical considerations.”
Still, the trial “provides evidence, but not strong evidence, of benefits with routine immediate PCI during the index procedure as compared with staged outpatient PCI,” said Dr. Byrne, Mater Private Hospital and RCSI University of Medicine and Health Sciences, Dublin.
MULTISTARS-AMI randomly assigned patients at 37 European sites to undergo same-session CR (418 patients) or CR staged 19-45 days later (422 patients).
Rates for the primary endpoint at 1 year were 8.5% and 16.3%, respectively, for a risk ratio of 0.52 (95% confidence interval, 0.38-0.72). The difference was driven by fewer instances of nonfatal MI, 0.36 (95% CI, 0.16-0.80), and unplanned ischemia-driven revascularization, 0.42 (95% CI, 0.24-0.74), in the immediate-CR group.
The wee hours
Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health System, New York, said that, in general at his center, patients who are stable with a good primary PCI outcome and whose lesions aren’t very high risk are often discharged to return later for the staged CR procedure.
But after the new insights from MULTISTARS-AMI, Dr. Rao, who is not connected to the study, said in an interview that immediate same-session CR is indeed likely preferable to staged CR performed weeks later.
Same-session CR, however, may not always be practical or wise, he observed. For example, some patients with STEMI can be pretty sick with MVD that involves very complex lesions that might be better handled later.
“The wee hours of the night may not be the best time to tackle such complex lesions,” Dr. Rao observed. If confronted with, for example, a bifurcation that requires a complex procedure, “2 o’clock in the morning is probably not the best time to address something like that.”
So the trial’s more realistic translational message, he proposed, may be to perform CR after the primary PCI but during the same hospitalization, “unless there are some real mitigating circumstances.”
The trial was supported by Boston Scientific. Dr. Stähli had no disclosures. Dr. Byrne discloses research funding to his institution from Abbott Vascular, Translumina, Biosensors, and Boston Scientific. Dr. Rao had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
FIRE a win for physiology-guided MI complete revascularization in older patients
(MVD) in a large, randomized trial.
In the study with more than 1,400 patients, CR was guided by assessments of the functional effect of coronary lesions other than the MI culprit, a process that selects or excludes the lesions, regardless of angiographic profile, as targets for percutaneous coronary intervention (PCI).
Such physiology-guided CR led to a significant 27% drop in risk for a composite primary endpoint over 1 year in the trial, called FIRE (Functional Assessment in Elderly MI Patients with Multivessel Disease), compared with the culprit-only approach. The endpoint included death, MI, stroke, or ischemia-driven revascularization.
Risk for cardiovascular (CV) death or MI fell by 36% in the trial, and all-cause mortality declined 30%. The differences were significant, although the study wasn’t powered for those secondary endpoints. Safety outcomes were similar for the two revascularization approaches.
FIRE was noteworthy for entering only patients with ST-segment elevation or non–ST-segment elevation MI (STEMI or NSTEMI) who were age 75 years or older, a higher-risk age group poorly represented in earlier CR trials. Such patients in practice are usually managed with the culprit lesion–only approach because of a lack of good evidence supporting CR, observed Simone Biscaglia, MD, the study’s principal investigator.
“This is the first trial actually showing a benefit” from physiology-guided CR in older patients with acute MI that is similar to what the strategy can offer younger patients, said Dr. Biscaglia, from Azienda Ospedaliera Universitaria S. Anna, Ferrara, Italy.
Biscaglia made the comments at a media briefing on FIRE held during the annual congress of the European Society of Cardiology, where he presented the study. He is also lead author on its publication in the New England Journal of Medicine.
“This is a remarkable trial that adds substantially to prior studies that examined the topic of complete versus culprit-only revascularization,” Deepak L. Bhatt, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
It shows “quite clearly” that physiology-guided CR is superior to the culprit-only approach in patients with acute MI, said Dr. Bhatt, who is also director of Mount Sinai Heart at Mount Sinai Hospital and not connected to FIRE.
The primary findings applied to a range of different patient subgroups, including those older than 80. That’s important, he said, because “it is sometimes incorrectly assumed that patients who are older may not benefit from complete revascularization in this setting.”
And the trial’s finding of reduced risk for CV death or MI in the CR group “really should make the complete revascularization approach the standard of care in MI patients without contraindications,” Dr. Bhatt said. And certainly, “age per se should no longer be considered a contraindication.”
“First and foremost, the FIRE trial confirms the benefit of complete revascularization that has been observed in previous trials and provides additional evidence for this approach in older patients,” wrote the author of an editorial accompanying the published report.
The mortality reduction with CR at 1 years “is particularly notable” and underscores that CR should be considered in all patients with acute MI, “regardless of age,” wrote Shamir R. Mehta, MD, McMaster University, Hamilton, Ont., and Hamilton Health Sciences.
Dr. Mehta was principal investigator for the 2019 COMPLETE trial, which made the case for CR, guided by standard angiography, in patients with MVD and STEMI; their age averaged about 62 years.
FIRE definitely ought to sway practice toward greater use of physiology-guided CR regardless of age, observed Vijay Kunadian, MBBS, MD, invited discussant for the Biscaglia presentation. “My oldest patient is 98,” she said, “and it is beneficial without a doubt.”
But Dr. Kunadian, from Newcastle (England) University, said that the trial results can’t be generalized to all older patients. That’s because their outcomes after CR could vary depending on, for example, their different frailties or comorbidities, cognition, or CV history. “So, there is an absolute need to individualize care.”
FIRE enrolled patients 75 years or older with MVD, about 64% male, who had been admitted with acute STEMI or NSTEMI at 34 sites in Italy, Spain, and Poland. All underwent successful culprit-lesion PCI using, as “strongly” recommended, the same model of sirolimus-eluting stent.
Patients were randomly assigned to physiology-guided CR of nonculprit lesions, at the same session or at least during the same hospitalization, or to no further revascularization: 720 and 725 patients, respectively.
The hazard ratio for the composite primary outcome, CR versus culprit-only PCI was 0.73 (95% confidence interval, 0.57-0.93; P = .01). The benefit was driven by reductions in three individual components of the primary endpoint: death, MI, and revascularization, but not stroke.
The HR for CV death or MI was 0.64 (95% CI, 0.47-0.88) and for death from any cause was 0.70 (95% CI, 0.51-0.96).
There was no significant difference in the primary safety outcome, a composite of contrast-related acute kidney injury, stroke, or Bleeding Academic Research Consortium grade 3 to 5 bleeding at 1 year. The rates were 22.5% in those assigned to CR and 20.4% in the culprit-only group.
The functional effect of individual lesions was assessed by either of two methods, crossing them with a standard “pressure wire” or by angiographic derivation of their quantitative flow ratio.
The choice was “left to operator discretion,” Dr. Biscaglia said in an interview, “because we wanted to mirror clinical practice at the participating centers.” Still, the CR primary benefit was independent of the physiology-guidance method.
FIRE’s sponsor – the nonprofit Consorzio Futuro in Ricerca, Italy – received grant support from Sahajanand Medical Technologies, Medis Medical Imaging systems, Eukon, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr. Biscaglia had no other disclosures. Dr. Mehta reported receiving grants from Abbott Vascular and personal fees from Amgen, Janssen, and Bristol Myers Squibb. Dr. Bhatt reported numerous disclosures with various companies and organizations. Dr. Kunadian had no disclosures.
A version of this article first appeared on Medscape.com.
(MVD) in a large, randomized trial.
In the study with more than 1,400 patients, CR was guided by assessments of the functional effect of coronary lesions other than the MI culprit, a process that selects or excludes the lesions, regardless of angiographic profile, as targets for percutaneous coronary intervention (PCI).
Such physiology-guided CR led to a significant 27% drop in risk for a composite primary endpoint over 1 year in the trial, called FIRE (Functional Assessment in Elderly MI Patients with Multivessel Disease), compared with the culprit-only approach. The endpoint included death, MI, stroke, or ischemia-driven revascularization.
Risk for cardiovascular (CV) death or MI fell by 36% in the trial, and all-cause mortality declined 30%. The differences were significant, although the study wasn’t powered for those secondary endpoints. Safety outcomes were similar for the two revascularization approaches.
FIRE was noteworthy for entering only patients with ST-segment elevation or non–ST-segment elevation MI (STEMI or NSTEMI) who were age 75 years or older, a higher-risk age group poorly represented in earlier CR trials. Such patients in practice are usually managed with the culprit lesion–only approach because of a lack of good evidence supporting CR, observed Simone Biscaglia, MD, the study’s principal investigator.
“This is the first trial actually showing a benefit” from physiology-guided CR in older patients with acute MI that is similar to what the strategy can offer younger patients, said Dr. Biscaglia, from Azienda Ospedaliera Universitaria S. Anna, Ferrara, Italy.
Biscaglia made the comments at a media briefing on FIRE held during the annual congress of the European Society of Cardiology, where he presented the study. He is also lead author on its publication in the New England Journal of Medicine.
“This is a remarkable trial that adds substantially to prior studies that examined the topic of complete versus culprit-only revascularization,” Deepak L. Bhatt, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
It shows “quite clearly” that physiology-guided CR is superior to the culprit-only approach in patients with acute MI, said Dr. Bhatt, who is also director of Mount Sinai Heart at Mount Sinai Hospital and not connected to FIRE.
The primary findings applied to a range of different patient subgroups, including those older than 80. That’s important, he said, because “it is sometimes incorrectly assumed that patients who are older may not benefit from complete revascularization in this setting.”
And the trial’s finding of reduced risk for CV death or MI in the CR group “really should make the complete revascularization approach the standard of care in MI patients without contraindications,” Dr. Bhatt said. And certainly, “age per se should no longer be considered a contraindication.”
“First and foremost, the FIRE trial confirms the benefit of complete revascularization that has been observed in previous trials and provides additional evidence for this approach in older patients,” wrote the author of an editorial accompanying the published report.
The mortality reduction with CR at 1 years “is particularly notable” and underscores that CR should be considered in all patients with acute MI, “regardless of age,” wrote Shamir R. Mehta, MD, McMaster University, Hamilton, Ont., and Hamilton Health Sciences.
Dr. Mehta was principal investigator for the 2019 COMPLETE trial, which made the case for CR, guided by standard angiography, in patients with MVD and STEMI; their age averaged about 62 years.
FIRE definitely ought to sway practice toward greater use of physiology-guided CR regardless of age, observed Vijay Kunadian, MBBS, MD, invited discussant for the Biscaglia presentation. “My oldest patient is 98,” she said, “and it is beneficial without a doubt.”
But Dr. Kunadian, from Newcastle (England) University, said that the trial results can’t be generalized to all older patients. That’s because their outcomes after CR could vary depending on, for example, their different frailties or comorbidities, cognition, or CV history. “So, there is an absolute need to individualize care.”
FIRE enrolled patients 75 years or older with MVD, about 64% male, who had been admitted with acute STEMI or NSTEMI at 34 sites in Italy, Spain, and Poland. All underwent successful culprit-lesion PCI using, as “strongly” recommended, the same model of sirolimus-eluting stent.
Patients were randomly assigned to physiology-guided CR of nonculprit lesions, at the same session or at least during the same hospitalization, or to no further revascularization: 720 and 725 patients, respectively.
The hazard ratio for the composite primary outcome, CR versus culprit-only PCI was 0.73 (95% confidence interval, 0.57-0.93; P = .01). The benefit was driven by reductions in three individual components of the primary endpoint: death, MI, and revascularization, but not stroke.
The HR for CV death or MI was 0.64 (95% CI, 0.47-0.88) and for death from any cause was 0.70 (95% CI, 0.51-0.96).
There was no significant difference in the primary safety outcome, a composite of contrast-related acute kidney injury, stroke, or Bleeding Academic Research Consortium grade 3 to 5 bleeding at 1 year. The rates were 22.5% in those assigned to CR and 20.4% in the culprit-only group.
The functional effect of individual lesions was assessed by either of two methods, crossing them with a standard “pressure wire” or by angiographic derivation of their quantitative flow ratio.
The choice was “left to operator discretion,” Dr. Biscaglia said in an interview, “because we wanted to mirror clinical practice at the participating centers.” Still, the CR primary benefit was independent of the physiology-guidance method.
FIRE’s sponsor – the nonprofit Consorzio Futuro in Ricerca, Italy – received grant support from Sahajanand Medical Technologies, Medis Medical Imaging systems, Eukon, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr. Biscaglia had no other disclosures. Dr. Mehta reported receiving grants from Abbott Vascular and personal fees from Amgen, Janssen, and Bristol Myers Squibb. Dr. Bhatt reported numerous disclosures with various companies and organizations. Dr. Kunadian had no disclosures.
A version of this article first appeared on Medscape.com.
(MVD) in a large, randomized trial.
In the study with more than 1,400 patients, CR was guided by assessments of the functional effect of coronary lesions other than the MI culprit, a process that selects or excludes the lesions, regardless of angiographic profile, as targets for percutaneous coronary intervention (PCI).
Such physiology-guided CR led to a significant 27% drop in risk for a composite primary endpoint over 1 year in the trial, called FIRE (Functional Assessment in Elderly MI Patients with Multivessel Disease), compared with the culprit-only approach. The endpoint included death, MI, stroke, or ischemia-driven revascularization.
Risk for cardiovascular (CV) death or MI fell by 36% in the trial, and all-cause mortality declined 30%. The differences were significant, although the study wasn’t powered for those secondary endpoints. Safety outcomes were similar for the two revascularization approaches.
FIRE was noteworthy for entering only patients with ST-segment elevation or non–ST-segment elevation MI (STEMI or NSTEMI) who were age 75 years or older, a higher-risk age group poorly represented in earlier CR trials. Such patients in practice are usually managed with the culprit lesion–only approach because of a lack of good evidence supporting CR, observed Simone Biscaglia, MD, the study’s principal investigator.
“This is the first trial actually showing a benefit” from physiology-guided CR in older patients with acute MI that is similar to what the strategy can offer younger patients, said Dr. Biscaglia, from Azienda Ospedaliera Universitaria S. Anna, Ferrara, Italy.
Biscaglia made the comments at a media briefing on FIRE held during the annual congress of the European Society of Cardiology, where he presented the study. He is also lead author on its publication in the New England Journal of Medicine.
“This is a remarkable trial that adds substantially to prior studies that examined the topic of complete versus culprit-only revascularization,” Deepak L. Bhatt, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
It shows “quite clearly” that physiology-guided CR is superior to the culprit-only approach in patients with acute MI, said Dr. Bhatt, who is also director of Mount Sinai Heart at Mount Sinai Hospital and not connected to FIRE.
The primary findings applied to a range of different patient subgroups, including those older than 80. That’s important, he said, because “it is sometimes incorrectly assumed that patients who are older may not benefit from complete revascularization in this setting.”
And the trial’s finding of reduced risk for CV death or MI in the CR group “really should make the complete revascularization approach the standard of care in MI patients without contraindications,” Dr. Bhatt said. And certainly, “age per se should no longer be considered a contraindication.”
“First and foremost, the FIRE trial confirms the benefit of complete revascularization that has been observed in previous trials and provides additional evidence for this approach in older patients,” wrote the author of an editorial accompanying the published report.
The mortality reduction with CR at 1 years “is particularly notable” and underscores that CR should be considered in all patients with acute MI, “regardless of age,” wrote Shamir R. Mehta, MD, McMaster University, Hamilton, Ont., and Hamilton Health Sciences.
Dr. Mehta was principal investigator for the 2019 COMPLETE trial, which made the case for CR, guided by standard angiography, in patients with MVD and STEMI; their age averaged about 62 years.
FIRE definitely ought to sway practice toward greater use of physiology-guided CR regardless of age, observed Vijay Kunadian, MBBS, MD, invited discussant for the Biscaglia presentation. “My oldest patient is 98,” she said, “and it is beneficial without a doubt.”
But Dr. Kunadian, from Newcastle (England) University, said that the trial results can’t be generalized to all older patients. That’s because their outcomes after CR could vary depending on, for example, their different frailties or comorbidities, cognition, or CV history. “So, there is an absolute need to individualize care.”
FIRE enrolled patients 75 years or older with MVD, about 64% male, who had been admitted with acute STEMI or NSTEMI at 34 sites in Italy, Spain, and Poland. All underwent successful culprit-lesion PCI using, as “strongly” recommended, the same model of sirolimus-eluting stent.
Patients were randomly assigned to physiology-guided CR of nonculprit lesions, at the same session or at least during the same hospitalization, or to no further revascularization: 720 and 725 patients, respectively.
The hazard ratio for the composite primary outcome, CR versus culprit-only PCI was 0.73 (95% confidence interval, 0.57-0.93; P = .01). The benefit was driven by reductions in three individual components of the primary endpoint: death, MI, and revascularization, but not stroke.
The HR for CV death or MI was 0.64 (95% CI, 0.47-0.88) and for death from any cause was 0.70 (95% CI, 0.51-0.96).
There was no significant difference in the primary safety outcome, a composite of contrast-related acute kidney injury, stroke, or Bleeding Academic Research Consortium grade 3 to 5 bleeding at 1 year. The rates were 22.5% in those assigned to CR and 20.4% in the culprit-only group.
The functional effect of individual lesions was assessed by either of two methods, crossing them with a standard “pressure wire” or by angiographic derivation of their quantitative flow ratio.
The choice was “left to operator discretion,” Dr. Biscaglia said in an interview, “because we wanted to mirror clinical practice at the participating centers.” Still, the CR primary benefit was independent of the physiology-guidance method.
FIRE’s sponsor – the nonprofit Consorzio Futuro in Ricerca, Italy – received grant support from Sahajanand Medical Technologies, Medis Medical Imaging systems, Eukon, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr. Biscaglia had no other disclosures. Dr. Mehta reported receiving grants from Abbott Vascular and personal fees from Amgen, Janssen, and Bristol Myers Squibb. Dr. Bhatt reported numerous disclosures with various companies and organizations. Dr. Kunadian had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Recall for Impella RP Flex labeling short on safety cautions
The current labeling doesn’t adequately describe safety precautions clinicians can take when patients’ clotting times are below the recommended range, according to the company and cited by the FDA.
The action applies to 65 units of the Impella RP Flex with SmartAssist, model number 1000323, first distributed by the company in November 2022, the statement notes.
Twelve related injuries but no deaths have been reported, the agency said.
The devices themselves are not part of the recall; clinicians may continue to use them, the FDA says.
However, “the use of affected catheters may cause serious adverse health consequences, including the risk of blood clots or particle deposits forming or death,” says the statement, which outlines instructions for mitigating the risk.
Abiomed says it is revising the label’s Instructions for Use section “to clarify the risk factors and recommendations related to the potential of thrombus formation or deposition.”
A version of this article first appeared on Medscape.com.
The current labeling doesn’t adequately describe safety precautions clinicians can take when patients’ clotting times are below the recommended range, according to the company and cited by the FDA.
The action applies to 65 units of the Impella RP Flex with SmartAssist, model number 1000323, first distributed by the company in November 2022, the statement notes.
Twelve related injuries but no deaths have been reported, the agency said.
The devices themselves are not part of the recall; clinicians may continue to use them, the FDA says.
However, “the use of affected catheters may cause serious adverse health consequences, including the risk of blood clots or particle deposits forming or death,” says the statement, which outlines instructions for mitigating the risk.
Abiomed says it is revising the label’s Instructions for Use section “to clarify the risk factors and recommendations related to the potential of thrombus formation or deposition.”
A version of this article first appeared on Medscape.com.
The current labeling doesn’t adequately describe safety precautions clinicians can take when patients’ clotting times are below the recommended range, according to the company and cited by the FDA.
The action applies to 65 units of the Impella RP Flex with SmartAssist, model number 1000323, first distributed by the company in November 2022, the statement notes.
Twelve related injuries but no deaths have been reported, the agency said.
The devices themselves are not part of the recall; clinicians may continue to use them, the FDA says.
However, “the use of affected catheters may cause serious adverse health consequences, including the risk of blood clots or particle deposits forming or death,” says the statement, which outlines instructions for mitigating the risk.
Abiomed says it is revising the label’s Instructions for Use section “to clarify the risk factors and recommendations related to the potential of thrombus formation or deposition.”
A version of this article first appeared on Medscape.com.